Abstract
Background:
We aimed to investigate the antibacterial activity of Persian Gulf microalgae extracts on some Gram-positive and negative bacterial species in order to find new compounds with antibacterial activity.
Methods:
After sampling microalgae from December 2020 to April 2021 from the northernmost part of Qeshm Island in Persian Gulf, the antibacterial activity of methanolic and ethyl acetate extract of microalgae were tested in three concentrations of 125, 250, and 500 mg/ml on Gram-positive bacteria including Staphylococcus aureus, Bacillus cereus, and Gram-negative bacteria including Pseudomonas aeruginosa and Escherichia coli by disk-diffusion assay and the results were compared with two standard antibiotics including ciprofloxacin and streptomycin. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were assessed spectrophotometrically using microplate and enzyme-linked immunosorbent assay (ELISA) reader.
Results:
Methanolic and ethyl acetate extracts had antibacterial effects against Gram-positive and negative bacteria. Compared to ethyl acetate extract, the methanolic extract showed stronger effects on both Gram-positive and negative bacteria. The most antibacterial effect was related to methanolic extract with a concentration of 500 mg/ml on S. aureus by 14.6 mm inhibition zone. Evidence from MIC also confirmed that the lowest MIC was belonged to methanolic extract by 0.75 mg/ml against S. aureus. Interestingly, both of these extracts showed more antibacterial activity on Gram-positive bacteria than Gram-negative bacteria.
Conclusion:
The investigation proved the efficacy of microalgae extracts isolated from Persian Gulf as natural antimicrobials and suggested the possibility of employing them in medicines as antimicrobial agents.
Keywords: Microalgae, Pathogenic bacteria, Persian Gulf, Gram-positive, Gram-negative
Introduction
Microalgae have developed a very attractive source of antibacterial agents and contribute various advantages for antimicrobial investigations due to their fast growth rate and great biodiversity (1). Microalgae are novel origins of bioactive compounds in pharmaceutical production (2, 3). Some microalgae provide anti-cancer, anti-inflammatory, and anti-oxidative compounds (4). The cell extracts and effective ingredients of various microalgae have been shown to carry antibacterial activity on Gram-positive and Gram-negative bacteria (5). Microalgal biomass and compounds produce an extensive variety of plausible applications from animal feed to human health and nutrition products (6, 7). Antibiotic-resistance bacteria has become a serious problem in human health and has turned even further problems to become newly-evolving harmful bacteria (8, 9). Various researches proposed that microalgae can provide various chemical compounds with different biological activities (10). These components can hinder the growth of harmful bacteria and other microorganisms, or eradicate them (11–13).
There is an endless demand to discover novel chemical structures and antimicrobial compounds with a novel mechanism of action because of the improvement of resistance to antibiotics (14, 15). Among the important bioactive components of microalgae with exhibited antimicrobial potential, polysaccharides, proteins, polyunsaturated fatty acids (PUFAs) such as Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA), antioxidants such as polyphenols, carotenoids, and flavonoids, and amino acids are the most important ones (1, 11, 16).
Aquatic microalgae and microorganisms generate various secondary metabolites underinvestigated and remain potential sources of lead compounds to inhibit pathogens (17). Microalgae, algae, and seaweeds are possible sources adopted as antimicrobial agents (18, 19). A large number of findings have been published around compounds derived from microalgae and algae with antibacterial activity, such as aliphatic compounds, halogenated, terpene, and acrylic acid (20). Nevertheless, the detecting and finding of compounds immediately responsible for the antimicrobial potential of microalgae is an attractive field of research, principally owing to the different varieties of compounds found in recent years (21–23).
Here we investigate the in vitro antibacterial activity of methanol and ethyl acetate extracts of microalgae isolated from the Persian Gulf against two Gram-positive and two Gram-negative bacteria (S. aureus, B. cereus, P. aeruginosa, and E. coli). We determined the zone of inhibition by agar disk-diffusion method, also minimum inhibitory concentration and minimum bactericidal concentration by microplate reader.
Materials and Methods
Bacterial Culture
The standard test bacteria for antibacterial activity involved the S. aureus (ATCC 25923), B. cereus (ATCC 14579), E. coli (ATCC 10586) and P. aeruginosa (ATCC 27853) obtained from Pasteur Institute of Iran. All the bacterial strains were subcultured from the original culture, stored at −70 °C and kept on Müller-Hinton (MH) agar plates at 4 °C, and grown at 37 °C when required.
