Abstract
Candida auris is emerging as a major global threat to human health. C. auris infections are associated with high mortality due to intrinsic multi-drug resistance. Currently, therapeutic options for the treatment of C. auris infections are rather limited. We aim to provide a comprehensive review of current strategies, drug candidates, and lead compounds in the discovery and development of novel therapeutic agents against C. auris. The drug resistance profiles and mechanisms are briefly summarized. The structures and activities of clinical candidates, drug combinations, antifungal chemosensitizers, repositioned drugs, new targets, and new types of compounds will be illustrated in detail, and perspectives for guiding future research will be provided. We hope that this review will be helpful to prompting the drug development process to combat this fungal pathogen.
Key words: Candida auris, Antifungal agents, Drug resistance, Virulence factors, Antifungal targets
Graphical abstract
Strategies for the discovery and development of novel antifungal agents against Candida auris infections, including investigational antifungal agents, antifungal chemosensitizers, drug repurposing, new targets, and new chemotypes.
1. Introduction
There are approximately 200 species in the genus Candida, and these are the main causal agents (e.g., Candida albicans) of worldwide invasive fungal infections (IFIs)1. Candida auris was first isolated in 2009 and since then has rapidly spread globally2. C. auris is characterized by a high level of multi-drug resistance and has emerged as a major and urgent healthcare threat3. C. auris infections have been reported in more than 45 countries and have caused serious hospital outbreaks, with crude mortality rates as high as 72%4. C. auris can be transmitted by direct or indirect contact5. Persistent skin colonization, environmental adaptation and contamination, and nosocomial transmission have contributed to the global pandemic of C. auris6.
C. auris is a member of the Candida haemulonii clade and is distantly related to common fungal pathogens such as C. albicans and Candida glabrata7,8. To date, the origins of C. auris are still largely unknown. Based on genetic studies using whole-genome sequencing (WGS), C. auris strains are classified into four major geographic clades, namely, clade I (the South Asian clade), clade II (the East Asian clade), clade III (the South African clade), and clade IV (the South American clade)9. Recently, a potential fifth clade of C. auris was isolated from Iran10. Considerable differences in genetics and phenotypes have been observed among C. auris strains from different clades.
The characteristics and mechanisms of C. auris infections slightly differ from those of other Candida species. The body sites of C. auris colonization mainly include skins, mucosa and gastrointestinal tract, overlapping with those of other Candida species, such as C. albicans, C. glabrata, Candida parapsilosis, and Candida tropicalis11, 12, 13. However, C. auris exhibits a stronger capacity for skin colonization than other Candida species. The number of colonized patients was 2–3 folds more than that of infected patients, which causes clonal inter- and intra-hospital transmission and healthcare-associated infections14, 15, 16. The colonization of C. auris on skin or other sites may not cause infections, which could possibly lead to the contamination of the nosocomial and healthcare environment and pose a risk on immunocompromised individuals12,17. Thus, guidelines on the prevention of spread of C. auris are much stricter than those of other Candida species. Routine screening on colonization sites of patients and medical staff and improved environmental decontamination may interrupt healthcare-associated transmission18. Chlorine-containing disinfectants and 2% chlorhexidine are currently used in clinical practice for environmental decontamination and skin decolonization, respectively19. Moreover, recent studies showed that C. auris may behave differently as other Candida species to induce innate immune responses20, 21, 22. C. auris tends to significantly reduce the innate immunoinflammatory response than C. albicans due to the thicker mannan layer of the cell wall21, facilitating its colonizations and infections in hosts.
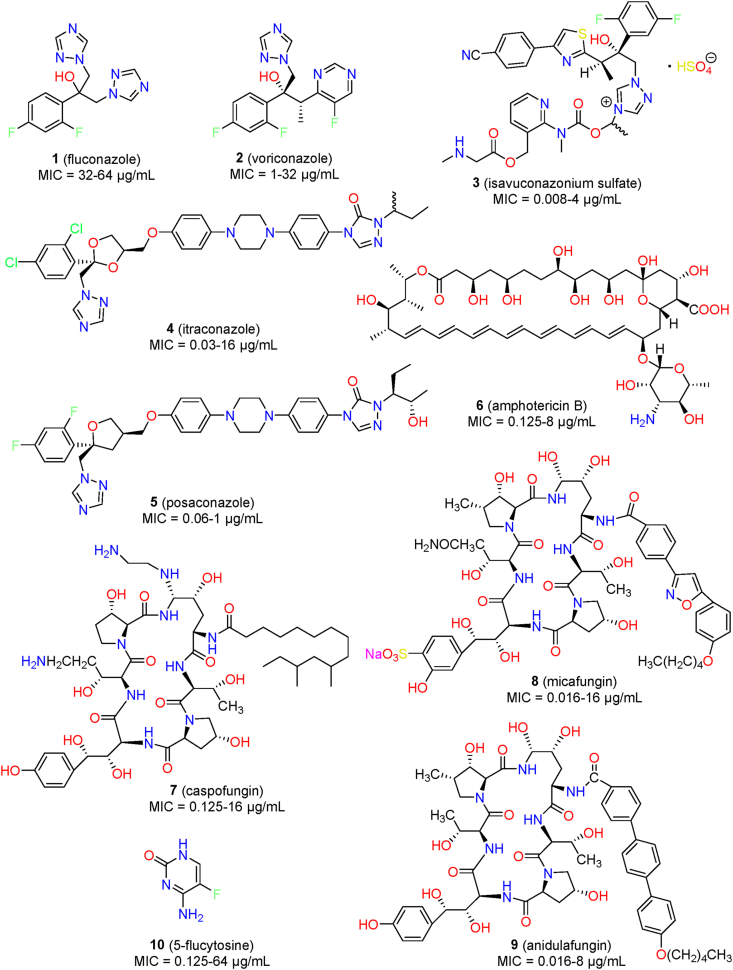
Unfortunately, therapeutic options for the treatment of C. auris infections are rather limited. Only three major classes of antifungal agents (Fig. 1), namely, azoles (1–5), polyenes (6), and echinocandins (7–9), are clinically available for the treatment of IFIs. Additionally, the nucleoside analogue 5-flucytosine (10) is generally used in adjunctive therapy. However, most C. auris strains were reported to be resistant to fluconazole (1), and multi-drug resistance has also observed against two, three, or even four classes of antifungal agents15. C. auris is the only Candida species in which several isolates have been identified to be resistant to all four classes of antifungal drugs11. The order of drug resistance is fluconazole > amphotericin B (6) > echinocandins23. Thus, echinocandins (e.g., caspofungin, 7) are commonly recommended as the first-line therapy for the treatment of C. auris infections24. Even so, several cases of deaths were reported for patients after the administration of echinocandins17,25. In addition to intrinsic resistance, rapid development of multidrug resistance has also been documented during antifungal treatments15. Thus, there is an urgent need to develop effective therapeutics to treat life-threatening and multi-drug resistant C. auris infections.
Figure 1.
Chemical structures of clinically available antifungal agents.
The biology, pathogenicity, epidemiology, resistance mechanisms and active compounds of C. auris have been reviewed previously3,26, 27, 28, 29, 30, 31, 32, 33, 34. Continuing our efforts in the discovery of novel antifungal agents against resistant fungal pathogens35,36, this review focuses on the small molecules and potential drug targets with which to tackle C. auris infections. After a brief introduction of resistance profiles and mechanisms, the activity of clinical candidates and drug combinations is discussed. Then, we provide a detailed illustration of drug discovery strategies and active lead compounds for combating C. auris infections, focusing on antifungal chemosensitizers, drug repurposing, new targets, and new chemotypes. Finally, perspectives for future research on drug development for this superbug fungal pathogen are provided.
2. Susceptibility of C. auris to antifungal agents
Several studies have investigated the susceptibility of C. auris to antifungal agents using different sets of isolates23,37. On the basis of a susceptibility test against 350 isolates collected in India, 90% of the isolates were resistant to fluconazole (MIC: 32–64 μg/mL); 8% were resistant to amphotericin B (MIC: 2 μg/mL), and 2% were resistant to echinocandins (MIC: 8 μg/mL)23. In another test of 296 C. auris isolates, a similar resistance trend was observed in which 80% of the strains were resistant to fluconazole, 23% to amphotericin B, and 7% to micafungin (8)37. Notably, 24% of the tested strains were resistant to at least two classes of antifungal agents, and 1% were resistant to all three of the classes37. The resistance profiles appeared to be clade-specific. For example, C. auris isolates in clade III were reported to be more resistant to fluconazole and voriconazole (2) than isolates in clade I. Newer azoles such as posaconazole (5, MIC range: 0.06–1 μg/mL) and isavuconazole (3, MIC range: 0.008–4 μg/mL) showed improved in vitro activity against C. auris11,38. Elevated MIC values were observed for the new azoles (e.g., voriconazole) compared with those against other Candida species39.
3. Resistance mechanisms of C. auris
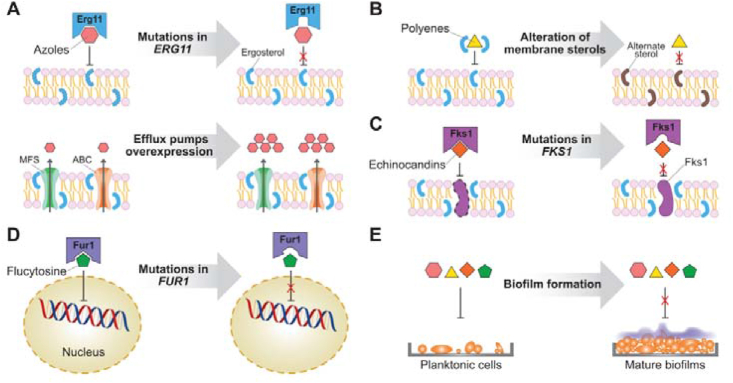
The antifungal drug resistance mechanisms of C. auris are similar to those observed in other Candida species, including overexpression or mutation of the drug target, overexpression of efflux pumps, reductions of drug intake, and biofilm formation (Fig. 2)32. Azole antifungal agents act by inhibiting lanosterol 14α-demethylase (CYP51, encoded by the ERG11 gene), a key enzyme in the biosynthesis of ergosterol of the fungal cell membrane. In C. auris strains resistant to azoles, no significant overexpression of the ERG11 gene was observed, and substitution mutations in CYP51 were generally clade-dependent: F126T (clade III), Y132F (clade IV), and Y132F or K143R (clade I)9,23,37,40,41. Higher expression of multidrug efflux pumps was also involved in decreased susceptibility of C. auris to azoles42. The ATP-binding cassette (ABC) family and the major facilitator superfamily (MFS) are two major transporters associated with antifungal resistance43 that are conserved in C. auris9. Increased expression of the CDR1 gene of the ABC transporter and the MDR1 gene of the MFS transporter contributed to the azole resistance of C. auris42,44,45.
Figure 2.
Resistance mechanisms to antifungal agents in C. auris. Mutation of targets, overexpression of efflux pumps, and alteration of membrane components are associated with the resistance of C. auris to azoles (A), polyenes (B), echinocandins (C), and flucytosine (D). The formation of biofilms is a general mechanism of antifungal resistance (E).
Amphotericin B exerts fungicidal activity by binding to ergosterol in fungal cell membranes and thereby altering the membrane permeability, resulting in the leakage of vital cytoplasmic components. Overexpression of genes involved in ergosterol biosynthesis, such as ERG1, ERG2, ERG6, and ERG13, was reported to be related to amphotericin B resistance in C. auris strains9. Although significant mutation for amphotericin B resistance is rare, a point mutation in transcription factor FLO8 has been observed in a resistant C. auris isolate46.
Echinocandins act on the fungal cell wall via inhibition of 1,3-β-glucan synthase (encoded by the FKS1 gene). In C. auris, FKS1 substitution mutations S639F, S639P, S639Y, and S652Y were responsible for echinocandins resistance23,47,48. The compound 5-flucytosine inhibits fungal DNA and RNA synthesis and is activated in fungal cells by Fur1. In C. auris, a substitution mutation F211I in the FUR1 gene was detected in an isolate resistant to 5-flucytosine41.
The increased expression of ABC and MFS transporters also contributes to the formation of biofilms that are highly resistant to antifungal agents49. Most antifungal agents, such as fluconazole, voriconazole, and amphotericin B, showed higher MIC values against C. auris biofilms than against planktonic cells50. Although planktonic cells are susceptible to echinocandins, these compounds are ineffective against biofilms50. Similar to other Candida species, C. auris is able to form biofilms that are largely composed of mannan polysaccharides and glucan50,51. C. auris formed significantly less biofilm than C. albicans with a limited amount of extracellular matrix52. C. auris seems to be unable to form true hyphae, and its biofilms consist largely of yeast cells50,53. The phenotypic, biochemical, and functional features of C. auris biofilms seem to be clade- or strain-specific. Differences in the extent of biofilm formation were observed among various C. auris isolates. Compared with C. albicans, C. auris formed more consistent biofilms in colonization models, suggesting higher virulence and resistance54.
