Abstract
Monkeypox is a zoonotic disease caused by the monkeypox virus (MPXV), which is a potential biological warfare agent of bioterrorism and poses the greatest threat to the world’s public biosafety and health after variola virus (VARV). While the coronavirus disease 2019 (COVID-19) pandemic has not ended yet, monkeypox is spreading menacingly. The first case of monkeypox in a nonendemic country was confirmed on May 6th, 2022, while the first imported case from Asia was found on June 21st. There were more than 16 thousand reported cases as of July 23rd, the day the World Health Organization (WHO) declared the global monkeypox outbreak a public health emergency of international concern (PHEIC) at the same level as smallpox and COVID-19; while there were more than 53 thousand cases as of September 1st. Therefore, we will propose relevant biosafety prevention and control strategies after analyzing the etiology of the 2022 multi-country monkeypox outbreak from the biological feature, transmissibility, epidemic, and variability of MPXV.
Keywords: Monkeypox virus, Re-emerging infectious disease, Biological characteristics, Biosafety strategy
1. Biological feature
1.1. Classification
Monkeypox virus (MPXV), as a double-stranded DNA (dsDNA) virus, belongs to the Poxviridae family, Chordopoxvirinae subfamily, and Orthopoxvirus genus (OPXV) and is closely related to variola virus (VARV), vaccinia virus (VACV) and cowpox virus (CPXV) [1]. There are two distinct phylogenetic clades of MPXV, Clade I (Congo Basin/Central African clade, C.B. clade) and Clade II (West African clade, W.A. clade). Clade II consists of two subclades, Clade IIa and Clade IIb. It showed an overall nucleotide identity of 99% in the same region while only 95% nucleotide identity across different geographical groupings [2].
1.2. Pathogenic features
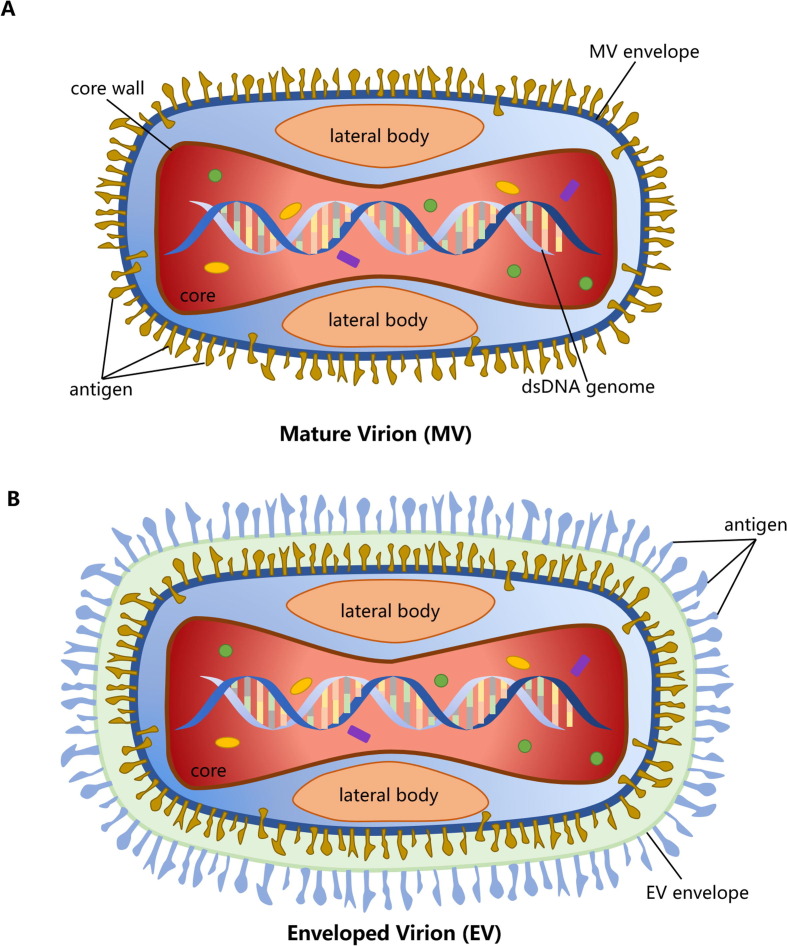
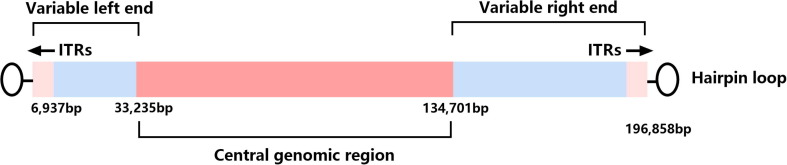
MPXV and other OPXVs, such as VARV, have high similarity in biological characteristics, morphological structure, and antigenicity [3] (Table 1 ). With the brick shape and typical size of other known poxviruses (200 nm by 250 nm), MPXV has two infectious forms, intracellular mature virion (MV) and extracellular enveloped virion (EV) [3], [4]. Microscopely, the core of MPXV is described as biconcave, and there is a lateral body (L.B.) on each side [3], [4] (Fig. 1 ). Meanwhile, the genomes are ≈ 197 kb long, containing approximately 190 non-overlapping open reading frames (ORFs) and encoding > 200 proteins [5], [6]. Moreover, the outer membrane of MV contains 20 proteins. Like other OPXVs, MPXV has a highly conserved and flanked central coding region sequence (CRS) for replication and assembly machinery, and the proteins encoded by CRS are from C10L to A25R. The variable ends containing 6397 kb long inverted terminal repeats (ITRs) [6] that cover genes involved in host range determination and virulence [7], [8] (Fig. 2 ). While ITRs composed of short tandem repeats (54 bp/ repeat), hairpin loops (80 bp long, and situated in both side terminal), NR1 and NR2 regions, and some ORFs [9], [10]. In addition, a comparative analysis of genomes between MPXV and VARV has shown that the CRS of these two viruses are nearly identical, whereas the ITRs are substantially different, and MPXV exhibits considerable differences in virulence genes from VARV finally [10], [11]. MPXV shares common antigens, such as soluble antigens, nucleoprotein antigens, and hemagglutinins (H.A.), with VARV, VACV, and CPXV, such that cross-reactivity on the challenge should be observed [3]. In terms of physical and chemical properties, MPXV placed them in an intermediate position between VARV and VACV. MPXV is resistant to drying and can be quickly inactivated by formalin, sodium dodecyl sulfate (SDS), chloroform, methanol, phenol, and other chemical reagents. Moreover, 20 min of heating at 50 °C or 56 °C can cause a complete loss of infectivity, and it can be stored stably at 4 °C or −70 °C for a long time [3].
