Abstract
During colonization of the respiratory tract by Bordetella pertussis, virulence factors contribute to adherence of the bacterium to the respiratory tract epithelium. In the present study, we examined the roles of the virulence factors filamentous hemagglutinin (FHA), fimbriae, pertactin (Prn), and pertussis toxin (PT) in the adherence of B. pertussis to cells of the human bronchial epithelial cell line NCI-H292 and of the laryngeal epithelial cell line HEp-2. Using B. pertussis mutant strains and purified FHA, fimbriae, Prn, and PT, we demonstrated that both fimbriae and FHA are involved in the adhesion of B. pertussis to laryngeal epithelial cells, whereas only FHA is involved in the adherence to bronchial epithelial cells. For PT and Prn, no role as adhesion factor was found. However, purified PT bound to both bronchial and laryngeal cells and as such reduced the adherence of B. pertussis to these cells. These data may imply that fimbriae play a role in infection of only the laryngeal mucosa, while FHA is the major factor in colonization of the entire respiratory tract.
Whooping cough, or pertussis, is a highly contagious infection of the respiratory tract in humans. The main causative microorganism is Bordetella pertussis, a gram-negative coccobacillus that colonizes the respiratory tract and secretes a variety of virulence factors. Some of these factors, including pertussis toxin (PT), adenylate cyclase, tracheal cytotoxin, dermonecrotic toxin, and lipopolysaccharide, exert a toxic effect. Other virulence factors, such as filamentous hemagglutinin (FHA), fimbriae, pertactin (Prn), and PT, have been reported to mediate the adherence of B. pertussis to various mammalian cells, including human epithelial cells (11, 14, 47, 49, 52) and human monocytes (17, 36). However, most of these studies pertain to the role of one or more of these virulence factors in the binding of B. pertussis to cells (4, 12, 13, 28, 32, 38, 47, 49, 51); a comparative study of the most relevant adhesion factors was lacking until now.
FHA has a filamentous structure composed of subunits with a molecular mass of 220 kDa. It is loosely associated with the outer membrane of B. pertussis and is secreted into culture medium during growth. FHA has at least three distinct binding sites: one for glycosaminoglycans (15), one for carbohydrates (35, 48), and an arginine-glycine-aspartic acid (RGD) sequence which after binding to the leukocyte response integrin/integrin-associated protein complex promotes adherence to complement receptor 3 (CD11b/CD18) (20, 36). B. pertussis produces two serologically distinct types of fimbriae, designated serotypes 2 and 3, composed of the major subunits Fim2 and Fim3, respectively, and one minor fimbrial subunit, FimD (51). The major subunits binds to glycosaminoglycans (13, 14), and the minor one binds to the α5β1-integrin very late antigen 5 (VLA-5; CD49e/CD29) (18), which is expressed on a variety of cells, including epithelial cells and human monocytes. Prn is a 69-kDa protein associated with the bacterial outer membrane. It contains an RGD sequence (23), but ligands for Prn have not been identified. PT is a hexameric protein toxin composed of five subunits (S1 to S5) with the A-B conformation, characteristic for many bacterial toxins (44). It has an A-protomer, harboring the enzymatic toxic activity, and a B-oligomer, mediating binding of the toxin to eukaryotic cells. PT binds to the glycosaminoglycan heparin (27) and has carbohydrate recognition domains which mediate adherence to lectin-like glycolipids such as lactosylceramide and gangliosides (42) which are expressed on many different types of mammalian cells. PT, like FHA, is secreted into the medium during culture of the bacteria.
In the present study, we investigated the contributions of the four virulence factors FHA, fimbriae, Prn, and PT to the adherence of these bacteria to cells of two human respiratory tract epithelial cell lines derived from bronchial and laryngeal epithelia.
MATERIALS AND METHODS
Bacteria and purified bacterial proteins.
Bacterial strains used in this study are listed in Table 1. Bacteria were cultured for 2 days on Bordet-Gengou agar plates (Difco Laboratories, Detroit, Mich.) supplemented with 15% sheep blood. Before use, bacteria were harvested from the plates and suspended in phosphate-buffered saline (PBS; pH 7.4). The number of bacteria was determined with a spectrophotometer at 600 nm and adjusted to 108 CFU/ml in HAP medium (PBS containing 3 mM glucose, 150 nM CaCl2, 500 nM MgCl2, 0.3 U of aprotinin per ml, and 0.05% [wt/vol] human serum albumin) and confirmed by colony counts after plating on Bordet-Gengou agar.
TABLE 1.
Strains of B. pertussis used in this study
| Strain | Relevant phenotype or genotype | Refer-ence |
|---|---|---|
| W28 | Wellcome 28 (wild type) | 39 |
| BBC9 | BBC8 prn::Km; Prn− derivative of W28 | 38 |
| Tohama I | Virulent phase (wild type) | 41 |
| BP338 | Nalr derivative of Tohama I | 50 |
| BP536 | Strepr derivative of 338 | 37 |
| BP-TOX6 | ptxΔ6; PT− derivative of 536 | 37 |
| BP348 | hly-1::Tn5; HLY−/Ac− derivative of 338 | 50 |
| BP349 | hly-2::Tn5; HLY− derivative of 338 | 50 |
| BP101 | fhaBΔ101; FHA− derivative of 536 | 37 |
| GR4 | fhaB; FHA− derivative of 536 | 24 |
| B52 | fim2::SacI, fim3::Kan; Fim2− Fim3− derivative of 536 | 32 |
| B316 | fimB::Kan; Fim2− Fim3− FimD− derivative of 536 | 13 |
| BP347 | vir-1::Tn5; Vir− derivative of 338 | 50 |
Purified FHA, Prn, and PT used in this study were kindly provided by R. Rappuoli (Biocine SpA, Siena, Italy); purified fimbriae were kindly provided by A. Robinson (Centre for Applied Microbiology & Research, Porton Down, United Kingdom).
