Graphical abstract
Keywords: Hydroxychloroquine, Eco-Scale Assessment, National Environmental Methods Index, Green Analytical Procedure Index, Green Analytical Chemistry, Analytical Greenness metrics
Abstract
Hydroxychloroquine is a drug that has been widely used during the early stages of COVID-19 pandemic. Different liquid chromatographic methods have been reported for the analysis of hydroxychloroquine in various biological matrices such as human plasma, serum, whole blood, oral fluid, rat plasma and tissues. In this comparative study, the most popular tools used for assessing the greenness profile: National Environmental Methods Index (NEMI), Eco-Scale Assessment (ESA), Green Analytical Procedure Index (GAPI) and Analytical Greenness metric (AGREE) were utilized to evaluate the ecological impact of eighteen liquid chromatographic methods developed for the bioanalysis of COVID-19 drug; hydroxychloroquine.
NEMI is the simplest tool for evaluating the greenness profile of developed methods, but it is the least informative approach as all the reported methods had the same NEMI pictograms. On the other hand, GAPI is a dependable tool providing a complete picture about the method greenness starting from sampling until the final determination. ESA and AGREE tools are digitally presented and more easily applied. Therefore, their utilization for greenness assessment is highly recommended. Selection of the highest eco-friendly analytical procedure is of a paramount importance for protecting human health and the environment. Considering the greenness of the analytical procedures is highly recommended before proceeding to routine use in order to minimize the chemical hazards to the environment. The most eco-friendly analytical procedures for the analysis of hydroxychloroquine in biological samples according to ESA, GAPI and AGREE tools will be highlighted and discussed.
1. Introduction
Applying the concept of green analytical chemistry (GAC) in analytical laboratories is of great importance. Making liquid chromatographic methods greener becomes a necessary goal for many researchers and practitioners aiming at reducing organic solvent consumption and consequently waste production. In this context, many approaches have been developed to make pharmaceutical analysis more environmentally benign [1], [2], [3].
Hydroxychloroquine is an antimalarial drug that has been used to treat malaria for many years [4]. This drug has been used for malaria, rheumatic diseases and viral infections as well [5]. Hydroxychloroquine has an important role in the treatment of systemic lupus erythematosus. It has been reported that hydroxychloroquine can help preventing the occurrence of disease flares and reducing the risk of complications, thus improving quality of life and survival [5]. During Corona virus outbreak (covid-19), several researchers have proposed the use of hydroxychloroquine and chloroquine on the novel virus because of the similarities between covid-19 and SARS-CoV [6], [7]. Several in-vitro and in-vivo studies have proven the effectiveness of hydroxychloroquine on severe acute respiratory syndrome virus. For example, Yao et al. has tested the effect of hydroxychloroquine and chloroquine in vitro and concluded that hydroxychloroquine is more effective in vitro than chloroquine for both prophylaxis and treatment of severe acute respiratory syndrome viruses [8].
Several liquid chromatographic methods have been developed to determine hydroxychloroquine in biological fluids such as human blood [9], [10], [11], [12], [13], [14], [15], [16], [17], [18] and rat blood [19]. Moreover, hydroxychloroquine was quantified with its metabolites either in human blood [20], [21], [22], [23] and rat plasma [24], [25]. Therapeutic drug monitoring of hydroxychloroquine with other drugs was also reported [26].
Most of classical analytical methods developed for the analysis of pharmaceuticals consume large volumes of harmful organic solvents and consequently produce large volumes of waste. With the awareness about the practitioners’ health and the environment as well, substituting traditional analytical methods with green alternatives via avoiding the use or reducing the amount of organic solvents without negatively affecting the chromatographic performance has been receiving increasing attention [27].
Implementing the GAC concept in analytical laboratories becomes more popular and the desire to replace the traditional analytical methods with more eco-friendly ones is becoming more widely accepted [28], [29]. Different strategies have been proposed to make pharmaceutical analysis more environmentally-friendly [27]. Reducing the chromatographic separation times, using green benign solvents and miniaturizing the analytical devices are the most widely applied approaches for greening liquid chromatographic methods [28]. Decreasing the analysis time is a straight forward approach for greening pharmaceutical analysis as it could significantly reduce solvent consumption and consequently waste generation [30], [31], [32]. Substituting harmful solvents with green solvents is an effective approach aiming at minimizing the detrimental effect on the environment. For example, acetonitrile has been replaced by environmental-friendly and less harmful alternatives such as water and ethanol for the liquid chromatographic determination of methylxanthines [33] and parabens [34]. Also, the use of miniaturized analytical columns could significantly minimize solvent consumption and waste generation [32]. For example, by reducing the diameter of LC column from 4.6 to 3 mm, the flow rate could be decreased by a factor of 0.43 which results in reducing the solvent consumption by 57 % [32].
A compromise between successful analyses and safe procedures is of a paramount importance during method development. Different tools have been developed in order to assess the greenness level of analytical procedures such as national environmental method Index (NEMI) [35], analytical Eco-Scale assessment tool (ESA) [36], green analytical procedure index (GAPI) [37] and analytical greenness metric approach (AGREE) [38]. For example, Shaaban and Mostafa [33] utilized NEMI and ESA for evaluating the greenness level of UPLC-UV method developed for simultaneous determination of methylxanthine in tea samples. The total penalty points assigned for the method was 10 and the final Eco-scale score was 90 (out of 100) which indicated that the proposed method was excellent green. The combination of two or more assessment tools for providing a comprehensive overview on the method ecological impact was also documented in the literature. For example, Mostafa et al. [39] employed GAPI and AGREE tools to assess the greenness level of UPLC-UV method developed for trace analysis of sulfonamides in water samples. The proposed method gained seven green pictograms for GAPI tool and a total AGREE score of 0.64 which indicated the high level of method greenness.
