Abstract
Serum-free culture filtrates of six Candida species and Saccharomyces cerevisiae were found to contain chemoattractants for human polymorphonuclear leukocytes (PMNs) and a mouse macrophage-like cell line, J774. The chemotactic factors differed for the PMN and J774 cells, however, in terms of heat stability, kinetics of liberation by the yeast cells, and divalent cation requirements for production. The chemoattractant in Candida albicans culture filtrates appeared to act through the formyl peptide receptor (FPR) of PMNs, since it was found to induce chemotaxis of Chinese hamster ovary (CHO) cells that were expressing the human FPR but did not induce chemotaxis of wild-type CHO cells. The C. albicans culture filtrates also induced migration of PMNs across confluent monolayers of a human gastrointestinal epithelial cell line, T84; migration occurred in the basolateral-to-apical direction but not the reverse direction, unless the epithelial tight junctions were disrupted. J774 cells did not migrate toward the formylated peptide (fMet-Leu-Phe; fMLF), and chemotaxis toward the C. albicans culture filtrate was not inhibited by an FPR antagonist (t-butoxycarbonyl-Met-Leu-Phe), suggesting that a different receptor mediated J774 cell chemotaxis. In conclusion, we have identified a receptor by which a non-serum-dependent chemotactic factor (NSCF) produced by C. albicans induced chemotaxis of PMNs. Additionally, we have shown that NSCF was active across epithelial monolayers. These findings suggest that NSCFs produced by C. albicans and other yeast species may influence host-pathogen interactions at the gastrointestinal tract mucosal surface by inducing phagocytic-cell infiltration.
The number of systemic Candida infections continues to increase among humans due to factors such as immunosuppressive therapeutic regimes, long-term catheterization, broad-spectrum antibiotic use, and increased survival time of immunologically compromised individuals (30, 44, 55). The gastrointestinal (GI) tract is believed to be one site of entry for Candida albicans into the blood stream of immunocompromised individuals (12, 25). C. albicans is commonly found as a commensal in the GI tracts of humans (12), but dissemination from this site is uncommon without immunosuppression, such as suppression of the normal GI bacterial flora and neutropenia (25). The containment of Candida spp. to the GI mucosa as a commensal in immunologically healthy individuals is not fully understood (13, 25, 36, 37).
Both nonspecific and specific immune defenses play roles in protection against disseminated candidiasis. Polymorphonuclear leukocytes (PMNs) have been shown to be the primary components of the host’s innate immune defenses against disseminated candidiasis in in vitro studies, animal models, and studies of neutropenic patients (16). A protective role for macrophages in disseminated candidiasis has also been suggested (2, 3, 42). In a murine model, promotion of a TH1 cell response by the release of interleukin-12 (IL-12) and IL-10 from professional phagocytic cells has been correlated with resistance to systemic candidiasis (43). Additionally, antibodies have been shown to play a protective role against systemic and vaginal candidiasis (9, 21, 31, 32), and they may act by enhancing the activity of professional phagocytic cells (7, 22). Thus, a better understanding of the interactions between C. albicans and professional phagocytic cells would provide valuable insights into how the body protects itself against this opportunistic pathogen. In particular, it is important to further characterize factors released from C. albicans that are recognized by phagocytic cells and attract them to the site of infection or colonization.
In this paper, we describe the production of a culture filtrate containing non-serum-dependent chemotactic factors (NSCFs) from a variety of Candida spp. yeast forms and Saccharomyces cerevisiae. A C. albicans culture filtrate induced the attraction of both human PMNs and the murine macrophage-like cell line J774. Using an in vitro model of PMN migration across the intestinal epithelium (39, 40) (simulating the transmigration of PMNs into the GI lumen), we have observed transmigration of PMNs toward culture filtrates containing the NSCF. We also determined that formyl peptide receptor (FPR)-mediated chemotaxis of human PMNs was responsible for a significant portion of the observed PMN chemotaxis. The results presented in this study suggest that there are at least two NSCFs produced by C. albicans. One NSCF attracts macrophages and the other attracts PMNs.
MATERIALS AND METHODS
Mammalian cell culture.
T84 epithelial cells were purchased from the American Type Culture Collection. J774, clone 8, and wild-type Chinese hamster ovary (CHO-WT) cells were a kind gift from Ira Mellman, Yale University, New Haven, Conn. T84 epithelial cell monolayers were grown in tissue culture dishes and on 0.33-cm2 permeable supports as previously described (10, 40). The cells were maintained in a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F-12 medium supplemented with 50 U of penicillin/ml, 0.05 mg of streptomycin/ml, 5% fetal bovine serum (FBS), and 15 mM HEPES. CHO-WT cells and CHO cells transfected with the human FPR (CHO-FPR) (35) were grown as adherent monolayers in Eagle’s α-modified minimum essential medium (α-MEM) supplemented with 50 U of penicillin/ml, 0.05 mg of streptomycin/ml, and 5% FBS. J774 cells were grown in spinner flasks (Bellco Glass, Inc., Vineland, N.J.) in DMEM containing 50 U of penicillin/ml, 0.05 mg of streptomycin/ml, and 5% FBS.
Isolation of human PMNs.
Normal human PMNs were isolated from noncoagulated citrated blood by a gelatin sedimentation technique, which gave approximately 90% pure PMNs as determined by microscopic evaluation (40). PMNs were suspended at a concentration of 5 × 107/ml, not counting mononuclear cells, in modified Hanks’ balanced salt solution (Sigma Chemical Co., St. Louis, Mo.) containing (per liter) 0.185 g of CaCl2, 0.098 g of MgSO4, 0.4 g of KCl, 0.06 g of KH2PO4, 8 g of NaCl, 0.048 g of Na2HPO4, and 0.01 g of glucose and 10 mM HEPES (pH 7.4; 4°C) [HBSS(+)]. The PMNs were kept on ice for up to 2 h until use.
Yeast.
All yeast strains were from the stock collection at Montana State University and included C. albicans CA-1, A9, 105, 222a, LGH1095Y, and ATCC 64550. Other yeast species used in this study included Candida lusitaniae (ATCC 64125), Candida parapsilosis (ATCC 90018), Candida tropicalis (ATCC 750), Candida glabrata (ATCC 90030), and S. cerevisiae (2180WT). The identification of all strains was confirmed with API 20C yeast identification strips (Analytab Products, Plainview, N.Y.). All isolates were cultured from glycerol stock cultures held at −70°C and plated onto Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.) for 48 h at 37°C. C. albicans CA-1 was used in all experiments except where otherwise noted. This strain was originally characterized by Hasenclever’s original antiserum as a serotype A strain (23, 24). However, by the Candida Check system (Iatron Laboratories Inc., Tokyo, Japan), CA-1 was a serotype B strain. Others have noted discrepancies between the two methods of serotyping (21).
Production of NSCF by yeast isolates.
