Abstract
Inula viscosa is a perennial herbaceous plant native to the Mediterranean Basin, which is used topically for the treatment of various diseases in folk medicine. This study aimed to evaluate the in vivo intestinal anti-inflammatory activity of the ethanolic extract of I. viscosa (EEIV) and to test its effect on a colorectal cancer cell line. EEIV was administered to rats orally and daily at 100 and 200 mg/kg body weight for 7 days, and then colitis was induced by intrarectal instillation of 2 ml of 4% (v/v) acetic acid (AA) solution. At the end of the experiment, clinical examinations of the rats were conducted by evaluating macroscopic and histological signs of colonic tissues and measuring erythrocyte sedimentation rate (ESR) and the levels of C-reactive protein, fibrinogen, myeloperoxidase (MPO), malondialdehyde (MDA) and nitric oxide (NO). Using MTS assay, the antiproliferative effect of EEIV against human colon carcinoma HT29 cells and cytotoxicity on nondifferentiated Caco-2 cell line was evaluated. EEIV significantly decreased the ESR and fibrinogen levels as compared to control colitic rats (P < 0.001). It also significantly decreased the NO, MDA, and MPO levels in the colon tissue compared with the untreated colitic group (P < 0.001). These results were confirmed by macroscopic and histological examination, which showed significant protection against AA-induced ulcerative colitis. Furthermore, EEIV at a concentration of 369.88 μg/ml did not show cytotoxicity on confluent Caco-2 cells, with significant inhibition of colorectal cancer cell (HT29) growth (EC50 = 62.39 μg/ml). These results demonstrate that EEIV plays a potential role as a pharmacological tool in the management of inflammatory bowel disease and prevention of colorectal cancer.
Keywords: Inula viscosa, ulcerative colitis, colorectal cancer, anti-inflammatory, antiproliferative
Introduction
Ulcerative colitis (UC) is one of the chronic idiopathic inflammatory bowel diseases (IBDs), and its clinical symptoms include blood in the stool, diarrhea, and abdominal pain (Kornbluth et al., 2010; Joseph et al., 2014). It is characterized by diffuse and continuous involvement of the mucosa and submucosa and begins in the rectum and extends up the colon (Kornbluth et al., 2010; Joseph et al., 2014).
UC is a complex and multifactorial disease and involves a multitude of factors such as personal genetic susceptibility, environment, intestinal microbiota, and immune response, and these factors play an important role in the pathogenesis of UC (Khor, 2011; Loddo and Romano, 2015). Nevertheless, specific pathways that lead to cellular damage in UC are not completely known. In both experimental animals and human subjects, oxidative stress has been demonstrated to play a critical role in the pathophysiology of UC (Balmus et al., 2016). Moreover, during the inflammation process, UC is associated with the overproduction of reactive oxygen species (ROS) and reactive nitrogen species (Rezaie et al., 2007).
In previous studies, different biomarkers have been explored in patients with IBD for diagnosis and assessment of disease activity as well as the risk of complications. One of these studies has proposed the combination of clinical, radiology, endoscopy, and biochemical markers as the most useful tool for the prediction or assessment of UC (Fengming and Jianbing, 2014).
Direito et al. (2017) have reported that colitis-associated colorectal cancer (CACC) is the most serious complication of IBD and the most common cause of colorectal cancer (CRC) in the context of chronic inflammation. Patients with UC are subject to an increased risk of CRC with a 20- to 30-fold higher risk than the general population (Romano et al., 2016). In addition, the incidence of CACC increases mainly after 8–10 years of UC diagnosis (Romano et al., 2016).
In IBD therapy, anti-inflammatory and immunosuppressive agents are primarily administered, including corticosteroids, antibiotics, and antitumor necrosis factor (TNF-α agent), but the use of these drugs may not be effective in all patients and may often result in a certain number of adverse effects that limit their long-term use (López-Posadas et al., 2010; Triantafyllidi et al., 2015). In this regard, it is of scientists’ increasing interest to develop alternative and coadjuvant therapeutic strategies with less or no adverse effects. In recent years, attention has been paid to the effects of plant secondary metabolites in the treatment of IBD and CRC. In particular, phenolic compounds have been speculated to contribute to the inhibition of inflammatory processes and reduce the risk of colon cancer development (Ding et al., 2020).
Inula viscosa L. (Asteraceae) is a perennial herb native to the Mediterranean Basin. It grows on hillslopes, damp habitats, and roadsides (Wang et al., 2004). This plant has sticky leaves and bright yellow flowers that bloom between August and November (Danino et al., 2009). It has been considered one of the most important medicinal plants around the Mediterranean Sea. In Algeria, I. viscosa leaves have been traditionally used as a decoction for the treatment of numerous diseases such as bronchitis and diabetes and as cataplasm for injuries and rheumatic pain (Baba Aissa, 2000; Haoui et al., 2015). Previous studies have demonstrated that crude extracts from different parts of I. viscosa possess many pharmacological properties, including antioxidant (Chahimi et al., 2015; Kheyar et al., 2018), antimicrobial (Tailib et al., 2012), antiulcerogenic (Alkofahi et al., 1999), antihelminthic (Oka et al., 2001), and antidiabetic effects (Hernàndez et al., 2007). Furthermore, the biological activities of this plant have been related to its abundance of a diverse range of phytochemicals, especially phenolic compounds, such as hydroxycinnamic acids and flavonoids (Hernández et al., 2007; Danino et al., 2009). It has been reported that these compounds exert a variety of biological functions such as antioxidant, anti-inflammatory, and anticancer activities (In et al., 2016). In a more recent study, I. viscosa has been reported to induce apoptosis in CRC cells in vitro and in vivo (Bar-Shalom et al., 2019). In our previous work, the chemical composition of an ethanolic extract from I. viscosa was characterized and, for the first time, 26 compounds were identified, including phenolic acids, flavonoids, tannins, and terpenoids (Kheyar et al., 2018). In the present study, the protective effect of EEIV against acetic acid (AA)-induced UC in rats and its antiproliferative effect were evaluated using a human colorectal cell line.
Materials and methods
Plant material
Leaves of I. viscosa were collected from Bejaia area (N 36°45′00″, E 5°04′00″) in the Northeast of Algeria. A voucher specimen (Reference No. IV017) was deposited at the Laboratory of Plant Biotechnology and Ethnobotany, University of Bejaia, Bejaia, Algeria. The ethanolic extract of I. viscosa (EEIV) was obtained as described in our previous study (Kheyar et al., 2018). Briefly, fresh I. viscosa leaves were air-dried in the shade and ground into a fine powder of 63 μm, and 500 g of powder was extracted with ethanol (1 : 4, w : v) under continuous stirring at room temperature for 24 h. The resulting supernatant was collected and dried until the crude extract was obtained. The obtained extract was used for biological tests on animals and cell lines.
Animals
Male Wistar rats (220–250 g) and Balb/c mice (23–25 g) were used in this study. The animals were obtained from Pasteur Institute (Algiers, Algeria) and were housed in rack-mounted cages in an air-conditioned atmosphere with a 12 h light–dark cycle. The animals had ad libitum access to water and standard food. All animals were acclimatized for 10 days and were fasted for 16 h before testing. The experimental procedures were carried out in accordance with the Animal Ethics Committee of the University of Bejaia (Algeria), in strict compliance with the internationally accepted principles for the handling of laboratory animals (directive N° 2010/63/EU of September 22, 2010).
