Abstract
There exist several viable first-line treatment options for metastatic renal cell carcinoma, making the choice of initial therapy difficult. Metrics other than patient factors, such as cost, may assist in selecting the most appropriate therapy. Using data from CheckMate 214, KEYNOTE 426, and JAVELIN Renal 101 and including costs of medication administration, monitoring, and adverse events, a cost-effectiveness analysis revealed that nivolumab + ipilimumab is more cost-effective than both pembrolizumab + axitinib and avelumab + axitinib.
Background:
There now exist several viable first-line treatment options for metastatic renal cell carcinoma, making the choice of initial therapy difficult. Considering metrics other than patient factors may be necessary to select the most appropriate therapy. We aimed to assess the cost-effectiveness of the three combination therapies currently approved in treatment-naïve advanced or metastatic renal cell carcinoma—nivolumab + ipilimumab (NI), pembrolizumab + axitinib (PA), and avelumab + axitinib (AA)—from a US payer perspective.
Patients and Methods:
Our analysis was performed based on previously obtained data derived from progression-free survival and overall survival curves from CheckMate 214, KEYNOTE 426, and JAVELIN Renal 101.
Results:
The total costs of each treatment were found to be $437,556.12 for NI, $450,597.15 for PA, and $542,882.34 for AA, with quality-adjusted life-year (QALY) values of 4.04, 3.77, and 2.95 for each combination, respectively. The incremental cost-effectiveness ratio (ICER) of NI versus PA was ($47,504.73/QALY); for NI versus AA, it was ($96,533.11/QALY); for PA versus AA, it was ($113,015.87/QALY). Net health benefit scaled against a willingness-to-pay threshold of $150,000 per QALY was positive for NI versus PA at 0.36 and versus AA at 1.79, and this index was also positive for PA versus AA at 1.43, indicating that the additional value of these therapies versus their alternatives is greater than the extra cost.
Conclusion:
NI was found to be the most cost-effective treatment option compared with the other considered therapies. PA was found to be cost effective compared to AA. When patient factors such as social issues and pre-existing conditions do not dictate their first-line therapy, clinicians may use this additional information to make financially conscious choices.
Keywords: Cost–benefit analysis, Health care costs, Immunotherapy, Monoclonal antibodies, Protein tyrosine kinases
Introduction
Renal cell carcinoma (RCC) is expected to be diagnosed in nearly 74,000 patients this year, accounting for approximately 4.2% of newly diagnosed cancer in the United States1 The 5-year relative survival for advanced RCC remains low, and better treatments and outcomes are needed. Recently, immunotherapy agents have overtaken tyrosine kinase inhibitors (TKIs) as first-line therapy for metastatic renal cell carcinoma. Immunotherapy-based combinations (nivolumab + ipilimumab, pembrolizumab + axitinib, avelumab + axitinib) represent current options for recommended first-line treatment of metastatic RCC. The treatment choice is primarily driven by patient-specific factors; however, in situations where multiple options are feasible, additional metrics to guide the choice of combination therapy are needed.
Nivolumab is a monoclonal antibody that binds to the programmed death 1 (PD-1) receptor, and ipilimumab is a monoclonal antibody that binds to the cytotoxic T-lymphocyte antigen 4, both working to restore T-cell immune activity. CheckMate 214 was a randomized phase 3 study that evaluated nivolumab + ipilimumab compared to sunitinib, the prior TKI standard of care in patients with previously untreated advanced RCC.2 Based on the 42-month follow-up updates presented at the European Society of Medical Oncology (ESMO) 2020 Virtual Congress, the trial demonstrated a significant improvement with nivolumab + ipilimumab in objective response rate (ORR; 41.9% for nivolumab + ipilimumab vs. 26.8% for sunitinib), complete responses (10.4% vs. 1.4%), and median overall survival (OS; 48.1 vs. 26.6 months) in patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate- and poor-risk disease.3 In the intention to treat (ITT) population, nivolumab + ipilimumab still had a significant OS benefit over sunitinib (not reached vs. 38.4 months), but progression-free survival (PFS) did not differ significantly between the groups. The combination of nivolumab + ipilimumab was approved in 2018 by the US Food and Drug Administration (FDA) for first-line treatment of previously untreated intermediate- and poor-risk advanced RCC, and it has become the new standard of care.4–6
Pembrolizumab is also a monoclonal antibody against PD-1, and axitinib is a TKI with action against the vascular endothelial growth factor pathway. KEYNOTE 426 was a randomized controlled, phase 3 study evaluating the combination of pembrolizumab + axitinib compared to sunitinib in patients with previously untreated advanced RCC, regardless of IMDC risk categorization7. The 23-month follow-up updates presented at the American Society of Clinical Oncology 2020 Annual Conference demonstrated a significant improvement with pembrolizumab + axitinib versus sunitinib in PFS (15.4 vs. 11.1 months), ORR (60.2% vs. 49.9%), complete responses (9% vs. 3%), and median OS (not reached vs. 35.7 months) in patients with advanced RCC.8 In 2019, the US FDA approved this combination for first-line treatment of any-risk advanced RCC, and it represents another standard of care option.5,6,9
Avelumab is an anti-programmed death ligand 1 (PD-L1) monoclonal antibody. JAVELIN Renal 101 was a phase 3 randomized study that assessed the efficacy of avelumab + axitinib compared to sunitinib in patients with advanced RCC.10 Although there has not been a reported benefit in OS, this study demonstrated a significant improvement with avelumab + axitinib versus sunitinib in PFS in the PD-L1-positive population (13.8 vs. 7.0 months, respectively) and in the overall population (13.3 vs. 8.0 months, respectively), as well as in the ORR of the overall population (52.5% vs. 27.3%, respectively) in patients with advanced RCC, based on updated data most recently presented at ESMO 2020.11 The combination of avelumab + axitinib was approved by the US FDA in 2019 for first-line treatment of advanced RCC, regardless of IMDC risk.5,12
Given the range of options now approved and available for first-line treatment of advanced RCC, the objective of this study was to estimate the cost-effectiveness of the three systemic combination therapies available in the first-line treatment setting for any-risk advanced RCC from the US payer perspective.