Culture filtrates containing NSCF were prepared the same way for all yeast isolates. A single yeast colony from a Sabouraud dextrose agar plate was inoculated into glucose (2%)–yeast extract (0.3%)–peptone (1%) broth (GYEPB), incubated to stationary phase at 37°C for 24 h with aeration, and subcultured to fresh 2% GYEPB and incubated to stationary phase. Yeast cells were then harvested by centrifugation, washed three times in HBSS(+), and suspended in HBSS(+) at 5 × 108/ml or other cell concentrations as indicated. The yeast suspensions were incubated for various times at 37°C under vigorous aeration by rotation at approximately 200 rpm (Controlled Environment Incubator Shaker model M52, New Brunswick Scientific Co., Inc. Edison, N.J.). The yeast cells were removed by centrifugation, and the culture supernatant containing the NSCF was filtered through a sterile 0.2-μm-pore-size cellulose acetate filter (Corning Costar Corp., Cambridge, Mass.). The culture filtrates were kept on ice or stored at 4°C until use. No loss of chemotactic activity was observed for culture filtrates stored for up to 1 month. For filtration experiments, 1- and 0.5-kDa cutoff filters (Amicon Inc., Beverly, Mass.) were used.
PMN transmigration across an epithelial monolayer.
For transmigration experiments, T84 intestinal epithelial cell monolayers were grown in cell culture inserts on permeable polycarbonate filters (Corning Costar Corp.) with 5.0-μm-diameter pores exactly as previously described (39). T84 monolayers were cultured both in the standard (apical surface upward) configuration and in the inverted (basolateral surface upward) configuration to permit transepithelial migration in the apical-to-basolateral and basolateral-to-apical directions. The confluence and tight-junction formation of monolayers were determined by measuring the transepithelial resistance with an EVOM epithelial voltohmmeter (World Precision Instruments, Sarasota, Fla.) (27). Prior to the addition of PMNs, the monolayers were washed extensively with HBSS(+) to remove the tissue culture medium containing FBS. For apical-to-basolateral transmigration experiments, 2 × 106 PMNs were added to the top chamber and allowed to transmigrate into the lower well containing either 1 μM fMet-Leu-Phe (fMLF) (as a positive control) or the yeast culture filtrate. In a subset of experiments, transmigration was performed with T84 monolayers in the standard configuration after treatment of the monolayers with 2 mM EDTA for 12 min at 37°C (38) to disrupt the tight junctions. Such experiments were performed exactly like the standard apical-to-basolateral assays except that half the concentration of PMNs was added. For basolateral-to-apical transmigration experiments, 106 PMNs were added to the top well and allowed to transmigrate into the lower well containing either 100 nM f-MLF (as a positive control) or the yeast culture filtrate. After incubating for 110 min at 37°C, the transmigrated cells and cells contained within the T84 cell monolayer were quantified by a myeloperoxidase assay, as previously described (40).
Chemotaxis across a membrane filter.
Cell culture inserts with 5.0- and 8.0-μm-diameter pore sizes were used for chemotaxis of PMN and CHO or J774 cells, respectively. PMNs (106) were suspended in HBSS(+) for chemotaxis assays or in culture filtrate or fMLF (10 nM) for chemokinetic experiments and placed directly in the upper well of the filter insert. For some experiments, the PMNs were treated with t-butoxycarbonyl-Phe-Leu-Phe-Leu-Phe (tBoc-FLFLF) (33 μM final concentration) or dimethyl sulfoxide (DMSO) (0.3% [vol/vol] final concentration) for 10 min at 4°C before being added to the upper well. The PMNs migrated into the lower well containing either 10 nM fMLF (as a positive control) or the yeast culture filtrate. After 110 min at 37°C, the number of PMNs in the lower well was determined by the myeloperoxidase assay. The results were normalized by setting the average value for fMLF-induced chemotaxis to 100.
At 14 to 16 h before they were used in the chemotaxis experiments, 6 mM (final concentration) Na-butyrate was added to the adherent CHO cells to increase cellular protein expression (20). The CHO cells were then harvested from the tissue culture plate surface with 1× trypsin-EDTA (Sigma) in phosphate-buffered saline without Ca2+ and Mg2+ and suspended in α-MEM containing FBS (5%) for 1 h at 37°C. The CHO cells were washed two times with serum-free α-MEM containing 10 mM HEPES (pH 7.4) and suspended to 2 × 106/ml in serum-free α-MEM containing 10 mM HEPES (pH 7.4). For J774 cell chemotaxis experiments, the cells were removed from the spinner flasks, washed two times with serum-free DMEM containing 10 mM HEPES (pH 7.4), and suspended to 2 × 106/ml in serum-free DMEM containing 10 mM HEPES (pH 7.4). For both CHO and J774 cell chemotaxis experiments, 3 × 105 cells (150 μl) were placed in the upper well and yeast culture filtrate or controls were placed in the lower well. For some experiments CHO-WT and CHO-FPR cells were treated with tBoc-Met-Leu-Phe (tBoc-MLF) (33 μM final concentration) or DMSO (0.3% [vol/vol] final concentration) for 10 min before being added to the upper well. As a positive fibroblast migration control, fibronectin (20 μg/ml) was used for both CHO-WT and CHO-FPR cells (1, 33). In chemotaxis experiments, 1 nM fMLF served as the positive control for CHO-FPR cells. Zymosan A (Sigma Chemical Co.) complement-activated human serum was used as the positive control for J774 cell chemotaxis. Zymosan A (1 mg/ml) was added to serum (30 min; 37°C) and pelleted from solution, and the supernatant was used as the positive control. CHO and J774 cell migration was carried out for 4 h at 37°C, after which the filters were fixed with 2.5% paraformaldehyde (Sigma Chemical Co.) for 2 h at room temperature or overnight at 4°C. The cells on the upper side were removed from the filter with a cotton swab, and those that had migrated to the underside were stained with hematoxylin (Sigma Chemical Co.). After being stained, the upper side of the filter was again swabbed with cotton. For analysis, the filter was removed from the plastic holder. Quantification of cells was performed with a computerized image analysis system (Imaging Research M4 True Color; Imaging Research, St. Catherines, Ontario, Canada) to determine the average cell area (in square micrometers) per field from an average of 10 randomly chosen (avoiding the periphery) 40× fields. The results are equalized by setting the average value for chemotaxis induced by fMLF or zymosan A-activated serum to 100.
RESULTS
Production of NSCF from C. albicans yeast.
