Abstract
Objective
Extramammary Paget's disease (EMPD) is a rare cutaneous malignant disease. Due to its rarity, there is a paucity of data regarding best treatment strategy. EMPD primarily affects apocrine gland-bearing skin areas such as the vulva, scrotum, and penis. Our objective was to provide a present-day rationale for diagnosis, pathogenesis, and treatment of EMPD with a focus on recent progress in workup and management of the disease.
Methods
Literature on EMPD until February 2022 was assessed through PubMed, MEDLINE databases, and Google scholar. A narrative review of the most relevant articles was provided.
Results
EMPD usually presents with indolent growth while usually being diagnosed primarily as carcinoma in situ. The foundation of EMPD treatment centers around prompt and accurate diagnosis, wide local or Mohs micrographic surgical excision with proper management towards the margin status, and careful consideration for lymphadenectomy in patients with regionally positive disease. Conventional chemotherapies are alternative treatments modality for patients with distant metastases; however, they sometimes have suboptimal efficacy. At present, there is no agreement regarding adjuvant or systemic therapies, although recent studies have shown several insights into the molecular pathogenesis, tumor biology, and genomics of the development and advancement of EMPD, which may lead to novel and targeted treatment approaches for metastatic EMPD in the future.
Conclusion
Patients with EMPD should seek care from physicians with expertise in disease management and patient counseling. These patients should be surveilled with close follow-up to evaluate them for disease recurrence or progression. Global collaborations with groups such as the Global Society for Rare Genitourinary Tumors, and especially patient support groups are crucial in designing clinical trials to help elucidate more robust data in this orphan disease.
Keywords: Extramammary Paget's disease, Extramammary Paget's malignancy, Genitourinary Paget's disease, Rare genitourinary tumors
1. Introduction
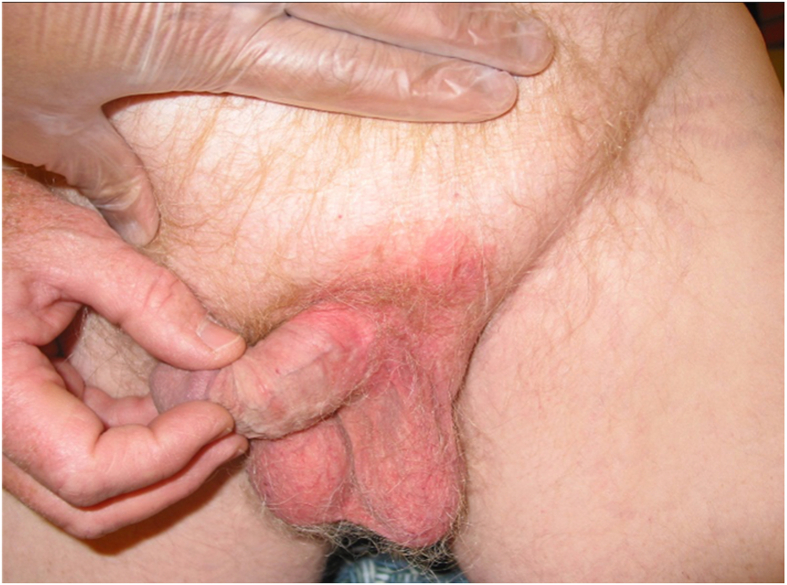
Extramammary Paget's disease (EMPD) is a rare malignancy of skin. It primarily affects apocrine gland-bearing skin areas such as vulva, scrotum, and penis. The disease was first described by Dr. Crocker in 1889 [1]. The lesions generally manifest as infiltrative erythema with crust and scale resembling skin disorders such as eczema (Fig. 1). Given its rarity, EMPD is frequently considered an orphan diagnosis. At present there is no consensus regarding the optimal adjuvant or systemic therapies, although recent studies have shown several insights into the molecular pathogenesis, tumor biology, and genomics of the disease, which may lead to development of novel treatment options in the future.
Figure 1.
Initial presentation of an ill-defined eczematous lesion in the apocrine gland-bearing penoscrotal area. The patient signed informed written consent to procedures and publication.
2. Epidemiology
EMPD is a rare malignancy specifically developing in genital and perianal skin, in addition to other sites with abundant apocrine glands [2]. A recent study by Yin et al. [3] reported the crude prevalence of EMPD in mainland China to be 0.4 per 1 000 000 population. Similarly, a study from 2012 by van der Zwan et al. [4] reported that the crude incidence rate in Europe was 0.7 per 1 000 000 population. However, the true incidence of the disease is still unknown, but it has been estimated to be as low as 0.12 per 100 000 [[5], [6], [7]]. Most individuals are between 50 and 80 years old with the peak age at diagnosis being 66 years old [7]. Gender demographics differ between Western and Asian populations. In studies with Caucasian populations, the disease showed female predominance, with male-to-female ratios ranging from 1:2 to 1:7 [5] while two studies by Ghazawi et al. [8] and Cheng et al. [9] revealed male-to-female ratios of 1.5:1 and 3.5:1, respectively.