Table 1.
The biological characteristics of VARV, MPXV, and VACV.
| OPXVs | Structure | Genomes | Antigenicity | Resistance | References |
|---|---|---|---|---|---|
| VARV | Brick-shaped, with 300 × 200 nm in size | ≈ 186 kb long, encodes ≥ 65 proteins but lacks ORFs in the ITR region | Some proteins are similar to other OPXVs, and cross-reactivity with other OPXVs can be observed because of hemagglutinins (H.A.) | Stronger than MPXV, resistant to drying and hypothermia | [3], [10], [11] |
| MPXV | Brick-shaped, with 200 by 250 nm in size | ≈ 197 kb long, encodes > 200 proteins and contains at least 4 ORFs in the ITR region | Some proteins are similar to other OPXVs, and cross-reactivity with other OPXVs can be observed | Between VARV and VACV | [3], [6], [10] |
| VACV | Brick-shaped, with 350 × 250 nm in size | ≈ 192 kb long, encodes > 200 proteins and contains at least 9 ORFs in the ITR region | Some proteins are similar to other OPXVs, and cross-reactivity with other OPXVs can be observed | Weaker than MPXV | [3], [8] |
Acbbreviations: OPXV = Orthopoxvirus; VARV = variola virus; VACV = vaccinia virus; MPXV = monkeypox virus; ORF = open reading frame; ITR = inverted terminal repeat; H.A. = hemagglutinins.
Fig. 1.
Schematic diagram of monkeypox virus (MPXV) structure. A) The structure of intracellular mature virion (MV). B) The structure of extracellular enveloped virion (EV).
Fig. 2.
Genomic organization of monkeypox virus (MPXV). Abbreviations: ITRs = inverted terminal repeats.
2. Clinical characteristics and pathogenicity
An exanthemata disease, both in humans and other animals, induced by MPXV is named monkeypox disease, and several monkeypox outbreaks have been reported, whereby monkeypox is just considered an emergent zoonotic viral disease. As a self-limiting disease, most of the clinical characteristics of human monkeypox mirror those of smallpox, where both can cause severe systemic diseases, such as fever, headache, myasthenia, and fatigue, accompanied by skin lesions that are distinguished from the diseases caused by other OPXVs [12]. The specific lesions of rashes often present as macular initially; then as papules, blisters, and pustules; and finally, as scabs that fall off [4], [13], [14], [15]. The rashes often grow on the face and limbs first and extend to the human body. However, the rashes appeared in untypical positions, like sexual contact positions [16]. In addition, the clinical presentation of lymphadenopathy is a notable feature of monkeypox that differentiates it from smallpox, chickenpox, and measles [4]; hence, lymphadenopathy can be used for clinical diagnosis.
In the past, the case fatality ratio (CFR) of VARV, which caused smallpox to be considered the most frightening disease, reached 30%, while the average CFR of MPXV was approximately 8.7%. Two clades of MPXV exhibit distinguish characters in pathogenicity. The CFR of Clade I/CB clade and Clade II/WA clade are 10.6% and 3.6%, respectively [17], [18]. Death due to MPXV infection is mainly related to complications, including secondary infection, sepsis, and encephalitis. Complications during pregnancy may result in congenital fetal monkeypox or stillborn fetuses via the placenta. In the first half of 2022, five monkeypox deaths were reported, which are all in Africa [19], with a CFR of approximately 0.03%. The much lower CFR of the Clade II/WA clade has been regarded as being associated with the lower overall CFR (≈1%) in African youth. In addition, the contribution to lower CFR and slight disease than smallpox may be due to the presence of the IL-1β-binding protein in MPXV. It has been found that IL-1β-binding protein is correlated with fever and pathogenicity in VACV [20]. MPXV encodes a secreted IL-1β-binding protein and 3-β-hydroxy-δ-5-steroid dehydrogenase [21], [22], while VARV does not have intact versions of these coding sequences (CDS) [10]. Some genome-region deletions in the MPXV clade also can affect viral replication and pathogenicity [23]. Thus, although inducing unusual clinical symptoms, the pathogenicity of MPXV is still low because of some virulence-related genes and proteins.
3. Epidemiology and transmissibility
3.1. Host tropism
Host tropism significantly affects the distribution and transmission of viruses among infected hosts. If a virus can infect more hosts or has an adaptive mutation to humans, it will spread widely and even influence public health globally once it crosses the host barrier efficiently. Even though the reservoir natural host and intermediate host of MPXV have not been recognized thus far [13], the virus is maintained in various mammalian species in endemic areas, including humans, nonhuman primates, and rodents (such as rope squirrels, tree squirrels, Gambia kangaroos, and dormouse) [24], [25]. With a variable host spectrum, it seems like that MPXV is provided with the potential to threaten human health.
Since rodents were reported to be the source of many monkeypox outbreaks [26], [27], they are most likely to be the natural host and play a crucial role in the transmission and infection of MPXV. Besides, MPXV is presumed to have a broader range of animal hosts; for example, the first monkeypox case of a dog that might have been acquired through human transmission was reported recently and demonstrated that domesticated dogs could be a vector for MPXV [28]. Additionally, based on currently available data, it appears that VACV and MPXV both have a broad host range, while VARV exhibits a very restricted host tropism (Table 2 ). The reason for such a difference remains mysterious. However, it is suggested that the answer might lie in the genetic diversity of those viruses, namely, host range genes. Compared with VARV infecting humans exclusively, MPXV has a broader host tropism and an increasing latent ability of widespread transmission genetically.
Table 2.