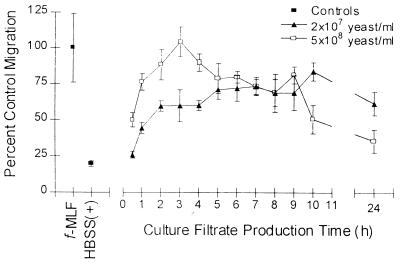
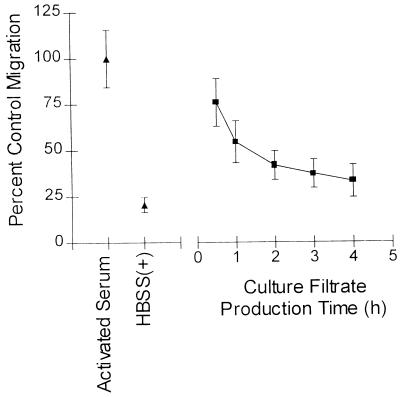
Samples of culture filtrates were taken at various times from HBSS(+) cultures inoculated with either 2 × 107 or 5 × 108 yeast cells/ml. As shown in Fig. 1, a time dependence for production of chemotactic activity in the culture filtrate was observed. Chemotactic activity consistently peaked at 1 to 3 h for culture filtrates from the 5 × 108 yeast cell/ml culture. Production of the chemotactic activity from the 2 × 107 yeast cell/ml culture was more delayed. The activity in the 2 × 107 yeast cell/ml culture leveled out at later time points and remained consistently high compared to the 5 × 108 yeast cell/ml culture, which showed a steady decrease after the early activity peak. The chemotactic activities in the cultures at the 24-h time point differed dramatically. In the high yeast concentration culture, the activity had dropped to almost the level of the negative control at 24 h, whereas the activity in the culture produced with a low concentration of yeast remained high at 24 h.
FIG. 1.
Production of NSCF by C. albicans is time and yeast concentration dependent. Chemotaxis of PMNs toward C. albicans culture filtrates produced with 2 × 107 yeast cells/ml or 5 × 108 yeast cells/ml for 0.5 to 24 h is shown. PMN chemotaxis was assessed by quantification of the total number of PMNs found in the lower reservoir by a myeloperoxidase assay. HBSS(+) was used as a negative control, and fMLF (10 nM) was used as a positive control. The data points are from a representative experiment. All samples were run in triplicate. The error bars indicate the standard deviations.
To confirm that the culture filtrate contained a chemotactic factor rather than a chemokinetic factor, a checkerboard analysis was performed (Tables 1 and 2). PMN migration was found to be maximal when undiluted culture filtrate was used. With the addition of culture filtrate into the upper well, a decrease in migration was observed, suggesting that the activity was mostly due to chemotaxis rather than chemokinesis (Table 1). Similar results were obtained when PMNs were allowed to migrate toward fMLF in the presence or absence of fMLF in the upper well (Table 2). These results suggest that the C. albicans culture filtrate stimulated PMN chemotaxis and contained chemokinetic activity at a level similar to that of 10 μM fMLF.
TABLE 1.
Checkerboard assay of PMN chemotaxis toward C. albicans culture filtratea
| Culture filtrate dilution in lower well | No. of PMNs (104) at culture filtrate dilution in upper well of:
|
||||
|---|---|---|---|---|---|
| 0 | 1:8 | 1:4 | 1:2 | Undil. | |
| 0 | 1.7 ± 0.5 | 1.2 ± 0.2 | 1.4 ± 0.3 | 2.3 ± 0.6 | 1.8 ± 0.3 |
| 1:8 | 3.7 ± 0.4 | 1.3 ± 0.2 | 1.8 ± 0.3 | 1.5 ± 0.2 | 1.7 ± 0.3 |
| 1:4 | 6.2 ± 0.6 | 1.5 ± 0.3 | 1.1 ± 0.2 | 2.9 ± 0.8 | 2.8 ± 0.6 |
| 1:2 | 19.1 ± 0.7 | 3.7 ± 0.7 | 3.5 ± 0.5 | 7.0 ± 0.9 | 7.7 ± 0.8 |
| Undil. | 32.9 ± 0.6 | 15.6 ± 2.7 | 12.7 ± 3.0 | 22.7 ± 1.2 | 14.3 ± 0.8 |
C. albicans culture filtrate produced by 5 × 108 yeast cells/ml for 1 h. Samples were diluted with HBSS(+). PMN chemotaxis was assessed by quantification of the total number of PMNs found in the lower reservoir by a myeloperoxidase assay. The results shown are the number of PMNs migrated (104). The results are the average of samples run in triplicate ± the standard deviation. Undil., undiluted.
TABLE 2.
Checkerboard assay of fMLF-induced PMN chemotaxisa
| fMLF concn in lower well (nM) | No. of PMNs (104) at fMLF concn (nM) in upper well of:
|
|
|---|---|---|
| 0 | 10 | |
| 0 | 1.7 ± 0.5 | 1.2b |
| 10 | 12.4 ± 0.8 | 4.7b |
Samples were diluted with HBSS(+). PMN chemotaxis was assessed by quantification of the total number of PMNs found in the lower reservoir by a myeloperoxidase assay. The results are the number of PMNs migrated (104). The results are the average of samples run in triplicate ± the standard deviation.
Samples run in duplicate only.
Characterization of NSCF.
NSCF stability, size range, and culture parameters were determined (Table 3). Culture filtrate from a 1-h culture retained NSCF activity for over 1 month at 4°C. NSCF activity was lost following filtration of active culture filtrates through a cellulose acetate filter with a glass prefilter, presumably due to adherence to the glass filter (data not shown). NSCF activity in the culture filtrate was not retained by a 1-kDa cutoff filter, and only partial activity for PMNs was recovered in the retentate when a 0.5-kDa cutoff filter was used (Table 3). These results suggest that the NSCF is a small molecule with an apparent molecular mass between 0.5 and 1 kDa. The chemotactic activity of C. albicans NSCF was concentration dependent (Table 1). Dilution analyses of culture filtrates made with 5 × 108 yeast cells/ml for 0.5 and 1 h revealed that the chemotactic activity decreased to that of the negative control at a greater-than-eightfold dilution (data not shown). It was also determined that glucose was not required for the production of NSCF. However, no activity was detected when yeast culture filtrates were produced without Ca2+ and Mg2+ (Table 3). Addition of the divalent cations to the culture filtrate did not restore NSCF activity (data not shown).
TABLE 3.
NSCF characteristicsa
| Characteristic | Conditions | PMN chemotaxis | J774 chemotaxis |
|---|---|---|---|
| Stabilityb | 4°C; ≥1 mo | Yes | Yes |
| 56°C; 30 min | Yes | Yes | |
| Boiling; 10 min | No | Yes | |
| Sizec | 1-kDa retentate | 21.9 ± 6.1 | 22.5 ± 14.8 |
| 1-kDa filtrate | 95.2 ± 27.6 | 119.7 ± 18.0 | |
| 0.5-kDa retentate | 37.0 ± 5.0 | NDe | |
| 0.5-kDa filtrate | 59.8 ± 21.8 | ND | |
| Production requirementsd | Glucose | Chemotactic | Chemotactic |
| Divalent cations | Not chemotactic | Chemotactic |
Characterization of the NSCF in a C. albicans 1-h culture filtrate. PMN chemotaxis was assessed by quantification of the total number of PMNs found in the lower reservoir by a myeloperoxidase assay.
