Abstract
Nano-drug delivery strategies have been highlighted in cancer treatment, and much effort has been made in the optimization of bioavailability, biocompatibility, pharmacokinetics profiles, and in vivo distributions of anticancer nano-drug delivery systems. However, problems still exist in the delicate balance between improved anticancer efficacy and reduced toxicity to normal tissues, and opportunities arise along with the development of smart stimuli-responsive delivery strategies. By on-demand responsiveness towards exogenous or endogenous stimulus, these smart delivery systems hold promise for advanced tumor-specificity as well as controllable release behavior in a spatial-temporal manner. Meanwhile, the blossom of nanotechnology, material sciences, and biomedical sciences has shed light on the diverse modern drug delivery systems with smart characteristics, versatile functions, and modification possibilities. This review summarizes the current progress in various strategies for smart drug delivery systems against malignancies and introduces the representative endogenous and exogenous stimuli-responsive smart delivery systems. It may provide references for researchers in the fields of drug delivery, biomaterials, and nanotechnology.
KEY WORDS: Pharmaceutics, Smart drug delivery system, Stimuli-responsive, Receptor-ligand-based delivery, Nano-drug delivery systems, Precise therapy, Toxicity, Cancer
Graphical abstract
The development of smart drug delivery strategies and relevant drug delivery systems could provide solutions for the insufficient specificity and toxicity concerns in anticancer therapy.
1. Introduction
Medications against malignancies are usually hindered by poor specificity and subsequent concerns of toxicity, and their therapeutic effects may also be challenged by deficient concentrations at tumor sites, drug resistances, etc1. Despite the availability of many other tactics for anticancer treatments, e.g., surgeries and radiation therapies, their limited ranges of application still urge the development of modern anticancer delivery strategies2. Nano-drug delivery systems (NDDS) provide promising platforms for anticancer therapy, holding potential for versatile improvements in the in vivo distributions, behaviors, and performances of therapeutic agents3. Smart drug delivery systems have been a highlighted field in NDDS, which could provide targeting specificity, controlled release, as well as the ability to cross biological barriers, giving rise to enhanced therapeutic effects with minimized systemic side effects4.
The development of functionalized modules and materials, including modern biomaterials such as peptides and nucleotides, has supported the design and research of various smart delivery strategies. Stimuli-responsiveness towards endogenous or exogenous factors offers on-demand transitions of vehicle structures or properties, enabling spatial-, temporal- or dosage-specific deliveries5. On the other hand, the diverse nano-drug delivery vehicles also facilitate the rational design based on proper therapeutic agents and pro-drugs, as well as the applications of combinational therapies, multi-responsive platforms, etc.
This review focuses on the recent advances in smart drug delivery strategies and systems. In this review, we highlighted the main strategies and mechanisms of smart drug loading and release for anticancer therapies, and also summarized the current delivery systems supporting these strategies.
2. Strategies and mechanisms of smart drug loading and release
Smart nanoparticles have constituted an excellent platform for achieving efficient cancer therapy, which is considered an extensively explored stimuli-responsive approach to specifically release the cargoes at the tumor sites in response to endogenous (pH, enzymes, or redox gradients) or exogenous stimuli (light, temperature, ultrasound, magnetic field, and electric field). Such stimuli-responsive nanoparticles can provide on-demand drug release, thus achieving more delicate therapeutic effects and preventing drug leakage in blood circulation for avoiding off-target side effect6.
2.1. Endogenous stimulus-responsive DDSs
The intrinsic biological factors of tumor tissues differ significantly from healthy tissues, mainly including low pH values, over-expression of specific enzymes, increased redox-potential and hypoxia, etc7. Based on these differences, the pH-, enzyme-, and redox-responsive drug delivery systems (DDS) have been rationally engineered for smart drug loading and spatiotemporal release in specific targets for enhanced therapeutic efficacy.
2.1.1. pH-Responsive DDSs
Currently, there are two main strategies for the development of pH-responsive DDSs. One strategy is based on the structure or solubility change of polymers containing ionizable functional groups8. Various ionizable groups (e.g., carboxylic acids and amines) in the nanoparticles can be protonated upon pH variations, disrupting the hydrophilic–hydrophobic equilibrium and triggering a dramatic change of structure or solubility of the nanoparticles, thereby realizing pH-responsive drug release9. The other strategy is based on the cleavage or the degradation of acid-labile bonds. Chemical bonds such as hydrazone, ester, imine, oxime, and ketal bonds are stable at neutral pH but can be cleaved under acidic conditions. Therefore, constructing nanocarriers with pH-cleavable chemical bonds or using these bonds for drug conjugation can achieve prompt drug release in acidic environment10. Through the above strategies, various pH-responsive DDSs differentiating the pathophysiological pH gradients in the body have been designed for cancer therapy with high efficacy and low toxicity.
The pH gradients throughout the body can be divided into three levels: organ, tissue, and cellular levels. At the organ level, the most significant pH variation is that of the gastrointestinal tract (GIT). Different segments of the GIT have their own characteristic intraluminal pH levels, from the acidic stomach (pH 1–3) to the alkaline intestine and colon (pH 6.5–7.5)11. Therefore, the pH difference of these parts can be exploited to design DDS for gastric retention or specific targeted intestinal drug delivery. Currently, colorectal cancer is a common cancer type and the third leading cause of death among the malignancies in the United States12. However, therapeutic drug molecules such as proteins and peptides are vulnerable to acidic and enzymatic degradation in the stomach, raising challenges for their oral administration. Fortunately, oral pH-responsive colon-targeted DDSs hold great promise for colorectal cancer therapy13. Some pH-sensitive polymers, e.g., cellulose acetate phthalates (CAP) and methacrylate-based Eudragit® polymers, could withstand the acidic pH of the stomach and be dissolved in the alkaline milieu of the colon, which could be adopted to protect the encapsulated anticancer drugs from degradation in the harsh condition of the stomach. For instance, a colon-targeted, oral nanoparticle vaccine was constructed by encapsulating protein vaccine into poly(D,L-lactic-co-glycolic acid) (PLGA) nanoparticles, and then loading PLGA nanoparticles into Eudragit FS30D microparticles14. The mucosal uptake of microparticles was impeded due to their large diameters (>10 μm) until they disintegrated into PLGA nanoparticles in the colon (pH > 7). Therefore, the microparticle could avoid premature degradation and uptake of vaccines and precisely deliver vaccines into the colon, thus inducing effective protective immunity.
At the tissue level, the extracellular pH (pHe) of the tumor microenvironment (pH 6.7–7.1) is slightly lower than that of healthy tissue and blood (pH 7.4). Tumor cells exhibit vigorous growth and metabolism, and the irregular vasculature in tumors is insufficient to supply nutrients and oxygen for the fast-growing tumors, thus the tumors shift towards a glycolytic metabolism and produce acidic metabolites such as lactic acid and CO2, lowering the pH of tumor interstitium15. However, there is only a subtle pH change in the tumor microenvironment as compared with the normal physiological environment. Thus, the ideal tumor pHe-responsive DDS should have a sharp response to pH variation. Intriguingly, based on the cooperative self-assembly of block copolymers with ionizable tertiary amine groups, Ma and coworkers16 pioneered the development of ultra-pH-sensitive (UPS) nanotechnology for cancer theranostics. The UPS nanoparticles micelles exhibited ultra pH sensitivity within 0.25 pH unit, tunable pH transition (pHt) ranging from 4.4 to 7.1, ultra-fast pH-triggered dissociation (<5 ms), and exponential signal amplification (>100-fold) upon pH-driven micelle dissociation, with extensive utilization for cancer imaging and image-guided cancer surgery17. Based on the UPS nanotechnology, ONM-100, an indocyanine green (ICG)-encoded fluorescence nanoprobe is in phase II clinical trial for image-guided cancer resection and metastatic lymph node mapping. Moreover, the UPS nanoplatform has also been widely used in chemical drug, gene, and vaccine delivery18.
At the cellular level, the pH of endocytic organelles is much lower than that of cytoplasm (pH 7.2). Following endocytosis, endosomal acidification occurs rapidly due to the vacuolar-type ATPase (V-ATPase) proton pump-mediated proton influx into the lumen of the organelles. Accordingly, the pHs of early endosome, late endosome, and lysosome are dropped from about 6.3 to 5.5, and finally around 4.719. The low pH of endocytic organelles is a widely used trigger for pH-responsive DDS which can realize organelle-specific activation and on-demand drug release. For instance, an EGFR-targeted ultra-pH-sensitive nano-photosensitizer was developed by Yan and coworkers for endocytic organelles-specific photodynamic therapy (PDT)20. The photoactivity of photosensitizer Ce6 was “OFF” at physiological pH due to the FRET (Fluorescence Resonance Energy Transfer) quenching at micelle state, which could be turned “ON” upon micelle dissociation in the acidic endocytic organelles for cancer cell killing. A UPS nanoparticle with sequential targeting ability of early endosome and mitochondria was also developed for exponential amplification of PDT efficacy21. The nanoparticle could immediately dissociate at the acidic environment of the early endosome, enabling the photoactivity activation of the mitochondria-targeted photosensitizer TPP-PPa. Then, the activated TPP-PPa quickly escaped from the early endosome and targeted the mitochondria for effective PDT upon laser irradiation. However, the acidic environment and harsh enzymatic activity of endocytic organelles are also harmful to some therapeutic macromolecules such as proteins, small interfering RNA (siRNA), and DNA. Therefore, to achieve the maximum therapeutic effects of macromolecules, various pH-responsive DDS are also designed for endosomal escape and cytosolic delivery based on the “proton sponge” effect22, with cationic materials such as poly(ethylene imine)s (PEIs)23.
2.1.2. Enzyme-responsive DDSs
The up-regulation of specific enzymes in the tumor microenvironment and inside the tumor cells has been exploited as important triggers for smart drug delivery. Many enzymes like proteases (e.g., matrix metalloproteinase/MMP and cathepsin B), phospholipases (e.g., phospholipase A2), and peptidases (e.g., aminopeptidase), etc. have been investigated to construct enzyme-responsive DDS for tumor-tropism drug delivery24. The enzyme-responsive DDS could act in the following ways: (i) Enzyme-triggered drug release either by constructing nanocarriers with a structural scaffold susceptible to specific enzymes or by using an enzyme-sensitive linker between the nanocarrier and therapeutics. Du et al.25 developed a pH/cathepsin B hierarchical-responsive micelle for the programmed delivery of docetaxel (DTX). The micelle remained stable in blood circulation, while dissociated into polymer-DTX conjugates under acidic tumor microenvironment for deep tumor penetration and enhanced cellular uptake. After being endocytosed into the lysosomes, the Gly–Phe–Leu–Gly (GFLG) tetrapeptide linker of polymer-DTX conjugates was cleaved by lysosomal cathepsin B, then bioactive DTX was effectively released into the cytoplasm for enhanced antitumor efficacy. (ii) Prodrugs, ligands, and probes activation through the cleavage of enzyme-sensitive bonds. Zheng et al.26 conjugated a photosensitizer (Pyro) and 1O2 quencher (BHQ3) with a matrix metalloproteinase-7 (MMP-7)-cleavable peptide linker, enabling effective 1O2 quenching of Pyro through FRET effect. Therefore, this construct was photodynamically inactive until entering the tumor with high MMP-7 expression. Upon MMP-7-induced cleavage, the photoreactivity of Pyro was recovered, thus producing cytotoxic 1O2 under light irradiation. (iii) Enzyme-activated detachable PEGylation layer for prolonged circulation and increased cellular internalization at the target site. Han and coworkers27 prepared a dual-enzyme-responsive, gemcitabine-loaded (GEM) nanovector by conjugation of matrix metalloproteinase-9 (MMP-9)-detachable poly(ethylene glycol) (PEG) protection layer, cathepsin B-cleavable GEM, and tumor-homing motif cRGD peptide to quantum dots (QDs). The nanovectors exhibited prolonged blood circulation due to the PEG decoration. Then, the PEG shield layer could be removed by the overexpressed MMP-9 after accumulation in tumor tissue, enabling the exposure of cRGD for enhanced cellular internalization. Once endocytosed into cancer cells, the GEM could be released by elevated lysosomal cathepsin B for tumor inhibition. Enzyme-responsiveness could assist in the development of size-changeable nanoparticle systems, realizing aggregation and retention at target sites, optimized targeting, and internalization, etc28, 29, 30. Despite the huge progress made in the development of enzyme-responsive DDS for cancer therapy, several challenges remain to be solved. Firstly, some enzymes share similar active sites and catalytic mechanisms, leading to their similar substrate preferences31. Secondly, the enzyme expression level varies greatly not only in different cancer types, but also in tumors with a similar type yet in different individuals, and in different parts of the tumor as well32.
2.1.3. Redox responsive DDSs
Redox homeostasis is critical for cell survival with diverse cellular processes involved, and glutathione (GSH), a tripeptide that can scavenge excess reactive oxygen species (ROS) through the transformation of its heterogeneous forms (GSH/GSSG), plays a key role in maintaining the intracellular redox balance33. Due to the highly reductive and hypoxic microenvironment of tumor tissues, the intracellular GSH level in tumor cells was four times higher than that in normal cells34. In addition, the concentration of GSH varies greatly between the intracellular environments (about 2–10 mmol/L) and the extracellular environments (about 2–10 μmol/L). The remarkable concentration gradient of GSH (about 100‒1000 times) serves as an attractive stimulus for tumor-targeted intracellular drug delivery, especially for cytoplasmic delivery of proteins and genes (DNA or siRNA). Various redox responsive DDS have been developed by introducing reduction-sensitive bonds (disulfide bonds, diselenide bonds, ditelluride bonds, etc.) into the nanocarrier backbones or for drug conjugation35. The disulfide bond (S–S), prone to fast cleavage by GSH, is the most commonly used linker for the fabrication of redox-responsive DDS, due to its easy introduction into polymers and drugs36. For instance, Wu and coworkers37 prepared a nanocomplex (siRNA/DOX@HMONs-ss-PAE) for redox-responsive gene delivery to reverse the multidrug resistance (MDR). The P-glycoprotein (P-gp) modulator siRNA and anticancer drug doxorubicin (DOX) were loaded into the hollow mesoporous organosilica nanoparticles (HMONs), which were capped with poly (β-amino esters) (PAE) through a disulfide bond. When endocytosed into tumor cells, the nanocomplex could translocate from the endo/lysosome to the cytoplasm via the “proton sponge” effect of cationic PAE, and then the encapsulated DOX and siRNA were released due to the cleavage of the disulfide bond between HMONs and capping PAE in the highly reductive intracellular microenvironment. Thus, the P-gp-mediated MDR would be reversed by siRNA, leading to enhanced intracellular DOX concentration for efficient chemotherapy of cancer cells.
In addition, tumor cells also have elevated ROS (e.g., H2O2, superoxide, and hydroxyl radicals) generation than normal cells due to their hypermetabolism38. Hence, various types of ROS-responsive DDS have been explored for tumor-specific drug delivery on the basis of the high ROS level in tumor tissues. Many ROS-responsive linkages are frequently used in cancer therapy, including thioketal, thioether, peroxalate ester, boronic ester, etc39. Xu et al.40 developed an innovative ROS-responsive poly-prodrug nanoparticle for targeted cancer therapy. The nanoparticle was composed of a poly-prodrug inner core of ROS-responsive mitoxantrone (MTO) for ROS-triggered drug release, a PEG outer shell for prolonged blood circulation, and an iRGD targeting ligand for deep tumor penetration and specific tumor targeting. Upon exposure to high-level ROS in tumor cells, the thioketal bond in the poly-prodrug was cleaved, leading to the release of intact drug molecules for efficient tumor inhibition.
2.2. Exogenous stimulus-responsive DDSs
In some cases, endogenously stimuli-responsive nanoparticles fail to overcome the biological barriers in tumors due to insufficient and intractable responses to some subtle alterations of the above endogenous factors in the tumor microenvironment. Reasonably, exogenously stimuli-responsive DDSs are considered alternative nanoplatforms that are of great importance due to target-specific and controlled drug release at the target sites.
Exogenous stimuli-responsive DDSs are smart drug delivery systems that can actively release the cargo in response to external stimuli including light, temperature, ultrasound, magnetic field, electric field, and other stimuli, maximizing therapeutic efficacy of cargos while reducing adverse side effects. The exogenous stimuli can undergo a chemical or physical change to cause the alteration of structures and surface properties of DDSs, which ensure their tumor targeting, penetration, cellular uptake, and intracellular drug delivery to be manipulated in a controllable manner7. There are various advantages of exogenous stimuli-responsive DDSs in cancer therapy including (i) the location and intensity can be precisely controlled by exogenous stimuli (e.g., light, magnetic or electric field); (ii) the exogenous stimuli can be flexibly applied or removed; (iii) multiple exogenous stimuli can be integrated into a single nanoplatform for providing multifunctional properties, although such DDSs would be unsuitable for treating distal or metastatic cancer since their tumor locations are unknown.