Collection of Microalgae Samples
Microalgae sample were collected from December 2020 to April 2021 from the northernmost part of Qeshm Island in Persian Gulf. The collected sample was isolated during the separation process under an inverted microscope based on morphology and differences in pigment color. The isolated microalgae were cultured in 10 ml dark and 14 h light conditions in 250 ml and then 500 ml Erlenmeyer flasks containing RM medium and sea salt.
Microalgae Extraction
Kellam and Walker modified method was used to prepare microalgae extracts (24). For extraction with organic solvents, 500 mg of the frozen bio-mass was poured into a glass tube and extracted with 3 ml of 100% hexane for 24 h at room temperature. It was then centrifuged and the supernatant placed in a 40 °C oven for 24 to 48 h to dry. Then other solvents such as ethyl acetate and methanol were poured on the microalgae deposit and extracted. Finally, all the supernatants were placed in the oven to dry and the desired weight was determined.
Agar Disk-Diffusion Assay
Antimicrobial susceptibility test was performed according to the standard CLSI guideline (25). The agar disc diffusion assay was carried out to assess the antimicrobial activity of microalgae extracts. A stock solution of extracts was provided by dissolving 0.5 g of extracts with 100 mL of their respective solvents (ethyl acetate and methanol) to generate a final concentration of 500 mg/mL. The stock solution was then diluted to concentrations of 500, 250, and 125 mg/mL of extracts. 20 μL of each dilution was impregnated into blank, sterile discs 6 mm in diameter. Both sides of the discs were spotted alternately with 5 μL of the extract and led to dry before the next 5μL was spotted to make sure accurate impregnation. Ethyl acetate and methanol-loaded discs were used as negative controls for ethyl acetate and methanol extracts, respectively. All discs were completely dried before the treatment on the bacterial lawn. The positive controls utilized were ciprofloxacin and streptomycin antibiotic discs for all bacteria strains. Antibacterial activity was assessed by measuring the diameter of the inhibition zone (IZ) around each disc. For all tested bacteria, three replicates were carried out. Antibacterial activity was stated as the mean zone of inhibition diameters (mm) formed by the microalgae extracts.
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
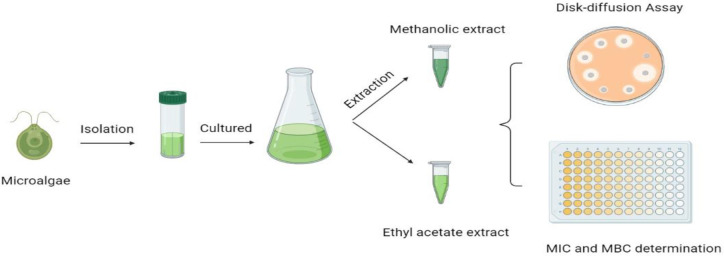
A popularly accepted precise serial dilution microplate assay (26) was employed to determine the minimum inhibitory concentration (MIC) of the microalgae extracts against four bacterial strains. This biological method was taken because of its sensitivity, reproducibility, simplicity, and almost low cost while being a fast method at the same time. Bacterial cultures grown overnight were adjusted to 0.5 McFarland standard, equivalent to 3.2 x 108 cfu/ml P. aeruginosa, 3.7 x 108 cfu/ml E. coli, 1.3 x 108 cfu/ml B. cereus, and 3.0 x 108 cfu/ml S. aureus. The extracts were dissolved in ethyl acetate and methanol to a concentration of 96 mg/ml and 100 μl was added to the first well of a 96-well microtitre plate and serial dilution 1:1 performed with water. 100 μl of bacterial cultures were added to each well. Starting with an extract concentration of 24 to 0.25 mg/ml, the bacteria were consequently subjected to final concentrations of 32 to 2 μg/ml. Streptomycin was used as positive control and methanol and ethyl acetate were used as solvent control. To determine the MBC, bacterial suspension from the wells comprising extract concentrations equal or higher than the MIC were inoculated in each well and incubated for 24 h at 37 °C in a 5% CO2 atmosphere. MBC was considered the lowest concentration that inhibited completely bacterial growth. Each extract was examined in triplicate; each trial was carried out twice (Fig. 1).
