Abstract
Most animal models of sepsis induced high mortality or early recovery and do not mimic the long-lasting catabolic state observed in patients. The purpose of this study is to develop a model of sepsis which reproduces these disorders, especially the long-lasting muscle wasting. This report summarizes our observations in a series of seven experiments using this model with rats to study the route of live Escherichia coli administration, dose of bacteria, reproducibility of the model, bacterial count in tissues, comparison of injection of live or dead bacteria, metabolic perturbations linked to infection, and potential role of tumor necrosis factor alpha (TNF-α) in muscle wasting. After intravenous infection, animals were anorexic and the catabolic state was long-lasting: body weight loss for 2 to 3 days followed by a chronic wasting state for several days. Liver, spleen, lung protein content, and plasma concentration of α2-macroglobulin were increased 2 and 6 days after infection. At 6 days, muscle protein content was substantially (−40%) reduced. The plasma TNF-α level measured 1.5 h after infection correlated with body weight loss observed 9 days later. The inhibition of TNF-α secretion by administration of pentoxifylline 1 h before infection reduced muscle wasting and activation of proteolysis at day 2 and abolished them at day 6. This septic model mimics in rats the prolonged protein metabolism alterations and muscle atrophy characteristics of infected patients and thus is useful for studying the impact of nutritional support on outcome.
Despite intensive care, sepsis is still a major cause of death throughout the world. Sepsis is associated with a very large range of disorders, such as profound hemodynamic and nutritional derangements, organ failure and multiple metabolic alterations with increased energy substrate turnover, altered hormonal pattern, and intensive protein catabolism. Among these disturbances, one of the most dramatic is the loss of lean body mass, especially muscle proteins. This problem is complicated by interactions with nutritional state (4). Moreover, these alterations can be maintained for weeks in patients with trauma and sepsis. It is therefore of primary importance to obtain a relevant model suitable for prolonged metabolic studies and sufficiently long-lasting to test the efficiency of nutrition on outcome. Numerous models have been described, but most of them provide only short time observations because the majority of animals die within 24 to 48 h.
Most of the proposed models are very severe and present a high degree of mortality. Cecal ligation with puncture (CLP) has been extensively used, but animals die in 24 to 48 h (22, 36). Prolonged survival has been described in a modification of the CLP model that uses only one small puncture (35), but standardization of this model is very difficult for metabolic studies due to a large variability in the severeness of disease (13); furthermore, body weight loss is limited (35). In an effort to standardize the bacterial strain used, peritonitis has been provoked with known bacteria entrapped in a gelatin capsule or a fecal pellet made of sterile rat feces (12, 24), but high mortality was still observed. More recently, this latter model has been successfully used for studying the effect of septic abscess on protein synthesis rate measured in skeletal muscle 5 days after operation of animals (33). However, animals began to exhibit growth recovery 4 days after implantation of the fecal-agar pellet, and nutritional status was different from clinical conditions since animals were not anorexic. Alexander et al. (2) induced peritonitis by means of continuous infusion of live bacteria with an osmotic pump, but body weight loss of control rats was similar to that of septic animals. In fact, all of these models involve an invasive surgical procedure which contributes to the catabolic state, and differentiation of the effect of sepsis from the effect of surgery is often difficult.
The administration of a high dose of endotoxin induced an overwhelming aggression (22, 36). In contrast, moderate doses of endotoxins are not lethal, and growth recovery of the animals occurred after only 48 h. Prolonged or repeated administration leads to endotoxin tolerance (3, 10, 20), which is also observed with continuous infusion of endotoxin (11). Intravenous (i.v.) administration of live bacteria has been criticized by numerous authors because of the high mortality observed in few hours (24, 27, 28, 38). However, Perbellini et al. (26) and more recently Shaw and Wolfe (30), clearly reproduced in dogs a situation that hormonally, hemodynamically, and metabolically resembles human sepsis. They provoked sepsis by injection of live Escherichia coli organisms in a sublethal dose but gave no data on body weight loss of the animals. With the same approach of a single i.v. injection of live E. coli, we describe a highly reproducible sepsis model which induces acute and prolonged body weight loss and muscle atrophy and provides a new tool for studying the impact of nutritional support in sepsis.
MATERIALS AND METHODS
Animal care.
Male Sprague-Dawley rats (Iffa Credo, Saint Germain sur l’Arbresle, France) weighing about 250 g were individually housed in wire-bottom cages in a temperature-controlled room (22 to 23°C) with a 12 h-12 h light-dark cycle. After 6 days of acclimatization, animals were randomized into groups for injection of live bacteria or saline. During the acclimatization period, all rats had free access to water and to a semisynthetic diet containing 12% protein described previously (25).
Preparation of bacteria.
An E. coli serotype O153:K−:H− strain isolated from calf septicemia was used. Bacteria were grown in 10 ml of Minca broth (18) and incubated overnight in a shaking incubator at 37°C. Chloramphenicol (Sigma, L’Isle d’Abeau Chesnes, France) was added to culture medium to avoid loss of the plasmid encoding antibiotic resistances (Kanr, Strr, Cmpr, Tetr, Sulr) and virulence properties (aerobactin [37] and surface protein CS31A [14]). The morning after, the bacterial suspension was used to inoculate fresh Minca broth. After a 2-h incubation at 37°C, optical density of the bacterial suspension was measured at 600 nm and the bacterial concentration was estimated, assuming that an optical density of 1 represents 4 × 108 E. coli per ml. Bacteria were collected in the logarithmic growth phase and centrifuged for 15 min at 6,000 × g, and the pellet was resuspended in saline. Then, viable bacteria were counted by serial 10-fold dilutions plated in duplicate on deoxycholate agarose (DCA; Difco, OSI, Paris, France). Plates were incubated at 37°C for 15 h, and colonies were counted.
Experiments.
Experiment 1 was designed to compare the effects of different routes of bacterial administration. A 0.5-ml volume of the same preparation of bacteria (1.3 × 109 bacteria per ml) was injected either intraperitoneally (i.p.) or i.v. into a lateral tail vein. Since rats in the i.v. group were highly anorexic, a pair-fed (PF) control group was injected with saline and fed with the same restricted food intake as the i.v. group. Rectal temperature was measured at different times before and after infection. Rats were studied for 2 days postinfection.