3. Classification and pathogenesis
Wilkinson and Brown [10] previously proposed classification for vulvar EMPD as either primary or secondary with distinctive prognosis. Primary or intraepidermal EMPD is associated with apocrine gland ducts with favorable prognosis but if left untreated can become invasive. It is further classified into intraepithelial cutaneous EMPD with invasion and intraepithelial cutaneous EMPD with manifestation of underlying skin-appendage adenocarcinoma [5]. There are few reports that EMPD may occasionally arise from adnexal stem cells or Toker cells [9,10]. Secondary EMPD refers to a metastatic epidermal spread usually derived from a separate primary tumor representing about 25% of total cases. Secondary EMPD can be related to prostate, bladder, urethra, rectum, cervix, stomach, and kidney cancers with further sub-divisions into other origins like anorectal and urothelial [5,11].
Stasenko et al. [12] in 2020 presented genomic sequencing results from 26 patients with vulvar EMPD [12]. They identified the most common gene mutations being PIK3CA (35%), ERBB2 (27%), and TP53 (27%). Other studies have found mutations in RAS, rapidly accelerated fibrosarcoma pathway genes, and AKT1 genes [13]. The epidermis reveals the presence of Paget cells, characterized as large, atypical cells with abundant, clear, and sometimes eosinophilic cytoplasm in hematoxylin and eosin staining [5]. The epidermal layer can often show acanthosis with hyperkeratosis, parakeratosis, or ulceration. These cells can be found as either singular or colonized by non-neoplastic dendritic melanocytes. Most common histopathology features for EMPD are carcinoma in situ, nodular growth patterns, and glandular formation [12].
4. Diagnosis
EMPD lesions usually present as ill-defined eczematous lesions in the apocrine gland-bearing skin areas. Symptoms are usually non-specific and include severe pruritus, erythema, and in some cases hyperpigmentation with ulcerative lesions (Fig. 1). Differentiating between the types of EMPD can be done by using different types of staining and immunohistochemistry techniques. Irrespective of the type, Paget cells are usually positive for the diastase-periodic acid-Schiff reaction, mucicarmine, and zirconyl hematoxylin, indicating the presence of neutral mucopolysaccharides [14]. CK7 and CK20 play a vital role in distinguishing between primary and secondary EMPD [5]. Additional factors like gross cystic-disease fluid protein-15 can assist with differentiation but the odds for a positive result in primary EMPD has been reported to be 30.0%–52.6% [15]. Other distinguishing markers could be CDX-2, and uroplakin II and III, which can provide an accurate discrimination of primary and secondary EMPD [5].
Common symptomatology with benign skin conditions and lack of established diagnostic criteria may delay the diagnosis and treatment. Most patients present with failed topical therapies such as corticosteroids or antifungal medications [14]. Therefore, a history of failed topical therapy warrants further investigation and biopsy. Poor prognosis is linked with delayed diagnosis, depth of invasion more than 1 mm, lympho-vascular invasion, and lymph node metastasis at the time of diagnosis [16].
5. Management
Management strategies for EMPD include non-surgical and surgical approaches as well as systemic therapy. A summary of these approaches is presented in Table 1.
Table 1.
A summary of different management strategies for EMPD.
| Management | Modality | Summary |
|---|---|---|
| Non-surgical management | Topical treatment: imiquimod | |
| Photodynamic therapy: photoreactive drugs, such as aminolaevulinic acid |
|
|
| Radiation therapy |
|
|
| Laser ablation: Neodym:YAG, CO2, and holmium lasers |
|
|
| Surgical management | Surgical excision, punch biopsy, and Mohs micrographic surgery |
|
| Systemic therapy | Combination drug therapies: FP, FECOM, and PET therapy |
|
CR, complete remission; EMPD, extramammary Paget's disease; FECOM: 5-FU, epirubicin, carboplatin, vincristine, and mitomycin C; FP, 5-fluorouracil and cisplatin; PET, cisplatin, epirubicin, and paclitaxel; IL-6, interleukin 6; IFN-alpha, interferon alpha; TNF-alpha, tumor necrosis factor-alpha.