4. Investigational antifungal agents for the treatment of C. auris infections
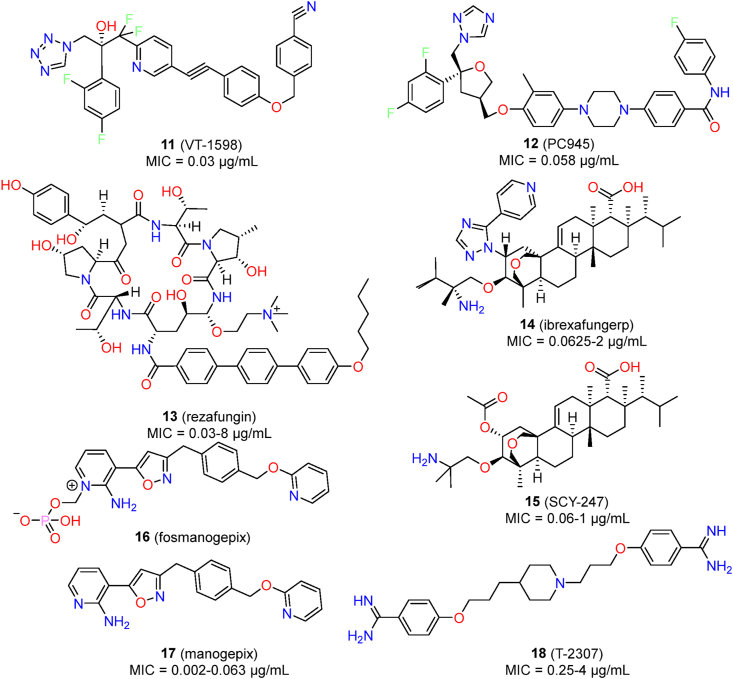
Currently, several new antifungals have entered into the clinical research, including VT-1598 (11), PC945 (12), rezafungin (13), ibrexafungerp (14), SCY-247 (15), fosmanogepix (16), manogepix (17) and T-2307 (18), which have demonstrated promising results against C. auris (Fig. 3). Herein the in vitro and in vivo anti-C. auris activities of these investigated antifungal agents are discussed. The antifungal assays and expression levels of activity are summarized in Table 1.
Figure 3.
Chemical structures of investigated antifungal agents for the treatment of C. auris infections.
Table 1.
Assays and expression of the activity for the research and development of novel antifungal agents against C. auris.
| Activity | Assay | Ref. |
|---|---|---|
| In vitro susceptibilitya | Clinical and Laboratory Standards Institute (CLSI) | 55 |
| European Committee on Antimicrobial Susceptibility Testing (EUCAST) | 55 | |
| Synergistic activityb | Fractional inhibitory concentration index (FICI) | 56 |
| Bliss independence model | 57 | |
| Biofilm inhibitionc | Inhibition of biofilm formation: XTT reduction assay | 50 |
| In vivo potencyd | Caenorhabditis elegans infections model (preliminary screen) | 58 |
| Galleria mellonella infections model (preliminary screen) | 59 | |
| C. auris candidemia mouse model: survival curve, reduction of fungal burden (log10 CFU/g) and ED50 | 60 | |
| Pharmacokinetic/pharmacodynamic (PK/PD) index | 61 | |
| Guinea pig cutaneous infections model | 62 |
In vitro activity was generally expressed by minimum inhibitory concentration (MIC); MIC50, MIC80, MIC90: the lowest concentration inhibiting fungal growth by 50%, 80%, and 90%, respectively; geometric mean (GM) MIC, mode MIC; MFC: minimum fungicidal concentration.
FICI < 0.5: synergism; 0.5 ≤ FICI ≤4; FICI >4: antagonism.
Sessile MIC (SMIC50): the concentration inhibiting 50% of biofilm formation; XTT: 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
ED50: doses required to produce 50% of the maximal effect; PK/PD index: AUC (concentration–time curve)/MIC value.
4.1. New CYP51 inhibitors: Triazole antifungal agents VT-1598 and PC945
VT-1598, a tetrazole-based fungal CYP51 inhibitor, has entered the clinical evaluation63. Currently, phase I clinical trial of VT-1598 has been completed and no more clinical trial is ongoing. Compared with traditional triazole antifungal agents, VT-1598 showed better selectivity between fungal CYP51 and mammalian cytochrome P450 enzymes, resulting in reduced drug–drug interactions64. VT-1598 demonstrated potent in vitro activity against a collection of 100 C. auris isolates (MIC range: 0.03 μg/mL; MIC50 = 0.25 μg/mL; MIC90 = 1 μg/mL)65. VT-1598 also showed dose-dependent in vivo efficacy in a neutropenic murine model of C. auris infections. At the doses of 15 and 50 mg/kg (once daily), oral VT-1598 treatment achieved significant improvement in survival, with median survival of 15 days and >21 days, respectively65. Moreover, VT-1598 also significantly reduced kidney and brain fungal burdens, suggesting that VT-1598 deserved further evaluation as a potential option for treating C. auris infections.
PC945 is a novel triazole antifungal derivative designed for inhaled administration of Aspergillus fumigatus infections66. PC945 also showed excellent antifungal activity against a collection of 50 C. auris clinical isolates, with GM MIC, MIC50 and MIC90 values of 0.058, 0.063, and 0.25 μg/mL, respectively67. PC945 also completely inhibited C. auris growth, with GM MIC and MIC90 values of 0.16 and 0.5 μg/mL, respectively. Notably, PC945 showed better anti-C. auris activity than fluconazole, voriconazole, and posaconazole.
4.2. New glucan synthase inhibitors: Rezafungin and ibrexafungerp
Rezafungin (CD101), an optimized echinocandin derivative, is currently under clinical development68. Compared with marketed echinocandin-like antifungal agents (e.g., caspofungin and micafungin), rezafungin possessed a better safety profile and improved pharmacokinetic properties such as an longer half time (t1/2 > 130 h) and higher plasma drug exposure, enabling once-weekly intravenous therapy69. Several studies have confirmed that rezafungin had excellent in vitro and in vivo activities against C. auris infections70, 71, 72, 73, 74, 75. In a susceptibility assay of a collection of 100 C. auris isolates, the MIC values of rezafungin ranged from 0.03 to 8 μg/mL70. The MIC50 and MIC90 values were 0.125 and 0.5 μg/mL, respectively70. Similar in vitro activity was observed in a test of rezafungin against 122 Indian C. auris isolates (MIC range: 0.016–16 μg/mL; MIC50 = 0.25 μg/mL; MIC90 = 1 μg/mL)73. In a mouse model of disseminated C. auris infections, rezafungin (20 mg/kg ip) showed potent in vivo efficacy and effectively reduced the fungal burden71. In particular, rezafungin showed superior activity compared to amphotericin B and micafungin, even with less frequent dosing71. The PK/PD advantage of rezafungin was further validated in a C. auris neutropenic mouse model72. The PK/PD index of rezafungin suggested that the clinically evaluated dose (400 mg, iv, once a week) may be a useful option to treat patients infected with C. auris infections, although further clinical trials are warranted72.
Ibrexafungerp (SCY-078) is an orally active inhibitor of glucan synthase that exhibited in vitro and in vivo inhibitory activity against Candida species, including echinocandin-resistant isolates76. Ibrexafungerp differs from echinocandin-like glucan synthase inhibitors in that it can be administrated both orally and intravenously, and it is active against the most common mutations of the target gene FKS77. A susceptibility assay indicated that the MIC values of ibrexafungerp ranged from 0.0625 to 2 μg/mL against a collection of 100 C. auris isolates, with MIC50 and MIC90 values of 0.5 and 1 μg/mL, respectively78,79. Furthermore, ibrexafungerp showed similar MIC values against C. auris isolates resistant to echinocandin antifungal agents78. Larkin et al.52 and Arendrup et al.79 reported similar MIC results for ibrexafungerp. Ibrexafungerp was able to completely inhibit the growth of C. auris, with an MIC90 value of 1 μg/mL52. Moreover, ibrexafungerp interrupted cell division of C. auris and inhibited biofilm formation (0.5–4 μg/mL) by reducing metabolic activity and biofilm thickness52. In a neutropenic murine model of C. auris infections, oral treatment with ibrexafungerp (20, 30, and 40 mg/kg, twice daily) resulted in dose-dependent improvements of survival and reductions in fungal burden, while caspofungin showed similar potency, and fluconazole was ineffective80. In an in vivo guinea pig cutaneous model of C. auris infections, oral dosing with ibrexafungerp (10 mg/kg) was effective in controlling skin infections and significantly reduced the fungal burden and the severity of lesions62. Ibrexafungerp is currently in phase II open-label clinical trials to evaluate efficacy and safety in patients infected with C. auris (identifier: NCT03363841). In an emergency-use phase III clinical trial, ibrexafungerp therapy successfully cured two patients without drug-related adverse events, highlighting its potential for further clinical evaluation81.
SCY-247 is an analogue of ibrexafungerp that showed broad-spectrum antifungal activity and an excellent safety profile, and it is suitable for both intravenous and oral administration82. Ghannoum’s group83 compared in vitro anti-C. auris activity between SCY-247 and ibrexafungerp. In a panel of 44 C. auris isolates, SCY-247 (MIC range: 0.06–1 μg/mL, MIC50 = 0.5 μg/mL, MIC90 = 0.5 μg/mL) showed similar MIC values to ibrexafungerp (MIC range: 0.06–2 μg/mL, MIC50 = 0.5 μg/mL, MIC90 = 0.5 μg/mL)83. The fungicidal activity of SCY-247 (MFC90 = 4 μg/mL) was slightly better than that of ibrexafungerp (MFC90 = 8 μg/mL)83. The in vivo potency of SCY-247 against C. auris infections has not been reported. However, SCY-247 (40 mg/kg) exhibited a 100% survival rate in a murine model of disseminated infections of C. albicans83, suggesting that the efficacy of SCY-247 to treat C. auris deserves further evaluation.
4.3. Fungal cell wall Gwt1 inhibitor: Manogepix
Manogepix (APX001A) is an inhibitor of fungal Gwt1 (glycosylphosphatidylinositol-anchored wall transfer protein 1) that showed broad-spectrum antifungal activity84. Fosmanogepix (APX001), the prodrug of manogepix, is currently being evaluated in clinical trials to treat various fungal infections85. The efficacy of APX001A to treat C. auris infections has been well characterized61,86, 87, 88, 89, 90, 91. Hager et al.88 determined the inhibitory activity of APX001A against 16 C. auris clinical strains. APX001A had MIC values in the range of 0.002–0.063 μg/mL (MIC50 = 0.004 μg/mL; MIC90 = 0.031 μg/mL), values that demonstrated greater potency than 10 tested antifungal agents88. The excellent activity of APX001A was further confirmed in a large collection of C. auris containing 100 geographically distinct isolates87. The MIC values ranged from <0.005 to 0.015 μg/mL, and the MIC50 and MIC90 values were 0.002 and 0.008 μg/mL, respectively87. Zhu et al.90 evaluated the in vitro inhibitory activity of APX001A against 200 New York C. auris isolates. APX001A demonstrated lower MIC values (MIC range: 0.004–0.06 μg/mL; MIC50 = 0.03 μg/mL; MIC90 = 0.03 μg/mL) than 10 clinical antifungal agents90. APX001 also showed potent in vivo efficacy to treat C. auris infections and was more effective than caspofungin and anidulafungin (9)61,88,89. In a murine model of disseminated C. auris infections, APX001 effectively prolonged the survival time of the treated mice (100% survival, 78 mg/kg TID) and significantly reduced the fungal burden of kidney, lung, and brain88. In a pharmacokinetics (PK) and pharmacodynamics (PD) study of APX001, the ED50 (50% of the maximum effect) to treat C. auris infections was 77 mg/kg61. Even delayed therapy with fosmanogepix showed good potency, significantly reducing the kidney fungal burden at the dose of 260 mg/kg (BID)89. These in vitro and in vivo data supported further clinical evaluation of fosmanogepix as an anti-C. auris agent. An open-label clinical study of APX001 for the treatment of patients with candidemia caused by C. auris was started in 2019 (identifier: NC-T04148287). Although the trial was terminated due to the impact of COVID-19, the objectives of the study were successfully met. However, the clinical data have not been disclosed to date.
4.4. Fungal mitochondria modulator: T-2307
T-2307 is an antifungal agent currently under clinical development for the treatment of IFIs92,93. T-2307 acts by targeting respiratory chain enzymatic complexes III and IV and selectively disrupting yeast mitochondrial function, leading to the collapse of the mitochondrial membrane potential94. In vitro activity of T-3207 against 23 C. auris isolates revealed that the MIC values ranged from ≤0.008 μg/mL to 0.015 μg/mL using 50% inhibition as the endpoint95. The MIC values were clearly higher when 100% inhibition was used as the endpoint (0.25 μg/mL to 4 > μg/mL)95. Overall, the geometric mean MIC of T-2307 (0.011 μg/mL) was significantly lower than those of fluconazole (14.6 μg/mL) and caspofungin (0.24 μg/mL). In a neutropenic mouse model with C. auris, treatment with T-2307 (3 mg/kg, subcutaneous, once daily) significantly improved median (21 days) and percent of survival (70%)95. T-2307 (3 mg/kg) also effectively reduced kidney and brain fungal burden, and the effect was more potent than with caspofungin (10 mg/kg).