Experiment 2 was designed to study the dose effects of i.v. injection of E. coli with the objective of finding a sublethal dose which induced a long-lasting catabolic state. Three suspensions containing theoretically 4.4 × 108, 1.3 × 109, and 4 × 109 bacteria per ml were prepared, and 0.5 ml of one of these suspensions was injected. Each syringe was weighed before and after injection, and the quantity injected into each rat was calculated. The rats were divided into three groups of eight rats each: one given (2.5 ± 0.3) × 108 (low-dose group [LDG]), one given (6.8 ± 0.9) × 108 (medium-dose group [MDG]), and one given (2.0 ± 0.5) × 109 (high-dose group [HDG]) bacteria per rat. A blood sample was taken 90 min after infection for measurement of tumor necrosis factor alpha (TNF-α) in plasma. In a preliminary kinetic study, we confirmed that the TNF-α plasma concentration peaked 90 min after infection. Rats were weighed every morning and studied for up to 10 days after infection. The medium dose of bacteria was used in subsequent experiments.
Experiment 3 was performed to establish the reproducibility of body weight loss after infection with different bacterial preparations. The infection was repeated four times with a medium dose of 6.6 × 108 bacteria per rat. Animals were kept for 10 days.
The aim of experiment 4 was to evaluate the number of bacteria in blood, liver, and spleen. Five rats were sacrificed on each of days 2, 6, and 10 after infection for bacterial counts in these organs.
Experiment 5 was designed to indirectly estimate the potential effect of the administration of the amount of lipopolysaccharide (LPS) contained in the bacteria injected. Six rats received 7.0 × 108 live bacteria, and six rats received the same bacterial suspension heated for 30 min at 60°C to kill the bacteria without altering the structure of the LPS. After heating, no colony was detected on the dilution 1:10 plated in duplicate on DCA after incubation at 37°C for 15 h. Body weights were recorded for 6 days postinfection.
Experiment 6 was performed to study metabolic disturbances induced by E. coli infection. Since we observed in experiment 2 that inoculated rats lost weight for 3 or 4 days and then maintained their body weight for a similar period of time, we chose to study metabolic perturbations 2 and 6 days after infection. In this experiment, the dose of bacteria was (8.3 ± 0.8) × 108 CFU per rat. Results for infected rats were compared to those for a control noninfected group which received saline and had free access to food for 6 days and to those for control groups injected with saline and pair-fed to infected groups (see “Food intake” below). Therefore, this protocol used five groups of animals (n = 6 to 8): control rats killed 6 days after saline injection; infected rats killed 2 and 6 days after infection; and PF counterparts of the infected rats killed 2 and 6 days after saline injection.
After anesthesia with sodium pentobarbital (6.0 mg/100 g of body weight; Sanofi Santé Animale, Libourne, France), blood was taken from the abdominal aorta for hematocrit determination and biochemical analyses. Immediately after the rats were killed, liver, lung, spleen, kidney, heart, gastrocnemius, and soleus muscle were quickly excised, weighed, frozen in liquid nitrogen, and stored at −20°C until analysis.
Experiment 7 was designed to study the role of cytokines, and particularly TNF-α, in muscle wasting. At an initial body weight of 300 g, rats were divided into four groups: infected rats (INF group) and their PF controls, and infected rats treated with pentoxifylline (PX; Torental; Hoechst, Paris, France) (PX-INF) and their PF controls treated with PX (PX-PF). The INF group received saline i.p. 1 h before injection of E. coli (7 × 108 bacteria per rat) into a lateral tail vein. PF animals received an i.p. injection of saline 1 h before an i.v. saline injection and were pair-fed the intake of infected rats. In the PX-INF group, PX (100 mg/kg) was injected i.p. 1 h before administration of bacteria. PX-PF animals received an i.p. injection of PX 1 h before an i.v. injection of saline. Because PX treatment increases voluntary food consumption in infected rats (5), the PX-PF control group was pair-fed the intake of PX-INF animals. Blood samples were taken 1.5 and 3 h after infection for cytokine measurements. Between six and eight animals of each group were studied on days 2 and 6 after infection. After anesthesia with sodium pentobarbital (6.0 mg/100 g body weight), the epitrochlearis muscles were dissected intact for incubation (see below). Gastrocnemius and soleus muscles were also dissected and weighed.
Food intake.
A controlled amount of food was distributed in six equal meals given at 03:00, 07:00, 11:00, 15:00, 19:00, and 23:00 by an automatic device. Infected rats were highly anorexic for 2 or 3 days and ate only 60% of the normal intake on day 7 after infection. In this context, pair-feedings were performed in experiments 1, 5, and 6. Since the food intake of PF rats was highly restricted, the time schedule distribution was used to maintain food intake throughout the day.
Food intake of ad libitum-fed rats was determined in a previous study, and the same daily amount of food was offered to infected animals. Food intake of each inoculated group was measured daily, and the same quantity of food was offered to PF rats with a time lag of 2 days after feeding of infected groups, except in experiment 6. To incubate simultaneously muscles of infected and control rats, the intake of controls was based on the intake of infected rats measured in previous experiments.
Analytical procedures.
TNF-α and interleukin-1β (IL-1β) plasma concentrations were measured by using enzyme-linked immunosorbent assay kits as instructed by the manufacturers (Genzyme [Cambridge, Mass.] and Amersham [Buckinghamshire, England], respectively). Biological activity of IL-6 was estimated in a bioassay using the B-9 hybridoma cell line (1). Briefly, B-9 cells (5,000/100 ml) were cultured in 96-well microtiter plates with serial dilutions of test samples (15). The IL-6 standard was human recombinant IL-6 (catalog no. 89/548; National Institute for Biological Standards and Control, Hertfordshire, England), which was serially diluted. After 48 h of incubation at 37°C with 5% CO2, 20 ml of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (2 mg/ml; Interchim, Montluçon, France), was added to each well in the presence of phenazine methosulfate, and the mixture was incubated for an additional 2 h to determine cell proliferation (7). The water-soluble formazan product was quantitated at 490 nm in an MR 700 microplate reader (Dynatech Laboratories, Inc., Guernsey, England).
Plasma glucose was assayed by a glucose oxidase method (Boehringer, Mannheim, Germany), plasma insulin was assayed by radioimmunoassay with a commercial kit (SB-INSI-1; CEA, Gif-sur-Yvette, France), plasma triglycerides were assayed by the glycerol phosphate oxydase–amino-4-antipyrine method (Roche Diagnostic Systems, Neuilly sur Seine, France), plasma proteins were assayed by the biuret method (Roche Diagnostic Systems), plasma lactate was assayed by enzymatic determination (lactate dehydrogenase from Boehringer, Mannheim, Germany), and tissue nitrogen contents were assayed by the Kjeldahl method. Tissue proteins were expressed as nitrogen X 6.25.