5.1. Non-surgical management
Clinicians should appropriately consider and counsel patients about all available treatment modalities including surgical options, even though primary EMPD can often be managed with nonsurgical approaches. The foremost non-surgical treatment modalities include topical imiquimod, photodynamic therapy (PDT), radiation therapy, and laser ablation.
5.1.1. Topical treatment
Imiquimod is an immunomodulatory topical therapy and works by innate immune pathway stimulation and inducing inflammatory cytokines such as interleukin-6, interferon-alpha, and tumor necrosis factor-alpha producing an antitumor effect [17,18]. Imiquimod also functions by binding to toll-like receptors, monocytes, and dendritic cells resulting in apoptosis [5]. A 2020 study by Snast et al. [18] investigated the utility of imiquimod for EMPD involving a total of 110 participants. The participants were provided with different doses from once a day to twice per week. Complete remission (CR) was reported in 54% of patients (95% confidence interval [CI] 40%–67%), and 85% of patients experienced 50% or more clinical regression (95% CI 74%–90%). However unfortunately, around 40% of participants who showed CR had disease recurrence highlighting the importance of continued follow-up [18].
Another available topical agent is 5-fluorouracil (5-FU) which functions through interfering with DNA and RNA synthesis [19]. A case series published in 2019 by Molina et al. [19] studied the application of 5% 5-FU cream with 0.005% calcipotriene (a vitamin D analogue) twice per day on patients with refractory EMPD. No patient was found to have a CR; however, they had minimal degree of clinical improvement.
5.1.2. PDT
PDT is a non-invasive treatment utilizing photoreactive drugs, such as aminolevulinic acid, which are selectively taken up by tumor cells. This is achieved by exposing the target area to the appropriate wavelength of light, creating toxic-free radicals that destroy tumor cells [5]. Overall research showed that PDT may not be curative but is more beneficial to be used as a palliative treatment to reduce symptoms associated with EMPD lesions [5]. The most common side effects include pain and photosensitivity [5]. However, a recent open-label, single arm study assessed the efficacy and safety of PDT using hematoporphyrin derivatives in EMPD patients. At a mean follow-up of 17.4 months, 72.7% (8/11) of the subjects showed CR with different degrees of scar formation [20]. This pilot study indicates PDT with hematoporphyrin derivatives could be a potential therapy for patients with EMPD.
5.1.3. Radiation therapy
Radiation therapy can be used as both primary as well as adjuvant therapy [21]. A systematic review evaluated the use of radiotherapy in 67 patients [18]. Radiation doses ranged from 10 Gy to 64 Gy delivered in 1–57 fractions. This review found that overall, 97% of patients attained CR, with all patients achieving at least a 50% improvement. Approximately 34% of patients (95% CI 23%–47%) who reached CR had disease recurrence [18]. The most common side effects included mucosal and dermatological toxicities, leukopenia, and variable degree of colitis, cystitis, and urethritis [18].
5.1.4. Laser ablation
Superficial EMPD can be treated with the use of Neodym:YAG, CO2, and holmium lasers. A study published by Li et al. [22] reported data from 61 patients with non-subcutaneous invasive EMPD. The patients were divided into two treatment groups with similar demographics and similarly sized lesions, but the cohorts were nonrandomized. Fifty percent of the cohort were treated with a traditional wide local excision and the remaining 50% were treated with a holmium laser with treatment parameters of 1–2 J at 20 Hz [22]. Cohort treated with holmium laser had a shorter operative duration and less bleeding but with longer wound healing period. The report showed no significant differences between the groups with respect to recurrence-free survival or disease-specific survival. The downside to this study was inconsistent follow-up duration (ranging from 5 to 60 months). Moreover, details regarding the type or nature of disease recurrence were not reported in this study [22]. Similar studies by Choi et al. [23] and Louis-Sylvestre et al. [24] showed higher recurrence rates of 67%–100% [23]. The major disadvantage of using this technique is the lack of additional histologic data for assessment of margins and depth of invasion. Overall, researchers have advocated the use of laser ablation after wide local surgical excision for the management of positive margins, or elderly patients with several comorbidities that are not appropriate surgical candidates [25].