The greenness level of newly developed analytical methods should be evaluated and the assessment tools should be adequately compared and included as a parameter in the development of a new eco-friendly analytical procedure. The selection of the most green analytical method is the responsibility of the researchers who should be aware about the environment and human safety. Therefore, clear planning and considering the greenness of the analytical procedure in method validation protocols is highly recommended before proceeding to routine use in order to reduce the environmental hazard.
In this regard, the main objective of this work is to assess the ecological impact of all liquid chromatographic methods developed for the determination of hydroxychloroquine in different biological samples using the most widely applied greenness evaluating tools including NEMI, ESA, GAPI, and AGREE. A comparative study will be constructed using the greenness level of the methods reported. Additionally, the most environmentally benign liquid chromatographic method for the bioanalysis of hydroxychloroquine will be highlighted.
2. Methodology
Several analytical methods have been developed for the determination of hydroxychloroquine with or without its metabolites in various matrices such as pharmaceutical dosage forms, tissues and biological fluids. Obviously some of these reported analytical procedures are considered to be greener than others due to the difference in solvents, columns and instruments used. Therefore, it is very important to assess the deleterious impacts of these analytical procedures on the environment.
In this study, all methods reported for the determination of hydroxychloroquine in the period of 2012–2022 were collected. For establishing a fair environmental comparison, the collected studies were sorted out according to the sample matrix and separation technique. The main criteria of method selection in this study are: 1) the method was applied for determination of hydroxychloroquine in biological fluids, 2) the method was based on liquid chromatography.
The analytical methods developed for the quantitative determination of hydroxychloroquine in pharmaceutical formulations were differentiated from those which developed for the determination of hydroxychloroquine in tissues or biological fluids (Because sample preparation of pharmaceutical preparations are simpler and less solvents consuming compared to the analysis in biological matrices). After excluding the analytical methods that did not fulfill our criteria, 18 liquid chromatographic methods reported for the determination of hydroxychloroquine in biological fluids were selected for this comparative study.
2.1. Greenness assessment tools used for evaluating the ecological impact of the reported methods
The greenness level of each analytical method was evaluated using the most widely applied greenness assessment tools such as National environmental Method index (NEMI), Analytical Eco-scale assessment method (ESA), Green Analytical procedure index (GAPI) and Analytical Greenness metric (AGREE). These approaches consider different aspects of environmental impact of the developed methods.
2.1.1. National environmental method index (NEMI)
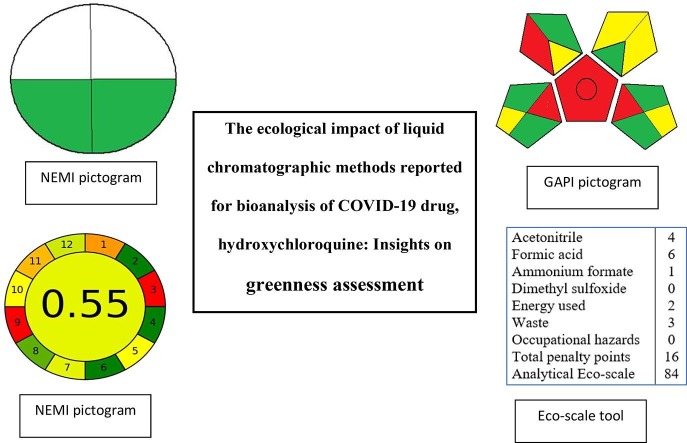
NEMI is one of the oldest tools used for evaluating the greenness level of analytical procedures. It is represented by a pictogram containing four quadrants expressing the reagents hazards, pH and generated waste. Each quadrant of the NEMI symbol is colored green or kept uncolored according to the method matched to the specific criterion. For example, NEMI quadrants are shaded green when the consumed solvents are not persistent, bio-accumulative, and toxic (PBT), not corrosive, not hazardous, pH is larger than 2 and lower than 12, and the waste generated amount is <50 g.
Although NEMI tool allows a quick comparison of the greenness of various analytical methods visually, it does not provide enough understanding about the amount of hazards compared with other assessment tools. For more information about NEMI pictograms, readers are referred to ref. [35].
Although the NEMI pictogram is a very fast approach to GAC metrics and allows fast comparison of the greenness level of different analytical methods, it does not provide sufficient information about the amount of hazards compared to other assessment tools. For more information about NEMI pictograms, readers are referred to ref. [35].
2.1.2. Analytical eco-scale assessment (ESA) tool
The analytical Eco-scale tool is based on assigning penalty points to different parameters such as the type and amount of consumed reagents, occupational hazard, waste generated and energy consumed. Then, the given points deducted from a total points of 100. The method is considered as excellent green method if the total Eco-scale points are greater than 75, the method is classified as acceptable green if the total points are larger than 50 and the method is classified as inadequately green, if the total value of Eco-scale is lower than 50. This approach of greenness assessment provides more information about the health, safety and ecological hazards of solvents and reagents used in the analytical methods compared to NEMI tool. For other information about Eco-scale assessment tool, readers are referred to ref. [36].
2.1.3. Green analytical procedure Index (GAPI)
GAPI is a recent greenness assessment tool that provides a comprehensive overview for the whole analytical procedures. The use of GAPI tool allows any research to compare the greenness of analytical methods, thus enabling quick selection of the greenest procedure for a particular study. It represents the greenness level of the method through five fields. Each field represents a certain aspect of the developed method. Fields can be shaded green, yellow or red depending on the ecological impact for each step. The field is shaded green if certain requirements are fulfilled. For more information about the description of GAPI parameters, readers are referred to ref. [37].