In experiment 3, blood, liver, and spleen were collected under sterile conditions for bacterial counts. The organs were immediately homogenized in sterile conditions, and 10-fold serial dilutions were done in duplicate in sterile saline. Each dilution was plated on 0.05% DCA agar medium (Difco), and colonies were counted after 15 h incubation at 37°C.
Muscle proteolysis was measured in experiment 6 as previously described (34). Briefly, epitrochlearis muscles were preincubated for 30 min in Krebs Henseleit buffer (120 mM NaCl, 4.8 mM KCl, 25 mM NaHCO3, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4 [pH 7.4]) supplemented with 5 mM glucose, 5 mM HEPES, 0.1% bovine serum albumin, 0.17 mM leucine, 0.20 mM valine, 0.10 mM isoleucine, 0.1 U of insulin per ml, and 0.5 mM cycloheximide. The medium was saturated with a 95% O2–5% CO2 gas mixture. Muscles were then transferred into fresh medium of the same composition for 60 min. At the end of the incubation, muscles were blotted and homogenized in 10% trichloroacetic acid (TCA). TCA-insoluble material was washed three times with 10% TCA and solubilized in 1 N NaOH at 37°C for determination of protein. Tissue protein mass was determined by the bicinchoninic acid (Pierce, Rockford, Ill.) procedure. Protein degradation was determined from tyrosine release in the incubation medium by a fluorimetric method as described previously (34). Tyrosine is not synthesized, degraded, or reused by muscle for protein synthesis since the incubation medium contained cycloheximide to block protein synthesis. Thus, the release of this amino acid from muscle into the incubation medium reflects the total rate of protein breakdown.
The statistical significance of differences between means was assessed by Student’s t test or by one-way analysis of variance. Differences were considered as significant at P < 0.05. All data were expressed as means ± standard deviations.
RESULTS
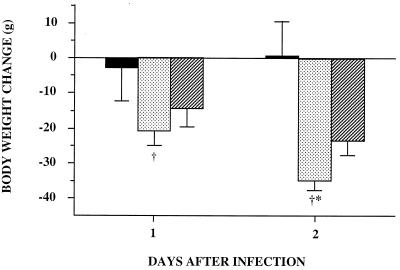
Experiment 1 revealed the importance of the route of administration of bacteria (Fig. 1). The i.p. group showed a very limited body weight loss (2.2 ± 9.6 g) on the day of infection, and growth recovery began as early as day 2, reaching a weight gain of 3.4 ± 3.8 g. In contrast, for the 2 days following infection, the i.v. group showed a marked body weight loss (of 34.8 ± 2.8 g on day 2). The PF mice of the i.v. group also showed body weight loss during the 2 days postinfection, but only 67% of that for the i.v. group. Food intake was very different between the two infected groups: rats in the i.p. group ate 73 and 85% of preinfection food intake on days 1 and 2 postinfection, respectively, while corresponding values for rats in the i.v. group were only 17 and 13%.
FIG. 1.
Effect of route of administration of bacteria on body
weight change (experiment 1). Rats were infected by administration of
live E. coli (6.5 × 108) either i.p. (■)
or i.v. (
). They had free access to alimentation. One control PF
group was injected i.v. with saline and ate the same quantity of food
as the i.v. group
( ). Body
weight changes were recorded for 2 days. Rats weighed 300 g at
infection. Data are means ± standard deviations. Intravenous
infection of rats resulted in the highest body weight loss: ∗,
P < 0.05 versus the PF group; †, P <
0.05 versus the i.p. group.
). Body
weight changes were recorded for 2 days. Rats weighed 300 g at
infection. Data are means ± standard deviations. Intravenous
infection of rats resulted in the highest body weight loss: ∗,
P < 0.05 versus the PF group; †, P <
0.05 versus the i.p. group.
Table 1 shows the effects of infection on rectal temperature. In the i.p. group, infection produced no change in rectal temperature. In contrast, the i.v. group showed a marked hypothermia 1.5 h after infection, which had normalized 4 h after infection. At 24 and 48 h, these rats exhibited a moderate fever (P < 0.05).
TABLE 1.
Effects of infection on rectal temperaturea
| Group | Mean rectal temp (°C) ± SD at indicated
time after infection
|
||||
|---|---|---|---|---|---|
| 0 h | 1.5 h | 4 h | 24 h | 48 h | |
| i.p. | 36.1 ± 0.7 | 35.5 ± 0.3 | 35.9 ± 0.9 | 36.6 ± 0.6 | 36.2 ± 0.7 |
| i.v. | 36.2 ± 0.5 | 34.0 ± 0.5b | 35.5 ± 0.5 | 37.0 ± 0.9b | 37.0 ± 0.5b |
Experiment 1; rats received, by the i.p. or i.v. route, 6.5 × 108 bacteria.
P < 0.05 versus time zero.
Bacteria were given by the i.v. route in the following experiments, since i.p. administration failed to produce body weight loss. In these experiments, all infected rats showed symptoms of severe illness. After infection, they rapidly became lethargic and anorexic and exhibited strong piloerection. Most of the MDG animals and surviving HDG rats (experiment 2) exhibited chromodacryorrhea and diarrhea. There was an obvious mortality dose effect, since all LDG rats survived, only one MDG rat died (on day 1 postinfection), and all HDG rats died within 4 days (three on day 1, four on day 2, and one on day 4). Three days after inoculation, we observed an improvement of the clinical state of the rats: they became more active and no longer exhibited chromodacryorrhea and diarrhea.
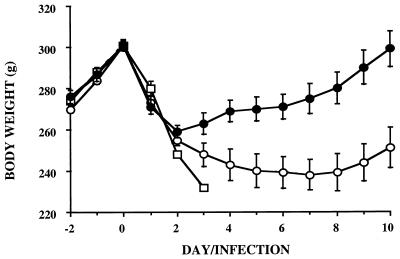
Both LDG and MDG animals exhibited acute body weight loss (of about 40 g) during the first 2 days postinfection (Fig. 2). From days 2 to 5 postinfection, MDG rats lost 13 g of body weight whereas LDG rats gained 12 g. This dose effect was amplified from days 6 to 8 postinfection; growth recovery persisted in LDG rats, but body weights of MDG rats plateaued around 60 g below the initial body weight. MDG rats did not begin growth recovery until day 9 postinfection. They were 46 g below their day 0 body weight on day 10, while LDG rats had recovered their initial body weight. Body weight change of the MDG rats followed a triphasic course that can be summarized as follows: acute loss for 3 or 4 days (acute phase), stability from day 3 or 4 to 8 (chronic phase), and progressive recovery between days 8 and 10 postinfection (late phase).
FIG. 2.