The comparison of VARV, MPXV, and SARS-CoV-2 in epidemiology and transmissibility.
| Host tropism | Transmission route | R0 | References | |
|---|---|---|---|---|
| VARV | Narrow (humans are the sole host) | Respiratory route and contact transmission | 5.0–7.0 | [11], [44] |
| MPXV | Broder (infects mammalian species like humans, nonhuman primates, and rodents) | Close contact with infectious substances or via respiratory droplet particles to transmission | 0.6–1.0 | [14], [24], [25], [29], [30], [31], [45], [46] |
| SARS-CoV-2 | Broder (including humans and a variety of animals, like bats, pangolins, minks, or other animals) | Close contact, respiratory route, and aerosol transmission | Original strain is between 1.4 and 3.9; Delta strain is 5.1; Omicron BA.1 is 9.5; BA.2 is 13.3, and B.A. 4/5 is much higher (18 is not a correct estimation) | [47], [51], [52], [53], [54], [55] |
Abbreviations: R0 = the primary case reproduction number; VARV = variola virus; MPXV = monkeypox virus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
3.2. Transmission
In the past 50 years, monkeypox has never been considered to spread among human beings. However, at the second meeting of the International Health Regulations (2005) (IHR) Emergency Committee [19], the concept changed when the World Health Organization (WHO) regarded the global monkeypox outbreak as the highest-level warning event of public global health emergency of international concern (PHEIC) because that monkeypox has spread to many nonendemic countries on a large scale from May 2022. Respiratory droplets, close contact with body fluids, a contaminated environment or items, skin lesions or ulcers of infected persons, and mother-to-child transmission are associated with interpersonal transmission [14], [29], [30], [31]. Infected animals can also infect people by biting, scratching, and even using or eating contaminated animal products [32]. Transmission via respiratory droplet particles usually requires prolonged face-to-face contact. In addition, the predominance of rising cases in many countries could suggest the possibility of community transmission, such as through family gatherings [1], [33], [34].
Apart from what was mentioned above, the transmission pattern of MPXV seems to be multiple and distinctive. Whether Monkeypox can be disseminated by sexual transmission through semen or vaginal discharge, as seen in human immunodeficiency virus (HIV), or transmitted by sexual contact, as seen in human papillomavirus (HPV), remains a mystery. Recently, some study reports on monkeypox cases in the United Kingdom (UK), Portugal, Italy, and Australia were published in Euro Surveillance [16], [35], [36], [37]. It suggested that MPXV is equipped with the potential transmission pattern by sexual contact [38]. For instance, Australian researchers found that rash symptoms first appeared at the sexual contact position in male patients [16], and four patients traced by Italian researchers reported that the rash and skin damage was limited to genital organs and perianal areas [35]. Furthermore, although the infectious pathogen had not been isolated at the time of reporting, it was the first time to be detected the MPXV nucleic acid in semen [35], showing that MPXV is very likely to have a pattern of sexual transmission as a sexually transmitted disease (STD).
On the other hand, many monkeypox patients are coinfected with HIV [12], [39], [40]; for example, 14 of 27 MPXV-positive individuals had a confirmed HIV infection [36], and one tested sample in an investigation in Spain was identified as having co-infection with HIV [41], [42]. These HIV patients may be acquired MPXV through sexual transmission, but this speculation needs tracing and investigation to support in the future. Overall, MPXV should no longer be considered a rare disease geographically limited to western and central Africa, and the transmission pattern through sexual transmission and sexual contact might answer why the 2022 monkeypox outbreak has specific features that have not been seen before.
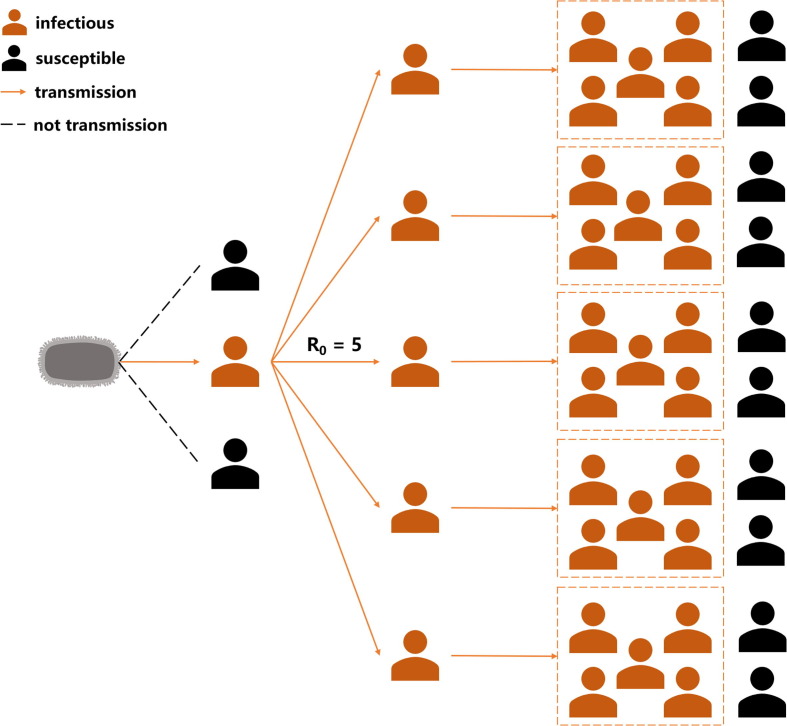
3.3. Transmissibility
The contact between infected and susceptible persons in the population leads to the transmission of infectious diseases. If infectious transmission does not occur, the pathogen infections will progressively disappear from the population. Thus, the concept of “basic case reproduction number (R0)”, the average number of secondary cases that contract an infection from an infected population, has been proposed by Macdonald [43]. As an indicator to evaluate the contagiousness of pathogens, R0 can reflect the biological mechanism of transmission and the rate of contact or interaction between members of the host population [44] and is estimated at mass action equations. Therefore, R0 can be used to describe the spreading potential for an infection in a population, and infectious period, contact rate, and mode of transmission are the main factors when estimating R0 (Fig. 3 ). The transmissibility, spread of infections and impact on the global public health in the occurrence of the epidemic can be observed via R0. If R0 > 1, the disease will spread interpersonal transmission and become an epidemic. According to statistics previously, R0 of MPXV is estimated to be in the range of 0.6–1.0 [45], [46] as the result of appearing to be a self-limited transmission. Meanwhile, the R0 of VARV reaches 5–7 [44], and the related SARS-CoV-2 Omicron B.A. 4/5 strain is much higher [47]. In sum, MPXV is not considered to have the same high infectivity and transmissibility as VARV because of its limiting transmission routes and the lower R0. Therefore, monkeypox represents a minor threat to humans in terms of its transmissibility, infection rate, and influence. Additionally, the epidemiology and transmissibility of MPXV are compared with VARV (causes smallpox, the most threatening disease in 16th − 18th century) and SARS-CoV-2 (induces COVID-19 that has still been affected human public health around the world nowadays). As a result, a more intuitive understanding of the transmissibility of MPXV can be found (Table 2).
Fig. 3.
The assumption diagram of transmissibility. The primary case reproduction number (R0) is presumed to be 5.
