Abstract
Objective
The aim of the current review is to summarize the available evidence to aid clinicians in the surveillance, treatment and follow-up of the different primary tumors developed by patients diagnosed with von Hippel-Lindau (VHL) syndrome.
Methods
A non-systematic narrative review of original articles, meta-analyses, and randomized trials was conducted, including articles in the pre-clinical setting to support relevant findings.
Results
VHL disease is the most common rare hereditary disorder associated with clear cell renal cell carcinoma. Affected individuals inherit a germline mutation in one VHL allele, and any somatic event that disrupt the other allele can trigger mutations, chromosomal rearrangements, or epigenetic regulations leading to oncogenesis. From a clinical perspective, patients continuously develop multiple primary tumors.
Conclusion
Because VHL is considered a rare disease, very limited evidence is available for diagnosis, surveillance, active treatment with local or systemic therapy and follow-up.
Keywords: Von Hippel-Lindau disease, Rare tumor, Genetic syndrome, Clear cell renal cell carcinoma
1. Introduction
Von Hippel-Lindau (VHL) syndrome is an autosomal dominant disease with an incidence around 1/36 000 live births [1] and is the genetic etiology most commonly associated with kidney cancer [2]. The onco-suppressor gene VHL is located in the chromosome 3p25.3 and it codes for an ubiquitin ligase. This protein is used to degrade the hypoxia inducible transcription factor-2alpha (HIF-2α). The inactivation of VHL genes leads to the accumulation of HIF-2α, which through the activation of other genes, including vascular endothelial growth factor (VEGF), leads to oncogenesis [3].
Multiple tumors can develop over the course of VHL syndrome, but the most prevalent ones are hemangioblastomas (HBs) of the retina and central nervous system (CNS), clear cell renal cell carcinomas (RCCs), pheochromocytomas, pancreatic islet tumors, endolymphatic sac tumors, renal and pancreatic cystadenomas, epididymal cystadenomas in men, and broad ligament of the uterus cystadenomas in women [1]. In 80% of sporadic clear cell RCC, VHL gene alterations are seen [4]. VHL patients present with a single allele VHL germline mutation and a subsequent somatic mutation affecting the remaining VHL allele increases risk of uncontrolled proliferation [5]. Individuals diagnosed with VHL suffer from multiple tumors, but patients’ quality of life is mainly influenced by RCC [3], while the main cause of death is still CNS HB [6,7].
Because VHL is considered a rare disease, very limited evidence is available for diagnosis, surveillance, active treatment with local or systemic therapy and follow-up; hence the aim of the current review is to summarize the available evidence to aid clinicians in the surveillance, treatment, and follow-up of patients diagnosed with VHL syndrome.
2. CNS HBs
2.1. Clinical features
CNS HBs are rare neoplasms that may occur sporadically or in association with VHL syndrome. HBs are generally considered indolent tumors composed of neoplastic stromal cells and a prominent capillary network composed of endothelium and pericytes. According to 2021 World Health Organization classification of CNS tumors, HBs are graded as 1 and are enclosed in the “vascular” subcategory of the “mesenchymal non-meningothelial” tumor subgroup [8].
There is a slight male predominance (male:female ratios of 1.3–2.6:1), with a peak incidence between 30 and 60 years old in sporadic cases and at a younger age (30 years old) in VHL families [9]. Isolated HBs are considered sporadic in 75% of cases, although family history should be always questioned, and genetic test is recommended. HBs in a VHL setting represent the remaining 25% of total HBs cases; they are frequently multiple, with a tendency to develop throughout the course of a patient's life [10]. VHL-associated HBs are tend to appear about 10 years earlier than their sporadic counterparts, often growing in unusual CNS regions; in fact, 50% of them are located in spinal cord and 40% in the cerebellum [11]. VHL subtypes 1, 2A, and 2B entail a higher risk of developing CNS disease occurrence or progression [12]. Recently new pathogenic variants at intron 2 in VHL-associated HBs were observed. According to gene sequencing, CNS HBs can arise from both exonic and intronic mutations [13].
Signs and symptoms are related to the affected anatomic region. Cerebellar HBs can cause headache (75%), gait ataxia (55%), dysmetria (29%), hydrocephalus (28%), and nausea and vomiting (28%); other symptoms such as slurred speech, nystagmus, labile hypertension, and positional vertigo have also been described. Brainstem HBs can cause hypoesthesia (55%), gait ataxia (22%), dysphagia (22%), hyperreflexia (22%), and headache (11%). Spinal cord HBs typically produce hypesthesia (83%), weakness (65%), gait ataxia (65%), hyperreflexia (52%), and pain (17%) [11]. Other symptoms such as paraneoplastic erythrocytosis can occur in 15%–20% of patients with HBs. The elevated values of hematocrit and red cell mass seem to be the result of excessive erythropoietin production by the stromal cells [14].
By the time of clinical presentation, the majority of mass effect, and related symptoms, derive from the cystic components, rather than the solid nodule generating them, mainly because the pace of cystic enlargement is way faster than for solid tumor progression. Cystic HBs are more prone, compared to solely solid tumors, to become symptomatic. Spontaneous edema is exceptional in the cerebellum, while relatively frequent in the spinal cord [15]. Hemorrhages occur more frequently in the peripheric nervous than in the CNS [16].
2.2. Diagnostic examinations
Magnetic resonance imaging (MRI) represents the imaging of choice in terms of neuroradiological evaluation and monitoring of VHL patients, since it provides extremely detailed images of CNS and orbital HBs [17,18] without ionizing radiation.
MRI evaluation of a patient affected by VHL is usually acquired on a 1.5 Tesla scan and includes scans of brain, the orbits, and a whole spine MRI. Brain MRI scan protocol comprises of axial T2-weighted images (WIs), three-dimensional fluid-attenuated inversion recovery, diffusion-WIs, and pre- and post-contrast three-dimensional T1WIs. T2WIs and fluid-attenuated inversion recovery sequences are useful in the evaluation and follow-up of the cystic part of the lesion and of adjoining brain edema or post-surgical changes. Post-contrast T1WIs are rather mandatory to monitor the progressive enlargement of the vascular nodular component of the lesion and to detect small (<2 mm in size) HBs [19]. HBs are most commonly primary intra-axial infra-tentorial tumor, but 5% can be supratentorial (typically in the optic radiations or the sellar region) [20]. Eventually, a T2WI coronal sequence can be added to study the pituitary gland region, or a T2 DRIVE sequence to better discriminate small infratentorial HBs of the vermis or rarely of the cerebellopontine angle.
As detailed, HBs in VHL patients are typically located in the retina, but can rarely occur in other regions of the visual pathway or in the CNS, such as in the optic nerve or chiasm, especially in case of clinical symptoms [21]. Orbital MRI scan protocol includes axial and coronal Dixon T2WI, diffuse WI, axial and coronal T1WIs, and post-contrast imaging.
Whole spine MRI scan consists of sagittal T2- and short-TI inversion recovery WI, and pre- and post-contrast T1WIs. Axial T2WI and axial and coronal post-contrast T1WI are also used to better evaluate small HBs that can be located eccentrically or may have an exophytic component along the dorsum of the cord [22]. Only 25% of spine HBs are entirely intramedullary; even if rare, some of those can be located extramedullary intradural along the cauda equine [23]. T2WI allows for a better definition of associated tumor cyst or syrinx (50%–100%) [24], as well in the assessment of eventual post-surgical spinal cord changes.
Overall, the time of acquisition to correctly perform this type of MRI evaluation is 1 h. Motion artifacts on acquired images can be present or patients can feel the burden of an extended imaging scan, so each examination must be tailored depending on the clinical status of the patient and the necessity of definition of specific imaging findings. When necessary, MRI scan can be performed with deep sedation.
For CNS screening, baseline MRI of the brain and spine is performed at 20 years of age, followed by annual neurologic examinations, with a shorter threshold for repeated imaging if there are any suspicious signs or symptoms. In absence of strong recommendations about surveillance, it has been proposed to perform annual MRI to monitor CNS in VHL patients [25].
2.3. Indications for active treatments
Guidelines for the optimal management of CNS HBs are not available yet. It has been reported that CNS HBs alternate period of quiescence (mean 25 months) with periods of growth (mean 13 months) [11]. Consequently, HBs frequently remain asymptomatic and do not require treatment for long periods of time; however, over time most patients become symptomatic and active treatment is indicated.
2.4. Therapeutic options
Different therapeutic options are conceivable, and the decision is tailored based on signs and symptoms, number of lesions, localization and depending on the sporadic or familial pattern. HBs en-bloc total surgical excision with closure of arterial feeders and dissection of the tumor margins from their pial attachments are usually regarded as the optimal treatment since it generally provides long-term tumor control and frequently results in definitive cure. Recurrence is usually associated with partial or complete removal of the mass in the sporadic group while VHL patients, unfortunately, develop new lesions with a frame rate of about 2 years, independently from surgical radicality. Surgery is associated to a generic risk of intraoperative bleeding which may be reduced by preoperative embolization in cases of large tumors and/or presence of several distal feeding arteries. Most lesions are well defined, have a small nidus together with a large cyst and few small feeding arteries and could be easily dissected from cerebellar parenchyma making embolization unneeded in most cases. Intraoperative use of indocyanine green angiography to demonstrate residual vascularity seems to be a valid option in case of remnants and could be employed in selected cases to accomplish gross total resection.