.
2.1.4. Analytical greenness metric (AGREE)
AGREE tool is the most recent greenness assessment approach. This approach is based on using a simple automated software developed by by Pererira et al in 2020 [38]. The AGREE software is freely available and can be downloaded through a free website link provided in an AGREE article [38]. The AGREE pictogram contains twelve sections equivalent to the twelve principles of GAC. Each section and the middle zone of the AGREE pictogram can be colored from red to green based on the level of the method greenness. The total score of the method is automatically calculated ranging from zero to one according to the method greenness and the score appeared in the middle zone of the AGREE pictogram. For more information about AGREE pictograms, readers are directed to ref. [38].
2.2. Applying the greenness assessment tools to liquid chromatographic methods reported for the bioanalysis of hydroxychloroquine
A detailed overview of each liquid chromatographic method reported for the determination of hydroxychloroquine such as sample matrix, analytical column, liquid chromatographic conditions, detection method and analysis time are illustrated in Table 1 .
Table 1.
Chromatographic conditions for liquid chromatographic methods reported for the analysis of hydroxychloroquine in biological samples.
| Method | Matrix | Analytes | Stationary phase | Mobile phase | Detector | Mode of elution | Flow rate | Temp. | Run time | Waste generated (mL) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Human plasma | Azithromycin and two metabolites (desethyl-hydroxychloroquine and bisdesethyl-chloroquine) | Pursuit pentafluoro phenyl (PFP) (50 mm × 2.0 mm, 3 μm) | Water and acetonitrile both containing 0.05 % trifluoroacetic acid | MS/MS | Gradient | 0.5 mL/min | – | 3.5 min | 1.75 | [17] |
| 2 | Rat blood | Hydroxychloroquine and three metabolites (bisdesethylchloroquin, desethylchloroquine and desethylhydroxychloroquine) | ZORBAX SB-C8 (3.5 μm, 2.1 mm × 150 mm) | Water containing 0.2 % formic acid and 10 mmol/L ammonium acetate: methanol | MS/MS | Gradient | 0.25 mL/min | 40 °C | 4 min | 3.25 | [21] |
| 3 | Human plasma | Hydroxychloroquine | Octadecyl silane Hypersil C18 column (250 mm × 6 mm, 5 µm) | Acetonitrile:methanol, (50:50, v/v) with ratio of 75:25, v/v of sodium 1-pentanesulfonate (96 mg/1000 mL of mobile phase) |
UV at 343 nm | Isocratic | 2 mL/min | 30° | 7 min | 14 | [6] |
| 4 | Rat plasma | Hydroxychloroquine enantiomers | Chiralpak AD-H (4.6 mm × 150 mm, 5 μm particle size) | n-hexane–isopropanol (93:7, v/v) plus 0.5 % diethyl amine into hexane | UV at 343 nm | Isocratic | 0.8 mL/min | 20 °C | 29 min | 23.2 | [16] |
| 5 | Oral Fluid and whole blood | Three metabolites (bisdesethylchloroquine, desethylchloroquine and desethylhydroxychloroquine) | Pentafluorophenyl reverse phase column (Accucore PFP 80 A, 50 mm × 2.1 mm, 2.6 mm) | Methanol, acetonitrile, ultrapure water, and formic acid (50:40:9.5:0.5, v/v) | MS/MS | Gradient | 1 mL/min | 40 °C | 8 min | 8 | [20] |
| 6 | Whole blood, plasma, and serum | Hydroxychloroquine | Accucore C18 (50 mm × 2.1 mm, 2.6 μm) | 10 mM ammonium formate and 0.4 % formic acid in water (mobile phase A) and 0.4 % formic acid in acetonitrile (mobile phase B) | HRMS | Gradient | 0.5 mL/min | – | – | [7] | |
| 7 | Human blood | Hydroxychloroquine | ZORBAX Eclipse XDB – C8 analytical HPLC column (50 mm × 2.1 mm, 5 µm) | Water containing 0.1 % formic acid–acetonitrile (94:6, v/v) | MS/MS | Isocratic | 0.5 mL/min | 25 °C | 3 min | 1.5 | [8] |
| 8 | Human serum | Hydroxychloroquine | HypersilGold C8, 50 mm × 2.1 mm, 5 µm) | Water and methanol, both containing 0.1 % formic acid and 10 mmol/l ammonium formate | MS/MS | Gradient | 0.7 mL/min | 70 °C | 2 min | 18.9 | [9] |
| 9 | Human plasma and whole blood | Hydroxychloroquine | Luna Omega Polar C18 column (100 mm ·×2.1 mm , 1.6 mm) | Water containing 0.01 mol/L ammonium formate and 0.1 % formic acid: acetonitrile 95/5 (vol/vol) | MS/MS | Gradient | 0.4 mL/min | 40 °C | 4 min | 1.6 | [10] |