5.2. Surgical management
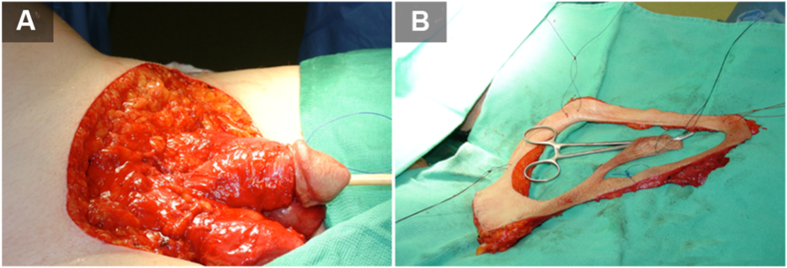
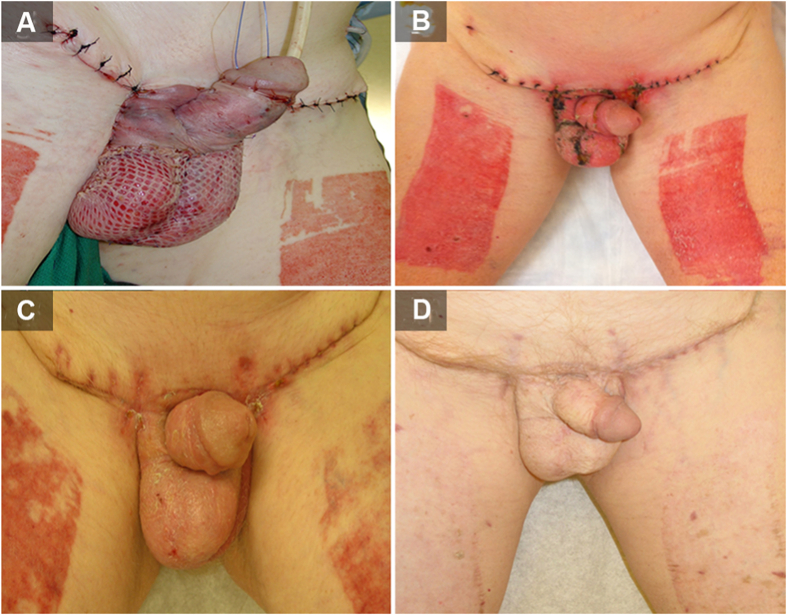
The primary treatment option includes wide local surgical excision of the target area (Fig. 2). Major obstacles for surgery are tumor border irregularities resulting in unclear margins and cases where satellite lesions are present. In a study conducted by Murata and Kumano [26], analysis was done by surgically treating with a 1 cm incision margin. It was determined that clinically determined border of well-defined EMPD lesions corresponded well to the histopathologic border and well-demarcated lesions of EMPD can be adequately treated with 1 cm margin resection [26]. Another case study by Adashek et al. [27] described using a punch biopsy-based mapping technique for determination of true borders of the lesion, by marking the skin surrounding the lesion and obtaining 2 mm punch biopsies circumferentially every 1 cm out to 5 cm from the visible edges of the lesion. Contrary to this approach, some have advocated for smaller margins between 1 cm and 2 cm [27,28]. Closer margins presented higher recurrence rates but could minimize wound defects that might require subsequent skin graft or perineal reconstruction procedure (Fig. 3) [29]. A study by Long et al. [30] with 154 cases of EMPD concluded that females were more likely than males to have a positive margin, and Mohs micrographic surgery resulted in an over 10-fold reduction in the risk of positive margins compared to the standard treatment (wide local excision) [5,29,30]. They also concluded that recurrence risk was 3.5 times more in those with positive pathologic margins compared to those with negative margins (95% CI 1.7–7.2, p<0.001) [30].
Figure 2.
Surgical management of extramammary Paget's disease. (A) Grossly involved skin is resected first; (B) The margin is marked and resected separately for pathologic assessment. The patient signed informed written consent to procedures and publication.
Figure 3.
Post-operative wound healing following resection and skin graft for extramammary Paget's disease. (A) Immediate; (B) 1-month; (C) 2-month; (D) 1-year. The patient signed informed written consent to procedures and publication.
Although EMPD is an intraepithelial malignancy, it may be accompanied with dermal invasion and clinically positive regional lymph nodes. Lymph node dissection is the standard of care in such cases, although level I evidence showing that lymph node dissection improves survival is still lacking [5]. A multicenter retrospective study by Fujisawa et al. [31] involving 151 patients with invasive disease between 1998 and 2012 investigated the role of sentinel lymph node biopsy in EMPD. Among this cohort, 107 had no clinically apparent lymphadenopathy and all these patients underwent sentinel biopsies. Fifteen percent of these patients who were clinically negative were finally found to have lymph node metastasis. This was associated with a greater depth of invasion and lympho-vascular invasion of the primary specimen [31]. The results of this study suggested that sentinel lymph node biopsy should be sought in patients with invasive disease in the absence of clinically evident lymphadenopathy. This study raised the possibility that early detection and lymph node dissection may improve the survival of patients with early-stage lymph node metastasis [31].