2.2.1. Light-responsive DDSs
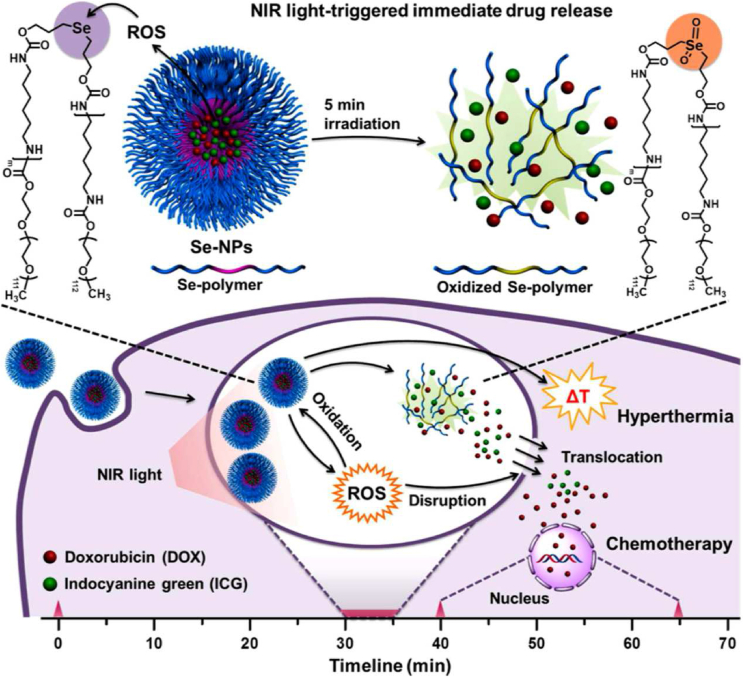
Light-responsive DDSs for cancer therapy have been extensively developed, since light [e.g., near-infrared (NIR) and visible light] is an attractive exogenous stimulus with a possibility to remotely control the irradiation power and exposure time for selectively treating local tumors. Lights can induce physicochemical changes, such as photoisomerization/photocleavage reactions and photothermal/photodynamic effects, thus improving the performance of light-responsive DDSs. Compared with ultraviolet (UV) and visible lights, NIR light with relatively deep tissue penetration ability is of particular interest in cancer therapy. For instance, the NIR light-responsive selenium-contained polymeric nanoparticles loading indocyanine green and doxorubicin (I/D-Se NPs) were designed for synergistic thermo-chemotherapy41. The prepared I/D-Se NPs could produce ultrafast irreversible disassembly via light-mediated selenium oxidation, thus promoting the NIR light-responsive drug release in cell cytoplasm for synergistic cancer therapy (Fig. 1)41. Zhao et al.42 reported the bifunctional light-responsive platinum nanocomplexes (PtNCs) that produced abundant heat via photothermal conversion from the Pt0 core upon irradiation and simultaneously caused a rapid release of chemotherapeutic Pt2+ ions surrounding the Pt0 core, thus leading to a light-triggered synergistic chemo-photothermal therapy. In another study, the light-responsive palladium nanocrystals-assimilated nanoscale metal-organic framework (NPMOF) nanoparticles were developed for synergistic hydrogen and photodynamic therapy, with significant light-triggered singlet oxygen (1O2) generation and persistent reductive hydrogen release, causing an adequate disturbance in tumor milieu for synergistic tumor therapy43. Recently, Chen et al.44 synthesized NIR-light modulated BF2-azadipyrromethene (aza-BODIPY) nanoaggregates that were actively transformed from wormlike nanofibers into spherical nanoparticles in vivo under light irradiation, with prolonged circulation, deep tumor penetration, and subsequent enhanced antitumor efficacy. More recently, aggregation-induced emission (AIE) luminogen-encapsulated lipid nanoparticles with robust NIR-I two-photon absorption were developed for spatiotemporal deep-tumor imaging, and simultaneously producing toxic hydroxyl radicals (•OH) and singlet oxygen (1O2) upon light irradiation for tumor ablation45. Zwitterionic luminogen nanodots with AIE features also showed enhanced photothermal conversion efficiency (35.76%) and ROS generation performance under NIR-light irradiation, providing synergistic phototherapy against breast cancer46. In another study, Ren and co-workers47 successfully developed a light-responsive multifunctional Bi2Se3-based nanoplatform loaded with glucose oxidase and oxygenated perfluorocarbon, for improved tumor starvation and light-mediated photothermal therapy (PTT). Light irradiation not only enabled Bi2Se3 nanoshell to generate local hyperthermia, but also triggered the release of capsulized oxygen to recede local hypoxia.
Figure 1.
Light-responsive polymeric nanoparticles (I/D-Se-NPs) with fast drug release and an efficient cytoplasmic translocation for synergistic thermo-chemotherapy. Reprinted with the permission from Ref. 41. Copyright © 2017 American Chemical Society.
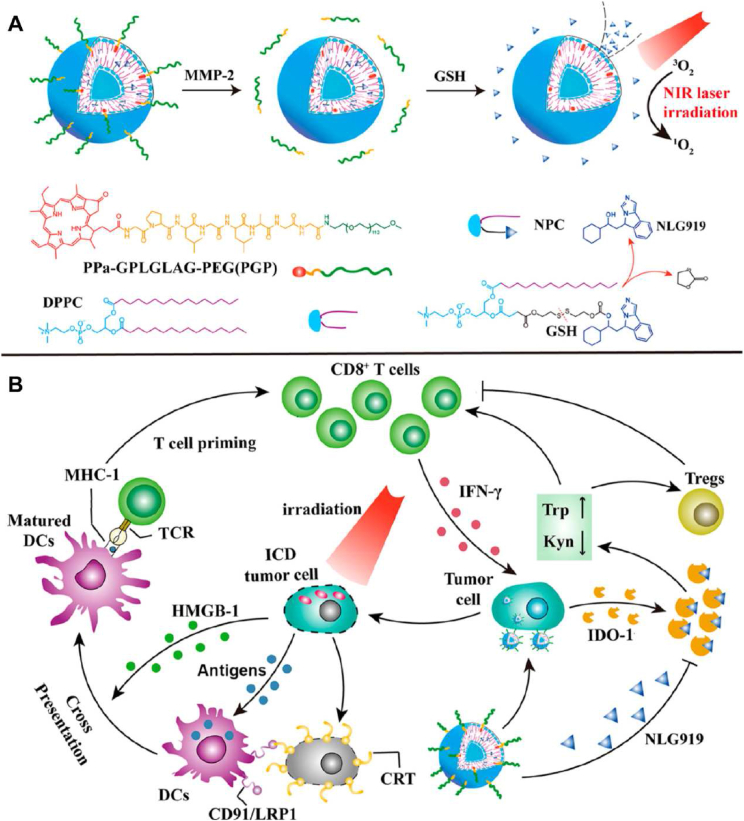
Specifically, drug resistance has been a vital challenge in anticancer therapies. Insufficient drug delivery to the tumor cells and sublethal chemotherapeutic concentration could lead to acquired chemo-resistance48, 49, 50, 51, and the recruitment of tumor-infiltrating cytotoxic T lymphocytes (CTLs) and intratumoral secretion of proinflammatory cytokine interferon gamma (IFN-γ), which consequently eliciting surface levels of immune regulators including programmed death ligand 1 (PD-L1) could hamper immunotherapeutic effects52, 53, 54, 55, 56. NIR-responsive nanoparticles were also reported in dealing with chemo- and immuno-resistance of the tumor through single or combination of multiple mechanisms including hyperthermia effects, photothermal ablations, immunogenic cell death (ICD) induced by laser irradiation, spatiotemporally tunable release, etc., exhibiting prospects for comprehensive anticancer therapy50,51,57, 58, 59, 60, 61, 62 (Fig. 2, reprinted from Ref. 57).
Figure 2.
(A) MMP-2-sheddable and GSH-activatable prodrug vesicle for combined photodynamic immunotherapy. (B) The mechanism of photodynamic immunotherapy against cancer combating IDO-1-induced adaptive immune resistance. Reprinted with the permission from Ref. 57. Copyright © 2019 American Chemical Society.
Researchers have also been pursuing photodynamic therapies with further reduced dark toxicity and side effects63. AIE has emerged as a solution to the unwanted phototoxicity at non-target sites, and smart delivery strategies also proved to be effective by providing more specified location, on-demand activation or release at the target sites64. Increased selectivity could be realized by the conjugation of photosensitizers with targeting moieties, for example, and the photosensitizer antibody-drug conjugates have been extensively reviewed in previous articles, with highlights on highly specific bioconjugation techniques65. Dual-responsiveness with multiple triggers instead of single light-responsiveness also add to the specificity of photosensitizers, and the on-demand activation strategies with various on-off mechanisms further alleviated the dark toxicity. Dong et al.66 prepared a calcium carbonate-polydopamine composite hollow nanoparticle, and the loaded photosensitizer was quenched by polydopamine and could be released in acidic environment. Feng et al.67 developed a nanoassembly system with pyridinium-functionalized tetraphenylethylene encapsulated in the cavity of water-soluble calixarene, which only exhibits yellow fluorescence upon light triggering due to the restriction of intramolecular motion. The photosensitizer could be displaced by 4,4′-benzidine dihydrochloride and translocate to mitochondria with restored photoactivity. Another work also concerns photosensitizer activation by displacement, the photosensitizer was quenched by macrocyclic amphiphile, and the overexpressed adenosine triphosphates on tumor sites competitively bind to the vehicle macrocyclic amphiphile, realizing release and activation of the photosensitizer at the tumor site68.
2.2.2. Temperature-responsive DDSs
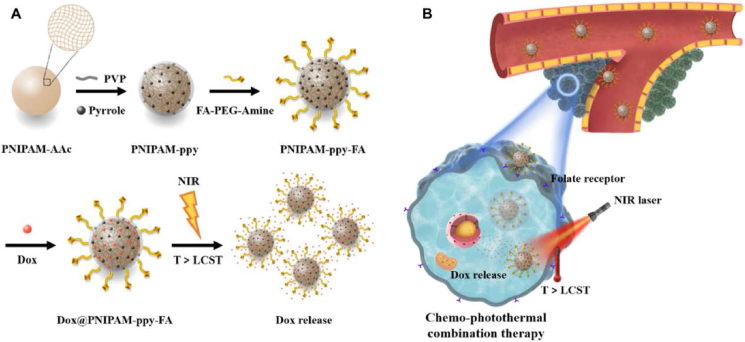
Temperature-responsive DDSs have also been extensively applied as drug delivery vehicles in cancer therapy. Generally, the DDSs are required to be stable and to retain the cargoes in normal body temperature (up to 37 °C), however sensitively release the cargoes at higher temperature (e.g., >40 °C) through significant physico-chemical changes in response to a narrow temperature increase7. The most popular temperature-sensitive polymeric materials include poly(amidoamine) (PAMAM), poly[2-(2-methoxyethoxy)ethyl methacrylate] (PMEO2MA), poly(N-isopropylacrylamide) (PNIPAM) and poly(2-oxazoline)s (POxs). In response to lower critical solution temperature (LCST), the temperature-sensitive polymers often show drastic changes in their aqueous solubility69. Above their LCST, these polymers become insoluble in water, and subsequently, the DDSs are destructed to release the cargo. For example, PNIPAM-based nanocomposites were found to actively release doxorubicin in response to increased temperature, as PNIPAM endured a reversible phase change beyond their LCST (Fig. 3)70. In another study, PNIPAM-based thermo-sensitive polypyrrole nanoplatforms were developed for synergistic photothermal-chemotherapy71. Additionally, some cancerous tissues exhibit abnormal increases (generally 1–2 °C) in temperature compared with normal tissues. Such temperature change could be a potential endogenous stimulus in drug delivery, which raises higher requirements for the precise and tunable responsiveness of the materials. Current thermosensitive polymers and nanocarriers have been intensively reviewed in Ref. 72.
Figure 3.
(A) Synthesis and thermo-triggered drug release from DOX@PNIPAM-ppy-FA nanocomposites. (B) The mechanism of temperature-responsive release of doxorubicin and enhanced thermo-chemotherapy in breast cancer. Reprinted with the permission from Ref. 70. Copyright © 2020, Springer Nature.
Besides, temperature-responsive DDSs were also developed by incorporating thermo-sensitive cargos inside the nanovehicles. For instance, gold nanorods and iron oxide nanoparticles-incorporated liposomes could release doxorubicin upon hyperemia (50 °C) caused by light irradiation due to the collapse of the liposomes, leading to the promoted tumor destruction73. Despite the extensive progress in temperature-responsive DDSs, only very few thermo-sensitive vectors are currently considered for clinical translation in addition to liposomes and gold nanoparticles. It is highly important to develop temperature-sensitive materials and vectors with high biosafety.
2.2.3. Ultrasound-responsive DDSs
Ultrasound-responsive DDSs have various applications in cancer therapy as they could specifically release payloads at the tumor site in response to externally applied ultrasound, showing promise for targeting ability, deep tumor penetration, and reduced side effects by adjusting their frequency in a specific range. For instance, high-frequency ultrasound waves (>20 kHz) could be used to trigger drug release or improve the tumor permeability of nanoparticles, while low ultrasound frequencies (<20 kHz) were usually applied for imaging7,74. Ultrasound also induces several physical effects such as cavitation, acoustic fluid streaming, and local hyperthermia, commonly adopted as triggers for ultrasound-responsive cargoes release (e.g., anticancer agents, imaging probes) from DDSs at the desired tumor sites75.
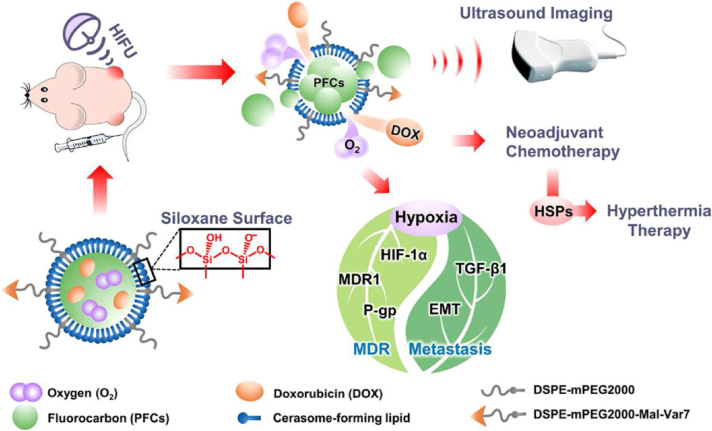
Many researches have supported the effectiveness of ultrasound triggering in accelerating drug release, endosomal escape, tumor accumulation, and significant tumor growth reduction75, 76, 77, and gene delivery has become a highlight in ultrasound-assisted delivery against solid tumors78. Meng et al.79 designed an ultrasound-responsive, self-healing hydrogel system loaded with nanovaccines, realizing remote release control and repeated vaccine release for durable anticancer therapy. Ultrasound-induced mild hyperthermia could also be utilized in DDS design, and Ma et al.77 constructed doxorubicin and oxygen-encapsulated cerasomal perfluorocarbon nanodroplets, which remained stable during blood circulation, while ultrasound-induced mild hyperthermia upon sonication could cause the disturbance and gasification of perfluorocarbon, producing drug release channels in cerasomes that significantly accelerating the release of doxorubicin and oxygen at the tumor site (Fig. 4)77. Besides the modulation of DDSs, ultrasound has also been widely applied in modulating the delivery barriers and assisting deep penetration into the tumor sites, as well as inducing immunological responses, as reviewed in Refs. 80 and 81.
Figure 4.
Ultrasound-triggered release of doxorubicin and oxygen from cerasomal perfluorocarbon nanodroplets. Reprinted with the permission from Ref. 77. Copyright © 2020 American Chemical Society.
2.2.4. Magnetic field-responsive DDSs
Magnetic field-responsive DDSs have gained considerable interest in the field of cancer therapy, due to their targeting potentials from the intrinsic tropism towards magnetic fields, and efficient local hyperthermia under external alternating magnetic field (AMF) for promoting on-demand drug release and effective cancer imaging and therapy. Magnetic field-responsive DDSs are generally comprised of a magnetic core with materials such as magnetite (Fe3O4), maghemite (Fe2O3), hybrid iron oxide (graphene/Au/Fe3O4), and other magnetic materials (ZnFe2O4), as well as a coating outlayer with materials including polymers, lipids, proteins, and mesoporous silica, etc. Superparamagnetic iron oxide nanoparticles (SPIONs) are predominantly adopted due to their targeting performance in external magnetic fields without retaining any residual magnetism after its withdrawal82. Besides magnetic materials, anticancer drugs, contrast agents, photosensitizers, plasmids, and antibodies could also be assimilated inside the magnetic field-responsive DDSs for achieving multimodal therapeutic activities. For example, doxorubicin and bioactive proteins were incorporated into magnetic Mn–Zn ferrite polymeric nanoparticles for thermo-chemotherapy under AMF83. Furthermore, the AMF-induced hyperthermia could also trigger on-demand drug release from the magnetic field-responsive DDSs in the diseased regions84. Generally, the magnetic field-responsive DDSs are often required to provide target-specific delivery, because they might affect healthy organs or tissues that are distributed with magnetic field-responsive DDSs when exposed to the AMF.
2.2.5. Electric field-responsive DDSs
Based on the electro-responsive materials such as conductive polymers (e.g., doped polypyrrole, polyaniline), polymer complexes with conductive materials (e.g., graphene, metallic nanoparticles) or hydrogel materials (e.g., alginate, chitosan), as well as installable electrical drug delivery devices, the electric field also proves to be a potential trigger for on-demand drug release85. DDSs comprised of conductive materials could provide controlled drug release through electro-chemical oxidation/reduction and the movement of charged moieties86. Various researches have been done on electro-responsive hydrogels, e.g., an injectable chitosan-graft-polyaniline copolymer crosslinked with oxidized dextran with dual-responsive drug release to both pH and electric field87, and different electroactive moieties (e.g., aniline trimer88, graphene oxide89, polyacrylamide89, etc.) were studied, which was reviewed by Carayon et al.90 Besides the activation of DDSs, electric field could also exhibit various physiological effects on tissues and cells, assisting the delivery of therapeutic agents. Electroporation, for example, could transiently promote the permeability of drugs through cell membranes, with intense research in the delivery of macromolecules such as proteins and genes91,92, while strong electric fields could further realize ablation or cytotoxicity on tumor cells and microorganisms by themselves93. Additionally, distinct electric fields could be generated endogenously by injured or pathological tissues compared with normal sites, e.g., Ying et al.94 developed an angiopep–2-modified electro-responsive hydrogel nanoparticle system, with enhanced brain accumulation and in situ drug release triggered by electroencephalograph epileptiform abnormalities.
2.2.6. Other exogenous stimuli-responsive DDSs
Various other exogenous stimuli-responsive DDSs have also been developed for remote-controlled cancer therapy, such as high-energy radiation (X-rays and gamma-rays). For example, the diselenide block copolymers-based nanoparticles loaded with doxorubicin were constructed to trigger drug release under a reduced dose (2 Gy) of X-ray radiation at the tumor site95. Besides, X-ray exposure can also enhance tumor accumulation and cellular uptake of albumin nanoparticles by increasing the expression of caveolin-1 on tumor cells96.
Moreover, exogenous stimuli-responsive DDSs also offer opportunities to overcome biological barriers such as reversing MDR and promoting lysosomal/endosomal escape. Besides, they can also integrate some novel therapeutic modalities, including photodynamic therapy, phototherapy, ultrasound, and magnetic hyperthermia, which are good alternatives to conventional cancer therapy.
2.3. Receptor-ligand-based smart DDS
Targeted delivery also contributes to modern smart DDSs. In general, tumor-targeting drug delivery systems can be constructed through two strategies: passive targeting and active targeting. In the past, numerous studies were based on the passive targeting strategy, but with decades of intensive research, there are increasingly controversial opinions on the efficiency of passive targeting based on the EPR effect. In 2017, Li et al.97 demonstrated that the active and passive effects made different contributions to the total accumulation of nanoparticles (NPs) in tumors, and they found that the receptor-mediated targeting contributed more than the EPR effect over time. In addition, NP transportation through the inter-endothelial cell gaps in the tumor blood vessels was considered to be one of the dominant factors of the EPR effect. Recently, however, Sindhwani et al.98 proved that the overall gap coverage was only 0.048% of the blood vessel surface area, which was 60-fold less than the required amount to explain the observed NP accumulation. Therefore, the concept that the passive effect occupies a central role in targeting drug delivery was challenged, while both passive and active targeting are still under intense research in cancer nanomedicine.