5. Synergistic drug combinations to treat C. auris
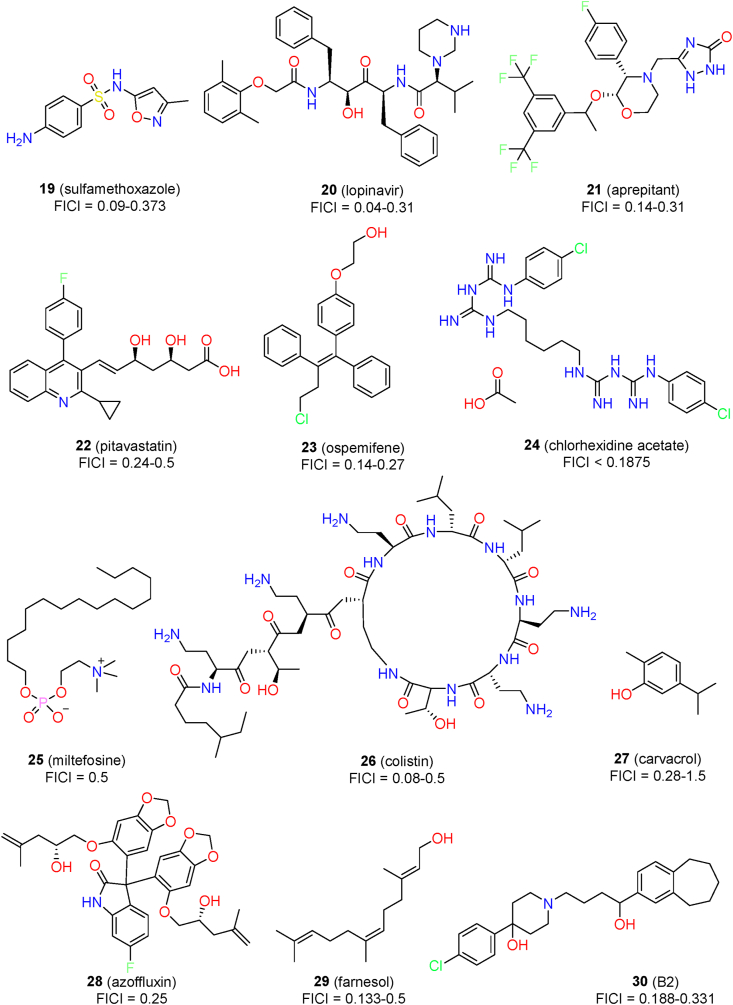
Due to limited therapeutic options for C. auris, the identification of effective drug combinations provides an alternative for clinical treatment (Fig. 4). Taking advantage of different mechanisms of action, such drug combinations are expected to achieve synergistic effects, thus increasing therapeutic efficacy and overcoming the resistance of C. auris to antifungal agents. Currently, more than 100 drug combinations have been evaluated for synergistic effects against C. auris infections96. The combinations have included antifungal agents, non-antifungal agents, and bioactive compounds (synthetic molecules and natural products).
Figure 4.
Chemical structures of marketed drugs and chemosensitizers for the combination treatment of C. auris infections.
5.1. Combinations of antifungal agents
Among the combinations of antifungal agents, voriconazole with micafungin, flucytosine with amphotericin B, and flucytosine with micafungin have shown synergistic effects regarding inhibition of the growth of C. auris (Table 2). Fakhim et al.56 evaluated the synergism between echinocandins and azoles against 10 multidrug-resistant C. auris clinical isolates. Synergistic effects were observed for the combination of micafungin and voriconazole against all of the tested isolates (FICI range: 0.15–0.5)56. Another study systematically evaluated 864 antifungal drug combinations against 15 C. auris isolates97. Flucytosine (1.0 μg/mL) was able to potentiate the activity of other antifungal agents, including azoles, echinocandins, and amphotericin B97. However, in another study by Bidaud et al.98, indifferent interactions between flucytosine and other antifungal agents were observed. Thus, the therapeutic effects of drug combinations remain to be further evaluated by in vivo studies.
Table 2.
Synergistic combinations of antifungal drugs against C. auris.
| Drug | Antifungal drug | FICI | Isolatea | Ref. |
|---|---|---|---|---|
| Antifungal drug | ||||
| Micafungin | Voriconazole | 0.15–0.5 | 10/10 | 56 |
| Anidulafungin | Isavuconazole | 0.25–0.38 | 11/36 | 38 |
| Voriconazole | 0.25–0.38 | 5/36 | 38 | |
| Amphotericin B | Caspofungin | NDb | 6/6 | 100 |
| Anidulafungin | ND | 6/6 | 100 | |
| Non-antifungal drug | ||||
| Sulfamethoxazole | Fluconazole | 0.156 | 1/1 | 101 |
| Voriconazole | 0.09–0.5 | 3/8 | 101 | |
| Itraconazole | 0.186–0.373 | 3/4 | 101 | |
| Lopinavir | Itraconazole | 0.04–0.09 | 10/10 | 58 |
| Voriconazole | 0.19–0.31 | 6/10 | 58 | |
| Fluconazole | 0.13–0.31 | 3/10 | 58 | |
| Aprepitant | Itraconazole | 0.14–0.31 | 8/10 | 102 |
| Pitavastatin | Fluconazole | 0.25–0.5 | 5/5 | 103 |
| Ospemifene | Itraconazole | 0.14–0.27 | 5/5 | 104 |
| Chlorhexidine acetate | Fluconazole | <0.1875c | 2/2 | 105 |
| Miltefosine | Amphotericin B | 0.5 | 3/12 | 106 |
| Colistin | Caspofungin | 0.08–0.14 | 15/15 | 107 |
| Isavuconazole | 0.3125–0.5 | 15/15 | 107 | |
| Chemosensitizer | ||||
| Azoffluxin | Fluconazole | 0.25 | 1/1 | 108 |
| Carvacrol | Amphotericin B | 0.28–1.5 | 7/25 | 109 |
| Farnesol | Caspofungin | 0.156–0.5 | 3/4 | 110 |
| Micafungin | 0.133–0.281 | 4/4 | 110 | |
| Anidulafungin | 0.14–0.375 | 4/4 | 110 | |
| B2 | Itraconazole | 0.188 | 1/3 | 111 |
| Voriconazole | 0.311 | 1/3 | 111 | |
Number of isolates: active isolates/tested isolates.
ND: not determined.
Synergistic effect against biofilm inhibition.
More recently, the synergistic activity of isavuconazole and voriconazole in combination with anidulafungin was evaluated against a collection of 36 C. auris isolates38. The isavuconazo-le–anidulafungin combination (active in 11/36 isolates) showed stronger synergistic effects than the voriconazole–anidulafungin combination (active in 5/36 isolates). Similar synergistic interactions between isavuconazole and echinocandin-like antifungal agents were also observed in an assay with six clinical C. auris isolates99. In addition to the synergistic effects against planktonic isolates, isavuconazole was also able to potentiate the activity of caspofungin in inhibiting the biofilm formation of C. auris (FICI range: 0.023–0.5, 12/14 sessile isolates)57. In a mouse infections model of C. auris, single uses of caspofungin (1 mg/kg, daily) or isavuconazole (20 mg/kg, daily) were statistically ineffective. When used in combination, the kidney fungal burden was significantly decreased by more than three log volumes57. Thus, isavuconazole had direct and synergistic activity against C. auris, providing a promising option for further evaluation.
In a time–kill curve assay against six C. auris isolates, monotherapy with echinocandins (anidulafungin and caspofungin) was ineffective, while high concentrations of amphotericin B (≥1 μg/mL) only showed fungistatic activity100. When used in combination, higher fungal killing activity was observed100. Lower doses of amphotericin B (0.5 mg/L) and anidulafungin or caspofungin (2 mg/L) achieved rapid synergism with potent fungicidal activity100.
5.2. Combinations of antifungal agents and non-antifungal agents
Seleem’s group performed systemic screening of known drugs and identified several synergists that were able to potentiate the activity of antifungal agents against C. auris58,101, 102, 103, 104. Inspired by the effects of sulfa antibacterial drugs to reverse azole resistance against C. alibicans112, the sulfa drugs were also confirmed to possess synergistic activity with azole antifungals to inhibit the growth of C. auris isolates101. Among them, sulfamethoxazole (19) exhibited the best synergistic activity with voriconazole (FICI range: 0.09–0.5) and itraconazole (4, FICI range: 0.186–0.373)101. Sulfamethoxazole alone was inactive against C. auris (MIC ≥256 μg/mL). When sulfamethoxazole (128 μg/mL) was used in combination with voriconazole (0.5 μg/mL), the survival of the infected nematodes was prolonged by about 70% in an in vivo model using C. elegans. The underlying mechanisms of the synergism was possibly associated with interference with the expression of the target protein CYP51 and the fungal folate pathway101.
After screening 1547 compounds, Seleem’s group found that the antiviral agent lopinavir (20) was a potent chemosensitizer that could potentiate the activity of fluconazole against resistant C. auris isolates58. At a therapeutically acceptably concentration (10 μg/mL), lopinavir showed good synergistic effects with fluconazole, voriconazole, and itraconazole (Table 2). The strongest synergism was observed between lopinavir and itraconazole (FICI range: 0.04–0.09). The drug combination also showed good in vivo efficacy in a C. auris-infected model using C. elegans, improving the survival rate by 90% and reducing the fungal burden by 88.5%. The mechanism of the synergism was investigated by comparative transcriptomic analysis. The drug combination may act by interfering with the expression of several transporters that are related to glucose permeation and drug efflux58.
The same group identified aprepitant (21, an antiemetic agent) as a potent synergist of itraconazole by assaying the azole chemosensitizing activity of a compound library containing about 1600 FDA-approved drugs102. Aprepitant was able to reduce the MIC value of itraconazole by up to eight-fold against C. auris (FICI range: 0.14–0.31). The drug combination was fungicidal and significantly inhibited biofilm formation (95%) and mature biofilms (52%). The combination of aprepitant and itraconazole also showed in vivo activity in a C. elegans infections model, significantly prolonging the survival rate by ∼90% and reducing the fungal burden by ∼92%. The mechanism of synergistic effects was associated with interfering with metal ion homeostasis and the ROS detoxification ability of C. auris102.
In a screen of synergists against azole-resistant C. albicans, the pitavastatin (22)-fluconazole combination was identified to have broad-spectrum synergistic activity103. In particular, pitavastatin displayed potent fluconazole chemosensitizing activity against 5 C. auris isolates (FICI range: 0.25–0.5). The combination of pitavastatin-fluconazole effectively inhibited the biofilm-forming abilities and reduced the CFU burden by 14%–92% in an in vivo C. elegans model with C. auris103. The mechanism of synergism was associated with interference with the efflux machinery.
Eldesouky et al.104 assayed nine stilbene compounds for their synergistic activity with azole drugs against azole-resistant fungal isolates. The ospemifene (23)‒itraconazole combination displayed the most potent chemosensitizing activity against a variety of fungal pathogens, including C. auris (FICI range: 0.14–0.27). The drug combination reduced C. auris CFU burden by 96% in a C. elegans infections model. Ospemifene exerted synergistic activity by directly interfering with fungal efflux systems such as ABC and MFS transporters and facilitating the entry of azoles into fungal cells.
Chlorhexidine acetate (24) is a broad-spectrum antibacterial agent. When used alone, its MIC80 values against C. auris isolates CBS12373 and CBS10913 were 8 and 2 μg/mL, respectively105. When chlorhexidine acetate was used in combination with fluconazole, significant synergism was observed in the growth curve assay105. In particular, the drug combination showed strong synergism against the biofilm formation of C. auris strains (FICI <0.1875).
The antileishmanial drug miltefosine (25) possessed both in vitro and in vivo antifungal activities, with an MIC value of 2 μg/mL against 12 C. auris isolates106. When used in combination with amphotericin B, miltefosine showed marginal synergistic effects against 3 out of 12 isolates (FICI = 0.5)106. In contrast, indifferent interaction was observed for the miltefosine and fluconazole combination against all of the tested isolates.
Colistin (26), an antibiotic, had synergistic activity with caspofungin against several azole-resistant Candida spp113. For C. auris, colistin used alone was totally ineffective (MIC >64 μg/mL)107. Synergistic activities were observed for the combination of colistin and caspofungin, with FICI values in the range of 0.08 to 0.14107. In contrast, the combination of colistin with micafungin showed indifferent interactions (FICI range: 0.51–1.01). Colistin also had in vitro synergistic interactions with amphotericin B (FICI range: 0.1563–0.375)114 and isavuconazole (FICI range: 0.3125–0.5)115 against C. auris strains.
5.3. Chemosensitizers potentiating the activity of antifungal agents
Cowen’s group108 screened a diverse chemical library and identified azoffluxin (28, a bis-benzodioxolylindolinone derivative) as an effective synergist with fluconazole against C. auris. Azoffluxin exerted species-selective synergistic activity against C. auris that reduced the MIC value of fluconazole more than eight-fold (FICI = 0.25). The synergistic activity was also observed in C. auris clades I, II, and IV, whereas azoffluxin was ineffective against clade III. In a mouse infections model of systemic C. auris, azoffluxin (10 mg/kg, subcutaneously, four times daily) significantly enhanced fluconazole (32 mg/kg, intraperitoneally, twice daily) activity in reducing the fungal burden. Unexpectedly, azoffluxin alone also reduced the fungal burden despite it showing no in vitro inhibitory activity against C. auris growth. Further mechanistic studies revealed that the inhibition of the efflux pump Cdr1 was associated with the potency of azoffluxin. Thus, Cdr1 may be an effective target for development of novel therapeutics.
Shaban et al. evaluated the anti-C. auris activity of four phenolic natural products, and carvacrol (27) was found to be the most potent compound109. Carvacrol had direct activity against at the highest concentration (125 μg/mL) and exerted synergistic and additive effects in combination with fluconazole, caspofungin, amphotericin B, and nystatin. Carvacrol also inhibited virulence factors of C. auris, including proteinase production and adherence ability. Although echinocandins were used as the first-line therapy for the treatment of C. auris infections, their activity against C. auris biofilms was significantly lower than that against C. albicans50. Farnesol (29) is a quorum-sensing antibacterial molecule that has been demonstrated to enhance the activity of echinocandins against C. auris biofilms110. The synergism was observed for caspofungin (FICI range: 0.156–0.5), micafungin (FICI range: 0.133–0.281), and anidulafungin (FICI range: 0.14–0.375).
The antipsychotic drug haloperidol exhibited direct inhibitory effects against C. albicans116. Our group designed a series of haloperidol derivatives that showed improved antifungal activities111,117. The compound B2 (30) exhibited potent synergistic activity against C. auris when used in combination with itraconazole (FICI = 0.188) or voriconazole (FICI = 0.313)111.
6. Drug repurposing
Drug repurposing has become an effective approach to rapidly identifying new therapeutics for emerging infectious disease118, 119, 120. Three independent HTS studies have been performed to identify potential agents against C. auris from among marketed drugs (Fig. 5)121, 122, 123. Several hits were shown to possess potent anti-C. auris activity when used alone or in combination with antifungal agents (Table 3).
Figure 5.
Chemical structures of marketed drugs and derivatives with inhibitory activity against C. auris infections.