Acute toxicity experiment
The acute oral toxicity of EEIV was tested in accordance with acute-toxic-class method guideline no. 423 of the Organization for Economic Cooperation and Development. Briefly, Balb/c mice were divided into control and test groups (six animals in each group). EEIV extract dissolved in 3% Tween 80 in 0.9% sodium chloride was administered orally at single doses of 100, 200, 500, 1000, and 2000 mg/kg. Vehicle (3% Tween 80 in 0.9% sodium chloride) alone was administered to the control group. All animals were observed for the appearance of the signs of toxicity or death for 14 days.
In vivo AA-induced colitis assay
Induction of colitis
Using the method of Dey et al. (2016), the protective effect of EEIV was investigated against AA-induced UC. Briefly, male rats were randomly divided into five groups (n = 6 rats per group):
Group 1: rats received 10 ml/kg vehicle (3% Tween 80 in 0.9% NaCl) for 5 days and then a single dose of 2 ml 0.9% NaCl intrarectally on day 6 to serve as the negative control (non colitic group).
Group 2: rats received 10 ml/kg vehicle (3% Tween 80 in 0.9% NaCl) for 5 days and then a single dose of AA (2 ml, 4% (v/v) in 0.9% NaCl) intrarectally on day 6 to serve as the control colitic group.
Group 3: rats received 100 mg/kg EEIV (in 3% Tween 80 in 0.9% NaCl) for 5 days and then a single dose of AA (2 ml, 4% (v/v) in 0.9% NaCl) intrarectally on day 6.
Group 4: rats received 200 mg/kg of EEIV (in 3% Tween 80 in 0.9% NaCl) for 5 days and then a single dose of AA (2 ml, 4% (v/v) in 0.9% NaCl) intrarectally on day 6.
Group 5: rats received dexamethasone at the dose of 2.4 mg/kg (in 3% Tween 80 in 0.9% NaCl) for 5 days and then a single dose of AA (2 ml, 4% (v/v) in 0.9% NaCl) intrarectally on day 6.
At the end of day 7, the rats were anesthetized and blood samples were collected. The rats were sacrificed by cervical dislocation, and their colons were cut close to the ileocecal junction and the proximal rectum.
Blood sampling and analysis
Using retro-orbital puncture under anesthesia, blood samples were collected and centrifuged at 3000g for 10 min. Serum was stored at 4°C until analyses. According to the manufacturer’s recommendations, the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and fibrinogen protein levels were determined, using standard methods at the medical laboratory of Franz Fanon Hospital, Bejaia (Algeria).
Colon sampling and analysis
Colon tissues were removed immediately after the rats were sacrificed, gently washed with ice-cold saline, and examined under a microscope at 40 × magnification, and the images of lesions were recorded. The macroscopically visible damage was rated on a 0–10 scale (Bell et al., 1995). Then, colons were weighed and their length was measured. Samples from the colon tissue were obtained and fixed in 10% buffered formalin for histological assessment, and the remaining colon samples were subsequently frozen and kept at −80°C until further use.
For histological examination, the colon tissues were embedded in paraffin, sectioned with a thickness of 5 μm, and then stained with hematoxylin and eosin (Cardiff et al., 2014). According to the damage index adapted from Fattahian et al. (2016), the histological analysis was based on inflammation severity, inflammation extent, and crypt damage.
Nitric oxide (NO)
As described by Mei et al. (2005), the NO level was analyzed in the colonic tissue by colorimetric quantitative test using the Griess reaction. The colon tissue samples were homogenized in ice-cold phosphate buffer saline (pH 7.4), the homogenates were centrifuged at 4000 g at 4°C for 30 min, and the supernatant was stored at −80°C until levels were determined. A volume of 100 μl of each sample was added to 50 μl of Griess reagent (5% N -1-naphthyl-ethylenediamine in 20% HCl, 5% sulfanilamide in 20% HCl (v/v)). Simultaneously, the standard (Sigma-Aldrich, St. Louis, MO, USA) for calibration curves was treated similar to the samples. After incubation at 37°C for 20 min, the absorbance of the blue chromogen formed was measured at 540 nm, revealing the presence of nitrite ions.
Malondialdehyde (MDA)
Using the thiobarbituric acid-reactive substances method previously described by Ohkawa et al. (1979), lipid peroxidation (LPO) was evaluated by measuring the MDA levels in tissue homogenate. Briefly, in 5 ml of 1.15% cold KCl, 500 mg of tissue was homogenized. Then, to 0.5 ml of homogenate, 0.5 ml of 20% TCA and 1 ml of 0.67% TBA were added and shaken well. The mixture was incubated for 15 min at 100°C and cooled immediately on ice; then, 4 ml of n -butanol was added to the mixture. Centrifugation was performed at 3000 rpm and 4°C for 15 min, and the absorbance of the supernatant was measured at 530 nm. With each set of samples, a standard curve was run simultaneously by using 1,1,3,3-tetraetoxypropane as an external standard. The results were expressed as nmol/g wet tissue weight.
Myeloperoxidase (MPO) activity
To evaluate neutrophil accumulation, MPO activity was determined in the colonic tissue. This was done based on the reaction with hydrogen peroxide and o-dianisidine dihydrochloride as substrates, in accordance with the method previously described by Rodrigues-Palacios et al. (2015). Briefly, 100 mg of colonic tissue was mixed with 2 ml of phosphate buffer containing 0.5% hexadecyltrimethyl ammonium bromide (HTAB). The samples were then centrifuged for 10 min at 3000 rpm and 4°C, and the supernatant was collected. Subsequently, 0.1 ml of the supernatant was combined with 2.9 ml of phosphate buffer containing o-dianisidine and 0.0005% hydrogen peroxide. The mixture was stirred for 5 min, and 0.1 ml of HCl (1.2 M) was added. The intensity of the resulting orange color was measured at 460 nm. MPO activity was expressed as U/g tissue, which was calculated using the following equation: MPO activity = ΔA450 – 0.5 – 0.0113 – 0.05, where 0.0113 is MPO constant; 0.5 is the time interval (30 s or 0.5 min), and 0.05 is the dilution factor of the sample: HTAB lysis buffer (0.05 g of tissue per 1000 ml, or 50 mg/1 ml).
In vitro colon cancer cell assays
Cell culture
Human colorectal adenocarcinoma cell lines Caco-2 and HT29 were purchased from DSMZ (Deutsche Sammlung von Microorganismen und Zellkulturen, Germany) and ATCC (American Type Culture Collection, USA), respectively. Before the start of the experiment, the cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% of heat-inactivated fetal bovine serum (FBS) and 2 mM of glutamine for 24 h. The cells were grown as a monolayer in 175-cm2 culture flasks and incubated at 37°C with 5% CO2 in a humidified incubator.