Labeling of bacteria.
Bacteria were labeled with fluorescein isothiocyanate (FITC; Sigma Chemical Co., St. Louis, Mo.) as described previously (17, 53). Briefly, bacteria (108/ml) were incubated in a solution of FITC (1 mg/ml), 50 mM sodium carbonate, and 100 mM NaCl (pH 9.0) for 20 min at room temperature, washed four times, and resuspended in HAP medium to a final concentration of 108/ml. The bacteria were kept for 30 min at 37°C until use.
To assess the validity of FITC labeling, bacteria were labeled with SYTO 9 (Molecular Probes Inc., Eugene, Oreg.) as recommended by the manufacturer. Briefly, bacteria (109/ml) were incubated for 15 min in 0.01 mM SYTO 9 in saline (pH 7.4) at room temperature, washed four times, and resuspended in HAP medium to a final concentration of 108/ml. The bacteria were kept for 30 min at 37°C until use. Neither FITC nor SYTO 9 labeling of bacteria affected the viability of B. pertussis Tohama I and W28 (data not shown).
Cells.
The human bronchial epithelial cell line NCI-H292 (ATCC CRL-1848; American Tissue Culture Collection, Rockville, Md.) (6) and the human laryngeal epithelial cell line HEp-2 (ATCC CCl 23) (33) were used. The cells were cultured in RPMI 1640 (Gibco, Grand Island, N.Y.) containing 1,000 U of sodium penicillin G per ml, 50 μg of streptomycin per ml, 2 mM l-glutamine, and 10% heat-inactivated fetal calf serum (Gibco) in uncoated tissue culture flasks (Greiner Labortechnik, Frickenhausen, Germany). Before use in the adherence assay, cultures of NCI-H292 and HEp-2 cells were detached with 1 mM EDTA in PBS at 37°C for 5 min and washed, and 5 × 103 cells per well were subcultured overnight in protein-free medium (Ultradoma-PF; Boehringer Ingelheim/Biowhittaker, Verviers, France) supplemented with sodium penicillin G (1,000 U/ml) and streptomycin (50 μg/ml) in Terasaki plates (Greiner Labortechnik).
Mouse sera against B. pertussis virulence factors.
Mice (BALB/c/Rivm) were immunized on days 0 and 28 intraperitoneally with 5 μg of purified FHA, PT, fimbriae, or Prn adsorbed to aluminum hydroxide (25% Alu-Gel-S; Serva, Heidelberg, Germany) in PBS. Antibody levels in serum samples collected 2 weeks after the second immunization were determined by enzyme-linked immunosorbent assay. Immune sera contained high titers of specific antibodies (Abs) against the respective purified B. pertussis virulence factors. Titers were comparable in the endpoint titration curves, and there was no cross-reactivity between the virulence factors (data not shown).
MAbs.
The following monoclonal Abs (MAbs) against human cell surface proteins were used as culture supernatants or purified immunoglobulin (Ig). W6/32 (IgG2a; 23 μg/ml) against HLA class I antigens A, B, and C (34) and 7.5.10.1 (IgG2a; 2.8 mg/ml) against HLA class II antigens DP, DQ, and DR (22) were kindly provided by F. Koning (Department of Immunohematology and Bloodbank, University Hospital, Leiden, The Netherlands); RR1/1.1 (IgG1; 1.9 mg/ml) against ICAM-1 (CD54) was donated by T. A. Springer (Dana Farber Cancer Institute, Boston, Mass.) (40); 4B9 (IgG1; 20 mg/ml) against VCAM-1 (CD106) was obtained from J. M. Harlan (Department of Medicine, University of Washington, Seattle) (9); MY43 (IgM) against FcαR (CD89) was provided by J. G. J. van de Winkel (Department of Immunology, University Hospital, Utrecht, The Netherlands) (26); 32.2 (IgG1; 10 μg/ml; ATCC HB 469) against FcγR-I (CD64) (3), IV.3 (IgG2b; 27 μg/ml; ATCC HB 217) against FcγR-II (CD32) (25), CLB-149 (IgG2a; 50 μg/ml) against FcγR-III (CD16) (29), CLB-170 (IgG1; 250 μg/ml) against the integrin β2 subunit (CD18) (30), CLB-37 (IgG1; 100 μg/ml) against the integrin β3 subunit (CD61) (45), NKI-M9 (IgG1; 200 μg/ml) against the integrin αv subunit (CD51) of the vitronectin receptor (10), and 15A8 (IgG1; 200 μg/ml) against the integrin α4 subunit of VLA-4 (CD49d) (19) were all obtained from CLB (Amsterdam, The Netherlands); 11G5 (IgG1; 100 μg/ml) against the integrin α3 subunit of VLA-3 (CD49c), SAM-1 (IgG2b; 500 μg/ml) against the integrin α5 subunit of VLA-5 (CD49e) (46), and rat MAb G0H3 (IgG2a; 2.7 mg/ml) against the integrin α6 subunit of VLA-6 (CD49f) (43) were obtained from Sanbio (Uden, The Netherlands); and K-20 (IgG2a; 200 μg/ml) against the integrin β1 subunit (CD29) was obtained from Immunotech (Marseille, France) (2). The FITC-conjugated Abs used were G(F(ab′)2)αM-Ig/FITC (Southern Biotechnology Associates, Inc., Birmingham, Ala.) and RαRa-Ig/FITC (Dako, Glostrup, Denmark).