Materials and Methods
Model Structure
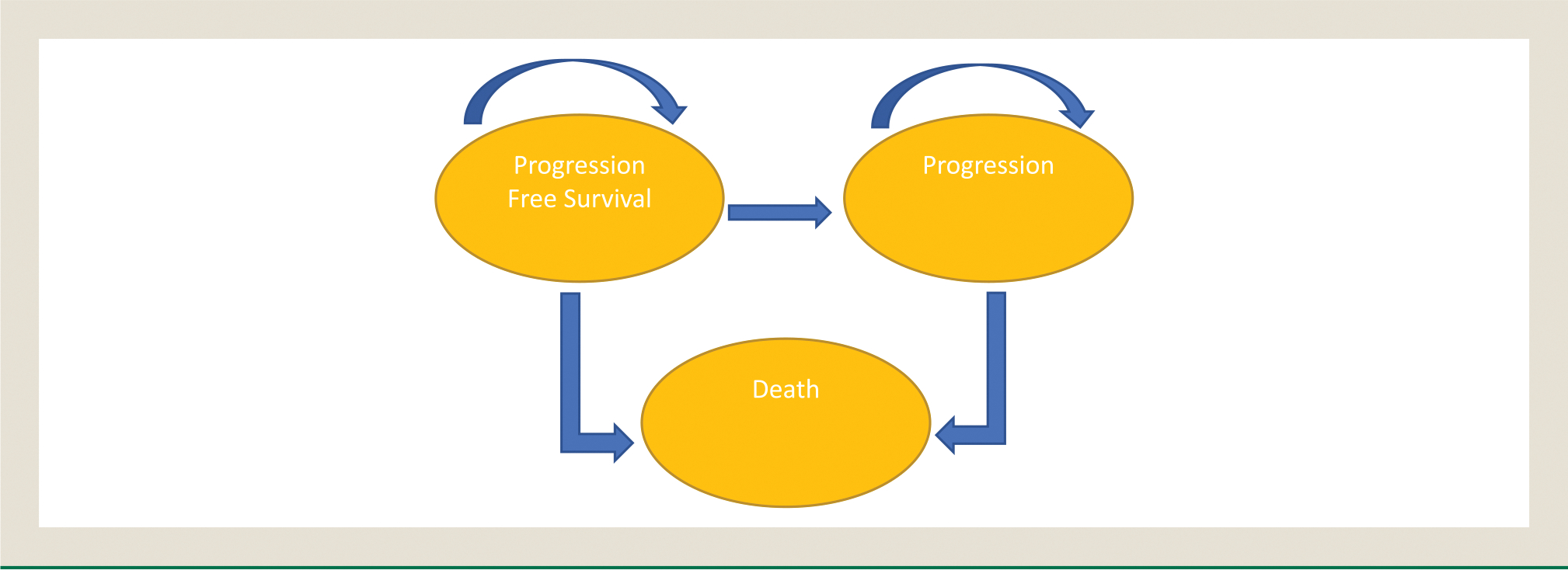
A Markov model was developed using TreeAge Pro 2018 (TreeAge, Williamstown, MA) and Excel (Microsoft Corporation, Redmond, WA) to evaluate the cost-effectiveness among the three available systemic therapy combinations. The three health states in the model were PFS, disease progression, and death (Figure 1). The initial health state was PFS. Patients in the progression-free state were assumed to receive one of the combination therapies until progression or death. Upon progression, patients were assumed to receive cabozantinib, the most frequent second-line therapy as identified in the KEYNOTE 426 and Javelin RENAL 101 trials. This cost was assigned proportional to the amount of patients that received second-line therapy in the CheckMate 214, KEYNOTE 426, and JAVELIN Renal 101 trials for the median duration noted in the METEOR Trial.13 Each model cycle represented 6 weeks, and the time horizon was set at 10 years. Half-cycle correction was applied for costs and health outcomes in the model. Only direct medical care costs were included. A 3% discount rate per year was applied across costs and life-year outcomes. The threshold utilized for willingness-to-pay (WTP) was $150,000/quality-adjusted life-year (QALY).14
Figure 1. Health States with Transitions.
