Abstract
Natural products, and especially the active ingredients found in traditional Chinese medicine (TCM), have a thousand-year-long history of clinical use and a strong theoretical basis in TCM. As such, traditional remedies provide shortcuts for the development of original new drugs in China, and increasing numbers of natural products are showing great therapeutic potential in various diseases. This paper reviews the molecular mechanisms of action of natural products from different sources used in the treatment of inflammatory diseases and cancer, introduces the methods and newly emerging technologies used to identify and validate the targets of natural active ingredients, enumerates the expansive list of TCM used to treat inflammatory diseases and cancer, and summarizes the patterns of action of emerging technologies such as single-cell multiomics, network pharmacology, and artificial intelligence in the pharmacological studies of natural products to provide insights for the development of innovative natural product-based drugs. Our hope is that we can make use of advances in target identification and single-cell multiomics to obtain a deeper understanding of actions of mechanisms of natural products that will allow innovation and revitalization of TCM and its swift industrialization and internationalization.
Key words: Natural products, Traditional Chinese medicine, Molecular mechanism, Inflammatory diseases, Cancer, Target identification, Single-cell multiomics, Biosynthesis
Graphical abstract
This review proposes new strategies that focus on elucidating the mechanisms of natural products and their target proteins, hoping to provide insights into the development of new drugs based on natural products.
1. Introduction
Natural medicines are defined as chemical substances with pharmacological or biological activities that are produced in nature by living organisms, such as plants, animals, insects, marine organisms, and microorganisms. These natural products are precious treasures gifted to us by nature and serve as key sources of substances for the prevention and treatment of human diseases. As such, they also have an important role and irreplaceable status in drug development and design. More than 10,000 species of medicinal plants are used in China, and their use in traditional Chinese medicine (TCM) is guided by a complex theoretical system with a history of clinical use of more than 2000 years. The identification of the active ingredients in these medicines is an important component of China's drug research, as natural products provide useful shortcuts in new drug development.
New drugs are needed to cope with the changes in the modern human population, in which accelerating aging and altered dietary habits and lifestyles are now increasing the incidence of inflammatory diseases, making these disorders one of the major threats to human health worldwide. Inflammation is associated with many infections, autoimmune diseases, malignant tumors, neurodegenerative diseases, cardiovascular diseases, diabetes, and other major chronic non-communicable diseases, and these disorders are spreading globally and becoming important public health problems that seriously endanger both human health and sustainable socioeconomic development. Natural products have made great contributions to the prevention and treatment of inflammatory diseases and cancer, and scientists have been greatly encouraged to invest in the development of natural product-based drugs by the discovery of the potent antimalarial natural product artemisinin by Youyou Tu, a Chinese scientist who won the 2015 Nobel Prize in Physiology or Medicine. This paper reviews the important research progress made in the use of natural products for the treatment of inflammatory diseases and cancer, summarizes methods for target identification and validation, and reports on the application of frontier technologies in natural product research aimed at elucidating the targets and mechanisms of natural product action and promoting the development of new drugs to combat major diseases.
2. Natural products in the treatment of inflammatory diseases and cancer
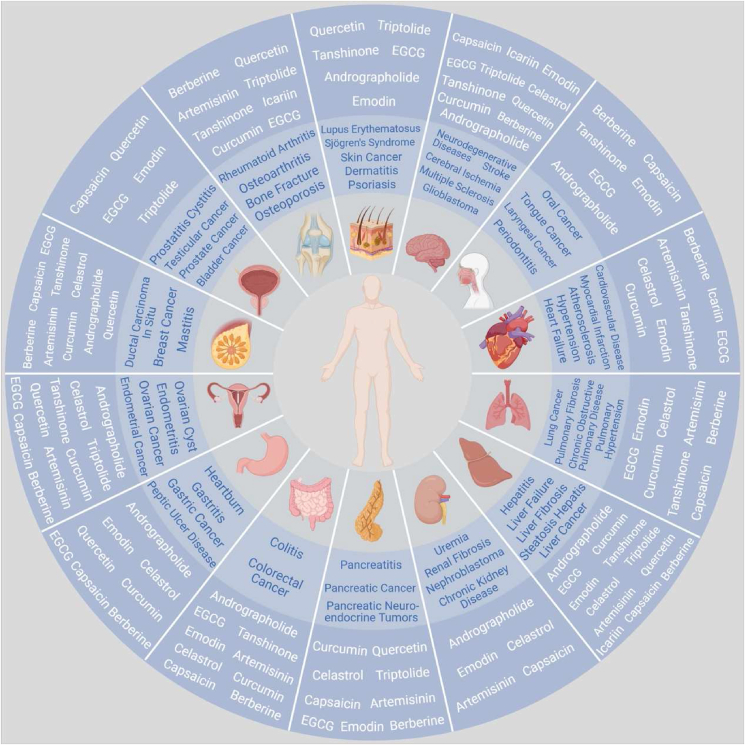
Natural products, especially of plants, animals, marine and mineral origins, play an important role in inflammatory diseases and cancer, as outlined in Fig. 1.
Figure 1.
Schematic representation of natural products (in white) improving inflammatory diseases and cancer (in blue). This figure is created with biorender.com.
2.1. Plant natural products
Plants are vital components of TCM, as they serve as important guarantees of the quality of the natural product library. Herbal remedies have played a major role in the treatment of various diseases since Shennong first tasted all kinds of herbs. Plant-derived natural products are characterized by diverse chemical structures and activities, wide ranges of action, and a low occurrence of toxic side effects.
2.1.1. Berberine
Berberine is an isoquinoline alkaloid extracted from Coptis chinensis Franch., Phellodendron chinense Schneid. and other plants of the C. Salisb. group. C. chinensis Franch. was first recorded in the “Shennong Ben Cao Jing” as having roots that taste extremely bitter, and impart a cold sensation, with clearing heat, drying dampness, and firing detoxification. It is commonly used in the treatment of dysentery, thirst, carbuncle swelling, and poisoning. Berberine is a broad-spectrum antibacterial drug that is clinically used for the treatment of gastrointestinal diseases caused by bacterial infections. It is also approved for the treatment of hyperlipidemia in several countries.
Jiang and colleagues1,2 found that berberine has hypolipidemic atherosclerosis-improving effects and can be used as a complement to statins, an undervalued attribute of this drug in the prevention and treatment of hyperlipidemia, diabetes, and cardiovascular diseases. Berberine can inhibit the action of the potassium voltage-gated channel subfamily H member 6 (KCNH6) potassium channel, which has a newly determined high glucose-dependent pro-insulin secretion effect, so berberine can greatly reduce the risk of hypoglycemia3 and may be used to develop a whole new class of hypoglycemic drugs in the future. Oral administration of berberine activates the gut–brain axis and enhances tyrosine hydroxylase activity by triggering the biosynthesis of tetrahydrobiopterin in the gut microbiota. This, in turn, elevates blood and brain dopamine concentrations to produce l-dopa, thereby ultimately improving Parkinson's disease progression4. Berberine can also promote osteoblast proliferation and differentiation5, as well as inhibit osteoclast differentiation6, to improve osteoporosis. Berberine administration can also improve the effects of organ ischemia–reperfusion injury, ischemic stroke7, peritoneal adhesions8, nonalcoholic fatty liver9, and oral diseases10.
Berberine has significant effects on many common cancers11 (e.g., lung, breast, colon, and liver cancer). Fang and colleagues12 have shown that berberine could also reduce cancer recurrence after endoscopic resection of adenoma, a precancerous colorectal disease.
2.1.2. Capsaicin
Capsaicin is the main pungent secondary metabolite component of the fruit of Capsicum annuum L. and is known for its analgesic effects. According to the “Yao Jian” C. annuum L. can “dispel wind and blood, dispel cold and relieve depression, induce stagnation, stop diarrhea, and wipe out ringworm”. Modern medicine proves that C. annuum L. has pharmacological effects, such as analgesic, antipruritic, hypolipidemic, hypoglycemic, antibacterial, and antitumor activities.
The United States Food and Drug Administration (FDA) has approved the Qutenza (capsaicin) 8% patch for the treatment of neuropathic pain caused by postherpetic neuralgia13 and neuropathic foot pain associated with diabetic peripheral neuropathy (DPN) in adult patients14. Capsaicin reversibly desensitizes and defunctionalizes the TRPV1 receptor, which plays a key role in pain signaling15. Years of research of Zhu's team on capsaicin systems16, 17, 18, 19, 20, 21 have provided an important scientific basis for the prevention of cardiovascular metabolic diseases by spicy diets. Capsaicin also reportedly exerts anti-obesity effects by altering gut microbial composition, reducing intestinal permeability, and regulating the gut microbe–brain axis22. Capsaicin also activates TRPV1 ion channels to improve glucose homeostasis in the body, thereby preventing and improving prediabetic insulin resistance, diabetes, and its complications23. Capsaicin also has a therapeutic effect on allergic rhinitis by disrupting the TRPV1–substance P nociceptive signaling pathway in the nasal mucosa24. Oral administration of capsaicin was also shown to activate somatosensory nerves by binding to TRPV1, thereby greatly alleviating the acute vaso-occlusive episodes in sickle cell disease mice and significantly preventing chronic liver and kidney damage25.
Capsaicin also has significant antitumor activity. Han's group26 used an ingenious combination of natural capsaicin and CaCO3 as a nanocarrier to exploit this activity. The nanocarrier undergoes rapid cleavage in the acidic tumor microenvironment and releases capsaicin, which then specifically activates TRPV1 channels and increases the calcium ion concentration within the tumor. This causes mitochondrial damage and increases the intracellular levels of reactive oxygen species, ultimately leading to apoptosis of tumor cells and inhibition of tumor growth26. Capsaicin can increase the level of calcitonin gene-related peptide (CGRP) in bone marrow extracellular fluid and promote the movement of hematopoietic stem cells from bone marrow to blood vessels, indicating its promise as a therapy for hematologic tumors27.
2.1.3. Quercetin
Quercetin is a plant secondary metabolite widely found in the bark, flowers, leaves, buds, seeds, and fruits of many plants. Quercetin is a dietary polyphenol that has protective effects when consumed in the diet or as a food supplement.
Senolytics is a drug combination composed of dasatinib and quercetin, which can selectively kill senescent cells28 in a variety of affected organs, repair physiological functions, and delay aging29, 30, 31, 32, 33, 34. Liu et al.35 showed that quercetin not only delayed the aging of UV-irradiated human primary dermal fibroblasts, but it also delayed the aging of HES1-deficient human primary dermal fibroblasts. Quercetin regulates the gut microbiota and affects the progression of non-alcoholic fatty liver disease (NAFLD) in mice via the intestinal–hepatic axis36.
Quercetin can also enhance the anticancer effects of adriamycin chemotherapy in hepatocellular carcinoma cells while protecting normal hepatocytes37. Quercetin combined with cisplatin nanoparticles significantly inhibited the progression of bladder cancer38. Quercetin activated gastric cancer cell autophagy through regulating Akt–mTOR signaling and hypoxia-inducible factor 1α (HIF-1α) signaling, thereby inhibiting gastric cancer progression39. The synergistic effect induced by the co-administration of quercetin and alantolactone as a colorectal cancer treatment was able to reactivate antitumor immunity by inducing immunogenic cell death (ICD), causing cytotoxicity, and modulating the immunosuppressive tumor microenvironment40.