Body weight changes after an i.v. injection of various doses of live E. coli (experiment 2). ●, low dose ([2.5 ± 0.3] × 108); ○, medium dose ([6.8 ± 0.9] × 108); □, high dose ([2.0 ± 5] × 108). With the high dose, only one rat survived until day 4.
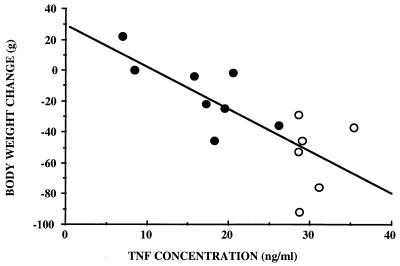
Plasma TNF-α concentrations measured 1.5 h after infection (experiment 2) were significantly higher (P < 0.05) in rats that died (30.3 ± 4.6 ng/ml) than in rats that survived (22.0 ± 8.5 ng/ml). Plasma TNF-α concentrations were significantly lower in LDG rats (14.8 ± 5.4 ng/ml) than in MDG rats (29.0 ± 2.7 ng/ml), but MDG and HDG rats showed similar TNF-α concentrations (30.7 ± 4.9 ng/ml in HDG rats). In surviving animals, there was a significant correlation between TNF-α concentration measured 1.5 h postinfection and body weight change between day 0 (just before infection) and any day between days 6 and 10. The strongest relationship was found between TNF-α concentration and body weight change observed nine days after infection (Fig. 3).
FIG. 3.
Correlation between TNF concentration measured 1.5 h after infection and body weight change observed 9 days later (experiment 2). y = 26.11 − 2.73x, r = 0.576; P < 0.01. Rats were infected by i.v. injection of live E. coli. ●, low dose ([2.5 ± 0.3] × 108); ○, medium dose ([6.8 ± 0.9] × 108); n = 14.
To test the reproducibility of the model in term of body weight changes (experiment 3), four groups of eight rats were infected with different bacterial preparations, using the medium dose of experiment 2. The amount of bacteria in the inoculum was between 88 and 103% of the theoretical dose. Two days after infection, body weight losses were 44 ± 5, 40 ± 3, 38 ± 4, and 34 ± 4 g for the four groups. The maximum weight losses were similar in the four groups, about 57 g. The days on which growth recovery began were also similar in the four groups (between days 7 and 9). Furthermore, repetition of administration of 15 successive preparations of bacterial inoculum allowed us to obtain a standard deviation of 20% around the mean value of CFU (data not shown).
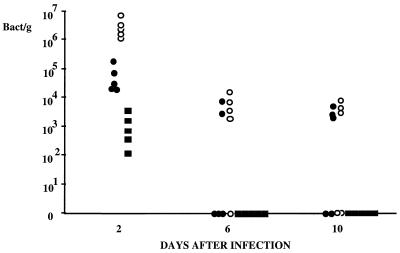
In experiment 4, bacterial counts were determined in blood, liver, and spleen (Fig. 4). Bacteria disappeared quickly from blood, being found in all rats 2 days after infection but in no animals at 6 or 10 days. Bacteria were also detected in spleens and livers of all animals on day 2, the level observed in spleen being 100 times greater than that in liver. At 6 and 10 days after infection, we observed a great animal-to-animal variability in the number of bacteria in liver and spleen. For two animals, bacteria were still present in the spleen but not the liver on day 6; this could indicate better clearance of bacteria in the liver than in the spleen.
FIG. 4.
Number of bacteria in different tissues after infection (experiment 4). Fifteen rats were infected by i.v. injection of live E. coli. Animals were sacrificed 2, 6, and 10 days after infection (n = 5 at each time), and tissue samples were taken for bacterial count. Each point represent one rat. ■, blood; ●, liver; ○, spleen.
The administration of dead bacteria failed to produce long-lasting body weight loss, since rats began growth recovery as soon as day 2 after injection (experiment 5 [Fig. 5]). Six days after administration of bacteria, rats injected with live bacteria had lost 50.5 ± 10.0 g of body weight, and rats injected with dead bacteria had gained 17.0 ± 6.4 g.
FIG. 5.
Body weight changes after an i.v. injection of live or dead bacteria (experiment 5). Rats received an i.v. injection of 7 × 108 live E. coli (●) or the same amount of bacteria heated for 30 min at 60°C (○). Data are means ± standard deviations (n = 6). ∗, P < 0.05 versus the group injected with live bacteria.
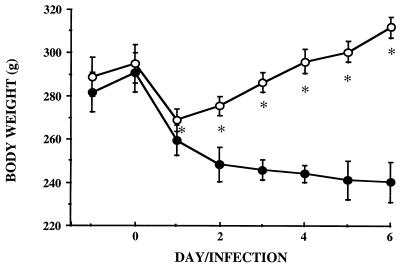
Experiment 6 was designed to characterize organic and metabolic disturbances induced by the i.v. model of infection. Comparative variations in body weight change of control rats, infected rats, and PF rats of the infected group are given in Fig. 6. Body weight loss observed 6 days after infection (58.3 ± 16.6 g) was similar to that observed in rats of experiment 2 that received the same dose of bacteria (59.0 ± 16.7 g). During the same period, control rats exhibited a gain of 31.4 ± 4.8 g. PF animals showed the same loss as infected rats on the first day of pair-feeding. This was probably due to digestive tract emptying, since the food intake of these rats was greatly restricted (80% less than on the day before infection [Table 2]). As soon as day 2 postinfection, PF animals lost significantly less weight than infected rats.
FIG. 6.
Effect of infection on body weight change (experiment
6). ■, control rats given an i.v. injection of saline and free access
to food;
, infected rats given an i.v. injection of E.
coli ([8.3 ± 0.8] × 108) and free access to
food;  , PF
counterparts of infected rats given an i.v. injection of saline. All
measurements were performed over the 6 days following injection of
bacteria or saline. Data are means ± standard deviations. ∗,
P < 0.05 versus the PF group.
, PF
counterparts of infected rats given an i.v. injection of saline. All
measurements were performed over the 6 days following injection of
bacteria or saline. Data are means ± standard deviations. ∗,
P < 0.05 versus the PF group.
TABLE 2.
Effects of infection on food intakea
| Day postinfection | Food intake (g of dry matter/day)
|
||
|---|---|---|---|
| Control rats | Infected ratsb | PF ratsb | |
| 1 | 21.4 ± 0.2 | 6.3 ± 3.3 | 6.0 ± 0.3 |
| 2 | 21.0 ± 0.2 | 2.7 ± 5.1 | 2.1 ± 0.2 |
| 3 | 21.3 ± 0.2 | 4.1 ± 5.7 | 4.9 ± 0.4 |
| 4 | 20.9 ± 0.2 | 8.5 ± 6.8 | 8.0 ± 0.2 |
| 5 | 20.9 ± 0.6 | 9.8 ± 5.3 | 9.2 ± 0.3 |
| 6 | 21.6 ± 0.4 | 10.5 ± 4.9 | 10.0 ± 0.3 |
Experiment 6; control rats were fed ad libitum; infected rats received an i.v. injection of 8.3 × 108 bacteria.