5.3. Systemic therapy
Metastatic disease has a poor 5-year overall survival rate of less than 10% [32]. Currently, there is no standard systemic treatment regimen for such patients [33]. Available combination therapy includes 5-FU and cisplatin (FP therapy), 5-FU, epirubicin, carboplatin, vincristine, and mitomycin C (FECOM therapy), cisplatin, epirubicin, and paclitaxel (PET therapy), or combination S-1 (combination of tegafur, 5-FU, and 5-chloro-2-4-dihydroxypyridine) and docetaxel, S-1 monotherapy, or docetaxel monotherapy [[33], [34], [35], [36]]. Although overall median survival is less than 1 year, patients on FP and FECOM regimens have demonstrated a median progression-free survival of 5.2 months and 6.5 months, respectively [33,36]. Several studies have focused on HER2-PI3K/ERK signaling but not all EMPD cases showed overexpression of HER2. Only 15%–58% of patients exhibited HER2 overexpression on immunohistochemistry and fluorescence in situ hybridization studies in both primary and lymph node metastatic lesions [33,36]. HER2 over-expression was discernible in invasive EMPD cases and was associated with multiple lymph node metastases [36]. Therefore, monoclonal antibodies targeting HER2 which were originally used in breast cancer, such as trastuzumab, may have role in treatment of metastatic EMPD. Some hormone receptors such as the estrogen receptor have also been investigated [37]. EMPD is usually expressed with a low 4% estrogen receptor -positivity rate but has a high 54%–90% androgen receptor-positivity [38]. Androgen receptor expression is more commonly associated with invasive EMPD [38]. Further investigations have been made in androgen blockade agents that are commonly used in advanced prostate cancer. Use of combination of androgen blockade, an antiandrogen (bicalutamide), and a luteinizing hormone-releasing hormone agonist (leuprolide acetate) was reported in one case study [39]. Combination efficacy was only seen for a six-month period and in a single case with wide metastatic EMPD which resulted in reduction of multiple EMPD bony metastases [39]. There have been few studies investigating the expression of programmed death-ligand 1 (PD-L1) to aid in better characterizing the immune landscape for these tumors. Karpathiou et al. [40] investigated the expression of CD3, PD-L1, and CTLA-4 in 22 patients with EMPD, involving the vulva, anus, and inguinal region. None of these cases were found to express PD-L1, but CTLA-4 expression was identified in nine of those cases with most cases showing stromal CD3 and intra-epithelial CD3 expression [40]. Another study by Goto et al. [41] investigated 39 cases to identify PD-L1 and programmed cell death protein 1 staining. Out of 90% of the cohort with genital EMPD, 59% of cases with carcinoma in situ and 41% cases with invasive disease, none of which were found to express PD-L1, respectively [41]. Fujimura et al. [42] in 2016 reported that three of six cases of EMPD expressed PD-L1, even though the data collected from several studies suggest that PD-L1 expression is exceedingly rare suggesting involvement of other immune pathways. Guercio et al. [32] published one case report describing management of metastatic EMPD with combination of ipilimumab and nivolumab, after progressing on cytotoxic chemotherapy. This combination therapy resulted in a partial response lasting 7 months. Such case report represents one of earliest in the literature utilizing combination systemic immunotherapy for metastatic EMPD.
A summary of studies included in this review article is provided in Table 2.
Table 2.
A summary of studies included in this review article.
