Dear Editor,
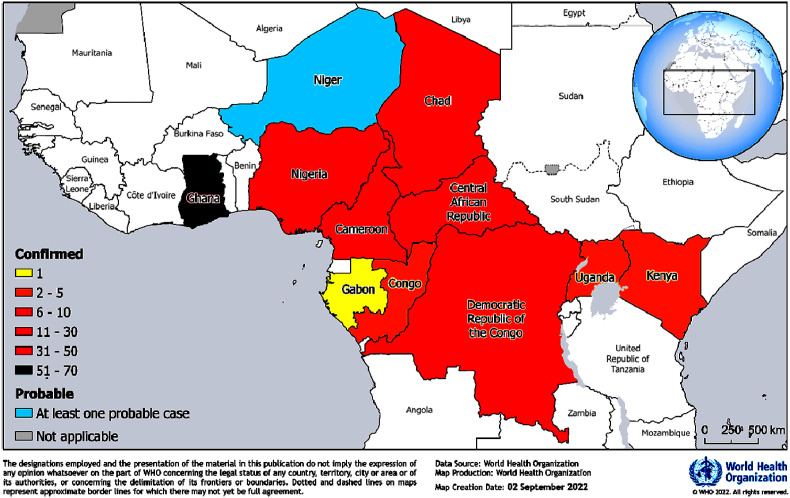
Yellow fever is endemic to the African subcontinent and has been frequently reported from this region. The Eliminate Yellow Fever Epidemics global strategy classifies 27 African countries as high-risk [1]. A total of 12 countries in this region have confirmed 184 cases and 274 probable cases with 21 deaths from January 2021 to August 26, 2022 (Fig. 1 ) [1]. Nine African countries, namely, Cameroon, Ghana, Nigeria, Chad, Côte d'Ivoire, Central African Republic, Democratic Republic of Congo, Gabon and the Republic of Congo reported 151 confirmed yellow fever cases in 2021. Six countries out of the nine continued to report confirmed cases in 2022 with ongoing transmission. With Ghana reporting the majority (33%) of the confirmed cases, two additional countries of the region (Kenya and Uganda) also reported confirmed cases, and Niger reported probable cases in 2022 [[1], [2], [3]]. 73% out of the 184 confirmed cases were ≤30 years of age with an 1.2 male-to-female ratio [1] (Fig. 1).
Fig. 1.
Confirmed and probable yellow fever cases in the African Region during January 2021 and August 26, 2022, as per the WHO report [1].
Caused by an arbovirus, yellow fever transmits to humans by bites of infected Aedes and Haemagogus mosquitoes, and the disease incubates for 3–6 days [1]. Fever, headache, muscle pain with prominent backache, loss of appetite and nausea are the most common symptoms, and in most cases it remains asymptomatic. With systemic infection, few cases may reach to a toxic phase that affects the liver and kidneys. Such cases may experience severely high fever, abdominal pain, vomiting, jaundice and dark urine with acute liver and kidney failure [1], even leading to bleeding from nose, eyes, mouth or stomach. About half of such cases with severe symptoms may progress to death in 7–10 days.
WHO provides coordination and technical support to conduct comprehensive investigations and respond to outbreaks. It highly recommends to strengthen the surveillance and laboratory capacity (laboratory testing and capacity building) with focussed training on yellow fever case investigation and case classification (to ascertain the epidemiology of the cases) to health personnels. Medical transcription and data management must be strengthened. There have been seven reactive vaccination campaigns (RVC) in this region as a part of the outbreak response. The International Coordination Group (ICG) has vaccinated 3,991,568 persons, particularly in Cameroon, Central African Republic, Chad, Ghana and Kenya as of August 26, 2022 [1]. Additional RVC has been approved by the ICG and is scheduled for September 2022. Areas at high risk of transmission and inadequate herd immunity may need preventive mass vaccination (PMV).
As the infections are recurring in areas that had been free from yellow fever for decades, it has become a matter of great concern. It is probably due to the rapid spread of the mosquito vectors and evolutionary dynamics of the virus involving non-human primates. In their pathogeographic assessment, Aliaga-Samanez and coworkers reported that enzootic cycles based on primate assemblages could be amplifying the risk of yellow fever viral infections [4]. Critical regions for the possible spread of yellow fever are the Amazon basin in South America, and southern Brazil where forest fragmentation could activate enzootic cycles next to the urban areas [4]. Such a study helps to identify risk areas in prioritising vaccination and deep surveillance for yellow fever in South American and African primates.
As per the WHO, 27 countries in the African region are at high-risk with regard to the transmission potential and the urban reach. With continued risk of spread, the current outbreak and active virus circulation across international boundaries may increase morbidity and mortality. Yellow fever may be prevented by a single dose vaccine for sustained life-long immunity, thereby helping to reduce future outbreak risk. Yellow fever vaccination is safe and highly effective, which could provide immunity effectively in 10 days in 80–100% of vaccinated people, and within 30 days in more than 99% cases [1]. Routine child immunisation coverage in the region was 47% for yellow fever, which is much lower than the required threshold of 80% as per the WHO and UNICEF. This means that a larger population still remains susceptible to yellow fever with a risk of horizontal transmission. While Uganda has planned to introduce yellow fever vaccine into routine immunisation from August 2022, the immunisation coverage in Ghana is reportedly the highest (94%) and Kenya the lowest (7%).