Key Concepts
The primary outputs of the model included total cost, QALYs, incremental cost-effectiveness ratios (ICERs), and net monetary/health benefit. The ICER is a calculated ratio comparing two different scenarios that results in the incremental cost per unit of effectiveness. In this case, the chosen unit was the QALY, a measure of disease burden, in both the quantity and quality of life lived. It ranges from 0 to 1, with 1 representing perfect health.
Progression and Mortality Estimates
Transition probabilities for each immunotherapy combination were derived from the PFS and OS curves for the overall population utilizing the most recent data from the CheckMate 214, KEYNOTE 426, and JAVELIN Renal 101 trials. Engauge Digitizer software (http://markummitchell.github.io/engauge-digitizer) was used to extract the probabilities from the curves. Using the extracted data, pseudo-individual patient events and censoring were simulated using the method derived by Hoyle and Henley.15 A Weibull distribution was fit to all models, as it provided the most consistent best fit according to the Akaike information criterion. Background mortality from all causes was assumed to be present in the OS estimates.
Utility Estimates
QALYs were calculated by combining survival time and health-related quality of life (QOL). The health utility score was based on QOL data obtained via Functional Assessment of Cancer Therapy–Kidney Symposium Index 19 (FKSI-19) questionnaires in each trial, as well as from previously published data regarding the second-line therapy of cabozantinib. FKSI-19 scores were divided by 76 (the maximum value of a FKSI-19 score) to best estimate the utility score. In the model, average utilities of 0.828 for nivolumab + ipilimumab, 0.87 for pembrolizumab + axitinib, and 0.82 for avelumab + axitinib were utilized.2,7,16–18 Previously published data were used to determine QOL for second-line therapies, and an average utility of 0.79 for cabozantinib was selected.19
Cost Estimates
We considered only direct medical care costs in the analysis, including the drugs and their administration, management of adverse events, monitoring via physical exams, imaging, and lab tests. The cost of each combination was calculated based on the dosing and administration schedule defined in each trial. Per the Centers for Disease Control and Prevention (CDC), the mean US patient weight was 77.34 kg for women and 89.72 kg for men; we chose to use 85 kg as an average to account for both the higher prevalence of RCC among males and weight loss effects.14,20 We used average wholesale prices of drugs, as published by UpToDate,21 to estimate the unit price of all drugs. Intravenous administration costs were obtained from the Centers of Medicare & Medicaid Services (CMS).22
Our model accounted for wastage and no vial sharing (see Supplemental Materials). Given the wide range of second-line therapies used as described in each trial and the fact that they often were not given as single agents mutually exclusively, we made the assumption that all patients who went on to receive subsequent therapy received cabozantinib alone.
The costs of imaging were considered fixed across the different regimens and thus were not included in the analysis. However, physician visits and lab studies were likely to differ among the treatment options due to the dosing schedule and were accounted for accordingly. Physician visit and lab costs were estimated based on data obtained from the CMS.22 We included in the model grade 3 and 4 adverse drug events (ADEs), with an incidence of >1%, that might have contributed an economic impact. An ADE having an economic impact was defined as an adverse event that would require management with medication or procedures or assessment with lab work, imaging, or further procedures. Details of the included ADEs can be found in the Supplemental Materials. Drug costs, lab costs, and hospitalization costs via diagnosis-related groups were estimated based on data obtained from the CMS.22
Sensitivity Analysis
One-way sensitivity analysis was performed to evaluate the influence of parameter uncertainty. Each parameter was assessed separately for its effect on the ICER. All costs were varied by ±20%, the model parameters were varied by ±15%, and utilities were varied by ±10%. Probabilistic sensitivity analysis was conducted using 10,000 Monte Carlo simulations fluctuating the model parameters over realistic probability distributions.
Results
The total costs of each treatment, inclusive of the medications, their administration, monitoring studies, and any ADEs, were found to be $437,556.12 for the combination of nivolumab + ipilimumab, $450,597.15 for pembrolizumab + axitinib, and $542,882.34 for avelumab + axitinib (Table 1). Both cost and QALY results were found to be hierarchical in that treatment costs increased with declining QALYs. Due to this relationship, nivolumab + ipilimumab was found to be the dominant treatment over both pembrolizumab + axitinib and avelumab + axitinib. Correspondingly, pembrolizumab + axitinib was the dominant treatment compared to avelumab + axitinib. Both nivolumab + ipilimumab and pembrolizumab + axitinib outperformed avelumab + axitinib in terms of cost and QALYs (Table 2). Observing the net monetary benefits, adjusted using the prespecified WTP threshold, further compounds this finding. The positive results in each comparison (Table 2) indicate that the additional value of these therapies versus their alternatives is greater than the extra cost. Thus, nivolumab + ipilimumab was found to be the most cost-effective treatment option over the other considered therapies.
Table 1.