Cytotoxicity assay
Using Cell Titter® aqueous one solution cell proliferation (MTS) assay (Rodrigues et al., 2016), the cytotoxicity effect of EEIV was evaluated on confluent and nondifferentiated Caco-2 cells. Briefly, the cells were incubated for 24 h in 96-well plates (at a density of 2 × × 104 cells/well), and the medium was changed every 48 days. After 7 or 8 days of culture, confluent Caco-2 cells were treated with EEIV dissolved in 5% ethanol (0.5%, v/v) at 2.5–250 μg/ml concentrations. The control cells were incubated with a culture medium or ethanol (0.5%, v/v) at 37°C. After 24 h of incubation, cell viability was measured using PrestoBlue® Viability Reagent (Sigma-Aldrich, USA) according to the manufacturer’s protocol. The results were expressed as percentage of cellular viability relative to control (%). Experiments were performed in triplicate using at least two independent assays. The half-maximal inhibitory concentration (IC50) was also calculated from dose–response curves using the GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Antiproliferative assay
The antiproliferative cell assay was performed as described by Rodrigues et al. (2016). Briefly, the cells were seeded at a density of 1 × 104 cells/well in 96-well microplates. After 24-h incubation at 37°C in 5% CO2 atmosphere, the medium of each well was removed, and the cells were incubated with noncytotoxic concentrations of EEIV diluted in RPMI medium (2 g/l) with 0.5% FBS. The medium was removed after 24 h, and cell viability was determined using the MTS assay. The experiments were performed in triplicate using three independent assays. The results were expressed as percentage of cell viability relative to the control. Effective concentration values (EC50 concentrations that inhibited HT29 cell proliferation by 50%) were obtained from dose–responses curves using the GraphPad Prism software (GraphPad software, Inc., La Jolla, CA).
Statistical analysis
Statistical analysis of the results was carried out using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA) by one-way analysis of variance, followed by Tukey’s test. The results were expressed as mean ± standard deviation for in vitro cancer cell models and as mean ± standard error for animal colitis model. Data were considered statistically significant at P < 0.05.
Results
Toxicity assay for EEIV
Animals were weighed, and their visual changes, mortality, behavior, physical appearance, and signs of disease for 14 days were examined daily. No toxic symptoms or death was observed for EEIV at doses up to 2000 mg/kg in any treated rat during the 14 consecutive days of the treatment. Hence, it seems that the minimum lethal dose (LD50) of EEIV should be above 2000 mg/kg.
Macroscopic and functional signs of colitis injury
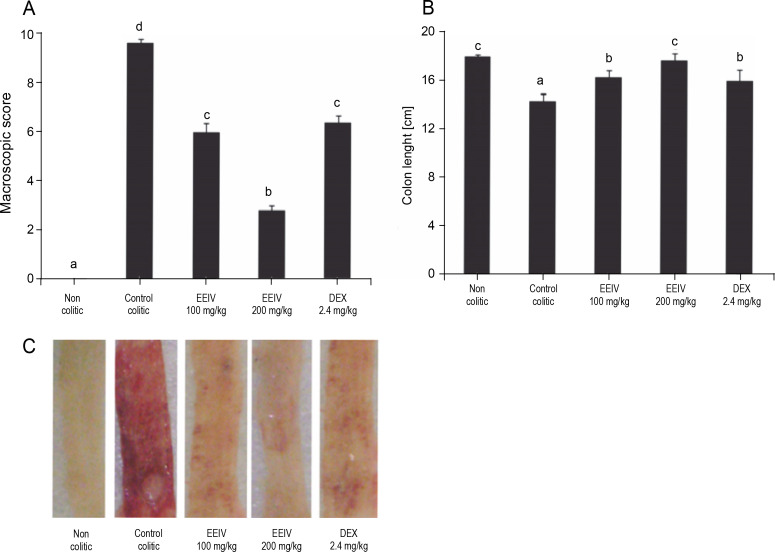
Highly significant clinical disorders were observed in rats after colonic instillation of AA, including increment in diarrhea severity, decrease in colon length, and increase in the extent of visible damage (ulcer formation). The control colitic group showed important macroscopic signs of colon injury with values of 9.6 (8–10). Interestingly, macroscopic scores were found to be significantly decreased to 6 (4–8) and 2.8 (2–4) after treatment with EEIV at a dose of 100 and 200 mg/kg (P < 0.001), respectively (Fig. 1A). In fact, rats treated with 200 mg/kg EEIV showed the lowest macroscopic scores. These differences were evident in macroscopic observation of the fresh and rinsed colon immediately after collection at the end of the experiments (Fig. 1C).
Fig. 1.
Effects of EEIV and dexamethasone administration on A) the macroscopic score, B) length of the colon, and C) colonic mucosa in the acetic acid model of colitis; data are expressed as means ± SEM (n = 6); groups with different letters statistically differ (P < 0.05)
Dexamethasone administration had a positive impact on colonic inflammation. In the present study, based on the analysis of macroscopic scores, animals treated with EEIV at 100 mg/kg showed similar results to those treated with dexamethasone, which showed macroscopic scores of 6.2 (4–6) (Fig. 1A). This confirms the antiinflammatory properties of EEIV. In addition, when compared with the non colitic group, EEIV at 200 mg/kg preserved the integrity of colon length (Fig. 1B).
Microscopic and histological features of colitis injury
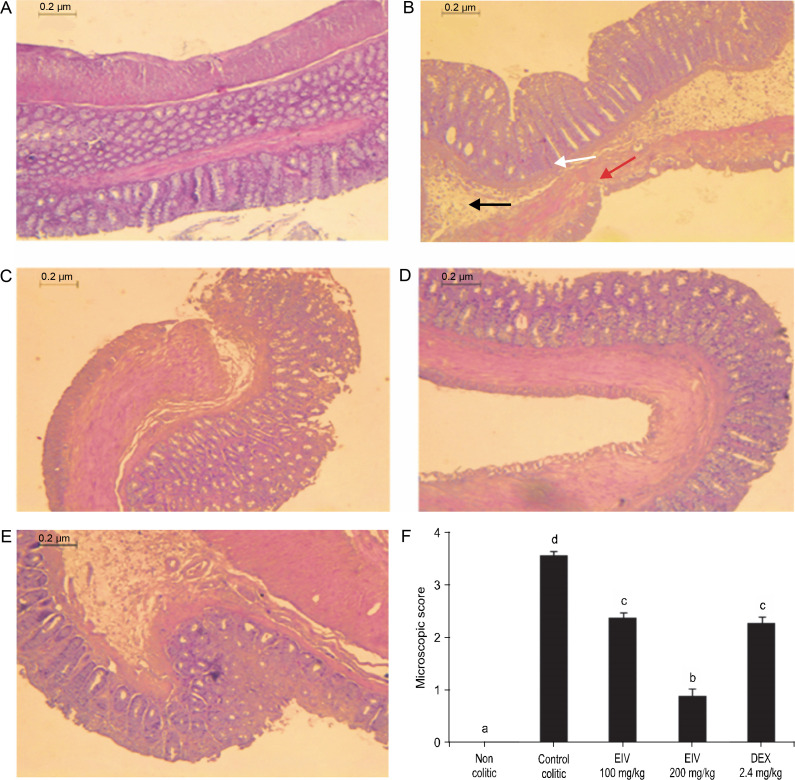
A normal colon with intact epithelium and normal crypt architecture, with no signs of inflammation or necrosis, were observed in the non colitic group (Fig. 2A). On the other hand, the control colitic group showed severe ulceration with significant histological changes, including inflammatory infiltration, loss of crypts, and thinner mucosa with marked neutrophil infiltration in lamina propria (Fig. 2B), with a microscopic score of a median of 6.33 (5.0–7.0) (Fig. 2F).