Culture filtrate was held at 4°C (for ≥1 month), 56°C (30 min), and boiling (10 min) and then tested for PMN chemotactic activity.
Culture filtrate was passed through 1- and 0.5-kDa cutoff filters. The retentate was diluted to the original volume with HBSS(+) to avoid concentration effects and then tested for PMN chemotactic activity. The values given are the percentages of activity retained in the retentate compared to the nonfiltered 1-h culture filtrate sample value that was set to 100. Migration of PMNs toward HBSS(+) was 14.2 ± 6.8. Migration of J774 cells toward HBSS(+) was 15.5 ± 5.3.
A C. albicans 1-h culture filtrate was produced by using HBSS(+) without glucose and HBSS without Ca2+ and Mg2+ [HBSS(−)]. Divalent cations were added back to the HBSS(−) culture filtrate (0.185 g of CaCl2/liter and 0.098 g of MgSO4/liter) before determination of PMN chemotaxis.
ND, not determined.
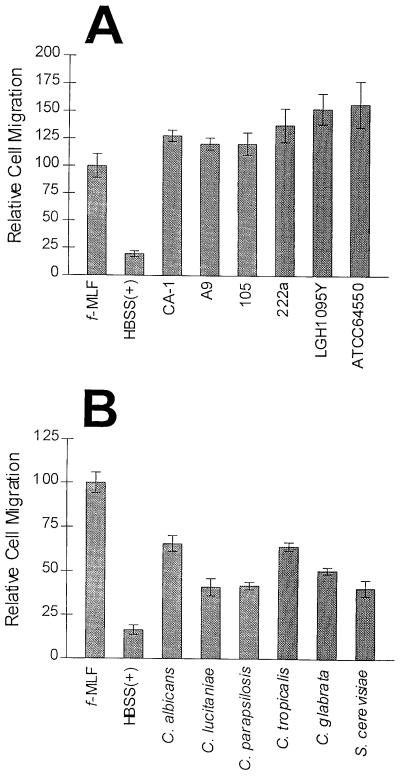
To examine whether the production of the NSCF was strain or species dependent, we tested the production of the factor by six different strains of C. albicans, a strain of S. cerevisiae, and a variety of Candida spp. (Fig. 2). For all strains and species, 1-h culture filtrates stimulated PMN chemotaxis significantly more than the negative control (Student’s t test; P values ranged from 0.003 to <0.0001). Chemotaxis differences observed in Fig. 2 are possibly due to PMN donor variability. Differences in the degree of chemotaxis toward the same chemoattractants by different donors’ PMNs were routinely observed.
FIG. 2.
NSCF production is not species or strain dependent. (A) Chemotaxis of PMNs toward 1-h culture filtrates produced by six different C. albicans strains. (B) Chemotaxis of PMNs toward 1-h culture filtrates produced by various Candida spp. and S. cerevisiae yeast forms. The C. albicans strain is CA-1. Chemotaxis was assessed by quantification of the total number of PMNs found in the lower reservoir by a myeloperoxidase assay. The data are expressed as the mean ± standard error of the mean from three different experiments, with samples run in triplicate. The data points were equalized by setting the migration toward fMLF (10 nM) to 100.
C. albicans NSCF stimulates chemotaxis by binding to the FPR.
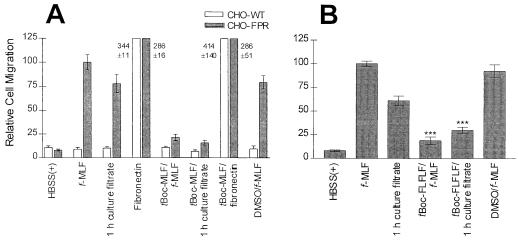
We previously generated a CHO cell line stably expressing the human FPR (CHO-FPR) and showed that these cells migrated toward a gradient of formylated peptides (34). Our preliminary characterization revealed that the size of the NSCF appeared to be similar to those of formylated peptides; thus, we performed experiments to determine if the NSCF could stimulate CHO-FPR chemotaxis. As seen in Fig. 3C and 4A, CHO-FPR cells displayed chemotaxis toward the 1-h culture filtrate whereas CHO-WT cells did not (Fig. 4A). Both CHO-FPR and CHO-WT cells displayed chemotaxis toward fibronectin (20 μg/ml), a natural chemoattractant for fibroblasts (1, 33) (Fig. 4A). Chemotaxis of CHO-FPR cells toward the culture filtrate was inhibited by the FPR antagonist tBoc-MLF, confirming that the CHO-FPR cell chemotaxis was mediated by FPR. In addition, the FPR antagonist tBoc-FLFLF inhibited the chemotaxis of PMNs toward the 1-h culture filtrate by approximately 51% (Fig. 4B).
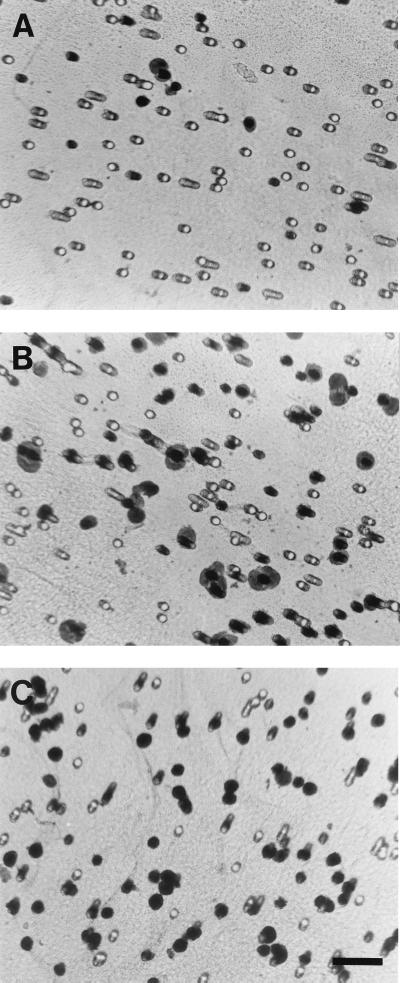
FIG. 3.