| Management | Modality | Study | Case, n | Outcome | Best clinical use |
|---|---|---|---|---|---|
| Non-surgical management | Imiquimod | Snast et al., 2020 [18] | 110 |
|
|
| 5-FU | Molina et al., 2019 [19] | 3 (recurrent refractory EMPD cases) product used: 5% 5-FU cream with 0.005% calcipotriene |
|
||
| PDT | Ishizuki and Nakamura, 2021 [5] | NA |
|
|
|
| Radiation doses ranged from 10 Gy to 64 Gy delivered in 1–57 fractions | Snast et al., 2020 [18] | 67 |
|
|
|
| Holmium laser | Li et al., 2014 [22] | 61 |
|
|
|
| Carbon dioxide laser | Choi et al., 2001 [23] and Louis-Sylvestre et al., 2004 [24] | 3 |
|
|
|
| Surgical management | Wide excision (1 cm incision margins) | Murata et al., 2005 [26] | 46 |
|
|
| Mohs micrographic surgery | Long et al., 2017 [30] | 154 |
|
|
|
| Punch biopsy | Adashek et al., 2019 [27] | 41 |
|
||
| Sentinel lymph node biopsy | Fujisawa et al., 2015 [31] | 151 |
|
|
|
| Systemic therapy | FECOM therapy | Oashi et al., 2014 [33] | 7 |
|
|
| HER2 | Tanaka et al., 2013 [34] | 104 |
|
|
|
| Combination chemotherapy of low-dose FP | Tokuda et al., 2015 [36] | 22 |
|
|
|
| Trastuzumab | Fukuda and Funakoshi, 2018 [37] | 3 |
|
|
|
| Trastuzumab+paclitaxel | Fukuda and Funakoshi, 2018 [37] | 3 |
|
|
|
| Docetaxel+S-1 | Fukuda and Funakoshi, 2018 [37] | 4 |
|
|
CI, confidence interval; CR, complete remission; EMPD, extramammary Paget's disease; 5-FU, 5-fluorouracil; FECOM, 5-FU, epirubicin, carboplatin, vincristine, and mitomycin C; FP, 5-FU and cisplatin; HER2, human epidermal growth factor receptor 2; PDT, photodynamic therapy; PR, partial response; S-1, 1-(2-tetrahydrofuryl)-5-fluorouracil + 5-chloro-2, 4-dihydroxypyridine; SD, stable disease; NA, not available.
6. Conclusion
EMPD is a rare disease and as a result, lacks standardization of treatment guidelines. Treatment currently centers on accurate diagnosis, surgical excision with negative margins, and consideration of lymphadenectomy in patients with regionally positive disease. Conventional chemotherapies are an alternative treatment modality for patients with distant metastases. At present there is no standard adjuvant or systemic therapy although recent studies have shown several insights into the molecular pathogenesis, tumor biology, and genomics of the development and advancement of EMPD, which may lead to novel treatment approaches in the future. Patients with EMPD should be referred to experienced centers and physicians with expertise in disease management and patient counseling. Global collaborations with supportive groups can be considered crucial in designing clinical trials and effective database evaluation.
Author contributions
Study concept and design: Reza Nabavizadeh, Behnam Nabavizadeh, Vikram M. Narayan, Viaj A. Master.
Data acquisition: Khushali B. Vashi.
Data analysis: Khushali B. Vashi.
Drafting of manuscript: Khushali B. Vashi.
Critical revision of the manuscript: Reza Nabavizadeh, Behnam Nabavizadeh.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Crocker H.R. Paget's disease affecting scrotum and penis. Trans Path Soc Lond. 1889;40:187–191. [Google Scholar]
- 2.Yao H., Xie M., Fu S., Guo J., Peng Y., Cai Z., et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403. doi: 10.1186/s12885-018-4257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin S., Xu L., Wang S., Feng J., Liu L., Liu G., et al. Prevalence of extramammary Paget's disease in urban China: a population-based study. Orphanet J Rare Dis. 2021;16:134. doi: 10.1186/s13023-021-01715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Zwan J.M., Siesling S., Blokx W.A., Pierie J.P., Capocaccia R. Invasive extramammary Paget's disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214–221. doi: 10.1016/j.ejso.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Ishizuki S., Nakamura Y. Extramammary Paget's disease: diagnosis, pathogenesis, and treatment with focus on recent developments. Curr Oncol. 2021;28:2969–2986. doi: 10.3390/curroncol28040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayan V.M., Master V.A. Extramammary Paget's disease of genitourinary origin. Curr Opin Urol. 2022;32:211–215. doi: 10.1097/MOU.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 7.Leong J.Y., Chung P.H. A primer on extramammary Paget's disease for the urologist. Transl Androl Urol. 2020;9:93–105. doi: 10.21037/tau.2019.07.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazawi F.M., Iga N., Tanaka R., Fujisawa Y., Yoshino K., Yamashita C., et al. Demographic and clinical characteristics of extramammary Paget's disease patients in Japan from 2000 to 2019. J Eur Acad Dermatol Venereol. 2021;35:e133–e135. doi: 10.1111/jdv.16868. [DOI] [PubMed] [Google Scholar]