Fig. 2.
Effects of EEIV on histological appearance of colonic tissue in rats (magnification × 40): A) non colitic group with normal colonic mucosa, no lesions, and normal crypt architecture, B) control colitic group with very severe ulceration, mucosal necrosis (red arrow) with loss of crypts (white arrow), and markedly increased infiltration of neutrophils (black arrow, score 3), C) colitic group treated with EEIV at 100 mg/kg showing colon with mild lesions, partial crypt loss, and moderate inflammatory neutrophil infiltration (score 2), D) colitic group treated with EEIV at 200 mg/kg with normal colonic structure, reduction in the inflammatory infiltrate, mild edema limited mainly to the lamina propria, and minimal ulceration, E) colonic mucosa of 2.4 mg/kg dexamethasone-treated rats, F) microscopic score assigned to the different groups according to the criteria described by Fattahian et al. (2016); data are expressed as median (range) (n = 6); groups with different letters statistically differ (P < 0.05)
The impact of the AA-induced colonic damage was attenuated with treatment with EEIV. Insignificant histopathological changes and significant reduction in the microscopic scores were observed in groups treated with EEIV when compared with the control colitic group with values of 3.66 (3.0–5.0) (100 mg/kg) and 2.16 (2.0–3.0) (200 mg/kg) (P < 0.05 vs. colitic control) (Fig. 2F). In fact, in rats treated with a high dose of EEIV (200 mg/kg), normal colonic structures and reduction in inflammatory infiltrates, limited mild edema mainly to the lamina propria, and minimal ulceration were observed (Fig. 2D).
Dexamethasone administration to colitic rats showed a similar effect on colonic histology to that observed in rats treated with EEIV at a dose of 100 mg/kg with a value of 2.83 (2.0–4.0) (Fig. 2F).
Inflammatory serum biomarkers
As shown in Table 1, the intestinal inflammatory process, induced by AA in rats, was also characterized by the measurement of serum ESR, CRP, and fibrinogen levels. The results showed a significant increase (P < 0.001) in ESR, after 1 and 2 h, and fibrinogen levels in the control colitic group (13 mm/h, 28.25 mm/h, and 2.83 g/l, respectively) when compared with the non colitic group (2.75 mm/h, 7.5 mm/h, and 1.45 g/l, respectively).
Table 1.
Effects of EEIV (100 and 200 mg/kg) on serum biomarkers (ESR, CRP, and fibrinogen levels)
| Group | ESR [mm/h] | CRP [mg/l] | Fibrinogen [g/l] | |
|---|---|---|---|---|
| 1 h | 2 h | |||
| Non colitic | 2.75 ± 0.5 a | 7.5 ± 0.81 a | – | 1.45 ± 0.08 a |
| Control colitic | 14.32 ±0.41 d | 28.25 ± 1.50 d | – | 2.83 ± 0.35 c |
| EEIV 100 mg/kg | 8.50 ± 0.41 c | 16.12 ± 1.11 c | – | 2.07 ± 0.43 b |
| EEIV 200 mg/kg | 5.25 ± 0.40 b | 11.25 ± 1.08 b | – | 1.57 ± 0.08 a |
| DEX 2.4 mg/kg | 4.25 ± 0.95 b | 9.5 ± 1.73 ab | – | 1.70 ± 0.09 ab |
Data are expressed as mean ± SEM (n = 6); groups in the same column with different letters statistically differ (P < 0.05); (–) – absence of agglutination indicates CRP concentration 6 mg/l
Treatment with EEIV at 100 mg/mg caused a significant decrease (P < 0.001) in serum ESR, after 1 and 2 h, and fibrinogen levels (9.25 mm/h, 19.5 mm/h, and 2.07 g/l, respectively). However, when compared with the control colitic group, EEIV at 200 mg/kg resulted in a nearly twofold and threefold decrease in fibrinogen markers (1.57 g/l) and ESR levels (4 and 7.12 mm/h after 1 and 2 h, respectively), (Table 1). Similarly, after treatment with dexamethasone, these markers were significantly ameliorated. CRP values in the serum showed no significant difference between different groups of rats (CRP < 6 mg/l) (Table 1).
Biochemical markers of colitis injury
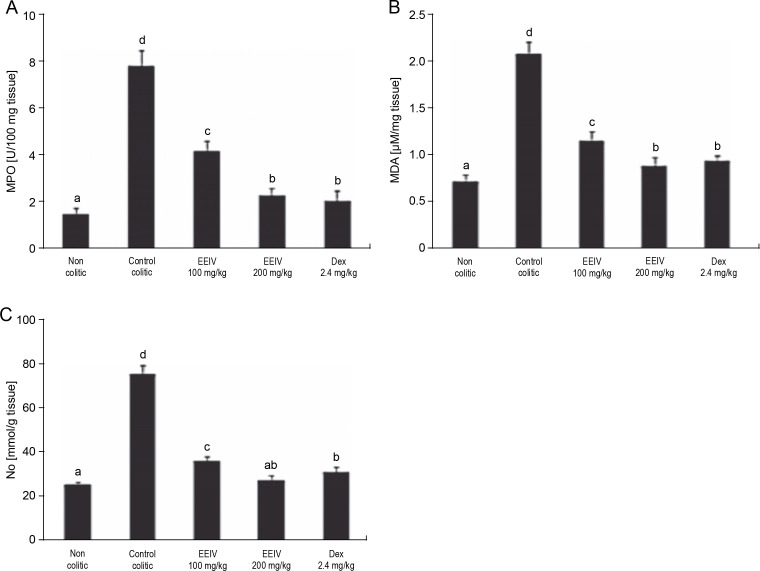
To evaluate the levels of MPO, MDA, and NO metabolites (nitrites and nitrates) in the colonic tissue, a biochemical analysis was performed (Fig. 3). As revealed by the results presented in Fig. 3A, MPO activity of the control colitic group significantly increased (P < 0.001) (7.82 ± 0.61 U/100 g of tissue) in comparison with that of the non colitic group (1.54 ± 0.24 U/100 g of tissue), thus confirming the induction of severe UC. After pretreatment with EEIV at concentrations of 100 and 200 mg/kg, the MPO activity was significantly (P < 0.001) decreased with values of 4.18 ± 0.42 and 2.29 ± 0.32 U/100 g of tissue, respectively. Similarly, after pretreatment of rats with dexamethasone, the reduction in MPO activity was statistically significant (2.07 ± 0.43 U/100 g of tissue). This result is similar to that obtained with rats pretreated with EEIV at 200 mg/kg.
Fig. 3.
Effects of EEIV administration on A) MPO enzyme activity, B) MDA level, and C) NO production in the colonic tissue; data are expressed as mean ± SEM (n = 6); groups with different letters are statistically different (P < 0.05)
Colon MDA levels were significantly increased (P < 0.001) in the control colitic group (2.09 ± 0.12 μM/mg of tissue) compared with the non colitic group (0.73 ± 0.07 μM/mg of tissue). In addition, a significant decrease in the MDA level was observed after treatment with EEIV and dexamethasone. There was no significant variation in MDA in rats pretreated with EEIV at 200 mg/kg and dexamethasone (0.89 ± 0.09 to 0.95 ± 0.05 μM/mg of tissue, respectively).