Fig. 1:
Graphical abstract showing schematic procedures of the study
Results
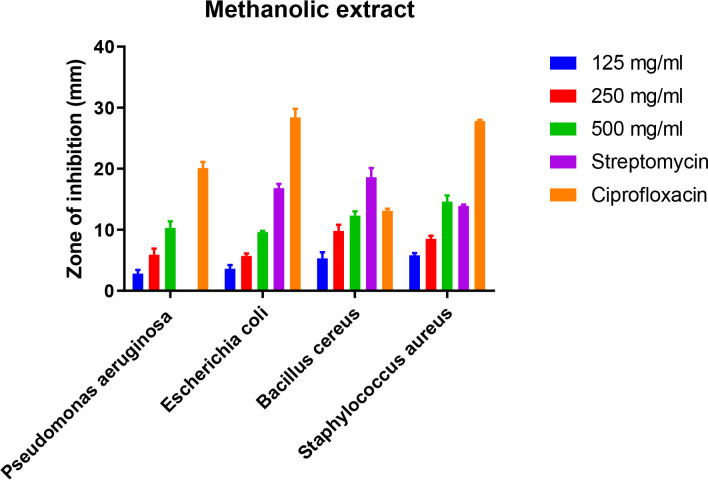
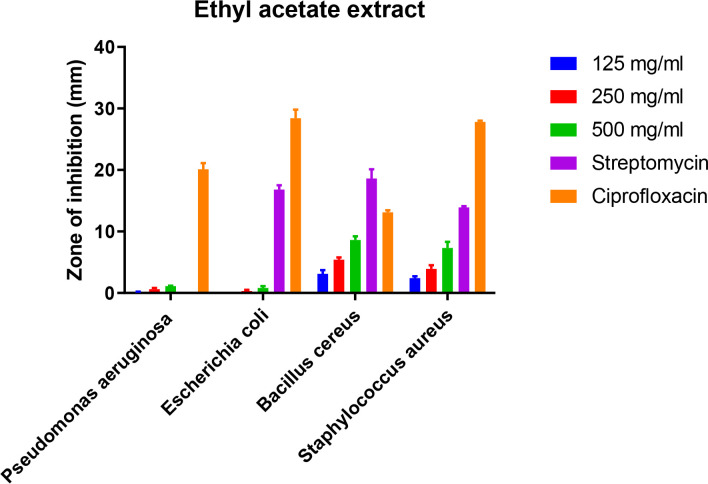
Results obtained from the effects of microalgae extracts with methanol and ethyl acetate solvents at concentrations of 125, 250, 500 mg/ml as well as two conventional antibiotics ciprofloxacin and streptomycin (5 μg/ml) by disk-diffusion method throughout determining the diameter of the growth inhibition zone, MIC and MBC determination of microalgae extracts on the strains of S. aureus, B. cereus, P. aeruginosa, and E. coli (Fig. 2 and 3).
Fig. 2:
Zone of inhibition against some Gram-positive and Gram-negative bacteria strains by methanolic extract of microalgae isolated from Persian Gulf
Fig. 3:
Zone of inhibition against some Gram-positive and Gram-negative bacteria strains by ethyl acetate extract of microalgae isolated from Persian Gulf
Antibacterial studies showed that methanolic and ethyl acetate extracts had antimicrobial effects on both Gram-positive (S. aureus and B. cereus) and Gram-negative (P. aeruginosa and E. coli) bacteria. Comparison of antimicrobial effect of methanolic and ethyl acetate extracts showed that methanolic extract at a concentration of 500 mg/ml had a significant inhibitory effect on S. aureus and B. cereus by a 14.6 mm and 12.3 mm halo diameter, respectively. While Gram-negative bacteria showed a less inhibitory effect at the same concentration of methanolic extracts, 10.3 and 9.6 mm for P. aeruginosa and E. coli, respectively. These data for concentrations of 250 and 125 mg/ml show similar regular results of 500 mg/ml (Table 1). Interestingly, streptomycin did not show any effect on P. aeruginosa, while on other Gram-negative bacteria (E. coli) showed an average halo diameter of 16.8 mm. Unlike streptomycin, ciprofloxacin had an inhibitory effect on all of bacterial test strains.