Table 3.
Natural peptides and synthetic derivatives with inhibitory activity against C. auris.
| Peptides | Description | Antifungal activity | Ref. |
|---|---|---|---|
| Crotamine | Natural peptide | MIC range: 40–80 μmol/L | 164 |
| Myr-B | Myristoylated lipopeptide | MIC: 16 μg/mL; MIC range: 16–32 μg/mL; In vivo potency in a Galleria mellonella infection model. | 165 |
| Peptide 3 | Cyclic temporin L peptide analogue | MIC: 50 μmol/L; MFC: 50 μmol/L; 50% biofilm inhibition at 6.25 μmol/L; In vivo potency on the infected G. mellonella larvae without significant toxicity. | 59 |
| Pom-1 | A fragment of Closticin 574 | Planktonic cells IC50: 13.8 μg/mL Biofilm IC50: 4.2 μg/mL |
166 |
| Pom-2 | A fragment of cecropin D-like peptide | Planktonic cells IC50: 8.4 μg/mL Biofilm IC50: 2.2 μg/mL |
166 |
| NCR169C17-38 | A derivative of specific cysteine-rich (NCR) peptide | MIC: 6.25 μmol/L; Additive effect with fluconazole | 167 |
| NCR335C17-33 | A derivative of specific cysteine-rich (NCR) peptide | No direct activity; Synergic effect with fluconazole FICI: 0.375 |
167 |
| Cm-p5 | Natural peptide | MIC: 11 μg/mL | 168 |
| Dimer 1 and 2 | Cyclic and helical-stabilized analogues of the antifungal peptide Cm-p5 | Inactive against planktonic cells Biofilm IC50: 10–21 μg/mL |
169 |
| CR-184 | Cathelicidin-inspired AMPs | Abolish metabolic activity at the concentration <1 μmol/L | 170 |
| θ-Defensins | 18-Amino-acid macrocyclic peptides | MIC range: 3.125–6.25 μg/mL | 171 |
| AF4 | Lipopeptide homologues | MIC: 3.48 μg/mL; MFC: 3.48 μg/mL; Inhibition of biofilm formation: SMIC50 = 6.96 μg/mL |
172 |
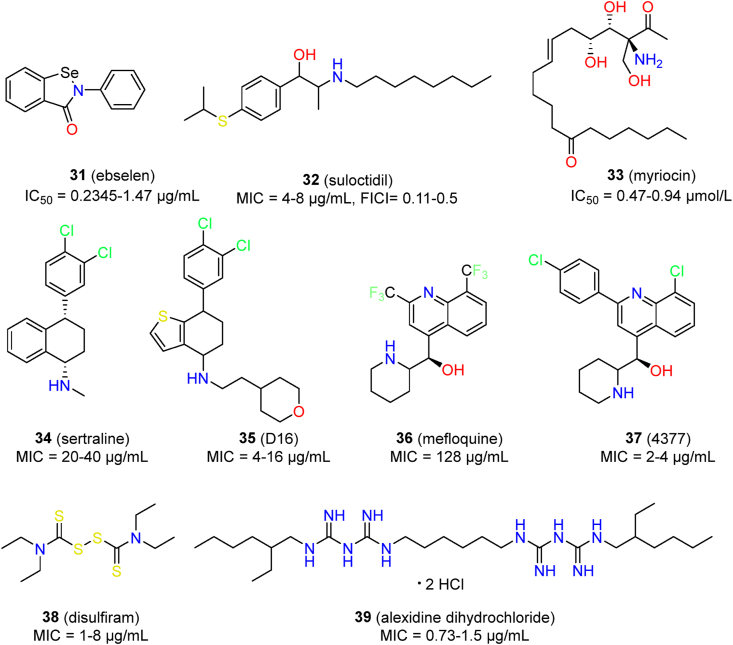
Among such drugs, the effects of ebselen (31) have been well characterized in two screens122,123. Ebselen is an antioxidant agent with diverse biological activities; it is currently undergoing clinical trials for various applications124,125. Ebselen had IC50 values in the range 0.2345–1.47 μg/mL against 10 C. auris clinical isolates123. Moreover, ebselen effectively inhibited biofilm formation of C. auris (IC50 range: 5.864–9.781 μg/mL)123. Ebselen was unable to synergize with fluconazole, amphotericin B, or caspofungin123, while it showed moderate synergism with anidulafungin122. However, the in vivo potency of ebselen against C. auris has not been reported. In addition to C. auris, ebselen also showed broad-spectrum antifungal activity123,126,127. The antifungal target and mechanism of ebselen have not been fully characterized. The diverse activity of ebselen may be related to its electrophilic nature, meaning that it could interact with cysteine-rich proteins. In fungal cells, the antifungal activity of ebselen was associated with the inhibition of plasma membrane H+-ATPase, regulation of glutathione (GSH), and reactive oxygen species (ROS) production126, 127, 128.
Suloctidil (32), an antiplatelet drug, has been reported to be active against C. albicans and C. neoformans129,130. Suloctidil also showed significant inhibitory activity against C. auris, with MIC values ranging from 4 to 8 μg/mL122. In addition, suloctidil was able to synergize with voriconazole against C. auris, with FICI values ranging from 0.11 to 0.5. The synergistic antifungal activity of suloctidil may be due to vacuolar biogenesis and membrane trafficking131.
Myriocin (33), a serine palmitoyltransferase inhibitor, showed IC50 values of 0.94 and 0.47 μmol/L against C. auris 0384 and C. auris 0385, respectively. Moreover, myriocin demonstrated a synergistic effect with flucytosine against 13 clinical isolates of C. auris (FICI range: 0.49–0.53)121.
Sertraline (34), an antidepressant agent, was reported to possess broad spectrum antifungal activity, including against C. auris132, 133, 134, 135. Sertraline significantly inhibited the growth of C. auris, with MIC values ranging from 20 to 40 μg/mL132. Sertraline displayed fungicidal activity against C. auris and effectively inhibited virulence factors such as the yeast to hyphae formation and biofilm formation132. The possible mechanism of action of sertraline was associated with cell membrane damage in C. auris132. CYP51, the target of azole antifungal agents, was suggested to be the target of sertraline by molecular docking. However, there is still a lack of experimental evidence to support this hypothesis. Recently, our group designed a series of sertraline derivatives by scaffold hopping136. Compound D16 (35) showed potent activity against three C. auris isolates (MIC range: 4–16 μg/mL). Antifungal mechanistic studies revealed that compound D16 blocked the biosynthesis of ergosterol through the inhibition of Δ5,6–desaturase, a potential target for the development of anti-C. auris therapeutics136.
Mefloquine (36) is an antimalarial agent that was reported to possess moderate antifungal activity137. Montoya et al. further evaluated the activity of mefloquine derivatives, and they identified several compounds with improved potency. Among these, compound 4377 (37) showed the best activity against five C. auris isolates (MIC range: 2–4 μg/mL)138. However, this compound was still less active than caspofungin and amphotericin B. Mefloquine derivatives acted by multi-targeting mechanisms in which interference with the functions of mitochondria and vacuoles was preliminarily confirmed138.
Disulfiram (38), an aldehyde dehydrogenase enzyme inhibitor for the treatment of alcohol dependence, was identified as an antifungal agent against C. auris139. Disulfiram exhibited superior activity against C. auris over fluconazole, with MIC values ranging from 1 to 8 μg/mL. In addition, disulfiram showed inhibitory activity against biofilm formation of C. auris by increasing fungal cell aggregation, with SMIC50 values ranging from 32 to 128 μg/mL139. Preliminary mechanism studies indicated that disulfiram could combat drug resistance by inhibiting the ABC transporter proteins140.
Alexidine dihydrochloride (39), an anticancer drug act by inhibiting mitochondrial tyrosine phosphatase, has been reported to possess antifungal and anti-biofilm activity against C. auris141. Alexidine dihydrochloride had MIC values in the range of 0.73–1.5 μg/mL against C. auris, and displayed low toxicity on lung epithelial cells and HUVECs (IC50 > 7.37 μg/mL). Moreover, alexidine dihydrochloride effectively inhibited biofilm formation and mature biofilm of C. auris, with SMIC50 values of 6 and 3 μg/mL, respectively141.
7. New targets for the development of anti-C. auris agents
7.1. Phosphatidylinositol–phosphatidylcholine transfer protein: Sec14p
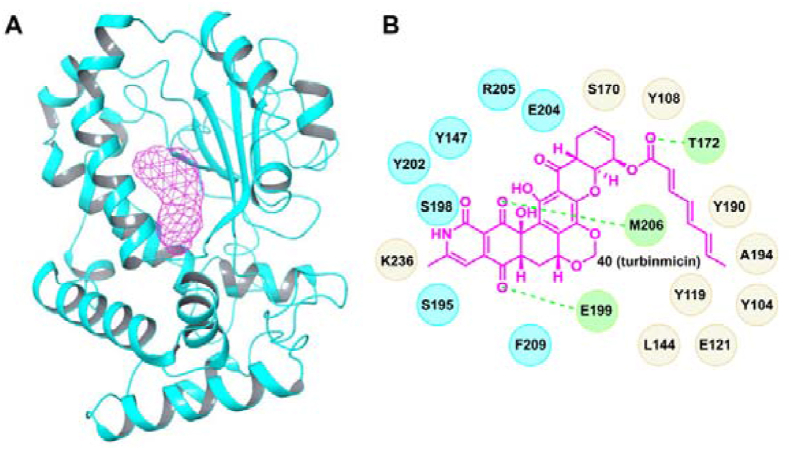
Bugni’s group performed anti-C. albicans high-throughput screening of the microbiomes of marine animals through an integrated platform of metabolomic and genomic tools142. Turbinmicin (40), a highly oxidized polyketide, was identified to possess broad-spectrum inhibitory activity against Candida spp., Fusarium spp., Scedosporium spp., and Rhizopus spp. (MIC range: 0.03–0.5 μg/mL). In particular, turbinmicin was effective against C. auris (strain number: B11211) with an MIC value of 0.25 μg/mL. Further evaluation indicated that turbinmicin was fungicidal with low toxicity, and the maximum tolerated dose (MTD) in a mouse model was above 256 mg/kg. Turbinmicin also showed dose-dependent in vivo potency for the treatment C. auris infections. At the dose of 4 mg/kg, turbinmicin treatment led to a 3.6 log10 reduction in fungal burden compared with a blank control. The mode of action of turbinmicin was preliminary clarified by screening knockdown and knockout gene libraries of Saccharomyces cerevisiae. Sec14p, a phosphatidylinositol–phosphatidylcholine transfer protein, was validated as the molecular target of turbinmicin (Fig. 6A). Turbinmicin binds to the phospholipid binding pocket of Sec14p through hydrophobic and hydrogen bonding interactions (Fig. 6B).
Figure 6.
Crystal structure of Sec14p (A, PDB code: 6F0E) and a proposed binding model of turbinmicin with Sec14p (B). The magenta mesh indicates turbinimicin, and the dashed green lines represent hydrogen bonding interactions.
The promising in vitro and in vivo antifungal activity and favo-rable mammalian safety profile have made turbinmicin a valuable lead compound. However, turbinmicin was administrated by intraperitoneal injection; this limited its further clinical development. After removal of the side chain by ester hydrolysis, the antifungal activity was reduced. Thus, structure optimization of turbinmicin into an orally active antifungal agent is required. To facilitate extensive SAR investigation, the difficulty involved in total synthesis should be solved. However, Sec14p may be further exploited as a drug target to design drug-like inhibitors against C. auris infections. Ergoline143, benzamide144, and picolinamide144 derivatives have been reported to be fungal Sec14p inhibitors. However, the antifungal activities of these Sec14p inhibitors were rather weak143,144. Fortunately, the crystal structure of Sec14p has been solved144; this could improve the efficiency of designing potent Sec14p inhibitors.
7.2. Casein kinase: Yck2
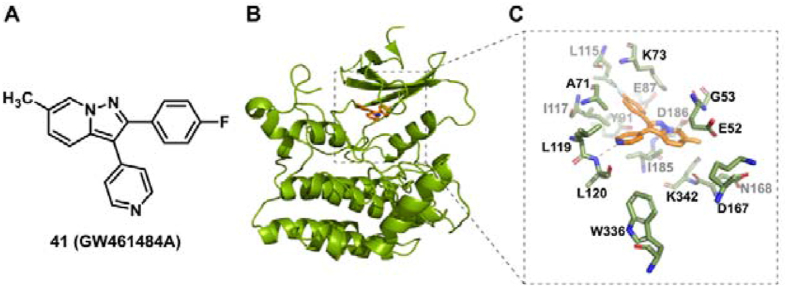
By screening a library of 736 protein kinase inhibitors, the arylpyrazolopyridine derivative GW461484A (41) was identified as a sensitizer to reverse caspofungin resistance against C. albicans145. Interestingly, GW461484A also potentiated the activity of caspofungin against a multidrug-resistant C. auris isolate with an FIC80 (fractional inhibitory concentration 80 index) value lower than 0.156, whereas it had little effect on the anti-C. auris activity of fluconazole. Furthermore, Yck2 was identified to be the molecular target of GW461484A by chemogenomic and biochemical profiling145. Yck2 belongs to the protein family of CK1 (casein kinase 1) that has been associated with the morphogenesis and virulence of C. albicans. Structural biology studies indicated that GW461484A interacted with the ATP-binding pocket of Yck2 through hydrogen bonding and hydrophobic interactions (Fig. 7). Although the biological functions of Yck2 are still unknown, Yck2 represents a valuable target for the development of C. auris therapeutics. In vivo antifungal potency of GW461484A was not determined due to poor metabolic stability. The pyrazolopyridine Yck2 inhibitors remain to be further optimized and evaluated against C. auris infections.
Figure 7.
Chemical structure of GW461484A (A), crystal structure of Yck2 (B, PDB code: 6U6A), and the binding mode of GW461484A with Yck2 (C). The orange molecule indicates compound GW461484A.