| 10 | Human blood | Hydroxychloroquine and its two metabolites (desethylchloroquine and desethylhydroxychloroquine) | YMC-Triart C18 column (250 mm × 4.6 mm, 5 μm) | 20 mM sodium phosphate buffer solution containing 0.25 % triethylamine (pH 8.0)—acetonitrile (60:40, v/v) | Fluorescence excitation wavelength at 337 nm and emission wavelength at 405 nm |
Isocratic | 1.0 mL/min | 40 °C | 8 min | 8 | [18] |
| 11 | Human plasma | Hydroxychloroquine | Discovery C18 analytical HPLC column (15 cm × 4.6 mm, 5 µm) | Acetonitrile-phosphate buffer (85:15, v/v) | UV at 260 nm | Isocratic | 0.7 mL/min | 30 °C | – | [11] | |
| 12 | Human whole blood | Three metabolites (monodesethylhydroxychloroquine, desethylchloroquine, bisdesethylchloroquine) | Hypersil Gold aQ column (50 mm × 3 mm, 3 µm) |
Water and methanol acidified with 0.1 % formic acid | MS/MS | Gradient | 0.5 mL/min | – | 3.3 min | 1.65 | [19] |
| 13 | Human whole blood | One metabolite (desethyl hydroxychloroquine) | Kinetex PFP 100A column (50 mm × 4.6 mm, 2.6 µm) | 0.01 mol/L of ammonium formate in methanol containing 0.1 % formic acid along with 0.005 mol/L of ammonium formate in water containing 0.2 % formic acid (70:30) | MS/MS | Isocratic | 0.45 mL/min | 45 ℃ | 2.2 min | 0.99 | [12] |
| 14 | Human whole blood | Two metabolites (desethylhydroxychloroquine and desethylchloroquine) | XTerra phenyl® column (250 mm × 4.6 mm, 5 μm) | Glycine buffer/sodium chloride (pH 9.7, 100 mM) and methanol (46:54; v/v) | Fluorescence detection with excitation wavelength at 320 nm and emission wavelength at 380 nm | Isocratic | 1.2 mL/min | 50 °C | 18 min | 21.6 | [13] |
| 15 | Human serum | Hydroxychloroquine, minocycline and doxycycline | Acquity BEH Phenyl (2.1 mm × 50 mm 1.7 μm) reversed phase column | 1 % Triethylamine and 1 mM oxalic acid in water adjusted to pH 2.4 with orthophosphoric acid at 85 %. | UV detection at 343 nm | Gradient | 0.5 mL/min | 35 °C. | 2.5 min | 1.25 | [23] |
| 16 | Venous blood | Hydroxychloroquine and its metabolites; desethylhydroxychloroquine, desethylchloroquine, and bisdesethylchloroquine | Kinetex C8 column (2.1 mm × 50 mm, 2.6 µm) | 0.1 % Formic acid and 0.01 % triethylamine in water (or acetonitrile) | MS/MS | Gradient | 0.5 mL/min | – | 2.1 min | 1.05 | [14] |
| 17 | Whole blood | Hydroxychloroquine and its metabolites: desethylhydroxychloroquine, desethylchloroquine, and bisdesethylchloroquine | Octadecyl silane Luna C18 (150 mm × 4.6 mm, 5 μm) | Water– methanol–acetonitrile (47:10:43, v/v/v) mixture containing 3.2 mM sodium dodecyl sulfate | Fluorescence excitation and emission wave lengths were set respectively at 320 and 370 nm | Isocratic | 1.0 mL/min | 40 °C | 17 min | 17 | [15] |
| 18 | Mouse blood and tissues | Hydroxychloroquine and its metabolites: desethylchloroquine, bisdesethylchloroquine, and monodesethylhydroxychloroquine | Thermo Aquasil C18 (50 mm × 4.6 mm, 3 µ) column | 0.2 % Formic acid and 0.1 % formic acid in methanol | MS/MS | Gradient | 0.5 mL/min | 40 ℃ | 7.5 min | 3.75 | [22] |
The four greenness assessment tools were individually utilized for assessing the greenness level of the 18 selected analytical methods. In NEMI approach, the level of method greenness is presented in a figure in which green color indicates that the method is eco-friendly. In eco-scale approach, only number values are provided without using any figures. The calculations of eco-scale scores of all investigated methods are given in Table 2 . In GAPI approach, three colors (green, yellow, and red) were used to present the results where green color represents the most ecofriendly method and red color indicates a non-ecofriendly method. The detailed calculations for each method using GAPI tool are presented in Table 3 . Similar to GAPI, the AGREE approach is expressed by 3 colors with different degree of color saturation according to the level of the method eco-friendliness. The final score for AGREE is given in the middle of the pictogram. The pictograms of AGREE approach for each investigated method for bioanalysis of hydroxychloroquine are displayed in Table 2.
Table 2.
Assessment of greenness level of reported liquid chromatographic methods for the bioanalysis of hydroxychloroquine using NEMI, Eco-scale, GAPI and AGREE tools.