Cystic drainage alone and/or partial removals or even mere biopsies should be avoided due to inconsistency and risk of recurrence, although they can be accepted when combined with a radiation treatment of the remaining lesion. Draining the cyst before surgery can relief neural structure and allow for a natural corridor to better dissect each HBs from the parenchyma. Generally, a second and third surgical look may be considered in HBs larger than 3 cm, mainly located in posterior fossa, in case of hemorrhage or when a new enlarging cyst is growing to prevent further neurologic sequelae. Surgical techniques should be tailored according to HB localization and center experience; intraoperative neuronavigation paired with augmented reality software's as well as intraoperative ultrasound (US) scans are useful to define the mass, identify the shorter and safer surgical trajectory, restrict the corticectomy size, and preserve cerebellum nuclei.
Although the main therapy of HB is surgical resection, for patients with sub-totally excised, unresectable lesions, or miliary HB diffusion, radiotherapy (RT) and/or stereotactic or radiosurgery (SRS) are effective options. Conventional RT has been associated with good rates of local control in the 60%–90% range depending on the extension, localization, and number of HBs. Concerning volumes of interest for RT planning, the clinical target volume should include the operative bed with an isotropic margin of 1 cm. Prescription dose is 50–60 Gy at 1.8 Gy or 2.0 Gy per fraction, which has been associated with improved local tumor control. The target for SRS in HB has typically been identified as the contrast-enhancing tumor without margin, and prescription doses are typically among 15–20 Gy [26]. Depending on cyst sizes, different irradiation techniques may be adopted: generally, the cyst must be included in the gross tumor volume, and whenever possible, cyst should be drained in advance to reduce volume, especially if larger than 2 cm in diameter. Since higher control rates have been reported for smaller volumes and higher doses, a stereotactical cyst evacuation after the placement of a catheter connected to an Ommaya reservoir just before irradiation is generally advocated.
Radiosurgical experience especially in the management of brainstem HBs is limited because of the frequent presence of symptomatic cystic components and the eloquence of the normal tissue surrounding the lesion, especially for lower brainstem locations, thus raising some concern on long-term efficacy and safety of the procedure. SRS is potentially attractive for patients with VHL syndrome where multiple HBs may develop either concurrently or sequentially and may be difficult to treat or retreat with repeated surgery and/or conventional radiation techniques without excessive cumulative toxicity.
The only known factor predictive of recurrence for long-term tumor control among patients treated with gamma knife SRS is the coexistence of multiple lesions; thereof, patients with multiple HBs are less likely to exhibit long-term tumor control of treated lesions following SRS. In different published series, HBs treated with SRS resulted in excellent tumor control reaching 80%–90% at 5 years, with improvement of symptoms likewise. Factors associated with longer tumor control on univariate analysis were solid tumor characteristics on imaging, small treatment volume, and tumors associated with VHL.
3. Retinal HBs
3.1. Clinical features
Retinal capillary hemangioblastoma (RCH) is one of the most common clinical manifestations of VHL syndrome and is commonly considered as its earliest presenting condition [[27], [28], [29], [30]]. HBs occur in approximately 68% of VHL patients [31,32]. According to a large cross-sectional study, RCHs presented unilaterally in 42% of VHL patients, while 58% presented bilaterally, regardless of age and sex [33]. A recent study found VHL to be the underlying cause of 84% retinal hemangiomas, emphasizing the importance of VHL screening in patients diagnosed with RCH [34].
The exact pathophysiology of the lesion is not fully understood. It has been proposed to be either a congenital, hamartomatous-like lesion or a benign vascular neoplastic process [31]. Histopathological studies displayed that the tumor is characterized by a neovascular plexus, with numerous communicating, irregular vessels (i.e., the result of the benign proliferation of endothelial cells and pericytes), separated by collagen fibers and vacuolated “stromal” cells [[35], [36], [37]].
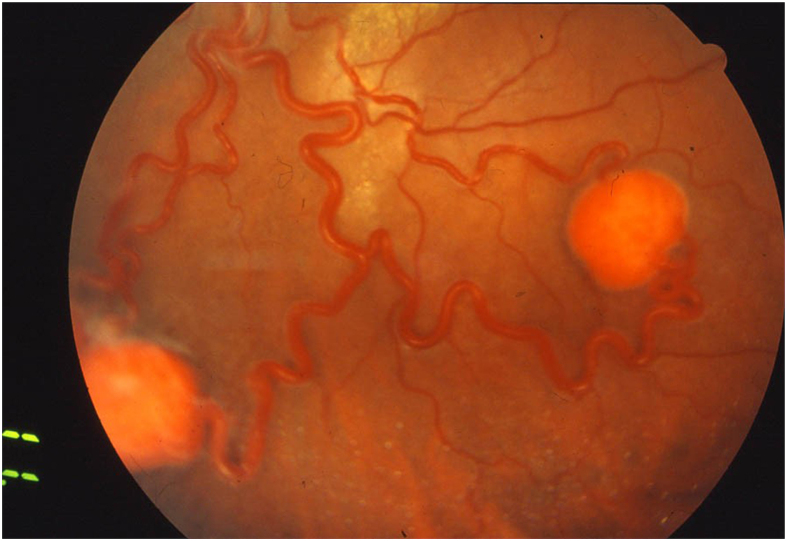
RCH is usually a well-circumscribed, round bulging with a red to orange color and a variable appearance depending upon whether the tumor is endophytic, sessile, or exophytic [28]. RCH can arise at the level of the optic disc (designated as juxtapapillary or epipapillary RCH), or within retina elsewhere (designated as extrapapillary RCH) [38]. Extrapapillary RCH usually starts as a tiny red or gray intraretinal dot no larger than a few hundred microns, appearing on ophthalmoscopic examination like a microaneurysm or punctate intraretinal hemorrhage. As the tumor grows, RCH displays more distinctive prototypical features, such as increasing nodularity, dilated and tortuous feeding and draining vessels, associated with exudation and/or retinal edema [28] (Fig. 1). Noteworthy, peripheral tumors can lead to marked retinal edema and hard exudates in the macular region. Conversely, juxtapapillary RCH may exhibit more subtle features, sometimes appearing only as a localized fullness of the inner retina at the border of the neural rim, while feeding and draining vessels are usually absent. A majority of RCHs grow over time, while only few untreated tumors remain static for a long period and rarely regress spontaneously [39]. Complications, such as exudation of subretinal or intraretinal spaces, are often limited to the perilesional region, but can also be far enough to produce macular star exudates. Without treatment, large or multiple adjacent RCHs might grow to displace retinal structures and cause an exudative retinal detachment. In large RCHs, fibrosis of the epiretinal space, followed by posterior hyaloid contraction, may lead to macular epiretinal membrane with macular thickening, vitreoretinal traction, or tractional retinal detachment. As a rare complication, neovascularization of the iris can also occur, eventually leading to neovascular glaucoma [40]. Symptoms of RCH are generally secondary to macular exudation or retinal detachment. Patients frequently complain of gradual vision loss or metamorphopsia.
Figure 1.
Two retinal capillary hemangioblastomas with dilated and tortuous feeding and draining vessels.
3.2. Diagnostic examinations
Nowadays, multimodal retinal imaging plays a key role in detecting early signs of ocular involvement in VHL syndrome. Fluorescein angiography, particularly with the use of wide-field imaging, is a sensitive tool for identifying small or subtle tumors, which exhibit early hyper-fluorescence of the feeder artery during the arterial phase and cumulative hyper-fluorescence within the entire tumor during the later phases of the exam [27]. For larger tumors, B-scan ultrasonography can be used to corroborate the size of the lesion and to detect signs of exudative retinal detachment [41]. On optical coherence tomography scans, RCH appears as a mass within the inner retinal, with hyper-reflective thickening of the inner layers and displacement of the outer layers with increasing size of the lesion [42].
Further studies, with optical coherence tomography angiography, showed that eyes with VHL syndrome are characterized by an abnormal retinal vascular pattern of the macular region compared with eyes without VHL syndrome [43,44]. These findings strongly support the role of optical coherence tomography angiography as a novel imaging tool to detect potential ocular complications of VHL syndrome.
RCH may be one of the first manifestations of VHL. The role of the ophthalmologist can potentially be lifesaving in educating the patient and the physicians as to the possibility of VHL. This is an important reminder to ophthalmologists to make that first hypothesis of systemic disease. It is recommended that patients with positive genetic testing or positive family history of VHL have ophthalmologic examinations, starting at infancy [31]. Screening recommendations suggest yearly eye examinations with indirect ophthalmoscope to rule out retinal lesions, associated with annual neurological screening for nystagmus or strabismus, hearing impairment, and blood pressure abnormalities [27].
3.3. Indications for active treatments
Since therapy of small angiomas is extremely successful, early diagnosis and treatment are essential to prevent progression in evolved forms of the disease. Active surveillance is recommended just for juxtapapillary HBs and peripheral micro-HBs smaller than 500 μm that do not cause exudation or subretinal fluid [29].