- 9.Cheng P.S., Lu C.L., Cheng C.L., Lai F.J. Significant male predisposition in extramammary Paget disease: a nationwide population-based study in Taiwan. Br J Dermatol. 2014;171:191–193. doi: 10.1111/bjd.12851. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson E.J., Brown H.M. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol. 2002;33:549–554. doi: 10.1053/hupa.2002.124788. [DOI] [PubMed] [Google Scholar]
- 11.Hegarty P.K., Suh J., Fisher M.B., Taylor J., Nguyen T.H., Ivan D., et al. Penoscrotal extramammary Paget's disease: the University of Texas M. D. Anderson Cancer Center contemporary experience. J Urol. 2011;186:97–102. doi: 10.1016/j.juro.2011.02.2685. [DOI] [PubMed] [Google Scholar]
- 12.Stasenko M., Jayakumaran G., Cowan R., Broach V., Chi D.S., Rossi A., et al. Genomic alterations as potential therapeutic targets in extramammary Paget's disease of the vulva. JCO Precis Oncol. 2020;4:1054–1060. doi: 10.1200/PO.20.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Z., Xu F., Zhang Q.A., Wu Z., Zhang X., Xu J., et al. Oncogenic mutations in extramammary Paget's disease and their clinical relevance. Int J Cancer. 2013;132:824–831. doi: 10.1002/ijc.27738. [DOI] [PubMed] [Google Scholar]
- 14.Phyo A.K., Mun K.S., Kwan K.C., Ann C.C., Kuppusamy S. Genitourinary extramammary Paget's disease: review and outcome in a multidisciplinary setting. Int J Clin Exp Pathol. 2020;13:2369–2376. [PMC free article] [PubMed] [Google Scholar]
- 15.Ohnishi T., Watanabe S. The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget's disease. Br J Dermatol. 2000;142:243–247. doi: 10.1046/j.1365-2133.2000.03291.x. [DOI] [PubMed] [Google Scholar]
- 16.Shu B., Shen X.X., Chen P., Fang X.Z., Guo Y.L., Kong Y.Y. Primary invasive extramammary Paget disease on penoscrotum: a clinicopathological analysis of 41 cases. Hum Pathol. 2016;47:70–77. doi: 10.1016/j.humpath.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bubna A.K. Imiquimod—its role in the treatment of cutaneous malignancies. Indian J Pharmacol. 2015;47:354–359. doi: 10.4103/0253-7613.161249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snast I., Sharon E., Kaftory R., Noyman Y., Oren-Shabtai M., Lapidoth M., et al. Nonsurgical treatments for extramammary Paget disease: a systematic review and meta-analysis. Dermatology. 2020;236:493–499. doi: 10.1159/000506832. [DOI] [PubMed] [Google Scholar]
- 19.Molina G.E., Khalifian S., Mull J.L., Chen L., Rosman I.S., Faulkner-Jones B.E., et al. Topical combination of fluorouracil and calcipotriene as a palliative therapy for refractory extramammary Paget disease. JAMA Dermatol. 2019;155:599–603. doi: 10.1001/jamadermatol.2018.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Wang P., Li C., Zhou Z., Zhang L., Zhang G., et al. Efficacy and safety of HpD-PDT for extramammary Paget's disease refractory to conventional therapy: a prospective, open-label and single arm pilot study. Photodiagnosis Photodyn Ther. 2022;37 doi: 10.1016/j.pdpdt.2021.102670. [DOI] [PubMed] [Google Scholar]
- 21.Hata M., Koike I., Wada H., Miyagi E., Kasuya T., Kaizu H., et al. Radiation therapy for extramammary Paget's disease: treatment outcomes and prognostic factors. Ann Oncol. 2014;25:291–297. doi: 10.1093/annonc/mdt478. [DOI] [PubMed] [Google Scholar]
- 22.Li Z., Yang D., Che X., Zong H., Adnan H., Wang J., et al. Clinical research of holmium laser therapy in extramammary Paget's disease. Laser Med Sci. 2014;29:1907–1912. doi: 10.1007/s10103-014-1599-z. [DOI] [PubMed] [Google Scholar]
- 23.Choi J.B., Yoon E.S., Yoon D.K., Kim D.S., Kim J.J., Cho J.H. Failure of carbon dioxide laser treatment in three patients with penoscrotal extramammary Paget's disease. BJU Int. 2001;88:297–298. doi: 10.1046/j.1464-410x.2001.02326.x. [DOI] [PubMed] [Google Scholar]
- 24.Louis-Sylvestre C., Haddad B., Paniel B.J. Paget's disease of the vulva: results of different conservative treatments. Eur J Obstet Gynecol Reprod Biol. 2001;99:253–255. doi: 10.1016/s0301-2115(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 25.Christodoulidou M., Alnajjar H.M., Parnham A., Khetrapal P., Freeman A., Haider A., et al. Multidisciplinary approach for the management of penoscrotal extramammary Paget's disease—an eUROGEN study. Urol Oncol. 2021;39 doi: 10.1016/j.urolonc.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Murata Y., Kumano K. Extramammary Paget's disease of the genitalia with clinically clear margins can be adequately resected with 1 cm margin. Eur J Dermatol. 2005;15:168–170. [PubMed] [Google Scholar]