Among the frequently discussed issues of active targeting, widespread concerns have been raised about the unwanted interactions of DDSs with non-target sites including nonspecific interactions, and specific interactions whereby both target sites and non-target sites could express relevant receptors, nevertheless at different expression levels (usually referred to as on-target off-tumor effect). Various strategies have been proposed accordingly. For example, Wang et al.50 developed tumor acidity-responsive nanoparticles for reversible shielding of the targeting ligand. The iRGD moiety was shielded from interactions with non-target sites in systemic circulation, and was responsively exposed to the acidic tumor microenvironment, assisting in tumor penetration and cellular uptake.
2.3.1. The effect of spatial distribution of ligands on drug delivery
Traditionally, ligands have been attached onto drug delivery systems (liposomes, nanoparticles, micelles, etc.) with required quantities, and the targeting effect might be increased by elevating the density of ligands within certain limits99. However, ligands are usually present on the surface of vehicles in a random distribution pattern due to their symmetrical structure, resulting in a limited ability to recognize receptors and incomplete use of ligand materials. Moreover, it will increase the probability of forming protein coronas with the plasma binding protein during the transport process in the blood circulation if the density of the ligand is greatly increased. For instance, folic acid-modified liposomes will adsorb large amounts of natural IgM after intravenous injection, leading to a series of unexpected off-target effects, rapid clearance and enhanced immunogenicity100.
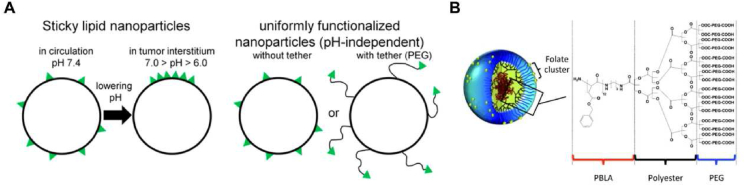
The above problems can be effectively relieved by accurately controlling the presentation mode of multivalent ligands on the carrier to construct a non-uniform distribution of ligands. In this way, efficient utilization of ligands and the enhancement of the effectiveness and specificity of targeted delivery could be realized. Sempkowski et al.101 designed lipid-based vesicles (Fig. 5A) containing HER-2-targeting short peptides (KCCYSL), and sticky vesicles were constructed so that ligands (shown in green) were uniformly distributed over the surface of the vesicle during circulation (pH 7.4), and preferably partitioned within the lipid phase-separated domain in the acidic tumor interstitium (7.0 > pH > 6.0), resulting in low reactivity in the circulation and high reactivity even in cells with few copies of targeted receptors. This strategy increased the local ligand density of lipid vesicles and their ability to recognize target tissues in acidic environments, and decreased specific interactions with normal cells with low receptor expressions. Moreover, Poon et al.102 constructed self-assembling linear dendritic polymers (LDPs) by hydrophilic/hydrophobic interaction (Fig. 5B). The experiments demonstrated that the titer relationship did not increase linearly but reached saturation dynamics. Once passing the allowable number of ligand-receptor binding events within the binding space, the presence of excess ligands clustered in a small binding area and would result in steric binding interference, lowering the binding energy. Their findings indicated that changing the focus from ligand density to specific manipulation of ligand cluster presentation on molecularly targeted NPs may have important implications for cell targeting, leading to the development of more smart and effective targeted delivery systems.
Figure 5.
(A) pH-tunable sticky vesicles (left) and conventionally functionalized nanoparticles with pH-independent uniform distributions of targeting ligands (right). Reprinted with the permission from Ref. 101. Copyright © 2016, American Chemical Society. (B) Chemical structure and self-assembly of linear dendritic polymers (LDP). Reprinted with the permission from Ref. 102. Copyright © 2010, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In addition, Janus nanoparticles (JPs) are composed of two or more anisotropic compartments103. It makes them ideally suitable for modification with different functional molecules to achieve asymmetrical multivalent ligand modification. Recently, Liu et al.104 developed an entirely synthetic, multivalent, Janus nanotherapeutic platform named Synthetic Nanoparticle Antibodies (SNAbs). For myeloid-derived immunosuppressor cells (MDSCs)-targeting SNAbs, they modified onto one “face” of the JPs with G3 peptides which efficiently targeted MDSCs, and cp33 peptide was modified on the opposite “face” for the binding to Fcγ receptors (FcγRs) on immune effector cells. The anisotropy in biological functions of the different faces enabled the effective pairing of target cells with effector cells.
2.3.2. The effect of ligand-receptor binding/dissociation kinetics on drug delivery
Even if ligand-modified drug delivery systems are demonstrated efficient in vitro, the cellular uptake and intracellular transport of NPs are still challenging in pursuing satisfactory in vivo performances. Various extracellular physiological barriers exist, and the competitive affinities of non-target regions for the ligands act as a specific extracellular barrier sequestrating ligand-modified drug delivery systems. In the past, most studies focused only on the affinity constant (KD) in the assessment of this competitiveness. However, ligand-receptor interaction is a dynamic process orchestrated by several parameters including binding (Kon), dissociation (Koff), and KD = Koff/Kon, thus similar affinities can be indicative of a completely different process of interaction. Therefore, a more comprehensive discussion of the binding/dissociation process is necessary.
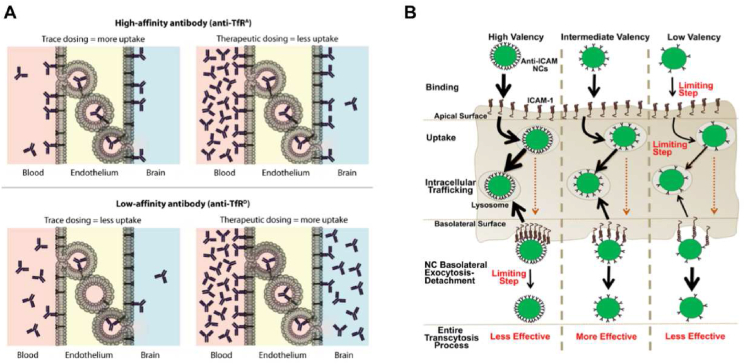
A high affinity for the cell membranes or the membrane receptors is commonly believed to be beneficial to the targeted delivery of anti-tumor drugs. In the extracellular process, however, when the binding affinity with non-target regions becomes too strong, the extracellular affinity barrier occurs with as-increased strength, impeding the subsequent journey to the target sites105. Many solid tumors such as pancreatic ductal adenocarcinoma (PDA), non-small cell lung cancer (NSCLC) and certain breast cancers, display tumor-associated fibroblast cells (TAFs) in their microenvironment, which could also form a major component of the binding site barrier (BSB)106, intercepting in the way of vehicle delivery and reducing their final accumulation in tumor cells107,108. Miao et al.109 demonstrated that NPs were preferentially distributed in fibroblasts due to the strong binding affinity between NPs and TAFs (KD = 10.11 nmol/L). Therefore, the number of NPs uptaken by tumor cells was reduced and the barrier effect of TAFs-induced BSB was increased. Typically, for brain tumor-targeted delivery, previous efforts have exploited the transferrin (Tf)/transferrin receptor (TfR) pathway to enhance the brain uptake of macromolecules through receptor-mediated transcytosis. However, Yu et al.110,111 demonstrated that a reduction in Tf/TfR affinity could enhance the receptor-mediated transcytosis of anti-TfR across the mouse blood–brain barrier and achieve the drug concentration needed for treatment (Fig. 6A). In a therapeutic circumstance, the enhanced peripheral antibody concentrations could compensate for the relatively reduced affinity towards the TfR, ensuring the final interaction with the target cells and the overall internalization. Strategies such as cleavable ligand modification have been proposed according to such problems112. For example, Lei et al.113 developed a nanocleaner modified with KLVFF peptide and acid-cleavable DAG peptide, which could assist in cellular uptake and detach in acidic endosomes, promoting the transcytosis of the DDS from endothelial cells into brain.
Figure 6.
(A) A model in which the affinity of anti-TfR is inversely proportional to the absorption of TfR in the brain. Reprinted with the permission from Ref. 111. Copyright © 2011, The American Association for the Advancement of Science. (B) Model for the role of nanocarrier (NC) affinity on transcytosis and lysosomal trafficking. (The thickness of each black arrow represents the efficiency of the step indicated, where increasing arrow thickness corresponds to faster rate. The orange dotted lines indicate that the effect of NC valency on the efficiency of the secretion part of transcytosis was not assessed.) Reprinted with the permission from Ref. 117. Copyright © 2020, Elsevier B.V.
The dynamic characteristics of ligand-receptor interaction can be used to design the corresponding smart drug delivery system for different tumor tissues. In the case of solid tumors above, it would be an effective solution to construct a fast-binding/fast-unbinding model to circumvent the affinity barrier and BSB. But for tumor cells at dynamic or flowing state, such as the leukemia cells and circulating tumor cells, the presence of fluidic shear stress in blood circulation might be not favorable for the binding of ligand-modified nanodrugs with their target receptor, so it would be necessary to construct a fast-binding/slow-unbinding model. Song et al.114 used two αvβ3 ligands (RGDm7 and DT4) with different binding rates to assemble dual-targeting nanovesicles. RGDm7 served as a “slow-binding/slow-unbinding” ligand (KD = 6.193 μmol/L) and contributed to the stable binding with αvβ3115. In the meantime, DT4 showed rapid association and dissociation (KD = 0.797 μmol/L) and made the vesicles adhere quickly to the flowing tumor cells and transfer the drug to the nucleus116. It was demonstrated that the potency of the dual-targeting vesicles for flowing tumor cells was superior to that for static tumor cells.
Similarly, the unsuitable affinity of receptor-ligand could also impede the intracellular transport process. The design of appropriate affinity could result in rational kinetic processes and regulations of intracellular transport, thus achieving a better therapeutic outcome. Manthe et al.117 used polystyrene nanocarriers targeting the intercellular adhesion molecule-1 (ICAM-1) to investigate the relationship between cellular uptake and intracellular transport of the nanocarriers with varying degrees of affinity (Fig. 6B). The results indicated that nanocarriers with high affinity (KD = 177.2 pmol/L) had clear advantages in binding and uptake, but the nanocarrier-receptor detachment post-transport was more compromised, and this led to enhanced lysosomal transport from the basolateral side. In contrast, the low-affinity (KD = 401.4 pmol/L) nanocarriers, which could detach more easily from the basolateral cell surface and traffic more slowly to lysosomes, would also experience lower binding and uptake on the apical side. Improving the first steps in this process could hinder the latter steps, and vice versa. Therefore, it was important to achieve a balance where nanocarriers had an intermediate affinity (KD = 218.7 pmol/L) for apical binding and uptake without hindering the basolateral detachment or facilitating massive lysosomal trafficking. This is in accordance with the discussions in the previous section. In other words, only appropriate number of ligand modification and intermediate affinity can achieve satisfactory targeted delivery of anti-tumor drugs.
3. Carrier-based smart drug delivery
The development of varied carriers or vehicles have enabled versatile design and modification for the interface between therapeutic agents and patients. Both conventional and newly-developed carrier platforms provide fundamental supports for the various smart drug delivery strategies. In this part, several representative drug carriers and their recent developments are discussed, including lipid-based carriers, polymeric nanoparticle-based carriers, micelles, self-assembled chemical drugs-based DDSs, nucleotide-based DDSs, self-assembled peptide-based systems, and cell-derived/biomimetic delivery systems, with multiple points of view.
3.1. Lipid-based carriers for smart drug delivery
Lipid-based carriers have been under intensive investigations in anticancer delivery, demonstrating satisfactory performance in drug delivery, with relatively mature synthesis of lipid materials as well as scale-up manufacturing processes118,119. Besides the conventional liposomes, various other lipid vehicles have emerged, e.g., lipid nanoparticles120, 121, 122. Lipid-based carriers have seen various successful clinical translations, and their capacity in cargo loading and functionalizations enabled the further development of lipid-based smart delivery systems118.
Liposome is the most common vehicle in lipid-based nanocarriers, and have been widely applied for chemotherapeutic delivery due to their unique ability to encapsulate hydrophilic and hydrophobic agents and targeting characteristics caused by the enhanced permeability and retention (EPR) effect or ligand modification123. Since 1995, liposomal doxorubicin, Doxil® was first approved by U.S. Food and Drug Administration (FDA) to treat ovarian cancer and AIDS-related Kaposi's sarcoma124. Subsequently, liposomal daunorubicin (DaunoXome®), mifamurtide liposomes (Mepact®), and vinCRIStine sulfate liposomes (Marqibo®), etc. were developed for various cancers125.
Liposomes make a promising platform to improve tumor deposition and achieve on-demand drug release. Many pH-sensitive liposomes, for example, are designed to disintegrate in the acidic lysosomal environment after internalization, releasing encapsulated drugs in the desired target. Furthermore, some pH-sensitive liposomes were reported to exhibit enhanced tumor accumulation and cellular internalization, caused by changes in physicochemical properties such as size and surface charge126,127. Additionally, other stimuli-responsive smart liposomes in response to internal triggers (e.g., enzymes, and redox potential) and external guides (e.g., magnetic field, ultrasound, and NIR excitation) have emerged as promising drug delivery systems pioneering in antitumor smart drug delivery5. Alpizar et al.128 designed light-triggered liposomes for target therapy, which could successfully evade innate immune cells and then be internalized with surface potential switching from neutral to positive upon in situ irradiation. Ji et al.129 constructed a matrix metalloproteinase-2 (MMP-2)-responsive peptide-hybrid liposome to specifically release pirfenidone at the pancreatic tumor site and down-regulate the multiple components of extracellular matrix (ECM), enhancing the antitumor efficacy of encapsulated gemcitabine. These stimuli-responsive liposomes exhibit promising application in enhancing drug delivery and achieving on-demand drug release in targets.
Liposomes could also serve as combination delivery platforms for multiple therapeutic agents, enabling smart combinational therapy strategies such as chemo-photothermal therapy. Photothermal ablation can synergistically enhance the therapeutic effect of chemotherapeutics by elevating cell membrane permeability and triggering drug release at the target site123. ICG is a quintessential NIR dye for several clinical applications approved by FDA. Liposomal co-encapsulation of chemotherapeutics and ICG remarkably prolonged the blood circulation time and enhanced the tumor accumulation, proving satisfactory chemo-photothermal combinational therapeutic effect130. Liposomes with chemo-photothermal therapy are expected to become the next clinically effective anti-tumor strategy.
Lipid nanoparticle (LNP) is another lipid-based carrier with a solid core structure, and has become a promising platform for the delivery of hydrophobic and hydrophilic small-molecule drugs as well as biological agents such as oligonucleotides, peptides, and vaccines120,131. Various LNP vaccines have been quickly developed and played vital roles in the COVID-19 pandemic, e.g., the Moderna vaccine132, and the application of LNP in nucleic acid (NA) delivery has also been highlighted133. Endosomal escape is a vital process for many NA drugs, and the utilization of ionizable lipids and functional helper lipids could provide essential pH-responsiveness in the endosome134. Onpattro® (anti-transthyretin siRNA-loaded LNP) contains an ionizable cationic lipid (DLin–MC3–DMA) which could convert the potential of liposomes to positive in the endosomal environment, resulting in the release of siRNA in target cells135,136. With the accumulating library of lipid materials with various functions and characteristics, LNPs holds great potential for versatile delivery of a universal selection of therapeutic agents, with greatly shortened time for formulation screening and optimization. To this end, however, the actual quality of relevant lipids in both laboratory research and scaled-up production requires more emphasis.
Besides liposomes and LNPs, lipids assist in various promising platforms such as nanostructured lipid nanocarriers (NLCs), lipid-drug conjugates, self-emulsifying systems, etc137, 138, 139, 140. Lipid-based smart vehicles promise a bright future for cancer treatment in the next decade or so. They might become a major arsenal for safer and more efficient treatments by ensuring proper drug localization in tumors and on-demand drug release. Although substantial advances have been made, efforts are still needed to solve the problems of scale-up and clinical verification to transfer lipid-based therapy from laboratories to clinics.
3.2. Polymeric nanoparticles-based smart drug delivery
Nanoparticles based on biocompatible and biodegradable polymers such as polylactic acid (PLA), PLGA, PEG, and N-(2-hydroxypropyl) meth-acrylamide (HPMA) have gained popularity for nanocarrier fabrication. Through chemical conjunction or physical encapsulation, polymer nanoparticles could deliver chemotherapeutics, proteins, and nucleic acids to the tumor site141. Several polymeric nanoparticles have been approved for clinical application, e.g., Eligard® (Tolmar) containing poly(d,l-lactide-co-glycolide) has been approved by the FDA for prostate cancer therapy142. The clinical trials of BIND-014 (docetaxel-loading polylactide core with PEG corona and ACUPA-targeting ligands), CRLX101 (nanoparticle with camptothecin-conjugated polymer backbone based on cyclodextrin and PEG), and AZD-2811 Accurin (AZD-2811-encapsulated PLA–PEG block copolymer nanoparticle) also provide valuable experiences for the translation of smart polymeric nanoparticles. In recent years, combined with specific ligands and responsive groups, smart polymeric nanoparticles are at the forefront of antitumor drug delivery with their unique advantages.
Due to high synthetic versatility and ease of conjugations, polymers were suitable for functionalization and ligand modification. For example, Qiu et al.143 synthesized an IL12 plasmid (pIL12)-loaded polyplex constructed with esterase-responsive cationic polymer PQDEA, which was further coated with various lipids as well as DSPE–PEG conjugated with tumor-targeting ligand AEAA. Responsive to the high-level esterase in tumor cells and tumor-associated macrophages (TAMs), these smart nanoparticles turned anionic and quickly released the cargo, thus efficiently producing IL-12, activating anticancer immune responses, and remodeling the tumor microenvironment. Zou et al.144 functionalized cNGQGEQc peptide onto reversibly crosslinked PEG–P(TMC–DTC)–PEI and cNGQ–PEG–P(TMC–DTC) chimeric polymersomes (cNGQ/RCCP), achieving prolonged circulation, efficient targeting, and GSH-responsive release. Yin et al.145 took advantage of the affinity of hyaluronic acid (HA) for CD44 to create HA–ss–PTX, which could be specifically absorbed by tumor cells via CD44-mediated endocytosis and disrupted by reducing glutathione to release PTX.