3.4. Therapeutic options
To date, the most effective treatment remains thermal laser photocoagulation [45]. Vascular lesions, such as RCHs, absorb yellow laser more than other laser wavelengths, based on the absorption range of oxyhemoglobin; therefore, yellow and green wavelengths are the most used. For angiomas smaller than the optic disc diameter, the treatment is performed in a single session, with low power values and long exposure to prevent bleeding complications. Larger angiomas firstly require a perilesional barrage, followed by direct photocoagulation of the tumor. After 20–30 days from the barrage, time necessary for the spots to meet scarring, direct photocoagulation could be performed in several sessions. Furthermore, it is suggested—in more voluminous angiomas—first to proceed with the photocoagulation of the feeder vessels to reduce blood supply to the angioma and to reduce the risk of bleeding [46].
To date, cryotherapy is used only for large size angiomas, located in the extreme periphery, in the presence of lens opacities or with evidence of neuroepithelial detachment around the lesion [47]. Surgical treatment is considered for those cases of partial or non-response to laser photocoagulation or cryotherapy [48]. Via pars plana vitrectomy is performed when angiomas cause fibrous or tractional retinal detachment, epiretinal or vascular proliferation affecting central vision, but require careful attention to several risks [49]. Eyes with RCH presenting with exudation, hemorrhage or epiretinal proliferation are at high risk of developing proliferative vitreoretinopathy after surgery.
Notably, intravitreal anti-VEGF have recently been used to reduce exudation resulting from RCH [[50], [51], [52]]. There is hope that better understanding of the molecular basis of VHL syndrome will allow rational design of non-ablative strategies to reduce RCH growth, with concrete improvement on visual outcomes.
The most frequent complications of RCH photocoagulation are hemorrhages and fibrotic contraction, which can occur during and after therapy [53]. Marked adverse side effects, including altitudinal defects and central scotomas in the visual field, are also associated with photocoagulation. Notably, both laser and cryotherapy of peripheral tumors may lead to massive retinal hard exudate accumulation and macular edema, contributing to further decrease in vision after treatment. Indeed, early diagnosis and prompt treatment of RCH may significantly impact both on short- and long-term visual outcomes of the patients.
4. Pancreatic neuroendocrine tumors and pancreatic cistoadenomas
4.1. Clinical features
Pancreatic lesions develop in approximately 70% of patients with VHL syndrome and comprise pancreatic neuroendocrine neoplasms (PanNENs) as well as pancreatic cystic lesions, including simple cysts, serous cystadenomas (SCs), intraductal papillary mucinous neoplasms, and mixed tumors (cystic PanNENs) [54,55]. Pancreatic cyst is the most frequent kind of pancreatic involvement and appear in about half of VHL patients [56], showing a benign biological behavior. Since pancreatic cysts are extremely rare in the general population [57], their detection during screening examination may help in identification of gene carriers. PanNENs diagnosis occurs in 10%–20% of patients, with a slight female predominance [58,59]. Patients with VHL syndrome typically develop multiple, non-functioning PanNENs [60], which are more frequently located in the pancreatic head or uncinate process. VHL-related PanNENs usually display a lower malignant potential compared to sporadic neoplasms, with a consequently reduced rate of high-grade and metastatic lesions (9%–12%) [60]. Symptoms are uncommon and mainly related to compression of the bile duct and the increased risk of pancreatitis.
4.2. Diagnostic examinations
At computed tomography (CT), pancreatic cysts are seen as hypodense lesions (fluid density) without enhancement while SCs appear as multicystic lesions (usually composed of more than six cysts) with each cyst measuring less than 2 cm. A central scar with delayed enhancement and sometimes stellate calcification (20% of cases) may be seen. MRI shows a T1 hypointense and T2 hyperintense “cluster of cysts”; its central fibrotic scar may have hypointense signal on both T1WI and T2WI with delayed enhancement. The absence of communication with the Wirsung duct permits differentiation of SCs from intraductal papillary mucinous neoplasms, while it could be difficult to distinguish SCs from a cluster of closely apposed simple cysts; however, it has no clinical relevance since both are benign lesions requiring no intervention [61].
Unrelated to pancreatic cysts, PanNENs occur in 5%–17% of patients [[61], [62], [63]], and are usually multiple and more common in patients with pheochromocytomas [17,64,65]. If CT and US could be used for screening purpose, MRI is the imaging method that better depicts PanNENs. At US, PanNEN appears as a well-defined mass, hypoechoic relative to pancreatic parenchyma. They are homogeneous and hypo- or iso-attenuating relative to the rest of the pancreas at unenhanced CT and hypointense on T1-weighted and slightly hyperintense on T2-weighted MRI scans [65,66]. They typically show intense arterial enhancement at both CT and MRI scans [57]. Calcification, areas of necrosis, or cystic degeneration may be evident in large tumors [65]. Combining anatomic and functional imaging is essential in PanNEN. In fact, fluorine-18 fluorodeoxyglucose positron emission tomography/CT and gallium-68 somatostatin receptor positron emission tomography/CT may play a role in identification of lesion occulted to anatomic imaging and in prognostication [67,68].
VHL-related PanNENs usually appear during the fourth decade, although several cases have been described in teenage as well as in elderly patients. Given this wide range of disease onset, current guidelines recommend initiating the radiological screening, performed on a 2-year basis, no later than the age of 15 years and to suspend it at the age of 65 years if no pancreatic lesions have appeared [59,69]. Gadolinium-enhanced MRI represents the technique of choice for the surveillance of VHL-related pancreatic lesions, due to lower radiation exposure compared to CT, especially considering the lifelong radiological follow-up required in VHL patients [70]. However, the choice of the best imaging technique is complex, and it should be carefully weighted also considering the high risk of other visceral neoplasms. In this setting, additional imaging should be avoided, favoring a single radiological examination to investigate at the same time all the organs that might be involved. VHL-related PanNENs measuring less than 1.5 cm and remaining stable in at least two consecutive scans are considered as low-risk and can be managed by active surveillance, with serial imaging every 1 or 2 years [56,59,71].
4.3. Indications for active treatments
The management of VHL-associated PanNENs should be carefully tailored based on the unique clinical course of each lesion, to avoid unjustified surgical resections. Likewise, the presence of extrapancreatic manifestations should be always considered to establish the optimal treatment sequence.
Patients with VHL-related cystic lesions that are symptomatic or cannot be distinguished from other (pre)malignant lesions should be managed by surgery [59]. When the surgical indication is represented by diagnostic uncertainty, parenchyma-sparing resection with nodal sampling is suggested. On the other hand, when biliary or gastrointestinal obstruction represents the indication for surgery, endoscopic stent placement or surgical bypass can be considered [72]. Finally, radiological drainage or cyst marsupialization can be regarded as alternative treatment options for simple cysts [58].
Surgical resection should be considered for patients with VHL-related PanNENs at high risk of developing metastatic disease. Tumor size more than 3 cm predicts disease aggressiveness and represents the major risk-stratifying parameter in these patients [60,73]. Therefore, surgical resection is currently recommended for all the patients with VHL-related PanNENs more than 3 cm [[56], [59]]. The management of PanNENs measuring between 2 and 3 cm represents a “grey zone” [74] and depends on multiple factors. If the lesion is in the pancreatic body or tail, surgical resection is recommended. Conversely, when the lesion is located in the pancreatic head, a lower dimensional threshold is applied and the patient is elected for surgery earlier (tumor size ≥2 cm), when enucleation, rather than pancreaticoduodenectomy, is still technically feasible [54,56,59]. A rapid tumor growth during follow-up (i.e., doubling time <500 days) has also been shown to be associated with an increased risk of distant metastases. Therefore, this parameter represents an additional indication for surgical resection. Finally, germline mutations in exon 3 are considered as predictors of poor prognosis [74,75]. In any case, the oncological benefit should be always carefully balanced with the surgical risk and sequelae, considering that VHL patients frequently undergo multiple surgeries due to various manifestations of their genetic condition.
4.4. Therapeutic options
When surgical resection is recommended, treatment options include formal or parenchyma-sparing resections. Parenchyma-sparing surgery (i.e., enucleation) should be preferred over formal resections (i.e., pancreaticoduodenectomy) for treating patients with VHL-related PanNENs and cystic lesions [56,59]. Enucleation is an attractive surgical option, especially considering the high risk of recurrent PanNENs and the potentially compromised pancreatic function due to concomitant cystic disease. Enucleation requires the tumor to be located at more than 3 mm from the main pancreatic duct and to harbor a low-risk metastatic disease [76]. Total pancreatectomy should be considered only when all the other options for limited pancreatic resection have been ruled out. Pancreatic endocrine and exocrine insufficiency represent the major sequelae of formal pancreatic resections. The risk of this long-term functional impairment is reduced in patients submitted to parenchyma-sparing resections, although the rate of postoperative pancreatic fistula is higher [76].
Patients with advanced VHL-related PanNENs are managed following the guidelines for sporadic PanNENs [77]. Tyrosine kinase inhibitors, including sunitinib and pazopanib, have been evaluated as possible therapeutic options in this setting. In particular, a phase 2 study reported the efficacy of pazopanib in reducing the size of VHL-related unresectable PanNENs [78]. However, the most promising therapeutic option is represented by HIF-2α inhibitors (i.e., belzutifan). This drug prevents HIF-2α or HIF-1beta dimerization in conditions of hypoxia or non-functional VHL protein, reducing the expression of HIF-2α target genes and controlling the growth of VHL-associated tumors [79]. HIF-2α inhibitor has shown good preliminary results in the treatment of VHL-related RCC and its use has recently been approved for the treatment of VHL-associated PanNENs [80].