Seemingly, the transmissibility of infectious pathogens is also related to hosting tropism. Moreover, the spillover of pathogens often follows the more vital transmission ability and broader host range. In recent years, the study of viral host range genes and the interactions of their products with host cells have provided an outlook on the nature of poxvirus tropism [48]. Some proteins encoded by ankyrin repeats which comprise the most prominent OPXVs gene family have been identified as important host range factors in MPXV (like D7L and C1L) [7], [49]. In addition, some deletion or truncation in this gene family could be investigated between VARV and MPXV, such as D1L, D6L, D7L, C1L, and O3L in VARV and B19R in MPXV [10], which might affect host range and transmissibility of the virus. However, the presence or absence of host range genes does not determine the host tropism in poxvirus [50]; thereby, the host range genes may be related to different means of evolution molecularly and culminate in different pathogenesis, host tropism, transmissibility and immunoregulation ultimately.
4. Epidemics
4.1. History
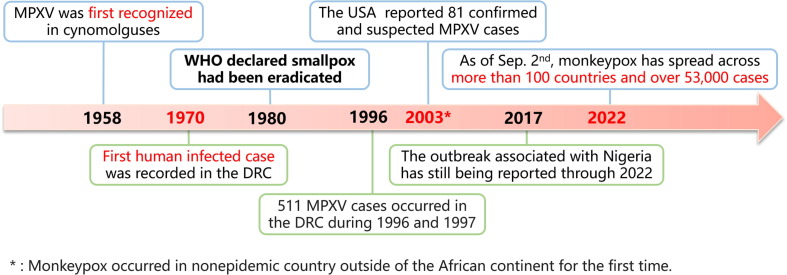
Monkeypox has been known for 64 years since the first identification of MPXV. Because of geological limitations, it has existed on the African continent narrowly for an extended period, but there were also several monkeypox outbreaks before 2022 (Fig. 4 ). Monkeypox was first recognized in cynomolguses in 1958 [56], [57]. Nevertheless, it was not until 1970 that the first human case infected with MPXV was recorded in the Democratic Republic of the Congo (DRC) [58], [59]. With consideration of regional limitations and low CFR, several monkeypox outbreaks and sporadic cases were reported only in central and western African countries after that [32], and there were 511 cases occurred in the DRC between 1996 and 1997 [60]; hence, the public gave little attention to monkeypox for a long time. However, this situation did not last long. Monkeypox emerged in the United States of America (USA) in 2003, with 81 confirmed and suspected MPXV cases in six states, and the source was determined to be imported African rodents [61]; these cases were the first human MPXV cases occurring outside of the African continent and reported in the western hemisphere. Then, an outbreak associated with travel to Nigeria was transported to many countries, namely, the UK, Israel, and Singapore, from 2017 to 2019 [17], [18], with cases still being reported through 2022. Although MPXV has never disappeared, the multi-national transmission of MPXV demonstrated that the virus could adapt to new ecological and geographical circumstances.
Fig. 4.
The historical outbreaks of mokeypox virus (MPXV). Abbreviations: MPXV = monkeypox virus; WHO = World Health Organization; the DRC=the Democratic Republic of the Congo; the USA = the United States of America. *: Monkeypox occurred in nonepidemic country outside of the African continent for the first time.
4.2. Epidemic development of the multi-country monkeypox outbreak in 2022
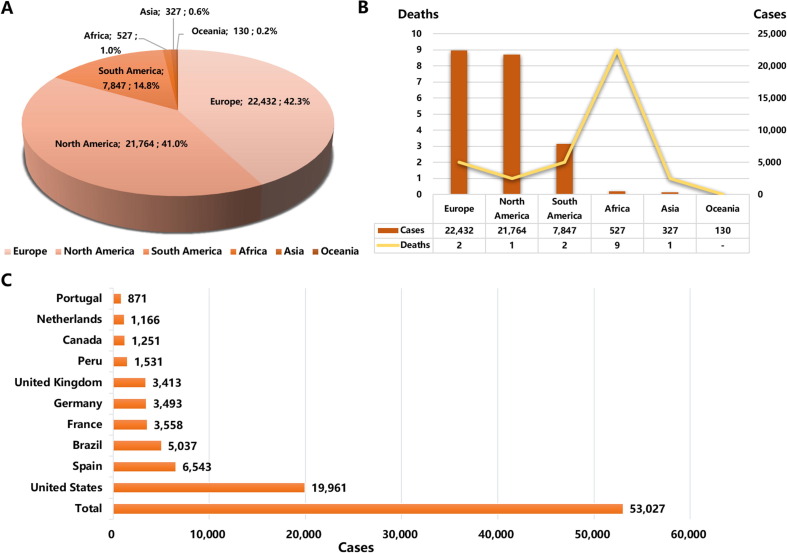
A monkeypox outbreak has been spread across many countries worldwide since May 2022, with a more comprehensive transmitted range, more extensive geographical distribution, more confirmed cases, and atypical symptoms, which are different from departed monkeypox outbreaks. The earliest case in this outbreak was identified in the UK on May 6th, and as of September 2nd, Monkeypox has spread across 100 countries and regions around six continents, with 53,027 confirmed cases and 15 deaths [62]. The highest numbers of cases mainly prevailed over Europe and North America (Fig. 5 A-B), including the United States, Spain, Brazil, France, Germany, the UK, Peru, Canada, Netherlands, and Portugal. Meanwhile, the number of cases in these ten countries accounts for 85.4% of the total number (Fig. 5C). Compared with the period of July, when the number of cases increased to >10,000, the period of August presented a threefold-larger growth rate and saw an increase to >30,000 cases. Notably, many infected people are men, especially those who have sex with men [32], [61]. For example, almost all 27 people surveyed in Portugal consider themselves MSM [36], and the Centers for Disease Control and Prevention in the United States pointed out that gay, bisexual, and MSM account for most male patients [61]. Moreover, there remain the difficulties of uncertain infectious origin and unconfirmed transmission patterns.
Fig. 5.
The global situation of monkeypox confirmed cases. A) The proportion of monkeypox confirmed cases in different continents. B) Confirmed cases and deaths of monkeypox in different continents. C) Top ten countries with the highest monkeypox cases. The source of data statistics from CDC in the United States (https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html) as of September 2nd, 2022.