The acidic tumor microenvironment provided the basis for intelligent response at low pH. For example, dual sensitive dual drug backboned shattering polymer self-assembled nanoparticle (DD-NP) was triggered intracellularly to break down and release the dual drugs payload in a chain-shattering manner under the intracellular acidic microenvironment for optimal anticancer efficacy. Cong et al.146 claimed that DD-NP could be a good example of nanomedicine tackling the major challenges together, including precise composition, direct fate monitoring of drug, drug evaluation, and screening on reliable cancer models, validating the possible use of DD-NP in the clinic. In another example, Tang et al.147 synthesized charge-switchable polymeric nanoparticles, which were consisted of PEG–block–poly[(1,4-butanediol)-diacrylate-β-5-amino-1-pentanol] (PEG–PDHA), and conjugated with 2,3-dimethyl maleic anhydride (DMA). In the slightly acidic environment of tumor tissues, the anionic shell was removed, inducing the conversion of the surface charge from negative to positive, which resulted in higher intra-tumor accumulation, more efficient cellular uptake, and stronger cytotoxicity. These charge-convertible polymers represented a class of typical intelligent polymers that can afford tunable physical and structural changes that are envisioned to address critical issues in response to the stimulus at the tumor site148.
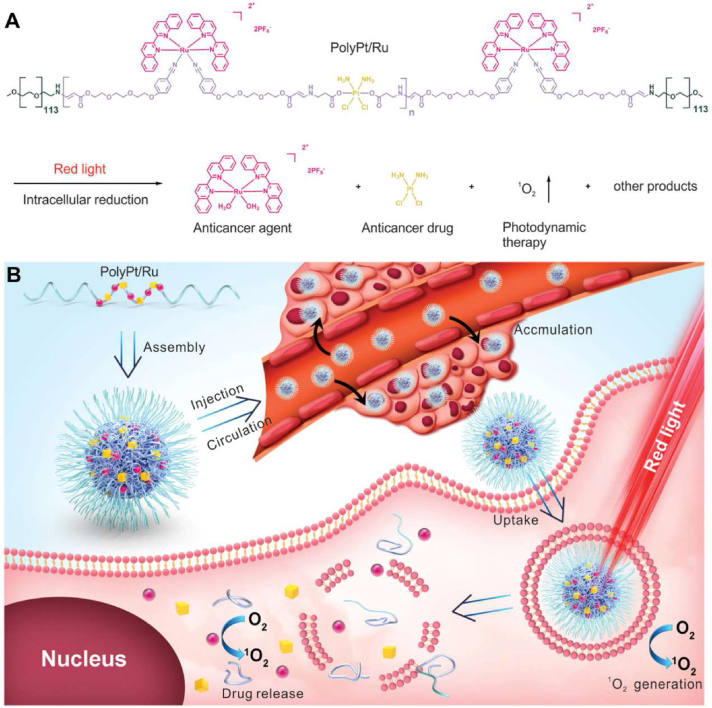
Photoactivatable polymeric nanoparticles enabled remotely controlled drug and imaging agent delivery. For example, Senthilkumar reported photo-responsive poly(p-phenylene vinylene) conjugated polymer nanoparticles (CPNs) functionalized with donor-acceptor Stenhouse adduct (DASA) and folic acid units for drug delivery and imaging149. Notably, drug-loaded CPNs exhibited excellent biocompatibility in the dark, indicating perfect control of the light trigger over drug release. In another work, Zeng et al.150 reported the synthesis of an amphiphilic triblock copolymer, PolyPt/Ru, consisting of biocompatible PEG, reduction-responsive Pt(IV), and red-light-responsive Ru(II) moieties, which further improved the selectivity of the cancer treatment, though photoactivation at the irradiated tumor tissue (Fig. 7).
Figure 7.
Illustration of amphiphilic triblock copolymer PolyPt/Ru (A) and the self-assembly, circulation and internalization process of PolyPt/Ru (B). Reprinted with the permission from Ref. 150. Copyright © 2020, Wiley-VCH GmbH.
Functionalization always leads to a more complex structure and changes the original characteristics of the polymer. Polymeric nanoparticles without biodegradability may cause serious side effects after their accumulation at the tumor site. With the rapid emergence of novel polymeric nanoparticles, safety evaluation is important to identify polymeric candidates.
3.3. Micelle-based smart drug delivery
Many chemotherapeutics are hydrophobic small molecules, and the hydrophobicity often becomes a major barrier for their systemic delivery to solid tumors. Over the past few decades, polymeric micelles (PMs) have provided a promising platform for the delivery of poorly soluble drugs. PMs are usually self-assembled from amphiphilic block copolymers with nanoscale size (commonly 10–100 nm). Among these polymers, poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), poly(N-vinyl-2-pyrrolidone) (PVP), poly[N-(2-hydroxypropyl) methacrylamide] (PHPMA), and polysaccharides are commonly used as core-forming segments, enabling the encapsulation of hydrophobic molecules. Besides, polyesters and poly(amino acid) (PAAs) have drawn broad attention for shell forming151, and the hydrophilic outer shell could protect the drug-loaded core. The core-shell structure of micelles has enhanced the encapsulation and selective tumor targeting of many therapeutic agents. For example, PEG–b–PLA micelles for PTX have realized enhanced drug solubilization, biocompatibility, and dose escalation152.
Up to now, various micelle formulations including Paclical, Genexol-PM, and Nanoxel-PM have been approved for PTX delivery. Also, hydrophilic polypeptides have been developed to mimic the conformation of synthetic hydrophilic polymers. Banskota et al.153 developed new unstructured polypeptides called zwitterionic polypeptides (ZIPPs), which could self-assemble into micelles after conjugation with hydrophobic paclitaxel, with a 17-fold-longer half-life compared to free paclitaxel in the HT-29 colon cancer model.
Recently, intelligent polymeric micelles have been developed to respond to different stimuli, including light-, ultrasound-, temperature-, pH-, enzyme-, redox-sensitive systems, for achieving spatiotemporal control of their therapeutic effects. For example, Li and coworkers154 fabricated a micelle drug delivery system based on poly(AAm–co–AN)–g–PEG with an upper critical solution temperature (UCST) of 43 °C, with thermal-sensitive drug release combined with microwave hyperthermia. Su et al.155 developed a pH and MMP-2 dual-responsive Azide–PEG–PAsp (Dip/Bz) copolymer for antitumor drug delivery, spatiotemporally controlling the release of PD-1 and PTX from MMP-2 enriched tumor tissues. Additionally, surface modification of PMs with ligand molecules such as peptides, antibodies, and small molecules also has wide applications for advanced tumor therapy. For example, iRGD-modified micelles were developed for αvβ integrin and neuropilin-1-mediated blood–brain barrier penetration and targeted delivery to glioma cells156. PMs system has also been regarded as a promising platform for imaging and diagnosis, such as tumor micro-environment and tumor tissue imaging, etc. Among these materials, star polymer-based unimolecular micelles are widely investigated, which could be obtained through rational structure design, and different kinds of imaging probes can be encapsulated or labeled157.
The past decade has witnessed explosive development of biodegradable micelles for targeted and controlled anticancer drug delivery. Considering their advantages such as reduced immunogenicity, biocompatibility, biodegradability, highly structural and chemical variability, amphiphilic block copolymers like PHPMA, PVA and polyesters have the potential to achieve clinical translation. Nevertheless, the clinical translation is a lengthy, costly, and complex process, successful examples of PMs from bench to bed are few. Several challenges associated with their use concern the disassembly, degradation, clearance, and metabolism of PMs in the blood stream, besides, the long-term effects of some PMs remain to be studied, especially following repeated and cumulative administration151. Thus, polymers for stable micellar structure, active targeting ligands, and combination delivery of multiple drugs are potentially effective approaches.
3.4. Self-assembled chemical drugs-based smart drug delivery
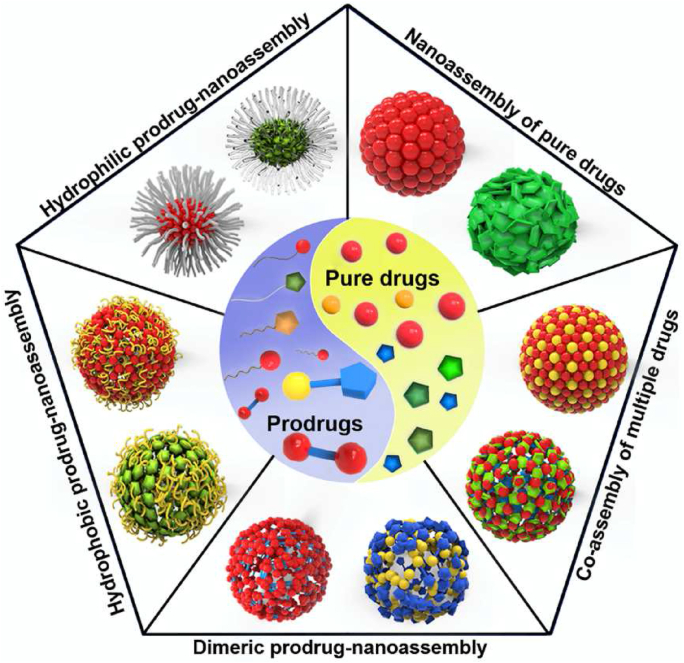
Conventional organic nanocarriers usually consist of lipids or polymeric materials, such as liposomes, vesicles, micelles, and polymeric nanoparticles (NPs)158. Interestingly, some chemical drugs or prodrugs are also adopted to participate in the construction of these nanocarriers themselves159, 160, 161. The most remarkable feature of chemical drug-based nanoassembly is its self-delivery capacity, namely drugs or prodrugs could simultaneously serve as both cargoes and vehicle materials in the nanosystems159,160,162. As a result, chemical drug-based nanoassembly usually shows ultrahigh drug loading capacity, sometimes even reaching 50%159,160,162. Recently, this kind of nanocarrier has drawn more and more attention as a promising and efficient drug delivery system. In this section, we focus on the latest trends in chemical drug-based nanoassembly (Fig. 8), including pure drug-based nanoassembly and prodrug-based nanoassembly.
Figure 8.
Schematic diagram of chemical drug-based nanoassembly.
3.4.1. Pure drug-based nanoassembly
Over the years, several anticancer drugs have been formulated into nanomedicine with the help of carrier materials, such as paclitaxel, doxorubicin, and irinotecan158. However, low drug-loading efficiency and carrier material-related toxicity have been widely regarded as the main obstacles to the clinical translation of nanomedicines. Therefore, the rational design of carrier-free nanomedicines has always been a high priority. Several chemotherapeutic drugs have been recently found to show self-assembly characteristics in water, such as 10-hydroxycamptothecin, doxorubicin, and curcumin159,163, 164, 165. Moreover, photosensitizers such as 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) and zinc meso-tetra(4-pyridyl) porphyrin (ZnTPyP) were also found to successfully self-assemble into stable NPs for imaging-guided photothermal therapy (PTT) or photodynamic therapy (PDT)166,167. In addition to single drug nanoassembly, the co-assembly of two or more drugs was also explored for multimodal cancer therapy168, 169, 170.
The self-assembly or co-assembly of drugs was found to be driven by multiple interactions, including hydrophobic force, hydrogen bonding, π–π stacking, and electrostatic interactions, etc.159. Despite the self-assembly capacity of hydrophobic drug molecules, amphipathic polymers or PEG were usually utilized in these systems to further enhance the colloidal stability and prolong the circulation time159. Distinguished from polymeric micelles, PEG polymers mainly acted as PEGylation modifiers instead of carrier materials in pure drug-nanoassembly159.
3.4.2. Prodrug-based nanoassembly
Despite their simple fabrication process and ultrahigh drug loading capacity, it is still challenging for pure drug-based nanoassemblies to achieve tumor stimuli-responsive drug release. In contrast, rational design of small-molecule prodrugs and prodrug-nanoassemblies could not only modify the physicochemical properties of drugs but also realize selective drug release, e.g., by inserting tumor-specific stimuli-sensitive linkers in the prodrug molecules160. Moreover, many drugs are not able to form nanoassemblies themselves, but proper chemical modifications could endow them with self-assembly capability160.
According to the molecular structures of prodrugs, small molecular prodrug-based nanoassembly could be summarized as three types: (i) nanoassembly of amphiphilic prodrugs; (ii) nanoassembly of hydrophobic prodrugs; and (iii) nanoassembly of dimeric prodrugs160,171. Among them, relatively few studies have been focused on amphiphilic prodrug-based nanoassembly, due to their relatively large critical aggregation concentrations (CACs) and unsatisfactory dilute stability in blood160,171. An increasing number of hydrophobic drugs and prodrugs have been found to have self-assembly capacity, despite the past views that they could hardly form stable NPs in water159,160,171. Notably, the nanoassembly of hydrophobic prodrugs showed excellent stability after modification with a small amount of PEG polymers. The self-assembly ability and good stability of hydrophobic drug nanoassemblies could be attributed to multiple intermolecular interactions/forces including hydrophobic forces, hydrogen bonding, π–π stacking, and electrostatic interactions; while the assembly of amphiphilic prodrugs is mainly considered to be driven by hydrophobic forces159,160,171.
Despite the specific self-assembly features, the effective hydrolysis of hydrophobic prodrugs remains challenging. To address this problem, tumor stimuli-responsive strategies by inserting chemical linkers in the prodrugs has been proved to be effective160. Recently, a wide range of redox-responsive prodrug-nanoassemblies have been developed by utilizing reduction-, oxidation- or redox dual-sensitive chemical linkers, such as thioether bond, disulfide bond, trisulfide bond, thioketal bond, and diselenide bond, etc172, 173, 174, 175, 176, 177. In addition to triggering the tumor-specific activation of prodrugs, the bond angles and/or dihedral angles of these redox-responsive linkers play crucial roles in the stability and in vivo drug delivery efficiency of prodrug-nanoassemblies174, 175, 176, 177.
In addition to monomeric prodrug-based nanosystems, the nanoassembly of dimeric prodrugs represents another unique nanoplatform, including homodimer-nanoassembly and heterodimer-nanoassembly171. The nanoassemblies of homodimer synthesized by conjugating two identical drug molecules could exhibit higher drug loading efficiency than monomeric prodrug-nanoassemblies, sometimes even up to 80%171,175,176. Moreover, the nanoassembly of heterodimers synthesized by coupling two different therapeutic agents provides a new platform for combination cancer treatment171,178. Notably, most dimeric prodrugs demonstrated relatively poor assembly ability when compared with monomeric prodrugs, especially the homodimers with symmetric molecular structures175. Moreover, although heterodimer-nanoassembly provides a natural platform for combination cancer therapy, the molar ratios of drugs were strictly limited to 1:1, which is detrimental to exerting the synergistic effects in different cases171. Still, heterodimers of two drugs with different therapeutic modes might be more flexible in producing synergistic effects, such as chemo-photodynamic heterodimers178.
3.5. Nucleotide-based smart drug delivery
The nucleotide is a type of organic molecule consisting of a nucleobase, a sugar ring, and a phosphate, while such simple structure is highly important in biology. It is the basic element of endogenous nucleic acids for a broad range of physiological functions, such as DNA, mRNA, and microRNA. From a chemical standpoint, DNA has ∼106-fold higher stability than RNA due to its deoxyribose structure179. As such, DNA is preferably chosen by nature as the carrier to store genetic information, forming a Watson–Crick base pairing structure as discovered in 1953. Later in the 1980s, it was found that DNA was able to form much more complicated architectures than merely linear duplexes, based on which the concept “structural DNA nanotechnology” was coined to describe the higher-order DNA structures. Since then, various elegant DNA nanostructures have been designed with arbitrary sizes, shapes, and functions, such as DNA polyhedra, DNA origami, DNA dendrimer, DNA nano-train, DNA nano-flower, providing versatile vehicles for smart drug delivery161,180. Besides the evolution of structure, the functions of DNA have also been extensively expanded by virtue of the rapid progress of molecular biology and chemical biology, resulting in the discovery of various types of functional nucleic acids181. One typical example is the aptamer, which is artificially obtained through a combinatorial process called SELEX. Theoretically, aptamers can be isolated to selectively bind any target of interest, and currently, a wide range of aptamers have been discovered with binding substrates of metal ions, small molecules, proteins, and even the whole cells182. Among them, the tumor cells recognition aptamers have attracted particular attention, which elicits the development of both tumor diagnostic probes and tumor-targeting drug delivery systems183.
Compared with many other synthetic polymers, the endogenously derived DNA has various appreciable advantages as drug loading carriers for in vivo delivery. Owing to the development of the solid-phase synthesis technique, DNA is readily commercially available for large-scale synthesis via automated phosphoramidite chemistry with relatively low prices, making it accessible to most ordinary research labs. In addition, DNA with various modifications for subsequent conjugation as well as fluorophore-labeling can be easily designed, which significantly expanded the functionalities of the DNA-assembled nanostructures, such as the delivery of multiple drugs, active targeting modifications, and imaging-guided tumor therapy. The biomimetic DNA is highly biocompatible and biodegradable, which meets the stringent demand as drug carriers for in vivo applications. More importantly, DNA has a well-characterized conformation and geometry, in which the double-stranded B-DNA is ∼2 nm in diameter with a helical repeat of 3.4 nm for every 10.5 base pairs. Through the precise A/T and G/C base-pairing, DNA nanostructures with different sizes, shapes, and dimensions can be rationally designed via tuning the building block DNA sequences and incubation conditions161. Besides base-pairing-based assembly, DNA nanostructures can also be formed by more elegant strategies, such as polymerase chain reaction (PCR), rolling circle amplification (RCA), enabling the in situ generation of nanoparticles with a high level of reproducibility and efficiency161.
While the nucleotide has quite a simple chemical structure, the nucleotide-based nanoassemblies are able to deliver a wide range of therapeutic drugs, including chemotherapies, photodynamic agents, radio-therapeutics, oligonucleotides-based, and even protein-based drugs161,180. The drugs can be loaded into DNA nano-architectures via either physisorption or chemical conjugation. Among various antitumor drugs, the most commonly used example is DOX, which can readily sandwich into two adjacent pairs of bases (especially G/C pairing), achieving ultra-high drug loading into DNA nano-assembly through a simple preparation procedure184. This is inspired by the antitumor mechanism of DOX, which enters the cell nucleus and intercalates among base pairs in genetic DNA, thereby preventing DNA replication and ultimately inhibiting protein expression. A similar binding pattern was also employed to load porphyrin derivative (TMPyP4), a photodynamic therapy agent185. Overall, such strategy is simple, cost-effective, yet highly efficient, while it has specific requirements for the loaded drugs, such as planar or extended planar components within their structures. More generalized methods to load the payloads are through sequence hybridization and chemical conjugations. For nucleic acids-based drugs, direct hybridization is the most straightforward way to integrate into DNA nanostructure, which has been used to deliver siRNA, microRNA, DNAzyme, and CRISPR/Cas9 system186. For many other drugs, however, tedious chemical conjugations are required to incorporate them into DNA nanostructures. Although DNA itself has limited functional groups for chemical conjugations, the chemical solid-phase synthesis generates ester, amide, and disulfide bonds within DNA, allowing for subsequent conjugation of drugs with different chemical properties. However, chemical modifications are only feasible for limited lengths of DNA sequences, and the following drug conjugations need complicated chemical synthesis with low yield. In some cases, the combination of different strategies was employed to construct multiple-model therapeutic systems to conquer the extreme complexity and heterogeneity of tumor187. To enable tumor targeting delivery, the aptamers and other active ligands have been equipped into DNA nanostructures for targeted tumor therapy188,189.