Table 1:
Zone of inhibition against some Gram-positive and Gram-negative bacteria strains by 500 mg/ml methanolic and ethyl acetate extract of microalgae isolated from Persian Gulf
| Bacteria | P. aeruginosa | E. coli | B. cereus | S. aureus |
|---|---|---|---|---|
| Samples | Zone of inhibition (mm) | Zone of inhibition (mm) | Zone of inhibition (mm) | Zone of inhibition (mm) |
| Ciprofloxacin (5 μg/ml) | 20.1 ± 1 | 28.4 ± 1.4 | 13.1 ± 0.5 | 27.8 ± 0.5 |
| Streptomycin (5 μg/ml) | - | 16.8 ± 0.7 | 18.6 ± 1.5 | 13.9 ± 0.2 |
| Methanolic | 10.3 ± 1.1 | 9.6 ± 0.2 | 12.3 ± 0.7 | 14.6 ± 1 |
| Ethyl acetate | 1.1 ± 0.1 | 0.8 ± 0.3 | 8.6 ± 0.6 | 7.3 ± 1 |
Comparison of antimicrobial effect of the 500 mg/ml methanolic extract on Gram-positive and Gram-negative bacteria (Table 1):
S. aureus (14.6 mm) > B. cereus (12.3 mm) > E. coli (10.3 mm) > P. aeruginosa (9.6 mm)
Methanolic extract had the highest antimicrobial effect on S. aureus Gram-positive bacteria (14.6 mm), but ethyl acetate extract had the most antimicrobial effect on B. cereus bacteria (8.6 mm). In the present study, Gram-negative bacteria E. coli and P. aeruginosa showed the highest resistance to extracts against Gram-positive bacteria. Comparison of antimicrobial effect of 500 mg/ml ethyl acetate extract on Gram-positive and Gram-negative bacteria (Table 1):
B. cereus (8.6 mm) > S. aureus (7.3 mm) > P. aeruginosa (1.1 mm) > E. coli (0.8 mm)
The results of the study of the minimum inhibitory concentration and the minimum bactericidal concentration of methanolic and ethyl acetate microalgae extracts on the studied Gram-positive and Gram-negative bacteria shown in Table 1. The lowest MIC against the studied bacteria was related to methanolic extract by 0.75 mg/ml and was observed against S. aureus which is a Gram-positive bacterium. In other words, the most susceptible bacteria among the studied microorganism was S. aureus and the MBC of microalgae extracts against this pathogen was double of its MIC (equivalent to 1.5 mg/ml), while the highest MIC in this study was 24 mg/ml belong to P. aeruginosa, a Gram-negative bacterium. Therefore, the most resistant bacteria to methanolic and ethyl acetate microalgae extracts was observed in P. aeruginosa. The MBC of ethyl acetate extract against P. aeruginosa was equal to its MIC of 24 mg/ml. The MIC of the methanolic extract against Gram-positive bacterium B. cereus was 1.5 mg/ml and the susceptibility of this bacterium was in the next rank after S. aureus. MBC of this extract was observed against B. cereus was equal to its MIC (1.5 mg/ml). Methanolic and ethyl acetate extracts in different doses of 125, 250 and, 500 mg/ml showed similar regular results, which shows that the antibacterial effect of these extracts is dose-dependent (Table 2).
Table 2:
Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Methanolic and Ethyl acetate extracts against Gram-positive and Gram-negative bacteria
| Bacteria | P. aeruginosa | E. coli | B. cereus | S. aureus | ||||
|---|---|---|---|---|---|---|---|---|
| Samples | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Methanolic (mg/ml) | 6 | 6 | 3 | 3 | 1.5 | 1.5 | 0.75 | 1.5 |
| Ethyl acetate (mg/ml) | 24 | 24 | 12 | 12 | 3 | 6 | 6 | 6 |
| Streptomycin (μg/ml) | - | - | 8 | 8 | 4 | 8 | 16 | 16 |
Discussion
Death due to microbial infection and enhancing the resistance of microorganisms to antibiotics prompted humans to think of ways to combat these microorganisms (13). One of these alternatives is compound extracted from algae and microalgae, considered antimicrobial compounds and alternatives to synthetic drugs (27). Microalgae have an extensive range of biologically active compounds and numerous extracts or extracellular products of microalgae have antimicrobial activity. As shown in the results methanolic extract had great inhibitory effects on both Gram-positive and Gram-negative in comparison to ethyl acetate extract (Table 1). Salem et al. examined the antibacterial effect of methanolic and ethyl acetate extracts of 8 species of seaweed and concluded that methanolic extract was more effective than ethyl acetate extract (28). Moreover, Verrier et al. used some solvents for extraction and stated that among these solvents, the methanolic extract showed the best results for both Gram-positive and Gram-negative bacteria (29).