7.3. Acetohydroxyacid synthase
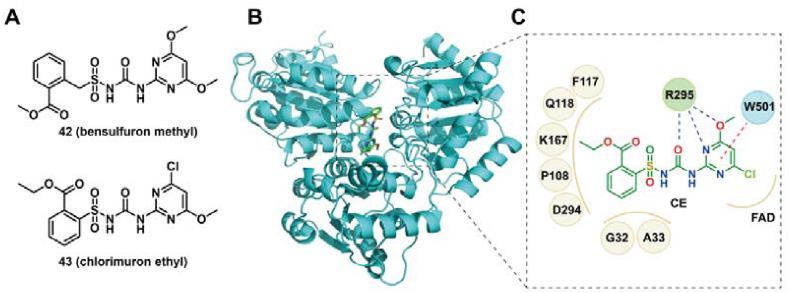
Acetohydroxyacid synthase (AHAS), an enzyme in the biosynthesis pathway of branched-chain amino acid, has been demonstrated as a promising target for the development of antifungal agents against C. auris146,147. Guddat et al.147 expressed and obtained the AHAS from C. auris (CauAHAS), and identified several sulfonylurea and triazolopyrimidine herbicides as potent antifungal inhibitors against C. auris (MIC50 < 5 μmol/L), with the Ki values of < 2 μmol/L for CauAHAS. Among them, bensulfuron methyl (BSM, 42), a sulfonylurea inhibitor, exhibited the best fungicidal potency with the MIC50 values of 0.09 μmol/L. BSM was also an excellent inhibitor for preventing the biofilms formation of C. auris (SMIC50 = 0.6 μmol/L). Cell viability assays revealed that BSM was non-cytotoxic to human embryonic kidney (HEK)-293 cells at the concentrations of < 100 μmol/L147. The possible binding model of these inhibitors with CauAHAS was identified by homology modelling based on the crystal complex of C. albicans AHAS with chlorimuron ethyl (CE, 43), an analogue of compound 42. CE interacted with the binding sites of CauAHAS by hydrophobic, hydrogen bonding and π‒π stacking interactions (Fig. 8). These data indicated that CauAHAS was a viable target for treating C. auris infections.
Figure 9.
Chemical structures of new chemotypes with inhibitory activity against C. auris.
Figure 8.
Chemical structure of BSM and CE (A), crystal structure of AHAS (B, PDB code: 6DEL), and the binding mode of CE with AHAS (C). The green molecule indicate compound CE. Solid brown lines, dashed blue lines, and dashed red lines represent hydrophobic, hydrogen bonding and π‒π stacking interactions, respectively.
7.4. New chemical scaffolds against C. auris
7.4.1. Rocaglates
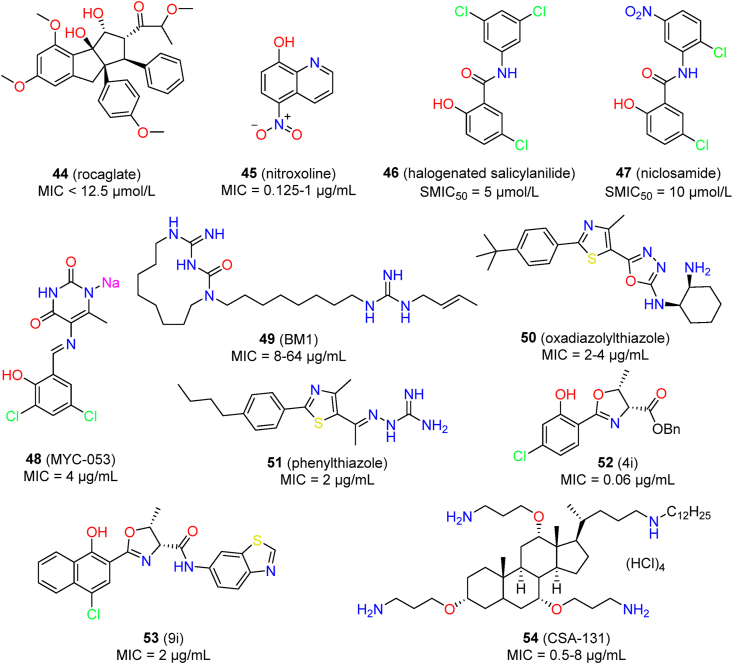
Cowen’s group screened a library containing 2454 compounds to identify anti-C. auris compounds, and the hits shared a common rocaglate scaffold (44)148. Representative compound CMLD010515 (Fig. 9) displayed inhibitory activity against C. auris (active concentration: < 12.5 μmol/L) and was demonstrated to be fungicidal. Interestingly, the anti-C. auris activity was species-specific, because the rocaglates were inactive against pathogenic related Candida species such as C. albicans. The antifungal mechanisms of rocaglates were preliminary elucidated; these involved inhibition of translation initiation in C. auris, triggering an apoptosis-like cell death program and blocking vacuolar homeostasis148.
7.4.2. Hydroxyquinolines: Nitroxoline
The hydroxyquinoline derivate nitroxoline (45) is an antibacterial agent used for urinary tract infections. It also has shown inhibitory activity against C. auris, with MICs ranging from 0.125 to 1 μg/mL (35 isolates: MIC50 = 0.25 μg/mL, MIC90 = 0.5 μg/mL)149. It was more potent than amphotericin B (MIC > 1 μg/mL in 4/35 isolates) and fluconazole (MIC > 4 μg/mL in 31/35 isolates). Nitroxoline was proposed as a potential treatment option for C. auris candiduria. However, its in vivo efficacy remains to be confirmed.
7.4.3. Halogenated salicylanilides
In an antivirulence phenotypic screen, halogenated salicylanilides 1 and its analog niclosamide (46, 47) exhibited potent inhibitory activities against C. albicans filamentation and biofilm formation150. Both were also active against the biofilms of C. auris in a dose-dependent manner150. Mechanistic studies revealed that the mitochondrial protein import machinery may be involved in the activity of halogenated salicylanilides.
7.4.4. Pyrimidinedione: MYC-053
The pyrimidinedione derivative MYC-053 (48) showed broad spectrum effects against Candida spp., Cryptococcus spp., and Pneumocystis spp. It had an MIC value of 4 μg/mL against 5 C. auris isolates, and it was also active against several strains resistant to fluconazole and caspofungin151.
7.4.5. Macrocyclic amidinoureas: BM1
BM1 (49) is a derivative of macrocyclic amidinoureas whose chemical structure features an amphiphilic macrocycle, a methylene linker, and a terminal alkenyl guanidine152. BM1 showed potent inhibitory activity against various fluconazole-sensitive and fluconazole-resistant Candida spp., including C. auris isolates. The MIC value of BM1 against 18 C. auris isolates was in the range of 8 μg/mL to 64 μg/mL153. However, the antifungal activity of BM1 against C. auris was significantly lower than that against C. albicans (MIC range: 0.125–2 μg/mL). The activity of BM1 against resistant fungi was associated with the overexpression of ABC transporters153. BM1 showed in vivo efficacy for treating infections by drug-resistant C. albicans152, whereas the in vivo potency against C. auris is still unknown.
7.4.6. Oxadiazolylthiazoles
Hagras et al. synthesized a series of oxadiazolylthiazole derivatives and identified selective antifungal agents154. Diaminocyclohexyl derivative 50 showed broad-spectrum in vitro antifungal activity, including against C. auris. It had MIC values of 4, 2, and 2 μg/mL against C. auris 381, C. auris 383, and C. auris 384, respectively. Moreover, compound 50 showed low toxicity against human colorectal adenocarcinoma (Caco-2) and monkey fibroblast-like kidney epithelial (Vero) cells, with CC50 values larger than 64 μg/mL.
7.4.7. Phenylthiazoles
Mohammad et al. assayed 85 synthetic phenylthiazole derivatives for inhibitory activity against drug-resistant C. albicans155. Thiazole-aminoguanidine derivative 51 showed the most potent antifungal activity, with a broad spectrum. Compound 51 had an MIC value of 2 μg/mL against eight C. auris isolates, a value that was more potent than fluconazole (MIC > 64 μg/mL) and comparable to amphotericin B (MIC range: 0.50–2 μg/mL). Compound 51 showed rapid fungicidal activity against C. auris viability within 30 min. At the concentration of 2 μg/mL, compound 51 effectively inhibited biofilm formation of C. auris (91.2% reduction), and it was equally effective as amphotericin B (92.4% reduction). In contrast, the cytotoxicity of compound 51 against mammalian cells was significantly lower than that of amphotericin B. In a C. elegans model with C. auris, compound 51 prolonged the survival of infected nematodes by about 70% at the concentration of 10 μg/mL.
7.4.8. 2-Aryloxazolines
Stefani’s group synthesized a series of 2-aryloxazoline derivatives and assayed these for inhibitory activity against C. albicans156. Most compounds showed comparable or superior antifungal activity to fluconazole. The compounds 4i (52) and 9i (53) were also effective against C. auris isolates CBS 10913 (MIC = 0.06 μg/mL) and CBS 12766 (MIC = 2 μg/mL).
8. Antimicrobial peptides
Antimicrobial peptides (AMPs) are emerging as an attractive area in antifungal therapy due to the important roles in human innate immunity and host defense and the low risk of inducing MDR157. Several antifungal AMPs have also shown potent activity against C. auris (Table 3).
Histatin 5 (Hst 5) was reported to possess good antifungal activity against C. albicans158. In a susceptibility assay of 10 C. auris clinical isolates, Hst 5 showed fungicidal activity against the majority of tested isolates, killing 55%–90% of C. auris cells at the concentration of 7.5 μmol/L159. The high tolerance of C. auris strains to oxidative stress was possibly associated with the killing effect of Hst 5159.
Human cathelicidin peptides LL-37 showed both direct and synergistic activities against C. auris160. The growth inhibitory activity of LL-37 was moderate in a collection of 10 clinical strains (MIC range: 25–100 μg/mL; MFC range: 50–200 μg/mL). LL-37 also effectively synergized with antifungal agents such as fluconazole (80% of strains, FICI range: 0.27–0.5), amphotericin B (100% of strains, FICI range: 0.13–0.31), and caspofungin (100% of strains, FICI range: 0.13–0.26). The antifungal mechanistic studies revealed that LL-37 acted by disrupting the cell membrane, causing oxidative stress, and arresting the S phase of cell cycle of C. auris160.
AMPs are generally the substrates for proteases and are prone to be degraded in vivo. Thus, non-peptide AMP mimics were designed to overcome the limitations of peptide molecules. Ceragenins feature a bile acid scaffold and a lipid chain that mimics the common amphiphilic secondary structure of AMPs and that has shown broad-spectrum antifungal activity161,162. The compound ceragenin CSA-131 (54) had potent fungistatic and fungicidal activity against a set of 100 C. auris clinical isolates (MIC range: 0.5–8 μg/mL; MFC range: 2–64 μg/mL) and was generally more potent than fluconazole, caspofungin, and amphotericin B163. The antifungal activity of CSA-131 was clade independent without variation between the four clades (overall mode: 1 μg/mL; MIC90 = 1 μg/mL). Notably, no loss of inhibitory activity was observed for those isolates resistant to fluconazole and/or echinocandin. CSA-131 also effectively inhibited the activity of C. auris biofilm formation (SMIC50 range: 2–4 μg/mL). In an ex vivo infections model of mucosal tissues, topical use of CSA-131 (2% gel and cream formulations) resulted in significant reductions of fungal burdens.
9. Perspectives and conclusions
Although some progress has been made, we still do not clearly know where C. auris originated or why C. auris has independently and simultaneously spread worldwide. Our understanding of the virulence, risk factors, and mechanisms of drug resistance remains in its infancy. In some cases, the results obtained from different clades (strains) of C. auris are controversial or contradictory. Thus, there is still a long way to go before we can fully understand this novel fungal pathogen. To tackle the threat of C. auris, improvement in early diagnosis, control measures, and education of healthcare providers will help to reduce the incidence of infections. More importantly, the development of effective therapeutics is urgently needed to improve clinical outcomes and decrease mortality.
Although echinocandins have been recommended as the first-line therapy, the options for effective treatment of C. auris infections are rather limited. Several antifungal agents in clinical development have been shown to be effective against C. auris through in vitro and in vivo evaluations. These compounds have also shown favorable pharmacokinetic and safety profiles with a low risk of drug–drug interactions in clinical trials. However, only two clinical trials have been started for C. auris infections (ibrexafungerp and APX001) with as yet undisclosed data. Therefore, more clinical studies are required to validate the potential usefulness of these candidates in clinical practice.
Synergistic drug combinations have been suggested as a potential option for the treatment of C. auris infections. Although a number of active combinations have been reported, it is premature to predict their clinical utility, because most have only been evaluated in vitro. Additional evaluation in animal models or eventually in clinical trials is required to identify useful combinations for pan-resistant C. auris.
Drug repurposing has been demonstrated to be a useful approach to accelerate the drug development process, particularly for emerging infectious diseases. Several “non-antifungal” drugs have been shown be active in inhibiting the growth of C. auris when used alone or in combination the antifungal agents. However, such known drugs could hardly be used directly in clinical application due to limited potency and side effects. An important value of drug repurposing is to offer drug-like lead compounds for the optimization of therapeutic efficacy and safety profiles. For example, our group has designed a series of new derivatives of sertraline and piperidol as antifungal agents, and it has also identified new leads with improved antifungal activity and reduced original activity of approved agents111,136. Thus, further structural optimization and exploration of the SARs of the hits from anti-C. auris screening may improve the efficiency of drug development. Also, the clinical values of broad-spectrum antifungal agents or C. auris selective inhibitors still remain to be further explored.