2.1.4. Icariin
Icariin, an isopentenyl flavonoid glycoside compound from Epimedium Linn., shows antioxidant, anti-inflammatory, and antitumor pharmacological effects and has extensive therapeutic capabilities for the treatment of cardiovascular diseases41, neurodegenerative diseases42, and tumors. Icariin can also reduce morbidity in MRL/Lpr mice43.
Icariin Softgel, an original new drug in TCM, was approved for marketing by the State Drug Administration in China on January 10, 2022, for unresectable hepatocellular carcinoma in patients who are unsuitable for or have refused standard treatment and have not previously received systemic therapy. Mechanistically, icariin inhibits the IKK–NF-κB inflammatory pathway by directly binding to MyD88/IKKα, thereby reducing the production of inflammatory factors, such as TNF-α and IL-6, inhibiting the effects of PD-L1 expression and MDSC, and activating IFN-γ positive CD8+ T cells to exert antitumor effects. Icariin can also induce mitochondrial autophagy and synergize with adriamycin to induce immunogenic cell death in hepatocellular carcinoma44.
2.1.5. Artemisinin
Artemisinin, a sesquiterpene lactone containing a peroxy-bridge structure, was first discovered by Chinese scientist Youyou Tu in the 1970s, who then pioneered the design of antimalarial drugs with peroxy-bridges as the active group. In addition to its use as a first-line antimalarial drug, artemisinin has received increasing attention for its other potential pharmacological effects, including antiviral, antifungal, anti-inflammatory, and anticancer activities.
Artemisinin and its derivatives exhibit cytotoxic effects against a variety of cancers, both in vivo and in vitro, and also play roles in the prevention and treatment of carcinogenesis and tumor metastasis45,46. Wang's team47, 48, 49 has been researching the antitumor effects of artemisinin and its derivatives for more than ten years, and has elucidated that artemisinin and its derivatives can exert anticancer effects by inhibiting tumor growth and cycle progression, promoting apoptosis of tumor cells, and sensitizing tumor cells to the therapeutic effects of clinical chemotherapeutic and target drugs. Dihydroartemisinin, the main in vivo metabolite of artemisinin drugs, is the active form, and it exerts antitumor effects by targeting autophagy and ferroptosis in tumor cells50. Wang's team51 was the first to discover the molecular mechanism by showing that dihydroartemisinin selectively inhibits PDGFRα-positive tumor cell growth, metastasis, and the epithelial–mesenchymal transition by targeting PDGFRα and promoting its ubiquitous degradation, as well as having a co-sensitizing effect on clinical PDGFRα inhibitors.
Youyou Tu's group52 also found that dihydroartemisinin is uniquely effective in treating lupus erythematosus. In 2016, Kubicek and colleagues53 demonstrated the efficacy of artemisinin as a diabetes treatment. The ability of artemisinin to achieve α-cell to β-cell transition has identified a new and surprising treatment for type I diabetes. Artemisinin can exert anti-atherosclerosis effects by inhibiting inflammatory responses in macrophages through the AMPK/NF-κB/NLRP3 signaling pathway54. Artemisinin has also displayed great potential in the fight against fibrosis55.
2.1.6. Triptolide
Triptolide, an epoxide diterpene lactone compound, is one of the main active ingredients of Tripterygium wilfordii Hook. f. (family Euonymusaceae). Triptolide shows the anti-inflammatory and immunosuppressive effects. Tripterygium tablets and tripterygium glycoside tablets are mainly used in the treatment of rheumatoid arthritis.
Triptolide can covalently bind XPB and inhibit its DNA-dependent adenosine triphosphatase (ATPase) activity, thereby inhibiting RNA polymerase II-mediated transcription. This, in turn, leads to the inhibition of cell activation and proliferation and explains its pharmacological activity as well as its extreme cytotoxicity56. Shen and colleagues57 found that triptolide can target TAB1 in macrophages and modulate inflammatory diseases by regulating the MAPK signaling pathway.
As part of its wide range of antitumor effects, triptolide inhibits the activity of genes such as SLC7A11, inhibits the Nrf 2-associated glutathione synthesis pathway, and allows IDH1-mutated cancer cells to die from oxidative stress, thereby offering hope for the treatment of IDH1-mutated cancers, such as glioma, acute myeloid leukemia, and pancreatic cancer58. However, its serious systemic toxicity and poor water solubility greatly hinder its clinical application; therefore, improving its solubility and bioavailability and reducing adverse effects are the focus of current research related to its antitumor effects. Hui et al.59 developed a pH-sensitive folic acid-coated triptolide nanoform designed to release the drug in the acidic microenvironment of cancer cells. The drug promoted overexpression of the folic acid receptor in some hepatocellular carcinoma cells, which not only significantly inhibited the growth of hepatocellular carcinoma but also significantly reduced the cytotoxicity to normal hepatocytes. Liu's group60 found that glutriptolide 2, a glucose–triptolide conjugate, achieved targeted delivery and showed durable anti-prostate cancer activity. Tan's group61 coupled triptolide with the amino-modified aptamer AS1411 to obtain an aptamer triptolide conjugate that significantly inhibited the progression of triple negative breast cancer in vitro and in vivo. This conjugate is expected to become a broad-spectrum and efficient antitumor targeting drug.
2.1.7. Celastrol
Celastrol, another major active ingredient in T. wilfordii Hook. F., originates from the root bark and has remarkable anti-rheumatoid, anti-oxidant, analgesic, and anti-tumor effects.
In 2015, Ozcan and colleagues62 provided the first demonstration that celastrol is a potentially effective herbal medicine for weight loss. They found that the celastrol-enhanced leptin sensitivity and weight loss effects required IL1R1 mediation, as the absence of IL1R1 completely eliminated the weight loss and antidiabetic effect of celastrol on obese mice63. Zhang's team64 found that celastrol was effective in inhibiting high-fat diet-induced weight gain in mice and that the mechanism of action involved binding of celastrol to the orphan nuclear receptor Nur 77 in the nucleus and promotion of the selective clearance of damaged mitochondria, with a resulting inhibition of the inflammatory response and obesity. This team subsequently found that celastrol promoted ubiquitination of the LBD structural domain at the C-terminus of Nur77 to interact with the UBA structural domain of the autophagy receptor P62, promoted the size and mobility of the P62 phase-separated particles, and completed the clearance of damaged mitochondria in the lysosome65. Celastrol also activates the heat-stimulating factor HSF1, which increases energy expenditure and helps mice resist high-fat diet-induced obesity66. Celastrol can antagonize obesity by altering the distribution of intestinal microbiota under a high-fat diet and reducing lipid absorption in the intestine67. In addition, celastrol improved metabolism, which may help improve glucose metabolism in obese patients, such as diabetics68. Our group69 found that celastrol targeted adenylyl cyclase associated protein 1 (CAP1) to inhibit macrophage inflammation and improve metabolic syndrome in mice. Liang's team70 revealed that celastrol inhibits angiotensin (Ang II)-induced hypertensive heart injury by binding to STAT3, thus exerting a protective effect on heart function. They also found that celastrol could directly bind to and inhibit PRDX2 protein activity, which in turn upregulated oxidative stress levels and finally achieved the effects of killing gastric cancer cells71. In addition, celastrol also improves liver fibrosis72. Celastrol has some limitations in its clinical translational application, such as poor water solubility, short biological half-life, and unknown optimal dose interval; therefore, high-technology research and development is needed on molecular modifications and new dosage forms.
2.1.8. Tanshinone
Tanshinone is a lipophilic diterpenoid isolated from the rhizome of Salvia miltiorrhiza. Tanshinone is used in TCM to treat heart disease, stroke, and vascular diseases. Tanshinone IIA, the most studied active component of tanshinone, has anti-inflammatory and antioxidant activities73,74. Clinically, tanshinone IIA sodium sulfonate injection is mainly used for the treatment of coronary heart disease, angina pectoris, myocardial infarction, and premature ventricular contractions.
Renshaw's group75 found that tanshinone IIA reduces inflammation by inducing neutrophil apoptosis and promoting the reverse migration of neutrophils, offering hope for inflammatory diseases such as chronic obstructive pulmonary disease. Fan's group76 conducted scRNA-seq assays of the hearts of myocardial infarction model mice and found that the administration of tanshinone IIA attenuated myocardial infarction progression by inhibiting early infiltrating macrophage subpopulations. Tanshinone IIA also protected neurons and improved cognitive impairment in patients with Alzheimer's disease77,78 and Parkinson's disease79,80. Tanshinone IIA also shows considerable antitumor activity81,82.
2.1.9. Andrographolide
Andrographolide is a well-known natural lactone with a range of pharmacological actions in TCM. Andrographolide is reported to improve radiation-induced pneumonia and pulmonary fibrosis by inhibiting the activation of the AIM2 inflammasome83. Andrographolide improves lipopolysaccharide-induced acute lung injury in mice by inhibiting the NF-κB pathway84. Andrographolide reversed the colitis–colon cancer transformation by inducing mitophagy in macrophages85. Andrographolide ameliorated TNBS-induced colitis in mice by inhibiting Th1/Th17-mediated immune response, and it could downregulate P38, MAPK, STAT3, and NF-κB to improve mouse sepsis86. Andrographolide mediated apoptosis by binding Bax to restore the sensitivity of drug-resistant colon cancer cells to 5-fluorouracil87. Andrographolide alleviated the symptoms of an N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse Parkinson's model by targeting the mitochondrial division protein DRP188. Andrographolide improved the symptoms of Alzheimer's disease in mice by regulating mitochondrial homeostasis89. Andrographolide improved imiquimod-induced psoriasis-like skin inflammation in mice by inducing the autophagic degradation of MyD8890. These series of studies provide a theoretical basis for the new use of old drugs in clinical preparations containing andrographolide as the main component. A recent review has systematically summarized the structure–activity relationship analysis, pharmacokinetics, new andrographolide delivery systems, and the protective functions of andrographolide against inflammatory diseases and cancer91.
2.1.10. Emodin
Emodin is an anthraquinone natural compound extracted from the rhizomes of several Chinese herbs, including Rheum palmatum L., Fallopia multiflora (Thunb.) Harald., and Reynoutria japonica Houtt. In traditional studies, emodin is mostly considered an active ingredient of laxatives, but modern pharmacological studies have found that emodin has significant anti-inflammatory, antioxidant, antibacterial, and antitumor effects.
Song et al.92 developed ferromagnetic responsive rhodopsin-loaded micelles to realize magnetic resonance imaging (MRI)-guided magnetothermal–chemotherapy combination therapy for malignant tumors. The micelles could target tumor sites under external magnetic field guidance and release emodin to kill significant numbers of tumor cells at very low doses. Emodin can selectively inhibit 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which can effectively limit the effect of glucocorticoids and improve diabetes and insulin resistance93,94. Emodin nanoparticles can specifically release emodin in the diseased colon and effectively enhance the anti-colitis effect of emodin related to improving the intestinal wall barrier. Improvements in the therapeutic efficacy and reduction of side effects will make emodin a novel alternative to oral colon-targeted ulcerative colitis therapy95. In addition, emodin could alleviate the progression of cardiac fibrosis96.
Emodin also had a highly significant ameliorative effect on neurodegenerative changes in Alzheimer's disease mice, including inhibition of Alzheimer's disease pathology and enhancement of learning memory capacity97. An AI-based drug discovery scheme for Alzheimer's disease treatment, which is efficient and feasible, has been proposed to provide rapid development of anti-Alzheimer's disease drugs and represents a major breakthrough in the modernization of TCM research.