The horizontal spread risk factors include low herd immunity, anthropogenic movements, viral transmission dynamics and the climatic and ecological factors that are critical in the spread of mosquito vectors. Medical facilities in the African countries are below par and they are overwhelmed due to the concurrent COVID-19, Ebola virus disease, cholera, meningitis, malaria, monkeypox, poliovirus type 2, chikungunya, leishmaniasis, plague, Lassa fever, and other outbreaks [5,6]. Furthermore, many African countries are faced with political instability and insecurity that may stifle the efforts towards case investigation, surveillance and early response [1]. Monitoring the situation closely with active cross-border coordination and information sharing is recommended to address the risk across international boundaries. Enhanced surveillance and laboratory testing of suspects are recommended. The greatest yellow fever virus (YFV) transmission risk being during the day and early evening, targeted vector control measures may help to counter the transmission particularly in the urban areas. Mosquito bite may be prevented by using repellents and insecticide treated mosquito nets as a general precaution. Keeping travellers well informed of risks and preventive measures about yellow fever symptoms and signs is also necessary. Whenever a competent vector is present, the returning infected travellers may pose a risk of local transmission cycle.
Despite the significant effect of yellow fever virus on human lives in the 18th and 19th centuries, there is a lot to understand about it [7]. Yellow fever virus is a neglected tropical disease and may be a potential future threat. The recent 2016–2018 outbreaks in areas with no historical cases have raised serious concerns. There are gaps in understanding the human host interaction with yellow fever virus and manifestation of the disease, which needs to be urgently addressed [7]. Yellow fever virus can potentially infect numerous vertebrate hosts and remain in the environment through vectors [8]. Owing to its ability to affect liver, kidneys and the heart, yellow fever can lead to a high (25–50%) mortality rate. The infection could also result in multi-organ failure [9]. The envelope (E) protein of the yellow fever virus helps in virus attachment to host cells (including the hepatic cells), contributing to invasion and manifestation of its pathogenicity. Live attenuated 17D strain vaccine may not protect against viscero-tropism probably owing to mutations [10]. Animal experiments confirmed the mutation potential of yellow fever virus may allow the virus to adapt to a new host [11]. Despite low infection reports in new hosts, the virus may acquire enough virulence and replication abilities. Such observations point to the fact that the virus can defy host specificity barrier and could potentially lead to widespread transmission. Thus, the yellow fever virus can be a potential agent for a later zoonotic pandemic [12].
Provenance and peer review
Not commissioned, internally peer-reviewed.
Ethical approval
This article does not require any human/animal subjects to acquire such approval.
Funding
No funding received.
Author contribution
RKM: conceptualised and made the first draft; LVSK, AA, VK, updated the manuscript; SM edited the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Research registration Unique Identifying number (UIN)
1. Name of the registry: NA.
2. Unique Identifying number or registration ID: NA3. Hyperlink to your specific registration (must be publicly accessible and will be checked): NA.
Guarantor
All authors.
Data statement
Data not available/not applicable.
Declaration of competing interest
No conflicts to declare.
References
- 1.WHO Yellow fever - east, west, and central Africa, 2 september 2022. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON405
- 2.WHO Yellow fever – Kenya, 25 march 2022. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON361
- 3.WHO Yellow fever – Uganda, 25 april 2022. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON367
- 4.Aliaga-Samanez A., Real R., Segura M., Marfil-Daza C., Olivero J. Yellow fever surveillance suggests zoonotic and anthroponotic emergent potential. Communications Biology. 2022;5:530. doi: 10.1038/s42003-022-03492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohapatra R.K., Tuli H.S., Sarangi A.K., Chakraborty S., Chandran D., Chakraborty C., Dhama K. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat? Int. J. Surg. 2022;103 doi: 10.1016/j.ijsu.2022.106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohapatra R.K., Sarangi A.K., Kandi V., Chakraborty S., Chandran D., Alagawany M., Chakraborty C., Dhama K. Recent re-emergence of Marburg virus disease in an African country Ghana after Guinea amid the ongoing COVID-19 pandemic: Another global threat? Current knowledge and strategies to tackle this highly deadly disease having feasible pandemic potential. Int. J. Surg. 2022;106:106863. doi: 10.1016/j.ijsu.2022.106863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douam F., Ploss A. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018;26(11):913–928. doi: 10.1016/j.tim.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y.-J.S., Higgs S., Vanlandingham D.L. Arbovirus-mosquito vector-host interactions and the impact on transmission and disease pathogenesis of Arboviruses. Front. Microbiol. 2019;10:22. doi: 10.3389/fmicb.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes R.L., Pinto J.R., da Silva Junior G.B., Santos A.K.T., Souza M.T.O., Daher E.F. Kidney involvement in yellow fever: a review. Rev. Inst. Med. Trop. Sao Paulo. 2019;61:e35. doi: 10.1590/S1678-9946201961035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis E.H., Wang B., White M., et al. Impact of yellow fever virus envelope protein on wild-type and vaccine epitopes and tissue tropism. npj Vaccines. 2022;7:39. doi: 10.1038/s41541-022-00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klitting R., Roth L., Rey F.A., de Lamballerie X. Molecular determinants of YFV pathogenicity in Syrian Golden Hamsters: one mutation away from virulence. Emerg. Microb. Infect. 2018;7(1):51. doi: 10.1038/s41426-018-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinert R.D.V., Montoya-Diaz E., Khera T., Welsch K., Tegtmeyer B., Hoehl S., Ciesek S., Brown R.J.P. Yellow fever: integrating current knowledge with technological innovations to identify strategies for controlling a Re-emerging virus. Viruses. 2019;11(10):960. doi: 10.3390/v11100960. [DOI] [PMC free article] [PubMed] [Google Scholar]