The extraction method and the type of solvent used have an important role in the antimicrobial activity of the extract on certain species of microorganisms. Rosaline et al. used methanol, ethyl acetate, acetone and hexane for extraction. The acetone extract obtained from the studied algae has a greater antibacterial effect than other extracts (30). Besides, methanolic extract of Scenedesmus microalgae had antimicrobial activity against the bacterium Xanthomonas oryzae, while ethanolic and hexane extracts showed no activity at different concentrations tested that shows the effect of different solvents on antimicrobial activity well (31). Besides, antimicrobial compounds derived from microalgae include various groups of chemicals such as aliphatic compounds, halogenates, macrolides, cyclic peptides, proteins, polylactides, terpenes, saponins, carbohydrates, phenols, and fatty acids, which the amount of their dissolution in different solvents can be different (32, 33).
The results of MIC and MBC were in accordance with the results of antibacterial properties by the disk-diffusion method. Both methanol and ethyl acetate extracts have better antimicrobial effects on Gram-positive bacteria. The difference in the susceptibility of these microorganisms to antimicrobials is probably due to the different structures of their cell walls (34, 35). B. cereus and S. aureus are Gram-positive bacteria that, unlike Gram-negative bacteria, do not have an outer layer in their wall, which in turn can cause the active compounds to penetrate better (13, 34, 36). We had some limitations in the study. In this phase we were not able to select and screen some representative strains with high antimicrobial resistance from clinical cases to subject in antimicrobial assays. We also suggest using of microalgae active ingredients instead of their extracts for the next studies.
Conclusion
Some of the natural products possessed great potential for inhibition applications against some microorganism. Extracts with high antimicrobial activity against Gram-positive bacteria do not surely have high activity against Gram-negative bacteria. The activity of some antibacterial agents is related to the differences in the cell wall structure. Moreover, solvents play a crucial role in the extraction of antibacterial compounds and consequently on bacterial inhibition. Methanolic extracts had good antimicrobial activity in comparison to ethyl acetate extract. Microalgae isolated from Persian Gulf had a good inhibitory effect on Gram-positive bacteria in comparison to Gram-negative bacteria. Further investigation is needed on the Persian Gulf microalgae for detecting special antimicrobial compounds on these microalgae.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
Thanks to guidance and advice from “Clinical Research Development Unit of Baqiyatallah Hospital”, Tehran, Iran.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Amaro H M, Guedes A C, Malcata F X. (2011). Antimicrobial activities of microalgae: an invited review. Science against microbial pathogens communicating current research and technological advances 3, pp.: 1272–84. [Google Scholar]
- 2.Mimouni V, Ulmann L, Pasquet V, et al. (2012). The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr Pharm Biotechnol, 13 (15): 2733–50. [DOI] [PubMed] [Google Scholar]
- 3.Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K. (2016). Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J, 109: 282–96. [Google Scholar]
- 4.Lauritano C, Andersen J H, Hansen E, et al. (2016). Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci, 3: 68. [Google Scholar]
- 5.Kokou F, Makridis P, Kentouri M, Divanach P. (2012). Antibacterial activity in microalgae cultures. Aquac Res, 43(10): 1520–27. [Google Scholar]
- 6.Spolaore P, Joannis-Cassan C, Duran E, Isambert A. (2006). Commercial applications of microalgae. J Biosci Bioeng, 101(2): 87–96. [DOI] [PubMed] [Google Scholar]
- 7.Świątkiewicz S, Arczewska-Włosek A, Józefiak D. (2015). Application of microalgae biomass in poultry nutrition. Worlds Poult Sci J, 71(4): 663–72. [Google Scholar]
- 8.Jafari H, Jahromi St, Zargan J, et al. (2021). Cloning and Expression of N-CFTX-1 Antigen from Chironex fleckeri in Escherichia coli and Determination of Immunogenicity in Mice. Iran J Public Health, 50(2):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aminnezhad S, Kermanshahi R K, Ranjbar R. (2015). Evaluation of synergistic interactions between cell-free supernatant of Lactobacillus strains and amikacin and genetamicin against Pseudomonas aeruginosa. Jundishapur J Microbiol, 8(4):e16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda Y, Yoshino T, Matsunaga T, Matsumoto M, Tanaka T. (2018). Marine microalgae for production of biofuels and chemicals. Curr Opin Biotechnol, 50: 111–20. [DOI] [PubMed] [Google Scholar]