5. Pheochromocytomas
5.1. Clinical features
The reported range of prevalence of pheochromocytomas is wide, from 0 to 60% [64], with a higher prevalence in families with a lower frequency of RCC and cerebellar HB [17]. VHL pheochromocytomas tend to be multiple and bilateral in 50%–80% of cases [66], with a very low proportion of malignancy [64]; ectopic sites (including the organ of Zuckerkandl, glomus jugulare, carotid body, and periaortic, perisplenic, and intrarenal lesions) are more common, occurring in 15%–18% of cases [66]. In tumors secreting predominantly epinephrine or dopamine, symptoms of orthostatic hypotension may be more prevalent [81]. Non-specific symptoms such as weakness and chronic fatigue are also quite common. Leukocytosis and gastrointestinal manifestation (constipation, nausea, and vomiting) are among the other less common presentations. Uncontrolled hypertension can lead to other manifestations such as stroke, seizures, and focal neurologic deficits [82]. Larger tumors may present with other cardiovascular incidents such as cardiogenic shock, acute pulmonary edema, myocardial infarction, cardiac arrhythmias, and cardiomyopathy [83]. Recently, a newly discovered cryptic exon (E1′) of VHL gene was observed in VHL patients. Patients with a clinical suspicion of VHL should be screened for VHL E1′ cryptic exon mutations, which in a recent study was observed to account for 1.32% (1/76) of a “VHL-like” cohort [84].
5.2. Diagnostic examinations
At CT pheochromocytomas present as solid or complex cystic masses; calcifications, areas of necrosis or hemorrhage may be present. Pheochromocytomas typically show strong enhancement during arterial phase [85]. However, this pattern of enhancement does not help in distinguishing pheochromocytomas from adenomas and hyper vascular adrenal metastases since absolute and relative percentage CT washout values may overlap [86]. MRI is superior in diagnosis pheochromocytomas even in ectopic sites [17]. In 95%–100% of cases, they showed low or intermediate signal intensity on T1WIs and high signal intensity on T2WIs (similar to that of cerebrospinal fluid) [17,64,66] with a marked gadolinium enhancement. Radionuclide imaging (e.g., iodine 131 or iodine 123 metaiodobenzylguanidine scintigraphy) with its high specificity (98%–100%) may help in tumor localization and occult metastatic disease detection. However, because of a relatively low sensitivity (75%–95%), a negative image does not exclude the diagnosis of pheochromocytoma [87].
In addition to other forms of surveillance, VHL patients should, starting at 2 years of age, commence with blood pressure checks at every medical visit, and with annual plasma or urine metanephrine levels [88].
5.3. Indications for active treatments
If after imaging and clinical evaluations the diagnosis of pheochromocytoma is established, the definitive therapy is complete surgical resection. It is important to highlight that in the case of acute hyperadrenergic crisis immediate surgery is not recommended [89].
5.4. Therapeutic options
To optimize the patient's condition prior to surgery, careful pharmacologic treatment is essential. The control on blood pressure and heart rate is of essential importance prior to surgery in order to prevent surgery-related catecholamine excess [90]. Alpha-adrenergic antagonists and combined alpha- and beta-adrenergic blockers are the most commonly used drugs and is typically started 7–21 days prior to surgery [91]. Adrenalectomy, which consists in the removal of the entire gland, remains the primary treatment of pheochromocytoma. Laparoscopic techniques are used in over 90% of cases and are preferred over open surgery due to improved postoperative morbidity, duration of hospital stay, and cost, especially for tumors less than 10 cm in diameter. Open techniques are recommended in cases of complex dissections and malignant disease. Operative survival rates are as high as 98%–100% with an experienced surgeon, anesthesiologist, and adequate preoperative management [92]. As previously stated, VHL has a high incidence of bilateral disease with less metastatic potential. Cortex sparing or partial adrenalectomies are preferred in such cases to prevent permanent glucocorticoid deficiency [93].
Among the different immediate postoperative complications, hypotension and hypoglycemia are the most common. Persistent non-responding hypotension, especially in the setting of bilateral adrenal manipulation, should raise suspicion for adrenal insufficiency and be treated with stress doses of glucocorticoids and intravenous pressors. In 10%–15% of patients after pheochromocytoma resection due to excess catecholamines removed from circulation and the consequent interruption of suppression of insulin, hypoglycemia occurs, which should be carefully monitored for and treated with intravenous glucose [94].
6. Clear cell RCCs
6.1. Clinical features
Renal masses present at an early age as multicentric renal cysts of various complexity and are found in approximately 42%–63% of patients; age at presentation tends to be earlier than sporadic clear cell RCC, with a mean age of 37 years [95]. RCC is diagnosed in approximately 40%–70% of patients with VHL syndrome. Similarly to renal cysts, the median age at the time of diagnosis is 39 years [96]. The majority of RCCs in VHL syndrome are identified as clear cell histology, and patients with VHL type 1 and type 2B are at significantly increased risk of developing RCC compared to patients with other types [5,17].
With the increasing use of routine imaging for many different diseases, most patients with RCC are identified by chance. Among the 30% of patients diagnosed, physical examination has a limited role in the diagnostic workup of RCC. The presence of a palpable abdominal mass, new-onset varicocele, or lower extremity edema should always lead to additional imaging assessments. Moreover, at all stages RCC might produce multiple hormone-like or cytokine-like biologically active products that lead to clinically important paraneoplastic syndromes [97].
6.2. Diagnostic examinations
RCCs may arise as degeneration of a cystic lesion or completely de novo [98]. Periodic screening is fundamental because it can early detect any malignant transformation of cystic lesion and because untreated RCCs carry a poor prognosis [1]. US can easily distinguish a solid from a cystic lesion and can be used for surveillance purpose given its wide availability and lack of radiation exposure. RCCs can present as either multicentric and bilateral solid masses or complex cystic lesion with thick septa and mural nodules. Renal lesions are usually further explored with CT or MRI. CT has a higher sensitivity than US for small lesions (<2 cm) detection [17] and it is also useful when renal architecture has been distorted by multiple renal cysts [99]. At CT, cysts are hypodense lesion with little or no wall enhancement; solid nodules show vivid enhancement after contrast material administration [100]. On the other hand, solid RCCs tend to be heterogeneous with early avid enhancement and washout in the delayed phase. However, renal cysts (particularly if <1 cm) are not easily differentiated from small RCCs at CT, owing to the phenomenon of pseudo-enhancement [101].
MRI helps to solve this problem and has several advantages compared to CT. It has no radiation exposure (particularly important for young patient), could be used in patients with an allergy to iodinated contrast agents, and has a better accuracy for evaluating small renal lesion, even when contrast medium could not be used because of renal impairment [25]. Simple cysts show hypointense signal on T1WI and hyperintense signal on T2WI, without enhancement. Solid RCCs are usually hypointense on T1WIs; however, if there are areas of hemorrhage, they appear as T1 hyperintense foci. RCCs typically demonstrate T2 hyperintense signal and intense heterogeneous enhancement. Of note, even if characterized by presence of intracellular lipid, RCCs may not show signal loss in out-of-phase chemical shift MRI imaging sequences [5].
Regarding RCC, older studies and international guidelines suggest an initial screening based on abdomen MRI or CT [102], which should be repeated every 2 years until the largest solid neoplasm reaches 3 cm [98]. It is recommended to start renal mass screening at the age of 15 years using MRI scans and to continue the screening based on abdominal MRI every 2 years if no tumor is detected.
6.3. Indications for active treatments
In the case of detection of renal masses with dimension less than 3 cm, active surveillance evaluating tumor growth rate with MRI every 3–6 months for the first year is recommended. For renal masses bigger than 3 cm, nephron-sparing techniques are currently the gold standard in order to preserve renal function [103]. Patients with small tumors (3 cm is the commonly accepted threshold) who are at high peri-operative risk of complications and mortality can benefit from ablative techniques, including radiofrequency ablation and cryoablation [104]. Robust prior clinical researches are currently lacking. These voids must be filed with well-structured prospective trials.
6.4. Therapeutic options
To date, the treatment options after the diagnosis of RCC are being managed with active surveillance, multiple session of renal surgery, or ablative therapies since there are not any validated standardized protocols [96]. Patient mortality is mainly associated to renal failure, complications of dialysis or systemic progression, and cancer-specific mortality [105].
The different surgery strategies include radical nephrectomy, partial nephrectomy (nephron-sparing surgery), and ablative techniques. While radical nephrectomy implicates the removal of the whole kidney, in nephron-sparing surgeries only the renal mass is excised and as much of the normal renal parenchyma as possible is maintained. Observations regarding nephron-sparing surgery suggested oncological control similar to radical nephrectomy [106] but with the additional benefit of renal preservation [107]. These implications are of profound importance since VHL patients are usually managed with active surveillance, multiple session of renal surgery, or ablative therapies without any validated standardized protocol [96], and about one out of four patients who underwent multiple surgeries to the kidney will develop chronic kidney disease, which often is the key determinant of patients’ survivorship together with RCC progression [105].
In the setting of nephron-sparing surgery, image-guided ablative procedures with different possible approach (percutaneous or intraoperative) may play an important role in the management of VHL-related RCCs [108]. Many investigations reported up to a 100% 5-year cancer-specific survival rate for non-cystic RCCs measuring 2–3 cm [109]. The presence of major abdominal structure is a relative contraindication for ablative procedure, as cystic RCCs [109]. However, it should be noted that ablative procedure may have higher recurrence rates compared with surgery, and therefore, close monitoring is required [109].