With better understanding of the virulence and resistant mechanisms of C. auris, the discovery and identification of new targets is highly important for developing effective therapeutics with new modes of action. Sec14p and Yck2 have been preliminarily identified as potential targets for C. auris infections. These targets were identified through chemogenomic profiling of active compounds. Biology-inspired discovery of new targets is still rare, possibly due to limited knowledge concerning C. auris. Another problem for the new antifungal targets is the inconsistency between molecular and antifungal activity. After proof-of-concept validation, extensional medicinal chemistry exploration of the inhibitors would contribute to identifying selective and drug-like inhibitors. Recently, a large number of anti-C. auris compounds were identified by phenotypic antifungal screening, and their molecular targets are mostly unknown. These bioactive compounds could be used as chemical probes to look for new targets by chemogenomic profiling; this would provide an alternative for target discovery in C. auris. With a better understanding of C. auris, increased medicinal chemistry effort, and more preclinical and clinical trials, highly effective antifungal drugs will become a reality for the treatment of patients with severe C. auris infections.
Acknowledgments
This work was supported by the National Natural Science Foundation (81725020, 82003591 and 81973175, China), the Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-07-E00073, China), and Science and Technology Commission of Shanghai Municipality (20S11900400, China).
Author contributions
Yahui Huang and Wanzhen Yang performed the literature search and data collection. Chunquan Sheng conceived the concept of the study. Jie Tu designed and regenerated the conceptual pictures. Jie Tu, Na Liu and Chunquan Sheng prepared and revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
References
- 1.Lamoth F., Lockhart S.R., Berkow E.L., Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73:i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 3.Du H., Bing J., Hu T., Ennis C.L., Nobile C.J., Huang G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortegiani A., Misseri G., Fasciana T., Giammanco A., Giarratano A., Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by. Candida auris. J Intensive Care. 2018;6:69–82. doi: 10.1186/s40560-018-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh R.M., Bentz M.L., Shams A., Houston H., Lyons A., Rose L.J., et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol. 2017;55:2996–3005. doi: 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor D.M., Dangana T., Sexton D.J., Fukuda C., Yelin R.D., Stanley M., et al. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nat Med. 2021;27:1401–1409. doi: 10.1038/s41591-021-01383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Ami R., Berman J., Novikov A., Bash E., Shachor-Meyouhas Y., Zakin S., et al. Multidrug-resistant Candida haemulonii and C. auris, tel Aviv, Israel. Emerg Infect Dis. 2017;23:192–203. doi: 10.3201/eid2302.161486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S., Alampalli S.V., Nageshan R.K., Chettiar S.T., Joshi S., Tatu U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen. Candida auris. BMC Genomics. 2015;16:686–697. doi: 10.1186/s12864-015-1863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz J.F., Gade L., Chow N.A., Loparev V.N., Juieng P., Berkow E.L., et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. 2018;9:5346–5359. doi: 10.1038/s41467-018-07779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow N.A., de Groot T., Badali H., Abastabar M., Chiller T.M., Meis J.F. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis. 2019;25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhary A., Sharma C., Meis J.F. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhary A., Sharma C., Duggal S., Agarwal K., Prakash A., Singh P.K., et al. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis. 2013;19:1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo B., Melo A.S., Perozo-Mena A., Hernandez M., Francisco E.C., Hagen F., et al. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Navalkele B.D., Revankar S., Chandrasekar P. Candida auris: a worrisome, globally emerging pathogen. Expert Rev Anti Infect Ther. 2017;15:819–827. doi: 10.1080/14787210.2017.1364992. [DOI] [PubMed] [Google Scholar]
- 15.Osei Sekyere J. Candida auris: a systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen. 2018;7 doi: 10.1002/mbo3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohlenberg A., Struelens M.J., Monnet D.L., Plachouras D. Candida auris: epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Euro Surveill. 2018;23:18–136. doi: 10.2807/1560-7917.ES.2018.23.13.18-00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schelenz S., Hagen F., Rhodes J.L., Abdolrasouli A., Chowdhary A., Hall A., et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35–39. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadnum J.L., Shaikh A.A., Piedrahita C.T., Sankar T., Jencson A.L., Larkin E.L., et al. Effectiveness of disinfectants against Candida auris and other Candida species. Infect Control Hosp Epidemiol. 2017;38:1240–1243. doi: 10.1017/ice.2017.162. [DOI] [PubMed] [Google Scholar]
- 19.Abdolrasouli A., Armstrong-James D., Ryan L., Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with. Candida auris. Mycoses. 2017;60:758–763. doi: 10.1111/myc.12699. [DOI] [PubMed] [Google Scholar]
- 20.Bruno M., Kersten S., Bain J.M., Jaeger M., Rosati D., Kruppa M.D., et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen. Candida auris. Nat Microbiol. 2020;5:1516–1531. doi: 10.1038/s41564-020-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Zou Y., Chen X., Li H., Yin Z., Zhang B., et al. Innate immune responses against the fungal pathogen. Candida auris. Nat Commun. 2022;13:3553–3573. doi: 10.1038/s41467-022-31201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav B., Mora-Montes H.M., Wagener J., Cunningham I., West L., Haynes K., et al. Differences in fungal immune recognition by monocytes and macrophages: N-mannan can be a shield or activator of immune recognition. Cell surf. 2020;6:100042–100054. doi: 10.1016/j.tcsw.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhary A., Prakash A., Sharma C., Kordalewska M., Kumar A., Sarma S., et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 24.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azar M.M., Turbett S.E., Fishman J.A., Pierce V.M. Donor-derived transmission of Candida auris during lung transplantation. Clin Infect Dis. 2017;65:1040–1042. doi: 10.1093/cid/cix460. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Bustos V., Cabanero-Navalon M.D., Ruiz-Sauri A., Ruiz-Gaitan A.C., Salavert M., Tormo M.A., et al. What do we know about Candida auris? State of the art, knowledge gaps, and future directions. Microorganisms. 2021;9:2177–2197. doi: 10.3390/microorganisms9102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giacobbe D.R., Magnasco L., Sepulcri C., Mikulska M., Koehler P., Cornely O.A., et al. Recent advances and future perspectives in the pharmacological treatment of Candida auris infections. Expet Rev Clin Pharmacol. 2021;14:1205–1220. doi: 10.1080/17512433.2021.1949285. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes J., Fisher M.C. Global epidemiology of emerging. Candida auris. Curr opin microbiol. 2019;52:84–89. doi: 10.1016/j.mib.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Chaabane F., Graf A., Jequier L., Coste A.T. Review on antifungal resistance mechanisms in the emerging pathogen. Candida auris. Front Microbiol. 2019;10:2788–2796. doi: 10.3389/fmicb.2019.02788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montoya M.C., Moye-Rowley W.S., Krysan D.J. Candida auris: the Canary in the mine of antifungal drug resistance. ACS Infect Dis. 2019;5:1487–1492. doi: 10.1021/acsinfecdis.9b00239. [DOI] [PubMed] [Google Scholar]
- 31.Rossato L., Colombo A.L. Candida auris: what have we learned about its mechanisms of pathogenicity? Front Microbiol. 2018;9:3081–3087. doi: 10.3389/fmicb.2018.03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bravo Ruiz G., Lorenz A. What do we know about the biology of the emerging fungal pathogen of humans Candida auris? Microbiol Res. 2021;242:126621–126634. doi: 10.1016/j.micres.2020.126621. [DOI] [PubMed] [Google Scholar]
- 33.Bandara N., Samaranayake L. Emerging and future strategies in the management of recalcitrant. Candida auris. Med Mycol. 2022;60:8–39. doi: 10.1093/mmy/myac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billamboz M., Fatima Z., Hameed S., Jawhara S. Promising drug candidates and new strategies for fighting against the emerging superbug. Candida auris. Microorganisms. 2021;9:634–674. doi: 10.3390/microorganisms9030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han G., Liu N., Li C., Tu J., Li Z., Sheng C. Discovery of novel fungal lanosterol 14alpha-demethylase (CYP51)/histone deacetylase dual inhibitors to treat azole-resistant candidiasis. J Med Chem. 2020;63:5341–5359. doi: 10.1021/acs.jmedchem.0c00102. [DOI] [PubMed] [Google Scholar]
- 36.Li Z., Tu J., Han G., Liu N., Sheng C. Novel carboline fungal histone deacetylase (HDAC) inhibitors for combinational treatment of azole-resistant candidiasis. J Med Chem. 2021;64:1116–1126. doi: 10.1021/acs.jmedchem.0c01763. [DOI] [PubMed] [Google Scholar]
- 37.Chow N.A., Munoz J.F., Gade L., Berkow E.L., Li X., Welsh R.M., et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio. 2020;11 doi: 10.1128/mBio.03364-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaller M.A., Messer S.A., Deshpande L.M., Rhomberg P.R., Utt E.A., Castanheira M. Evaluation of synergistic activity of isavuconazole or voriconazole plus anidulafungin and the occurrence and genetic characterization of Candida auris detected in a surveillance program. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.02031-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kathuria S., Singh P.K., Sharma C., Prakash A., Masih A., Kumar A., et al. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol. 2015;53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lockhart S.R., Etienne K.A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N.P., et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes J., Abdolrasouli A., Farrer R.A., Cuomo C.A., Aanensen D.M., Armstrong-James D., et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen. Candida auris. Emerg Microbes Infect. 2018;7:43–58. doi: 10.1038/s41426-018-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S.H., Iyer K.R., Pardeshi L., Munoz J.F., Robbins N., Cuomo C.A., et al. Genetic analysis of Candida auris implicates Hsp90 in morphogenesis and azole tolerance and Cdr1 in azole resistance. mBio. 2019;10 doi: 10.1128/mBio.02529-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Oliveira Santos G.C., Vasconcelos C.C., Lopes A.J.O., de Sousa Cartagenes M.D.S., Filho A., do Nascimento F.R.F., et al. Candida Infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol. 2018;9:1351–1359. doi: 10.3389/fmicb.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rybak J.M., Doorley L.A., Nishimoto A.T., Barker K.S., Palmer G.E., Rogers P.D. Abrogation of triazole resistance upon deletion of CDR1 in a clinical isolate of. Candida auris. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.00057-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasi M., Khandelwal N.K., Moorhouse A.J., Nair R., Vishwakarma P., Bravo Ruiz G., et al. ABC transporter genes show upregulated expression in drug-resistant clinical isolates of Candida auris: a genome-wide characterization of ATP-binding cassette (ABC) transporter genes. Front Microbiol. 2019;10:1445–1461. doi: 10.3389/fmicb.2019.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escandon P., Chow N.A., Caceres D.H., Gade L., Berkow E.L., Armstrong P., et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis. 2019;68:15–21. doi: 10.1093/cid/ciy411. [DOI] [PubMed] [Google Scholar]
- 47.Kordalewska M., Lee A., Park S., Berrio I., Chowdhary A., Zhao Y., et al. Understanding echinocandin resistance in the emerging pathogen. Candida auris. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biagi M.J., Wiederhold N.P., Gibas C., Wickes B.L., Lozano V., Bleasdale S.C., et al. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis. 2019;6:ofz262. doi: 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kean R., Delaney C., Sherry L., Borman A., Johnson E.M., Richardson M.D., et al. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere. 2018;3 doi: 10.1128/mSphere.00334-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherry L., Ramage G., Kean R., Borman A., Johnson E.M., Richardson M.D., et al. Biofilm-forming capability of highly virulent, multidrug-resistant. Candida auris. Emerg Infect Dis. 2017;23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominguez E.G., Zarnowski R., Choy H.L., Zhao M., Sanchez H., Nett J.E., et al. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere. 2019;4 doi: 10.1128/mSphereDirect.00680-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larkin E., Hager C., Chandra J., Mukherjee P.K., Retuerto M., Salem I., et al. The Emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romera D., Aguilera-Correa J.J., Gadea I., Vinuela-Sandoval L., Garcia-Rodriguez J., Esteban J. Candida auris: a comparison between planktonic and biofilm susceptibility to antifungal drugs. J Med Microbiol. 2019;68:1353–1358. doi: 10.1099/jmm.0.001036. [DOI] [PubMed] [Google Scholar]