2.1.11. Curcumin
Curcumin is an acidic polyphenolic compound extracted from Curcuma longa L. and used in Chinese medicine to treat bruises and injuries, canker sores, and pain. Curcumin is the main component of C. longa L. and is widely used as a natural pigment in the food industry, TCM, and Indian medicine. Curcumin has antibacterial, antioxidant, anti-inflammatory, analgesic, antitumor, antidiabetic, and antihyperlipidemic activities and is traditionally used in the treatment of liver diseases.
Curcumin can function as an anti-inflammatory agent by blocking NF-κB and STAT3-mediated inflammatory response signaling pathways, and preventing the development of inflammation-related chronic diseases98,99. Curcumin significantly improves atherosclerosis100. Curcumin and its metabolites extend the lifespans of nematodes, drosophila, and mice. Curcumin also plays an important role in the prevention and treatment of aging-related diseases101 and it regulates the abundance and composition of gut microbiota and improves intestinal barrier function102. Banerjee et al.103 used X-ray crystallography and a specificity analysis technique with a kinase inhibitor to successfully reveal that curcumin binds and inhibits the kinase dual specific tyrosine regulated kinase 2 (DYRK2), thereby impeding the function of the cellular proteasome, impairing tumor cell proliferation, and inhibiting cancer progression. In addition, curcumin also showed quite good effect in anti-fibrosis104.
However, the low bioavailability and poor stability of curcumin due to its low solubility have affected its application in the pharmaceutical field. Therefore, in recent years, many studies have combined nanomedicine with curcumin to improve its pharmacological activity. Bao et al.105 established α-lactalbumin nanotubes that successfully overcame intestinal mucus and cellular barriers to improve the bioavailability of lipid-soluble curcumin and effectively promoted the colonic anti-inflammatory effect of curcumin. Yu's team106 fabricated nano-inducers composed of curcumin, iron oxide nanoparticles, and organic silica nanoparticles that significantly enhanced intracellular oxidative stress and endoplasmic reticulum stress to induce ICD and systemic antitumor immunity. In addition, gut microbiota can biotransform curcumin by demethylation and hydroxylation to produce derivatives that show improved bioavailability and bioactivity102.
2.1.12. Epigallocatechin gallate (EGCG)
EGCG is a catechin-like monomer isolated from tea leaves. It is the main component of tea polyphenols and is a well-known antioxidant. EGCG can prolong the lifespan by inducing mitochondrial reactions107. EGCG cross-linked in chitosan hydrogels has been shown to promote proliferation and remodeling processes, such as regeneration of the epidermis, dermis, and skin attachments, to accelerate skin wound healing108. EGCG shows neuroprotective effects in Alzheimer's disease and other neurodegenerative disorders by reducing Aβ its expression and inflammatory responses109,110. Oral administration of EGCG attenuated ulcerative colitis in mice by regulating gut microbiota111. EGCG-targeted action of TAK1 effectively alleviated joint swelling due to rheumatoid arthritis112. Liu et al.113 discovered a new regulator of cGAS, G3BP1, and showed that EGCG can ultimately inhibit the activation of cGAS by inhibiting the binding of G3BP1 to cGAS. They confirmed its effectiveness in autoimmune animal models and cells of AGS patients and suggested the use of EGCG as a therapeutic strategy option for autoimmune diseases, such as AGS syndrome, which currently lack therapeutic remedies113.
EGCG has inhibitory effects on a variety of tumors. EGCG reduces the risk of pancreatic cancer by inhibiting the activity of LDHA, thereby altering the metabolism of pancreatic cancer cells114. EGCG can inhibit tongue cancer progression in K-Ras transgenic mice by targeting the Notch pathway115. EGCG also acts as a drug delivery system to protect protein drugs from degradation while synergistically exerting its own antitumor effects116. Wang's group117 was the first to report that EGCG can directly interact with and cause conformational changes in P53 protein that disrupt its interaction with P53–MDM2. This promotes the apoptosis of cancer cells and provides a new concept of nutritional intervention in disease using dietary polyphenols117.
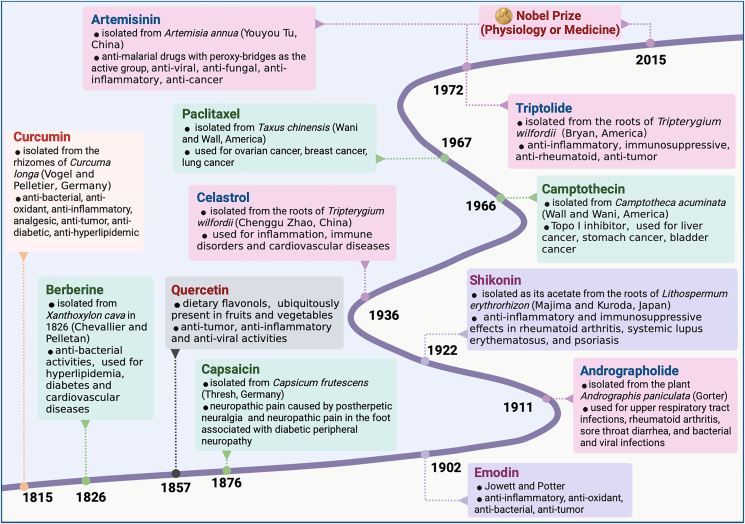
In addition, Wang et al.118 found that the natural glycoside product ginsenoside Rh2 could activate the pentose phosphate pathway after cancer pretreatment discontinuation to improve redox disorders in tumor cells. It also enhanced the antitumor effects of adriamycin by further inhibiting the growth of ovarian cancer spheroids118. Cheng's team119 found that the antitumor activity was much stronger for the rare ginsenoside protopanaxadiol (PPD) than for other common ginsenosides, making this a potentially more potent anticancer drug component. Ginseng polysaccharides enhanced the effect of anti-PD-1 monoclonal antibodies in improving lung cancer and modulated immunity by regulating the composition of gut microbiota and metabolites, which in turn enhanced responses to immunotherapy120. Ginseng extract enriched the intestinal microbiota with Escherichia faecalis, stimulated the thermogenic activity of brown adipose tissue, and induced the formation of beige adipose tissue to reduce fat accumulation and obesity121. The role of natural products in tumor, represented by paclitaxel and camptothecin, has been described in detail in the review by Huang et al.122. We will not reiterate them here. The discovery time and main efficacy of typical natural products from plants are shown in Fig. 2. The relationship between natural products and disease treatment mentioned in above are shown in Table 1.
Figure 2.
Milestones in the discovery of typical natural products for the treatment of inflammatory diseases and cancer. This figure is created with biorender.com.
Table 1.
Types of diseases treated by typical natural products.
| Natural product | Disease type |
|---|---|
| Berberine | Gastrointestinal diseases123, hypoglycemia3, atherosclerosis124, diabetes125, cardiovascular diseases2, Parkinson's disease4, ischemic stroke7, peritoneal adhesions8, nonalcoholic fatty liver9, oral diseases10, lung cancer126, breast cancer126, liver cancer126, colon cancer127 |
| Capsaicin | Neuropathic pain14, cardiovascular diseases18, obesity22, diabetes23, nasal mucosa24, hematologic tumors27, Lassa hemorrhagic fever128 |
| Quercetin | Aging34, non-alcoholic fatty liver disease36, hepatocellular carcinoma37, bladder cancer38, gastric cancer39, colorectal cancer40 |
| Icariin | Cardiovascular diseases41, neurodegenerative diseases42, hepatocellular carcinoma44 |
| Artemisinin | Lupus erythematosus52, diabetes53, atherosclerosis54, fibrosis55, breast cancer129 |
| Triptolide | Rheumatoid arthritis130, IDH1-mutated cancers58, hepatocellular carcinoma131, prostate cancer60, breast cancer61 |
| Celastrol | Rheumatoid arthritis132, obesity62, diabetes68, metabolic syndrome69, hypertensive heart injury70, gastric cancer71, liver fibrosis72, psoriasis133 |
| Tanshinone | Coronary heart disease134, angina pectoris134, myocardial infarction76, chronic obstructive pulmonary disease75, myocardial infarction76, Alzheimer's disease77, Parkinson's disease79 |
| Andrographolide | Pulmonary fibrosis83, acute lung injury84, colitis84, colon cancer87, Parkinson's disease88, Alzheimer's disease89, psoriasis90 |
| Emodin | Malignant tumor92, diabetes93, ulcerative colitis95, cardiac fibrosis96, Alzheimer's disease97 |
| Curcumin | Atherosclerosis100, aging-related diseases101, cancer progression103, fibrosis104, colitis105 |
| EGCG | Alzheimer's disease109, ulcerative colitis111, rheumatoid arthritis112, pancreatic cancer114, tongue cancer115 |
| Shikonin | Rheumatoid arthritis135, psoriasis136, bladder cancer137, pancreatic cancer138, pulmonary hypertension139, hypertrophic scars140 |
| Camptothecin | Liver cancer141, breast cancer142, bladder cancer143 |
| Paclitaxel | Ovarian cancer144, breast cancer145, lung cancer146 |
2.2. Animal natural products
Animal medicinal compounds are indispensable and important components of TCM. According to Chinese medicine, animal medicine is a “flesh and blood sentient product, which is able to run and pass, attack poison and dispel evil”. Animal-derived medicines have unique therapeutic effects on many difficult and miscellaneous diseases, and their clinical value is irreplaceable. The following is an example of the current research progress in animal medicines as treatments for inflammatory diseases and cancer.
Toad venom is the dried secretion from the skin glands of Bufo bufo gargarizans Cantor or Bufo melanostictus Schneider. In TCM practice, toad venom is considered an anti-infectious agent for the treatment of pyogenic infection-induced unconsciousness and is prescribed to patients with “heat and toxins” syndrome, which has cancer-like symptoms. Numerous pharmacological studies have revealed that the anti-inflammatory and antineoplastic effects of toad venom were due to its content of bioactive steroidal cardiac glycosides, called bufadienolides, including bufalin, cinobufagin, and gamabufotalin147.
The typical antitumor mechanisms included induction of apoptosis and proliferation through targeting of the IKKβ/NF-κB/COX-2 signaling pathway148, the AKT/mTOR pathway149 or Notch signaling pathway150, induction of G0/G1 or G2/M cell cycle arrest through HIF-1α- and NF-κB-mediated Plk1151,152, inhibition of invasion and metastasis through cortactin expression and nuclear translocation153 or RIP3-mediated necroptosis154, and reversal of multi-drug resistance by regulation of P-glycoprotein (P-gp)155, as demonstrated in vitro or in vivo in homograft/xenograft tumor models in mice. Several findings have also provided evidence that cardiac glycosides, including bufalin, can exert potent antineoplastic effects by targeting the Na+/K+-ATPase, hence the intracellular accumulation of Ca2+ ions156, and they appear to increase the immunogenicity of dying cancer cells157. Recent studies have indicated that AHSA1, identified as the targeted protein of bufalin, acts as a co-chaperone of HSP90A to activate CDK6 and PSMD2, thereby regulating multiple myeloma proliferation and proteasome inhibitor resistance, respectively158. Yang et al.159 found that bufalin directly targeted Syndecan-4 and increased its interaction with substrate protein DEAD-box helicase 23 to inhibit the progression of hepatocellular carcinoma. Other mechanisms associated with the anti-inflammation action of bufadienolides have included modulation of NF-κB signaling160 and enhancement of immune responses161.