| Method | NEMI tool | Eco-scale tool | GAPI | AGREE | |
|---|---|---|---|---|---|
| 1 [17] |
 |
Acetonitrile Trifluoracetic acid Methanol Sodium hydroxide Formic acid Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 4 6 2 6 2 3 0 27 73 |
 |
 |
| 2 [21] |
 |
Methanol Ammonium acetate Formic acid Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
6 1 6 2 3 0 18 82 |
 |
 |
| 3 [6] |
 |
Acetonitrile Methanol Sodium pentasulfonate Phosphoric acid Sodium hydroxide Ammonia Diethyl ether Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 6 1 2 2 8 4 1 5 0 33 67 |
 |
 |
| 4 [16] |
 |
n-hexane Isopropanol Diethyl amine Triethyl amine Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
8 4 6 6 1 5 0 30 70 |
 |
 |
| 5 [20] |
 |
Ammonium acetate Ammonium formate Methanol Acetonitrile Formic acid Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
1 1 6 4 6 2 3 0 23 77 |
 |
 |
| 6 [7] |
 |
Acetonitrile Formic acid Ammonium formate Dimethyl sulfoxide Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 6 1 0 2 3 0 16 84 |
 |
 |
| 7 [8] |
 |
Formic acid Methanol Acetonitrile Zinc sulphate Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
6 6 4 2 2 3 0 23 77 |
 |
 |
| 8 [9] |
 |
Perchloric acid Methanol Formic acid Ammonium formate Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
8 6 6 1 2 3 0 26 74 |
 |
 |
| 9 [10] |
 |
Ammonium formate Formic acid Acetonitrile Methanol Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
1 6 4 6 2 3 0 22 78 |
 |
 |
| 10 [18] |
 |
Acetonitrile Sodium phosphate Methanol Triethyl amine Sodium hydroxide Sodium dihydrogen phosphate Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 1 6 6 2 1 1 3 0 24 76 |
 |
 |
| 11 [11] |
 |
Acetonitrile Phosphate buffer Hexane Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 1 8 1 3 0 17 83 |
 |
 |
| 12 [19] |
 |
Formic acid Methanol Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
6 6 2 3 0 17 83 |
 |
 |
| 13 [12] |
 |
Ammonium formate Formic acid Methanol Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
1 6 6 2 3 0 18 82 |
 |
 |
| 14 [13] |
 |
Methanol Glycine buffer Cupric acid Sodium chloride Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
6 0 2 1 1 5 0 15 85 |
 |
 |
| 15 [23] |
 |
Ethyl acetate Methanol Triethyl amine Ascorbic acid Orthophosphoric acid Oxallic acid Sodium dihydrogen phosphate Sodium sulfite Sodium hydroxide Diethyl ether Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 6 6 0 2 4 1 2 2 4 1 3 0 35 65 |
 |
 |
| 16 [14] |
 |
Acetonitrile Methanol Formic acid Triethyl amine Perchloric acid Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
4 6 6 6 8 2 3 0 35 65 |
 |
 |
| 17 [15] |
 |
Methanol Diethyl ether Acetonitrile Sodium dodecyl sulphate Ammonia Dimethyl sulfoxide Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
6 4 4 6 6 1 1 5 0 33 67 |
 |
 |
| 18 [22] |
 |
Methanol Acetonitrile Formic acid Energy used Waste Occupational hazards Total penalty points Analytical Eco-scale |
6 4 6 2 3 0 21 79 |
 |
 |
Table 3.
The detailed calculations for the reported LC methods using GAPI tool.
| No. | Category | Method | GAPI pictograms |
|---|---|---|---|
| 1 | Sample preparation | [17] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatment (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 2 | Sample preparation | [21] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 3 | Sample preparation | [6] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatment (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≤1.5 kWh per sample (Yellow) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 4 | Sample preparation | [16] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatment (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | 10–100 mL (Yellow) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≤1.5 kWh per sample (Yellow) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | (Red) greater than 10 mL | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 5 | Sample preparation | [20] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 6 | Sample preparation | [7] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | Chemical preservation (Yellow) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under special conditions (Red) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | 10–100 mL (yellow) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 7 | Sample preparation | [8] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | Chemical preservation (Yellow) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under special conditions (Red) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatments (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 8 | Sample preparation | [9] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 9 | Sample preparation | [10] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatments (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 10 | Sample preparation | [18] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | 10–100 mL (yellow) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≤1.5 kWh per sample (Yellow) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 11 | Sample preparation | [11] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | None (Green) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Macro-extraction (Red) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatments (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 12 | Sample preparation | [19] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 13 | Sample preparation | [12] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (Yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 14 | Sample preparation | [13] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | 10–100 mL (yellow) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≤1.5 kWh per sample (Yellow) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | greater than10 mL (Red) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 15 | Sample preparation | [23] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatments (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 16 | Sample preparation | [14] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | None (Green) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | None (Green) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 17 | Sample preparation | [15] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | Chemical preservation (Yellow) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatments (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | 10–100 mL (yellow) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | greater than100 mL (Red) | ||
| Waste treatment (15) | Recycling possible (Green) | ||
| 18 | Sample preparation | [22] |  |
| Collection (1) | Offline (Red) | ||
| Preservation (2) | None (Green) | ||
| Transport (3) | Required (Yellow) | ||
| Storage (4) | Under normal conditions (Yellow) | ||
| Type of method: Direct or indirect (5) | Extraction required (Red) | ||
| Scale of extraction (6) | Micro-extraction (yellow) | ||
| Solvents/ reagents used (7) | Non-green solvents and reagents used (Red) | ||
| Additional treatments (8) | Simple treatments (Yellow) | ||
| Reagent and Solvents | |||
| Amount (9) | <10 mL (green) | ||
| Health hazard (10) | NFPA = 3, Moderate toxicity (Yellow) | ||
| Safety hazard (11) | NFPA = 2; high flammability (Yellow) | ||
| Instrumentation | |||
| Energy (12) | ≥1.5 Kwh per sample (red) | ||
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | ||
| Waste (14) | 1–10 mL (yellow) | ||
| Waste treatment (15) | Recycling possible (Green) |
3. Results and discussion
The main objective of green analytical chemistry (GAC) is to minimize or eliminate the harmful ecological effects caused by chemical procedures and to make the analytical procedure more eco-friendly without scarifying the method performance. For greening liquid chromatographic methods, different approaches could be implemented such as reducing organic solvents consumption and consequently waste generation. The overview on chromatographic conditions of the 18 methods reported for the determination of hydroxychloroquine in different biological samples (Table 1) illustrated that all the developed methods utilized hazardous non eco-friendly reagents or not biodegradable such as acetonitrile or methanol. To accurately evaluate the eco-friendliness of the 18 liquid chromatographic methods reported for the analysis of hydroxychloroquine in biological samples, greenness evaluation approaches such as NEMI, ESA, GAPI and AGREE were applied for each method.
By applying NEMI tool, it was found that all pictograms for all reported methods are the same having two blank and two green quarters. This finding is in accordance with a previous study conducted by Ahmed et al. for assessing the environmental impact of 16 chromatographic methods reported for determination of remdesivir [40]. It was indicated that NEMI is unreliable tool and is not suitable for comparing the greenness profile of the developed methods as all the methods have the same NEMI pictograms. Because NEMI is a poor, non-descriptive and less informative tool for providing a clear picture about the green status of each method in comparison with other highly informative tools such as GAPI and AGREE, this tool was excluded from the discrimination of the greenest liquid chromatographic method.