Variations in NO concentrations were observed. Indeed, a significant increase (P < 0.001) was observed in the colitic control group (76.37 ± 3.87 mmol/g tissue) compared with the non colitic group (25.69 ± 1.33 mmol/g tissue). On the other hand, the levels of NO were significantly reduced (36.76 ± 1.79, 27.64 ± 2.55, 31.67 ± 2.24 mmol/g tissue) in rats pretreated with EEIV at 100 and 200 mg/kg and dexamethasone, respectively. Nevertheless, no significant differences were observed in the levels of NO between EEIV- (200 mg/kg) and dexamethasone-treated groups.
Cytotoxic and antiproliferative effects of EEIV
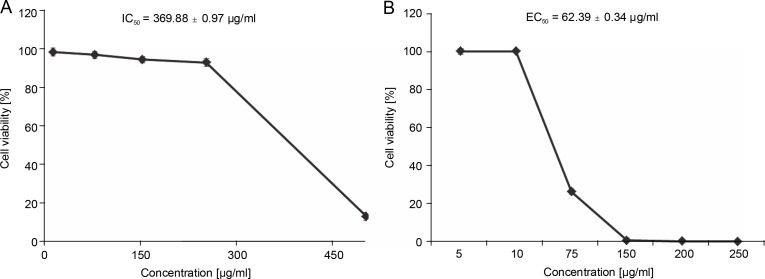
Before the antiproliferative assay, the cytotoxicity of EEIV was tested on the Caco-2 cell model. This cell model is widely used and considered as an acceptable intestinal model since it shares some characteristics with crypt enterocytes (Cano-Sancho et al., 2015; Rodrigues et al., 2016). After incubation of these cells for 24 h with different concentrations of EEIV (10, 75, 100, 150 and 250 μg/ml), the cell survival rate was found to be greater than 90% at all tested concentrations (Fig. 4A), except for the concentration of 500 μg/ml, which induced a significant (P < 0.001) cell toxicity (cell viability < 50%). Lower cytotoxicity was observed for the IC50 value obtained after 24 h of incubation time (IC50 = 369.88 ± 0.97 μg/ml). Based on the cytotoxicity results, non-cytotoxic concentrations were selected for the antiproliferative assay.
Fig. 4.
A) Effects of EEIV on Caco-2 cell viability and B) antiproliferative activity in HT-29 cells; data are expressed as mean ± SD
The effectiveness of EEIV in reducing the proliferation of a human colorectal adenocarcinoma cell line (HT29) was further analyzed. As shown in Fig. 4B, the exposure of HT29 cells to EEIV at noncytotoxic concentrations (5, 10, 75, 150, 200 and 250 μg/ml) for 24 h caused significant inhibition of cancer cell growth in a dose-dependent manner, with viability percentages of 100, 26.32, 0.72, and 0.12%, respectively. The EC50 value was found to be 62.39 ± 0.34 μg/ml (Fig. 4).
Discussion
Two different strategies were used in the present study to screen the anti-inflammatory and anticancer potency of EEIV: evaluation of the intestinal anti-inflammatory effect of EEIV in an experimental colitis model in rats and evaluation of the antiproliferative capacity of EEIV in a CRC cell line (HT29).
It is worth noting that various experimental models of colitis were established, by administration of dextran sodium sulfate (DSS), trinitrobenzene sulfonic acid (TNBS), or AA (Safarpour et al., 2015). AA-induced colitis is a well-established experimental model with several signs and symptoms of human UC, where weight loss, diarrhea, and rectal bleeding are some of the major symptoms (Hartmann et al., 2012). In the present study, the intestinal anti-inflammatory effects of EEIV in AA-induced colitis were investigated in rats. The results demonstrated that oral administration of EEIV significantly prevented the loss of colon length (Fig. 1B) and reduced both macroscopic (Fig. 1A) and microscopic scores (Fig. 2F) in a dose-dependent manner. In colitic groups, EEIV treatments significantly ameliorated the pathological manifestations by AA, including inflammatory cell infiltration with crypt abscesses and ulceration of the epithelium in the colonic mucosa (Fig. 2). This antiinflammatory effect was also clearly associated with a decrease in the levels of serum biomarkers (Table 1).
These findings might explain the intestinal anti-inflammatory properties of EEIV. Intrarectal administration of AA induced macroscopic damage of the colon (Fig. 1C) in the control colitic group, and the colonic mucosa appeared hemorrhagic and ulcerated. EEIV treatment of colitic rats at the doses of 100 and 200 mg/kg ameliorated the colon injury when compared with the untreated control group (Fig. 1C).
Acute and chronic inflammation in IBD is associated with the migration and filtration of leukocytes and activation of inflammatory mediators such as TNF-α, IL-1β, and IL-6, which have been considered to play a key role in the increase in the production of several proteins (Fengming and Jianbing, 2014). To examine and detect inflammatory disorders in plasma, serum ESR, CRP, and fibrinogen are the most widely used biomarkers. In this study, significant increases in the concentrations of fibrinogen and ESR were observed in the colitic control group (P < 0.001). Similarly, a significant increase in the levels of ESR and fibrinogen in IBD patients has been reported in several previous research works (Danese et al., 2007; Dolapcioglu et al., 2014). Interestingly, a remarkable reduction in the levels of serum ESR and fibrinogen was observed in colitic rats after treatment with different doses of EEIV.
CRP was found at concentrations lesser than 6 mg/l in all rats in the present study, which indicates that the levels of CRP were not affected during UC. These results are in agreement with those of earlier reports (Iskandar and Ciorba, 2012; Fengming and Jianbing, 2014). A previous study has demonstrated that serum CRP had a relatively low sensitivity in the detection of UC and is therefore not sufficient to identify patients with symptoms compatible with IBD (Lewis, 2011).
Several mechanisms are involved in the inflammatory responses that lead to tissue damage in IBD. Oxidative stress may play an important role in the initiation and progression of inflammation in IBD and which is associated with the overproduction of ROS or decrease in antioxidant defense systems (Balmus et al., 2016). The severity of inflammatory changes in colonic mucosa can be correlated with the levels of ROS and the infiltration of inflammatory cells. Previous studies reported that these cells produce large quantities of proinflammatory mediators such as TNF-α, IL-6, IL-1, IL-8, and IFN-γ, which can induce the overexpression of enzymes like inducible nitric oxide synthase (iNOS), via transcription factors especially NF-κB (Balmus et al., 2016).
To get more insight into the mechanisms responsible for the anti-inflammatory activity of EEIV, the effects of EEIV on NO production, LPO, and MPO activity in the inflamed colonic tissue were investigated.
This study demonstrated that the induction of colitis is associated with severe histological damage and increases in NO production in colonic tissue. In particular, in the intestine, NO produced by iNOS is the most important source of the evaluated nitrite level during experimental colitis (Direito et al., 2017). Peroxynitrite, a highly toxic species, can be generated by the combination of NO with superoxide anions, which can cause irreversible damage to the structure and function of proteins (Mei et al., 2005; Bribi et al., 2016).