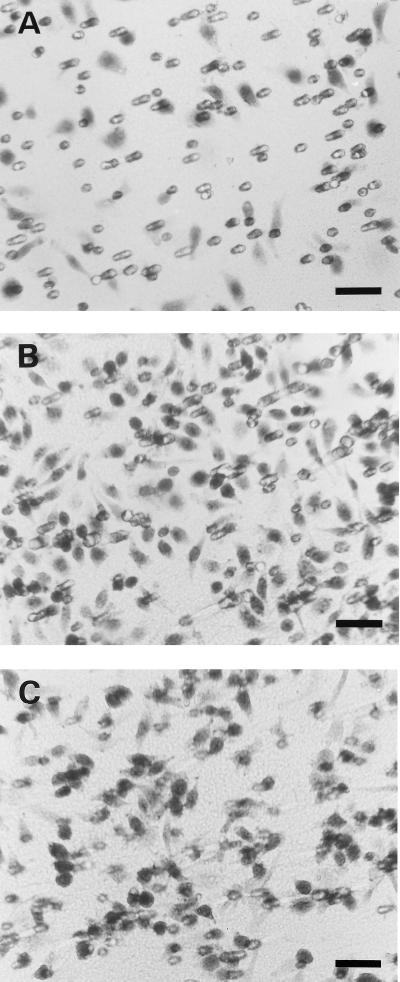
Migration of CHO-FPR cells toward C. albicans 1-h culture filtrate. CHO-FPR cells migrated through 8.0-μm semiporous supports for 4 h at 37°C toward HBSS(+) (A), fMLF (1 nM) (B), and 1-h culture filtrate (C). CHO-FPR cells adhering to the underside of the support were stained with hematoxylin. Bar, 50 μm.
FIG. 4.
C. albicans culture filtrate contains a chemotactic factor that activates chemotaxis through FPR. CHO-WT cells and CHO-FPR cells (A) or PMNs (B) were stimulated to migrate toward C. albicans 1-h culture filtrate or fMLF (1 nM). Fibronectin (0.02 mg/ml) was used as a positive control for chemotaxis of CHO cells. Some CHO cells and PMNs were preincubated with the FPR antagonist tBoc-MLF (10 μM; 10 min; 37°C) or tBoc-FLFLF (10 μM; 10 min; 4°C), respectively, before being placed in the upper well. The final concentration of DMSO in the wells containing agonist was 33 μM. This concentration of DMSO by itself did not significantly decrease chemoattractant-induced migration. (A) Chemotaxis of CHO cells was assessed by quantification of the average cell area of hematoxylin-stained cells that had migrated and adhered to the underside of the porous support. A total of 10 randomly chosen 40× fields were examined. The data are expressed as the mean ± standard error of the mean (SEM) from three different experiments. The data points were equalized by setting the migration of CHO-FPR cells toward fMLF to 100. (B) Chemotaxis of PMNs was assessed by quantification of the total number of PMNs found in the lower reservoir by a myeloperoxidase assay. The data are expressed as the mean ± SEM from three different experiments, with samples run in triplicate for each experiment. The data points were equalized by setting the migration toward fMLF (10 nM) to 100. ***, Student’s t test; P = 0.0001.
PMN transmigration across a cultured intestinal epithelial monolayer.
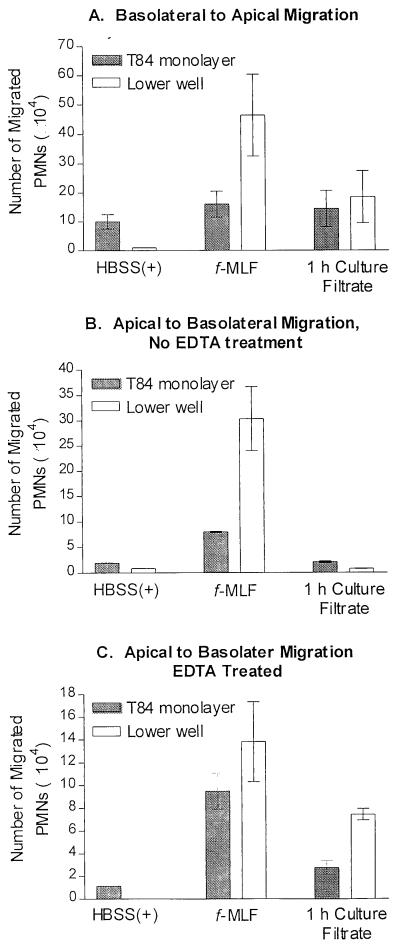
Since the GI tract is considered to be a major portal of entry to the bloodstream, we tested whether the culture filtrate could attract PMNs across a monolayer of T84 intestinal epithelial cells grown on permeable supports (39). As shown in Fig. 5A, the filtrate from a 1-h C. albicans culture stimulated basolateral-to-apical migration of PMNs. To determine if the polarity of the monolayer affected the PMN transmigration, we also examined the chemotaxis in the opposite direction (apical to basolateral). As expected, based on previous results of Parkos et al. (40), the 1-h culture filtrate was unable to induce transmigration in the nonphysiological direction, from the apical side to the basolateral side (Fig. 5B). However, transmigration in the apical-to-basolateral direction toward the culture filtrate could be induced after transient disruption of epithelial tight junctions by Ca2+ chelation (38) (Fig. 5C). Thus, NSCF can drive polarized transmigration in a physiologically relevant direction but fails to induce migration in the reverse direction unless barrier function is disrupted.
FIG. 5.
Transmigration through a monolayer of T84 cells toward 1-h C. albicans culture filtrate. (A) Transmigration in the physiological direction, basolateral to apical. (B) Transmigration in the nonphysiological direction, apical to basolateral, without the removal of extracellular Ca2+. (C) Transmigration of cells in the nonphysiological direction with prior treatment of the T84 cells with EDTA to remove extracellular Ca2+ that results in breaking epithelial tight junctions. PMN transmigration was assessed by quantification of the total number of PMNs found in the T84 cell monolayer and lower reservoir by the myeloperoxidase assay. The data are expressed as the mean ± standard deviation from a single experiment representative of three separate experiments. Determination of the number of PMNs contained within the monolayer was only performed for the data shown. For each experiment, all samples were run in triplicate.
Finally, to confirm that the culture filtrate itself did not damage the epithelial monolayer or alter the tight-junction permeability, we examined the monolayer resistance. After a 5-h incubation with culture filtrate in the apical compartment and HBSS(+) in the basolateral compartment, or vice versa, no change in resistance was observed (data not shown). Activation of superoxide production by PMNs may also effect the epithelial monolayer while at the same time altering the myeloperoxidase assay results. Therefore, we determined whether the C. albicans culture filtrate stimulated superoxide production from PMNs by using a cytochrome c microplate assay (11, 41). The culture filtrate did not stimulate the production of superoxide from PMNs, suggesting that the culture filtrate does not activate the NADPH oxidase (data not shown). Superoxide production was only observed after the addition of phorbol 12-myristate 13-acetate and did not occur in the presence of superoxide dismutase (data not shown).
Chemotaxis of the murine macrophage-like cell line J774.