In this scenario, systemic therapy has an experimental role only so far. In fact, current clinical research is focused on demonstrating the feasibility and the efficacy of systemic therapies for patients with VHL-related RCC, with the aim to possibly preserve kidney function and at least not reducing patient survival.
To date, a few trials have been published investigating the effect of molecules targeting different pathogenetic pathways. Firstly sunitinib, an oral tyrosine kinase inhibitor (TKI) which targets VEGF receptor, platelet-derived growth factor receptor, FMS-related receptor tyrosine kinase 3, and cKit, has been tested. A pilot study reported in 2011 evaluated its safety and efficacy in 15 patients with VHL [110]. Only six of 18 RCC lesions had at least a partial response with a mean decrease in volume of 14.4% by the end of the treatment. Another multicenter, phase II, open-label study investigated sunitinib in five VHL patients [111]. All five patients showed stable disease as best response at 6 months. Another TKI dovitinib has been tested in VHL patients. However, since all the six enrolled patients presented intolerable adverse events, the trial was stopped due to toxicity [112]. Pazopanib, another VEGF-TKI inhibitor, was tested in 37 patients with genetically confirmed or clinical features consistent with VHL syndrome [113]. Pazopanib had an objective response in 42% of patients as for Response Evaluation Criteria in Solid Tumors criteria: according to lesion sites responses were observed in 31 (53%) of 59 R CCs, 9 (53%) of 17 pancreatic lesions, and 2 (4%) of 49 CNS HBs. Another class of molecule that has been investigated is HIF-2α inhibitor. Recently, VHL patients were treated with 120 mg orally once daily of MK-6482 (belzutifan); overall response rate for clear cell RCC was 49% (95% confidence interval 36%−62%). Responses were also observed in patients with pancreatic lesions (77%) and CNS HBs (30%) [114].
Throughout their entire life, VHL patients undergo multiple surgical treatments to prevent systemic progression of clear cell RCC or to prevent, resolve, or even palliate symptoms caused by benign neoplasms (e.g., retinal and CNS HBs or pancreatic neuroendocrine tumors) [96]. To date, the determinants of VHL patients’ prognosis and survivorship are the multiple RCCs which most VHL patients eventually develop repeatedly over their life span. On the one hand, the almost inevitable consequence of multiple surgical treatments on the kidneys are renal failure and the consequent need for dialysis; on the other hand, if left untreated, RCCs are associated with dramatic systemic progression and cancer-specific mortality. The prevalence of chronic kidney disease in VHL patients is 16.5% before and 25.3% after renal treatment [105]. These proportions are very high given the young age of these patients. As such, the preservation of renal function, along with oncological safety, should be the most important goal to achieve in VHL patients undergoing kidney surgery, also given the high rate (46%) of patients diagnosed with low aggressive tumors (pT1a G1–2) [105].
7. Endolymphatic sac tumors and broad ligament cystadenomas
About 10%–16% of people with VHL syndrome develop endolymphatic sac tumors, and in some cases, the associated unilateral or bilateral hearing loss is the first sign of the illness [115]. Hearing loss usually develops suddenly, ranging in degree from severe to profound [116]. The presenting ailment is either vertigo or tinnitus. Individuals with endolymphatic sac tumors unrelated to VHL reported more severe hearing loss and higher tumor sizes at presentation compared to those with VHL-related endolymphatic sac tumors [117]. Other cranial nerves may be affected by large endolymphatic sac tumors. These tumors are rarely malignant [118]. A discussion of the potential side effect of total deafness must be initiated with the patient when deciding whether to remove these slow-growing tumors surgically. It has been demonstrated that early treatment of tiny tumors can preserve hearing and vestibular function [119].
Epididymal or papillary cystadenomas in men with VHL syndrome are relatively common. They rarely pose a concern, unless bilateral, in which case infertility could develop. A papillary cystadenoma of the wide ligament is the equivalent, far less frequent, lesion in women. Both tissues were presumably left behind after somatic VHL loss during development and have mesonephric origins [120]. Surgery is typically not necessary for epididymal or broad ligament papillary cyst adenomas unless they are symptomatic or endanger fertility [120].
8. Conclusions
In conclusion, VHL disease is one of the most frequent autosomal dominant syndromes leading to multiple tumors, among which the most important in terms of mortality and quality of life is clear cell RCC. To date, pre-clinical and clinical research on the subject is scarce and clinical guidelines are not supported by strong validation studies. Further studies are needed to develop precise clinical guidelines.
Author contributions
Study concept and design: Andrea Salonia.
Drafting of manuscript: Alessandro Larcher, Federico Belladelli, Giuseppe Fallara, Isaline Rowe, Umberto Capitanio, Laura Marandino, Daniele Raggi, Jody Filippo Capitanio, Michele Bailo, Rosangela Lattanzio, Costanza Barresi, Sonia Francesca Calloni, Maurizio Barbera, Valentina Andreasi, Giorgia Guazzarotti, Giovanni Pipitone, Paola Carrera.
Critical revision of the manuscript: Andrea Necchi, Pietro Mortini, Francesco Bandello, Andrea Falini, Stefano Partelli, Massimo Falconi, Francesco De Cobelli, Andrea Salonia.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Varshney N., Kebede A.A., Owusu-Dapaah H., Lather J., Kaushik M., Bhullar J.S. A review of von Hippel-Lindau syndrome. J Kidney Cancer VHL. 2017;4:20–29. doi: 10.15586/jkcvhl.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball M.W., An J.Y., Gomella P.T., Gautam R., Ricketts C.J., Vocke C.D., et al. Growth rates of genetically defined renal tumors: implications for active surveillance and intervention. J Clin Oncol. 2020;38:1146–1153. doi: 10.1200/JCO.19.02263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Considine B., Hurwitz M.E. Current status and future directions of immunotherapy in renal cell carcinoma. Curr Oncol Rep. 2019;21:34. doi: 10.1007/s11912-019-0779-1. [DOI] [PubMed] [Google Scholar]
- 4.Capitanio U., Montorsi F. Renal cancer. Lancet Lond Engl. 2016;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 5.Lonser R.R., Glenn G.M., Walther M., Chew E.Y., Libutti S.K., Linehan W.M., et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 6.Binderup M.L.M., Jensen A.M., Budtz-Jørgensen E., Bisgaard M.L. Survival and causes of death in patients with von Hippel-Lindau disease. J Med Genet. 2017;54:11–18. doi: 10.1136/jmedgenet-2016-104058. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B., Wang J., Liu S., Peng X., Hong B., Zhou J., et al. Hemangioblastoma instead of renal cell carcinoma plays a major role in the unfavorable overall survival of von Hippel-Lindau disease patients. Front Oncol. 2019;9:1037. doi: 10.3389/fonc.2019.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aksu G., Ulutin C., Fayda M., Saynak M. Cerebellar and multiple spinal hemangioblastomas and intraventricular meningioma managed with subtotal resection and external beam radiotherapy; report of a case with literature review. J BUON. 2005;10:405–409. [PubMed] [Google Scholar]
- 10.Neumann H.P., Eggert H.R., Weigel K., Friedburg H., Wiestler O.D., Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989;70:24–30. doi: 10.3171/jns.1989.70.1.0024. [DOI] [PubMed] [Google Scholar]
- 11.Wanebo J.E., Lonser R.R., Glenn G.M., Oldfield E.H. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 12.van der Horst-Schrivers A.N.A., Sluiter W.J., Kruizinga R.C., van Leeuwaarde R.S., Giles R., Olderode-Berends M.J.W., et al. The incidence of consecutive manifestations in von Hippel-Lindau disease. Fam Cancer. 2019;18:369–376. doi: 10.1007/s10689-019-00131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Zhou J., Li L., Yi Z., Lu R., Li C., et al. Intronic mutation of the VHL gene associated with central nervous system hemangioblastomas in two Chinese families with von Hippel-Lindau disease: case report. BMC Med Genet. 2020;21:191. doi: 10.1186/s12881-020-01126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesener M.S., Seyfarth M., Warnecke C., Jürgensen J.S., Rosenberger C., Morgan N.V., et al. Paraneoplastic erythrocytosis associated with an inactivating point mutation of the von Hippel-Lindau gene in a renal cell carcinoma. Blood. 2002;99:3562–3565. doi: 10.1182/blood.v99.10.3562. [DOI] [PubMed] [Google Scholar]