Results of Markov Model
| Treatment | Cost | QALY | ICER ($/QALY) |
||
|---|---|---|---|---|---|
| Nivolumab + Ipilimumab | Pembrolizumab + Axitinib | Avelumab + Axitinib | |||
| Nivolumab + ipilimumab | $437,556.12 | 4.04 | — | ($47,504.73) | ($96,533.11) |
| Pembrolizumab + axitinib | $450,597.15 | 3.77 | ($47,504.73) | — | ($113,015.87) |
| Avelumab + axitinib | $542,882.34 | 2.95 | ($96,533.11) | ($113,015.87) | — |
Abbreviations: ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
Table 2.
Net Monetary/Health Benefit
| Nivolumab + Ipilimumab vs. Pembrolizumab + Axitinib | Pembrolizumab + Axitinib vs. Avelumab + Axitinib | Nivolumab + Ipilimumab vs. Avelumab + Axitinib | |
|---|---|---|---|
| Incremental cost | ($13,041.03) | ($92,285.19) | ($105,326.22) |
| Incremental benefit | 0.27 | 0.82 | 1.09 |
| Incremental cost-effectiveness ratio | ($47,504.73) | ($113,015.87) | ($96,533.11) |
| Net monetary benefit | $54,219.14 | $214,770.44 | $268,989.58 |
| Net health benefit | 0.36 | 1.43 | 1.79 |
bilistic sensitivity analysis was conducted using 10,000 Monte Carlo simulations fluctuating the model parameters over realistic probability distributions.
Sensitivity Analysis
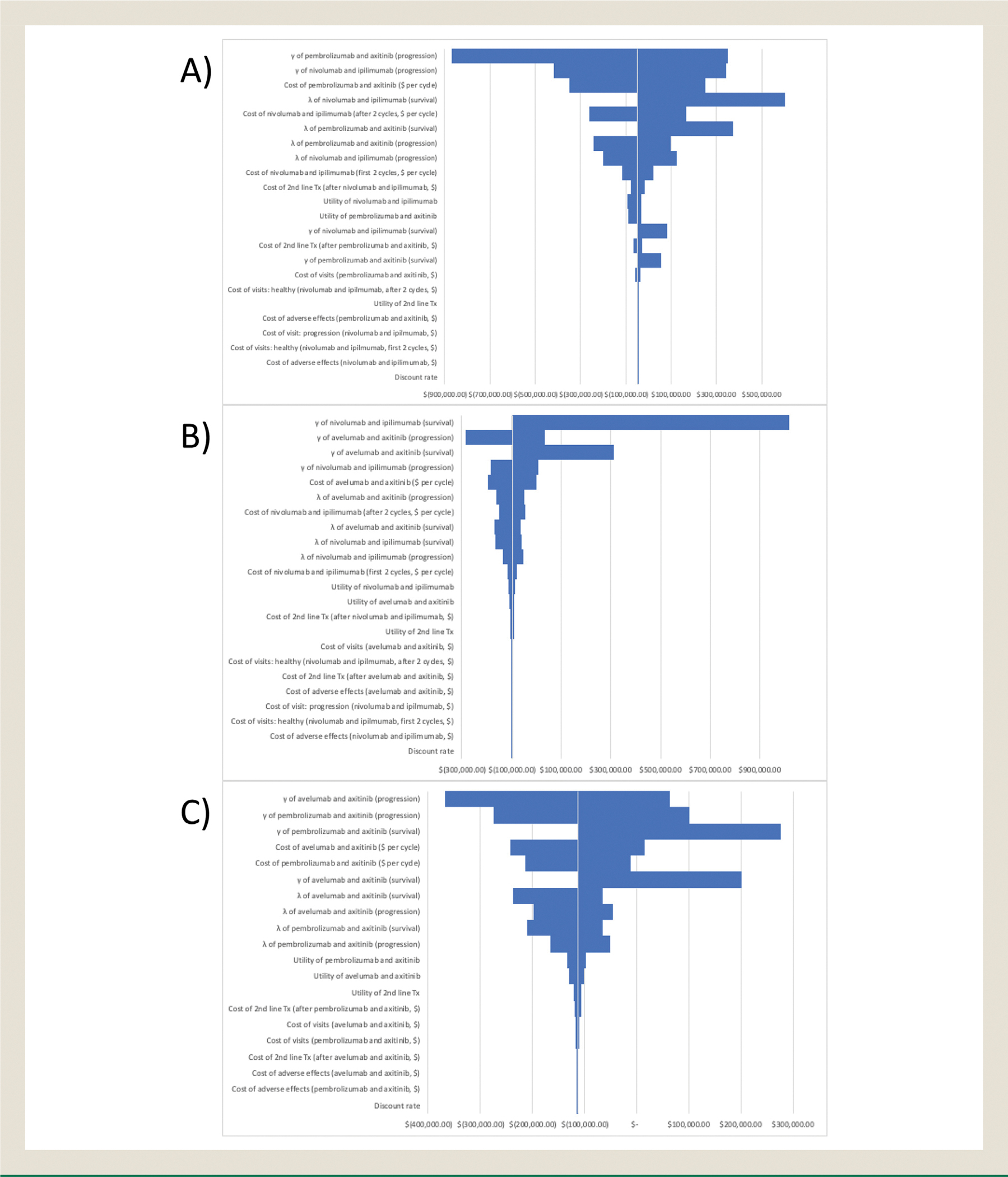
Tornado diagrams (Figures 2A–C) present the results of the univariable sensitivity analysis. The largest differences stemmed primarily from the parameters for the OS/PFS models. This behavior was expected due to the nature of obtaining these inputs. In comparing pembrolizumab + axitinib to the other therapies, another large influence on the ICERs were the drug costs. Unsurprisingly, given the similarity of their overall cost, taking the extremes of price for either pembrolizumab + axitinib or nivolumab + ipilimumab resulted in a different conclusion for the ICER. Other parameters such as cost outside of drug administration and utility were found to have negligible impact in comparison.