2.3. Marine natural products
Oceans cover more than 70% of the earth's surface and were the origin of life on the planet. Since 2008, more than 1000 new marine natural products (MNPs) had been found annually, and approximately 30% of them have bioactive properties162. Marine microorganisms have evolved special metabolic pathways due to long-term adaptation to special living environments, resulting in a large number of active substances with novel structures and unique functions, making natural products derived from marine microorganisms a hot spot in the development of new marine drugs. However, a relatively low number of MNPs have been approved in the clinical setting. Here, we provide an overview of the currently approved MNPs.
The marine natural nucleosides cytarabine (Ara-C; originally isolated from the Caribbean sponge Cryptotheca crypta) and vidarabine (Ara-A; originally isolated from the Caribbean sponge Tethya crypta) are well known as the first FDA-approved MNPs for anticancer treatment (in 1969) and antiviral treatment (in 1976), respectively163, 164, 165, 166. Ara-C and Ara-A work as inhibitors of tumor cell or viral DNA synthesis and replication167. Ziconotide (originally isolated from the venom of a marine snail) is a novel powerful antinociceptive drug that acts as a specific calcium channel blocker for the treatment of severe chronic pain, especially in patients refractory to opioids, but it still has the potential for systemic and central nervous system side effects168,169. Eribulin (originally isolated from the natural Japanese marine sponge Halichondria okada) is a macrocyclic ketone analog that acts as an anticancer drug by inducing irreversible mitotic blockade and is now used to treat people with locally advanced or metastatic breast cancer or unresectable liposarcoma170. Dolastatins (originally isolated from the sea hare Dolabella uricularia) are broad-spectrum cytotoxic anticancer pentapeptides that can impede tubulin assembly and induce cell apoptosis and are widely used in the treatment of lymphoma, and other carcinomas171,172. However, according to the strong adverse reactions observed in preclinical toxicology research, dolastatins are now used as payloads for antibody–drug conjugates (ADCs)173,174. ADC technology is based on the idea that the linking of a cytotoxic drug to a monoclonal antibody specific for antigens of cancer cells can deliver high doses of the cytotoxic drug specifically to cancer cells while sparing normal tissues175. Brentuximab vedotin is a CD30-directed ADC that consists of a human-specific CD30 antibody and the microtubule-disrupting agent monomethyl auristatin E (MMAE; a synthetic analog of the naturally occurring dolastatin 10) and is now used as a lymphoma treatment176,177. Cephalosporin C, the best known MNP, is a β-lactam type natural antibiotic derived from marine fungi and is widely used to treat bacterial infections by disrupting the synthesis of the peptidoglycan layer that forms the bacterial cell wall178. Omega-3 fatty acid, another well-known MNP, was originally derived from fish and fish oils in 1929. Dietary consumption of omega-3 fatty acids reduces the incidence of cardiovascular disease, osteoarthritis, and rheumatoid arthritis. Dietary supplementation with omega-3 fatty acids provides antioxidant activity by regulating the antioxidant signaling pathway and may modulate inflammatory processes179.
2.4. Mineral natural products
As one of the important components of TCM, mineral medicine has a long history and abundant resources. The earliest extant pharmacological work in China, the Shennong Ben Cao Jing, contains a total of 41 mineral drugs. Here, we use arsenic as an example to introduce the pharmacological effects of mineral drugs in diseases.
The arsenic-containing compound realgar (mainly As4S4) is a highly recognized and widely used TCM. Although realgar minerals contain large amounts of arsenic, the toxicity related to their structures is far less than that observed for other compounds, such as arsenolite (which contains arsenic trioxide, As2O3) and arsenite (NaAsO₂)180,181. Realgar is widely used in prescriptions for treating infectious inflammation symptoms, ranging from tonsillitis to delirium and allergy182. Several recent clinical studies have revealed that realgar provided therapeutic benefits as a cancer treatment, especially for adult and pediatric acute promyelocytic leukemia (APL), where encouraging responses were obtained, including a high complete remission rate, long disease-free survival period, and tolerable side effects183, 184, 185.
Numerous in vitro and in vivo pharmacological studies have demonstrated the anti-inflammation and antitumor mechanisms of realgar and realgar-containing preparations. Realgar has been reported to induce G2/M phase arrest, apoptosis, and autophagy in osteosarcoma through mechanisms related to the activation of the ROS/JNK and suppression of the Akt/mTOR signaling pathways186. Other studies have shown that realgar preparations inhibited breast cancer through downregulation of HIF-1α expression via the PI3K/Akt/mTOR pathway187 and reversed drug resistance by degrading the BCR–ABL fusion oncoprotein188. Several studies189, 190, 191 identified potential binding proteins of realgar in rat metabolism through interactions with sulfhydryl groups in specific proteins, such as pyruvate dehydrogenase, thioredoxin, DNA repair enzymes, and metallothionein, and suggested a high relationship with realgar anti-inflammation activity.
3. Mechanisms of natural products in inflammatory diseases
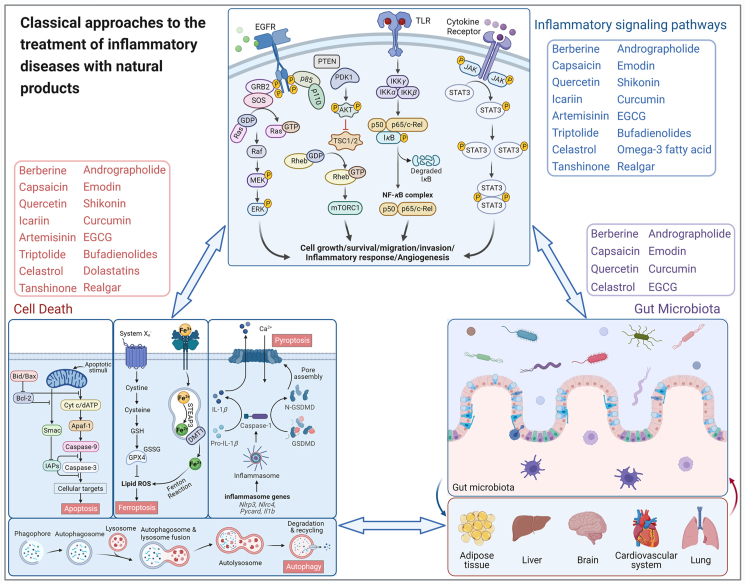
Exploration of the recent research progress in the use of natural products in inflammatory diseases has revealed three main categories of common mechanisms: inflammation-related signaling pathways, programmed cell death, and gut microbiota (Fig. 3).
Figure 3.
Molecular mechanisms of natural products ameliorating inflammatory diseases and cancer through inducing cell death, inhibiting inflammatory signaling and affecting gut microbiota. This figure is created with biorender.com.
The inflammation-related signaling pathways, such as MAPK, PI3K/AKT, NF-κB, and JAK/STAT, can transduce extracellular signals into cells and conduct cellular signals through cascade reactions to regulate cell proliferation, differentiation, and migration as well as inflammatory responses and vascular development192.
Programmed cell death can occur by apoptosis, ferroptosis, pyroptosis, or autophagy193 and describes the active extinction of cell responses to stimulation by certain signals or factors to maintain the stability of the internal environment. Programmed cell death removes unwanted cells, as well as infected or potentially tumorigenic cells, so it plays an important role in homeostasis, host defenses against pathogens, cancer, and a range of other pathologies. Apoptosis is a relatively “mild” form of cell death and generally does not elicit an immune or inflammatory response. Many natural products exert their antitumor effects by promoting apoptosis, mainly mediated by apoptotic caspases (caspase-2, 3, 6, 7, 8, 9, and 10). Pyroptosis is involved in the body's defense against pathogenic bacteria, occurs more rapidly, is accompanied by the release of large amounts of pro-inflammatory factors, and is mainly induced by inflammatory caspases (caspase-1, 4, 5, and 11). Ferroptosis is an iron-dependent, non-apoptotic, oxidative form of cell death caused by the failure or blockage of cellular glutathione-dependent antioxidant defenses. This leads to uninhibited lipid peroxidation and ultimately kills cells; consequently, inhibition of iron-related death by natural products has great potential in the treatment of tumors, diabetes, ischemic organ damage, and degenerative diseases associated with lipid peroxidation. Autophagy, the process by which cellular cargoes are transported to lysosomes and degraded, removes functionally abnormal intracellular proteins, organs, and microorganisms under normal conditions and is essential for maintaining cellular, tissue, and organ homeostasis. Autophagy is tightly regulated by autophagy-related genes, and mutations in these genes can induce a range of diseases, including neurodegenerative diseases, inflammation, and even cancer.
The gut microbiota represents the second largest genome in the body and is involved in a variety of physiological functions in the liver, intestines, brain, and other organs. Imbalance of the gut microbiota is associated with most diseases in the body, so the study of the gut microbiota has become a hot research topic in the field of Chinese medicine in recent years. Gut microbe interactions fit with the theory of TCM, but they also represent one of the important ways by which orally administered TCM can exert its medicinal effects194. A variety of gut microbes, especially Bacillus spp., Bifidobacterium spp., and Lactobacillus spp., can biotransform herbal components to improve the bioavailability and bioactivity of some difficult-to-absorb natural drug components and provide a theoretical basis for their remarkable therapeutic effects. The combination of gut microbiota with TCM can help modernize TCM and rejuvenate traditional medicine.