- 15.Capitanio J.F., Mazza E., Motta M., Mortini P., Reni M. Mechanisms, indications and results of salvage systemic therapy for sporadic and von Hippel-Lindau related hemangioblastomas of the central nervous system. Crit Rev Oncol Hematol. 2013;86:69–84. doi: 10.1016/j.critrevonc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Gläsker S., Berlis A., Pagenstecher A., Vougioukas V.I., Van Velthoven V. Characterization of hemangioblastomas of spinal nerves. Neurosurgery. 2005;56:503–509. doi: 10.1227/01.neu.0000153909.70381.c8. [DOI] [PubMed] [Google Scholar]
- 17.Choyke P.L., Glenn G.M., Walther M.M., Patronas N.J., Linehan W.M., Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194:629–642. doi: 10.1148/radiology.194.3.7862955. [DOI] [PubMed] [Google Scholar]
- 18.Haddad N.M.N., Cavallerano J.D., Silva P.S. Von Hippel-Lindau disease: a genetic and clinical review. Semin Ophthalmol. 2013;28:377–386. doi: 10.3109/08820538.2013.825281. [DOI] [PubMed] [Google Scholar]
- 19.Butman J.A., Linehan W.M., Lonser R.R. Neurologic manifestations of von Hippel-Lindau disease. JAMA. 2008;300:1334–1342. doi: 10.1001/jama.300.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills S.A., Oh M.C., Rutkowski M.J., Sughrue M.E., Barani I.J., Parsa A.T. Supratentorial hemangioblastoma: clinical features, prognosis, and predictive value of location for von Hippel-Lindau disease. Neuro Oncol. 2012;14:1097–1104. doi: 10.1093/neuonc/nos133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez R., Mastorakos P., Hogan E., Scott G., Lonser R.R., Wiley H.E., et al. Retrobulbar hemangioblastomas in von Hippel-Lindau disease: clinical course and management. Neurosurgery. 2021;88:1012–1020. doi: 10.1093/neuros/nyaa565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu B.C., Terae S., Hida K., Furukawa M., Abe S., Miyasaka K. MR findings in spinal hemangioblastoma: correlation with symptoms and with angiographic and surgical findings. AJNR Am J Neuroradiol. 2001;22:206–217. [PMC free article] [PubMed] [Google Scholar]
- 23.Blaty D., Malos M., Palmrose T., McGirr S. Sporadic intradural extramedullary hemangioblastoma of the cauda equina: case report and literature review. World Neurosurg. 2018;109:436–441. doi: 10.1016/j.wneu.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 24.Osborn A. Elsevier; London: 2016. Diagnostic imaging: brain; p. 593. [Google Scholar]
- 25.Apaydin M., Varer M., Oztekin O. Radiological considerations in von Hippel-Lindeau disease: imaging findings and the review of the literature. Radiol Oncol. 2010;44:164–167. doi: 10.2478/v10019-010-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan L., Wang E.M., Wang B.J., Zhou L.F., Zhang N., Cai P.W., et al. Gamma knife radiosurgery for hemangioblastomas. Stereotact Funct Neurosurg. 1998;70(Suppl. 1):179–186. doi: 10.1159/000056420. [DOI] [PubMed] [Google Scholar]
- 27.Ruppert M.D., Gavin M., Mitchell K.T., Peiris A.N. Ocular manifestations of von Hippel-Lindau disease. Cureus. 2019;11 doi: 10.7759/cureus.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karimi S., Arabi A., Shahraki T., Safi S. Von Hippel-Lindau disease and the eye. J Ophthalmic Vis Res. 2020;15:78–94. doi: 10.18502/jovr.v15i1.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atik S.Ş., Solmaz A.E., Öztaş Z., Eğrilmez E.D., Uğurlu Ş., Atik T., et al. Von Hippel-Lindau disease: the importance of retinal hemangioblastomas in diagnosis. Turk J Ophthalmol. 2017;47:180–183. doi: 10.4274/tjo.90912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher E.R., Neumann H.P.H., Richard S. Von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–623. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chew M.Y. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- 32.Webster A.R., Maher E.R., Moore A.T. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Arch Ophthalmol. 1999;117:371–378. doi: 10.1001/archopht.117.3.371. [DOI] [PubMed] [Google Scholar]
- 33.Wong W.T., Agrón E., Coleman H.R., Tran T., Reed G.F., Csaky K., et al. Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel-Lindau disease. Ophthalmology. 2008;115:181–188. doi: 10.1016/j.ophtha.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binderup M.L.M., Stendell A.S., Galanakis M., Møller H.U., Kiilgaard J.F., Bisgaard M.L. Retinal hemangioblastoma: prevalence, incidence and frequency of underlying von Hippel-Lindau disease. Br J Ophthalmol. 2018;102:942–947. doi: 10.1136/bjophthalmol-2017-310884. [DOI] [PubMed] [Google Scholar]
- 35.Grossniklaus H.E., Thomas J.W., Vigneswaran N., Jarrett W.H., 3rd Retinal hemangioblastoma. A histologic, immunohistochemical, and ultrastructural evaluation. Ophthalmology. 1992;99:140–145. doi: 10.1016/s0161-6420(92)32024-x. [DOI] [PubMed] [Google Scholar]
- 36.Annesley W.H.J., Leonard B.C., Shields J.A., Tasman W.S. Fifteen year review of treated cases of retinal angiomatosis. Trans Sect Ophthalmol Am Acad Ophtalmol Otolaryngol. 1977;83(3 Pt 1):OP446–OP453. PMID: 888257. [PubMed] [Google Scholar]
- 37.Chan C.C., Collins A.B.D., Chew E.Y. Molecular pathology of eyes with von Hippel-Lindau (VHL) disease: a review. Retina. 2007;27:1–7. doi: 10.1097/01.iae.0000244659.62202.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiley H.E., Krivosic V., Gaudric A., Gorin M.B., Shields C., Shields J., et al. Management of retinal hemangioblastoma in von Hippel-Lindau disease. Retina. 2019;39:2254–2263. doi: 10.1097/IAE.0000000000002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitson J.T., Welch R.B., Green W.R. Von Hippel-Lindau disease: case report of a patient with spontaneous regression of a retinal angioma. Retina. 1986;6:253–259. [PubMed] [Google Scholar]
- 40.Chen S., Chew E.Y., Chan C.C. Pathology characteristics of ocular von Hippel-Lindau disease with neovascularization of the iris and cornea: a case report. J Med Case Rep. 2015;9:66. doi: 10.1186/s13256-015-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansfield Smith S., Makam R., Sullivan L., Sandford R., Allen L. Is ultra wide-field retinal imaging alone appropriate for retinal angioma screening in lower risk subjects attending von Hippel-Lindau (VHL) clinics? Ophthalmic Genet. 2019;40:403–406. doi: 10.1080/13816810.2019.1678177. [DOI] [PubMed] [Google Scholar]
- 42.Chin E.K., Trikha R., Morse L.S., Zawadzki R.J., Werner J.S., Park S.S. Optical coherence tomography findings of exophytic retinal capillary hemangiomas of the posterior pole. Ophthalmic Surg Laser Imag. 2010:1–5. doi: 10.3928/15428877-20100215-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilotto E., Nacci E.B., Ferrara A.M., De Mojà G., Zovato S., Midena E. Macular perfusion impairment in von Hippel-Lindau disease suggests a generalized retinal vessel alteration. J Clin Med. 2020;9:2677. doi: 10.3390/jcm9082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Y., Wang J.C., Zeng R., Nagata T., Katz R., Mukai S., et al. Detection of retinal microvascular changes in von Hippel-Lindau disease using optical coherence tomography angiography. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krivosic V., Kamami-Levy C., Jacob J., Richard S., Tadayoni R., Gaudric A. Laser photocoagulation for peripheral retinal capillary hemangioblastoma in von Hippel-Lindau disease. Ophthalmol Retina. 2017;1:59–67. doi: 10.1016/j.oret.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Blodi C.F., Russell S.R., Pulido J.S., Folk J.C. Direct and feeder vessel photocoagulation of retinal angiomas with dye yellow laser. Ophthalmology. 1990;97:791–797. doi: 10.1016/s0161-6420(90)32509-5. [DOI] [PubMed] [Google Scholar]
- 47.Shields J.A. Response of retinal capillary hemangioma to cryotherapy. Arch Ophthalmol. 1993;111:551. doi: 10.1001/archopht.1993.01090040143049. [DOI] [PubMed] [Google Scholar]
- 48.Gaudric A., Krivosic V., Duguid G., Massin P., Giraud S., Richard S. Vitreoretinal surgery for severe retinal capillary hemangiomas in von Hippel-Lindau disease. Ophthalmology. 2011;118:142–149. doi: 10.1016/j.ophtha.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 49.Singh A.D., Nouri M., Shields C.L., Shields J.A., Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109:1799–1806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 50.Slim E., Antoun J., Kourie H.R., Schakkal A., Cherfan G. Intravitreal bevacizumab for retinal capillary hemangioblastoma: a case series and literature review. Can J Ophthalmol. 2014;49:450–457. doi: 10.1016/j.jcjo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal A., Kumari N., Singh R. Intravitreal bevacizumab and feeder vessel laser treatment for a posteriorly located retinal capillary hemangioma. Int Ophthalmol. 2016;36:747–750. doi: 10.1007/s10792-016-0183-x. [DOI] [PubMed] [Google Scholar]