5. Heredity and variation
5.1. Heredity of muti-country monkeypox outbreaks in 2022
Mature sequencing technology and the rich experience of COVID-19 have enabled scientists to address emerging and re-emerging infectious diseases more easily and quickly. Although sequencing could be completed within five days after sample acquisition [63], it took one month to release the first SARS-CoV-2 sequence after COVID-19 broke out [64]. Meanwhile, two weeks after the first MPXV case was reported, Dr. João Paulo Gomes, whose research group is from the National Institute of Health (Portugal), published the first draft genome sequence of MPXV on virological.org (https://virological.org/) on May 19th [42], [65]. The first rapid phylogenetic analysis of the draft genome indicated that the virus belongs to the Clade II/WA clade and is most closely related to viruses associated with the exportation of MPXV from Nigeria to several countries in 2018 and 2019 [17], [18]. On May 20th, the Institute of Tropical Medicine team in Antwerp, Belgium, released the first complete MPXV genome and reached a consistent conclusion as Dr. João Paulo Gomes [66], supporting the speculation about the existence of community transmission in Europe. Additionally, accounting for the relatively divergent branch detected within the USA there is likely to be another evolutionary branch that currently prevails in Europe and the USA [42].
5.2. Genetic diversity of muti-country monkeypox outbreaks in 2022
With the sharply rising number of confirmed MPXV cases, whole-genome sequencing has been attempted to analyze the genetic diversity of viruses, and many nonendemic countries with cases have performed sequencing analysis in succession. As a result, 1,592 human MPXV genome sequences have been uploaded to GISAID (https://gisaid.org/) by September 1st, 2022, of which 1,453 are associated with this outbreak [67]. In addition, 607 MPXV whole-genome sequences have been uploaded to the nucleoside database on National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) [68].
Although the phylogenetic analysis showed that the MPXV belongs to the Clade II/WA clade, the fact that the pandemic monkeypox outbreak is unusual gave rise to the conjecture that some genetic mutations have occurred. Gregorio Marañón University Hospital in Spain identified 46 single-nucleotide polymorphisms (SNPs) shared by 129 sequences and three amino acid changes (D209N, P722S, and M1741I) in the surface glycoprotein B21, which were not detected in the genomes of previous 2018 and 2019 strain [41]. The mutation of B21 is conducive to virus immune evasion and transmission. Researchers have also found signs of sustained microevolution of MPXV [42], and mutations occurring at important sites can enable the virus to obtain higher adaptability to the human host and stronger transmissibility to spread widely. Significantly, fewer than 6 SNPs were detected in past MPXV-related cases, and 21 SNPs were seen in unrelated samples [17]. The evolutionary rate of VARV has previously been estimated to be approximately 9 × 10-6 substitutions per site per year [69]; that is, VARV translates into approximately 1–2 nucleotide changes per year [70]. Therefore, it seems theoretically impossible to make approximately 50 substitutions unexpectedly in 3–4 years. Notwithstanding, the research group from France conducted the most profound sequencing (950 × mean depth) through Illumina, and 56 mutation differences and three deletions were measured [71]. However, the meanness of these genetic changes needs to be analyzed later, which might favor adaptation to a human host and increase transmissibility.
Moreover, a deaminase editing of the apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3) enzyme might drive hypermutation and evolution of MPXV, presenting a dinucleotide change bias from T.C. > T.T. or G.A. > A.A. [42], [70]. The antiviral activity of upregulated APOBEC3 can be identified with some viruses, such as HIV, hepatitis B virus (HBV), human papillomavirus (HPV), herpes simplex virus (HSV), and Epstein-Barr virus (EBV) [72]. Moreover, the expression of APOBEC3 is remarkably upregulated after HIV infection [73]. The variations mediated by APOBEC3 may not destroy the virus but increase the possibility of producing hypermutations with altered biological characteristics of transmission, infection, and pathogenicity in some cases [70]. A few adaptive mutations have already been discovered in the genomes of recent MPXV strains, but as a DNA virus, MPXV does not represent substantial variability as SARS-CoV-2 does and will not frequently mutate theoretically.
6. Biosafety requirements
Based on relative pathogenicity and the extent of harm to human health, etiologic agents are categorized into four risk groups (RG) for laboratory use recommended by WHO in the Laboratory Biosafety Manual [74]. MPXV has been assigned to be a Risk Group 4 (RG4) etiologic agent because that MPXV can pose a high individual risk of aerosol-transmitted laboratory infections and life-threatening disease. Moreover, four biosafety levels (BSLs) represent combinations of laboratory practices and techniques, safety equipment, and laboratory facilities, and MPXV should be conducted in biosafety level 3 and animal biosafety level (BSL-3/ABSL-3) 3 laboratories but not in biosafety level 4 or animal biosafety level 4 (BSL-4/ABSL-4) laboratories, including manipulations of virus culture, animal infection and uncultured material operation. While other pathogens assigned to RG4, such as VARV, are required to conduct in BSL-4/ABSL-4 laboratories [74].
The experimenters acquire MPXV laboratory infections mainly through needle stabbing, animal scratching or biting direct contact with infectious materials, and inhalation of aerosols. Therefore, laboratory personnel should wear personal protective equipment according to the corresponding biosafety level, such as a buoyant pressure personnel suit. In addition, all procedures should be conducted in biosafety containment devices carefully to minimize the production of aerosols and avoid the risk of infection. Furthermore, laboratory workers engaged in primate experiments are encouraged to be inoculated against MPXV.
7. Biosafety prevention and control strategies
Due to the increasingly frequent international communication, infectious pathogenic microorganisms can be transmitted to humans remotely by only one flight. Therefore, emerging and re-emerging infectious diseases are not just a public health concern for one country or continent but also a concern to global health by implication, such as Ebola in 2014 [75] and COVID-19 [76]. For this reason, humankind should keep high vigilance and give close attention to them. Currently, many countries have biosafety prevention and control measures for different infectious diseases. For instance, the Chinese government has proposed relative prevention and control strategies for COVID-19, like quarantine and dynamic zero-COVID policies, which extensively protect human health. Although the CFR of MPXV is lower than VARV, and the variability is not as substantial as SARS-CoV-2, monkeypox poses a threat to global health and biosecurity. However, whether it will become another infectious disease causing a worldwide pandemic is difficult to know, and great effort must be directed at the supervision, research, and prevention of monkeypox such that biosafety prevention and control strategies can stay one step ahead of the virus. Therefore, some biosafety prevention and control strategies are proposed following.
7.1. Establishment and improvement of the global biosafety network for monkeypox
Although naturally occurring smallpox was eliminated through the efforts of the WHO Global Eradication Program by 1980, the public does not pay close attention to MPXV subjectively. The biosafety monitoring networks of monkeypox in many countries are fragile or have been imperfect for a long time, resulting from the limitation of MPXV transmission, moderate symptoms of infection, and the lack of data on potential reservoir species. The biosafety monitoring network can be a form of disease reporting system; many countries have their reporting systems for some diseases, such as the National Electronic Disease Surveillance System (NEDSS) Base System (NBS) in the USA, the national monitoring information system for infectious diseases and public health emergencies in China, a new global hub (for pandemic and epidemic intelligence, data, surveillance, and analytics innovation) established by WHO and Germany in 2021. The hard work to monitor the epidemics of emerging and re-emerging infectious diseases has been done by WHO, but it lacks a mature global biosafety monitoring system network for rare infectious diseases. Thus, longer-term surveillance for monkeypox is required.