Figure 2. Tornado Diagram of A, Nivolumab + Ipilimumab Versus Avelumab + Axitinib; B, Avelumab + Axitinib Versus Pembrolizumab + AXITINIB; and C, Nivolumab + Ipilimumab Versus Pembrolizumab + Axitinib.
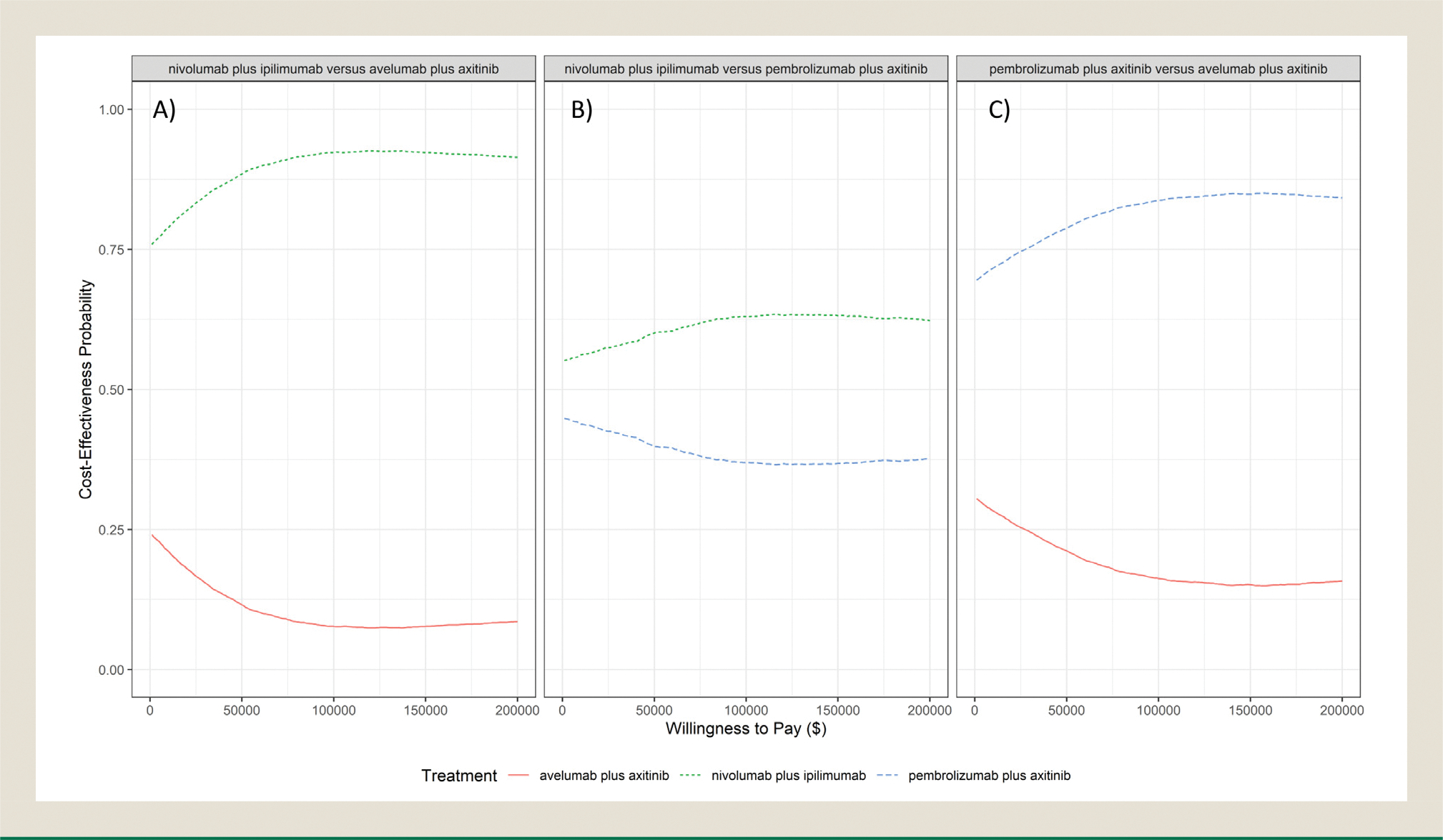
The results of the probabilistic sensitivity analysis support the dominance that both nivolumab + ipilimumab and pembrolizumab + axitinib exhibit over avelumab + axitinib (Figures 3A, B). In both cases, the probability of these therapies being cost effective exceeds 75% for almost the entire range of WTP thresholds. At a WTP threshold of $150,000, the probability of nivolumab + ipilimumab and pembrolizumab + axitinib being cost effective when compared to avelumab + axitinib was 92.3% and 84.9%, respectively. The comparison of nivolumab + ipilimumab and pembrolizumab + axitinib suggests more uncertainty regarding the results. Although nivolumab + ipilimumab has a higher probability of being cost effective over all considered WTP thresholds, it does not surpass 65% (Figure 3C).