- 54.Horton M.V., Johnson C.J., Kernien J.F., Patel T.D., Lam B.C., Cheong J.Z.A., et al. Candida auris forms high-burden biofilms in skin niche conditions and on porcine skin. mSphere. 2020;5:e00910–e00919. doi: 10.1128/mSphere.00910-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arendrup M.C., Prakash A., Meletiadis J., Sharma C., Chowdhary A. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fakhim H., Chowdhary A., Prakash A., Vaezi A., Dannaoui E., Meis J.F., et al. In vitro interactions of echinocandins with triazoles against multidrug-resistant. Candida auris. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagy F., Toth Z., Nyikos F., Forgacs L., Jakab A., Borman A.M., et al. In vitro and in vivo interaction of caspofungin with isavuconazole against Candida auris planktonic cells and biofilms. Med Mycol. 2021;59:1015–1023. doi: 10.1093/mmy/myab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eldesouky H.E., Salama E.A., Lanman N.A., Hazbun T.R., Seleem M.N. Potent Synergistic interactions between lopinavir and azole antifungal drugs against emerging multidrug-resistant. Candida auris. Antimicrob Agents Chemother. 2020;65 doi: 10.1128/AAC.00684-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellavita R., Maione A., Merlino F., Siciliano A., Dardano P., De Stefano L., et al. Antifungal and antibiofilm activity of cyclic temporin L peptide analogues against albicans and non-albicans Candida species. Pharmaceutics. 2022;14:454–479. doi: 10.3390/pharmaceutics14020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lepak A.J., Zhao M., Berkow E.L., Lockhart S.R., Andes D.R. Pharmacodynamic optimization for treatment of invasive Candida auris infection. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao M., Lepak A.J., VanScoy B., Bader J.C., Marchillo K., Vanhecker J., et al. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghannoum M., Isham N., Angulo D., Borroto-Esoda K., Barat S., Long L. Efficacy of ibrexafungerp (SCY-078) against Candida auris in an in vivo Guinea pig cutaneous infection model. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00854-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yates C.M., Garvey E.P., Shaver S.R., Schotzinger R.J., Hoekstra W.J. Design and optimization of highly-selective, broad spectrum fungal CYP51 inhibitors. Bioorg Med Chem Lett. 2017;27:3243–3248. doi: 10.1016/j.bmcl.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 64.Hargrove T.Y., Garvey E.P., Hoekstra W.J., Yates C.M., Wawrzak Z., Rachakonda G., et al. Crystal structure of the new investigational drug candidate VT-1598 in complex with aspergillus fumigatus sterol 14alpha-demethylase provides insights into its broad-spectrum antifungal activity. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00570-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiederhold N.P., Lockhart S.R., Najvar L.K., Berkow E.L., Jaramillo R., Olivo M., et al. The fungal CYP51-specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against. Candida auris. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.02233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colley T., Alanio A., Kelly S.L., Sehra G., Kizawa Y., Warrilow A.G.S., et al. In vitro and in vivo antifungal profile of a novel and long-acting inhaled azole, PC945, on Aspergillus fumigatus infection. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02280-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudramurthy S.M., Colley T., Abdolrasouli A., Ashman J., Dhaliwal M., Kaur H., et al. In vitro antifungal activity of a novel topical triazole PC945 against emerging yeast. Candida auris. J Antimicrob Chemother. 2019;74:2943–2949. doi: 10.1093/jac/dkz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ham Y.Y., Lewis J.S., 2nd, Thompson G.R., 3rd Rezafungin: a novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021;16:27–36. doi: 10.2217/fmb-2020-0217. [DOI] [PubMed] [Google Scholar]
- 69.Sandison T., Ong V., Lee J., Thye D. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berkow E.L., Lockhart S.R. Activity of CD101, a long-acting echinocandin, against clinical isolates of. Candida auris. Diagn Microbiol Infect Dis. 2018;90:196–197. doi: 10.1016/j.diagmicrobio.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 71.Hager C.L., Larkin E.L., Long L.A., Ghannoum M.A. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother. 2018;73:2085–2088. doi: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lepak A.J., Zhao M., Andes D.R. Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01572-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Helleberg M., Jorgensen K.M., Hare R.K., Datcu R., Chowdhary A., Arendrup M.C. Rezafungin in vitro activity against contemporary nordic clinical candida isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02438-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toth Z., Forgacs L., Locke J.B., Kardos G., Nagy F., Kovacs R., et al. In vitro activity of rezafungin against common and rare Candida species and. Saccharomyces cerevisiae. J Antimicrob Chemother. 2019;74:3505–3510. doi: 10.1093/jac/dkz390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovacs R., Toth Z., Locke J.B., Forgacs L., Kardos G., Nagy F., et al. Comparison of in vitro killing activity of rezafungin, anidulafungin, caspofungin, and micafungin against four Candida auris clades in RPMI-1640 in the absence and presence of human serum. Microorganisms. 2021;9:863–876. doi: 10.3390/microorganisms9040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jallow S., Govender N.P. Ibrexafungerp: a first-in-class oral triterpenoid glucan synthase inhibitor. J Fungi (Basel) 2021;7:163–182. doi: 10.3390/jof7030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jimenez-Ortigosa C., Paderu P., Motyl M.R., Perlin D.S. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother. 2014;58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berkow E.L., Angulo D., Lockhart S.R. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against clinical isolates of Candida auris. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00435-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arendrup M.C., Jorgensen K.M., Hare R.K., Chowdhary A. In vitro activity of ibrexafungerp (SCY-078) against Candida auris isolates as determined by EUCAST methodology and comparison with activity against C. albicans and C. glabrata and with the activities of six comparator agents. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02136-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiederhold N.P., Najvar L.K., Olivo M., Morris K.N., Patterson H.P., Catano G., et al. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.02694-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghannoum M., Arendrup M.C., Chaturvedi V.P., Lockhart S.R., McCormick T.S., Chaturvedi S., et al. Ibrexafungerp: a novel oral triterpenoid antifungal in development for the treatment of Candida auris infections. Antibiotics (Basel) 2020;9:539–552. doi: 10.3390/antibiotics9090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu S., Long L., McCormick T.S., Borroto-Esoda K., Barat S., Ghannoum M.A. A second-generation fungerp analog, SCY-247, shows potent in vivo activity in a murine model of hematogenously disseminated. Candida albicans. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.01989-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chu S., Long L., Sherif R., McCormick T.S., Borroto-Esoda K., Barat S., et al. A second-generation fungerp analog, SCY-247, shows potent in vitro activity against Candida auris and other clinically relevant fungal isolates. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.01988-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw K.J., Ibrahim A.S. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel) 2020;6:239–260. doi: 10.3390/jof6040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoenigl M., Sprute R., Egger M., Arastehfar A., Cornely O.A., Krause R., et al. The Antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs. 2021;81:1703–1729. doi: 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arendrup M.C., Chowdhary A., Jorgensen K.M., Meletiadis J. Manogepix (APX001A) in vitro activity against Candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00656-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berkow E.L., Lockhart S.R. Activity of novel antifungal compound APX001A against a large collection of. Candida auris. J Antimicrob Chemother. 2018;73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 88.Hager C.L., Larkin E.L., Long L., Zohra Abidi F., Shaw K.J., Ghannoum M.A. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiederhold N.P., Najvar L.K., Shaw K.J., Jaramillo R., Patterson H., Olivo M., et al. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Y., Kilburn S., Kapoor M., Chaturvedi S., Shaw K.J., Chaturvedi V. In vitro activity of manogepix against multidrug-resistant and panresistant Candida auris from the New York outbreak. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01124-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arendrup M.C., Chowdhary A., Astvad K.M.T., Jorgensen K.M. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiederhold N.P. Review of T-2307, an investigational agent that causes collapse of fungal mitochondrial membrane potential. J Fungi (Basel) 2021;7:130–138. doi: 10.3390/jof7020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abe M., Nakamura S., Kinjo Y., Masuyama Y., Mitsuyama J., Kaku M., et al. Efficacy of T-2307, a novel arylamidine, against ocular complications of disseminated candidiasis in mice. J Antimicrob Chemother. 2019;74:1327–1332. doi: 10.1093/jac/dkz020. [DOI] [PubMed] [Google Scholar]
- 94.Shibata T., Takahashi T., Yamada E., Kimura A., Nishikawa H., Hayakawa H., et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother. 2012;56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiederhold N.P., Najvar L.K., Jaramillo R., Olivo M., Patterson H., Connell A., et al. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against. Candida auris. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02198-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aghaei Gharehbolagh S., Izadi A., Talebi M., Sadeghi F., Zarrinnia A., Zarei F., et al. New weapons to fight a new enemy: a systematic review of drug combinations against the drug-resistant fungus. Candida auris. Mycoses. 2021;64:1308–1316. doi: 10.1111/myc.13277. [DOI] [PubMed] [Google Scholar]
- 97.O'Brien B., Chaturvedi S., Chaturvedi V. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02195-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bidaud A.L., Botterel F., Chowdhary A., Dannaoui E. In vitro antifungal combination of flucytosine with amphotericin B, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01393-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caballero U., Kim S., Eraso E., Quindos G., Vozmediano V., Schmidt S., et al. In vitro synergistic interactions of isavuconazole and echinocandins against. Candida auris. Antibiotics (Basel) 2021;10:355–367. doi: 10.3390/antibiotics10040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caballero U., Eraso E., Quindos G., Jauregizar N. In vitro interaction and killing-kinetics of amphotericin B combined with anidulafungin or caspofungin against. Candida auris. Pharmaceutics. 2021;13:1333–1343. doi: 10.3390/pharmaceutics13091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eldesouky H.E., Li X., Abutaleb N.S., Mohammad H., Seleem M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant. Candida auris. Int J Antimicrob Agents. 2018;52:754–761. doi: 10.1016/j.ijantimicag.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 102.Eldesouky H.E., Lanman N.A., Hazbun T.R., Seleem M.N. Aprepitant, an antiemetic agent, interferes with metal ion homeostasis of Candida auris and displays potent synergistic interactions with azole drugs. Virulence. 2020;11:1466–1481. doi: 10.1080/21505594.2020.1838741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eldesouky H.E., Salama E.A., Li X., Hazbun T.R., Mayhoub A.S., Seleem M.N. Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Sci Rep. 2020;10:7525–7537. doi: 10.1038/s41598-020-64571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eldesouky H.E., Salama E.A., Hazbun T.R., Mayhoub A.S., Seleem M.N. Ospemifene displays broad-spectrum synergistic interactions with itraconazole through potent interference with fungal efflux activities. Sci Rep. 2020;10:6089–6099. doi: 10.1038/s41598-020-62976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hao W., Wang Y., Xi Y., Yang Z., Zhang H., Ge X. Activity of chlorhexidine acetate in combination with fluconazole against suspensions and biofilms of. Candida auris. J Infect Chemother. 2022;28:29–34. doi: 10.1016/j.jiac.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 106.Wu Y., Totten M., Memon W., Ying C., Zhang S.X. In vitro antifungal susceptibility of the emerging multidrug-resistant pathogen Candida auris to miltefosine alone and in combination with amphotericin B. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02063-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bidaud A.L., Djenontin E., Botterel F., Chowdhary A., Dannaoui E. Colistin interacts synergistically with echinocandins against. Candida auris. Int J Antimicrob Agents. 2020;55:105901–105907. doi: 10.1016/j.ijantimicag.2020.105901. [DOI] [PubMed] [Google Scholar]
- 108.Iyer K.R., Camara K., Daniel-Ivad M., Trilles R., Pimentel-Elardo S.M., Fossen J.L., et al. An oxindole efflux inhibitor potentiates azoles and impairs virulence in the fungal pathogen. Candida auris. Nat commun. 2020;11:6429–6446. doi: 10.1038/s41467-020-20183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaban S., Patel M., Ahmad A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against. Candida auris. Sci Rep. 2020;10:1162–1170. doi: 10.1038/s41598-020-58203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagy F., Toth Z., Daroczi L., Szekely A., Borman A.M., Majoros L., et al. Farnesol increases the activity of echinocandins against Candida auris biofilms. Med Mycol. 2020;58:404–407. doi: 10.1093/mmy/myz057. [DOI] [PubMed] [Google Scholar]
- 111.Yang W., Tu J., Ji C., Li Z., Han G., Liu N., et al. Discovery of piperidol derivatives for combinational treatment of azole-resistant candidiasis. ACS Infect Dis. 2021;7:650–660. doi: 10.1021/acsinfecdis.0c00849. [DOI] [PubMed] [Google Scholar]
- 112.Eldesouky H.E., Mayhoub A., Hazbun T.R., Seleem M.N. Reversal of azole resistance in Candida albicans by sulfa antibacterial drugs. Antimicrob Agents Chemother. 2018;62:e00701–e00717. doi: 10.1128/AAC.00701-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeidler U., Bougnoux M.E., Lupan A., Helynck O., Doyen A., Garcia Z., et al. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J Antimicrob Chemother. 2013;68:1285–1296. doi: 10.1093/jac/dks538. [DOI] [PubMed] [Google Scholar]
- 114.Schwarz P., Nikolskiy I., Bidaud A.L., Sommer F., Bange G., Dannaoui E. In vitro activity of amphotericin B in combination with colistin against fungi responsible for invasive infections. J Fungi (Basel) 2022;8:115–131. doi: 10.3390/jof8020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schwarz P., Bidaud A.L., Dannaoui E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci Rep. 2020;10:21448–21456. doi: 10.1038/s41598-020-78588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stylianou M., Kulesskiy E., Lopes J.P., Granlund M., Wennerberg K., Urban C.F. Antifungal application of nonantifungal drugs. Antimicrob Agents Chemother. 2014;58:1055–1062. doi: 10.1128/AAC.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji C., Liu N., Tu J., Li Z., Han G., Li J., et al. Drug Repurposing of Haloperidol: discovery of new benzocyclane derivatives as potent antifungal agents against cryptococcosis and candidiasis. ACS Infect Dis. 2020;6:768–786. doi: 10.1021/acsinfecdis.9b00197. [DOI] [PubMed] [Google Scholar]