Smart delivery systems should release their payload after reaching the targeting site for action. DNA nanostructures can exploit various internal and external stimuli to trigger drug release. For example, pH and GSH are the most widely employed internal triggers for anti-tumor nano-delivery systems, by virtue of acidic tumor microenvironment and up-regulated GSH levels in cancer cells. It is known that cytosine-rich sequences (i-motif domain) undergo a conformational change in response to slightly acidic pH, based on which the pH-responsive DNA nano-delivery systems were formed by the integration of i-motif190. Li and coworkers191 developed a DNA hydrogel that was crosslinked via disulfide bonds, which was cleavable in presence of GSH, resulting in burst drug release inside cells. Likewise, a photodegradable DNA-cross-linked hydrogel was designed for drug loading, and external stimulus of light-induced gel-sol conversion to trigger the rapid release of the encapsulated molecules192. Compared with other polymers, the elegance of DNA as drug carriers is their capability to sense various biological molecules. For instance, complementary sequences can be designed to hybridize with target mRNA/microRNA193, and aptamers can recognize ATP and nucleolin194,195, all of which have been employed to construct DNA-based smart drug delivery system. The landmark work was reported by Li and coworkers195, who developed a DNA nanorobot to deliver thrombin via incorporating an aptamer that targeted nucleolin, a protein specifically expressed on tumor-associated endothelial cells. The aptamer served both as a targeting domain and also a molecular trigger to activate the DNA nanorobot, through which thrombin could be specifically delivered to tumor-associated blood vessels for intravascular thrombosis, resulting in tumor necrosis and tumor growth inhibition.
3.6. Self-assembled peptide-based smart drug delivery
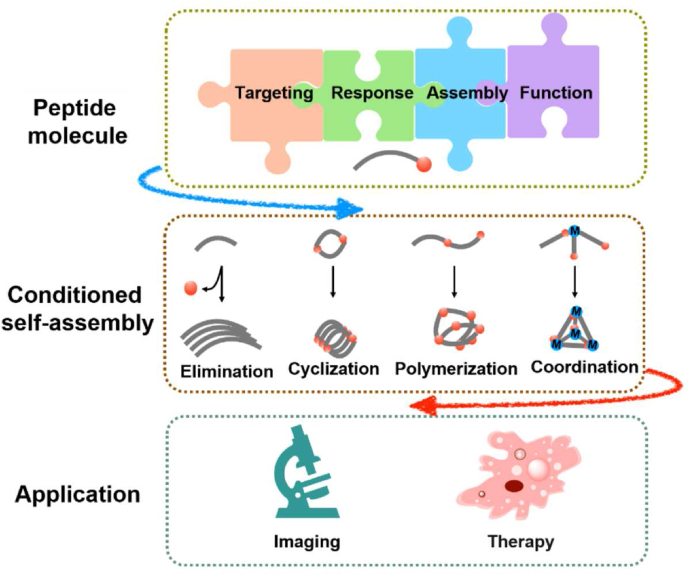
Biological and bio-inspired building blocks have attracted widespread attention in smart drug delivery technologies owing to their advanced functions for both nanotechnology and biomedicine. Among various building-block molecules, peptides with self-assembly characteristics are of particular interest, not only because of their biological origin and biodegradability, but also because of their specific bioactivity derived from rationally designed sequences196. In this section, we focus on the bioactivated in vivo assembly (BIVA) nanotechnology, the modular structures of peptide materials, and corresponding conditioned self-assembly mechanism, as well as their application in the imaging and therapies against tumor (Fig. 9). BIVA technology could provide active targeting and assembly, inducing tumor-specific accumulation and prolonged retention. Based on different modules, versatile functions could be endowed onto the BIVA system, including but not limited to prolonged circulation, targeting, self-assembly, imaging, etc. Ren et al.197 reported a BIVA optical nanofiber probe with five different functional modules which could self-assemble on the tumor surface, with prolonged tumor imaging and precise recognition of <2 mm orthotopic pancreatic tumor in vivo.
Figure 9.
Schematic illustration of bioactivated in vivo assembly (BIVA) nanomaterials including modular molecule designs, conditioned assembly mechanisms, and biomedical applications.
Modular design enables efficient production, maintenance, and customization across modern engineering technologies. In the terms of peptide design, modular peptide building blocks with various functions, such as targeting motif, response motif, self-assembly motif, and functional motif, are employed for the construction of delivery platforms, providing advanced scope and accuracy in their metabolic steps, associated enzymes, and biological function parts, and assisting in the programmable and controllable regulation of the assembled structure and the morphology198. Combining with molecular dynamics (MD) simulation, the pre-designed compound and its assembled behavior can be further predicted. Remarkably, the molecular assemblies can be synthesized, assembled, and characterized in a rapid manner with desirable performance199. Also, recent reports suggest the potential of biosynthesis for the efficient combination of diverse modules and cargos with diverse structures in anticancer immunotherapies and relevant other applications200.
Enzymatic triggers are commonly adopted self-assembly mechanisms in vivo, including enzymatic elimination, cyclization, and polymerization, as well as metal ion coordination. For in situ self-assembly of peptides in physiological conditions, a simple and efficient approach is to modify molecules with responsive molecular elimination to remove hydrophilic and steric hindrance parts, thus the overall balance of hydrophobicity and hydrophilicity could lead to in situ assembly201,202. Additionally, π–π interactions could also serve as a driving force to influence the reaction pathways of cyclization, altering self-assembly states203,204. Besides, the polymerization based on elongation of the peptide monomers-induced polymeric phase transition can provide an efficient route to form divers self-assembly. These nanoscale architectures and polymers have myriad possibilities when directly incorporated by polymerization and in situ assembly205,206. Coordination-based self-assembly, a strategy that uses metal coordination as an important force to direct the association of peptide molecules, has recently developed into an elegant method for the precise design of theranostic systems by combining the advantages of both peptide self-assembly and metal coordination interactions207. Assembly is an equilibrium process between the individual building units and their aggregated state, the practical self-assembly of molecules should be precisely controlled by concentration in solvent208.
The BIVA technology-based peptide candidates exhibit promising drug delivery capability and advanced drug substitute ability for cancer therapy209,210 and unique advantages, including reduced side effects, optimized pharmacokinetics and pharmacodynamics211, 212, 213.
3.7. Cell-derived/biomimetic smart drug delivery
Endogenous materials have been widely used in the research of drug delivery systems because of the advantages of good biocompatibility, low immunogenicity, and retention of endogenous biological functions. In recent years, endogenous materials such as biomembrane-coated vectors and extracellular vesicles (EVs) have received more and more attention. Compared with traditional nanomaterials, biomembrane-coated NDDSs can mimic many natural characteristics of their cells of origin214, 215, 216. The current biomembrane-coated nanomaterials in research mainly include red blood cell (RBC) membrane217, platelet membrane218, immune cell membrane219, tumor cell membrane220, and hybrid cell membrane221. RBCs and platelets have been intensively studied due to their non-nucleus characteristics. Yang et al.222 synthesized a thermal-sensitive nitric oxide (NO) donor S-nitrosothiols (SNO)-pendant copolymer [poly(acrylamide-co-acrylonitrile-co-vinylimidazole)-SNO copolymer, PAAV-SNO] with upper critical solution temperature (UCST), and RBCs were together adopted to fabricate an erythrocyte membrane-camouflaged nanobullet for codelivery of NIR-II photothermal agent IR1061 and indoleamine 2,3-dioxygenase 1 (IDO-1) inhibitor 1-methyl-tryptophan (1-MT), realizing stealth in the circulation as well as light- and heat-regulated release at the tumor site. Platelet membranes consist of CD47 and P-selectin, which could help escape from macrophages and provide high affinity with tumor cells, respectively223. Wang et al.224 utilized platelet membranes to coat the nanoparticles formed by chitosan oligosaccharide (CS)-PLGA copolymer (PCS-PLGA NPs) for delivering anticancer drug bufalin, which exhibited prolonged half-life and significant accumulation in the tumor site due to P-selectin. Immune cell membranes, e.g., macrophage, T cell, and natural killer cell membranes, etc., can effectively reduce opsonization and non-specific clearance, and provide cellular recognitions or targeting abilities225. Also, the application of hybrid cell membranes might retain the inherent functions of both cell membranes. Jiang et al.226 designed erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles using RBC membrane and MCF-7 membrane, the bionic nanoparticle exhibited prolonged blood circulation time and more accurate targeting to MCF-7 cells.
Exosomes are a type of EVs, containing components from the parent cell such as DNA, RNA, lipids, proteins, etc.227 Besides, exosomes possess natural composition, low immunogenicity, reduced cytotoxicity, favorable penetration through physiological barriers, and tumor cell-derived exosomes exhibit specific targeting towards parent tumor cells228. Also, extra characteristics and smart functions could be endowed on exosomes with ligand modification, cargo loading, etc.229 These exosomes were used as ideal carriers for delivering nucleic acids and proteins to tumor sites230. Towards the large-scale production of targeted and therapeutic exosomes, cell nanoporation technology was adopted by Yang et al.231, achieving increased exosome production and transcription of therapeutic mRNA, as well as the expressing of targeting peptide on the N terminus of exosomal CD47. The produced exosomes realized efficient penetration through the blood–brain barrier and targeted delivery to the glioma sites with enhanced therapeutic effect. The exosomes could also provide various biological properties for the modification of NPs232. In recent years, biomimetic drug delivery systems have been extensively studied, but for clinical applications, more in-depth researches are needed.
4. Future perspective
At present, with the emphasis on precision and comprehensiveness in clinical cancer treatments, new requirements are put forward for tumor-targeted NDDS, urging the development of flexible and adjustable formulation, controllable interval and dosage of administration, spatiotemporally-precise delivery, and easy clinical translations. The construction of NDDS by smart self-assembly has become an important trend in modern smart/stimuli-responsive drug delivery. Based on supramolecular in vivo self-assembly, a tumor-selective cascade activatable self-detained system (TCASS) could significantly enhance the smart accumulation of self-assembly NDDS on desired targets, and promote the penetration and retention at tumor sites via aggregation/assembly-induced retention (AIR) effect202. Additionally, on the combination of host-guest interactions233 and biomarker displacement activation (BDA)68 strategy, some overexpressed tumor markers in the tumor microenvironment may competitively bind with the macrocyclic host, and enable controllable release of the anti-tumor agent from its disguised form and restoring its anti-tumor activity. This host-guest complex exhibits high efficacy and low cytotoxicity, hence this kind of supramolecular system is expected to become an important development direction of multifunctional nanomedicine.
In recent years, the global trend of pharmaceutical products has shifted from chemicals to biopharmaceuticals. However, the performance of conventional NDDS proves insufficient to solve the problems of poor stability and low encapsulation in the delivery of biological macromolecules such as peptides, proteins, vaccines, and gene drugs. A strategy of supramolecular peptide therapeutics (SPT) was proposed for the improvement of the stability and anticancer efficacy of the peptides in vivo. The phenylalanine was introduced into the N terminal of anti-tumor peptide (KLAK), and the encapsulation efficiency of supramolecular peptide complex reached 97% through host-guest interactions between the phenylalanine and cucurbit uril234. Considering the huge demand for peptide-based pharmaceuticals for clinical purposes, SPT might provide a versatile and potential bridge for clinical translations of bioactive peptides and proteins. Nanotechnologies also have widespread applications in vaccinology to such an extent that there is a very active research area known as “nanovaccinology”, especially in tumor-targeting. However, conventional formulation strategies still have to face the difficulty of complicated preparation processes, low antigen loading, and weak immune response. A Nano-B5 platform was built through an in vivo production process combining the self-assembly capacities of pentamer domains from the bacterial AB5 toxin and the loading capacity of unnatural trimer peptides to encapsulate diverse antigens including peptides and polysaccharides. The full-biosynthesis process of protein skeleton could avoid the introduction of exogenous materials and ensure the safety of the vaccine, and the utilization of fusion expression or protein glycosylation modification strategies could easily achieve high-efficacy loading of different antigens without chemical coupling. Compared with pure antigen, nanovaccines could avoid the rapid clearance of antigen and achieve effective enrichment in lymph nodes, and rapidly activated antigen-presenting cells (APCs) for T cell activation without additional adjuvant, thus enhancing the level of subsequent immune response, and offering a platform technology for combining diverse modular chassis components and antigen cargos to generate various high-performance nanovaccines200.
In addition, various individualized gene therapy drugs have also been gradually developed. Traditionally, most gene therapy delivery systems focus only on one therapeutic target, such as DNA in the nucleus or mRNA in the cytoplasm. However, spatiotemporal features of transcription (nucleus) and translation (cytoplasm) of target genes differ widely. Thus, the utilization of branched DNA structure with circular supramolecule as the core can efficiently load components through base complementary pairing-based self-assembly of nucleic acids and supramolecular host-guest recognition mechanism, then the gene-editing (sgRNA/Cas9) and gene-silencing (antisense) components can achieve level-by-level responsive release. This strategy takes advantage of the responsiveness of the assembly structure to tumor markers, realizing the synergistic dual-drug codelivery for gene editing system and gene silencing system, and offering new strategies for the treatment of malignancies235.
Despite the great progress in smart NDDS, several challenges remain in the design of biologically functional controllable components, e.g., the prediction of in vivo stimuli-responsive behavior, the standardized evaluation criteria, clinical translation and industrial production. Although novel functional materials have been under continuous development, in-depth research of their in vivo degradation and safety issues remains in urgent need. Artificial intelligence (AI), big data and multi-scale simulation technology can be introduced into the construction of smart NDDS to change the paradigm of pharmaceutical research into data-driven mode236, 237, 238. Specifically, a structurally diverse hydrogel library with more than 2000 motifs239 can link the chemical features of carriers with their self-assembly properties and accurately predict the gel formation ability by deep learning. Hydrogels offer scalable and tunable delivery platforms which can load a variety of drugs by hydrophobic domains, affinity-mediated binding, or covalent integration240,241. These kinds of design and prediction tools represent a future direction of the smart anticancer NDDS. Carrier module libraries and drug libraries could be established in various combinations in terms of different patients and tumor classification. Accordingly, the tumor-targeting smart NDDSs are expected to be realized with simple formulation design, easy preparation, good biocompatibility, and benefit for advancing the precise treatment for cancer patients.
Acknowledgments
This review paper was supported by the projects of National Natural Science Foundation of China (No. 81973259, 82073789, 81803472) and the project for Innovative Research Group at Higher Educational Institutions in Chongqing (CXQT20006, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Chong Li, Email: chongli@swu.edu.cn.
Jiancheng Wang, Email: wang-jc@bjmu.edu.cn.