All values, P < 0.05 versus control rats.
Sepsis induced an anabolic effect on liver, spleen, and lung since the protein mass of these organs was significantly greater after infection than in PF rats and often in controls (P < 0.05 [Table 3]). Kidney protein mass was only slightly changed 6 days after infection.
TABLE 3.
Effects of infection on hematocrit, plasma parameters, and tissue protein contentsa
| Determination | Controls rats | Infected
rats
|
PF rats
|
||
|---|---|---|---|---|---|
| J2 | J6 | J2 | J6 | ||
| Hematocrit | 42.1 ± 3.0 | 42.1 ± 2.5 | 37.3 ± 3.6bc | 44.2 ± 2.0 | 46.0 ± 1.4b |
| Glucose (g/liter) | 1.70 ± 0.18 | 1.91 ± 0.23b | 1.53 ± 0.12 | 1.82 ± 0.04 | 1.69 ± 0.12 |
| Insulin (mU/ml) | 14.4 ± 5.1 | 29.9 ± 10.4bc | 8.7 ± 3.8 | 18.2 ± 4.9 | 7.9 ± 4.7 |
| Lactate (mM) | 3.08 ± 0.95 | 5.03 ± 2.04bc | 5.62 ± 1.17bc | 1.77 ± 1.03 | 2.36 ± 1.34 |
| Triglycerides (mM) | 1.87 ± 0.40 | 1.02 ± 0.19bc | 0.98 ± 0.11b | 0.45 ± 0.05b | 0.76 ± 0.15b |
| Proteins (g/liter) | 61.5 ± 3.5 | 57.8 ± 1.9 | 66.9 ± 7.0bc | 60.6 ± 2.8 | 57.1 ± 3.8 |
| α2-Macroglobulin (mg/liter) | 56 ± 9 | 1,868 ± 623bc | 1,177 ± 578b | 37 ± 5 | 47 ± 5 |
| Protein content | |||||
| Liver (mg) | 2.39 ± 0.09 | 2.62 ± 0.11bc | 2.18 ± 0.21bc | 1.99 ± 0.09b | 1.70 ± 0.13b |
| Spleen (mg) | 119 ± 14 | 196 ± 18bc | 261 ± 84bc | 143 ± 24 | 114 ± 18 |
| Lung (mg) | 201 ± 10 | 238 ± 9bc | 210 ± 30c | 203 ± 4 | 168 ± 20b |
| Kidney (mg) | 394 ± 21 | 372 ± 10 | 393 ± 35c | 381 ± 24 | 344 ± 14b |
| Gastrocnemius muscle (mg) | 501 ± 31 | 382 ± 14bc | 299 ± 38bc | 445 ± 29 | 422 ± 27b |
Experiment 6; control rats were fed ad libitum and studied 6 days after saline injection; infected rats received an i.v. injection of 8.3 × 108 bacteria and were studied either 2 days (J2) or 6 days (J6) after infection; PF rats received the same amount of food as infected rats and were studied either 2 days (J2) or 6 days (J6) after saline injection.
P < 0.05 versus control rats.
P < 0.05 versus PF rats.
A pronounced catabolic effect was seen in muscle. At 2 and 6 days after infection, protein content of the gastrocnemius was 14 and 29% less in infected rats than in the corresponding PF animals. Six days after infection, this protein loss was 40% compared to controls. The atrophy observed in other muscles from different metabolic types (extensor digitorum longus, soleus, and tibialis anterior) was quantitatively and qualitatively similar to that observed in the gastrocnemius (data not shown).
Glycemia was greater in infected rats than in controls, but there was no difference among other groups (Table 3). Two days after infection, insulinemia was greater in infected rats than in controls and PF rats, but this parameter was normalized 6 days after infection. Lactate was not significantly modified in PF rats but was increased by infection. Triglyceridemia was less in all treated groups than in controls, but 2 days after infection the levels of plasma triglycerides were higher in infected rats than in their PF controls. Levels of plasma proteins were the same in all groups, but an increase was observed 6 days postinfection in infected rats. In contrast, the concentration of α2-macroglobulin, a protein characteristic of inflammation in the rat, was dramatically elevated in infected animals regardless of the time after infection; this concentration peaked 2 days after infection.
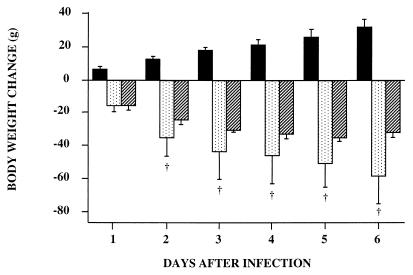
Experiment 7 was designed to further explore the role of TNF-α in our model in relation to the correlation presented in Fig. 3. Administration of PX 1 h prior to infection suppressed the rise of the plasma TNF-α level 1.5 h after infection (0.6 ± 0.6 versus 71.9 ± 19.0 ng/ml). IL-1β and IL-6 peaked 3 h after infection in our model (data not shown). PX treatment induced 84 and 61% reductions of plasma IL-1β and IL-6 concentrations, respectively, 3 h after infection (0.22 ± 0.14 versus 1.38 ± 1.36 ng/ml for IL-1β and 1.98 ± 1.50 versus 5.09 ± 2.09 mg/ml for IL-6). Pretreatment of animals with PX before infection reduced anorexia (Table 4). The body weight loss of rats in the PX-INF group was always similar to that of their PF controls. Moreover, PX-INF rats began to gain weight on day 3, although rats in the INF group continued to lose weight. Thus, 6 days after infection, septic rats had lost about 32 g of their initial body weight and infected rats treated with PX had regained about 10 g.
TABLE 4.