According to the results of this study, pretreatments with EEIV significantly reduced NO production in the inflamed colonic tissue. This may be due to antioxidant and anti-inflammatory properties of EEIV, such as inhibiting iNOS expression, blocking peroxynitrite formation, and eliminating NO in the inflamed colon.
The extent of mucosal damage is also associated with the level of LPO induced by ROS (Kumar et al., 2015). Results of the present study showed that the content of MDA was significantly decreased after the administration of EEIV, which may play a vital role in the mechanism of mucosal protection.
Findings of this study also showed that the severity of colitis was associated with neutrophil infiltration and increase in MPO activity. MPO activity was suppressed by the administration of EEIV in the colitis rat model through inhibition of neutrophil infiltration in inflamed mucosa. Therefore, it can be postulated that the inhibitory effect of EEIV on cell activation, degranulation, and production of hypochlorous acid would play a role in its anti-inflammatory activity. In summary, EEIV was able to attenuate AA-induced UC by inhibiting oxidative and inflammatory biomarkers. The major phenolics present in EEIV extract—chlorogenic acid and derivatives such as caffeoylquinic acids and dicaffeoylquinic acids (Kheyar et al., 2018) could probably be responsible for the observed anti-inflammatory effects of EEIV. A previous study reported that chlorogenic acid possesses strong antioxidant and anti-inflammatory effects, which can inhibit neutrophil migration and TNF-α expression (Chagas-Paula et al., 2011). Furthermore, another study of UC induced by DSS has reported that chlorogenic acid was able to suppress the inflammatory mediators IL-6, INF-γ, and TNF-α and the colonic infiltration of neutrophils via inhibition of the NF-κB translocation to the nucleus (Zhang et al., 2017). Similarly, di-O-caffeoylquinic acid reduced NO production and inhibited proinflammatory mediators in lipopolysaccharide-stimulated macrophages as well as prostaglandin E2 release (Chen et al., 2016). Moreover, the presence of quercetin, myricetin, catechin, isorhamnetin, naringenin, and luteolin derivatives in EEIV can account for the intestinal antiinflammatory activity (Kheyar et al., 2018). These molecules were already described as compounds exhibiting an anti-inflammatory effect by modulating a number of important metabolic pathways. In accordance with the literature, quercetin, myricetin, and luteolin exhibited a potential anti-inflammatory activity via reduction in the expression of proinflammatory cytokine IL-2, TNF-α, and inducible enzyme activities like cyclooxygenase 2 (COX-2) and iNOS, and stimuli of the secretion of anti-inflammatory cytokine interleukin 10 (García-Mediavilla et al., 2007; Mueller et al., 2010). Moreover, previous studies demonstrated that catechin exert anti-inflammatory effects by inhibiting the MPO activity and MDA level in TNBS-induced colitis model in rats (Sato et al., 1998), NF-κB activation (Jung et al., 2009), and production of proinflammatory cytokines IL-6 and TNF-α as well as activation of inducible enzymes such as iNOS and COX-2 have also been demonstrated in previous studies (Liu et al., 2014).
In this work, the potential antiproliferative effect of EEIV in human colon cancer cell line HT29 was examined. Cytotoxicity assays on nondifferentiated Caco-2 cells were carried out simultaneously to evaluate the intestinal safety of EEIV. As this cell model displays some characteristics of colonic crypt cells, it is considered as a good model of the intestinal barrier, and is often used to evaluate the effect of natural compounds and plant extracts on intestinal functions (Kuntz et al., 1999; Rodrigues et al., 2016).
EEIV was screened for cytotoxicity on Caco-2 cell lines, and was found to show a cytotoxic effect at a concentration of 500 μg/ml. EEIV extract resulted in a dosedependent decrease in the cell growth of HT29. The crude extracts of I. viscosa have already been studied in multiple cancer cells and have been shown to inhibit the proliferation of MCF-7 human breast carcinoma cells (Talib et al., 2012), Hep-2 human liver cancer (Talib and Mahsnem, 2010), and SiHa and HeLa cervical cancer cell lines (Benbacer et al., 2012).
The antiproliferative capacity of this extract could be due to its phenolic composition. As reported in our previous study, caffeoylquinic acid and flavonoids are the main phenolic compounds identified in the EEIV leaves, which were already reported to be effective in targeting colon cancer proliferation and other important hallmarks of cancer using cell-based assays (Kheyar et al., 2018). Furthermore, in several studies, it has been demonstrated that caffeoylquinic acid derivatives and flavonoids could inhibit colon cancer cell proliferation and induce cancer cell apoptosis (Kuntz et al., 1999). In Murad et al. (2015) study, chlorogenic acid inhibited the growth and proliferation of colon cancer cells (HT29) by inducing G0/G1 arrest and apoptosis. In Puangpraphant et al. (2011) study, it has been shown that di-O-caffeoylquinic acid promoted significant inhibition of the proliferation of HT29 by inducing apoptosis through caspase-3 and Bax activation in HT29 colon cancer cells. In addition, the capacity of naringenin to inhibit cell proliferation in human CRC cells (HCT116 and SW480) has been demonstrated by Song et al. (2015). Araújo et al. (2011) have reported that some flavonoids, such as quercetin, myricetin, and catechin, exhibited antiproliferative activity against human colon HT29 cells, by blocking cell migration and differentiation and inducing cell cycle arrest, apoptosis, and antimetastatic activity. As a consequence, the potent effect of EEIV could be related to its highest total phenolic content and its composition in caffeoylquinic acid derivatives and flavonoids. A synergic effect between all these has been also described (Gilani et al., 2005; Kheyar et al., 2018).
Conclusions
This study confirmed that the ethanol extract of I. viscosa exerted intestinal anti-inflammatory effects in an experimental AA-induced colitis model in rats. This extract was also able to inhibit CRC cell proliferation in HT29 cells. Overall, the bioactive compounds of I. viscosa have promising cancer-preventive properties and the extract of this plant can be a potential pharmacological tool in the treatment of patients with IBD.
Acknowledgments
This study was supported by grants from the Algerian Minister of Higher Education and Scientific Research and the iNOVA4Health—UIDB/04462/2020 and UIDP/04462/2020, a program financially supported by Fundação para a Ciência eTecnologia/Ministério da Ciência, Tecnologia e Ensino Superior, through national funds. Funding from INTERFACE Programme, through the Innovation, Technology and Circular Economy Fund (FITEC), is gratefully acknowledged. ATS also acknowledge Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior, for the Individual Grant CEECIND/04801/2017.