In addition to PMNs, macrophages are also important in host defense against disseminated candidiasis (42). A J774 cell line was chosen as a model of murine macrophages and tested for chemotactic activity toward the NSCF. J774 cells displayed chemotaxis toward C. albicans 1-h culture filtrate (Fig. 6 and 7). A trend in activity over time similar to that for PMNs was noted (Fig. 7), except that the highest chemotactic activity occurred at 0.5 h instead of at 1 h. The filtrate from a 1-kDa ultrafiltration induced chemotaxis of J774 cells, suggesting that the size of the NSCF for J774 cells was similar to that for PMNs (data not shown and Table 3). We were unable to induce chemotaxis of J774 cells toward fMLF (10−5 to 10−10 M) (data not shown), which corresponded with evidence that murine macrophages do not express a high-affinity FPR (19). Furthermore, we examined whether mannoproteins released from C. albicans were responsible for the migration of J774 cells, since J774 cells have previously been shown to express the mannose receptor (4). Chemotaxis experiments were performed with a wide concentration range of 2-β-mercaptoethanol (2-ME) C. albicans cell wall extract (10−4 to 103 μg/ml) diluted in HBSS(+). This extract contains the C. albicans cell wall phosphomannan complex, primarily mannan with about 3.5% protein, that has been identified as responsible for attachment of C. albicans to the splenic and lymph node macrophages in mice (24). No significant chemotaxis toward 2-ME extract above the negative control was observed for J774 cells (data not shown). In addition, pretreatment of J774 cells with the 2-ME extract did not decrease J774 chemotaxis toward the C. albicans 1-h culture filtrate.
FIG. 6.
J774 cells migrate toward C. albicans 1-h culture filtrate. Migration of J774 cells through 8.0-μm semiporous supports for 4 h at 37°C toward HBSS(+) (A), zymosan A-activated human serum (B), and 1-h culture filtrate (C) is shown. J774 cells adhering to the underside of the support were stained with hematoxylin. Bars, 50 μm.
FIG. 7.
C. albicans culture filtrate contains a chemotactic factor for the murine macrophage-like J774 cells. Chemotaxis of J774 cells towards C. albicans 0.5-, 1-, 2-, 3-, and 4-h culture filtrates (■) is shown. Chemotaxis was assessed by quantification of the average cell area of hematoxylin-stained J774 cells that adhered to the underside of the porous support. Activated serum was used as the positive control, and HBSS(+) was used as the negative control. The average cell area of 10 randomly chosen 40× fields was determined for each sample. The data are expressed as the mean ± standard error of the mean from three different experiments. The data points were equalized by setting the migration of J774 cells toward zymosan A-activated serum to 100.
The stability of the J774 NSCF was also noted to differ from that of the PMN NSCF (Table 2). Neither heating at 56°C for 30 min nor boiling for 10 min decreased the chemotactic activity. These results suggest that the NSCF stimulating J774 cell chemotaxis produced by C. albicans yeast cells is different than the C. albicans NSCF for PMNs and interacts with an unknown receptor on J774 cells.
DISCUSSION
Previous studies have shown that C. albicans yeast cells produce a NSCF for PMNs (5, 6, 15, 52). Our results confirmed and extended the scope of the previous findings. We showed that FPR-mediated chemotaxis accounted for approximately half of the PMN chemotaxis toward NSCF(s) in the C. albicans culture filtrate. Furthermore, we demonstrated that the NSCF(s) attracts PMNs across an intestinal epithelial cell monolayer in vitro. We also provide evidence for an additional chemotactic agent that stimulates chemotaxis of the murine macrophage-like cell line J774 by a different receptor.
We found similarities and differences between the NSCF described here and those described by others. Consistent with other studies that implied a role for a NSCF in cutaneous candidiasis (6), we found that the C. albicans NSCF is produced in the absence of glucose. NSCF chemotactic activity was found to peak at 1 h of incubation at a concentration of 5 × 108 yeast cells/ml, whereas others have demonstrated that up to 12 h is necessary for production of chemotactic activity (15). Such discrepancies might be due to differences in the concentration of yeast, the chemotactic assays, and the species from which PMNs were obtained. When a lower concentration of yeast was used, a longer incubation time was required for chemotactic activity to equal that of 5 × 108 yeast cells/ml. Others have noted a correlation between virulence and decreased stimulation of PMN chemotaxis by specific C. albicans strains (54). However, in the results reported here, S. cerevisiae (a species rarely implicated in disease) and all Candida spp. tested produced NSCFs for human PMNs. In addition to these findings, others have shown that Trichophyton mentagrophytes and Blastomyces dermatitidis release low-molecular-weight chemotactic substances for PMNs (48, 49). Therefore, a wide range of fungi can produce non-serum-dependent PMN chemoattractants.
The decrease in chemotactic activity observed with a higher dose of C. albicans might be expected if an inhibitor of chemotaxis was produced after the initial production of the NSCF. The decrease in activity did not result from saturating amounts of chemoattractant, since NSCF activity could not be rescued by dilution of a 4-h culture filtrate (data not shown). It is also possible that denaturation or degradation of the NSCF occurs such that it no longer has chemotactic activity.
Our data implicated FPR as a receptor for a C. albicans NSCF. Preincubation of CHO-FPR and PMN with FPR antagonists significantly decreased chemotaxis toward the culture filtrate (Fig. 4). Standard ligands for FPR consist of bacterial and mitochondrial peptides that are synthesized with N-formylmethionine as the starting residue (8, 28, 45–47). Some nonformylated peptides have also been shown to stimulate FPR-mediated chemotaxis, but with the exception of a few cases (18), the chemotactic activity of these peptides was significantly lower than that of their formylated counterparts (17, 51). If the NSCF that interacts with FPR is a formylated peptide, the most obvious place of origin would be the mitochondrion. C. albicans mitochondrial proteins may be actively released or released as a byproduct of yeast cell death during culture filtrate production. However, examination of cell death in cultures of 1 to 4 h does not support cell death as a source. Propidium iodide-stained cells analyzed by flow cytometry (29) showed that cell death did not increase with time but remained less than 1% throughout the production time, 1 to 4 h (data not shown). Experiments are currently underway to determine if the factor is in fact an N-formylated peptide and mitochondrial in origin. Because the antagonist inhibited PMN chemotaxis toward the culture filtrate by only approximately 50%, it is possible that other chemotactic factors contained in the culture filtrate and chemotactic receptors expressed by PMNs are involved in the observed chemotactic response. Other known chemotactic receptors expressed by human PMNs are C5a receptor, C3a receptor, platelet activating factor receptor, C-X-C chemokine receptors (such as IL-8 receptor A and IL-8 receptor B), and C-C chemokine receptor 1. The ligands for the chemokine receptors are about 8 to 10 kDa, suggesting that these receptors are unlikely candidates for binding NSCF unless they also bind low-molecular-mass factors. C5a receptor binds a 74-amino-acid peptide but has also been shown to bind smaller peptides with lower affinity (26). However, CHO cells expressing C5a receptor showed no chemotaxis toward the C. albicans 1-h culture filtrate (data not shown), suggesting that NSCFs do not act as agonists for C5a receptor-mediated chemotaxis.