Author contributions
All of the authors drafted the manuscript together. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Khan M.I., Hossain M.I., Hossain M.K., Rubel M.H.K., Hossain K.M., Mahfuz A., et al. Recent progress in nanostructured smart drug delivery systems for cancer therapy: a review. ACS Appl Bio Mater. 2022;5:971–1012. doi: 10.1021/acsabm.2c00002. [DOI] [PubMed] [Google Scholar]
- 2.Kenchegowda M., Rahamathulla M., Hani U., Begum M.Y., Guruswamy S., Osmani R.A.M., et al. Smart nanocarriers as an emerging platform for cancer therapy: a review. Molecules. 2021;27:146. doi: 10.3390/molecules27010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W.J.M., Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorn C.R., Howell P.L., Wozniak D.J., Prestidge C.A., Thomas N. Enhancing the therapeutic use of biofilm-dispersing enzymes with smart drug delivery systems. Adv Drug Deliv Rev. 2021;179 doi: 10.1016/j.addr.2021.113916. [DOI] [PubMed] [Google Scholar]
- 5.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 6.Li J.J., Kataoka K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: the next generation. J Am Chem Soc. 2021;143:538–559. doi: 10.1021/jacs.0c09029. [DOI] [PubMed] [Google Scholar]
- 7.Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–4588. doi: 10.7150/thno.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Y.F., Ding H.W. pH-Responsive nanoparticles for cancer immunotherapy: a brief review. Nanomaterials (Basel) 2020;10:1613. doi: 10.3390/nano10081613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham S.H., Choi Y.H., Choi J.H. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics. 2020;12:630. doi: 10.3390/pharmaceutics12070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S.S., Bharadwaj P., Bilal M., Barani M., Rahdar A., Taboada P., et al. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers (Basel) 2020;12:1397. doi: 10.3390/polym12061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazban-Shotorbani S., Hasani-Sadrabadi M.M., Karkhaneh A., Serpooshan V., Jacob K.I., Moshaverinia A., et al. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J Control Release. 2017;253:46–63. doi: 10.1016/j.jconrel.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Naeem M., Awan U.A., Subhan F., Cao J.F., Hlaing S.P., Lee J.H., et al. Advances in colon-targeted nano-drug delivery systems: challenges and solutions. Arch Pharm Res (Seoul) 2020;43:153–169. doi: 10.1007/s12272-020-01219-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.H., Bajracharya R., Min J.Y., Han J.W., Park B.J., Han H.K. Strategic approaches for colon targeted drug delivery: an overview of recent advancements. Pharmaceutics. 2020;12:68. doi: 10.3390/pharmaceutics12010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Q., Talton J., Zhang G.F., Cunningham T., Wang Z.J., Waters R.C., et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med. 2012;18:1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb B.A., Chimenti M., Jacobson M.P., Barber D.L. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 16.Ma X.P., Wang Y.G., Zhao T., Li Y., Su L.C., Wang Z.H., et al. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J Am Chem Soc. 2014;136:11085–11092. doi: 10.1021/ja5053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.G., Wang C.S., Li Y., Huang G., Zhao T., Ma X.P., et al. Digitization of endocytic pH by hybrid ultra-pH-sensitive nanoprobes at single-organelle resolution. Adv Mater. 2017;29 doi: 10.1002/adma.201603794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H.J., Zou Y.L., Jiang L., Yin Q., He X.Y., Chen L.L., et al. Induction of apoptosis in non-small cell lung cancer by downregulation of MDM2 using pH-responsive PMPC-b-PDPA/siRNA complex nanoparticles. Biomaterials. 2013;34:2738–2747. doi: 10.1016/j.biomaterials.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 19.Casey J.R., Grinstein S., Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y., Chen B.L., Wang Z.H., Yin Q.Q., Wang Y.Q., Wan F.J., et al. Sequential modulations of tumor vasculature and stromal barriers augment the active targeting efficacy of antibody-modified nanophotosensitizer in desmoplastic ovarian carcinoma. Adv Sci (Weinh) 2020;8 doi: 10.1002/advs.202002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi T., Chen B.L., Wang Z.H., Du H.L., Liu D.C., Yin Q.Q., et al. A pH-activatable nanoparticle for dual-stage precisely mitochondria-targeted photodynamic anticancer therapy. Biomaterials. 2019;213 doi: 10.1016/j.biomaterials.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Chen G.J., Wang Y.Y., Xie R.S., Gong S.Q. Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery. J Control Release. 2017;259:105–114. doi: 10.1016/j.jconrel.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jager M., Schubert S., Ochrimenko S., Fischer D., Schubert U.S. Branched and linear poly(ethylene imine)-based conjugates: synthetic modification, characterization, and application. Chem Soc Rev. 2012;41:4755–4767. doi: 10.1039/c2cs35146c. [DOI] [PubMed] [Google Scholar]
- 24.Faal Maleki M., Jafari A., Mirhadi E., Askarizadeh A., Golichenari B., Hadizadeh F., et al. Endogenous stimuli-responsive linkers in nanoliposomal systems for cancer drug targeting. Int J Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118716. [DOI] [PubMed] [Google Scholar]
- 25.Du H.L., Zhao S., Wang Y.Q., Wang Z.H., Chen B.L., Yan Y., et al. pH/cathepsin B hierarchical-responsive nanoconjugates for enhanced tumor penetration and chemo-immunotherapy. Adv Funct Mater. 2020;30 [Google Scholar]
- 26.Zheng G., Chen J., Stefflova K., Jarvi M., Li H., Wilson B.C. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc Natl Acad Sci U S A. 2007;104:8989–8994. doi: 10.1073/pnas.0611142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han H.J., Valdepérez D., Jin Q., Yang B., Li Z.H., Wu Y.L., et al. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano. 2017;11:1281–1291. doi: 10.1021/acsnano.6b05541. [DOI] [PubMed] [Google Scholar]
- 28.Ruan S.B., Xie R., Qin L., Yu M.N., Xiao W., Hu C., et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019;19:8318–8332. doi: 10.1021/acs.nanolett.9b03968. [DOI] [PubMed] [Google Scholar]
- 29.Hu C., He X.Q., Chen Y.X., Yang X.T., Qin L., Lei T., et al. Metformin mediated PD-L1 downregulation in combination with photodynamic-immunotherapy for treatment of breast cancer. Adv Funct Mater. 2021;31 [Google Scholar]
- 30.Yu W.Q., He X.Q., Yang Z.H., Yang X.T., Xiao W., Liu R., et al. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119309. [DOI] [PubMed] [Google Scholar]
- 31.Mu J., Lin J., Huang P., Chen X.Y. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem Soc Rev. 2018;47:5554–5573. doi: 10.1039/c7cs00663b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie A., Hanif S., Ouyang J., Tang Z.M., Kong N., Kim N.Y., et al. Stimuli-responsive prodrug-based cancer nanomedicine. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirhadi E., Mashreghi M., Faal Maleki M., Alavizadeh S.H., Arabi L., Badiee A., et al. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int J Pharm. 2020;589 doi: 10.1016/j.ijpharm.2020.119882. [DOI] [PubMed] [Google Scholar]
- 34.Ghorbani M., Hamishehkar H. Redox-responsive smart nanogels for intracellular targeting of therapeutic agents: applications and recent advances. J Drug Target. 2019;27:408–422. doi: 10.1080/1061186X.2018.1514041. [DOI] [PubMed] [Google Scholar]
- 35.Kumar P., Liu B., Behl G. A comprehensive outlook of synthetic strategies and applications of redox-responsive nanogels in drug delivery. Macromol Biosci. 2019;19 doi: 10.1002/mabi.201900071. [DOI] [PubMed] [Google Scholar]
- 36.Mollazadeh S., Mackiewicz M., Yazdimamaghani M. Recent advances in the redox-responsive drug delivery nanoplatforms: a chemical structure and physical property perspective. Mater Sci Eng C Mater Biol Appl. 2021;118 doi: 10.1016/j.msec.2020.111536. [DOI] [PubMed] [Google Scholar]
- 37.Wu M.Y., Meng Q.S., Chen Y., Zhang L.X., Li M.L., Cai X.J., et al. Large pore-sized hollow mesoporous organosilica for redox-responsive gene delivery and synergistic cancer chemotherapy. Adv Mater. 2016;28:1963–1969. doi: 10.1002/adma.201505524. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.J., Kim H.S., Seo Y.R. Understanding of ROS-inducing strategy in anticancer therapy. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D.D., Zhang R.H., Liu G.T., Kang Y., Wu J. Redox-responsive self-assembled nanoparticles for cancer therapy. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.202000605. [DOI] [PubMed] [Google Scholar]
- 40.Xu X.D., Saw P.E., Tao W., Li Y.J., Ji X.Y., Bhasin S., et al. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv Mater. 2017;29 doi: 10.1002/adma.201700141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y.Y., Deng Y.B., Luo H.H., Zhu A.J., Ke H.T., Yang H., et al. Light-responsive nanoparticles for highly efficient cytoplasmic delivery of anticancer agents. ACS Nano. 2017;11:12134–12144. doi: 10.1021/acsnano.7b05214. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H., Xu J.B., Huang W.J., Zhan G.T., Zhao Y.B., Chen H.B., et al. Spatiotemporally light-activatable platinum nanocomplexes for selective and cooperative cancer therapy. ACS Nano. 2019;13:6647–6661. doi: 10.1021/acsnano.9b00972. [DOI] [PubMed] [Google Scholar]
- 43.Chen J.J., Lin S.Y., Zhao D.D., Guan L., Hu Y.P., Wang Y.T., et al. Palladium nanocrystals-engineered metal-organic frameworks for enhanced tumor inhibition by synergistic hydrogen/photodynamic therapy. Adv Funct Mater. 2021;31 [Google Scholar]
- 44.Chen Y.F., Zhang X.H., Cheng D.B., Zhang Y.J., Liu Y., Ji L., et al. Near-infrared laser-triggered in situ dimorphic transformation of BF2-azadipyrromethene nanoaggregates for enhanced solid tumor penetration. ACS Nano. 2020;14:3640–3650. doi: 10.1021/acsnano.0c00118. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Tang R.B., Liu X.Y., Gong J.Y., Zhao Z.J., Sheng Z.H., et al. Bright aggregation-induced emission nanoparticles for two-photon imaging and localized compound therapy of cancers. ACS Nano. 2020;14:16840–16853. doi: 10.1021/acsnano.0c05610. [DOI] [PubMed] [Google Scholar]
- 46.Zhu W., Kang M.M., Wu Q., Zhang Z.J., Wu Y., Li C.B., et al. Zwitterionic AIEgens: rational molecular design for NIR-II fluorescence imaging-guided synergistic phototherapy. Adv Funct Mater. 2021;31 [Google Scholar]
- 47.Ren J.J., Zhang L., Zhang J.Y., Zhang W., Cao Y., Xu Z.G., et al. Light-activated oxygen self-supplied starving therapy in near-infrared (NIR) window and adjuvant hyperthermia-induced tumor ablation with an augmented sensitivity. Biomaterials. 2020;234 doi: 10.1016/j.biomaterials.2020.119771. [DOI] [PubMed] [Google Scholar]
- 48.Zhou F.Y., Feng B., Wang T.T., Wang D.G., Meng Q.S., Zeng J.F., et al. Programmed multiresponsive vesicles for enhanced tumor penetration and combination therapy of triple-negative breast cancer. Adv Funct Mater. 2017;27 [Google Scholar]
- 49.Feng Q., Liu J.P., Li X.Y., Chen Q.H., Sun J.S., Shi X.H., et al. One-step microfluidic synthesis of nanocomplex with tunable rigidity and acid-switchable surface charge for overcoming drug resistance. Small. 2017;13 doi: 10.1002/smll.201603109. [DOI] [PubMed] [Google Scholar]
- 50.Wang T.T., Wang D.G., Liu J.P., Feng B., Zhou F.Y., Zhang H.W., et al. Acidity-triggered ligand-presenting nanoparticles to overcome sequential drug delivery barriers to tumors. Nano Lett. 2017;17:5429–5436. doi: 10.1021/acs.nanolett.7b02031. [DOI] [PubMed] [Google Scholar]
- 51.Wang T.T., Wang D.G., Yu H.J., Wang M.W., Liu J.P., Feng B., et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano. 2016;10:3496–3508. doi: 10.1021/acsnano.5b07706. [DOI] [PubMed] [Google Scholar]
- 52.Yang B., Gao J., Pei Q., Xu H.X., Yu H.J. Engineering prodrug nanomedicine for cancer immunotherapy. Adv Sci (Weinh) 2020;7 doi: 10.1002/advs.202002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J., Wang W.Q., Pei Q., Lord M.S., Yu H.J. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol Sin. 2020;41:986–994. doi: 10.1038/s41401-020-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X.L., Hou B., Xu Z.A., Saeed M., Sun F., Gao Z.M., et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer. Adv Sci (Weinh) 2020;7 doi: 10.1002/advs.201903332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D.G., Wang T.T., Yu H.J., Feng B., Zhou L., Zhou F.Y., et al. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aau6584. [DOI] [PubMed] [Google Scholar]
- 56.Wang T.T., Wang D.G., Yu H.J., Feng B., Zhou F.Y., Zhang H.W., et al. A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nat Commun. 2018;9:1532. doi: 10.1038/s41467-018-03915-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao A., Chen B.F., Gao J., Zhou F.Q., Saeed M., Hou B., et al. Sheddable prodrug vesicles combating adaptive immune resistance for improved photodynamic immunotherapy of cancer. Nano Lett. 2020;20:353–362. doi: 10.1021/acs.nanolett.9b04012. [DOI] [PubMed] [Google Scholar]
- 58.Zhou F.Q., Gao J., Xu Z.A., Li T.L., Gao A., Sun F., et al. Overcoming immune resistance by sequential prodrug nanovesicles for promoting chemoimmunotherapy of cancer. Nano Today. 2021;36 [Google Scholar]
- 59.Sun F., Zhu Q.R., Li T.L., Saeed M., Xu Z.A., Zhong F.S., et al. Regulating glucose metabolism with prodrug nanoparticles for promoting photoimmunotherapy of pancreatic cancer. Adv Sci (Weinh) 2021;8 doi: 10.1002/advs.202002746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou B., Zhou L., Wang H., Saeed M., Wang D.G., Xu Z.A., et al. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2020;32 doi: 10.1002/adma.201907210. [DOI] [PubMed] [Google Scholar]
- 61.Zhou F.Y., Feng B., Yu H.J., Wang D.G., Wang T.T., Ma Y.T., et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31 doi: 10.1002/adma.201805888. [DOI] [PubMed] [Google Scholar]
- 62.Feng B., Hou B., Xu Z.A., Saeed M., Yu H.J., Li Y.P. Self-amplified drug delivery with light-inducible nanocargoes to enhance cancer immunotherapy. Adv Mater. 2019;31 doi: 10.1002/adma.201902960. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y., Zhang X.Q., Yang H.C., Yu L., Xu Y.J., Sharma A., et al. Advanced biotechnology-assisted precise sonodynamic therapy. Chem Soc Rev. 2021;50:11227–11248. doi: 10.1039/d1cs00403d. [DOI] [PubMed] [Google Scholar]
- 64.Chilakamarthi U., Giribabu L. Photodynamic therapy: past, present and future. Chem Rec. 2017;17:775–802. doi: 10.1002/tcr.201600121. [DOI] [PubMed] [Google Scholar]
- 65.Sandland J., Boyle R.W. Photosensitizer antibody-drug conjugates: past, present, and future. Bioconjug Chem. 2019;30:975–993. doi: 10.1021/acs.bioconjchem.9b00055. [DOI] [PubMed] [Google Scholar]
- 66.Dong Z.L., Feng L.Z., Hao Y., Chen M.C., Gao M., Chao Y., et al. Synthesis of hollow biomineralized CaCO3-polydopamine nanoparticles for multimodal imaging-guided cancer photodynamic therapy with reduced skin photosensitivity. J Am Chem Soc. 2018;140:2165–2178. doi: 10.1021/jacs.7b11036. [DOI] [PubMed] [Google Scholar]
- 67.Feng H.T., Li Y.Y., Duan X.C., Wang X.X., Qi C.X., Lam J.W.Y., et al. Substitution activated precise phototheranostics through supramolecular assembly of AIEgen and calixarene. J Am Chem Soc. 2020;142:15966–15974. doi: 10.1021/jacs.0c06872. [DOI] [PubMed] [Google Scholar]
- 68.Gao J., Li J., Geng W.C., Chen F.Y., Duan X.C., Zheng Z., et al. Biomarker displacement activation: a general host-guest strategy for targeted phototheranostics in vivo. J Am Chem Soc. 2018;140:4945–4953. doi: 10.1021/jacs.8b02331. [DOI] [PubMed] [Google Scholar]
- 69.Sponchioni M., Capasso Palmiero U., Moscatelli D. Thermo-responsive polymers: applications of smart materials in drug delivery and tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;102:589–605. doi: 10.1016/j.msec.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 70.Shin H.H., Choi H.W., Lim J.H., Kim J.W., Chung B.G. Near-infrared light-triggered thermo-responsive poly(N-isopropylacrylamide)-pyrrole nanocomposites for chemo-photothermal cancer therapy. Nanoscale Res Lett. 2020;15:214. doi: 10.1186/s11671-020-03444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geng S.N., Zhao H., Zhan G.T., Zhao Y.B., Yang X.L. Injectable in situ forming hydrogels of thermosensitive polypyrrole nanoplatforms for precisely synergistic photothermo-chemotherapy. ACS Appl Mater Interfaces. 2020;12:7995–8005. doi: 10.1021/acsami.9b22654. [DOI] [PubMed] [Google Scholar]
- 72.Bordat A., Boissenot T., Nicolas J., Tsapis N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Rev. 2019;138:167–192. doi: 10.1016/j.addr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Liu K.C., Arivajiagane A., Wu S.J., Tzou S.C., Chen C.Y., Wang Y.M. Development of a novel thermal-sensitive multifunctional liposome with antibody conjugation to target EGFR-expressing tumors. Nanomedicine. 2019;15:285–294. doi: 10.1016/j.nano.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 74.El-Sawy H.S., Al-Abd A.M., Ahmed T.A., El-Say K.M., Torchilin V.P. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: past, present, and future perspectives. ACS Nano. 2018;12:10636–10664. doi: 10.1021/acsnano.8b06104. [DOI] [PubMed] [Google Scholar]
- 75.Cao Y., Chen Y.L., Yu T., Guo Y., Liu F.Q., Yao Y.Z., et al. Drug release from phase-changeable nanodroplets triggered by low-intensity focused ultrasound. Theranostics. 2018;8:1327–1339. doi: 10.7150/thno.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei P., Sun M., Yang B., Xiao J.G., Du J.Z. Ultrasound-responsive polymersomes capable of endosomal escape for efficient cancer therapy. J Control Release. 2020;322:81–94. doi: 10.1016/j.jconrel.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 77.Ma X.T., Yao M.N., Shi J.Y., Li X.D., Gao Y., Luo Q., et al. High intensity focused ultrasound-responsive and ultrastable cerasomal perfluorocarbon nanodroplets for alleviating tumor multidrug resistance and epithelial-mesenchymal transition. ACS Nano. 2020;14:15904–15918. doi: 10.1021/acsnano.0c07287. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz M.R., Debski A.C., Price R.J. Ultrasound-targeted nucleic acid delivery for solid tumor therapy. J Control Release. 2021;339:531–546. doi: 10.1016/j.jconrel.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng Z.Q., Zhang Y.J., She J.L., Zhou X.F., Xu J., Han X., et al. Ultrasound-mediated remotely controlled nanovaccine delivery for tumor vaccination and individualized cancer immunotherapy. Nano Lett. 2021;21:1228–1237. doi: 10.1021/acs.nanolett.0c03646. [DOI] [PubMed] [Google Scholar]