Adherence assay.
Adherence of the bacteria to the surface of cultured NCI-H292 or HEp-2 cells was assessed as described previously (17, 53), with some minor modifications. Overnight cultures in Terasaki plates containing 5 × 103 cells per well were washed three times with warm PBS. Next, 5 × 105 FITC-labeled bacteria in HAP medium were added to each well and incubated for 45 min at 37°C. After five washes with warm PBS to remove nonadherent bacteria, the plates were fixed for 15 min with 0.05% glutaraldehyde (Polyscience Inc., Warrington, Pa.). After two additional washes with PBS at room temperature, plates were examined with fluorescence microscopy at a magnification of ×400. The number of bacteria adherent to 100 cells was determined. In some experiments, epithelial cells with adherent unlabeled B. pertussis were fixed with glutaraldehyde (Polyscience) and next stained with 0.01 mM SYTO 9 (Molecular Probes).
Binding of purified B. pertussis virulence factors to epithelial cells.
Detached epithelial cells were suspended in PBS containing 1% formaldehyde and incubated for 10 min at room temperature. Next, the cells were washed and resuspended in PBS containing 1% bovine serum albumin (PBS-BSA), and 106 cells in 100 μl were incubated with the purified B. pertussis antigen FHA, PT, fimbriae, or Prn at different concentrations for 30 min at 37°C. Next the cells were washed twice with PBS-BSA and incubated for 30 min at room temperature with sera obtained from mice immunized with the specific virulence factors. These cells were then washed twice with PBS-BSA and incubated with G(F(ab′)2)αM-Ig/FITC (Southern Biotechnology Associates). Thereafter, the cells were washed twice with PBS-BSA before MAb binding was assessed with flow cytometry in a FACScan (Becton Dickinson, San Jose, Calif.) equipped with an argon ion laser (excitation wavelength at 488 nm; laser power, 15 mW) and band-pass filter of 530 nm (width, 30 nm). The fluorescence of 10,000 cells gated in forward and side scatter was analyzed. Cells incubated with FITC-conjugated Ab alone served as a control to set background fluorescence.
Analysis of surface antigen expression on NCI-H292 and HEp-2 cells.
About 106 detached cells were incubated for 30 min on ice with 100 μl of MAb (2 μg/ml) directed against the various surface antigens. Next, the cells were washed twice with ice cold PBS-BSA and incubated with the appropriate FITC-conjugated Ab for 30 min on ice. The cells were washed twice with PBS-BSA before MAb binding was analyzed by flow cytometry in a FACScan (Becton Dickinson).
Viability of bronchial epithelial cells.
Viability of epithelial cells after 45 min incubation with B. pertussis Tohama I was assessed by trypan blue exclusion. In addition, the viability of epithelial cells was assessed by the mitochondrion-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan (16). In short, 105 epithelial cells per well in culture medium without antibiotics were incubated with 107 bacteria per well during various time periods; then 25 μl of MTT (5 mg/ml) was added to the cells, and incubation was continued for 2 h at 37°C; next, 100 μl of a mixture of 33% N,N-dimethylformamide and 20% sodium dodecyl sulfate (pH 4.7) was added to solubilize the intracellular formed formazan overnight at 37°C. The extent of reduction of MTT to formazan was quantitated by the measurement of optical density at 550 nm minus optical density at 650 nm to correct for remaining MTT. During the incubation of B. pertussis with epithelial cells, the bacteria remained viable and increased about 200% in 24 h.
Statistical analysis.
Differences between results of the various experiments were evaluated by means of analysis of variance (ANOVA), t test, or multivariate analysis of variance (MANOVA). Results are shown as means and standard deviations (SD).
RESULTS
Adherence of B. pertussis wild-type strains to epithelial cell lines.
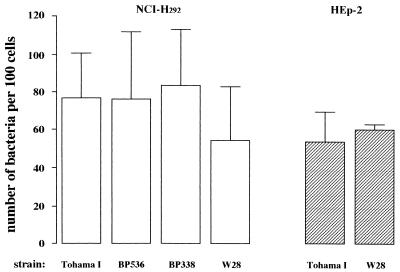
First, various wild-type strains of B. pertussis were compared for adherence to the epithelial cell lines NCI-H292 and HEp-2. The number of bacteria of the wild-type strain Tohama I that adhered to NCI-H292 cells was similar to that of its derivatives BP536 and BP338 and to that of the wild-type strain W28 (Fig. 1). Also, there was no significant difference (P > 0.05) in adherence of strains Tohama I and W28 for the two types of epithelial cells.
FIG. 1.
Adherence of different B. pertussis wild-type strains to the epithelial cell lines NCI-H292 and HEp-2. FITC-labeled B. pertussis strains were incubated with adherent cultures of both epithelial cell lines. After 45 min of incubation, the number of bacteria bound to 100 cells was determined. Values represent the mean ± SD of at least six separate experiments. Significance of difference in binding of the bacteria to these epithelial cell lines was determined by ANOVA. However, no significant differences were found.
The validity of FITC labeling of B. pertussis Tohama I and W28 was determined with the intracellular green fluorescent probe SYTO 9. Bacteria labeled with FITC or SYTO 9 and unlabeled bacteria were allowed to adhere to the epithelial cells. The numbers of bacteria of both wild-type strains labeled with FITC or SYTO 9 adherent to NCI-H292 cells or HEp-2 cells were similar and not different from the number of adherent unlabeled bacteria (Table 2).
TABLE 2.