To investigate the relevance of our finding with respect to the GI tract, we used an in vitro T84 cell monolayer system to examine whether the C. albicans NSCF can attract PMNs through an epithelial monolayer. Due to the complexity of the GI tract, a reductionistic approach to unraveling the interactions of C. albicans at the GI epithelium as a commensal and potential pathogen is necessary. As shown here, this in vitro-model system is useful for identification of putative C. albicans colonization factors and mechanisms of dissemination from the human GI tract. The C. albicans culture filtrate induced transmigration of PMNs in the physiological direction across a T84 epithelial cell monolayer (Fig. 5A), suggesting that the release of low-molecular-weight molecules by C. albicans helps to recruit PMNs into the gut. Since NSCFs induced chemotaxis of PMNs in the absence of the T84 cells, the factor(s) is not likely to be epithelium derived. However, it is possible that the NSCF induced T84 cells to release other chemotactic agents, such as IL-8, which stimulates PMN transmigration. Because fMLF has been shown to cross model intestinal epithelial monolayers by the paracellular pathway (50), it is highly probable that the NSCF contained in the culture filtrate is crossing the T84 monolayer by the paracellular pathway to stimulate the transepithelial migration of PMN. The production of secreted aspartyl proteinases by C. albicans has been suggested to facilitate hematogenous dissemination from the gut by digesting the mucin layer (14) and has also been shown to be chemotactic as well as chemokinetic for human PMNs (52). Thus, secreted aspartyl proteinases and the NSCFs described in this study may act together to stimulate PMN infiltration.
We also examined whether C. albicans culture filtrate stimulated the migration of the macrophage cell line J774. To our knowledge this is the first report of a NSCF produced by C. albicans for macrophages. Unlike the NSCF for PMNs, the production of the chemotactic factor for J774 cells peaked sooner and the activity remained stable when the 1-h culture filtrate was boiled for 10 min. In addition, despite using a wide concentration range of fMLF (10−5 to 10−10 M), we were unable to stimulate J774 cell migration, suggesting that the cells lack FPR expression and that the NSCF contains a factor which is a ligand for a separate chemotactic receptor. These results support existing data showing that murine macrophages may lack FPR expression (19, 53).
We provide evidence in this study that C. albicans, along with other yeast species, produces a NSCF for human PMNs which may have immunoregulatory activity at sites where there is decreased complement activity. The ability of the culture filtrate to induce transmigration of PMNs across the T84 monolayer from the basolateral to the apical side suggests that the NSCF influences the host-pathogen interactions in the GI tract by stimulating an infiltration of leukocytes.
ACKNOWLEDGMENTS
We thank Marcia Riesselman for providing yeast isolates and strain and species identification information.
This work was supported by a Medical Mycology Predoctoral Training Grant funded by the National Institute of Allergy and Infectious Diseases (5T32AW7465) and by Montana State University. Additionally, this work was partially supported by NIH grants AI40108-02, AI22735-10, HL54229, and HL60540; a biomedical grant; and an Arthritis Foundation investigator award.
REFERENCES
- 1.Aznavoorian S, Stracke M L, Drutzsch H, Schiffmann E, Liotta L A. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baghian A, Lee K W. Role of activated macrophages in resistance to systemic candidosis. J Leukoc Biol. 1988;44:166–171. doi: 10.1002/jlb.44.3.166. [DOI] [PubMed] [Google Scholar]
- 3.Bistoni F, Vecchiarelli A, Cenci E, Puccetti P, Marconi P, Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infections. Infect Immun. 1986;51:668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum J S, Stahl P D, Diaz R, Fiani M L. Purification and characterization of the d-mannose receptor from J774 mouse macrophage cells. Carbohydr Res. 1991;213:145–153. doi: 10.1016/s0008-6215(00)90605-0. [DOI] [PubMed] [Google Scholar]
- 5.Brasch J, Schröder J M, Christophers E. Serum-independent neutrophil chemotaxins in the yeast phase of Candida albicans. Mycoses. 1991;34:35–39. doi: 10.1111/j.1439-0507.1991.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 6.Brasch J, Schröder J M, Christophers E. Candida albicans grown in glucose-free media contains serum-independent chemotactic activity. Acta Derm Venereol. 1992;72:1–3. [PubMed] [Google Scholar]
- 7.Caesar-TonThat T C, Cutler J E. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect Immun. 1997;65:5354–5357. doi: 10.1128/iai.65.12.5354-5357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63:2619–2624. doi: 10.1128/iai.63.7.2619-2624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cereijido M, Sabatini D D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–876. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman-Kirkland E S, Wasvary J S, Seligmann B E. Superoxide anion production from human neutrophils measured with an improved kinetic and endpoint microassay. J Immunol Methods. 1991;142:95–104. doi: 10.1016/0022-1759(91)90296-r. [DOI] [PubMed] [Google Scholar]
- 12.Cole G T, Halawa A A, Anaissie E J. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis. 1996;22:S73–S88. doi: 10.1093/clinids/22.supplement_2.s73. [DOI] [PubMed] [Google Scholar]
- 13.Cole G T, Seshan K R, Lynn K T, Franco M. Gastrointestinal candidiasis: histopathology of Candida-host interactions in a murine model. Mycol Res. 1993;97:385–408. [Google Scholar]
- 14.Colina A, Aumont F, Deslauriers N, Belhumeur P, De Repentigny L. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect Immun. 1996;64:4514–4519. doi: 10.1128/iai.64.11.4514-4519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler J E. Chemotactic factor produced by Candida albicans. Infect Immun. 1977;18:568–573. doi: 10.1128/iai.18.3.568-573.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond R. Interactions of phagocytic cells with Candida and other opportunistic fungi. Arch Med Res. 1993;24:361–369. [PubMed] [Google Scholar]
- 17.Freer R J, Day A R, Radding J A, Schiffmann E, Aswanikumar S, Showell H J, Becker E L. Further studies on the structural requirements for synthetic peptide chemoattractants. Biochemistry. 1980;19:2404–2410. doi: 10.1021/bi00552a019. [DOI] [PubMed] [Google Scholar]
- 18.Gao J L, Becker E L, Freer R J, Muthukumaraswamy N, Murphy P M. A high potency nonformylated peptide agonist for the phagocyte N-formylpeptide chemotactic receptor. J Exp Med. 1994;180:2191–2197. doi: 10.1084/jem.180.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J L, Murphy P M. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- 20.Gorman C M, Howard B H. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983;11:7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, Cutler J E. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis. 1997;175:1169–1175. doi: 10.1086/516455. [DOI] [PubMed] [Google Scholar]