Based on previous serological data, the persons born after the cessation of smallpox vaccination within southeastern Sierra Leone had high OPXV-specific IgG values and positive IgM responses, suggesting that OPXV has circulated in this region consistently [77]. Given that the average number of SNPs between genomes is higher than that observed among previous outbreak strains [41], [71], MPXV may have evolved and undergone evolutionary adaptation in West Africa, Nigeria, and other countries through long-term concealed transmission. With no clear MPXV source pool identified to account for a large number of exported patients in the 2022 monkeypox outbreak, the naturally adaptive evolution of monkeypox also seriously threatens the health and life of humans. In summary, heightened public awareness and the initiation of monkeypox surveillance for the first time could help to establish and improve the global biosafety network for monkeypox. Meanwhile, some crucial behaviors are beneficial for the establishment and improvement of biosafety networks, including strengthening the monitoring, insisting on the detection of MPXV in customs, airports, and other points of entry and exit, reducing the number of imported animals, and enhancing the supervision and control of imported animals.
7.2. Increasing efforts on the research of MPXV variability, pathogenicity, and transmissibility
Although the infection biology, epidemiology, and immune response of MPXV have been widely studied [78], [79], [80], there are few studies on the multiple functions of genes. Furthermore, evidence or possible explanations for unimaginable rapid mutation, pathogenic infection mechanisms, and human-to-human transmission are lacking. Zheng et al. reported the structural predictions of the whole proteomes of three monkeypox variants, with the current annotation of potential small-molecule-binding regions of the proteins [81]. In terms of genetic evolution, some researchers have traced the origin and molecular evolution of OPXV [82], and other research has shown that the more potent virulence and infectivity of the Clade II/WA clade may be related to the interaction of multiple gene products, such as monkeypox inhibitor of complement enzymes (MOPICE) [80]. However, reasons for the diversity of pathogenicity between the two clades of MPXV, the regularity of the epidemic and transmission mechanisms of MPXV, and the relationship between the virus and the host are still unclear, which aggravated the difficulty of drug and vaccine research and development.
The exploration of the possibility of MPXV not having a temporary natural reservoir(s) but rather circulating in a wide variety of natural animal host species and to investigate the epidemiological factors that maintain and sustain the virus in ecosystems, a potential molecular mechanism for stepwise accumulation of genetic alterations, and the viral and host factors that modulate animal-to-human transmission and human-to-human transmission extensively are necessary. Moreover, scientists should drive a considerable effort to study the variability, pathogenicity, and transmissibility of MPXV prospectively.
7.3. Urgent need for effective prevention and control measures
As the global smallpox eradication and vaccination were terminated, the opportunity for other OPXVs infections and the risk of human-to-human transmission is increasing, the magnitude of the epidemic is further escalating, and human monkeypox infection has probably been exacerbated. Meanwhile, many mild infections or asymptomatic patients might not have been identified. Under the situation of no specific treatment or medicine for monkeypox infection, antiviral drugs used to treat smallpox can achieve a certain degree of treatment effects. These antiviral drugs include tecovirimat (TPOXX, ST-246), Tembexa (brincidofovir), Cidofovir (Vistide), and Vaccinia Immune Globulin Intravenous (VIGIV) [61], but some of them still lack clinical data to support their therapeutic effect on monkeypox effectively. Currently, the vaccines used for preventing monkeypox are second-generation and third-generation smallpox vaccines, including ACAM200 and MVA-BN (the only monkeypox vaccine approved by the Food and Drug Administration of the United States).
Although a retrospective study on the effectiveness of the smallpox vaccine during monkeypox in Congo during 1980–1984 showed that the protective efficacy of the smallpox vaccine against MPXV was 85% (not standard clinical data) [45]; while other studies approved the effectiveness of smallpox vaccine against monkeypox [83], [84], [85], [86]. Moreover, antiviral antibodies against MPXV can exist stably for 75 years, while the half-life of protective antibodies is 8–15 years [87]. Some studies showed that vaccination could relieve symptoms to a certain extent; for example, the general CFR decreased to 0.101% after COVID-19 vaccination in Singapore, which was the same as that of influenza virus infection [88]. The lessons learned from the epidemic outbreak in recent years have made humans aware that preparing and storing vaccines and drugs to handle emerging and re-emerging infectious diseases are of great importance. Effective prevention and protective measures are urgently needed to eliminate the further spread of monkeypox in this multi-country outbreak and reduce the impact on human health. In addition to focusing on the susceptible population, carrying out screening tests on time and reinforcing immunization campaigns, strengthening basic research, improving detection technology, and promoting vaccine and drug research and development are essential. Awareness about MPXV transmission should be raised, and the possible epidemiological links between cases should be explored to elucidate the factors that might have contributed to the exportation of MPXV from the African continent, whereby the prevalence has shifted to many more non-endemic countries.
8. Conclusion and discussion
Along with the mobilization of populations and international cooperation, emerging and re-emerging infectious diseases appear more frequently, spread to many nonendemic countries, and constitute a significant threat to human health and global biosafety. For example, the multi-country outbreak of monkeypox in 2022 has already “invaded” 6 continents and over 100 countries, with more than 53,000 people from different nations infected. MPXV, causing the 2022 multi-country monkeypox outbreak, is equipped with a faster speed of microevolution and multi-patterns of transmission between humans and animals. Presently, many countries are carrying out vaccination, and the research of therapeutic drugs to prevent and control MPXV infection is accelerating. Cutting off human-to-human transmission is our essential goal for preventing and controlling the monkeypox outbreak by keeping surveillance reservoirs and cases, improving detection technology, and tracing the evolution of MPXV. Our glorious vision is prevention preceding treatment, and strategy before the virus's evolution.
Acknowledgments
Acknowledgements
This work was supported by the Science and Technology Planning Project of Guangdong Province (2021B1212040017); the Science and Technology Innovation Project of the Ministry of Education (2022ZL01); and the Sun Yat-sen University Founded Program (2022_76220_B21127).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Chudan Liang: Writing – Original Draft. Jun Qian: Conceptualization , Writing – Review & Editing, Supervision. Linna Liu: Conceptualization, Writing – Review & Editing, Supervision.