Figure 3. Cost Effectiveness Acceptability Curves of A, Nivolumab + Ipilimumab Versus Avelumab + Axitinib; B, Nivolumab + Ipilimumab Versus Pembrolizumab + Axitinib; and C, Avelumab + Axitinib Versus Pembrolizumab + Axitinib.
Discussion
We performed a cost-effectiveness analysis of three combinations used in first-line treatment of metastatic or advanced RCC of any risk group from a US patient perspective. Our results suggest that nivolumab + ipilimumab offers the greatest cost-effectiveness of the drug combinations, as it is the dominant therapy by both cost and QALYs gained. The results of this analysis suggested a simple progression of monetary and health benefits, with nivolumab + ipilimumab offering a benefit when compared with pembrolizumab + axitinib, and pembrolizumab offering a benefit over avelumab + axitinib at our WTP threshold (Table 2). Our probabilistic sensitivity analysis also demonstrates a high likelihood for nivolumab + ipilimumab and pembrolizumab + axitinib being more cost effective than avelumab + axitinib. These findings from a US payer perspective may not apply to health jurisdictions outside of the United States.
Unlike prior analyses that used data from a single trial, we compared outcomes across three trials. Although cross-trial comparisons are generally discouraged, in this circumstance we felt it to be appropriate, as the baseline patient populations across all three studies were very similar (see Supplemental Table 5), the comparator arm in each study was sunitinib, and the method of QOL measurement (FKSI-19) was uniform across all three studies. We recognize this could be a weakness in our design, as our one-way sensitivity analysis suggested that the OS and PFS model parameters were very influential in determining ICERs. We also note that, even with the updated trial results, the information available for the PFS and OS curves covers a range of only 3 to 5 years; however, given the lack of data comparing the three therapies to each other, we believe this is a reasonable approach. Even comparing across multiple trials, we were able to obtain results similar to those seen in previously published cost-effectiveness analyses with regard to total cost per regimen and QALY,17,18,23,24,25 although a more recent network meta-analysis and cost-effectiveness analysis of the first-line treatments of advanced renal cell carcinoma that contain immune checkpoint inhibitors suggested that nivolumab + ipilimumab was the only cost-effective regimen for the overall population.26
This analysis was performed for the overall population, not only for the intermediate- or poor-risk patients as in CheckMate 214, and did not include a focus on PD-L1 positivity as in JAVELIN Renal 101. Furthermore, we did not perform subgroup analyses of these factors, nor other baseline patient characteristics used in the original publications (such as age, sex, history of nephrectomy or radiotherapy, locations of metastatic disease, body mass index, performance status, geographic region, or smoking status). This remains an area of further investigation, as controlling for these factors may have an influence, given the use of different study populations.
We also did not address treatment beyond progression in this analysis. Our approach was to incorporate only costs where there was expected to be a difference among the three treatment groups of patients, as this would have an impact on the ICER results. We therefore did not include imaging costs, such as magnetic resonance imaging, bone scans, or computed tomography scans, which bear significant costs and would have had an impact on increasing the overall total costs of each treatment. It should be noted that CMS Reimbursement was used to estimate the costs of the drugs, their administration, monitoring, and ADEs and may not represent the true cost to the patient or the system, or the costs may have changed since our analysis was performed. We also acknowledge that many providers now dose nivolumab in the maintenance phase of therapy every 4 weeks rather than every 2 weeks, as described in CheckMate 214, which would have an impact on the cost of a drug and its administration. Interestingly, we suspect that the cost of grade 3 or 4 toxicity management with nivolumab + ipilimumab is higher in reality than what we were able to include in this analysis, as the only reported adverse events from CheckMate 214 and its supplementary materials were those that had at least 15% incidence; for the other trials, we included adverse events that had >1% incidence. Ultimately, in our one-way sensitivity analysis, we did not see a particularly impactful influence of these costs.
Another approach to assessing the value of each regimen, and one that happens to coincide with the results of this analysis, would be to weigh the overall cost of regimens against their clinical benefit graded via the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS). This validated scale was developed as a standardized tool for grading clinical benefit, using metrics such as primary endpoints of OS, PFS, or disease-free survival in conjunction with QOL scores and presence of high-grade adverse events.27 When using this scale (graded 1 to 5, with 5 representing the highest clinical benefit), we calculated the ESMO-MCBS scores to be grade 5 for nivolumab + ipilimumab, grade 5 for pembrolizumab + axitinib, and grade 2 for avelumab + axitinib. When the grade for each regimen was matched to its cost, nivolumab + ipilimumab remained the most cost effective and avelumab + axitinib the least.