Adherence of labeled B. pertussis Tohama I and W28, with or without FITC or SYTO 9, to NCI-H292 and HEp-2 epithelial cells
| Labeling of bacteria | Mean no. of bacteria/100 epithelial cells ± SD (n ≥ 3)
|
|||
|---|---|---|---|---|
| NCI-H292
|
HEp-2
|
|||
| Tohama I | W28 | Tohama I | W28 | |
| FITC | 82 ± 14 | 52 ± 15 | 76 ± 2 | 53 ± 14 |
| SYTO 9 | 71 ± 11 | 52 ± 11 | 62 ± 9 | 48 ± 22 |
| Nonea | 94 ± 13 | 53 ± 19 | 70 ± 6 | 52 ± 20 |
After adherence of unlabeled bacteria to epithelial cells, the bacteria were stained with SYTO 9 (see Materials and Methods).
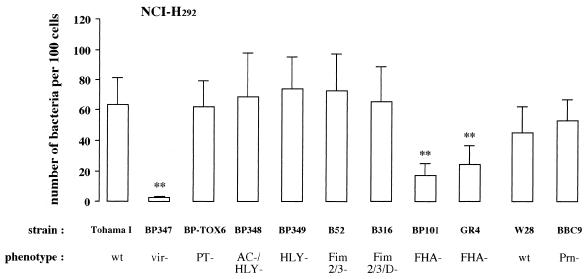
Role of virulence factors in adherence of B. pertussis to the bronchial epithelial cell line NCI-H292.
B. pertussis BP347, the avirulent derivative of strain Tohama I, did not bind the epithelial NCI-H292 cells, which indicates a role of virulence factors in the interaction between B. pertussis and epithelial cells. To assess the contribution of each virulence factor, the binding of several mutant strains to NCI-H292 was measured. The adherence of strains lacking PT (strain BP-TOX6), hemolysin activity (strain BP349), or hemolysin and adenylate cyclase activity (strain BP348) to NCI-H292 cells was not different from that of the parent strain Tohama I (Fig. 2). Adherence of strain B52, which lacks the major fimbrial subunits, and of strain B316, which lacks the major and minor fimbrial subunits, was also similar to that of Tohama I. Adherence of mutant strains BP101 and GR4, both defective in FHA, was significantly lower (P < 0.001) than that of strain Tohama I (73 and 62% reduction, respectively), while the adherence of these two mutant strains was the same but still considerably higher (P < 0.05) than that of the avirulent strain BP347. Strain BBC9, deficient in Prn, and its parental strain W28 showed approximately the same level of binding to NCI-H292, which was not significantly different from the level of adherence of strain Tohama I.
FIG. 2.
Contribution of virulence factors to the adherence of B. pertussis to epithelial NCI-H292 cells. Various B. pertussis wild-type (wt) and mutant derivative strains defective in expression of one or more virulence factors were allowed to adhere to the cell line. Values are the mean ± SD of at least six separate experiments. Difference in adherence of mutant B. pertussis strains compared to the respective wild-type strains was determined by ANOVA. Significance and P values were obtained by paired t test: ∗∗, P < 0.001 versus wild-type strain.
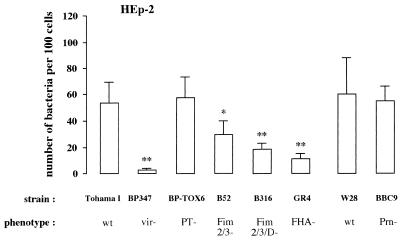
Role of virulence factors in the adherence of B. pertussis to the laryngeal epithelial cell line HEp-2.
In a similar way, the role of the virulence factors in binding of B. pertussis to HEp-2 cells was determined. Strain Tohama I adhered to HEp-2 cells, whereas the avirulent strain BP347 did not (Fig. 3). Strain GR4, lacking FHA, bound considerably less (79% reduction) to these cells than did strain Tohama I. Mutant strains B52 and B316, lacking fimbriae, also adhered less to HEp-2 cells (45 and 66% reduction, respectively), but their binding was still significant higher (P < 0.05) than that of the FHA-lacking strain GR4. In addition, strain B316, lacking both the major and minor fimbrial subunits, adhered significantly less (P < 0.05) to HEp-2 cells than strain B52, which lacks only the major fimbrial subunits. This result suggests a role for FimD in adherence. Adherence of strains BP-TOX6, deficient in PT, and BBC9, deficient in Prn, to HEp-2 cells was similar to that of their parent strains, Tohama I and W28. The levels of expression of Prn on B. pertussis Tohama I and W28 did not vary, as assessed by Western blotting (data not shown).
FIG. 3.
Contribution of virulence factors to the adherence of B. pertussis to epithelial HEp-2 cells. Various B. pertussis wild-type (wt) and mutant derivative strains defective in expression of one or more virulence factors were allowed to adhere to the cell line. Values are the mean ± SD of at least six separate experiments. Difference in adherence of mutant B. pertussis strains compared to the respective wild-type strains was determined by ANOVA. Significance and P values were obtained by paired t test: ∗, P < 0.05; ∗∗, P < 0.001 versus wild-type strain.
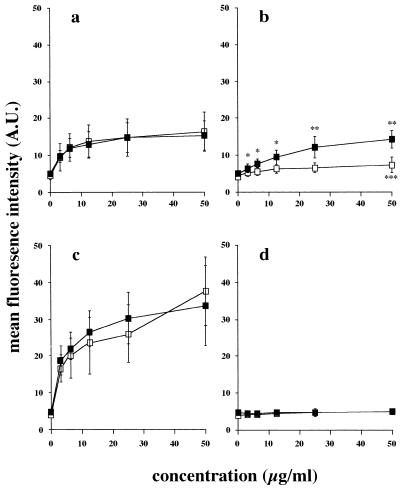
Binding of purified B. pertussis virulence factors to NCI-H292 and HEp-2 cells.