- 11.Falaise C, François C, Travers M-A, et al. (2016). Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar Drugs, 14(9):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranjbar R, Owlia P, Saderi H, et al. (2011). Characterization of Pseudomonas aeruginosa strains isolated from burned patients hospitalized in a major burn center in Tehran, Iran. Acta Med Iran, 49(10):675–9. [PubMed] [Google Scholar]
- 13.Jonaidi Jafari N, Kargozari M, Ranjbar R, et al. (2018). The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J Food Process Preserv, 42: e13336. [Google Scholar]
- 14.Cars O, Högberg L D, Murray M, et al. (2008). Meeting the challenge of antibiotic resistance. BMJ, 337:a1438. [DOI] [PubMed] [Google Scholar]
- 15.Raeispour M, Ranjbar R. (2018). Antibiotic resistance, virulence factors and genotyping of Uropathogenic Escherichia coli strains. Antimicrob Resist Infect Control, 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swapnil S, Bruno B, Udhaya R, et al. (2014). Bioactive compounds derived from microalgae showing antimicrobial activities. J Aquac Res Development, 5(3):224. [Google Scholar]
- 17.Dussault D, Vu K D, Vansach T, et al. (2016). Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem, 199: 114–18. [DOI] [PubMed] [Google Scholar]
- 18.Kausalya M, Rao G N. (2015). Antimicrobial activity of marine algae. J Algal Biomass Utln, 6(1): 78–87. [Google Scholar]
- 19.Pérez M J, Falqué E, Domínguez H. (2016). Antimicrobial action of compounds from marine seaweed. Mar Drugs, 14(3): 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Liu Y, Cao M-J, Liu G-M, et al. (2017). Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr Polym, 172: 294–305. [DOI] [PubMed] [Google Scholar]
- 21.Pina-Pérez M C, Rivas A, Martínez A, Rodrigo D. (2017). Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem, 235: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas V, Rivas L, Cárdenas C, Guzmán F. (2020). Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules, 25(24): 5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khomarlou N, Aberoomand-Azar P, Lashgari A P, et al. (2018). Essential oil composition and in vitro antibacterial activity of Chenopodium album subsp. striatum. Acta Biol Hung, 69(2): 144–55. [DOI] [PubMed] [Google Scholar]
- 24.Kellam S J, Walker J M. (1989). Antibacterial activity from marine microalgae in laboratory culture. Br Phycol J, 24(2): 191–94. [Google Scholar]
- 25.Wayne P. (2018). National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial disk susceptibility testing. Twelfth informational supplement (M100–S28).
- 26.Eloff J N. (1998). A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med, 64(8): 711–13. [DOI] [PubMed] [Google Scholar]
- 27.Karimi A, Majlesi M, Rafieian-Kopaei M. (2015). Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol, 4(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 28.El-deen N. (2011). Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr J Microbiol Res, 5(15): 2160–67. [Google Scholar]
- 29.Varier K M, Milton M, Arulvasu C, Gajendran B. (2013). Evaluation of antibacterial properties of selected red seaweeds from Rameshwaram, Tamil Nadu, India. J Acad Indus Res, 1(11): 667–70. [Google Scholar]
- 30.Rosaline X D, Sakthivelkumar S, Rajendran K, Janarthanan S. (2012). Screening of selected marine algae from the coastal Tamil Nadu, South India for antibacterial activity. Asian Pac J Trop Biomed, 2(1): S140–S46. [Google Scholar]
- 31.Krishnika A, Bhanupriya P, Nair B B. (2011). Antibacterial activity of eight marine microalgae against a few gram negative bacterial pathogens. J Pharm Innov, 5: 233. [Google Scholar]
- 32.Nur Syukriah A, Liza M, Harisun Y, Fadzillah A. (2014). Effect of solvent extraction on antioxidant and antibacterial activities from Quercus infectoria (Manjakani). Int Food Res J, 21(3): 1067–1073. [Google Scholar]
- 33.Rafińska K, Pomastowski P, Rudnicka J, et al. (2019). Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem, 289: 16–25. [DOI] [PubMed] [Google Scholar]
- 34.Münch D, Sahl H-G. (2015). Structural variations of the cell wall precursor lipid II in Gram-positive bacteria—Impact on binding and efficacy of antimicrobial peptides. Biochim Biophys Acta, 1848(11 Pt B):3062–71. [DOI] [PubMed] [Google Scholar]
- 35.Beveridge T J. (1999). Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol, 181(16):4725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silhavy T J, Kahne D, Walker S. (2010). The bacterial cell envelope. Cold Spring Harb Perspect Biol, 2(5): a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]