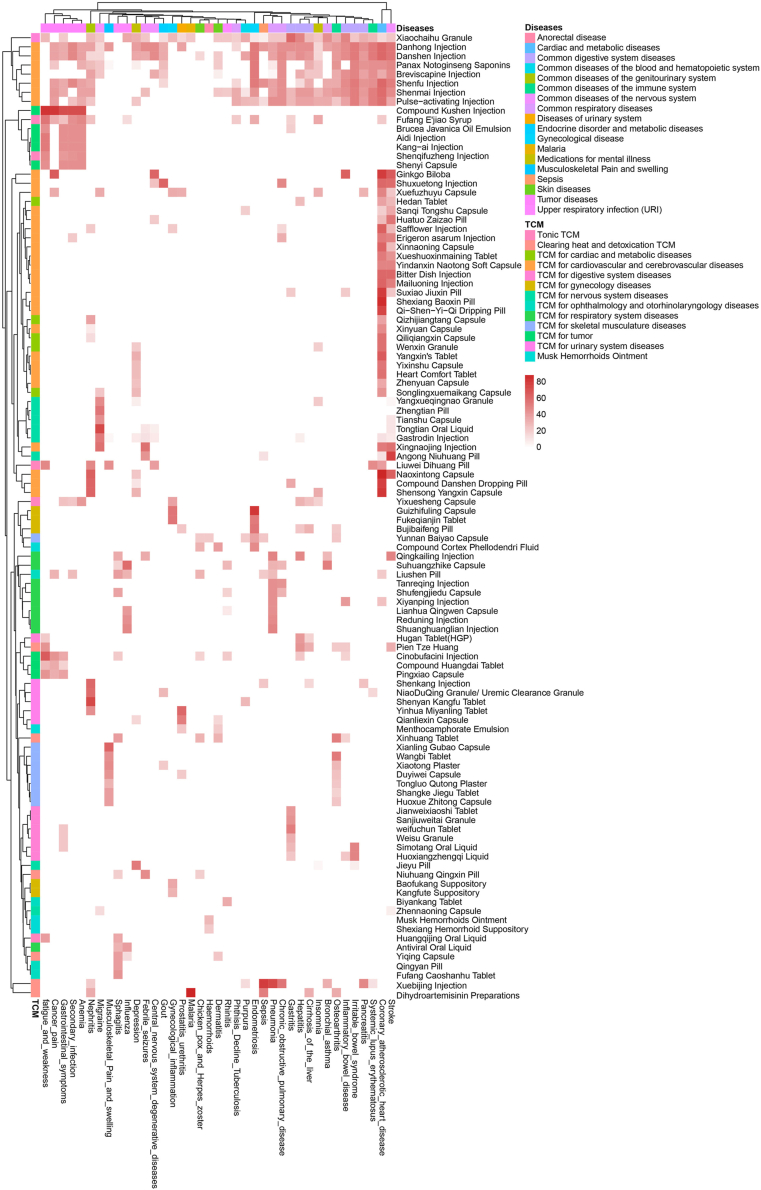
Active TCM ingredients show significant anti-inflammation and anti-tumor activities in vitro, but in clinical practice, TCM is usually supplied as compound prescriptions. We also systemically retrieved literature for 106 Chinese compound prescription preparations (Table 2) using preparation names as keywords and searching different electronic databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science (https://www.webofscience.com/wos), Chinese National Knowledge Infrastructure (CNKI, https://www.cnki.net), the Wanfang Database (https://new.wanfangdata.com.cn), and Pharmacopoeia of China. Here, we reorganized and evaluated the literature on 40 inflammatory diseases and cancer from five aspects: animal experiments, retrospective or real-world experiments, clinical randomized controlled trials, historical human usage, and treatment guideline inclusions or recommendations (Table 3). We graded the retrieved articles for quality and quantity, guideline inclusions or recommendation levels, and history of human use, and drew heatmaps according to the disease scores (Fig. 4). Generally, the preparations, and especially medicines for cardiovascular and cerebrovascular diseases, have been widely studied in inflammatory diseases. In the clinically related literature, retrospective or real-world experiments have been common, whereas few randomized controlled trials were conducted for TCM preparations. The combination of multiple ingredients in prescribed herbal medicinal compounds is consistent with the TCM concept and occurs widely in numerous preparations. In actual use, the key ingredients are likely to provide therapeutic effects, while the ancillary constituents might assist in dissolution or absorption.
Table 2.
Large varieties of TCM for treatment of inflammatory diseases and cancer.
| Category | TCM |
|---|---|
| Medicines for cardiovascular and cerebrovascular diseases | Danhong Injection134, Danshen Injection195, Shenfu Injection196, Shenmai Injection197, Pulse-activating Injection198, Panax Notoginseng Saponins199, Breviscapine Injection200, Erigeron asarum Injection201, Safflower Injection202, Ginkgo Biloba203, Bitter Dish Injection204, Shuxuetong Injection205, Mailuoning Injection206, Xingnaojing Injection207, Compound Danshen Dropping Pill208, Shensong Yangxin Capsule209, Naoxintong Capsule210, Xueshuoxinmaining Tablet211, Yixinshu Capsule212, Yangxin's Tablet213, Heart Comfort Tablet214, Xinyuan Capsule215, Shexiang Baoxin Pill216, Qi-Shen-Yi-Qi Dripping Pill217, Sanqi Tongshu Capsule218, Yindanxin Naotong Soft Capsule219, Xinnaoning Capsule220, Suxiao Jiuxin Pill221, Huatuo Zaizao Pill222, Zhenyuan Capsule223, Xuefuzhuyu Capsule224 |
| Medicines for cardiac and metabolic diseases | Wenxin Granule225, Songlingxuemaikang Capsule226, Hedan Tablet227, Qiliqiangxin Capsule228, Qizhijiangtang Capsule229 |
| Medicines for digestive system diseases | Hugan Tablet (HGP)230, Xiaochaihu Granule231, weifuchun Tablet232, Simotang Oral Liquid233, Weisu Granule234, Jianweixiaoshi Tablet235, Huoxiangzhengqi Liquid236, Sanjiuweitai Granule237 |
| Medicines for nervous system diseases | Angong Niuhuang Pill238, Yangxueqingnao Granule239, Tongtian Oral Liquid240, Zhengtian Pill241, Tianshu Capsule242, Gastrodin Injection243, Zhennaoning Capsule244, Jieyu Pill245 |
| Medicines for respiratory system diseases | Qingkailing Injection246, Reduning Injection247, Lianhua Qingwen Capsule248, Shuanghuanglian Injection249, Tanreqing Injection250, Xiyanping Injection251, Antiviral Oral Liquid252, Shufengjiedu Capsule253, Suhuangzhike Capsule254 |
| Medicines for urinary system diseases | Shenkang Injection255, NiaoDuQing Granule/Uremic Clearance Granule256, Shenyan Kangfu Tablet257, Qianliexin Capsule258, Yinhua Miyanling Tablet259 |
| Tonic medicines | Fufang E'jiao Syrup260, Yixuesheng Capsule261, Liuwei Dihuang Pill262, Huangqijing Oral Liquid263, Shenqifuzheng Injection264 |
| Medicines for tumor | Compound Kushen Injection265, Aidi Injection266, Shenyi Capsule267, Kang-ai Injection268, Brucea Javanica Oil Emulsion269, Cinobufacini Injection270, Compound Huangdai Tablet271, Pingxiao Capsule272 |
| Medicines for skeletal musculature diseases | Duyiwei Capsule273, Xiaotong Plaster274, Tongluo Qutong Plaster275, Shangke Jiegu Tablet276, Huoxue Zhitong Capsule277, Wangbi Tablet278, Xianling Gubao Capsule279, Yunnan Baiyao Capsule280 |
| Medicines for anorectal and dermatologic diseases | Musk Hemorrhoids Ointment281, Shexiang Hemorrhoid Suppository282, Menthocamphorate Emulsion283, Compound Cortex Phellodendri Fluid284 |
| Medicines for gynecology diseases | Guizhifuling Capsule285, Baofukang Suppository286, Kangfute Suppository287, Fukeqianjin Tablet288, Bujibaifeng Pill289 |
| Medicines for ophthalmology and otorhinolaryngology diseases | Qingyan Pill290, Fufang Caoshanhu Tablet291, Biyankang Tablet292, Liushen Pill293 |
| Clearing heat and detoxication medicines | Xinhuang Tablet294, Pien Tze Huang295, Yiqing Capsule296, Niuhuang Qingxin Pill297, Xuebijing Injection298, Dihydroartemisinin Preparations299 |
Table 3.
Descriptions of score calculated formula.
| Category | Aspects | Grade | Score |
|---|---|---|---|
| Evaluation aspect | (a) Animal experiment | – | 15 |
| (b) Retrospective or real-word experiment | – | 25 | |
| (c) Clinical randomized controlled trial | – | 35 | |
| (d) Human using history | – | 15 | |
| (e) Treatment guideline inclusions or recommendation | – | 10 | |
| Evaluation index | (m) Article quantity | (1) Very few (1–2 articles) | 0.2 |
| (2) Few (3–4 articles) | 0.4 | ||
| (3) General (5–6 articles) | 0.6 | ||
| (4) Much (7–8 articles) | 0.8 | ||
| (5) Very much (more than 8 articles) | 1.0 | ||
| (n) Article quality | (1) Chinese general periodical | 0.4 | |
| (2) Chinese core periodical | 0.6 | ||
| (3) Science citation index (SCI) included journal | 0.8 | ||
| (4) Very famous work (including impact factor>7) | 1.0 | ||
| (x) Human using history | (1) Modern preparation (<15 years) | 0.5 | |
| (2) Shorter history preparation (15–50 years) | 0.8 | ||
| (3) Long history preparation (>100 years) | 1.0 | ||
| (y) Guideline inclusion or recommendation level | (1) Recommendation | 1.0 | |
| (2) No recommendation | 0.0 | ||
| Computational formula | (a∗m+a∗n + b∗m + b∗n + c∗m + c∗n)/2 + d∗x + c∗y | ||
Figure 4.
Heatmap of literature scores of TCM for treating several diseases.
4. Target identification and validation of natural products
Natural products are an important source of new compounds for drug research and development300. At present, a considerable proportion of clinical drugs are directly or indirectly derived from natural drugs. A drug target is defined as a specific molecule in the human body that interacts with a given drug and confers its effects. Natural products with clear targets are not only conducive to clinical observation of drug metabolism but also further the exploration of mechanisms in related basic research fields. In addition, for natural small-molecule drugs with clear targets, the in vivo signal response pathways can be predicted. Therefore, by using appropriate antagonists and adjuvants, the associated pathways that induce adverse reactions can be inhibited, thereby enhancing the pharmacodynamic pathways and reducing the drug's side effects.
The identification of drug targets and related research has important theoretical significance and practical value in the field of pharmaceutical research. The discovery of new targets of natural active small molecules will also open up a broad research space for the treatment of related diseases. At present, the identification of natural product targets is becoming increasingly important in the biomedical field, and the identification methods are also diverse, each with its own advantages and disadvantages301. The current identification methods can be used both independently and complementarily, and their combined use may be more conducive to the identification of natural product targets.
4.1. Methods for target identification of natural products
4.1.1. Natural product–centric target identification strategies
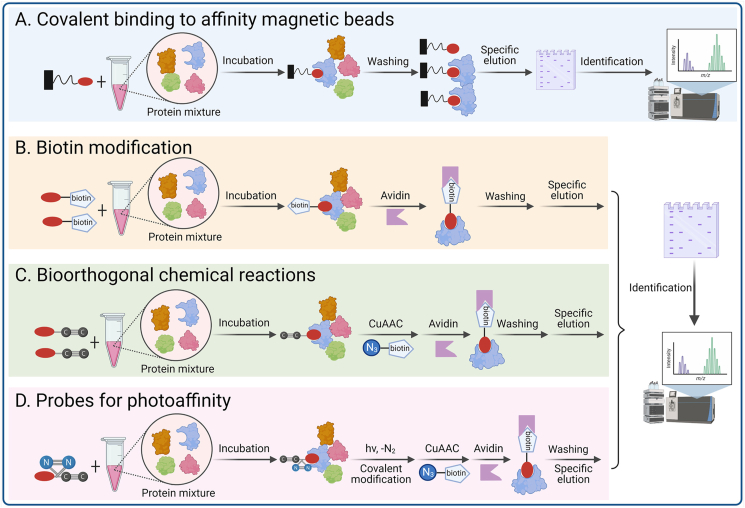
The method of coupling molecules with affinity probes is one of the main methods for discovering and identifying drug targets. The small molecule probe is mainly composed of a reporter group, a linking group, and an active group. The principle of action is that the active group part of the small molecule will tightly bind to its target, while the reporter group part can effectively label the target biological macromolecules. The targets can then be confirmed by a number of methods, such as chromatography, gel electrophoresis, and mass spectrometry (Fig. 5).
Figure 5.
Natural product–centric target identification strategies. (A) Covalent binding to affinity magnetic beads; (B) Biotin modification; (C) Bioorthogonal chemical reactions; (D) Probes for photoaffinity groups. This figure is created with biorender.com.