- 80.Snipstad S., Vikedal K., Maardalen M., Kurbatskaya A., Sulheim E., Davies C.L. Ultrasound and microbubbles to beat barriers in tumors: improving delivery of nanomedicine. Adv Drug Deliv Rev. 2021;177 doi: 10.1016/j.addr.2021.113847. [DOI] [PubMed] [Google Scholar]
- 81.Arsiwala T.A., Sprowls S.A., Blethen K.E., Adkins C.E., Saralkar P.A., Fladeland R.A., et al. Ultrasound-mediated disruption of the blood tumor barrier for improved therapeutic delivery. Neoplasia. 2021;23:676–691. doi: 10.1016/j.neo.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahle F.F., Gulfam M., Lowe T.L. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov Today. 2018;23:992–1006. doi: 10.1016/j.drudis.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montha W., Maneeprakorn W., Tang I.M., Pon-On W. Hyperthermia evaluation and drug/protein-controlled release using alternating magnetic field stimuli-responsive Mn-Zn ferrite composite particles. RSC Adv. 2020;10:40206–40214. doi: 10.1039/d0ra08602a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirvakili S.M., Ngo Q.P., Langer R. Polymer nanocomposite microactuators for on-demand chemical release via high-frequency magnetic field excitation. Nano Lett. 2020;20:4816–4822. doi: 10.1021/acs.nanolett.0c00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kolosnjaj-Tabi J., Gibot L., Fourquaux I., Golzio M., Rols M.P. Electric field-responsive nanoparticles and electric fields: physical, chemical, biological mechanisms and therapeutic prospects. Adv Drug Deliv Rev. 2019;138:56–67. doi: 10.1016/j.addr.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 86.Hosseini-Nassab N., Samanta D., Abdolazimi Y., Annes J.P., Zare R.N. Electrically controlled release of insulin using polypyrrole nanoparticles. Nanoscale. 2017;9:143–149. doi: 10.1039/c6nr08288b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qu J., Zhao X., Ma P.X., Guo B.L. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018;72:55–69. doi: 10.1016/j.actbio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 88.Qu J., Liang Y.P., Shi M.T., Guo B.L., Gao Y.Z., Yin Z.H. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int J Biol Macromol. 2019;140:255–264. doi: 10.1016/j.ijbiomac.2019.08.120. [DOI] [PubMed] [Google Scholar]
- 89.Peng L., Liu Y., Huang J.N., Li J.H., Gong J.H., Ma J.H. Microfluidic fabrication of highly stretchable and fast electro-responsive graphene oxide/polyacrylamide/alginate hydrogel fibers. Eur Polym J. 2018;103:335–341. [Google Scholar]
- 90.Carayon I., Gaubert A., Mousli Y., Philippe B. Electro-responsive hydrogels: macromolecular and supramolecular approaches in the biomedical field. Biomater Sci. 2020;8:5589–5600. doi: 10.1039/d0bm01268h. [DOI] [PubMed] [Google Scholar]
- 91.Frangoul H., Altshuler D., Cappellini M.D., Chen Y.S., Domm J., Eustace B.K., et al. CRISPR-Cas9 gene editing for sickle cell disease and beta-thalassemia. N Engl J Med. 2021;384:252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- 92.Lu Y., Xue J.X., Deng T., Zhou X.J., Yu K., Deng L., et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–740. doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- 93.Rahim M.A., Jan N., Khan S., Shah H., Madni A., Khan A., et al. Recent advancements in stimuli responsive drug delivery platforms for active and passive cancer targeting. Cancers (Basel) 2021;13:670. doi: 10.3390/cancers13040670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ying X.Y., Wang Y., Liang J., Yue J.X., Xu C.L., Lu L.N., et al. Angiopep-conjugated electro-responsive hydrogel nanoparticles: therapeutic potential for epilepsy. Angew Chem Int Ed Engl. 2014;53:12436–12440. doi: 10.1002/anie.201403846. [DOI] [PubMed] [Google Scholar]
- 95.Zhang L.X., Zhang S.T., Xu J.Y., Li Y.Y., He J.L., Yang Y., et al. Low-dose X-ray-responsive diselenide nanocarriers for effective delivery of anticancer agents. ACS Appl Mater Interfaces. 2020;12:43398–43407. doi: 10.1021/acsami.0c11627. [DOI] [PubMed] [Google Scholar]
- 96.Yi X., Zhou H.L., Zhang Z., Xiong S.S., Yang K. X-rays-optimized delivery of radiolabeled albumin for cancer theranostics. Biomaterials. 2020;233 doi: 10.1016/j.biomaterials.2020.119764. [DOI] [PubMed] [Google Scholar]
- 97.Li R., Zheng K., Yuan C., Chen Z., Huang M.D. Be active or not: the relative contribution of active and passive tumor targeting of nanomaterials. Nanotheranostics. 2017;1:346–357. doi: 10.7150/ntno.19380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J., et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 99.Azarmi S., Roa W.H., Lobenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev. 2008;60:863–875. doi: 10.1016/j.addr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Guan J., Shen Q., Zhang Z., Jiang Z.X., Yang Y., Lou M.Q., et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9:2982. doi: 10.1038/s41467-018-05384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sempkowski M., Zhu C., Menzenski M.Z., Kevrekidis I.G., Bruchertseifer F., Morgenstern A., et al. Sticky patches on lipid nanoparticles enable the selective targeting and killing of untargetable cancer cells. Langmuir. 2016;32:8329–8338. doi: 10.1021/acs.langmuir.6b01464. [DOI] [PubMed] [Google Scholar]
- 102.Poon Z.Y., Chen S.J., Engler A.C., Lee H.I., Atas E., von Maltzahn G., et al. Ligand-clustered “patchy” nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew Chem Int Ed Engl. 2010;49:7266–7270. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim K., Guo J.H., Liang Z.X., Fan D.L. Artificial micro/nanomachines for bioapplications: biochemical delivery and diagnostic sensing. Adv Funct Mater. 2018;28 [Google Scholar]
- 104.Liu J.Y., Toy R., Vantucci C., Pradhan P., Zhang Z.J., Kuo K.M., et al. Bifunctional janus particles as multivalent synthetic nanoparticle antibodies (SNAbs) for selective depletion of target cells. Nano Lett. 2021;21:875–886. doi: 10.1021/acs.nanolett.0c04833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Juweid M., Neumann R., Paik C., Perez-Bacete M.J., Sato J., van Osdol W., et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52:5144–5153. [PubMed] [Google Scholar]
- 106.Bordeau B.M., Yang Y., Balthasar J.P. Transient competitive inhibition bypasses the binding site barrier to improve tumor penetration of trastuzumab and enhance T-DM1 efficacy. Cancer Res. 2021;81:4145–4154. doi: 10.1158/0008-5472.CAN-20-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamashita M., Ogawa T., Zhang X., Hanamura N., Kashikura Y., Takamura M., et al. Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer. 2012;19:170–176. doi: 10.1007/s12282-010-0234-5. [DOI] [PubMed] [Google Scholar]
- 108.Ohlund D., Elyada E., Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miao L., Newby J.M., Lin C.M., Zhang L., Xu F.F., Kim W.Y., et al. The binding site barrier elicited by tumor-associated fibroblasts interferes disposition of nanoparticles in stroma-vessel type tumors. ACS Nano. 2016;10:9243–9258. doi: 10.1021/acsnano.6b02776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu Y.J., Atwal J.K., Zhang Y., Tong R.K., Wildsmith K.R., Tan C., et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6:261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 111.Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y.M., et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 112.Ruan S.B., Qin L., Xiao W., Hu C., Zhou Y., Wang R.R., et al. Acid-responsive transferrin dissociation and GLUT mediated exocytosis for increased blood-brain barrier transcytosis and programmed glioma targeting delivery. Adv Funct Mater. 2018;28 [Google Scholar]
- 113.Lei T., Yang Z.H., Xia X., Chen Y.X., Yang X.T., Xie R., et al. A nanocleaner specifically penetrates the blood–brain barrier at lesions to clean toxic proteins and regulate inflammation in Alzheimer's disease. Acta Pharm Sin B. 2021;11:4032–4044. doi: 10.1016/j.apsb.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song Y., Guo X.F., Fu J.J., He B., Wang X.Q., Dai W.B., et al. Dual-targeting nanovesicles enhance specificity to dynamic tumor cells in vitro and in vivo via manipulation of alpha v beta 3-ligand binding. Acta Pharm Sin B. 2020;10:2183–2197. doi: 10.1016/j.apsb.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park J.A., Lee Y.J., Lee J.W., Lee K.C., An G.I., Kim K.M., et al. Cyclic RGD peptides incorporating cycloalkanes: synthesis and evaluation as PET radiotracers for tumor imaging. ACS Med Chem Lett. 2014;5:979–982. doi: 10.1021/ml500135t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Latham K.R., Apriletti J.W., Eberhardt N.L., Baxter J.D. Development of support matrices for affinity-chromatography of thyroid-hormone receptors. J Biol Chem. 1981;256:12088–12093. [PubMed] [Google Scholar]
- 117.Manthe R.L., Loeck M., Bhowmick T., Solomon M., Muro S. Intertwined mechanisms define transport of anti-ICAM nanocarriers across the endothelium and brain delivery of a therapeutic enzyme. J Control Release. 2020;324:181–193. doi: 10.1016/j.jconrel.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grimaldi N., Andrade F., Segovia N., Ferrer-Tasies L., Sala S., Veciana J., et al. Lipid-based nanovesicles for nanomedicine. Chem Soc Rev. 2016;45:6520–6545. doi: 10.1039/c6cs00409a. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y.B., Sun C.Z., Wang C., Jankovic K.E., Dong Y.Z. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khosa A., Reddi S., Saha R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. doi: 10.1016/j.biopha.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 121.Bottger R., Pauli G., Chao P.H., Al Fayez N., Hohenwarter L., Li S.D. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev. 2020;154–155:79–101. doi: 10.1016/j.addr.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 122.Jiang T.Y., Wang T., Li T., Ma Y.D., Shen S.Y., He B.F., et al. Enhanced transdermal drug delivery by transfersome-embedded oligopeptide hydrogel for topical chemotherapy of melanoma. ACS Nano. 2018;12:9693–9701. doi: 10.1021/acsnano.8b03800. [DOI] [PubMed] [Google Scholar]
- 123.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blanco E., Shen H.F., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y., Miao Y.Q., Chen M.S., Chen X., Li F., Zhang X., et al. Stepwise targeting and responsive lipid-coated nanoparticles for enhanced tumor cell sensitivity and hepatocellular carcinoma therapy. Theranostics. 2020;10:3722–3736. doi: 10.7150/thno.42008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He S.F., Fan W.W., Wu N., Zhu J.J., Miao Y.Q., Miao X.R., et al. Lipid-based liquid crystalline nanoparticles facilitate cytosolic delivery of siRNA via structural transformation. Nano Lett. 2018;18:2411–2419. doi: 10.1021/acs.nanolett.7b05430. [DOI] [PubMed] [Google Scholar]
- 128.Arias-Alpizar G., Kong L., Vlieg R.C., Rabe A., Papadopoulou P., Meijer M.S., et al. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat Commun. 2020;11:3638. doi: 10.1038/s41467-020-17360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ji T.J., Lang J.Y., Wang J., Cai R., Zhang Y.L., Qi F.F., et al. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy. ACS Nano. 2017;11:8668–8678. doi: 10.1021/acsnano.7b01026. [DOI] [PubMed] [Google Scholar]
- 130.Li M.H., Teh C., Ang C.Y., Tan S.Y., Luo Z., Qu Q.Y., et al. Near-infrared light-absorptive stealth liposomes for localized photothermal ablation of tumors combined with chemotherapy. Adv Funct Mater. 2015;25:5602–5610. [Google Scholar]
- 131.Yan Y., Liu X.Y., Lu A., Wang X.Y., Jiang L.X., Wang J.C. Non-viral vectors for RNA delivery. J Control Release. 2022;342:241–279. doi: 10.1016/j.jconrel.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thi T.T.H., Suys E.J.A., Lee J.S., Nguyen D.H., Park K.D., Truong N.P. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines (Basel) 2021;9:359. doi: 10.3390/vaccines9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ickenstein L.M., Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv. 2019;16:1205–1226. doi: 10.1080/17425247.2019.1669558. [DOI] [PubMed] [Google Scholar]
- 134.Cheng X.W., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 135.Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 136.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 137.Plaza-Oliver M., Santander-Ortega M.J., Lozano M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv Transl Res. 2021;11:471–497. doi: 10.1007/s13346-021-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pinilla C.M.B., Lopes N.A., Brandelli A. Lipid-based nanostructures for the delivery of natural antimicrobials. Molecules. 2021;26:3587. doi: 10.3390/molecules26123587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salunkhe S.A., Chitkara D., Mahato R.I., Mittal A. Lipid based nanocarriers for effective drug delivery and treatment of diabetes associated liver fibrosis. Adv Drug Deliv Rev. 2021;173:394–415. doi: 10.1016/j.addr.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 140.Du Y.W., Ling L.B., Ismail M., He W., Xia Q., Zhou W.Y., et al. Redox sensitive lipid-camptothecin conjugate encapsulated solid lipid nanoparticles for oral delivery. Int J Pharm. 2018;549:352–362. doi: 10.1016/j.ijpharm.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 141.Shi J.J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanadgol N., Wackerlig J. Developments of smart drug-delivery systems based on magnetic molecularly imprinted polymers for targeted cancer therapy: a short review. Pharmaceutics. 2020;12:831. doi: 10.3390/pharmaceutics12090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qiu N.S., Wang G.W., Wang J.Q., Zhou Q., Guo M.Y., Wang Y.L., et al. Tumor-associated macrophage and tumor-cell dually transfecting polyplexes for efficient interleukin-12 cancer gene therapy. Adv Mater. 2021;33 doi: 10.1002/adma.202006189. [DOI] [PubMed] [Google Scholar]
- 144.Zou Y., Zheng M., Yang W.J., Meng F.H., Miyata K., Kim H.J., et al. Virus-mimicking chimaeric polymersomes boost targeted cancer siRNA therapy in vivo. Adv Mater. 2017;29 doi: 10.1002/adma.201703285. [DOI] [PubMed] [Google Scholar]
- 145.Yin S.P., Huai J., Chen X., Yang Y., Zhang X.X., Gan Y., et al. Intracellular delivery and antitumor effects of a redox-responsive polymeric paclitaxel conjugate based on hyaluronic acid. Acta Biomater. 2015;26:274–285. doi: 10.1016/j.actbio.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 146.Cong Y.W., Xiao H.H., Xiong H.J., Wang Z.G., Ding J.X., Li C., et al. Dual drug backboned shattering polymeric theranostic nanomedicine for synergistic eradication of patient-derived lung cancer. Adv Mater. 2018;30 doi: 10.1002/adma.201706220. [DOI] [PubMed] [Google Scholar]
- 147.Tang S., Meng Q.S., Sun H.P., Su J.H., Yin Q., Zhang Z.W., et al. Dual pH-sensitive micelles with charge-switch for controlling cellular uptake and drug release to treat metastatic breast cancer. Biomaterials. 2017;114:44–53. doi: 10.1016/j.biomaterials.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 148.Li Y., Yang H.Y., Thambi T., Park J.H., Lee D.S. Charge-convertible polymers for improved tumor targeting and enhanced therapy. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119299. [DOI] [PubMed] [Google Scholar]
- 149.Senthilkumar T., Zhou L.Y., Gu Q., Liu L.B., Lv F.T., Wang S. Conjugated polymer nanoparticles with appended photo-responsive units for controlled drug delivery, release, and imaging. Angew Chem Int Ed Engl. 2018;57:13114–13119. doi: 10.1002/anie.201807158. [DOI] [PubMed] [Google Scholar]
- 150.Zeng X.L., Wang Y.F., Han J.X., Sun W., Butt H.J., Liang X.J., et al. Fighting against drug-resistant tumors using a dual-responsive Pt(IV)/Ru(II) bimetallic polymer. Adv Mater. 2020;32 doi: 10.1002/adma.202004766. [DOI] [PubMed] [Google Scholar]
- 151.Cabral H., Miyata K., Osada K., Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 152.Tam Y.T., Gao J.M., Kwon G.S. Oligo(lactic acid)n-paclitaxel prodrugs for poly(ethylene glycol)-block-poly(lactic acid) micelles: loading, release, and backbiting conversion for anticancer activity. J Am Chem Soc. 2016;138:8674–8677. doi: 10.1021/jacs.6b03995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Banskota S., Saha S., Bhattacharya J., Kirmani N., Yousefpour P., Dzuricky M., et al. Genetically encoded stealth nanoparticles of a zwitterionic polypeptide-paclitaxel conjugate have a wider therapeutic window than abraxane in multiple tumor models. Nano Lett. 2020;20:2396–2409. doi: 10.1021/acs.nanolett.9b05094. [DOI] [PubMed] [Google Scholar]
- 154.Li W.S., Huang L.W., Ying X.Y., Jian Y., Hong Y., Hu F.Q., et al. Antitumor drug delivery modulated by a polymeric micelle with an upper critical solution temperature. Angew Chem Int Ed Engl. 2015;54:3126–3131. doi: 10.1002/anie.201411524. [DOI] [PubMed] [Google Scholar]
- 155.Su Z.W., Xiao Z.C., Wang Y., Huang J.S., An Y.C., Wang X., et al. Codelivery of anti-PD-1 antibody and paclitaxel with matrix metalloproteinase and pH dual-sensitive micelles for enhanced tumor chemoimmunotherapy. Small. 2020;16 doi: 10.1002/smll.201906832. [DOI] [PubMed] [Google Scholar]
- 156.Lu L., Zhao X.J., Fu T.W., Li K., He Y., Luo Z., et al. An iRGD-conjugated prodrug micelle with blood–brain barrier penetrability for anti-glioma therapy. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119666. [DOI] [PubMed] [Google Scholar]
- 157.Jin X., Sun P., Tong G.S., Zhu X.Y. Star polymer-based unimolecular micelles and their application in bio-imaging and diagnosis. Biomaterials. 2018;178:738–750. doi: 10.1016/j.biomaterials.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 158.Chen H.B., Gu Z.J., An H.W., Chen C.Y., Chen J., Cui R., et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. 2018;61:1503–1552. [Google Scholar]
- 159.Zhang X.B., Li N., Zhang S.W., Sun B.J., Chen Q., He Z.G., et al. Emerging carrier-free nanosystems based on molecular self-assembly of pure drugs for cancer therapy. Med Res Rev. 2020;40:1754–1775. doi: 10.1002/med.21669. [DOI] [PubMed] [Google Scholar]
- 160.Luo C., Sun J., Sun B.J., He Z.G. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol Sci. 2014;35:556–566. doi: 10.1016/j.tips.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 161.Jiang Q., Zhao S., Liu J.B., Song L.L., Wang Z.G., Ding B.Q. Rationally designed DNA-based nanocarriers. Adv Drug Deliv Rev. 2019;147:2–21. doi: 10.1016/j.addr.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Karaosmanoglu S., Zhou M.J., Shi B.Y., Zhang X.J., Williams G.R., Chen X.F. Carrier-free nanodrugs for safe and effective cancer treatment. J Control Release. 2021;329:805–832. doi: 10.1016/j.jconrel.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 163.Li W., Yang Y.L., Wang C., Liu Z., Zhang X.J., An F.F., et al. Carrier-free, functionalized drug nanoparticles for targeted drug delivery. Chem Commun (Camb) 2012;48:8120–8122. doi: 10.1039/c2cc33214k. [DOI] [PubMed] [Google Scholar]