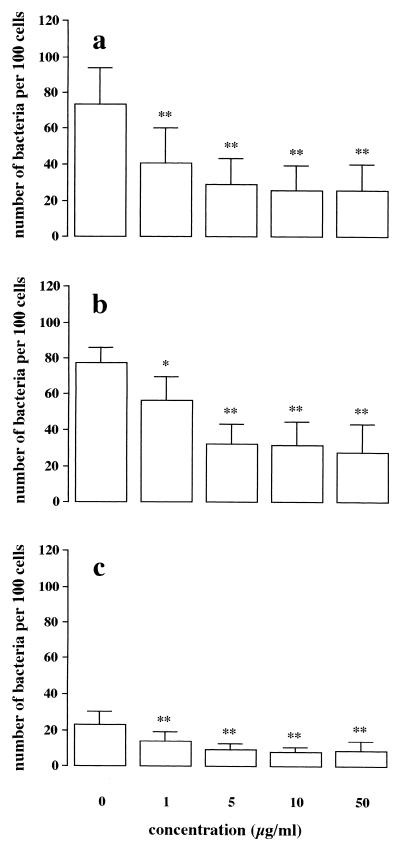
The above experiments showed differences in the involvement of virulence factors in the adhesion of B. pertussis to NCI-H292 and HEp-2 epithelial cells. To investigate the mechanisms underlying these differences, purified B. pertussis virulence factors were used to assess their ability to adhere to the surface of epithelial cells. To obtain optimal binding of purified virulence factors to detached epithelial cells, incubation must be performed at 37°C. To prevent under these conditions possible internalization of the virulence factors, cells were treated with a mild fixative. The results showed no difference in surface antigen expression on the surface of both NCI-H292 and HEp-2 cells in comparison to untreated cells by flow cytometric analysis (data not shown). Flow cytometric analysis showed that both NCI-H292 and HEp-2 cells bound purified FHA in a dose-dependent fashion (Fig. 4a). For fimbriae, binding to HEp-2 cells was significantly higher (P < 0.05) than binding to NCI-H292 cells (Fig. 4b). This binding was dose dependent for HEp-2 cells, whereas for NCI-H292 cells it was significantly increased (P < 0.05) only with a high (50 μg/ml) concentration of fimbriae. Both types of epithelial cell bound purified PT in a dose-dependent fashion (Fig. 4c), while they did not bind Prn (Fig. 4d).
FIG. 4.
Flow cytometric analysis of the binding of purified B. pertussis virulence factors to epithelial cell lines. Adherence of FHA (a), fimbriae (b), PT (c), or Prn (d) to NCI-H292 (□) or HEp-2 (■) cells was determined. Values are the mean ± SD of at least five separate experiments. Effects of concentration and virulence factor between the two epithelial cell lines were assessed by MANOVA. Significance and P values were obtained by paired t test. ∗, P < 0.05; ∗∗, P < 0.001 with respect to both epithelial cell lines used. ∗∗∗, P < 0.05 compared to no virulence factor added. AU, arbitrary units.
Expression of surface molecules on NCI-H292 and HEp-2 cells.
Because the two types of epithelial cells differed in the capacity to bind virulence factors, the expression of a variety of surface molecules on these cells was studied. Fluorescence-activated cell sorting analysis showed that cells of neither the bronchial epithelial cell line NCI-H292 nor the laryngeal epithelial cell line HEp-2 expressed HLA-II, VCAM-1, FcγR-I, FcγR-II, FcγR-III, FcαR, β2-integrin, β3-integrin, integrin α3 subunit of VLA-3, and integrin α4 subunit of VLA-4 (data not shown). The expression level of the β1-integrin family was higher on NCI-H292 cells than on HEp-2 cells (Table 3). On NCI-H292 cells, VLA-6 is the main β1-integrin expressed, whereas VLA-6 and VLA-5 are about equally expressed on HEp-2 cells. Furthermore, HEp-2 cells expressed higher levels of HLA-I molecules than did NCI-H292 cells. Both cell types equally expressed ICAM-1 and αv-integrin.
TABLE 3.
Expression of surface molecules on a human bronchial epithelial cell line (NCI-H292) and a laryngeal epithelial cell line (HEp-2) by flow cytometry
| Cell surface molecule | Mean fluorescence intensity ± SD (n ≥ 3)a
|
|
|---|---|---|
| NCI-H292 | HEp-2 | |
| HLA-I | 12.3 ± 4.0 | 21.3 ± 6.4 |
| ICAM-1 (CD54) | 10.8 ± 3.6 | 8.8 ± 2.3 |
| Integrin β1 subunit (CD29) | 35.8 ± 13.0 | 21.2 ± 3.1 |
| Integrin α5 subunit (CD49e) | 7.3 ± 1.0 | 11.0 ± 1.7 |
| Integrin α6 subunit (CD49f) | 38.3 ± 8.7 | 14.5 ± 2.7 |
| Integrin αv subunit (CD51) | 9.3 ± 3.8 | 10.7 ± 0.6 |
Background mean fluorescence intensity was 4.2 ± 0.79 (n = 10).
Role of purified PT in adherence of B. pertussis to NCI-H292 cells.
Since purified PT bound to epithelial cells and the adherence of strain BP-TOX6, lacking PT, to these cells was not reduced, the question of whether cell-bound PT would affect the adherence of B. pertussis was raised. To investigate this possibility, NCI-H292 cells were preincubated with increasing concentrations of purified PT for 30 min at 37°C, washed, and then incubated with strain Tohama I, BP-TOX6, or GR4. The results showed a dose-dependent reduction of adherence of the wild-type strain Tohama I (Fig. 5a), but the binding of strain BP-TOX6, which lacks PT, was also reduced in a dose-dependent fashion (Fig. 5b). Furthermore, the already low binding of strain GR4, lacking FHA, was further reduced after preincubation of NCI-H292 cells with purified PT (Fig. 5c).