- 52.Wong W.T., Liang K.J., Hammel K., Coleman H.R., Chew E.Y. Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease. Ophthalmology. 2008;115:1957–1964. doi: 10.1016/j.ophtha.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C., Tian Z., Lai K., Zhong X., Zhou L., Xu F., et al. Long-term therapeutic outcomes of photodynamic therapy-based or photocoagulation-based treatments on retinal capillary hemangioma. Photomed Laser Surg. 2018;36:10–17. doi: 10.1089/pho.2017.4296. [DOI] [PubMed] [Google Scholar]
- 54.Keutgen X.M., Hammel P., Choyke P.L., Libutti S.K., Jonasch E., Kebebew E. Evaluation and management of pancreatic lesions in patients with von Hippel-Lindau disease. Nat Rev Clin Oncol. 2016;13:537–549. doi: 10.1038/nrclinonc.2016.37. [DOI] [PubMed] [Google Scholar]
- 55.Sharma A., Mukewar S., Vege S.S. Clinical profile of pancreatic cystic lesions in von Hippel-Lindau disease: a series of 48 patients seen at a tertiary institution. Pancreas. 2017;46:948–952. doi: 10.1097/MPA.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 56.Howe J.R., Merchant N.B., Conrad C., Keutgen X.M., Hallet J., Drebin J.A., et al. The North American Neuroendocrine Tumor Society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas. 2020;49:1–33. doi: 10.1097/MPA.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hough D.M., Stephens D.H., Johnson C.D., Binkovitz L.A. Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol. 1994;162:1091–1094. doi: 10.2214/ajr.162.5.8165988. [DOI] [PubMed] [Google Scholar]
- 58.Hammel P.R., Vilgrain V., Terris B., Penfornis A., Sauvanet A., Correas J.M., et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 59.Laks S., van Leeuwaarde R., Patel D., Keutgen X.M., Hammel P., Nilubol N., et al. Management recommendations for pancreatic manifestations of von Hippel-Lindau disease. Cancer. 2022;128:435–446. doi: 10.1002/cncr.33978. [DOI] [PubMed] [Google Scholar]
- 60.Blansfield J.A., Choyke L., Morita S.Y., Choyke P.L., Pingpank J.F., Alexander H.R., et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs) Surgery. 2007;142:812–814. doi: 10.1016/j.surg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ganeshan D., Menias C.O., Pickhardt P.J., Sandrasegaran K., Lubner M.G., Ramalingam P., et al. Tumors in von Hippel-Lindau syndrome: from head to toe—comprehensive state-of-the-art review. Radiographics. 2018;38:849–866. doi: 10.1148/rg.2018170156. [DOI] [PubMed] [Google Scholar]
- 62.Tamura K., Nishimori I., Ito T., Yamasaki I., Igarashi H., Shuin T. Diagnosis and management of pancreatic neuroendocrine tumor in von Hippel-Lindau disease. World J Gastroenterol. 2010;16:4515–4518. doi: 10.3748/wjg.v16.i36.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karsdorp N., Elderson A., Wittebol-Post D., Hené R.J., Vos J., Feldberg M.A., et al. Von Hippel-Lindau disease: new strategies in early detection and treatment. Am J Med. 1994;97:158–168. doi: 10.1016/0002-9343(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 64.Hes F.J., Feldberg M.A. Von Hippel-Lindau disease: strategies in early detection (renal-, adrenal-, pancreatic masses) Eur Radiol. 1999;9:598–610. doi: 10.1007/s003300050717. [DOI] [PubMed] [Google Scholar]
- 65.Marcos H.B., Libutti S.K., Alexander H.R., Lubensky I.A., Bartlett D.L., Walther M.M., et al. Neuroendocrine tumors of the pancreas in von Hippel-Lindau disease: spectrum of appearances at CT and MR imaging with histopathologic comparison. Radiology. 2002;225:751–758. doi: 10.1148/radiol.2253011297. [DOI] [PubMed] [Google Scholar]
- 66.Taouli B., Ghouadni M., Corréas J.M., Hammel P., Couvelard A., Richard S., et al. Spectrum of abdominal imaging findings in von Hippel-Lindau disease. AJR Am J Roentgenol. 2003;181:1049–1054. doi: 10.2214/ajr.181.4.1811049. [DOI] [PubMed] [Google Scholar]
- 67.Satoh K., Sadowski S.M., Dieckmann W., Quezado M., Nilubol N., Kebebew E., et al. 18F-FDG PET/CT volumetric parameters are associated with tumor grade and metastasis in pancreatic neuroendocrine tumors in von Hippel-Lindau disease. Ann Surg Oncol. 2016;23(Suppl 5):714–721. doi: 10.1245/s10434-016-5541-4. [DOI] [PubMed] [Google Scholar]
- 68.Shell J., Tirosh A., Millo C., Sadowski S.M., Assadipour Y., Green P., et al. The utility of 68Gallium-DOTATATE PET/CT in the detection of von Hippel-Lindau disease associated tumors. Eur J Radiol. 2019;112:130–135. doi: 10.1016/j.ejrad.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kruizinga R.C., Sluiter W.J., de Vries E.G.E., Zonnenberg Bernard A., Lips C.J., van der Horst-Schrivers A.N.A., et al. Calculating optimal surveillance for detection of von Hippel-Lindau-related manifestations. Endocr Relat Cancer. 2014;21:63–71. doi: 10.1530/ERC-13-0308. [DOI] [PubMed] [Google Scholar]
- 70.Tirosh A., Journy N., Folio L.R., Lee C., Leite C., Yao J., et al. Cumulative radiation exposures from CT screening and surveillance strategies for von Hippel-Lindau-associated solid pancreatic tumors. Radiology. 2019;290:116–124. doi: 10.1148/radiol.2018180687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Mestier L., Gaujoux S., Cros J., Hentic O., Vullierme M.-P., Couvelard A., et al. Long-term prognosis of resected pancreatic neuroendocrine tumors in von Hippel-Lindau disease is favorable and not influenced by small tumors left in place. Ann Surg. 2015;262:384–388. doi: 10.1097/SLA.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 72.Medina D.C., Osorno R., Boutros C.N. Biliary and gastric drainage in advanced pancreatic serous cystadenoma and portal hypertension in von Hippel-Lindau syndrome. J Gastrointest Oncol. 2014;5:E50–E53. doi: 10.3978/j.issn.2078-6891.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Penitenti F., Landoni L., Scardoni M., Piredda M.L., Cingarlini S., Scarpa A., et al. Clinical presentation, genotype-phenotype correlations, and outcome of pancreatic neuroendocrine tumors in von Hippel-Lindau syndrome. Endocrine. 2021;74:180–187. doi: 10.1007/s12020-021-02752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krauss T., Ferrara A.M., Links T.P., Wellner U., Bancos I., Kvachenyuk A., et al. Preventive medicine of von Hippel-Lindau disease-associated pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2018;25:783–793. doi: 10.1530/ERC-18-0100. [DOI] [PubMed] [Google Scholar]
- 75.Tirosh A., Sadowski S.M., Linehan W.M., Libutti S.K., Patel D., Nilubol N., et al. Association of VHL genotype with pancreatic neuroendocrine tumor phenotype in patients with von Hippel-Lindau disease. JAMA Oncol. 2018;4:124–126. doi: 10.1001/jamaoncol.2017.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Partelli S., Bartsch D.K., Capdevila J., Chen J., Knigge U., Niederle B., et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 2017;105:255–265. doi: 10.1159/000464292. [DOI] [PubMed] [Google Scholar]
- 77.Pavel M., O'Toole D., Costa F., Capdevila J., Gross D., Kianmanesh R., et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 78.Jonasch E., McCutcheon I.E., Gombos D.S., Ahrar K., Perrier N.D., Liu D., et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol. 2018;19:1351–1359. doi: 10.1016/S1470-2045(18)30487-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metelo A.M., Noonan H., Iliopoulos O. HIF2α inhibitors for the treatment of VHL disease. Oncotarget. 2015;6:23036–23037. doi: 10.18632/oncotarget.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deeks E.D. Belzutifan: first approval. Drugs. 2021;81:1921–1927. doi: 10.1007/s40265-021-01606-x. [DOI] [PubMed] [Google Scholar]
- 81.Munakata M., Aihara A., Imai Y., Noshiro T., Ito S., Yoshinaga K. Altered sympathetic and vagal modulations of the cardiovascular system in patients with pheochromocytoma: their relations to orthostatic hypotension. Am J Hypertens. 1999;12:572–580. doi: 10.1016/s0895-7061(99)00026-6. [DOI] [PubMed] [Google Scholar]
- 82.Melmed S., Polonsky K.S., Larsen P.R., Kronenberg H.M. 12th ed. London: Elsevier; 2011. Williams textbook of endocrinology; p. 124. [Google Scholar]
- 83.Yu R., Nissen N.N., Bannykh S.I. Cardiac complications as initial manifestation of pheochromocytoma: frequency, outcome, and predictors. Endocr Pract. 2012;18:483–492. doi: 10.4158/EP11327.OR. [DOI] [PubMed] [Google Scholar]
- 84.Buffet A., Calsina B., Flores S., Giraud S., Lenglet M., Romanet P., et al. Germline mutations in the new E1’ cryptic exon of the VHL gene in patients with tumours of von Hippel-Lindau disease spectrum or with paraganglioma. J Med Genet. 2020;57:752–759. doi: 10.1136/jmedgenet-2019-106519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Northcutt B.G., Raman S.P., Long C., Oshmyansky A.R., Siegelman S.S., Fishman E.K., et al. MDCT of adrenal masses: can dual-phase enhancement patterns be used to differentiate adenoma and pheochromocytoma? AJR Am J Roentgenol. 2013;201:834–839. doi: 10.2214/AJR.12.9753. [DOI] [PubMed] [Google Scholar]