Because all the reported methods were used for quantitative bioanalysis of hydroxychloroquine, the quantitation part in GAPI pictograms are colored red for all reported methods. The ecological comparison for the reported 18 methods of hydroxychloroquine in biological samples according to AGREE and GAPI tools reveled that methods No. 2 [24] and 16 [26] are the best benign procedures. Both analytical methods have six green parts for GAPI and the highest value (0.59) of AGREE tool (Table 2). The second place is method No. 12 [22] having a high score (0.58) of AGREE tool and six green parts for GAPI. The thirdly ranked green methods are method No. 1 [20] and method No. 13 [15] in which the AGREE score is 0.58 and GAPI green parts are five. On the other hand, method No. 4 [19] and method No. 14 [16] were the least green methods according to the GAPI (4 green parts) and AGREE score of 0.45 and 0.47, respectively (Table 2). The GAPI and AGREE are reliable pictograms that could provide deep information about the GAC principles.
For the ecological comparison, numerical values of eco-scale tool were also used. ESA is a semi-quantitative tool that could help in greenness assessment. It gave penalty points for type and volume of used reagents and consumed energy as well. However, it was found that ESA tool gave a false green outcome when used for the environmental comparison of the 18 reported methods. For example, method No. 14 [16] had an ESA score of 85 points, however its AGREE score is 0.47 with 4 red parts of GAPI pictogram. On the other, method No. 16 [17] had a low ESA score of 65, whereas its AGREE score is relatively high (0.59) and its GAPI green parts are six. The false green outcome provided by ESA was also noticed by Gamal et al., [41] who developed a comparative study to select the greenest analytical method for analysis of hyoscine N-butyl bromide. It was noticed that one of the developed methods has a high ESA score (90), however the score of AGREE was low (0.55) and the GAPI pictograms had three red and nine yellow sections. The agreement noticed between GAPI and AGREE pictograms for the compared methods indicated that they are the most reliable greenness assessment tools that could provide integrated information on the whole procedures including all analytical steps for the studied methods. The agreement between the findings of the presented study and those reported in the other studies [40], [41] for assessing the environmental impact of analytical procedures indicated that combining at least two assessment tools is the best approach to improve the understanding of the greenness status of analytical methods. AGREE is a powerful greenness assessment tool that could be combined with GAPI tool for precise evaluation of greenness level of analytical procedures. Detailed results of greenness assessment using ESA, GAPI and AGREE methods are presented in Table 2.
4. Conclusion
Greening the analytical methods necessitates a proper planning prior to performing the analytical procedures in order to minimize the environmental impact. To find out the most environmentally friendly analytical method employed for the analysis of hydroxychloroquine in biological matrices, the greenness profile for each method was evaluated depending on the most applicable greenness assessment tools such as NEMI, ESA, GAPI and AGREE.
Applying the four greenness assessment tools for hydroxychloroquine determination in biological fluids indicated that the LC-MS/MS bioanalysis for hydroxychloroquine and its metabolites reported by Cui et al. [24] and Qu et al. [17] are considered the best analytical methods for the determination of hydroxychloroquine and its metabolites in biological fluids in terms of environmental impact according to GAPI and AGREE tools. NEMI is not a feasible tool to rely on for differentiating the green chromatographic methods from non eco-friendly ones. The ESA tool was less discriminative and its combination with AGREE or GAPI tools is an effective approach for greenness assessment. Applying all greenness assessment tools for evaluating the environmental impact of analytical procedures is practically unfeasible and gathering all detailed information regarding the developed procedures including sample preparation and chromatographic analysis is tedious and time consuming, therefore it is highly recommended to combine two or more greenness evaluating tools for assessing the greenness level of the analytical methods and providing comprehensive comparison.
Generally, the incorporation of greenness assessment of analytical procedures in method validation parameters is of a paramount importance to ensure practitioners’ safety and protect the environment from deleterious possible effects. Moreover, proper planning before implementation of analytical procedures in the laboratory in terms of selection of chemicals and reagents, instrumentation, derivatization, waste generation is highly recommended.
CRediT authorship contribution statement
Heba Shaaban: Conceptualization, Funding acquisition, Project administration, Visualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The author would like to thank the Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University, Saudi Arabia for the financial support (Grant No. 2016-218-Pharm).
Data availability
Data will be made available on request.
References
- 1.Hua D., Xiong R., Braeckmans K., Scheid B., Huang C., Sauvage F., De Smedt S.C. Concentration gradients in material sciences: methods to design and biomedical applications. Adv. Funct. Mater. 2021;31(15):2009005. doi: 10.1002/adfm.202009005. [DOI] [Google Scholar]
- 2.Lu T., Cui J., Qu Q., Wang Y., Zhang J., Xiong R., Ma W., Huang C. Multistructured electrospun nanofibers for air filtration: a review. ACS Appl. Mater. Interfaces. 2021;13(20):23293–23313. doi: 10.1021/acsami.1c06520. [DOI] [PubMed] [Google Scholar]
- 3.Xiong R., Xu R.X., Huang C., De Smedt S., Braeckmans K. Stimuli-responsive nanobubbles for biomedical applications. Chem. Soc. Rev. 2021;50(9):5746–5776. doi: 10.1039/c9cs00839j. [DOI] [PubMed] [Google Scholar]
- 4.Parhizgar A.R., Tahghighi A. Introducing new antimalarial analogues of chloroquine and amodiaquine: a narrative review, Iran. J Med. Sci. 2017;42(2):115–118. PMID: 28360437; PMCID: PMC5366359. [PMC free article] [PubMed] [Google Scholar]
- 5.Bajpaia J., Pradhan A., Singh A., kanta S. Hydroxychloroquine and covid-19– A narrative review. Indian J. Tuberc. 2020;67(4):S147–S154. doi: 10.1016/j.ijtb.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.S. Padmanabhan, Potential dual therapeutic approach against SARS-CoV-2/COVID-19 with Nitazoxanide and Hydroxychloroquine, (2020), 10.13140/RG.2.2.28124.74882.