FIG. 5.
Role of purified PT in the adherence of B. pertussis to NCI-H292 cells. NCI-H292 cells were preincubated with various concentrations of purified PT; after washing, the adherence of wild-type B. pertussis Tohama I (a) or the mutant strain BP-TOX6, lacking PT (b), or GR4, lacking FHA (c), to these cells was assessed. Values are the mean ± SD of at least five separate experiments. Difference in adherence of B. pertussis strains compared to the control (no endogenous PT added) was determined by ANOVA. Significance and P values were obtained by paired t test. ∗, P < 0.05; ∗∗, P < 0.001 versus control.
Role of B. pertussis toxins on the viability of NCI-H292 cells.
The toxic effects of B. pertussis toxins such as PT and adenylate cyclase during exposure of the bacteria to NCI-H292 cells were examined. After incubation of epithelial cells with virulent B. pertussis Tohama I for 45 min, more than 95 and about 93% of the cells remained viable, as determined by trypan blue exclusion and reduction of MTT, respectively. Similar percentages of viability were obtained when NCI-H292 cells were incubated with medium alone (>95%) or with the avirulent B. pertussis strain BP347 (89%). Incubation for up to 24 h of the epithelial cells with B. pertussis Tohama I or BP347 or with medium alone did not result in a large reduction of viable cells (76, 92, or 86% viable cells, respectively).
DISCUSSION
The major conclusions of this present study are that both fimbriae and FHA are involved in the adherence of B. pertussis to laryngeal epithelial HEp-2 cells and only FHA is involved in the adherence of these bacteria to bronchial epithelial NCI-H292 cells. These conclusions are based on our observations that mutant B. pertussis strains lacking either FHA or fimbriae adhere poorly to the laryngeal cells, whereas mutant strains lacking only FHA have reduced adherence to bronchial cells. Furthermore, it has been found that both purified FHA and purified fimbriae bind in a dose-dependent fashion to laryngeal cells, whereas FHA binds dose dependently to bronchial cells.
Both the major and minor fimbrial subunits are involved in the fimbria-mediated adherence of B. pertussis to laryngeal cells. A role for the major fimbrial subunits follows from the finding that the adherence of B. pertussis B52, which lacks the major fimbrial subunits but expresses the minor fimbrial subunit FimD, to laryngeal cells is lower than the binding of the wild-type B. pertussis Tohama I. Strain B316, which lacks all fimbrial subunits, adhered significantly less to laryngeal cells than did strain B52, indicating also a role for the minor fimbrial subunit in the adherence of B. pertussis to the laryngeal cells. It has been demonstrated that both major and minor fimbrial subunits bind to sulfated sugars (13, 14), while the minor fimbrial subunit adheres also to the β1-integrin VLA-5 (18). Therefore, the difference between bronchial and laryngeal cells in the ability to bind fimbriae, and in particular the fimbrial subunit FimD, suggests a difference in the expression of surface molecules on epithelial cells which may serve as receptors for fimbriae. Using flow cytometric analysis, we found that laryngeal cells expressed comparable levels of VLA-5 and VLA-6, whereas bronchial cells expressed lower levels of VLA-5 than of VLA-6. Thus, besides the binding of B. pertussis via fimbriae to sulfated sugars (13, 14), our results argue for a role of VLA-5 in the fimbria-mediated binding of B. pertussis to laryngeal cells.
In contrast to an earlier report describing a role for Prn in the adherence of B. pertussis to CHO cells and HeLa cells (23), no evidence was found for the contribution of Prn in the adherence of B. pertussis to either bronchial or laryngeal cells. Binding of strain BBC9, which lacks Prn, was similar to that of the wild-type strain W28, and purified Prn did not bind to either of the epithelial cell lines.
This study provides evidence that PT does not contribute to the adherence of B. pertussis to epithelial cells and even can inhibit such adhesion. This conclusion is supported by several observations. Although purified PT binds dose dependently to the surface of both epithelial cell lines, the adhesion of the PT-deficient strain BP-TOX6 to both the bronchial and laryngeal cells was similar to that of the wild-type strain Tohama I. Furthermore, preincubation of bronchial cells with purified PT inhibited the adherence of both Tohama I and BP-TOX6. The low residual adherence of the FHA deficient strain GR4 to epithelial cells was further reduced by purified PT. Since immunoelectron microscopic studies (5, 8) could not detect PT at the surface of B. pertussis, we assume that PT is released from the bacteria. Consequently, PT binds to the surface of the respiratory epithelium and interferes with the adherence of B. pertussis to these cells. How PT bound to epithelial cells eventually inhibits such bacterial adherence is not yet resolved.
FITC is a amine-reactive probe which covalently binds to α-amino groups at the N termini of proteins and ɛ-amino groups of lysines. To assess the validity of FITC labeling of B. pertussis, an intracellular green fluorescent probe (SYTO 9) was used. No significant differences were observed in the number of FITC- or SYTO 9-labeled wild-type B. pertussis strains Tohama I and W28 or unlabeled bacteria in adherence to either cell line. These data indicated that labeling of bacteria with FITC does not affect binding sites of virulence factors of B. pertussis.