References
- 1.Bunge E.M., Hoet B., et al. The changing epidemiology of human monkeypox - A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Likos A.M., Sammons S.A., et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86(Pt 10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 3.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol. Rev. 1973;37(1):1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 5.Kugelman J.R., Johnston S.C., et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014;20(2):232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shchelkunov S.N., Totmenin A.V., et al. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shchelkunov S.N., Safronov P.F., et al. The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology. 1998;243(2):432–460. doi: 10.1006/viro.1998.9039. [DOI] [PubMed] [Google Scholar]
- 8.Goebel S.J., Johnson G.P., et al. The complete DNA sequence of vaccinia virus. Virology. 1990;179(1):247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 9.Saxena S.K., Ansari S., Maurya V.K., Kumar S., Jain A., Paweska J.T., Tripathi A.K., Abdel-Moneim A.S. Re-emerging human monkeypox: A major public-health debacle. J. Med. Virol. 2022:e27902. doi: 10.1002/jmv.27902. [DOI] [PubMed] [Google Scholar]
- 10.Shchelkunov S.N., Totmenin A.V., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70. doi: 10.1016/s0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization, Scientific review of variola virus research, 1999–2010. https://www.who.int/publications/i/item/WHO-HSE-GAR-BDP-2010-3, 2010 (accessed 23 July 2022).
- 12.Ogoina D., Iroezindu M., et al. Clinical course and outcome of human monkeypox in Nigeria. Clin. Infect. Dis. 2020;71(8):e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 13.Sklenovska N., Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front. Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chieloka O., Amao L., Akinrogbe J., et al. Outbreak investigation of monkeypox in Akwa Ibom state: A matched case control study 14th - 24th October 2019. EAJHS. 2020;1:37–44. doi: 10.37284/eajhs.1.1.57. [DOI] [Google Scholar]
- 15.Fowotade A., Fasuyi T.O., Bakare R.A., et al. Re-emergence of monkeypox in Nigeria: a cause for concern and public enlightenment. Afr. J. Cln. Exper. Microbiol. 2018;19(4):307–313. doi: 10.4314/AJCEM.V19I4.9. [DOI] [Google Scholar]
- 16.Hammerschlag Y., MacLeod G., et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro. Surveill. 2022;27(22):2200411. doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauldin M.R., McCollum A.M., et al. Exportation of monkeypox virus from the African continent. J. Infect. Dis. 2022;225(8):1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yinka-Ogunleye A., Aruna O., et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect. Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO), WHO Director-General's statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of Monkeypox. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022, 2022 (accessed 23 July 2022).
- 20.Alcami A., Smith G.L. A mechanism for the inhibition of fever by a virus. Proc. Natl. Acad. Sci. U. S. A. 1996;93(20):11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spriggs M.K., Hruby D.E., et al. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- 22.Moore J.B., Smith G.L. Steroid hormone synthesis by a vaccinia enzyme: a new type of virus virulence factor. EMBO J. 1992;11(9):3490. doi: 10.1002/j.1460-2075.1992.tb05428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopera J.G., Falendysz E.A., et al. Attenuation of monkeypox virus by deletion of genomic regions. Virology. 2015;475:129–138. doi: 10.1016/j.virol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alakunle E., Moens U., et al. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falendysz E.A., Lopera J.G., et al. Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.) PLoS Negl. Trop. Dis. 2017;11(8):e0005809. doi: 10.1371/journal.pntd.0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faye O., Pratt C.B., et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect. Dis. 2018;18(3):246. doi: 10.1016/S1473-3099(18)30043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds M.G., Davidson W.B., et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg. Infect. Dis. 2007;13(9):1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seang S., Burrel S., et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400(10353):658–659. doi: 10.1016/S0140-6736(22)01487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis C.K., Carroll D.S., et al. Ecology and geography of human monkeypox case occurrences across Africa. J. Wildl. Dis. 2012;48(2):335–347. doi: 10.7589/0090-3558-48.2.335. [DOI] [PubMed] [Google Scholar]
- 30.Ihekweazu C., Yinka-Ogunleye A., et al. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev. Anti Infect Ther. 2020;18(5):389–392. doi: 10.1080/14787210.2020.1735361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogoina D., Izibewule J.H., et al. The 2017 human monkeypox outbreak in Nigeria-Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLos One. 2019;14(4):e0214229. doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO), Multi-country monkeypox outbreak: situation update. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON392, 2022 (accessed 23 July 2022).
- 33.Yinka-Ogunleye A., Aruna O., et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 2018;24(6):1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Besombes C., Gonofio E., et al. Intrafamily transmission of monkeypox virus, Central African Republic, 2018. Emerg. Infect. Dis. 2019;25(8):1602–1604. doi: 10.3201/eid2508.190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antinori A., Mazzotta V., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro. Surveill. 2022;27(22):2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez Duque M., Ribeiro S., et al. Ongoing monkeypox virus outbreak, Portugal, April 29th to May 23rd 2022. Euro. Surveill. 2022;27(22):2200424. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivancos R., Anderson C., et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro. Surveill. 2022;27(22):2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heskin J., Belfield A., et al. Transmission of monkeypox virus through sexual contact - a novel route of infection. J. Infect. 2022;85(3):334–363. doi: 10.1016/j.jinf.2022.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhunu C.P., Mushayabasa S., et al. Modelling HIV/AIDS and monkeypox co-infection. Appl. Math. Comput. 2012;218(18):9504–9518. doi: 10.1016/j.amc.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer H., Perrichot M., et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002;40(8):2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.S. Buenestado-Serrano, R. Palomino-Cabrera, et al. Second draft genome from Spain of the monkeypox virus 2022 outbreak, https://virological.org/t/second-draft-genome-from-spain-of-the-monkeypox-virus-2022-outbreak/846, 2022 (accessed 23 July 2022).