- 27.Adashek J.J., Leonard A., Nealon S.W., Krishnan A., Mosiello G.C., Dhillon J., et al. Extramammary Paget's disease: what do we know and how do we treat? Can J Urol. 2019;26:10012–10021. [PubMed] [Google Scholar]
- 28.Kaku-Ito Y., Ito T., Tsuji G., Nakahara T., Hagihara A., Furue M., et al. Evaluation of mapping biopsies for extramammary Paget disease: a retrospective study. J Am Acad Dermatol. 2018;78:1171–1177.e4. doi: 10.1016/j.jaad.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Wollina U., Goldman A., Bieneck A., Abdel-Naser M.B., Petersen S. Surgical treatment for extramammary Paget's disease. Curr Treat Options Oncol. 2018;19:27. doi: 10.1007/s11864-018-0545-x. [DOI] [PubMed] [Google Scholar]
- 30.Long B., Schmitt A.R., Weaver A.L., McGree M., Bakkum-Gamez J.N., Brewer J., et al. A matter of margins: surgical and pathologic risk factors for recurrence in extramammary Paget's disease. Gynecol Oncol. 2017;147:358–363. doi: 10.1016/j.ygyno.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Fujisawa Y., Yoshino K., Kiyohara Y., Kadono T., Murata Y., Uhara H., et al. The role of sentinel lymph node biopsy in the management of invasive extramammary Paget's disease: multi-center, retrospective study of 151 patients. J Dermatol Sci. 2015;79:38–42. doi: 10.1016/j.jdermsci.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Guercio B.J., Iyer G., Kidwai W.Z., Lacouture M.E., Ghafoor S., Rossi A.M., et al. Treatment of metastatic extramammary Paget disease with combination ipilimumab and nivolumab: a case report. Case Rep Oncol. 2021;14:430–438. doi: 10.1159/000514345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oashi K., Tsutsumida A., Namikawa K., Tanaka R., Omata W., Yamamoto Y., et al. Combination chemotherapy for metastatic extramammary Paget disease. Br J Dermatol. 2014;170:1354–1357. doi: 10.1111/bjd.12788. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka R., Sasajima Y., Tsuda H., Namikawa K., Tsutsumida A., Otsuka F., et al. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br J Dermatol. 2013;168:1259–1266. doi: 10.1111/bjd.12249. [DOI] [PubMed] [Google Scholar]
- 35.Tanese K., Nakamura Y., Hirai I., Funakoshi T. Updates on the systemic treatment of advanced non-melanoma skin cancer. Front Med. 2019;6:160. doi: 10.3389/fmed.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokuda Y., Arakura F., Uhara H. Combination chemotherapy of low-dose 5-fluorouracil and cisplatin for advanced extramammary Paget's disease. Int J Clin Oncol. 2015;20:194–197. doi: 10.1007/s10147-014-0686-2. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda K., Funakoshi T. Metastatic extramammary Paget's disease: pathogenesis and novel therapeutic approach. Front Oncol. 2018;8:38. doi: 10.3389/fonc.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liegl B., Horn L.C., Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget's disease. Mod Pathol. 2005;18:1283–1288. doi: 10.1038/modpathol.3800437. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama K., Kamada N., Kinoshita K., Kawashima T., Otani M., Endo H., et al. Androgen-deprivation regimen for multiple bone metastases of extramammary Paget disease. Br J Dermatol. 2005;153:853–855. doi: 10.1111/j.1365-2133.2005.06865.x. [DOI] [PubMed] [Google Scholar]
- 40.Karpathiou G., Chauleur C., Hathroubi S., Habougit C., Peoc'h M. Expression of CD3, PD-L1 and CTLA-4 in mammary and extra-mammary Paget disease. Cancer Immunol Immunother. 2018;67:1297–1303. doi: 10.1007/s00262-018-2189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goto H., Sugita K., Yamamoto O. Expression of programmed death-ligand 1 and programmed death-1 in patients with extramammary Paget's disease. Indian J Dermatol. 2021;66:169–173. doi: 10.4103/ijd.IJD_341_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujimura T., Kambayashi Y., Kakizaki A., Furudate S., Aiba S. RANKL expression is a useful marker for differentiation of pagetoid squamous cell carcinoma in situ from extramammary Paget disease. J Cutan Pathol. 2016;43:772–775. doi: 10.1111/cup.12743. [DOI] [PubMed] [Google Scholar]