To exclude toxic effects of PT and adenylate cyclase, bronchial cells were incubated with wild-type strain Tohama I. Since we observed no effect on the viability of the cells, we conclude that neither virulence factor has a toxic effect on respiratory tract epithelial cells. This observation is supported by an earlier report describing that tracheal cytotoxin instead of PT is the toxic virulence factor for primary human ciliated bronchial epithelium (52).
Various studies of the involvement of B. pertussis virulence factors in the adherence of these bacteria to host cells have been reported. These studies were performed with animal cells such as CHO (23, 37), Vero (12), baboon ciliated tracheal (12), and rabbit ciliated (31, 37) cells and with a murine infection model (14, 21, 32). Also, human-derived cell lines such as HeLa (11, 23, 28), WiDr (49), and HEp-2 (13, 38) have been used, and one report describes the interaction between B. pertussis and primary human ciliated respiratory tract epithelium (47). From these studies emerged a clear role for FHA as an adhesin of B. pertussis to epithelial cells. In none of these studies were the four virulence factors FHA, PT, fimbriae, and Prn, which are candidates to mediate bacterial adhesion, studied simultaneously under the same experimental conditions. Although it would be desirable to use primary human respiratory tract epithelial cells to study the role of B. pertussis virulence factors as possible adhesins, these epithelial cells show great morphological variance when subcultured, and the number of viable cells that can be obtained is very limited. Therefore, in the present study we used cell lines derived from human respiratory tract epithelium which maintain their surface characteristics during extensive subculture and optimally resemble the in vivo situation, especially with respect to the expression of surface molecules (1, 7).
In conclusion, the data from our study using human epithelial cell lines of different sites of the respiratory tract together with in vivo studies using mice (14, 21, 32) or rabbits (31) suggest that fimbriae play a role in a B. pertussis infection of laryngeal mucosa whereas FHA is the major factor in colonization of the entire respiratory tract. These results imply strong arguments for including fimbriae in acellular vaccines.
ACKNOWLEDGMENTS
We express our gratitude to Wouter L. W. Hazenbos for helpful discussions.
This work was financially supported by Preaventie Fonds grant 2825450.
REFERENCES
- 1.Albelda S M. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4:195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 2.Amiot M, Bernard A, Tran H C, Leca G, Kanellopoulos J M, Boumsell L. The human cell surface glycoprotein complex (gp 120,000) recognized by monoclonal antibody K20 is a component binding to phytohaemagglutinin on T cells. Scand J Immunol. 1986;23:109–118. doi: 10.1111/j.1365-3083.1986.tb01948.x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson C L, Guyre P M, Whitin J C, Ryan D H, Looney R J, Fanger M W. Monoclonal antibodies to Fc receptors for IgG on human mononuclear phagocytes—antibody characterization and induction of superoxide production in a monocyte cell line. J Biol Chem. 1986;261:12856–12864. [PubMed] [Google Scholar]
- 4.Arico B, Nuti S, Scarlato V, Rappuoli R. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proc Natl Acad Sci USA. 1993;90:9204–9208. doi: 10.1073/pnas.90.19.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashworth L A E, Dowsett A B, Irons L I, Robinson A. The location of surface antigens of Bordetella pertussis by immuno-electronmicroscopy. Dev Biol Stand. 1985;61:143–151. [PubMed] [Google Scholar]
- 6.Banks-Schlegel S P, Gazdar A F, Harris C C. Intermediate filament and cross-linked envelope expression in human lung tumor cell lines. Cancer Res. 1985;45:1187–1197. [PubMed] [Google Scholar]
- 7.Bloemen P G M, van den Tweel M C, Henricks P A J, Engels F, Wagenaar S S, Rutten A J J L, Nijkamp F P. Expression and modulation of adhesion molecules on human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1993;9:586–593. doi: 10.1165/ajrcmb/9.6.586. [DOI] [PubMed] [Google Scholar]
- 8.Blom J, Heron I, Hendley J O. Immunoelectron microscopy of antigens of Bordetella pertussis using monoclonal antibodies to agglutinogens 2 and 3, filamentous haemagglutinin, pertussis toxin, pertactin and adenylate cyclase toxin. APMIS. 1994;102:681–689. doi: 10.1111/j.1699-0463.1994.tb05220.x. [DOI] [PubMed] [Google Scholar]
- 9.Carlos T M, Schwarz B R, Kovach N L, Yee E, Rosso M, Osborn L, Chi-Rosso G, Neewman B, Lobb R, Harlan J M. Vascular cell adhesion molecule-1 (VCAM-1) mediates lymphocyte adhesion to cytokine-activated cultured human endothelial cells. Blood. 1990;76:965–970. [PubMed] [Google Scholar]
- 10.de Vries J E, Keizer G D, te Velde A A, Voordouw A, Ruiter D, Rumke P, Spits H, Figdor C G. Characterization of melanoma-associated surface antigens involved in the adhesion and motility of human melanoma cells. Int J Cancer. 1986;38:465–473. doi: 10.1002/ijc.2910380403. [DOI] [PubMed] [Google Scholar]
- 11.Ewanowich C A, Melton A R, Weiss A A, Sherburne R K, Peppler M S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989;57:2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funnel S G P, Robinson A. A novel adherence assay for Bordetella pertussis using tracheal organ cultures. FEMS Microbiol Lett. 1993;110:197–204. doi: 10.1111/j.1574-6968.1993.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 13.Geuijen C A W, Willems R J L, Mooi F R. The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect Immun. 1996;64:2657–2665. doi: 10.1128/iai.64.7.2657-2665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geuijen C A W, Willems R J L, Bongaerts M, Top J, Gielen H, Mooi F R. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65:4222–4228. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannah J H, Menozzi F D, Renauld G, Locht C, Brennan M J. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect Immun. 1994;62:5010–5019. doi: 10.1128/iai.62.11.5010-5019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 17.Hazenbos W L W, van den Berg B M, van’t Wout J W, Mooi F R, van Furth R. Virulence factors determine attachment and ingestion of nonopsonized and opsonized Bordetella pertussis by human monocytes. Infect Immun. 1994;62:4818–4824. doi: 10.1128/iai.62.11.4818-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazenbos W L W, van den Berg B M, Geuijen C A W, Mooi F R, van Furth R. Binding of FimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J Immunol. 1995;155:3972–3978. [PubMed] [Google Scholar]