As our study was reliant on three individual clinical trials, it was subject to their constraints. Assumptions were made regarding second-line therapy after discontinuation of the study drugs, as review of the second-line therapies administered often did not add up to the total proportion of patients who did receive the study drug; this suggested that the drugs were sometimes used in some combination and not as single agents. We therefore opted to use cabozantinib as a universal second-line therapy. We also made the assumption that axitinib was neither dose increased nor dose reduced throughout duration of therapy, which would have an impact on the medication costs. Furthermore, the use of an average QOL utility score may not necessarily reflect a patient’s condition over time. As QOL data over time were not present for all three therapies, it was determined that an equitable solution would be to utilize the average. Ultimately, data regarding QOL were neither obtained nor published from JAVELIN Renal 101, and we therefore assumed the utility score to be 0.82, as did Lu et al18 in their cost-effectiveness analysis of avelumab + axitinib versus sunitinib, using the utility score for nivolumab + ipilimumab previously published.13–15 However, the probabilistic sensitivity analysis and large value for the ICER estimates in the one-way sensitivity suggest that overall cost is the prohibitive factor to making avelumab + axitinib cost effective versus nivolumab + ipilimumab. Another consideration is that the long-term benefit of these combination therapies was extrapolated from the clinical data within the trials to a 10-year horizon and is subject to uncertainty, as long-term efficacy has not yet been reported. Finally, although they were all large and well-designed trials, our model was completely reliant on the validity and generalizability of the CheckMate 214, KEYNOTE 426, JAVELIN Renal 101, and METEOR data; therefore, any biases within the trials will be reflected in this analysis.
Conclusion
In this current climate, where there are multiple efficacious options for first-line systemic treatment of RCC, the data in this cost-effectiveness analysis can serve as further distinguishing factors among the therapies available. Although we recognize the limitations of this approach, we believe there is enough evidence through supporting publications and sensitivity analysis to support the trends we observed. From the perspective of US payers, the combination of nivolumab + ipilimumab is estimated to be cost effective when compared with both pembrolizumab + axitinib and avelumab + axitinib, and pembrolizumab + axitinib is estimated to be cost effective when compared with avelumab + axitinib for favorable-, intermediate-, and poor-risk patients with previously untreated advanced or metastatic RCC at a WTP threshold of $150,000 per QALY.
Clinically, providers take under significant consideration the disease characteristics and patient factors that may favor or discount one therapy over another: organ dysfunction, presence of underlying autoimmune disease, history of comorbidities such as uncontrolled hypertension coagulopathies, and limitations due to infusion center location, patient transportation, or dosing schedules. When these considerations are absent and not dictating the use of one combination over another, we hope that providers can use this type of model to aid in choosing the most appropriate therapy for their patients with cost-consciousness in mind.
Supplementary Material
Clinical Practice Points.
There now exist several viable first-line treatment options for metastatic renal cell carcinoma, making the choice of initial therapy difficult. Considering metrics other than patient factors may be necessary when selecting the most appropriate therapy.
We sought to assess the cost-effectiveness of the three combination therapies currently approved in treatment-naïve advanced or metastatic renal cell carcinoma: nivolumab + ipilimumab (NI), pembrolizumab + axitinib (PA), and avelumab + axitinib (AA).
Previously obtained data derived from progression-free survival and overall survival curves in the CheckMate 214, KEYNOTE 426, and JAVELIN Renal 101 trials were used for a cost-effectiveness analysis with endpoints of total cost of treatment, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and net monetary and health benefits using a willingness-to-pay (WTP) threshold of $150,000. The total costs of each treatment were found to be $437,556.12 for NI, $450,597.15 for PA, and $542,882.34 for AA, with QALYs of 4.04, 3.77, and 2.95 for each combination, respectively.
The ICER for NI versus PA was ($47,504.73/QALY); for NI versus AA, it was ($96,533.11/QALY); and for PA versus AA, it was ($113,015.87/QALY). Net health benefit scaled against a WTP threshold of $150,000 per QALY was positive for NI versus PA and versus AA, and this index was also positive for PA versus AA. NI was found to be the most cost-effective treatment option compared with the other considered therapies.
When patient factors such as social issues and pre-existing conditions do not dictate their first-line therapy, clinicians may use this additional information to make financially conscious choices.
Acknowledgments
We wish to acknowledge Derek Smith, MS, who assisted with software purchase and initially familiarizing A. Nicklawsky with the project.
Disclosure
A. Nicklawsky and D. Gao are partially supported by a grant from the University of Colorado Cancer Center (P30CA046934). R. Shay and E.T. Lam are partially supported by a grant from the University of Colorado Cancer Center (5P30CA046934–30). Institutional clinical trial funding was provided by Arrowhead Pharmaceuticals, Bristol Myers Squibb, Calithera Biosciences, Merck & Co., Pfizer, and Genentech/Hoffmann-La Roche. E.T. Lam serves as a consultant for Calithera Biosciences. The remaining authors have stated that they have no conflicts of interest.