Effects of PX treatment on body weight loss, muscle weight, and proteolysis 2 and 6 days postinfectiona
| Determination | 2 days
|
6 days
|
||||||
|---|---|---|---|---|---|---|---|---|
| PF | INF | PX-PF | PX-INF | PF | INF | PX-PF | PX-INF | |
| Body wt loss (g) | −24.3 ± 4.9 | −34.9 ± 4.4b | −11.1 ± 5.5 | −16.5 ± 13.0c | −1.5 ± 2.9 | −31.7 ± 14.6b | 15.6 ± 9.0c | 11.2 ± 6.1c |
| Total food intake (g) | 6.8 ± 1.6 | 4.8 ± 2.0 | 19.8 ± 1.5c | 17.5 ± 9.2c | 65.6 ± 6.8 | 67.9 ± 13.7 | 101.7 ± 3.7c | 101.8 ± 2.9c |
| Gastrocnemius (g) | 1.69 ± 0.02 | 1.44 ± 0.02b | 1.81 ± 0.03c | 1.56 ± 0.03bc | 1.83 ± 0.03 | 1.21 ± 0.04b | 1.91 ± 0.04 | 1.68 ± 0.03bc |
| Soleus (mg) | 125 ± 3 | 109 ± 2b | 128 ± 6 | 112 ± 4b | 121 ± 11 | 97 ± 4b | 124 ± 3 | 120 ± 2c |
| Epitrochlearis (mg) | 43.7 ± 6.6 | 33.7 ± 7.5b | 43.9 ± 10.4 | 39.5 ± 9.5 | 42.1 ± 6.7 | 30.2 ± 9.6b | 53.7 ± 15.9c | 51.9 ± 12.5c |
| Proteolysis (nmol of tyr/mg of protein/h) | 1.65 ± 0.24 | 2.17 ± 0.21b | 1.51 ± 0.25 | 1.74 ± 0.30bc | 1.37 ± 0.29 | 1.65 ± 0.21b | 1.27 ± 0.39 | 1.27 ± 0.36c |
Experiment 7; groups as described in Materials and Methods.
P < 0.05 versus PF control.
P < 0.05 versus non-PX-treated group.
As shown in experiment 5, weights of the various muscles studied were significantly lower in infected animals than in control rats (Table 4). By contrast, PX treatment reduced atrophy of the gastrocnemius 14 to 16% versus respective PF rats (P < 0.05) and abolished atrophy of the soleus and epitrochlearis muscles at the end of the experiment. To determine the reason for the variations in muscle mass, we measured proteolysis rates in incubated epitrochlearis muscles. Muscle atrophy reflects protein loss, since protein concentration (milligrams per milligram of muscle) was not modified (data not shown). Protein degradation was significantly increased, 32% on day 2 and 21% on day 6, in infected rats compared with their controls (Table 4). After PX treatment, the increase of proteolysis in infected rats compared to their controls reached only 15% on day 2 and was completely abolished on day 6 (Table 4).
DISCUSSION
Previous animal models of sepsis induce body weight loss and muscle atrophy for a period of time too short to test the effect of nutritional support on body protein loss and recovery from infection. Our model succeeds in this objective since rats were in a catabolic state for up to 9 days.
Peritonitis models generally induced body weight loss for only 1 or 2 days (12, 24, 35, 36) and always exhibited high mortality (2, 12, 22, 24, 36). Endotoxin models induce a low mortality rate, but animals become rapidly resistant to endotoxin and exhibit a rapid growth recovery (8, 10, 20). Similarly, our data show that the injection of dead bacteria produced only a transient body weight loss. Numerous authors have criticized sepsis models produced by i.v. administration of bacteria on the grounds that hosts were suddenly overwhelmed with a bacterial challenge so massive that they were unable to respond with the full expression of their defense mechanisms (22, 24, 36). These models were highly lethal (27, 28, 38) but were generally designed to study death rate (27) or hypometabolic shock (28, 38). Furthermore, such models using very high doses of live organisms probably reflect an intoxication similar to those observed with endotoxin challenge rather than the response of the organism to a pathogen which colonizes and replicates significantly following challenge. The choice of pathogen is therefore an important consideration since a limited number of serogroups have the characteristics allowing replication and dissemination of bacteria (8). The bacterial strain used in this study probably has this capacity, since bacteria can be detected in tissues 10 days after infection, and we frequently observed abscesses in kidneys or testes.
Septic canine models using i.v. injection of live bacteria that reproduce a number of clinical disorders typically found in human sepsis, including hypermetabolism and hormonal perturbations, have been described (26, 30). However, the first model was designed to examine mortality and was therefore a model of shock (26). The period examined in the second study did not exceed 24 h after infection (30). Moreover, body weight loss or muscle atrophy was not reported in these studies. Our model focused on the latter problem for two main reasons: muscle atrophy acutely weaken septic patients, and protein recovery requires intensive nutritional support with long and high-cost convalescence.
The fall in rectal temperature observed 1.5 h after i.v. infection seems to indicate that rats exhibited shock at this time. However, this reaction was quite transient, and rectal temperature returned to baseline as early as 4 h after infection. On the other hand, rats exhibited moderate fever on days 1 and 2 postinfection, a reaction often noted in rats due to a relatively high surface area-to-mass ratio (11). Moreover, in contrast to observations in human sepsis, a hypothermic reaction was observed in several septic rat models (11, 17, 24). Increases in plasma glucose and insulin levels have often been found in humans and are difficult to reproduce in small-animal models (12, 20, 22, 24). As observed in humans, glucose, insulin, lactate, and temperature were increased at the same time (2 days after infection), indicating that our animals were probably in the hypermetabolic flow phase. In any case, they were not in the agonal phase of shock that has often been described (18, 19) or criticized as an undesirable effect of bolus injection of live bacteria or endotoxin (12, 22, 24). Indeed, profound hypoglycemia is always observed in the preterminal phase (27).
Infection induced acute anorexia for several days. This anorexia has been largely observed in human sepsis. Interactions between metabolic disorders and nutrition are apparently present in the chronic (days 3 to 7) and late (after day 7) phases of our model, since infected rats ate about 32 g of dry matter between days 6 and 10 postinfection and gained only 4 g in the same time. Data for other experiments in our laboratory indicate that PF rats should gain 14 to 16 g with the same food intake. This indicates an inefficiency of food intake, well known in stress situations in humans. Our model gives the opportunity to study such nutritional disorders.
It is well established that even a brief infection causes some degree of malnutrition and that acute infection creates deficits in the nutritional stores of the body (4). These deficits are thought to be induced by the acute-phase response, which increases nutritional requirements of organs like the liver (6, 19, 24, 35). In the absence of correct nutritional supply (due to anorexia), the organism mobilizes muscle stores and induces a shift of amino acids from the periphery to central organs (3, 6, 20, 24). A good septic model for studying this problem should reproduce at the same time muscle atrophy, acute-phase response, and anorexia over a long period of time. Such long-lasting perturbations were observed in our model. Indeed, 6 days after infection, muscle protein mass was 60% of that for controls and 70% of that for PF rats, and liver protein mass was 129% of that for PF animals. The protein content of other tissues such as the spleen and lung increased after infection, suggesting an important role of these organs for defense of the organism. Part of the anabolic response of liver is thought to be associated with increase synthesis of acute-phase proteins. In this study, plasma levels of α2-macroglobulin, a characteristic protein of the acute-phase response in the rat, were increased 40- and 25-fold on days 2 and 6, respectively. Increased levels of acute-phase proteins are probably due to increased synthesis rates. Since the amino acid composition of acute-phase proteins is different from the mean amino acid composition of whole-body proteins, the liver acute-phase response could induce specific amino acid requirements (qualitatively and quantitatively) (6, 29).