- 7.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A. Singh, Roopkishora, C. L. Singh, R. Gupta, S. Kumar, M. Kumar, Development and Validation of Reversed-Phase High Performance Liquid Chromatographic Method for Hydroxychloroquine Sulphate, Indian J. Pharm. Sci. 77 (5) (2025) 586-91, doi: 10.4103/0250-474x.169038. PMID: 26798174; PMCID: PMC4700712. [DOI] [PMC free article] [PubMed]
- 10.Carlsson H., Hjorton K., Abujrais S., Rönnblom L., Åkerfeldt T., Kultima K. Measurement of hydroxychloroquine in blood from SLE patients using LC-HRMS—evaluation of whole blood, plasma, and serum as sample matrices. Arthritis Res. Ther. 2020;22(125):1–9. doi: 10.1186/s13075-020-02211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L.Z., Ong R.Y., Chin T.M., Thuya W.L., Wan S.C., Wong A.L., Chan S.Y., Ho P.C., Goh B.C. Method development and validation for rapid quantification of hydroxychloroquine in human blood using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012;61(5):86–92. doi: 10.1016/j.jpba.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Füzéry A.K., Breaud A.R., Emezienna N., Schools S., Clarke W.A. A rapid and reliable method for the quantitation of hydroxychloroquine in serum using turbulent flow liquid chromatography-tandem mass spectrometry. Clin. Chim Acta. 2013;421(5):79–84. doi: 10.1016/j.cca.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Doudka N., Giocanti M., Basso M., Ugdonne R., Barthelemy K., Lacarelle B., Blin O., Solas C., Guilhaumou R. Development and Validation of a Simple and Rapid Ultrahigh-Performance Liquid Chromatography Tandem Spectrometry Method for the Quantification of Hydroxychloroquine in Plasma and Blood Samples in the Emergency Context of SARS-CoV-2 Pandemic. Ther. Drug Monit. 2021;43(4):570–576. doi: 10.1097/FTD.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zohra S.F., Nassima L. Hydroxychloroquine serum concentration in coronavirus disease 2019 (COVID-19) patients: a retrospective study. Forensic Sci. Res. 2021;6(3):215–217. doi: 10.1080/20961790.2021.1936896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J. Y. Park, H. H. Song, Y. E. Kwon, S. J. Kim, S. Jang, S. S. Joo, Development and validation of LC-MS/MS for bioanalysis of hydroxychloroquine in human whole blood, J. Biomed. Transl. Res. 19(4):130-139, DOI:10.12729/jbtr.2018.19.4.130.
- 16.Qu Y., Noe G., Breaud A.R., Vidal M., Clarke W.A., Zahr N., Dervieux T., Costedoat-Chalumeau N., Blanchet B. Development and validation of a clinical HPLC method for the quantification of hydroxychloroquine and its metabolites in whole blood. Future Sci. OA. 2015;1(3) doi: 10.4155/fso.15.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu Y., Brady K., Apilado R., O'Malley T., Reddy S., Chitkara P., Ibarra C., Alexander R.V., Dervieux T. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. J. Pharm. Biomed. Anal. 2017;140:334–341. doi: 10.1016/j.jpba.2017.03.047. Epub 2017 Mar 27 PMID: 28391006. [DOI] [PubMed] [Google Scholar]
- 18.Charlier B., Pingeon M., Piaz F.D., Conti V., Valentini G., Filippelli A., Izzo V. Development of a novel ion-pairing HPLC-FL method for the separation and quantification of hydroxychloroquine and its metabolites in whole blood. Biomed. Chromatogr. 2018;32(8):e4258. doi: 10.1002/bmc.4258. [DOI] [PubMed] [Google Scholar]
- 19.Xiong X., Wang K., Tang T., Fang J., Chen Y. Development of a chiral HPLC method for the separation and quantification of hydroxychloroquine enantiomers. Sci. Rep. 2021;11(8017):1–7. doi: 10.1038/s41598-021-87511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sok V., Marzan F., Gingrich D., Aweeka F., Huang L. Development and validation of an LC-MS/MS method for determination of hydroxychloroquine, its two metabolites, and azithromycin in EDTA-treated human plasma. PLoS One. 2021;16(3):e0247356. doi: 10.1371/journal.pone.0247356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo X., Peng Y., Ge W. A Sensitive and optimized HPLC-FLD method for the simultaneous quantification of hydroxychloroquine and its two metabolites in blood of systemic lupus erythematosus patients. J. Chromatogr. Sci. 2020;58(7):600–605. doi: 10.1093/chromsci/bmaa023. [DOI] [PubMed] [Google Scholar]
- 22.Soichot M., Mégarbane B., Houzé P., Chevillard L., Fonsart J., Baud F.J., Laprévote O., Bourgogne E. Development, validation and clinical application of a LC-MS/MS method for the simultaneous quantification of hydroxychloroquine and its active metabolites in human whole blood. J. Pharm. Biomed. Anal. 2014;100:131–137. doi: 10.1016/j.jpba.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Duarte N.J.C., Kupa L.V.K., Ferreira-Filho J.C.R., Fontoura N., Chalom M.Y., Romano P., Ebner P.A.R., Silva C.A.A., Carvalho V.M., Bonfá E. UHPLC-MS/MS method for determination of hydroxychloroquine and its main metabolites in oral fluid and whole blood for therapeutic drug monitoring. J. Appl. Lab. Med. 2021;6(4):868–880. doi: 10.1093/jalm/jfab031. [DOI] [PubMed] [Google Scholar]