- 86.Schieda N., Alrashed A., Flood T.A., Samji K., Shabana W., McInnes M.D.F. Comparison of quantitative MRI and CT washout analysis for differentiation of adrenal pheochromocytoma from adrenal adenoma. AJR Am J Roentgenol. 2016;206:1141–1148. doi: 10.2214/AJR.15.15318. [DOI] [PubMed] [Google Scholar]
- 87.Blake M.A., Kalra M.K., Maher M.M., Sahani D.V., Sweeney A.T., Mueller P.R., et al. Pheochromocytoma: an imaging chameleon. Radiographics. 2004;24(Suppl. 1):S87–S99. doi: 10.1148/rg.24si045506. [DOI] [PubMed] [Google Scholar]
- 88.Druker H., Zelley K., McGee R.B., Scollon S.R., Kohlmann W.K., Schneider K.A., et al. Genetic counselor recommendations for cancer predisposition evaluation and surveillance in the pediatric oncology patient. Clin Cancer Res. 2017;23:e91–e97. doi: 10.1158/1078-0432.CCR-17-0834. [DOI] [PubMed] [Google Scholar]
- 89.Tsirlin A., Oo Y., Sharma R., Kansara A., Gliwa A., Banerji M.A. Pheochromocytoma: a review. Maturitas. 2014;77:229–238. doi: 10.1016/j.maturitas.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Mannelli M. Management and treatment of pheochromocytomas and paragangliomas. Ann N Y Acad Sci. 2006;1073:405–416. doi: 10.1196/annals.1353.044. [DOI] [PubMed] [Google Scholar]
- 91.Tauzin-Fin P., Sesay M., Gosse P., Ballanger P. Effects of perioperative alpha1 block on haemodynamic control during laparoscopic surgery for phaeochromocytoma. Br J Anaesth. 2004;92:512–517. doi: 10.1093/bja/aeh083. [DOI] [PubMed] [Google Scholar]
- 92.Shen W.T., Grogan R., Vriens M., Clark O.H., Duh Q.Y. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145:893–897. doi: 10.1001/archsurg.2010.159. [DOI] [PubMed] [Google Scholar]
- 93.Diner E.K., Franks M.E., Behari A., Linehan W.M., Walther M.M. Partial adrenalectomy: the national cancer institute experience. Urology. 2005;66:19–23. doi: 10.1016/j.urology.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Akiba M., Kodama T., Ito Y., Obara T., Fujimoto Y. Hypoglycemia induced by excessive rebound secretion of insulin after removal of pheochromocytoma. World J Surg. 1990;14:317–324. doi: 10.1007/BF01658514. [DOI] [PubMed] [Google Scholar]
- 95.Rednam S.P., Erez A., Druker H., Janeway K.A., Kamihara J., Kohlmann W.K., et al. Von Hippel-Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23:e68–e75. doi: 10.1158/1078-0432.CCR-17-0547. [DOI] [PubMed] [Google Scholar]
- 96.Maher E.R. Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management. World J Urol. 2018;36:1891–1898. doi: 10.1007/s00345-018-2288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sacco E., Pinto F., Sasso F., Racioppi M., Gulino G., Volpe A., et al. Paraneoplastic syndromes in patients with urological malignancies. Urol Int. 2009;83:1–11. doi: 10.1159/000224860. [DOI] [PubMed] [Google Scholar]
- 98.Choyke P.L., Glenn G.M., Walther M.M., Zbar B., Weiss G.H., Alexander R.B., et al. The natural history of renal lesions in von Hippel-Lindau disease: a serial CT study in 28 patients. AJR Am J Roentgenol. 1992;159:1229–1234. doi: 10.2214/ajr.159.6.1442389. [DOI] [PubMed] [Google Scholar]
- 99.Levine E., Lee K.R., Weigel J.W., Farber B. Computed tomography in the diagnosis of renal carcinoma complicating Hippel-Lindau syndrome. Radiology. 1979;130:703–706. doi: 10.1148/130.3.703. [DOI] [PubMed] [Google Scholar]
- 100.Choyke P.L., Glenn G.M., Walther M.M., Zbar B., Linehan W.M. Hereditary renal cancers. Radiology. 2003;226:33–46. doi: 10.1148/radiol.2261011296. [DOI] [PubMed] [Google Scholar]
- 101.Tappouni R., Kissane J., Sarwani N., Lehman E.B. Pseudoenhancement of renal cysts: influence of lesion size, lesion location, slice thickness, and number of MDCT detectors. AJR Am J Roentgenol. 2012;198:133–137. doi: 10.2214/AJR.10.6057. [DOI] [PubMed] [Google Scholar]
- 102.Patel J., Davenport M.S., Cohan R.H., Caoili E.M. Can established CT attenuation and washout criteria for adrenal adenoma accurately exclude pheochromocytoma? AJR Am J Roentgenol. 2013;201:122–127. doi: 10.2214/AJR.12.9620. [DOI] [PubMed] [Google Scholar]
- 103.Hamilton Z.A., Capitanio U., Lane B.R., Larcher A., Yim K., Dey S., et al. Should partial nephrectomy be considered “elective” in patients with stage 2 chronic kidney disease? A comparative analysis of functional and survival outcomes after radical and partial nephrectomy. World J Urol. 2019;37:2429–2437. doi: 10.1007/s00345-019-02650-9. [DOI] [PubMed] [Google Scholar]
- 104.Chahoud J., McGettigan M., Parikh N., Boris R.S., Iliopoulos O., Rathmell K.W., et al. Evaluation, diagnosis and surveillance of renal masses in the setting of VHL disease. World J Urol. 2021;39:2409–2415. doi: 10.1007/s00345-020-03441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Capitanio U., Rosiello G., Erdem S., Rowe I., Kara O., Roussel E., et al. Clinical, surgical, pathological and follow-up features of kidney cancer patients with von Hippel-Lindau syndrome: novel insights from a large consortium. World J Urol. 2021;39:2969–2975. doi: 10.1007/s00345-020-03574-5. [DOI] [PubMed] [Google Scholar]
- 106.Van Poppel H., Da Pozzo L., Albrecht W., Matveev V., Bono A., Borkowski A., et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 107.Scosyrev E., Messing E.M., Sylvester R., Campbell S., Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65:372–377. doi: 10.1016/j.eururo.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 108.De Cobelli F., Papa M., Panzeri M., Colombo M., Steidler S., Ambrosi A., et al. Percutaneous microwave ablation versus cryoablation in the treatment of T1a renal tumors. Cardiovasc Intervent Radiol. 2020;43:76–83. doi: 10.1007/s00270-019-02313-7. [DOI] [PubMed] [Google Scholar]
- 109.Matin S.F., Ahrar K., Wood C.G., Daniels M., Jonasch E. Patterns of intervention for renal lesions in von Hippel-Lindau disease. BJU Int. 2008;102:940–945. doi: 10.1111/j.1464-410X.2008.07718.x. [DOI] [PubMed] [Google Scholar]
- 110.Jonasch E., McCutcheon I.E., Waguespack S.G., Wen S., Davis D.W., Smith L.A., et al. Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Ann Oncol. 2011;22:2661–2666. doi: 10.1093/annonc/mdr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oudard S., Elaidi R., Brizard M., Rest C.L., Caillet V., Deveaux S., et al. Sunitinib for the treatment of benign and malignant neoplasms from von Hippel-Lindau disease: a single-arm, prospective phase II clinical study from the PREDIR group. Oncotarget. 2016;7:85306–85317. doi: 10.18632/oncotarget.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jonasch E. https://clinicaltrials.gov/ct2/show/results/NCT01266070?cond=Von+Hippel-Lindau+Disease&draw=2&rank=10
- 113.Jonasch E., McCutcheon I.E., Gombos D.S., Ahrar K., Perrier N.D., Liu D., et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol. 2018;19:1351–1359. doi: 10.1016/S1470-2045(18)30487-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jonasch E., Donskov F., Iliopoulos O., Rathmell W.K., Narayan V.K., Maughan B.L., et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med. 2021;385:2036–2046. doi: 10.1056/NEJMoa2103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Binderup M.L.M., Gimsing S., Kosteljanetz M., Thomsen C., Bisgaard M.L. Von Hippel-Lindau disease: deafness due to a non-MRI-visible endolymphatic sac tumor despite targeted screening. Int J Audiol. 2013;52:771–775. doi: 10.3109/14992027.2013.824117. [DOI] [PubMed] [Google Scholar]
- 116.Choo D., Shotland L., Mastroianni M., Glenn G., van Waes C., Linehan W.M., et al. Endolymphatic sac tumors in von Hippel-Lindau disease. J Neurosurg. 2004;100:480–487. doi: 10.3171/jns.2004.100.3.0480. [DOI] [PubMed] [Google Scholar]
- 117.Nevoux J., Nowak C., Vellin J.F., Lepajolec C., Sterkers O., Richard S., et al. Management of endolymphatic sac tumors: sporadic cases and von Hippel-Lindau disease. Otol Neurotol. 2014;35:899–904. doi: 10.1097/MAO.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 118.Muzumdar D.P., Goel A., Fattepurkar S., Goel N. Endolymphatic sac carcinoma of the right petrous bone in von Hippel-Lindau disease. J Clin Neurosci. 2006;13:471–474. doi: 10.1016/j.jocn.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 119.Friedman R.A., Hoa M., Brackmann D.E. Surgical management of endolymphatic sac tumors. J Neurol Surg B Skull Base. 2013;74:12–19. doi: 10.1055/s-0032-1329622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Leeuwaarde R.S., Ahmad S., Links T.P., Giles R.H. Seattle: University of Washington; 1993. Von Hippel-Lindau syndrome. GeneReviews®; p. 523.http://www.ncbi.nlm.nih.gov/books/NBK1463/ [Internet] [Google Scholar]