- 23.Hazen K C, Brawner D L, Riesselman M H, Jutila M A, Cutler J E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanbe T, Han Y, Redgrave B, Riesselman M H, Cutler J E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993;61:2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy M J. Regulation of Candida albicans populations in the gastrointestinal tract: mechanisms and significance in GI and systemic candidiasis. Curr Top Med Mycol. 1989;3:315–402. doi: 10.1007/978-1-4612-3624-5_11. [DOI] [PubMed] [Google Scholar]
- 26.Konteatis Z D, Siciliano S J, Van Riper G, Molineaux C J, Pandya S, Fischer P, Rosen H, Mumford R A, Springer M S. Development of C5a receptor antagonists; differential loss of functional responses. J Immunol. 1994;153:4200–4205. [PubMed] [Google Scholar]
- 27.Madara J L, Colgan S P, Nusrat A, Delp C, Parkos C A. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil-epithelial interactions. J Tissue Cult Methods. 1992;14:209–216. [Google Scholar]
- 28.Marasco W A, Phan S H, Krutzsch H, Showell H J, Feltner D E, Nairn R, Becker E L, Ward P A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984;259:5430–5439. [PubMed] [Google Scholar]
- 29.Martin E, Schlasius U, Bhakdi S. Flow cytometric assay for estimating fungicidal activity of amphotericin B in human serum. Med Microbiol Immunol. 1992;181:117–126. doi: 10.1007/BF00202051. [DOI] [PubMed] [Google Scholar]
- 30.Martino P, Girmenia C, Micozzi A, Dernardis F, Boccanera M, Cassone A. Prospective study of Candida colonization; use of empiric amphotericin B and development of invasive mycosis in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1994;13:797–804. doi: 10.1007/BF02111339. [DOI] [PubMed] [Google Scholar]
- 31.Matthews R C, Burnie J P. Acquired immunity to systemic candidiasis in immunodeficient mice: role of antibody to heat-shock protein 90. J Infect Dis. 1992;166:1193–1194. doi: 10.1093/infdis/166.5.1193. [DOI] [PubMed] [Google Scholar]
- 32.Matthews R C, Burnie J P, Howat D, Rowland T, Walton F. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy J B, Furcht L T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984;98:1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miettinen H M, Gripentrog J M, Jesaitis A J. Chemotaxis of Chinese hamster ovary cells expressing the human neutrophil formyl peptide receptor: role of signal transduction molecules and α5β1 integrin. J Cell Sci. 1998;111:1921–1928. doi: 10.1242/jcs.111.14.1921. [DOI] [PubMed] [Google Scholar]
- 35.Miettinen H M, Mills J S, Gripentrog J M, Dratz E A, Granger B L, Jesaitis A J. The ligand binding site of the formyl peptide receptor maps in the transmembrane region. J Immunol. 1997;157:4045–4054. [PubMed] [Google Scholar]
- 36.Odds F C. Candida and candidosis; a review and bibliography. Philadelphia, Pa: Bailliere Tindall; 1988. [Google Scholar]
- 37.Odds F C. Potential for penetration of passive barriers to fungal invasion in humans. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 287–295. [Google Scholar]
- 38.Parkos C A, Colgan S P, Bacarra A E, Nusrat A, Delp-Archer C, Carlson S, Su D H C, Madara J L. Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am J Physiol. 1995;268:C472–C479. doi: 10.1152/ajpcell.1995.268.2.C472. [DOI] [PubMed] [Google Scholar]
- 39.Parkos C A, Colgan S P, Madara J L. Interactions of neutrophils with epithelial cells: lessons from the intestine. J Am Soc Nephrol. 1994;2:138–152. doi: 10.1681/ASN.V52138. [DOI] [PubMed] [Google Scholar]
- 40.Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium. J Clin Investig. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- 42.Qian Q, Jutila M A, Van Rooijen N, Cutler J E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–5008. [PubMed] [Google Scholar]
- 43.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 44.Samonis G, Gikas A, Anaissie E J, Vrenzos G, Maraki S, Tselentis Y, Bodey G P. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob Agents Chemother. 1993;37:51–53. doi: 10.1128/aac.37.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffmann E, Corcoran B A, Wahl S M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffmann E, Showell H V, Corcoran B A, Ward P A, Smith E, Becker E L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975;114:1831–1837. [PubMed] [Google Scholar]
- 47.Shawar S M, Rich R R, Becker E L. Peptides from the amino-terminus of mouse mitochondrially encoded NADH dehydrogenase subunit 1 are potent chemoattractants. Biochem Biophys Res Commun. 1995;211:812–818. doi: 10.1006/bbrc.1995.1884. [DOI] [PubMed] [Google Scholar]
- 48.Tagami H, Natsume N, Aoshima T, Inoue F, Suehisa S, Yamada M. Analysis of transepidermal leukocyte chemotaxis in experimental dermatophytosis in guinea pigs. Arch Dermatol Res. 1982;273:205–217. doi: 10.1007/BF00409248. [DOI] [PubMed] [Google Scholar]
- 49.Thurmond L M, Mitchell T G. Blastomyces dermatitidis chemotactic factor; kinetics of production and biological characterization evaluated by a modified neutrophil chemotaxis assay. Infect Immunol. 1984;46:87–95. doi: 10.1128/iai.46.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tiehl T E, Stenson W F. Mechanisms of transit of lipid mediators of inflammation and bacterial peptides across intestinal epithelia. Am J Physiol. 1994;267:G687–G695. doi: 10.1152/ajpgi.1994.267.4.G687. [DOI] [PubMed] [Google Scholar]
- 51.Toniolo C, Crisma M, Moretto V, Freer R J, Becker E L. Nα-formulated and tert-butyloxycarbonylated Phe-(Leu-Phe)n and (Leu-Phe)n peptides as agonists and antagonists of the chemotactic formylpeptide receptor of the rabbit peritoneal neutrophil. Biochim Biophys Acta. 1990;1034:67–72. doi: 10.1016/0304-4165(90)90154-o. [DOI] [PubMed] [Google Scholar]
- 52.Tsuboi R, Ran Y-P, Ogawa H. Candida albicans proteinase acts as a chemoattractant for peripheral neutrophils. In: Suzuki S, Suzuki M, editors. Fungal cells in biodefense mechanisms. Tokyo, Japan: Saikon Publishing Co., Ltd.; 1996. pp. 111–115. [Google Scholar]
- 53.Walker B A M, Seiler A J, Owens C A, Hagenlocker B E, Ward P A. Absence of FMLF receptors on rat macrophages. J Leukoc Biol. 1991;50:600–606. doi: 10.1002/jlb.50.6.600. [DOI] [PubMed] [Google Scholar]
- 54.Weeks B A, Hamilton P B. Abstracts of the 75th Annual Meeting of the American Society for Microbiology 1975. Washington, D.C: American Society for Microbiology; 1975. Differential chemotaxis of polymorphonuclear leukocytes by strains of Candida albicans, abstr. F23; p. 89. [Google Scholar]
- 55.Wingard J R. Infections due to resistant Candida species in patients with cancer who are receiving chemotherapy. Clin Infect Dis. 1994;19:S49–S53. doi: 10.1093/clinids/19.supplement_1.s49. [DOI] [PubMed] [Google Scholar]