- 164.Yu C.T., Zhou M.J., Zhang X.J., Wei W.J., Chen X.F., Zhang X.H. Smart doxorubicin nanoparticles with high drug payload for enhanced chemotherapy against drug resistance and cancer diagnosis. Nanoscale. 2015;7:5683–5690. doi: 10.1039/c5nr00290g. [DOI] [PubMed] [Google Scholar]
- 165.Zhang J.F., Li S.L., An F.F., Liu J., Jin S.B., Zhang J.C., et al. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale. 2015;7:13503–13510. doi: 10.1039/c5nr03259h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang X.B., Sun B.J., Zuo S.Y., Chen Q., Gao Y.L., Zhao H.Q., et al. Self-assembly of a pure photosensitizer as a versatile theragnostic nanoplatform for imaging-guided antitumor photothermal therapy. ACS Appl Mater Interfaces. 2018;10:30155–30162. doi: 10.1021/acsami.8b10421. [DOI] [PubMed] [Google Scholar]
- 167.Wang D., Niu L.J., Qiao Z.Y., Cheng D.B., Wang J.F., Zhong Y., et al. Synthesis of self-assembled porphyrin nanoparticle photosensitizers. ACS Nano. 2018;12:3796–3803. doi: 10.1021/acsnano.8b01010. [DOI] [PubMed] [Google Scholar]
- 168.Feng B., Niu Z.F., Hou B., Zhou L., Li Y.P., Yu H.J. Enhancing triple negative breast cancer immunotherapy by ICG-templated self-assembly of paclitaxel nanoparticles. Adv Funct Mater. 2020;30 [Google Scholar]
- 169.Wei Z., Liang P.P., Xie J.Q., Song C.H., Tang C.C., Wang Y.F., et al. Carrier-free nano-integrated strategy for synergetic cancer anti-angiogenic therapy and phototherapy. Chem Sci. 2019;10:2778–2784. doi: 10.1039/c8sc04123g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liang C., Zhang X.L., Yang M.S., Wang W.J., Chen P., Dong X.C. Remodeling tumor microenvironment by multifunctional nanoassemblies for enhanced photodynamic cancer therapy. ACS Mater Lett. 2020;2:1268–1286. [Google Scholar]
- 171.Li S.M., Shan X.Z., Wang Y.Q., Chen Q., Sun J., He Z.G., et al. Dimeric prodrug-based nanomedicines for cancer therapy. J Control Release. 2020;326:510–522. doi: 10.1016/j.jconrel.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 172.Luo C., Sun J., Liu D., Sun B.J., Miao L., Musetti S., et al. Self-assembled redox dual-responsive prodrug-nanosystem formed by single thioether-bridged paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett. 2016;16:5401–5408. doi: 10.1021/acs.nanolett.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luo C., Sun J., Sun B.J., Liu D., Miao L., Goodwin T.J., et al. Facile fabrication of tumor redox-sensitive nanoassemblies of small-molecule oleate prodrug as potent chemotherapeutic nanomedicine. Small. 2016;12:6353–6362. doi: 10.1002/smll.201601597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun B.J., Luo C., Yu H., Zhang X.B., Chen Q., Yang W.Q., et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018;18:3643–3650. doi: 10.1021/acs.nanolett.8b00737. [DOI] [PubMed] [Google Scholar]
- 175.Yang Y.X., Sun B.J., Zuo S.Y., Li X.M., Zhou S., Li L.X., et al. Trisulfide bond-mediated doxorubicin dimeric prodrug nanoassemblies with high drug loading, high self-assembly stability, and high tumor selectivity. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zuo S.Y., Sun B.J., Yang Y.X., Zhou S., Zhang Y., Guo M.R., et al. Probing the superiority of diselenium bond on docetaxel dimeric prodrug nanoassemblies: small roles taking big responsibilities. Small. 2020;16 doi: 10.1002/smll.202005039. [DOI] [PubMed] [Google Scholar]
- 177.Sun B.J., Luo C., Zhang X.B., Guo M.R., Sun M.C., Yu H., et al. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat Commun. 2019;10:3211. doi: 10.1038/s41467-019-11193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Luo C., Sun B.J., Wang C., Zhang X.B., Chen Y., Chen Q., et al. Self-facilitated ROS-responsive nanoassembly of heterotypic dimer for synergistic chemo-photodynamic therapy. J Control Release. 2019;302:79–89. doi: 10.1016/j.jconrel.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 179.Li Y.F., Breaker R.R. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J Am Chem Soc. 1999;121:5364–5372. [Google Scholar]
- 180.Nishikawa M., Tan M.M., Liao W.Q., Kusamori K. Nanostructured DNA for the delivery of therapeutic agents. Adv Drug Deliv Rev. 2019;147:29–36. doi: 10.1016/j.addr.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 181.Micura R., Hobartner C. Fundamental studies of functional nucleic acids: aptamers, riboswitches, ribozymes and DNAzymes. Chem Soc Rev. 2020;49:7331–7353. doi: 10.1039/d0cs00617c. [DOI] [PubMed] [Google Scholar]
- 182.Rothlisberger P., Hollenstein M. Aptamer chemistry. Adv Drug Deliv Rev. 2018;134:3–21. doi: 10.1016/j.addr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 183.He F., Wen N.C., Xiao D.P., Yan J.H., Xiong H.J., Cai S.D., et al. Aptamer-based targeted drug delivery systems: current potential and challenges. Curr Med Chem. 2020;27:2189–2219. doi: 10.2174/0929867325666181008142831. [DOI] [PubMed] [Google Scholar]
- 184.Jiang Q., Song C., Nangreave J., Liu X.W., Lin L., Qiu D.L., et al. DNA origami as a carrier for circumvention of drug resistance. J Am Chem Soc. 2012;134:13396–13403. doi: 10.1021/ja304263n. [DOI] [PubMed] [Google Scholar]
- 185.Shieh Y.A., Yang S.J., Wei M.F., Shieh M.J. Aptamer-based tumor-targeted drug delivery for photodynamic therapy. ACS Nano. 2010;4:1433–1442. doi: 10.1021/nn901374b. [DOI] [PubMed] [Google Scholar]
- 186.Hu Q.Q., Li H., Wang L.H., Gu H.Z., Fan C.H. DNA nanotechnology-enabled drug delivery systems. Chem Rev. 2019;119:6459–6506. doi: 10.1021/acs.chemrev.7b00663. [DOI] [PubMed] [Google Scholar]
- 187.Pan Q.S., Nie C.P., Hu Y.L., Yi J.T., Liu C., Zhang J., et al. Aptamer-functionalized DNA origami for targeted codelivery of antisense oligonucleotides and doxorubicin to enhance therapy in drug-resistant cancer cells. ACS Appl Mater Interfaces. 2020;12:400–409. doi: 10.1021/acsami.9b20707. [DOI] [PubMed] [Google Scholar]
- 188.Wu C.C., Han D., Chen T., Peng L., Zhu G.Z., You M.X., et al. Building a multifunctional aptamer-based DNA nanoassembly for targeted cancer therapy. J Am Chem Soc. 2013;135:18644–18650. doi: 10.1021/ja4094617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee H., Lytton-Jean A.K., Chen Y., Love K.T., Park A.I., Karagiannis E.D., et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ren T., Deng Z.W., Liu H., Li X.F., Li J.B., Yuan J., et al. Co-delivery of DNAzyme and a chemotherapy drug using a DNA tetrahedron for enhanced anticancer therapy through synergistic effects. New J Chem. 2019;43:14020–14027. [Google Scholar]
- 191.Li J., Zheng C., Cansiz S., Wu C.C., Xu J.H., Cui C., et al. Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J Am Chem Soc. 2015;137:1412–1415. doi: 10.1021/ja512293f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kang H.Z., Liu H.P., Zhang X.L., Yan J.L., Zhu Z., Peng L., et al. Photoresponsive DNA-cross-linked hydrogels for controllable release and cancer therapy. Langmuir. 2011;27:399–408. doi: 10.1021/la1037553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Qiao G.M., Zhuo L.H., Gao Y., Yu L.J., Li N., Tang B. A tumor mRNA-dependent gold nanoparticle-molecular beacon carrier for controlled drug release and intracellular imaging. Chem Commun (Camb) 2011;47:7458–7460. doi: 10.1039/c1cc11490e. [DOI] [PubMed] [Google Scholar]
- 194.Sun W.J., Gu Z. ATP-responsive drug delivery systems. Expert Opin Drug Deliv. 2016;13:311–314. doi: 10.1517/17425247.2016.1140147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li S.P., Jiang Q., Liu S.L., Zhang Y.L., Tian Y.H., Song C., et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018;36:258–264. doi: 10.1038/nbt.4071. [DOI] [PubMed] [Google Scholar]
- 196.Li L.L., Qiao Z.Y., Wang L., Wang H. Programmable construction of peptide-based materials in living subjects: from modular design and morphological control to theranostics. Adv Mater. 2019;31 doi: 10.1002/adma.201804971. [DOI] [PubMed] [Google Scholar]
- 197.Ren H., Zeng X.Z., Zhao X.X., Hou D.Y., Yao H., Yaseen M., et al. A bioactivated in vivo assembly nanotechnology fabricated NIR probe for small pancreatic tumor intraoperative imaging. Nat Commun. 2022;13:418. doi: 10.1038/s41467-021-27932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li L.L., Ma H.L., Qi G.B., Zhang D., Yu F.Q., Hu Z.Y., et al. Pathological-condition-driven construction of supramolecular nanoassemblies for bacterial infection detection. Adv Mater. 2016;28:254–262. doi: 10.1002/adma.201503437. [DOI] [PubMed] [Google Scholar]
- 199.Li L.L., An H.W., Peng B., Zheng R., Wang H. Self-assembled nanomaterials: design principles, the nanostructural effect, and their functional mechanisms as antimicrobial or detection agents. Mater Horiz. 2019;6:1794–1811. [Google Scholar]
- 200.Pan C., Wu J., Qing S., Zhang X., Zhang L.L., Yue H., et al. Biosynthesis of self-assembled proteinaceous nanoparticles for vaccination. Adv Mater. 2020;32 doi: 10.1002/adma.202002940. [DOI] [PubMed] [Google Scholar]
- 201.Zhao X.X., Li L.L., Zhao Y., An H.W., Cai Q., Lang J.Y., et al. In situ self-assembled nanofibers precisely target cancer-associated fibroblasts for improved tumor imaging. Angew Chem Int Ed Engl. 2019;58:15287–15294. doi: 10.1002/anie.201908185. [DOI] [PubMed] [Google Scholar]
- 202.An H.W., Li L.L., Wang Y., Wang Z.Q., Hou D.Y., Lin Y.X., et al. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nat Commun. 2019;10:4861. doi: 10.1038/s41467-019-12848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liang G.L., Ren H.J., Rao J.H. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nat Chem. 2010;2:54–60. doi: 10.1038/nchem.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ye D.J., Shuhendler A.J., Cui L.N., Tong L., Tee S.S., Tikhomirov G., et al. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat Chem. 2014;6:519–526. doi: 10.1038/nchem.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li L.L., Qiao S.L., Liu W.J., Ma Y., Wan D., Pan J., et al. Intracellular construction of topology-controlled polypeptide nanostructures with diverse biological functions. Nat Commun. 2017;8:1276. doi: 10.1038/s41467-017-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Peng B., Zhao X., Yang M.S., Li L.L. Intracellular transglutaminase-catalyzed polymerization and assembly for bioimaging of hypoxic neuroblastoma cells. J Mater Chem B. 2019;7:5626–5632. doi: 10.1039/c9tb01227c. [DOI] [PubMed] [Google Scholar]
- 207.Song W.J., Tezcan F.A. A designed supramolecular protein assembly with in vivo enzymatic activity. Science. 2014;346:1525–1528. doi: 10.1126/science.1259680. [DOI] [PubMed] [Google Scholar]
- 208.Jeena M.T., Palanikumar L., Go E.M., Kim I., Kang M.G., Lee S., et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat Commun. 2017;8:26. doi: 10.1038/s41467-017-00047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Qiao S.L., Ma Y., Wang Y., Lin Y.X., An H.W., Li L.L., et al. General approach of stimuli-induced aggregation for monitoring tumor therapy. ACS Nano. 2017;11:7301–7311. doi: 10.1021/acsnano.7b03375. [DOI] [PubMed] [Google Scholar]
- 210.Cheng D.B., Zhang X.H., Gao Y.J., Ji L., Hou D.Y., Wang Z.Q., et al. Endogenous reactive oxygen species-triggered morphology transformation for enhanced cooperative interaction with mitochondria. J Am Chem Soc. 2019;141:7235–7239. doi: 10.1021/jacs.8b07727. [DOI] [PubMed] [Google Scholar]
- 211.Cai Q., Fei Y., Hu L.M., Huang Z.J., Li L.L., Wang H. Chemotaxis-instructed intracellular staphylococcus aureus infection detection by a targeting and self-assembly signal-enhanced photoacoustic probe. Nano Lett. 2018;18:6229–6236. doi: 10.1021/acs.nanolett.8b02286. [DOI] [PubMed] [Google Scholar]
- 212.Hu F., Mao D., Kenry, Cai X.L., Wu W.B., Kong D.L., et al. A light-up probe with aggregation-induced emission for real-time bio-orthogonal tumor labeling and image-guided photodynamic therapy. Angew Chem Int Ed Engl. 2018;57:10182–10186. doi: 10.1002/anie.201805446. [DOI] [PubMed] [Google Scholar]
- 213.Lu S.Z., Guo X.Y., Zou M.S., Zheng Z.Q., Li Y.C., Li X.D., et al. Bacteria-instructed in situ aggregation of AuNPs with enhanced photoacoustic signal for bacterial infection bioimaging. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.201901229. [DOI] [PubMed] [Google Scholar]
- 214.Li J.C., Zhen X., Lyu Y., Jiang Y.Y., Huang J.G., Pu K.Y. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12:8520–8530. doi: 10.1021/acsnano.8b04066. [DOI] [PubMed] [Google Scholar]
- 215.Shi Y.S., Qian H.Y., Rao P.S., Mu D., Liu Y., Liu G., et al. Bioinspired membrane-based nanomodulators for immunotherapy of autoimmune and infectious diseases. Acta Pharm Sin B. 2022;12:1126–1147. doi: 10.1016/j.apsb.2021.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Le Q.V., Lee J.W., Lee H.B., Shim G.Y., Oh Y.K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm Sin B. 2021;11:2096–2113. doi: 10.1016/j.apsb.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang D.T., Zhou C.F., Liu F., Ding T., Wang Z. Red blood cells membrane vehicle co-delivering DOX and IR780 for effective prostate cancer therapy. J Mater Res. 2020;35:3116–3123. [Google Scholar]
- 218.Hu Q.Y., Sun W.J., Qian C.E., Wang C., Bomba H.N., Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xuan M.J., Shao J.X., Dai L.R., Li J.B., He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 220.Yang R., Xu J., Xu L.G., Sun X.Q., Chen Q., Zhao Y.H., et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 221.Dehaini D., Wei X.L., Fang R.H., Masson S., Angsantikul P., Luk B.T., et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv Mater. 2017;29 doi: 10.1002/adma.201606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang Z., Gao D., Guo X.Q., Jin L., Zheng J.J., Wang Y., et al. Fighting immune cold and reprogramming immunosuppressive tumor microenvironment with red blood cell membrane-camouflaged nanobullets. ACS Nano. 2020;14:17442–17457. doi: 10.1021/acsnano.0c07721. [DOI] [PubMed] [Google Scholar]
- 223.Guo R., Deng M., He X., Li M.M., Li J.X., He P.H., et al. Fucoidan-functionalized activated platelet-hitchhiking micelles simultaneously track tumor cells and remodel the immunosuppressive microenvironment for efficient metastatic cancer treatment. Acta Pharm Sin B. 2022;12:467–482. doi: 10.1016/j.apsb.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang H.J., Wu J.Z., Williams G.R., Fan Q., Niu S.W., Wu J.R., et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnology. 2019;17:60. doi: 10.1186/s12951-019-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Parodi A., Quattrocchi N., van de Ven A.L., Chiappini C., Evangelopoulos M., Martinez J.O., et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jiang Q., Liu Y., Guo R.R., Yao X.X., Sung S.H., Pang Z.Q., et al. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. doi: 10.1016/j.biomaterials.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 227.Zhao Y., Li X.L., Zhang W.B., Yu L.L., Wang Y., Deng Z., et al. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharm Sin B. 2021;11:2114–2135. doi: 10.1016/j.apsb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cui J.W., Xu Y.X., Tu H.Y., Zhao H.C., Wang H.L., Di L.Q., et al. Gather wisdom to overcome barriers: well-designed nano-drug delivery systems for treating gliomas. Acta Pharm Sin B. 2022;12:1100–1125. doi: 10.1016/j.apsb.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yan Y., Du C.C., Duan X.X., Yao X.H., Wan J.J., Jiang Z.M., et al. Inhibiting collagen I production and tumor cell colonization in the lung via miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharm Sin B. 2022;12:939–951. doi: 10.1016/j.apsb.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Milman N., Ginini L., Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45:1–12. doi: 10.1016/j.drup.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 231.Yang Z.G., Shi J.F., Xie J., Wang Y.F., Sun J.Y., Liu T.Z., et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yong T.Y., Zhang X.Q., Bie N.N., Zhang H.B., Zhang X.T., Li F.Y., et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen Y.Y., Huang Z.H., Xu J.F., Sun Z.W., Zhang X. Cytotoxicity regulated by host-guest interactions: a supramolecular strategy to realize controlled disguise and exposure. ACS Appl Mater Interfaces. 2016;8:22780–22784. doi: 10.1021/acsami.6b08295. [DOI] [PubMed] [Google Scholar]
- 234.Wang H., Yan Y.Q., Yi Y., Wei Z.Y., Chen H., Xu J.F., et al. Supramolecular peptide therapeutics: host-guest interaction-assisted systemic delivery of anticancer peptides. CCS Chem. 2020;2:739–748. [Google Scholar]
- 235.Liu J.B., Wu T.T., Lu X.H., Wu X.H., Liu S.L., Zhao S., et al. A self-assembled platform based on branched DNA for sgRNA/Cas9/antisense delivery. J Am Chem Soc. 2019;141:19032–19037. doi: 10.1021/jacs.9b09043. [DOI] [PubMed] [Google Scholar]
- 236.Paul D., Sanap G., Shenoy S., Kalyane D., Kalia K., Tekade R.K. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26:80–93. doi: 10.1016/j.drudis.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jiang W.J., Luo J.T., Nangia S. Multiscale approach to investigate self-assembly of telodendrimer based nanocarriers for anticancer drug delivery. Langmuir. 2015;31:4270–4280. doi: 10.1021/la503949b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Upadhya R., Kosuri S., Tamasi M., Meyer T.A., Atta S., Webb M.A., et al. Automation and data-driven design of polymer therapeutics. Adv Drug Deliv Rev. 2021;171:1–28. doi: 10.1016/j.addr.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li F., Han J.S., Cao T., Lam W., Fan B., Tang W., et al. Design of self-assembly dipeptide hydrogels and machine learning via their chemical features. Proc Natl Acad Sci U S A. 2019;116:11259–11264. doi: 10.1073/pnas.1903376116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Webber M.J., Pashuck E.T. (Macro)molecular self-assembly for hydrogel drug delivery. Adv Drug Deliv Rev. 2021;172:275–295. doi: 10.1016/j.addr.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jiang T.Y., Ma Y.D., Xu X., Ji Q.C., Feng M.X., Cheng C., et al. Enzyme-instructed hybrid nanogel/nanofiber oligopeptide hydrogel for localized protein delivery. Acta Pharm Sin B. 2021;11:2070–2079. doi: 10.1016/j.apsb.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]