Plasma TNF-α concentration measured 1.5 h after infection was significantly higher in rats that died in the days after infection than in surviving animals. More interesting, in surviving animals, our data show a strong correlation between plasma TNF-α concentration measured 1.5 h after infection and body weight change observed 9 days later. This establishes a prognostic value of TNF-α concentration on outcome for rats. Clinical studies described controversial results concerning the prognostic value of TNF-α concentration in human sepsis. This is probably due to the difficulty in detecting sepsis enough early in patients. After infection, we found an acute but transient TNF-α peak, since plasma levels returned to baseline 4.5 h after infection. Such time-related variations have been reported for a murine endotoxemic model but not a peritonitis model obtained by CLP (9). It is possible that the CLP surgical procedure resulted in elevation of endogenous glucocorticoids which inhibited secretion of cytokines, and particularly of TNF-α (9). On the other hand, Martin et al. (23) demonstrated recently that intravascular plastic catheters potentiated TNF-α release and exacerbated complications associated with sepsis. Since cytokines are universally recognized as primary mediators of the septic syndrome, it is advisable to use models that do not require a surgical procedure.
To further explore the involvement of TNF-α in determining muscle wasting, as suggested by the results shown in Fig. 3, we used PX, which is known to inhibit the production of TNF-α (5). PX treatment minimized the difference in body weight between infected rats and their PF controls over the entire course of the study and reduced or abolished the muscle atrophy linked to infection. We showed previously that muscle atrophy was mainly due to a persistent activation of proteolysis (34). The present study demonstrates that PX treatment completely abolished the activation of proteolysis observed at day 6. Breuillé et al. (5) reported that PX treatment reduced the muscle protein synthesis inhibition observed in the septic acute phase, as previously found during chronic sepsis after administration of amrinone, another inhibitor of TNF-α secretion (21). Taken together, these data suggest that the PX-induced improvement of muscle nitrogen balance resulted from both reduced inhibition of protein synthesis and depressed activation of proteolysis. Since an important effect of PX treatment of septic rats is the suppression of the early appearance of TNF-α in plasma, our results lead us to conclude that TNF-α is an important determinant of muscle wasting and proteolysis activation in sepsis. This observation is consistent with results of Zamir et al. (39) showing in rats a decreased proteolysis activation after anti-TNF-α administration in acute sepsis induced by CLP. However, the decrease of IL-1β production can participate to the inhibition of muscle proteolysis in PX-treated septic animals, since reduced activation of muscle proteolysis was observed in septic animals treated with IL-1ra (40). On the other hand, our study suggest that IL-6 could be a minor determinant in sepsis-induced proteolysis. This contrasts to recent data suggesting that IL-6 could directly activate muscle proteolysis and especially the lysosomal and ATP-ubiquitin-dependent pathways (16, 32). The main point underlined by our results is that TNF-α secretion observed in the first hours after infection has a pivotal role in supporting activation of muscle proteolysis for days. However, the mode of action of TNF-α, direct or indirect through other cytokines, hormones, or unknown compounds, as found in cancer patients (31), remains to be determined.
In conclusion, we have developed a septic model in rats which reproduces sustained metabolic disturbances characteristics of sepsis. Our model is easy to use, since it requires no surgical procedure. Furthermore, we demonstrated that the response to a titrated dose of live E. coli was predictable and reasonably reproducible. Therefore, this model is suitable for studying possible nutritional interventions (prophylactic or therapeutic), especially the effect of specific nutritional support.
ACKNOWLEDGMENTS
This work was supported by INRA and Clintec Technologies.
We thank Jean Pierre Girardeau for helpful scientific discussions and Caroline Buffière, Philippe Denis, Corinne Pouyet, and Fabienne Rambourdin for technical assistance.
REFERENCES
- 1.Aarden L A, De Groot E R, Schaap O L, Lansdorp P M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J W, Gonce S J, Miskell P W, Peck M D, Sax H. A new model for studying nutrition in peritonitis. The adverse effect of overfeeding. Ann Surg. 1989;209:334–340. doi: 10.1097/00000658-198903000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ash S A, Griffin G E. Effect of parenteral nutrition on protein turnover in endotoxaemic rats. Clin Sci. 1989;76:659–666. doi: 10.1042/cs0760659. [DOI] [PubMed] [Google Scholar]
- 4.Beisel W R. Use of animals for the study of relations between nutrition and infectious diseases. In: Beynen A C, West C E, editors. Use of animal models for research in human nutrition. Comparative animal Nutrition. 6. S. Basel, Switzerland: Karger; 1988. pp. 33–55. [Google Scholar]
- 5.Breuillé D, Farges M C, Rosé F, Arnal M, Attaix D, Obled C. Pentoxifylline decreases the body weight loss and muscle protein wasting characteristics of sepsis. Am J Physiol. 1993;265:E660–E666. doi: 10.1152/ajpendo.1993.265.4.E660. [DOI] [PubMed] [Google Scholar]
- 6.Breuillé D, Rosé F, Arnal M, Melin C, Obled C. Sepsis modifies the contribution of different organs to whole-body protein synthesis. Clin Sci. 1994;86:663–669. doi: 10.1042/cs0860663. [DOI] [PubMed] [Google Scholar]
- 7.Cory A H, Owen T C, Barltrop J A, Cory J G. Use of aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 8.Cross A S, Opal S M, Sadoff J C, Gemski P. Choice of bacteria in animal models of sepsis. Infect Immun. 1993;61:2741–2747. doi: 10.1128/iai.61.7.2741-2747.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans G F, Snyder Y M, Butler L D, Zuckerman S H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989;29:279–290. [PubMed] [Google Scholar]