- 118.Yi D., Li Q., Wang H., Lv K., Ma L., Wang Y., et al. Repurposing of berbamine hydrochloride to inhibit Ebola virus by targeting viral glycoprotein. Acta Pharm Sin B. 2022 doi: 10.1016/j.apsb.2022.05.023. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan H., Sun J., Wang K., Wang H., Wu S., Bao L., et al. Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2. Acta Pharm Sin B. 2021;11:2850–2858. doi: 10.1016/j.apsb.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang G., Li L., Wang X., Li X., Zhang Y., Yu J., et al. Hypericin enhances β-lactam antibiotics activity by inhibiting sarA expression in methicillin-resistant. Staphylococcus aureus. Acta Pharm Sin B. 2019;9:1174–1182. doi: 10.1016/j.apsb.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng Y.S., Roma J.S., Shen M., Mota Fernandes C., Tsang P.S., Forbes H.E., et al. Identification of antifungal compounds against multidrug-resistant Candida auris utilizing a high-throughput drug-repurposing screen. Antimicrob Agents Chemother. 2021;65:e01305–e01320. doi: 10.1128/AAC.01305-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Oliveira H.C., Monteiro M.C., Rossi S.A., Pemán J., Ruiz-Gaitán A., Mendes-Giannini M.J.S., et al. Identification of off-patent compounds that present antifungal activity against the emerging fungal pathogen. Candida auris. Front Cell Infect Microbiol. 2019;9:83–93. doi: 10.3389/fcimb.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wall G., Chaturvedi A.K., Wormley F.L., Jr., Wiederhold N.P., Patterson H.P., Patterson T.F., et al. Screening a repurposing library for inhibitors of multidrug-resistant Candida auris identifies ebselen as a repositionable candidate for antifungal drug development. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Santi C., Scimmi C., Sancineto L. Ebselen and analogues: pharmacological properties and synthetic strategies for their preparation. Molecules. 2021;26:4230–4255. doi: 10.3390/molecules26144230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang J., Wang P., Dong C., Zhao Y., Zhou J., Yuan C., et al. Mechanisms of ebselen as a therapeutic and its pharmacology applications. Future Med Chem. 2020;12:2141–2160. doi: 10.4155/fmc-2019-0218. [DOI] [PubMed] [Google Scholar]
- 126.Billack B., Santoro M., Lau-Cam C. Growth inhibitory action of ebselen on fluconazole-resistant Candida albicans: role of the plasma membrane H+-ATPase. Microb Drug Resist. 2009;15:77–83. doi: 10.1089/mdr.2009.0872. [DOI] [PubMed] [Google Scholar]
- 127.Thangamani S., Eldesouky H.E., Mohammad H., Pascuzzi P.E., Avramova L., Hazbun T.R., et al. Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells. Biochim Biophys Acta Gen Subj. 2017;1861:3002–3010. doi: 10.1016/j.bbagen.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Azad G.K., Singh V., Mandal P., Singh P., Golla U., Baranwal S., et al. Ebselen induces reactive oxygen species (ROS)-mediated cytotoxicity in Saccharomyces cerevisiae with inhibition of glutamate dehydrogenase being a target. FEBS Open Bio. 2014;4:77–89. doi: 10.1016/j.fob.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Butts A., DiDone L., Koselny K., Baxter B.K., Chabrier-Rosello Y., Wellington M., et al. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot Cell. 2013;12:278–287. doi: 10.1128/EC.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng B., Li J., Wang Y., Chen P., Wang X., Cui J., et al. In vitro and in vivo effects of suloctidil on growth and biofilm formation of the opportunistic fungus. Candida albicans. Oncotarget. 2017;8:69972–69982. doi: 10.18632/oncotarget.19542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spitzer M., Griffiths E., Blakely K.M., Wildenhain J., Ejim L., Rossi L., et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol. 2011;7:499–513. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gowri M., Jayashree B., Jeyakanthan J., Girija E.K. Sertraline as a promising antifungal agent: inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action. in vitro. J Appl Microbiol. 2020;128:426–437. doi: 10.1111/jam.14490. [DOI] [PubMed] [Google Scholar]
- 133.Trevino-Rangel R.J., Villanueva-Lozano H., Mendez-Galomo K.S., Solis-Villegas E.M., Becerril-Garcia M.A., Montoya A.M., et al. In vivo evaluation of the antifungal activity of sertraline against. Aspergillus fumigatus. J Antimicrob Chemother. 2019;74:663–666. doi: 10.1093/jac/dky455. [DOI] [PubMed] [Google Scholar]
- 134.Lass-Florl C., Dierich M.P., Fuchs D., Semenitz E., Ledochowski M. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin Infect Dis. 2001;33:E135–E136. doi: 10.1086/324589. [DOI] [PubMed] [Google Scholar]
- 135.Rhein J., Huppler Hullsiek K., Tugume L., Nuwagira E., Mpoza E., Evans E.E., et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis. 2019;19:843–851. doi: 10.1016/S1473-3099(19)30127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li W., Yun Z., Ji C., Tu J., Yang W., Li J., et al. Discovery of novel sertraline derivatives as potent anti-cryptococcus agents. J Med Chem. 2022;65:6541–6554. doi: 10.1021/acs.jmedchem.1c01845. [DOI] [PubMed] [Google Scholar]
- 137.Kunin C.M., Ellis W.Y. Antimicrobial activities of mefloquine and a series of related compounds. Antimicrob Agents Chemother. 2000;44:848–852. doi: 10.1128/aac.44.4.848-852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Montoya M.C., Beattie S., Alden K.M., Krysan D.J. Derivatives of the antimalarial drug mefloquine are broad-spectrum antifungal molecules with activity against drug-resistant clinical isolates. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02331-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hao W., Qiao D., Han Y., Du N., Li X., Fan Y., et al. Identification of disulfiram as a potential antifungal drug by screening small molecular libraries. J Infect Chemother. 2021;27:696–701. doi: 10.1016/j.jiac.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 140.Khan S., Singhal S., Mathur T., Upadhyay D.J., Rattan A. Antifungal potential of disulfiram. Nippon Ishinkin Gakkai Zasshi. 2007;48:109–113. doi: 10.3314/jjmm.48.109. [DOI] [PubMed] [Google Scholar]
- 141.Mamouei Z., Alqarihi A., Singh S., Xu S., Mansour M.K., Ibrahim A.S., et al. Alexidine dihydrochloride has broad-spectrum activities against diverse fungal pathogens. mSphere. 2018;3 doi: 10.1128/mSphere.00539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang F., Zhao M., Braun D.R., Ericksen S.S., Piotrowski J.S., Nelson J., et al. A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science. 2020;370:974–978. doi: 10.1126/science.abd6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Filipuzzi I., Cotesta S., Perruccio F., Knapp B., Fu Y., Studer C., et al. High-resolution genetics identifies the lipid transfer protein Sec14p as target for antifungal ergolines. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pries V., Nocker C., Khan D., Johnen P., Hong Z., Tripathi A., et al. Target identification and mechanism of action of picolinamide and benzamide chemotypes with antifungal properties. Cell Chem Biol. 2018;25:279–290. doi: 10.1016/j.chembiol.2017.12.007. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caplan T., Lorente-Macias A., Stogios P.J., Evdokimova E., Hyde S., Wellington M.A., et al. Overcoming fungal echinocandin resistance through inhibition of the non-essential stress kinase Yck2. Cell Chem Biol. 2020;27:269–282. doi: 10.1016/j.chembiol.2019.12.008. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia M.D., Chua S.M.H., Low Y.S., Lee Y.T., Agnew-Francis K., Wang J.G., et al. Commercial AHAS-inhibiting herbicides are promising drug leads for the treatment of human fungal pathogenic infections. Proc Natl Acad Sci U S A. 2018;115 doi: 10.1073/pnas.1809422115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Agnew-Francis K.A., Tang Y., Lin X., Low Y.S., Wun S.J., Kuo A., et al. Herbicides that target acetohydroxyacid synthase are potent inhibitors of the growth of drug-resistant. Candida auris. ACS Infect Dis. 2020;6:2901–2912. doi: 10.1021/acsinfecdis.0c00229. [DOI] [PubMed] [Google Scholar]
- 148.Iyer K.R., Whitesell L., Porco J.A., Jr., Henkel T., Brown L.E., Robbins N., et al. Translation inhibition by rocaglates activates a species-specific cell death program in the emerging fungal pathogen. Candida auris. mBio. 2020;11 doi: 10.1128/mBio.03329-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fuchs F., Hof H., Hofmann S., Kurzai O., Meis J.F., Hamprecht A. Antifungal activity of nitroxoline against Candida auris isolates. Clin Microbiol Infect. 2021;27:1697. doi: 10.1016/j.cmi.2021.06.035. e7-e10. [DOI] [PubMed] [Google Scholar]
- 150.Garcia C., Burgain A., Chaillot J., Pic E., Khemiri I., Sellam A. A phenotypic small-molecule screen identifies halogenated salicylanilides as inhibitors of fungal morphogenesis, biofilm formation and host cell invasion. Sci Rep. 2018;8:11559–11564. doi: 10.1038/s41598-018-29973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tetz G., Collins M., Vikina D., Tetz V. In vitro activity of a novel antifungal compound, MYC-053, against clinically significant antifungal-resistant strains of Candida glabrata, Candida auris, Cryptococcus neoformans, and Pneumocystis spp. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01975-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Deodato D., Maccari G., De Luca F., Sanfilippo S., Casian A., Martini R., et al. Biological characterization and in vivo assessment of the activity of a new synthetic macrocyclic antifungal compound. J Med Chem. 2016;59:3854–3866. doi: 10.1021/acs.jmedchem.6b00018. [DOI] [PubMed] [Google Scholar]
- 153.Orofino F., Truglio G.I., Fiorucci D., D'Agostino I., Borgini M., Poggialini F., et al. In vitro characterization, ADME analysis, and histological and toxicological evaluation of BM1, a macrocyclic amidinourea active against azole-resistant Candida strains. Int J Antimicrob Agents. 2020;55:105865–105872. doi: 10.1016/j.ijantimicag.2019.105865. [DOI] [PubMed] [Google Scholar]
- 154.Hagras M., Salama E.A., Sayed A.M., Abutaleb N.S., Kotb A., Seleem M.N., et al. Oxadiazolylthiazoles as novel and selective antifungal agents. Eur J Med Chem. 2020;189:112046–112056. doi: 10.1016/j.ejmech.2020.112046. [DOI] [PubMed] [Google Scholar]
- 155.Mohammad H., Eldesouky H.E., Hazbun T., Mayhoub A.S., Seleem M.N. Identification of a phenylthiazole small molecule with dual antifungal and antibiofilm activity against Candida albicans and. Candida auris. Sci Rep. 2019;9:18941–18953. doi: 10.1038/s41598-019-55379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Argomedo L.M.Z., Barroso V.M., Barreiro C.S., Darbem M.P., Ishida K., Stefani H.A. Novel 2-aryloxazoline compounds exhibit an inhibitory effect on Candida spp., including antifungal-resistant isolates. ACS Med Chem Lett. 2020;11:2470–2475. doi: 10.1021/acsmedchemlett.0c00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Buda De Cesare G., Cristy S.A., Garsin D.A., Lorenz M.C. Antimicrobial peptides: a new frontier in antifungal therapy. mBio. 2020;11 doi: 10.1128/mBio.02123-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Puri S., Edgerton M. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot Cell. 2014;13:958–964. doi: 10.1128/EC.00095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pathirana R.U., Friedman J., Norris H.L., Salvatori O., McCall A.D., Kay J., et al. Fluconazole-resistant Candida auris is susceptible to salivary histatin 5 killing and to intrinsic host defenses. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01872-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rather I.A., Sabir J.S.M., Asseri A.H., Ali S. Antifungal activity of human cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in. Candida auris. J Fungi (Basel) 2022;8:204–221. doi: 10.3390/jof8020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Olekson M.A., You T., Savage P.B., Leung K.P. Antimicrobial ceragenins inhibit biofilms and affect mammalian cell viability and migration. in vitro. FEBS Open Bio. 2017;7:953–967. doi: 10.1002/2211-5463.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Durnas B., Wnorowska U., Pogoda K., Deptula P., Watek M., Piktel E., et al. Candidacidal activity of selected ceragenins and human cathelicidin LL-37 in experimental settings mimicking infection sites. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hashemi M.M., Rovig J., Holden B.S., Taylor M.F., Weber S., Wilson J., et al. Ceragenins are active against drug-resistant Candida auris clinical isolates in planktonic and biofilm forms. J Antimicrob Chemother. 2018;73:1537–1545. doi: 10.1093/jac/dky085. [DOI] [PubMed] [Google Scholar]
- 164.Dal Mas C., Rossato L., Shimizu T., Oliveira E.B., da Silva Junior P.I., Meis J.F., et al. Effects of the natural peptide crotamine from a South American rattlesnake on Candida auris, an emergent multidrug antifungal resistant human pathogen. Biomolecules. 2019;9:205–215. doi: 10.3390/biom9060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bugli F., Massaro F., Buonocore F., Saraceni P.R., Borocci S., Ceccacci F., et al. Design and characterization of myristoylated and non-myristoylated peptides effective against Candida spp. clinical isolates. Int J Mol Sci. 2022;23:2164–2187. doi: 10.3390/ijms23042164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raber H.F., Sejfijaj J., Kissmann A.K., Wittgens A., Gonzalez-Garcia M., Alba A., et al. Antimicrobial peptides Pom-1 and Pom-2 from pomacea poeyana are active against Candida auris, C. parapsilosis and C. albicans biofilms. Pathogens. 2021;10:496–505. doi: 10.3390/pathogens10040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Szerencses B., Gacser A., Endre G., Domonkos I., Tiricz H., Vagvolgyi C., et al. Symbiotic NCR peptide fragments affect the viability, morphology and biofilm formation of Candida species. Int J Mol Sci. 2021;22:3666–3686. doi: 10.3390/ijms22073666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vicente F.E.M., Gonzalez-Garcia M., Diaz Pico E., Moreno-Castillo E., Garay H.E., Rosi P.E., et al. Design of a helical-stabilized, cyclic, and nontoxic analogue of the peptide Cm-p5 with improved antifungal activity. ACS Omega. 2019;4:19081–19095. doi: 10.1021/acsomega.9b02201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kubiczek D., Raber H., Gonzalez-Garcia M., Morales-Vicente F., Staendker L., Otero-Gonzalez A.J., et al. Derivates of the antifungal peptide Cm-p5 inhibit development of Candida auris biofilms. in vitro. Antibiotics (Basel) 2020;9:363–370. doi: 10.3390/antibiotics9070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Eijk M., Boerefijn S., Cen L., Rosa M., Morren M.J.H., van der Ent C.K., et al. Cathelicidin-inspired antimicrobial peptides as novel antifungal compounds. Med Mycol. 2020;58:1073–1084. doi: 10.1093/mmy/myaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Basso V., Garcia A., Tran D.Q., Schaal J.B., Tran P., Ngole D., et al. Fungicidal potency and mechanisms of theta-defensins against multidrug-resistant Candida species. Antimicrob Agents Chemother. 2018;62:e00111–e00118. doi: 10.1128/AAC.00111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramachandran R., Shrivastava M., Narayanan N.N., Thakur R.L., Chakrabarti A., Roy U. Evaluation of antifungal efficacy of three new cyclic lipopeptides of the class bacillomycin from Bacillus subtilis RLID 12.1. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]