Declaration of conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Alkofahi A., Atta A.H. (1999) Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J. Ethnopharmacol. 67(3): 341–345. 10.1016/s0378-8741(98)00126-3 [DOI] [PubMed] [Google Scholar]
- Araújo J.R., Gonçalves P., Martel F. (2011) Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 31(2): 77–87. 10.1016/j.nutres.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Baba Aissa F. (2000) Encyclopédie des plantes utiles: flore d’Algérie et du Maghreb. Substances végétales d’Afrique d’Orient et d’Occident, Édas. (moderne Librairie ed). Rouiba, Algérie: 252–253. [Google Scholar]
- Balmus I.M., Ciobica A., Trifan A., Stanciu C. (2016) The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: clinical aspects and animal models. Saudi J. Gastroenterol. 22(1): 3–17. 10.4103/1319-3767.173753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shalom R., Bergman M., Grossman S., Azzam N., Sharvit L., Fares, F. (2019) Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Front. Oncol. 9: 227. 10.3389/fonc.2019.00227. eCollection 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.J., Gall D.G., Wallace J.L. (1995) Disruption of colonic electrolyte transport in experimental colitis. Am. J. Physiol. 268: G622–G630. 10.1152/ajpgi.1995.268.4.G622 [DOI] [PubMed] [Google Scholar]
- Benbacer L., Merghoub N., El Btaouri H., Gmouh S., Attaleb M., Morjani H., Amzazi S., El Mzibri M. (2012) Antiproliferative effect and induction of apoptosis by Inula viscosa L. and Retamamono sperma L. extracts in human cervical cancer cells. [in:] Topics on cervical cancer with an advocacy for prevention. 16: 267–284. 10.5772/30025 [DOI] [Google Scholar]
- Bribi N., Algieri F ., Rodriguez-Nogales A., Vezza T., Garrido-Mesa J., Utrilla M.P., Del Mar Contreras M., Maiza F., Segura-Carretero A., Rodriguez-Cabezas M.E. et al. (2016) Intestinal anti-inflammatory effects of total alkaloid extract from Fumaria capreolata in the DNBS model of mice colitis and intestinal epithelial CMT93 cells. Phytomedicine 15: 901–913. 10.1016/j.phymed.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Cano-Sancho G., González-Arias C.A., Ramos A.J., Sanchis V., Fernández-Cruz M.L. (2015) Cytotoxicity of the mycotoxins deoxynivalenol and ochratoxin A on Caco-2 cell line in presence of resveratrol. Toxicol. In Vitro 29: 1639–1646. 10.1016/j.tiv.2015.06.020 [DOI] [PubMed] [Google Scholar]
- Chagas-Paula D.A., Oliveira R.B., da Silva V.C., Gobbo-Neto L., Gasparoto T.H., Campanelli A.P., Faccioli L.H., Da Costa F.B. (2011) Chlorogenic acids from Tithoniadi versifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J. Ethnopharmacol. 22: 355–362. 10.1016/j.jep.2011.04.067 [DOI] [PubMed] [Google Scholar]
- Cardiff R.D., Miller C.H., Munn R.J. (2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2(6): 655–658. [DOI] [PubMed] [Google Scholar]
- Chen Y.L., Hwang T.L., Yu H.P., Fang J.Y., Chong K.Y., Chang Y.W., Chen C.Y., Yang H.W., Chang W.Y., Hsieh P.W. (2016) Ilex kaushue and its bioactive component 3,5-dicaffeoylquinic acid protected mice from lipopolysaccharideinduced acute lung injury. Sci. Rep. 6: 34243. 10.1038/srep34243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H., Lo Y., Lu F. (1993) Xanthine oxidase inhibitors from the leaves of Alsophila spinulosa (Hook) Tryon. J. Enzyme Inhib. 8: 61–71. 10.3109/14756369409040777 [DOI] [PubMed] [Google Scholar]
- Danese S., Papa A., Saibeni S., Repici A., Malesci A., Vecchi M. (2007) Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am. J. Gastroenterol. 102: 174–186. 10.1111/j.1572-0241.2006.00943.x [DOI] [PubMed] [Google Scholar]
- Danino O., Hugo E., Gottlieb S.G., Bergman M. (2009) Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 42: 1273–1280. 10.1016/j.foodres.2009.03.023 [DOI] [Google Scholar]
- Dey Y.N., Sharma G., Wanjari M.M., Kumar D., Lomashd V., Jadhav A.D. (2016) Beneficial effect of Amorphophallus paeoniifolius tuber on experimental ulcerative colitis in rats. Pharm Biol. 55: 53–62. 10.1080/13880209.2016.1226904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Xu S., Fang J., Jiang H. (2020) The protective effect of polyphenols for colorectal cancer. Front Immunol. 11: 1407. 10.3389/fimmu.2020.01407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direito R., Lima, A., Rocha R., Ferreira R.B., Mota J., Rebelo P., Fernandes A., Pinto R., Alves P., Bronze R., Sepodes B., Figueira M.-E. (2017) Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 46: 100–108. 10.1016/j.jnutbio.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Dolapcioglu C., Soylu A., Kendir T., Ince A.T., Dolapcioglu H., Purisa S., Bolukbas C., Sokmen H.M., Dalay R., Ovunc O. (2014) Coagulation parameters in inflammatory bowel disease. Int. J. Clin. Exp. Med. 7: 1442–1448. [PMC free article] [PubMed] [Google Scholar]
- Fattahian E., Hajhashemi V., Rabbani M., Minaiyan M., Mahzouni P. (2016) Anti-inflammatory effect of amitriptyline on ulcerative colitis in normal and reserpine-induced depressed rats. Iran J. Pharm. Res. 15: 125–137. [PMC free article] [PubMed] [Google Scholar]
- Fengming Y., Jianbing, W. (2014) Biomarkers of inflammatory bowel disease. Disease Markers. 2014: 710915. 10.1155/2014/710915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mediavilla V., Crespo I., Collado P.S., Esteller A., Sánchez-Campos S., Tuñón M.J., González-Gallego J. (2007) The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappa B pathway in Chang Liver cells. Eur. J. Pharmacol. 28: 221–229. 10.1016/j.ejphar.2006.11.014 [DOI] [PubMed] [Google Scholar]
- Gilani A.H, Rahman A.U. (2005) Trends in ethnopharmacology. J. Ethnopharmacol. 100(1–2): 43–49. 10.1016/j.jep.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Haoui I.E., Derriche R., Madani L., Oukali Z. (2015) Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arabian J. Chem. 8: 587–590. 10.1016/j.arabjc.2011.05.005 [DOI] [Google Scholar]
- Hartmann R.M., Morgan Martins M.I., Tieppo J., Fillmann H.S., Marroni N.P.(2012) Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Dig. Dis. Sci. 57: 2038–2044. 10.1007/s10620-012-2134-3 [DOI] [PubMed] [Google Scholar]
- Hernández V., Recio M.C., Manez S., Giner R.M., Riou J.F. (2007) Effects of naturally occurring dihydro flavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 81: 480–488. 10.1016/j.lfs.2007.06.006 [DOI] [PubMed] [Google Scholar]
- In J.K., Kim J.K., Oh J.S., Seo D.W. (2016) 5-Caffeoylquinic acid inhibits invasion of non-small cell lung cancer cells through the inactivation of p70S6K and Act activity: Involvement of p53 in differential regulation of signaling pathways. Int. J. Oncol. 48: 1907–1912. 10.3892/ijo.2016.3436 [DOI] [PubMed] [Google Scholar]