- 42.Isidro J., Borges V., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022;28(2022):1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macdonald G. Oxford University Press; 1957. The Epidemiology and Control Of Malaria; p. 201. [Google Scholar]
- 44.Fine P.E. Herd immunity: History, theory, practice. Epidemiol. Rev. 1993;15(2):265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 45.Fine P.E., Jezek Z., et al. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 46.McMullen C.L., Mulembekani P., et al. Human monkeypox transmission dynamics thirty years after smallpox eradication in the Sankuru district, Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2015;93(4_Suppl):212. doi: 10.4269/ajtmh.2015.93.1. [DOI] [Google Scholar]
- 47.Houriiyah T., Monika M., et al. Continued emergence and evolution of Omicron in south Africa: new BA.4 and BA.5 lineages [Preprint] medRxiv. 2022 doi: 10.1101/2022.05.01.22274406. [DOI] [Google Scholar]
- 48.Oliveira G.P., Rodrigues R.A.L., et al. Poxvirus host range genes and virus-host spectrum: A critical review. Viruses. 2017;9(11):1–17. doi: 10.3390/v9110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shchelkunov S.N., Blinov V.M., et al. Ankyrin-like proteins of variola and vaccinia viruses. FEBS Lett. 1993;319(1–2):163–165. doi: 10.1016/0014-5793(93)80059-4. [DOI] [PubMed] [Google Scholar]
- 50.McFadden G. Poxvirus tropism. Nat. Rev. Microbiol. 2005;3(3):201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou P., Shi Z.L. SARS-CoV-2 spillover events. Science. 2021;371(6525):120–122. doi: 10.1126/science.abf6097. [DOI] [PubMed] [Google Scholar]
- 52.The Geography of Transport Systems, Basic Reproduction Number (R0) of Major Infectious Diseases. https://transportgeography.org/contents/applications/transportation-pandemics/basic-reproduction-number-r0-of-major-infectious-diseases/, 2022 (accessed 14 September 2022).
- 53.Liu Y., Rocklov J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Travel Med. 2021;28(7):taab124. doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Rocklov J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J. Travel Med. 2022;29(3):taac037. doi: 10.1093/jtm/taac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan Y., Li X., et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct. Target. Ther. 2022;7(1):1–11. doi: 10.1038/s41392-022-00997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arita I., Jezek Z., et al. Human monkeypox: A newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am. J. Trop. Med. Hyg. 1985;34(4):781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 57.Magnus P.V., Andersen E.K., et al. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959;46(2):156–176. doi: 10.1111/j.1699-0463.1959.tb00328.x. [DOI] [Google Scholar]
- 58.Breman J.G., Kalisa R., et al. Human monkeypox, 1970-79. Bull. World Health Organ. 1980;58(2):165–182. [PMC free article] [PubMed] [Google Scholar]
- 59.Ladnyj I.D., Ziegler P., et al. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 60.Heymann D.L., Szczeniowski M., et al. Re-emergence of monkeypox in Africa: A review of the past six years. Br. Med. Bull. 1998;54(3):693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 61.The U.S Centers for Disease Control and Prevention (CDC), Monkeypox. https://www.cdc.gov/poxvirus/monkeypox/, 2022 (accessed 2 September 2022).
- 62.The U.S Centers for Disease Control and Prevention (CDC), 2022 Monkeypox outbreak global map. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html, 2022 (accessed 2 September 2022).
- 63.Ren L.L., Wang Y.M., et al. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. (Engl) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu F., Zhao S., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.J. Isidro, V. Borges, et al. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak, May 2022 (confirmed case in Portugal). https://virological.org/t/first-draft-genome-sequence-of-monkeypox-virus-associated-with-the-suspected-multi-country-outbreak-may-2022-confirmed-case-in-portugal/799, 2022 (accessed 23 July 2022).
- 66.P. Selhorst, AM. Rezende, et al., Belgian case of Monkeypox virus linked to outbreak in Portugal. https://virological.org/t/belgian-case-of-monkeypox-virus-linked-to-outbreak-in-portugal/801, 2022 (accessed 23 July 2022).
- 67.GISAID, Epipox. https://www.epicov.org/epi3/frontend#4bffa9, 2022 (accessed 2 September 2022).
- 68.NCBI, Nucleotide, monkeypox virus complete genome. https://www.ncbi.nlm.nih.gov/nuccore, 2022 (accessed 2 September 2022).
- 69.Firth C., Kitchen A., et al. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Mol. Biol. Evol. 2010;27(9):2038–2051. doi: 10.1093/molbev/msq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.A. Rambaut, Initial observations about putative APOBEC3 deaminase editing driving short-term evolution of MPXV since 2017. https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830, 2022 (23 July 2022).
- 71.D. Gregory, B. Antonin, et al., Illumina whole-genome sequence of monkeypox virus in a patient travelling from the Canary Islands to France. https://virological.org/t/illumina-whole-genome-sequence-of-monkeypox-virus-in-a-patient-travelling-from-the-canary-islands-to-france/829, 2022 (23 July 2022).
- 72.Pecori R., Di Giorgio S., et al. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat. Rev. Genet. 2022;23(8):505–518. doi: 10.1038/s41576-022-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris R.S., Liddament M.T. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 2004;4(11):868–877. doi: 10.1038/nri1489. [DOI] [PubMed] [Google Scholar]
- 74.WHO . Laboratory biosafety manual. fourth ed. World Health Organization; Geneva: 2020. [Google Scholar]
- 75.Tong Y.G., Shi W.F., et al. Genetic diversity and evolutionary dynamics of Ebola virus in Sierra Leone. Nature. 2015;524(7563):93–96. doi: 10.1038/nature14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Q., Guan X., et al. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Macneil A., Abel J., et al. Serologic evidence of human orthopoxvirus infections in Sierra Leone. BMC Res. Notes. 2011;4:465. doi: 10.1186/1756-0500-4-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu H., Bruneau R.C., et al. Battle royale: innate recognition of poxviruses and viral immune evasion. Biomedicines. 2021;9(7):765. doi: 10.3390/biomedicines9070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hammarlund E., Dasgupta A., et al. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. U S A. 2008;105(38):14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Estep R.D., Messaoudi I., et al. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 2011;85(18):9527–9542. doi: 10.1128/JVI.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng L.Z., Meng J.T., et al. Structure prediction of the entire proteome of monkeypox variants. Acta Mater. Med. 2022;1(2):260–264. doi: 10.15212/AMM-2022-0017. [DOI] [Google Scholar]
- 82.Babkin I.V., Babkina I.N., et al. An update of orthopoxvirus molecular evolution. Viruses. 2022;14(2):388. doi: 10.3390/v14020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trop. Med. Infect. Dis. 2016;1(1):8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petersen E., Abubakar I., et al. Monkeypox - enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nasir I.A., Dangana A., et al. Reminiscing the recent incidence of monkeypox in Nigeria: Its ecologic-epidemiology and literature review. Port Harcourt Med. J. 2018;12(1):1–9. doi: 10.4103/phmj.phmj_47_17. [DOI] [Google Scholar]
- 86.Adler H., Gould S., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammarlund E., Lewis M.W., et al. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 88.Watson O.J., Barnsley G., et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 2022;22(9):1293–1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]