4.1.1.1. Covalent binding to affinity magnetic beads
Li et al.53 coupled artemisinin to a solid support and performed pull-down experiments in the presence and absence of a competing free artemether and identified gephyrin as the most significantly enriched specific interacting protein by mass spectrometry. They showed that gephyrin is the mammalian target of this antimalarial drug and that the mechanism depends on the enhancement of GABAA receptor signaling. Zhang's group302 studied the anti-inflammatory effect and target protein of the natural sesquiterpene lactone IJ-5, which reacts with epoxy-activated Sepharose 6 B beads. They used the active hydroxyl group in the molecular structure of IJ-5 to form a covalent bond, and then bonded the IJ-5 molecule to the surface of a solid support. The interaction between IJ-5 and the target protein then identified the target protein as the ubiquitin ligase UbcH5. Further research determined that IJ-5 preferentially binds to its target protein through the active site cysteine by forming a covalent adduct. This prevents ubiquitin molecules from binding to UbcH5, thereby inhibiting the activation of NF-κB inflammatory signaling pathway and resulting in the observed anti-inflammatory effects302.
4.1.1.2. Biotin modification
The biotin affinity purification system is one of the most commonly used schemes, mainly because the avidin protein has a high ability to recognize its substrate biotin, and the binding energy of the two is almost as close as that of a covalent bond. Labeling a small molecule with biotin as a purification tag and then immobilizing avidin on a matrix as a purification matrix allows isolation of the probe-bound protein complex from a complex mixture for analysis and identification. Lei's group303 modified the structure of the natural anti-inflammatory active molecule ainsliadimer A by introducing biotin long-chain molecules to its hydroxyl group. They used the resulting biotin-ainsliadimer A and identified the target protein of its anti-inflammatory activity as IκB kinase (IKKα/β). Ainsliadimer A can selectively form a covalent bond with cysteine 46 of IKKα/β, thereby inhibiting the activity of IKKα/β and downregulating the NF-κB inflammatory signaling pathway to achieve anti-inflammatory effects. Liu et al.304 reported that adenanthin, a diterpenoid isolated from the leaves of Rabdosia adenantha, induces the differentiation of acute promyelocytic leukemia (APL) cells. Using biotin-tagged adenanthin, they found that adenanthin directly binds to the conserved cysteines of Prx I and Prx II and inhibits their peroxidase activity. They indicated that adenosine is the first lead natural compound for the development of Prx I- and Prx II-targeted therapeutics, and this may represent a promising approach to induce APL cell differentiation. Tu’ s team305 transformed the key active component of TCM sappanone A into a chemical probe, and used the reverse drug targeting strategy to “target fishing” drug target IMPDH2 in cells, which explained the molecular mechanism of its anti-inflammatory effect from the cellular and molecular level, and laid a theoretical foundation for the international promotion of TCM.
4.1.1.3. Bioorthogonal chemical reactions
Introducing affinity tags onto small-molecule compounds by derivatization is a very challenging task. For some compounds, the introduction of sterically hindered affinity tags easily leads to a loss of activity of the compounds. Fortunately, these problems can be resolved by bioorthogonal chemistry, such as Cu-catalyzed click reactions. Wang et al.306 described a novel approach that combined isobaric tags for the relative and absolute quantitation with clickable activity-based protein profiling to identify the targets of andrographolide, a natural product with known anti-inflammation and anticancer effects. They identified a series of specific targets for andrographolide, further deepening the understanding of the drug's mechanism of action.
4.1.1.4. Probes for photoaffinity groups
Most biologically active compounds usually bind with their respective target proteins through non-covalent interactions, which have a certain instability and are inconvenient to study. The introduction of photoactive groups enables the complex that forms based on a non-covalent interaction to undergo covalent cross-linking under light excitation and be transformed into a strong covalent conjugate, thereby improving the detection limit of the target protein.
Dai et al.307 discovered that baicalin acts as a natural allosteric activator of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme in the fatty acid β-oxidation (FAO) pathway. They designed and synthesized a photoaffinity probe for baicalin and found that baicalin directly binds to CPT1 and activates it to accelerate fatty acid degradation. Their study provided a mechanism that would explain the biological activity of baicalin, namely, its ability to reduce lipid accumulation. Matrine is a plant alkaloid that has shown potent anticancer activity, but with unknown molecular targets Wang et al.308, using a photoaffinity labeling approach, have recently identified annexin A2 as a direct binding target of matrine in cancer cells.
Despite the feasibility and effectiveness of the classical compound-centric design of molecular probes, the probes have certain limitations: (1) proteins with low intracellular abundance or that bind only weakly to the probes are difficult to identify; (2) nonspecific binding of the probes to protein impurities has a certain interference effect; (3) the structure–activity analyses of the compound are required, as the synthesized probe molecule should maintain its original biological activity and mechanism of action; and (4) connection of the compound to the linking chain and the reporter group usually requires the introduction of functional groups, such as amino, hydroxyl and carboxyl groups, to the core of the compound, and this may affect the activity of the compound309.
4.1.2. Label-free proteomics to identify the targets of natural products
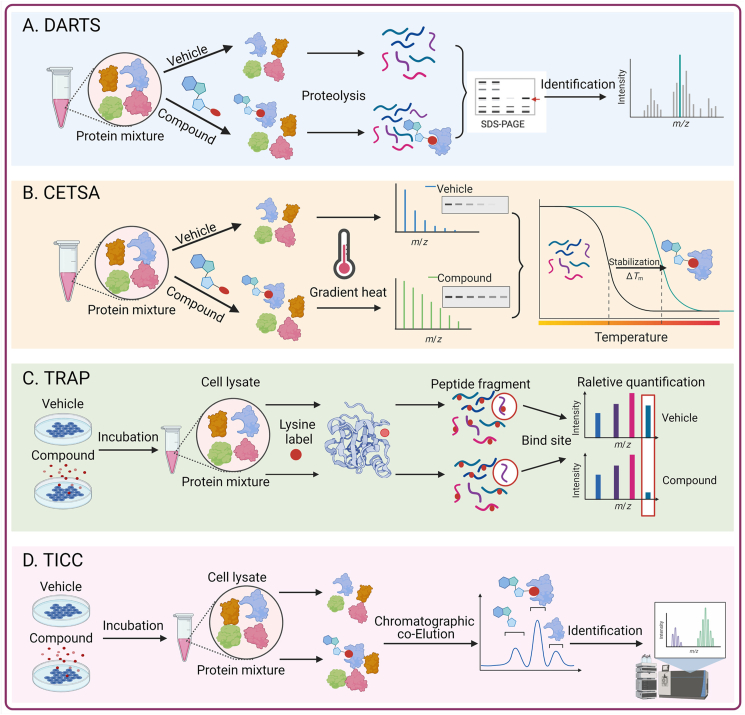
Identifying the target proteins of small-molecule drugs is crucial to understanding the mechanism of action of drugs. Methods based on chemical modifications have certain limitations, all of which require labeling or derivatization of small-molecule drugs, which may lack sites for covalent cross-linking, or chemical modifications. Therefore, the establishment of screening technology for small molecule drug target proteins without the need for chemical modifications is very important. At present, a variety of label-free methods have been developed to identify drug targets (Fig. 6).
Figure 6.
Method for target identification of natural products with Label-free. (A) Drug Affinity Responsive Target Stability (DARTS); (B) Cellular Thermal Shift Assay (CETSA); (C) Target-Responsive Accessibility Profiling (TRAP); (D) Target Identification by Chromatographic Co-Elution (TICC). This figure is created with biorender.com.
4.1.2.1. Drug affinity responsive target stability (DARTS)
In 2009, Lomenick et al.310, 311, 312 established the DARTS technology based on the principle that proteins are protected from protease degradation after binding to their ligands. The main strategy involves incubation of the small molecule drug with the sample protein for a certain time, and then adding protease for digestion. Because the small molecule drug can protect its target protein after binding to its target, the sensitivity of the target protein to proteases is reduced. Therefore, after electrophoresis gel staining, a comparison of the digested proteins in the absence and presence of the drug allows identification of the protected band, and the target protein can then be further identified by mass spectrometry.
The advantage of the DARTS method is that it does not require any chemical modification during the experiment, and it can theoretically be used for the interaction screening of any small molecule and its target protein. Using DARTS, Lomenick's group312 successfully identified the eIF4A protein as the target of resveratrol, a common plant natural product. Their findings pointed to eIF4A as a previously uncharacterized drug target for antiaging treatments. Zeng's group313 used Pull-down assay and DARTS assay to identify the ATP6V0D1 subunit in V-ATPase as the direct cellular target of natural small-molecule schisandrol A (SolA). SolA is significantly protective against AGEs-induced neuronal apoptosis by allosterically mediating ATP6V0D1 conformation targeting the unique cysteine 335 residue to activate V-ATPase-dependent lysosomal acidification. Similarly, Geng et al.88 used a DARTS method to identify Dynamin-related protein 1 (DRP1) as the target protein of andrographolide, and further study found that DRP1 is a key effector mediating mitochondrial fission. Andrographolide binds to DRP1 and inhibits its GTPase activity, thereby preventing the excessive mitochondrial fission and neuronal damage associated with Parkinson's disease. The protein samples used in DARTS technology can be purified proteins or whole cell lysates and can be used for low-affinity target screening because no washing is required during the experimental procedure. However, because this technique requires the use of gel staining for visual comparison, it has certain limitations when attempting to identify low-abundance target proteins310.
4.1.2.2. Cellular thermal shift assay (CETSA)
Martinez Molina et al.314,315 applied clinical drugs to four different target proteins in cell lysates and found that each of the four target proteins had its own unique melting curve. When drugs known to bind to these proteins were added to cell lysates, significant melting curve shifts were observed. This principle led to the development of the CETSA method, which can directly measure the extent of a drug that reaches target cells and allows the detection of the effect of small molecules in cell lysates and even in intact cell tissues. CETSA is based on the principle of ligand-induced changes in the thermodynamic stability of target proteins. This technique mainly identifies the possible targets of natural products based on the principle that drugs that combine with target proteins change the protein stability316.
Wang et al.317 used molecular docking and CETSA to demonstrate that two ginsenosides, Rg5 and Rk1, bind directly to annexin A2. This study was the first to demonstrate that G-Rg5 and G-Rk1 inhibit tumor cell growth by targeting annexin A2 and the NF-κB pathways. During a screening of natural products to identify STAT3 inhibitors, Jin et al.318 found a dose-dependent reduction in STAT3 enzymatic activity by geranylnaringenin (CG902). The interaction of CG902 with STAT3 was further verified by DARTS and CETSA, indicating that CG902 is a novel STAT3 pathway inhibitor. CETSA has a wide range of applications and can directly measure whether drug molecules reach their target at the cellular and whole-animal levels, thereby validating important clinical drug targets. However, the heat treatment during CETSA may affect the permeability of the cell membrane and could allow entry of drugs that normally would not enter the cells at physiological temperature, leading to false positives. Therefore, the conditions for heat treatment, including the shortest time and the most effective heating method, need improvement.
4.1.2.3. Target-responsive accessibility profiling (TRAP)
The DARTS and CETSA methods use the in vivo changes in the dynamic balance of the target proteins due to binding with the drug compounds and ligand-induced protein stability enhancement to identify drug targets. By contrast, TRAP identifies binding proteins for drug molecules in the cellular environment by monitoring the ligand-induced changes in lysine accessibility at the proteomic level. This method measures the steric hindrance induced in the protein targets due to ligand binding by global analysis of the accessibility changes to reactive lysine. Briefly, peptides that contain TRAP-induced and exhibit significant abundance changes in the presence of drug molecules are designated as target-responsive peptides319. Our research group used TRAP technology to identify the target of celastrol as adenylyl cyclase associated protein 1 (CAP1). Mechanistically, we found that celastrol interacts with CAP1 and resistin to inhibit the cAMP–PKA–NF-κB signaling pathway and ameliorate high-fat diet-induced metabolic syndrome in mice. Our study showed that celastrol binds to CAP1, inhibits the interaction between resistin and CAP1, effectively attenuates the subsequent inflammatory response, and ultimately improves metabolic syndrome69.