Abbreviations:
- RCC
renal cell carcinoma
- TKI
tyrosine kinase inhibitor
- PD-1
programmed death 1
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- ESMO
European Society of Medical Oncology
- ORR
objective response rate
- CR
complete response
- OS
overall survival
- IMDC
International Metastatic Renal Cell Carcinoma
- PFS
progression free survival
- ITT
Intention to Treat
- NR
not reached
- FDA
Food and Drug Administration
- VEGF
vascular endothelial growth factor
- ASCO
American Society of Clinical Oncology
- PD-L1
programmed death ligand 1
- QALY
Quality-adjusted life years
- ICER
incremental cost-effectiveness ratios
- WTP
willingness-to-pay
- QOL
quality of life
- FKSI-19
Functional Assessment of Cancer Therapy-Kidney Symposium Index
- AWP
average wholesale price
- CMS
Centers of Medicare & Medicaid Services
- ADE
adverse drug events
- DRGs
diagnosis related groups
- NMB
net monetary benefit
- PSA
Probabilistic sensitivity analysis
- ESMO-MCBS
European Society for Medical Oncology Magnitude of Clinical Benefit Scale
Footnotes
CRediT authorship contribution statement
Rebecca Shay: Conceptualization, Methodology, Resources, Writing - review & editing, Project administration. Andrew Nicklawsky: Conceptualization, Methodology, Software, Formal analysis, Review & editing, Supervision. Dexiang Gao: Methodology, Software, Review & editing, Supervision. Elaine T. Lam: Conceptualization, Review & editing, Supervision, Funding acquisition.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clgc.2021.01.009.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albiges L, Tannir N, Burotto M, et al. 711P nivolumab + ipilimumab (N+I) vs sunitinib (S) for first-line treatment of advanced renal cell carcinoma (aRCC) in CheckMate 214: 4-year follow-up and subgroup analysis of patients (pts) without nephrectomy. Ann Oncol. 2020;31:S559–S560. [Google Scholar]
- 4.US Food and Drug Administration. FDA approves nivolumab plus ipilimumab combination for intermediate- or poor-risk advanced renal cell carcinoma. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell. Accessed February 22, 2021.
- 5.National Comprehensive Cancer Network. NCCN Clinical Guidelines in Oncology. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed February 22, 2021.
- 6.Albiges L, Powles T, Staehler M, et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: immune checkpoint inhibition is the new backbone in firstline treatment of metastatic clear-cell renal cell carcinoma. Eur Urol. 2019;76:151–156. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 8.Plimack ER, Rini BI, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): updated analysis of KEYNOTE-426. J Clin Oncol. 2020;38:5001. [Google Scholar]
- 9.US Food and Drug Administration. FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma. Accessed February 22, 2021.
- 10.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. FDA approves avelumab plus axitinib for renal cell carcinoma. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma. Accessed February 22, 2021.
- 13.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renalcell carcinoma. N Engl J Med. 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarfaty M, Leshno M, Gordon N, et al. Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol. 2018;73:628–634. [DOI] [PubMed] [Google Scholar]
- 15.Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella D, Grünwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: a cost-effectiveness analysis. JAMA Oncol. 2019;5:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu P, Liang W, Li J, et al. A cost-effectiveness analysis: first-line avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. Front Pharmacol. 2020;11:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella D, Escudier B, Tannir NM, et al. Quality of life outcomes for cabozantinib versus everolimus in patients with metastatic renal cell carcinoma: METEOR phase III randomized trial. J Clin Oncol. 2018;36:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Centers for Disease Control and Prevention. Body measurements. Available at: https://www.cdc.gov/nchs/fastats/body-measurements.htm. Accessed February 22, 2021.
- 21.University of Alberta. Lexicomp. Available at: https://web.library.ualberta.ca/databases_help/lexicomp/. Accessed February 22, 2021.
- 22.Centers for Medicare & Medicaid Services. Website. Available at: CMS.gov. Accessed February 22, 2021.
- 23.Bensimon AG, Zhong Y, Swami U, et al. Cost-effectiveness of pembrolizumab with axitinib as first-line treatment for advanced renal cell carcinoma. Curr Med Res Opin. 2020;36:1501–1517. [DOI] [PubMed] [Google Scholar]
- 24.Reinhorn D, Sarfaty M, Leshno M, et al. A cost-effectiveness analysis of nivolumab and ipilimumab versus sunitinib in first-line intermediate- to poor-risk advanced renal cell carcinoma. Oncologist. 2019;24:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson TR, Gao X, Reynolds KL, et al. Cost effectiveness of pembrolizumab plus axitinib vs nivolumab plus ipilimumab as first-line treatment of advanced renal cell carcinoma in the US. JAMA Netw Open. 2020;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Y, Fu J, Du J, et al. First-line treatments for advanced renal cell carcinoma with immune checkpoint inhibitors: systematic review, network meta-analysis and cost-effectiveness analysis. Ther Adv Med Oncol. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015;26:1547–1573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