- 24.Cui L., Wang Z., Qiu S., Zhang M., Liu Y., Xu F., Song X., Gao S., Chen W. LC-MS/MS method for determination of hydroxychloroquine and metabolites: application in a pharmacokinetic study. J. Anal. Methods Chem. 2022;6058445 doi: 10.1155/2022/6058445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhonker Y.S., Sleightholm R.L., Li J., Oupický D., Murry D.J. Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC-ESI-MS/MS: An application for pharmacokinetic studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018;1072:320–327. doi: 10.1016/j.jchromb.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.N. Armstrong, M. Richez, D. Raoult, E. Chabriere, Simultaneous UHPLC-UV analysis of hydroxychloroquine, minocycline and doxycycline from serum samples for the therapeutic drug monitoring of Q fever and Whipple's disease, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1060 (166-172), doi: 10.1016/j.jchromb.2017.06.011. [DOI] [PubMed]
- 27.Shaaban H. New insights into liquid chromatography for more eco-friendly analysis of pharmaceuticals. Anal Bioanal Chem. 2016;408(25):6929–6944. doi: 10.1007/s00216-016-9726-2. [DOI] [PubMed] [Google Scholar]
- 28.Shaaban H., Gorecki T. Current trends in green liquid chromatography for the analysis of pharmaceutically active compounds in the environmental water compartments. Talanta. 2015;132:739–752. doi: 10.1016/j.talanta.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 29.Shaaban H. Eco-Friendly bio-Analytical techniques for pharmaceutical analysis. J. Clin. Bioanal. Chem. 2017;1(1):3–4. https://www.alliedacademies.org/articles/green-ecofriendly-bioanalytical-techniques-for-pharmaceutical-analysis.pdf [Google Scholar]
- 30.Shaaban H. High speed hydrophilic interaction liquid chromatographic method for simultaneous determination of selected pharmaceuticals in wastewater using a cyano-bonded silica column. J. Liq. Chromatogr. Relat. 2018;41(4):180–187. doi: 10.1080/10826076.2018.1429282. [DOI] [Google Scholar]
- 31.Shaaban H., Górecki T. Fused core particles as an alternative to fully porous sub-2 μm particles in pharmaceutical analysis using coupled columns at elevated temperature. Anal. Methods. 2012;4(9):2735–2743. doi: 10.1039/C2AY25202C. [DOI] [Google Scholar]
- 32.H. Shaaban, A. Mostafa, T. Górecki, Green gas and liquid capillary chromatography, in The Application of Green Solvents in Separation Processes, Elsevier (2017) 453-482. https://doi.org/10.1016/B978-0-12-805297-6.00015-2.
- 33.Shaaban H., Mostafa A. Sustainable eco-friendly ultra-high-performance liquid chromatographic method for simultaneous determination of caffeine and theobromine in commercial teas: evaluation of greenness profile using NEMI and eco-scale assessment tools. J. AOAC Int. 2018;101(6):1781–1787. doi: 10.5740/jaoacint.18-0084. [DOI] [PubMed] [Google Scholar]
- 34.Shaaban H., Mostafa A., Alhajri W., Almubarak L., AlKhalifah K. Development and validation of an eco-friendly SPE-HPLC-MS method for simultaneous determination of selected parabens and bisphenol a in personal care products: evaluation of the greenness profile of the developed method. J. Liq. Chromatogr. Relat. 2018;41(10):621–628. doi: 10.1080/10826076.2018.1499527. [DOI] [Google Scholar]
- 35.Tobiszewski M. Metrics for green analytical chemistry. Anal. Methods. 2016;8(15):2993–2999. doi: 10.1039/C6AY00478D. [DOI] [Google Scholar]
- 36.Gałuszka A., Migaszewski Z.M., Konieczka P., Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures, TrAC - Trends Anal. Chem. 2012;37:61–72. doi: 10.1016/j.trac.2012.03.013. [DOI] [Google Scholar]
- 37.Płotka-Wasylka J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta. 2018;181:204–209. doi: 10.1016/j.talanta.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 38.F. Pena-Pereira, W. Wojnowski, M. Tobiszewski, AGREE—Analytical GREEnness metric approach and software, 92 (14) (2020) 10076-10082. https://doi.org/10.1021/acs.analchem.0c01887. [DOI] [PMC free article] [PubMed]
- 39.Mostafa H., Shaaban A.M., Alqarni M., Alghamdi S., Alsultan J.S., Al-Saeed R. Ahmad, Vortex-assisted dispersive liquid–liquid microextraction using thymol based natural deep eutectic solvent for trace analysis of sulfonamides in water samples: assessment of the greenness profile using AGREE metric, GAPI and analytical eco-scale. Microchem. J. 2022;183 doi: 10.1016/j.microc.2022.107976. [DOI] [Google Scholar]
- 40.Ahmed B., Gamal M., Naguib I.A., Ali H.M., Abdallah F.F. Environmental impact of the reported chromatographic methods for the determination of the first FDA-approved therapy for COVID-19 patients, Remdesivir: a comparative study. Microchem. J. 2022;176 doi: 10.1016/j.microc.2022.107242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamal M., Naguib I.A., Panda D.S., Abdallah F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal. Methods. 2021;13(3):369–380. doi: 10.1039/D0AY02169E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.