4.1.2.4. Target identification by chromatographic co-elution (TICC)
TICC is a co-fractionation based on the formation of stable ligand–target complexes during native HPLC. The main premise is that binding to one or more target proteins changes the chromatographic properties of the compounds so that the ligand–target complexes exhibit different characteristic elution profiles relative to the free (unbound) drug. That is, the retention time of a compound is “transferred” to the retention time of its interacting protein partner. Binding proteins are then identified by high performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). Chan et al.320 have used TICC to reveal the sterol biosynthesis enzyme Erg6p as a novel putative antifungal target. The TICC target identification method is more suitable for TCM because it can identify multiple component targets at the same time. This method is also suitable for detecting low-abundance target proteins and low-affinity (micromolar) interactions. One limitation is the need to separate the unbound compounds from protein-bound compounds without using covalent bonding agents. The co-elution of proteins with similar retention properties may also complicate the identification of true targets190,321.
At present, the concept of a drug acting on multiple proteins has gradually been accepted. Drug development and synthesis are not conducted only for pathogenic genes and proteins; they are necessary to study the entire network of drug and pathogenic effects. This increases the need for the identification of drug targets. The continuous development of many disciplines, such as genomics, proteomics, bioinformatics, genetics, and biotechnology, will continue to improve existing methods, and new methods and strategies will continue to emerge. Currently, there are various methods for the identification of natural product targets, each with advantages and disadvantages (Table 4). It can be used independently or complementary, and the combination of the two may be more conducive to the identification of natural product targets. Many drug target proteins with unique structures and functions can be explored and discovered for natural products and will provide key theoretical information for subsequent innovative drug design.
Table 4.
Advantages and disadvantages of various target identification methods.
| Method | Principle | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Coupling molecules with affinity probes | Active group part of the natural products will tightly bind to its target, while the reporter group part can effectively label the target biological macromolecules |
|
|
53,302, 303, 304,306, 307, 308 |
| Drug Affinity Responsive Target Stability (DARTS) | Compounds that bind to their target proteins reduce the sensitivity of the target protein to proteases |
|
|
88,310, 311, 312, 313,318 |
| Cellular Thermal Shift Assay (CETSA) | Compounds that change protein stability after binding to target proteins, and the bound target protein has a unique melting curve | Wide range of applications and can directly measure whether drug molecules reach their target at the cellular and whole-animal levels | Heat treatment may affect the permeability of cell membranes and may allow the entry of drugs that would not normally enter cells at physiological temperatures, resulting in false positives | 314, 315, 316, 317, 318 |
| Target–Responsive Accessibility Profiling (TRAP) | TRAP identifies binding proteins for compounds in the cellular environment by monitoring the ligand-induced changes in lysine accessibility at the proteomic level |
|
No difference in lysine abundance at sites where compounds bind to target proteins | 69,319 |
| Target Identification by Chromatographic Co–Elution (TICC) | Compounds bind to the target protein alters the chromatographic properties of the compound so that the ligand–target complex exhibits a different characteristic elution profile relative to the free (unbound) compound |
|
|
190,320,321 |
4.2. Natural product target validation
The target identification methods used with a natural drug molecule usually deliver a series of target proteins. If several proteins are candidate targets, they need to be prioritized based on their known function and relevance to the phenotype induced by the drug molecule. For this prioritization, designing and implementing appropriate control experiments are essential to differentiate nonspecific binding. A further complexity that should be taken into account is that the identified protein may not be the direct target, but merely part of a protein complex. Therefore, confirmation of drug molecule targets is also crucial.
4.2.1. Binding experiments to validate target proteins
Determining the binding affinity of small molecules to their putative targets provides strong evidence for target validation. Several methods used to examine protein–protein interactions, such as surface plasmon resonance (SPR), isothermal titration calorimetry (ITC)322, fluorescence polarization (FP)323, homogeneous time-resolved fluorescence (HTRF)324 and microthermophoresis (MST)325, have been successfully used in small molecule–protein interaction studies. Most of these methods require purified target proteins, and some require fluorescent labeling326. If the drug molecule has a strong affinity for the target protein, the specific conformation and position of the drug molecule and the target protein can be further obtained through nuclear magnetic resonance, small angle scattering, and co-crystallization experiments to provide a structural basis for the development of new drugs that target specific diseases.
4.2.2. Biological function verification of target proteins
The binding of a small molecule to a protein does not necessarily modulate its function. For this reason, functional experiments are also required to confirm a protein target327. When the target has enzymatic activity, the modulation of this activity should be assessed with an enzymatic assay328. For confirmed targets, in vitro, RNA interference (siRNA/shRNA), and/or cDNA overexpression experiments should be performed, and both positive and negative aspects should be analyzed to verify whether the confirmed targets might affect the biological activity of the drugs. In vivo target validation can be achieved by breeding different Flox and Cre mice to achieve tissue/cell-specific knockout/knockin of specific genes. This type of analysis can determine the role of the target protein in the disease phenotype and establish whether the protein is the primary target that dictates the function of the drug molecule.
In conclusion, target validation of drug molecules is as important as target recognition. Validation of a target should not be limited to determining the binding affinity of the target to the ligand, but should also confirm the cellular context indicated by phenotypic screening. A combination of biophysical, biochemical, cell biology, and structural biology approaches will help to identify the final target protein. Broadly speaking, drug targets include proteins that directly interact with drugs, but they can also be intracellular signal-responsive molecules that are triggered by drug molecules. The discovery and research of these signal-responsive molecules have very important theoretical and practical value for understanding the mechanism of action of existing drugs and improving their clinical efficacy. Therefore, the identification of biologically active natural products is of great significance for advancing biomedical research. Identification of target proteins will aid in elucidating the mechanism of drug action, establishing the potential therapeutic value of the drug, and understanding its off-target-related side effects.
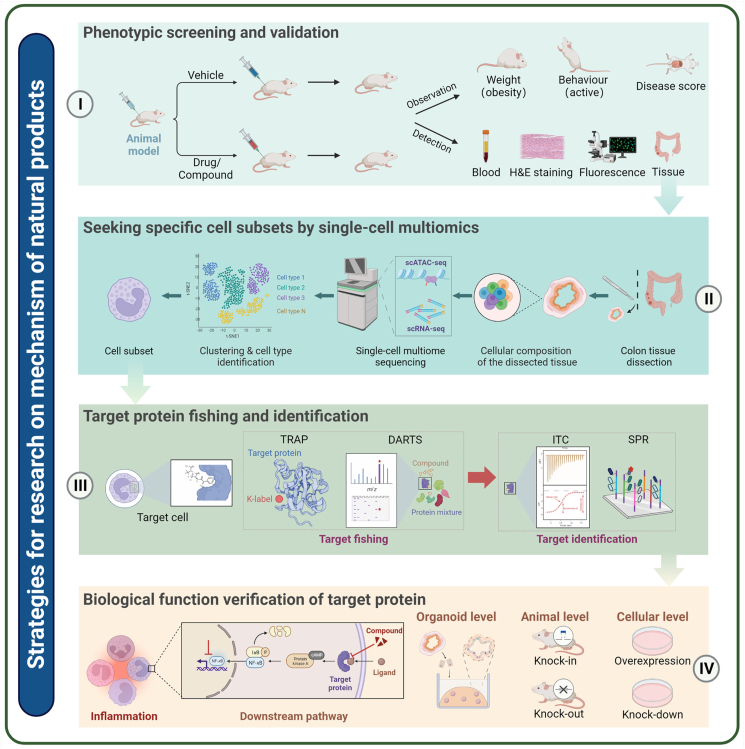
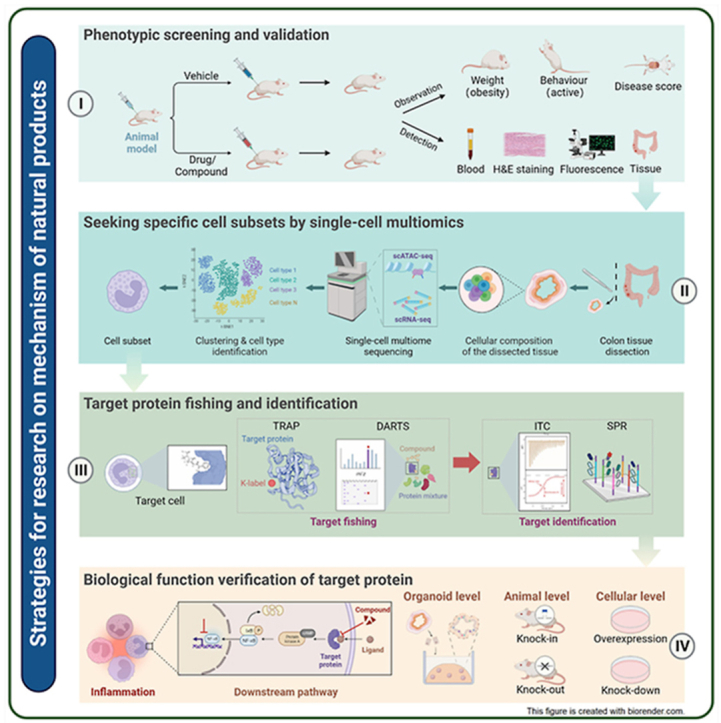
5. New techniques and strategies for researching the mechanism of natural small molecular compounds
5.1. Single-cell omics
Single-cell omics, as a rapidly developing frontier technology in life science, describes the genome sequencing, transcriptome sequencing, proteome detection, and metabolome detection in a single individual isolated cell from a sample329.
5.1.1. Single-cell transcriptome
The fundamental principle of single-cell sequencing (scRNA-seq) is similar to that of bulk RNA-seq, except that scRNA-seq is aimed at a single cell rather than a group of tissues. This imparts some particularity with respect to single cell isolation and capture, as well as trace RNA amplification. The main methods for single cell separation include fluorescence-activated cell sorting (FACS)330, microwell331, and microfluidic technology332. Recently, with the widespread use of SPLit-seq, the separation of individual cells has become a well-established method333. The appearance of the terminal tail method, Smart-seq334, Cel-seq335, and unique molecular identifiers (UMIs)336 has further improved the accuracy of single cell transcriptome quantification. The emergence of a variety of high-throughput, low-cost, automated commercial sequencing platforms, such as BD Rhapsody™, 10 × Genomics Chromium, and IlluminaBio-Rad, has greatly promoted the application of scRNA-seq.
The advent of scRNA-seq has opened up new avenues for studies on human physiology and disease pathologies, such as tumorgenicity337, inflammation, and immunity338, and now allows identification of cell subclusters339, heterogeneity of gene expression, cell development trajectories340, and cell–cell interactions341. It is also widely used in drug screening, efficacy evaluation, and pharmacological research. This technology was selected as “Method of the Year 2019” by NatureMethods342. In the study of the mechanisms of natural products, scRNA-seq can comprehensively and accurately describe the differences in cell types and molecular states between physiologically normal and pathological tissue prior to or following drug treatment to provide more information for the discovery of drug targets and pathways. For example, Fan's group76 investigated the systematic post-infarction dynamics of cardiac immune cells in the progression of myocardial infarction and found that macrophages Mø-5 and Mø-6, which express chemokines CCL7, CCL2, and PF4, were crucial for disease progression. Trajectory analysis revealed that the Mø-5 and Mø-6 macrophages were mainly derived from monocytic progenitors. The natural product tanshinone IIA significantly inhibited the expression of Mø-5 and Mø-6 and their chemokines.