- 10.Fink M P, Heard S O. Laboratory models of sepsis and septic shock. J Surg Res. 1990;49:186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- 11.Fish R E, Spitzer J A. Continuous infusion of endotoxin from an osmotic pump in the conscious, unrestrained rat: a unique model of chronic endotoxemia. Circ Shock. 1984;12:135–149. [PubMed] [Google Scholar]
- 12.Freund H R, James J H, LaFrance R, Gallon L S, Barcelli U O, Edwards L L, et al. The effect of indomethacin on muscle and liver protein synthesis and on whole body protein degradation during abdominal sepsis in the rat. Arch Surg. 1986;121:1154–1158. doi: 10.1001/archsurg.1986.01400100062012. [DOI] [PubMed] [Google Scholar]
- 13.Fried R C, Bailey P M, Nullen J L, Stein T P, Crosby L O, Buzby G P. Alterations in exogenous substrate metabolism in sepsis. Arch Surg. 1986;121:173–178. doi: 10.1001/archsurg.1986.01400020059007. [DOI] [PubMed] [Google Scholar]
- 14.Girardeau J P, Der Vartanian M, Ollier J L, Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia colistrains. Infect Immun. 1988;56:2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Givalois L, Dornand J, Mekaouche M, Solier M D, Bristow A F, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- 16.Goodman M N. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 17.Goris R J A, Boekholtz W K V, Van Bebber I P T, Nuytinck J K S, Schillings P H M. Multiple-organ failure and sepsis without bacteria. Arch Surg. 1986;121:897–901. doi: 10.1001/archsurg.1986.01400080039006. [DOI] [PubMed] [Google Scholar]
- 18.Guinée P A M, Jansen W H, Agterberg C M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coliisolates from calves and its correlation with enterotoxigenicity. Infect Immun. 1976;13:1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasselgren P O, Pedersen P, Sax H C, Warner P B W, Fischer J E. Current concepts of protein turnover and amino acid transport in liver and skeletal muscle during sepsis. Arch Surg. 1988;123:992–999. doi: 10.1001/archsurg.1988.01400320078016. [DOI] [PubMed] [Google Scholar]
- 20.Jepson M M, Pell J M, Bates P C, Millward D J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986;235:329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurassinski C V, Kilpatrick L, Vary T C. Amrinone prevents muscle protein wasting during chronic sepsis. Am J Physiol. 1995;268:E491–E500. doi: 10.1152/ajpendo.1995.268.3.E491. [DOI] [PubMed] [Google Scholar]
- 22.Lang C H, Bagby G J, Bornside G H, Vial L J, Spitzer J J. Sustained hypermetabolic sepsis in rats: characterization of the model. J Surg Res. 1983;35:201–210. doi: 10.1016/s0022-4804(83)80005-5. [DOI] [PubMed] [Google Scholar]
- 23.Martin L F, Vary T C, Davis P K, Munger B L, Lynch J C, Spangler S, et al. Intravascular plastic catheters—how they potentiate tumor necrosis factor release and exacerbate complications associated with sepsis. Arch Surg. 1991;126:1087–1093. doi: 10.1001/archsurg.1991.01410330041005. [DOI] [PubMed] [Google Scholar]
- 24.Nakatani T, Sato T, Marzella L, Hirai F, Trump B F, Siegel J C. Hepatic and systemic metabolic response to aerobic and anaerobic intra-abdominal abscesses in a highly reproducible chronic rat model. Circ Shock. 1984;13:271–294. [PubMed] [Google Scholar]
- 25.Obled C, Arnal M, Valin C. Variations through the day of hepatic and muscular cathepsin A (carboxypeptidase A; EC 3.4.12.2), C (dipeptidase; EC 3.4.14.1) and D (endopeptidase D; EC 3.4.23.5) activates and free amino acids of blood in rats: influence of feeding schedule. Br J Nutr. 1980;44:61–69. doi: 10.1079/bjn19800010. [DOI] [PubMed] [Google Scholar]
- 26.Perbellini A, Shatney C H, MacCarter D J, Lillehei R C. A new model for the study of septic shock. Surg Gynecol Obstet. 1978;147:68–74. [PubMed] [Google Scholar]
- 27.Pitcairn M, Schuler J, Erve P R, Holtzman S, Schumer W. Glucocorticoid and antibiotic effect on experimental gram-negative bacteraemic shock. Arch Surg. 1975;110:1012–1015. doi: 10.1001/archsurg.1975.01360140156030. [DOI] [PubMed] [Google Scholar]
- 28.Postel J, Schloerb P R. Metabolic effects of experimental bacteremia. Ann Surg. 1977;185:475–480. doi: 10.1097/00000658-197704000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeds P J, Fjeld C R, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 30.Shaw J H F, Wolfe R R. A conscious septic dog model with hemodynamic and metabolic responses similar to responses of humans. Surgery. 1984;95:553–561. [PubMed] [Google Scholar]
- 31.Todorov P, Cariuk P, McDvitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature. 1996;379:739–742. doi: 10.1038/379739a0. [DOI] [PubMed] [Google Scholar]
- 32.Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H, Monden M. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Investig. 1996;97:244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vary T, Kimball S R. Sepsis-induced changes in protein synthesis differential effects on fast-twitch and slow-twitch muscles. Am J Physiol. 1992;262:C1513–C1519. doi: 10.1152/ajpcell.1992.262.6.C1513. [DOI] [PubMed] [Google Scholar]
- 34.Voisin L, Breuillé D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, Obled C, Attaix D. Muscle wasting in a rat model of long lasting sepsis results from the activation of lysosomal, Ca2+-activated and ubiquitin-proteasome proteolytic pathways. J Clin Investig. 1996;97:1610–1617. doi: 10.1172/JCI118586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Allmen D, Hasselgren P O, Fischer J E. Hepatic protein synthesis in a modified septic rat model. J Surg Res. 1990;48:476–480. doi: 10.1016/0022-4804(90)90016-u. [DOI] [PubMed] [Google Scholar]
- 36.Wichterman K A, Baue A E, Chaudry I H. Sepsis and septic shock: a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 37.Williams P H, Warner P J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980;29:411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfe R R, Burke J F. Glucose and lactate metabolism in experimental septic shock. Am J Physiol. 1978;235:R219–R227. doi: 10.1152/ajpregu.1978.235.5.R219. [DOI] [PubMed] [Google Scholar]
- 39.Zamir O, Hasselgren P O, Kunkel S L, Frederick J, Higashiguchi T, Fischer J E. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. Arch Surg. 1992;127:170–174. doi: 10.1001/archsurg.1992.01420020052008. [DOI] [PubMed] [Google Scholar]
- 40.Zamir O, O’Brien W, Thompson R, Bloedow D C, Fischer J E, Hasselgren P O. Reduced muscle protein breakdown in septic rats following treatment with interleukin-1 receptor antagonist. Int J Biochem. 1994;26:943–950. doi: 10.1016/0020-711x(94)90088-4. [DOI] [PubMed] [Google Scholar]