- 19.Humphries M J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990;97:585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura A, Mountzouros K T, Relman D A, Falkow S, Cowell J L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990;58:7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koning F, Schreuder I, Giphart M, Bruning H. A mouse monoclonal antibody detecting a DR-related MT2-like specificity: serology and biochemistry. Hum Immunol. 1984;9:221–230. doi: 10.1016/0198-8859(84)90027-2. [DOI] [PubMed] [Google Scholar]
- 23.Leininger E, Roberts M, Kenimer F G, Charles I G, Fairweather N, Novotny P, Brennan M J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locht C, Geoffroy M, Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992;11:3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looney R J, Abraham G N, Anderson C L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986;136:1641–1647. [PubMed] [Google Scholar]
- 26.Maliszewski C R, March C J, Schoenborn M A, Gimpel S, Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990;172:1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mégret F, Alouf J E. A simple novel approach for the purification of pertussis toxin. FEMS Microbiol Lett. 1988;51:159–162. [Google Scholar]
- 28.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesion of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miedema F, Tetteroo P A T, Hesselink W G, Werner G, Spits H, Melief C J M. Both Fc receptors and lymphocyte-function-associated antigen 1 on human Tγ lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity) Eur J Immunol. 1984;14:518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- 30.Miedema F, Tetteroo P A T, Terpstra F G, Keizer G, Roos M, Weening R S, Weemaes C M R, Roos D, Melief C J M. Immunologic studies with LFA-1 and co 1-deficient lymphocytes from a patient with recurrent bacterial infections. J Immunol. 1985;134:3075–3081. [PubMed] [Google Scholar]
- 31.Mooi F R, van der Heide H G J, Walvoort H C, Brunings H, Jansen W H, Guinee P A M. The analysis of Bordetella pertussis fimbrial mutants in a rabbit model. In: Ron E Z, Rottem S, editors. Microbial surface components and toxins in relation to pathogenesis. New York, N.Y: Plenum Press; 1991. pp. 69–76. [Google Scholar]
- 32.Mooi F R, Jansen W H, Brunings H, Gielen H, van der Heide H G J, Walvoort H C, Guinee P A M. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb Pathog. 1992;12:127–135. doi: 10.1016/0882-4010(92)90115-5. [DOI] [PubMed] [Google Scholar]
- 33.Moore A E, Sabachewsky L, Wallace Toolan H. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955;15:598–602. [PubMed] [Google Scholar]
- 34.Parham P, Barnstable C J, Bodmer W F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A-B-C antigens. J Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- 35.Prasad S M, Yin Y, Rodzinski E, Tuomanen E I, Masure H R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993;61:2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 37.Relman D A, Domenighini M, Tuomanen E, Rappuoli R, Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci USA. 1989;86:2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts M, Fairweather N F, Leininger E, Pickard D, Hewlett E L, Robinson A, Hayward C, Dougan G, Charles I G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991;5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson A, Ashworth L A E, Baskerville A, Irons L I. Protection against intranasal infection of mice with Bordetella pertussis. Dev Biol Stand. 1985;61:165–172. [PubMed] [Google Scholar]
- 40.Rothlein R, Dustin M L, Marlin S D, Springer T A. A human intercellular adhesin molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 41.Sato Y, Aria H. Leukocytosis-promoting factor of Bordetella pertussis. I. Purification and characterization. Infect Immun. 1972;6:899–904. doi: 10.1128/iai.6.6.899-904.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saukkonen K, Burnette W N, Mar V L, Masure H R, Tuomanen E I. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci USA. 1992;89:118–122. doi: 10.1073/pnas.89.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonnenberg A, Modderman P W, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 44.Tamura M, Nogimori K, Murai S, Yajima M, Ito K, Katada T, Ui M, Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982;21:5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- 45.Tetteroo P A T, Lansdorp P M, Leeksma O C, von dem Borne K. Monoclonal antibodies against platelet glycoprotein IIIa. Br J Haematol. 1983;55:509–522. doi: 10.1111/j.1365-2141.1983.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 46.teVelde A A, Klomp J P G, Yard B A, de Vries J E, Figdor C. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988;140:1548–1554. [PubMed] [Google Scholar]
- 47.Tuomanen E, Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985;152:118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 48.Tuomanen E, Towbin H, Rosenfelder G, Braun D, Larson G, Hansson G, Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988;168:267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urisu A, Cowell J L, Manclark C R. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun. 1986;52:695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willems R J L, Geuijen C, van der Heide H G J, Matheson M, Robinson A, Versluis L F, Ebberink R, Theelen J, Mooi F R. Isolation of a putative fimbrial adhesin from Bordetella pertussis and the identification of its gene. Mol Microb. 1993;9:623–634. doi: 10.1111/j.1365-2958.1993.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 52.Wilson R, Read R, Thomas M, Rutman A, Harrison K, Lund V, Cookson B, Goldman W, Lambert H, Cole P. Effects of Bordetella pertussis infection on human respiratory epithelium in vivo and in vitro. Infect Immun. 1991;59:337–345. doi: 10.1128/iai.59.1.337-345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright S D, Jong M T C. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]