- Iskandar H.N., Ciorba M.A. (2012) Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl. Res. 159: 313–325. 10.1016/j.trsl.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D., Feuerstein M.D., Adam S., Cheifet M.D. (2014) Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin. Proc. 89: 1553–1563. 10.1016/j.mayocp.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Kheyar-Kraouche N., Bento da Silva A., Serra A.T., Bedjou F., Bronze M.R. (2018) Characterization by liquid chromatography–mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 156(2018): 297–306. https://doi.org10.1016/j.jpba.2018.04.047 [DOI] [PubMed] [Google Scholar]
- Khor B.G.A., Xavier R.J. (2011) Genetics pathogenesis of inflammatory bowel disease. Nature 474: 307–317. 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.R., Chang D.K. (2014) Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 7: 9872–9881. 10.3748/wjg.v20.i29.9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth A., Sachar D.B. (2010) Ulcerative colitis practice guidelines in adults: american college of gastroenterology, practice parameters committee. Am. J. Gastroenterol.105. 10.1038/ajg.2009.727 [DOI] [PubMed] [Google Scholar]
- Kumar C.S.V.S., Reddy K.K., Reddy A.G., Vinoth A.A., Chowdary C.H.S.R., Boobalan G., Rao G.S. (2015) Protective effect of Lactobacilus plantarum 21, a probiotic on trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. Int. Immunopharmacol. 25: 504–510. 10.1016/j.intimp.2015.02.026 [DOI] [PubMed] [Google Scholar]
- Kuntz S., Wenzel U., Daniel H. (1999) Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 38. 10.1007/s003940050054 [DOI] [PubMed] [Google Scholar]
- Lewis J.D. (2011) The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 140(6): 1817–1826. 10.1053/j.gastro.2010.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Lu T.H., Su C.C., Lay I.S., Lin H.Y., Fang K.M., Ho T.J., Chen K.L., Su Y.C., Chiang W.C., Chen Y.W. (2014) Lotus leaf (Nelumbo nucifera) and its active constituents prevent inflammatory responses in macrophages via JNK/NF-κB signaling pathway. Am. J. Chin. Med. 42: 869–889. 10.1142/S0192415X14500554 [DOI] [PubMed] [Google Scholar]
- Loddo I., Romano C. (2015) Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol. 2(6): 551. 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Posadas R., Ballester I., Mascaraque C., Suárez M.D., Zarzuelo A., Martínez-Augustin O., de Medina F.S. (2010) Flavonoids exert distinct modulatory actions on cyclooxygenase 2 and NF-κB in an intestinal epithelial cell line (IEC18). Br. J. Pharmacol. 160(7): 1714–1726. 10.1111/j.1476-5381.2010.00827.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Q., Xu J.M., Xiang L., Hu Y.M., Hu X.P., Xu Z.W. (2005) Change of nitric oxide in experimental colitis and its inhibition by melatonin in vivo and in vitro. Postgrad. Med. J. 81(960): 667–672. 10.1136/pgmj.2004.030817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M., Hobiger S., Jungbauer A. (2010) Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 122: 987–996. 10.1016/j.foodchem.2010.03.041 [DOI] [Google Scholar]
- Murad L.D., Soares N.C., Brand C., Monteiro M.C., Teodoro A.J. (2015) Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer 67(3): 532–542. 10.1080/01635581.2015.1004736 [DOI] [PubMed] [Google Scholar]
- OECD Guidelines for Testing of Chemicals : Guideline 423: Acute oral toxicity, toxic-class method office of economic and community development, Paris: 2001. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2): 351–358. 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- Oka Y., Ben-Daniel B., Cohen Y. (2001) Nematicidal activity of powder and extracts of Inula viscosa. Nematology 3: 735–742. [Google Scholar]
- Puangpraphant S., Berhow M.A., Vermillion K., Potts G., Gonzalez de Mejia E. (2011) Dicaffeoylquinic acids in Yerba mate (Ilex paraguariensis St. Hilaire) inhibit NF-κB nucleus translocation in macrophages and induce apoptosis by activating caspases-8 and -3 in human colon cancer cells. Mol. Nutr. Food Res. 55(10): 1509–1522. 10.1002/mnfr.201100128 [DOI] [PubMed] [Google Scholar]
- Rezaie A., Parker R.D., Abdollahi M. (2007) Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenonor the cause. Dig. Dis. Sci. 52(9): 2015–2021. 10.1007/s10620-006-9622-2 [DOI] [PubMed] [Google Scholar]
- Rodrigues L., Silva I., Poejo J., Serra A.T., Matias A.A., Simplício A.L., Bronze M.R., Duarte C.M.M. (2016) Recovery of antioxidant and antiproliferative compounds from watercress using pressurized fluid extraction. Royal Soc. Chem. 37: 30905–30918. [Google Scholar]
- Rodrigues-Palacios A., Aladyshkina N., Cominellial F. (2015) Stereomicroscopy and 3D-target myeloperoxidase intestinal phenotyping following a fecal flora homogenization protocol. Protocol Exchange 6: 75–77. [Google Scholar]
- Romano M., Francesco F., Zarantonello L., Ruffolo C., Ferraro G.A., Zanus G. (2016) From inflammation to cancer in inflammatory bowel disease: molecular perspectives. Anticancer Res. 36: 1447–1460. 10.1038/protex.2015.065 [DOI] [PubMed] [Google Scholar]
- Safarpour A.R., Hosseini S.V., Mehrabani D. (2015) Epidemiology of inflammatory bowel diseases in Iran and Asia. Iran. J. Med. Sci. 38: 140–149. [PMC free article] [PubMed] [Google Scholar]
- Sato K., Kanaawa A., Ota N., Nakamura T., Fujimotol K. (1998) Dietary supplementation of catechins and a-tocopherol accelerates the healing of trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. J. Nutr. Sci. Vitaminol. 44(6): 769–778. 10.3177/jnsv.44.769 [DOI] [PubMed] [Google Scholar]
- Serra A.T., Poejo J., Matias A.A., Bronze M.R., Duarte C.M.M. (2013) Evaluation of Opuntia spp. derived products as antiproliferative agents inhuman colon cancer cell line (HT29). Food Res. Int. 54: 892–901. 10.1016/j.foodres.2013.08.043 [DOI] [Google Scholar]
- Singh U.P., Singh B., Busbee N.P., Guan H., Singh B., Price R.L., Taub D.D., Mishra M.K., Nagarkatt M., Nagarkatti P.S. (2012) Alternative medicines as emerging therapies for inflammatory bowel diseases. Int.Rev.Immunol. 31(1): 66–84. 10.3109/08830185.2011.642909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.M., Park G.H., Eo H.J., Lee J.W., Kim M.K., Lee J.R., Lee H.M., Koo J.S., Jeong B.J. (2015) Anti-proliferative effect of naringenin through p38-dependent down regulation of cyclin d1 in human colorectal cancer cells. Biomol. Ther. 23(4): 339–344. 10.4062/biomolther.2015.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talib W.H., Mahasneh A.M. (2010) Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 78(1): 33–45. 10.3797/scipharm.0912-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talib W.H., Musa H., Zarga A., Mahasneh A.M. (2012) Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecule 17(3): 3291–3303. 10.3390/molecules17033291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Ben-Daniel B.H., Cohen Y. (2004) Control of plant diseases by extracts of Inula viscosa. Phytopathology 94(10): 1042–1047. 10.1094/PHYTO.2004.94.10.1042 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wu X., Cao S., Cromie M., Shen Y., Feng Y., Yang H., Li L. (2017) Chlorogenic acid ameliorates experimental colitis by promoting growth of Akkermansia in mice. Nutrients 9(7): 677. 10.3390/nu9070677 [DOI] [PMC free article] [PubMed] [Google Scholar]