5.1.2. Single-cell multiomics
Recent advances in molecular biology and systems biology have given rise to a multitude of multiomics technologies, which integrates different levels of information such as genes, mRNA, regulatory factors, proteins and metabolites, and then constructs gene regulatory networks and reveals the regulation and causal relationship between various molecules.
Bai's group343 integrated transcriptome, proteome and metabolome data and confirmed that P53 was a key target of ginsenoside, and 20(S)-protopanaxatriol directly targeted adjacent regions of the P53 DNA-binding pocket and promoted the stability of P53–DNA interactions with the application of affinity mass spectrometry (MS) screening and SPR. Liu's group344 integrated omics data including gene expression, DNA methylation and copy number alterations from TCGA, combined with bioinformatics including the similarity network fusion (SNF) method and the LASSO algorithm, to identify that SIRT3 and SF3B3 are potential autophagic regulators in invasive breast carcinoma. Li et al.345 integrated lipidomic and transcriptomic analysis revealed that SSa and SSd regulated TF-dependent gene expression to ameliorate non-alcoholic fatty liver disease.
For single cell level, multiomics enable a more comprehensive delineation of the state of single cells than is provided by single omics data based on multichannel molecular readouts346, and greatly promote the development of exploring rare cell types, identifying accurate cell types, and improving cell annotation information347,348.
Zhang et al.349 combined scRNA-seq, TCR-seq, and ATAC-seq to investigate immune cell dynamics of patients with triple-negative breast cancer (TNBC) treated with paclitaxel or paclitaxel plus atezolizumab, and found that CD8–CXCL13, CD4–CXCL13, Tregs, and Bfoc cells decreased in responders treated with paclitaxel, whereas they increased in those treated with paclitaxel plus atezolizumab. This difference in response indicated that the paclitaxel regimen could selectively reduce key antitumor immune cells while elevating immunosuppressive macrophages in TNBC, thereby providing novel insights into the treatment of TNBC by the combination of paclitaxel and atezolzumab.
Single-cell proteomics and single-cell metabolomics have also been used in pathological research. However, the technological development of both is still in the early stages, and their application is lagging, with no mature commercial solution at present350. In view of the similarities in the detection principles and analysis strategies, subsequent studies should use single-cell proteomics to analyze the protein expression profiles of single cells in the context of drug intervention to render comprehensive and accurate reflections of drug targets in different types of cells.
In view of the widely used single-cell sequencing technologies, we proposed new strategies that focused on elucidating the mechanisms of natural products and their target proteins (Fig. 7). Firstly, an appropriate animal model of disease was constructed to verify the therapeutic effect of natural products. Secondly, tissues from the animal models were selected as samples for single-cell multiome sequencing (including sRNA-seq and scATAC-seq) to identify the different cell subclusters that showed significant changes in response to drug treatment, which we defined as target cells (cell lines or primary cell cultures). Thirdly, approaches such as TRAP and DARTS were used to find the binding protein for a natural product in a target cell, with isothermal titration calorimetry (ITC), surface plasmon resonance (SPR), and microscale thermophoresis (MST) then used to confirm the binding of the target protein. Finally, the biological function of the target protein was verified by combining gene knockout/knockin at the animal level and knockdown/overexpression at the cellular level, followed by analysis of downstream pathways to determine the effects of natural product binding to the target protein on the progression of the disease. These new strategies are also applicable to research on the mechanisms of small molecules produced by chemical syntheses.
Figure 7.
Strategies for research on mechanism of natural products based on deciphering target protein. This figure is created with biorender.com.
5.2. Network pharmacology and artificial intelligence
Big data and artificial intelligence technologies will drive the rapid development of modernization of TCM. Integrative pharmacology-based traditional Chinese medicine (TCMIP) is a database- and algorithm-dependent research strategy, which form multidimensional association network with complex interaction types including constituent–target interactions, constituent–gut microbiota interactions, constituent–constituent interactions, gut microbiota–target interactions, and target–target interactions351. Protein three-dimensional structure analysis and computer virtual screening based on bioinformatics also provide directions for drug design for a variety of diseases including SARS-CoV-2352.
5.2.1. Network pharmacology
Network pharmacology is based on database data and builds a component–target–disease interaction network to predict drug substance efficacy, targets, and pathways. It breaks the model of “one disease–one target–one drug,” which is the main reason for the failure of 70% of new drugs in clinical trials. A variety of chronic diseases, such as tumors, cardio-cerebrovascular disease, and diabetes, are multi-gene and multi-factor diseases, so achieving a good therapeutic effect based on only a single target is difficult353. The technology of network pharmacology plays an important role in research into the prescription of TCM and natural products. For example, Wang's group354 used an integrated strategy combining metabolomics and network pharmacology to research the mechanism of hydroxysafflor yellow A (HSYA) in counteracting acute brain injury. Metabolomics of the brain tissues identified differentially expressed metabolites, while network pharmacology was applied to unearth potential targets against traumatic brain injury (TBI). Their findings revealed that HSYA alleviated the neurological deficits of TBI and that NOS1, AChE, and Ptgs2 were the key regulatory genes.
5.2.2. Artificial intelligence (AI)
Recent improvements in computer data processing ability and the rapid accumulation of multi-group data have laid the foundation for the development of more accurate drug target reasoning algorithms, making the combination of AI and new experimental technologies an efficient method for drug discovery. AI plays an important role in all preclinical stages of drug development, including cell sorting, cell classification, quantum mechanics calculation of compound properties, computer-aided organic synthesis, designing new molecules, developing assays, predicting the 3D structures of target proteins, and many others355. For example, Jiang's group356 proposed a novel Siamese spectral-based graph convolutional network (SSGCN) model based on gene transcriptional profiles. The model successfully predicted and verified the potential host targets of nelfinavir (NFV)-cyclophile A, and revealed the possible mechanism of interaction with SARS-CoV-2. The AI technology represented by a SSGCN model can significantly improve the prediction accuracy of drug targets, and therefore represents a powerful tool for the study of drug mechanisms and confirmation of targets. This method is also suitable for the prediction of the molecular targets of natural products.
5.3. Biosynthesis of natural products
In the past 20 years, advances in synthetic biology and the emergence of microbial cell factories have provided a novel idea for efficient and sustainable production of natural products. Through the metabolic transformation of microbial cells, the relevant metabolic enzyme components were transferred into microbial cells, so as to obtain the improved genetic engineering bacteria, and then the engineering bacteria were used to transform cheap carbon and nitrogen sources into natural products357.
Triterpenes are a large class of natural products with a wide range of bioactivities. More than 20,000 kinds of triterpenes have been identified and over 400 kinds of proprietary medicines, including many important steroid drugs and ginsenosides widely used in clinic. Their biosynthesis begins when squalene synthase catalyzes dimerization of farnesyl pyrophosphate (FPP) to squalene, which is further oxidized to epoxy squalene, and then triterpene synthase (TrTSs) catalyzes the cyclization of squalene or epoxy squalene to holene or lanosterol. The huge structural diversity is catalyzed by many different modifying enzymes358. Recently, Tao et al.359 found that two fungal chimeric class I TrTSs-TvTS and MpMS can directly synthesize triterpene core skeletons from IPP and DMAPP or HexPP, which broke the inherent understanding that the triterpene skeleton can only be synthesized with squalene as the starting unit.
6. Conclusions and perspectives
Natural products are components or metabolites of living organisms that have evolved over a long period in nature, and they are often structurally diverse and have unique pharmacological and biological activities. Currently, more than 60% of the pharmaceuticals on the market are related to the structure and information derived from natural products, and natural product-based drug development strategies still dominate in modern new drug development. The Nobel Prize awarded to Youyou Tu for her research on artemisinin set off a wave of interest in TCM and natural products among scientists. Liu and Shen's group360 led the research and development of “Moringa alba tablets” for the treatment of type 2 diabetes, and this drug has been approved for marketing by the State Drug Administration. It represents the first original natural drug for lowering blood sugar in China, and the first new TCM in this field approved in China in the past ten years. Icariin Softgels have also been approved for marketing in China as a first-line treatment for hepatocellular carcinoma, and are original innovative TCM drugs in China. The use of TCM active ingredients to develop new drugs is a special path that China can follow for a long time.
Natural products have some problems, such as poor water solubility, low bioavailability, and poor stability, and the development of new dosage forms using nanotechnology is a hot research area at present. Many difficulties still remain to be overcome before clinical application. Among them, TCM and natural products are still quite good choices for ADC drugs.
Knowledge of drug targets is a prerequisite for the identification of innovative drugs, and the targets have a source innovation significance for new drug development. An urgent need exists to establish an in vivo method for finding drug targets directly in the real physiological environment, as this is important for promoting biomedical research by elucidating the mechanism of action of drugs and discovering their potential therapeutic value, as well as for further discovery of the side effects unrelated to the targets. The present use of TCM is mostly in the form of compounding, and the identification and characterization of the targets of the chemicals that are combined in TCM compounding is also one of the difficulties. The identification of the targets and the interpretation of the clinical efficacy of the complex system of TCM prescriptions will help to explain the profound laws of TCM in a more scientific way and will play an important role in the development of TCM. Natural products, especially TCM products with a long history of use, have a rich foundation of clinical applications, which should be bestowed and continuously innovated with the help of advanced science and technology to boost the development of innovative drugs in China and to realize TCM industrialization and internationalization in the near future.
Single-cell multiomics technology has a profound impact on the development of life science. The research paradigm of “based on advanced science and technology, driven by data and knowledge” is of great significance for the modernization of TCM. Single-cell technology makes the precision medicine from tissue and organ to a single cell, revealing the dynamic regulation of natural products on different cell types. The application of single-cell multiomics also provides insights into the molecular mechanisms underlying the precise regulation of key molecules at various levels during disease onset and drug intervention. Drug target hooking and validation at the single cell level is of great significance for identifying sensitive cell types and studying the mechanism of drug resistance, especially for the research on tumor microenvironment, and provides insights for the clinical usage of anti-tumor drugs and the mechanism of drug resistance. The application of single-cell multiomics analysis is still in its early stage, with the great breakthroughs of experimental techniques of target identification and the improvement of multiomics analysis methods, it will drive a new era of disease intervention and drug discovery.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81872877, 81730100, 91853109, 82073975), School of Life Science (NJU)–Sipimo Joint Funds, Characteristic Innovation Project of Guangdong Provincial Education Department (Nos. 2019GKTSCX039; 2020KTSCX295, China), School-Level Scientific Research Project of Shenzhen Polytechnic (No. 6021310023K, China), Natural Science Research of Jiangsu Higher Education Institutions of China (No. 22KJB360005), and Fundamental Research Funds for the Central Universities (No. 020814380174, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Qiang Xu, Email: qiangxu@nju.edu.cn.
Hongyue Ma, Email: hongyuema@njucm.edu.cn.
Yang Sun, Email: yangsun@nju.edu.cn.
Author contributions
Yang Sun, Qiang Xu, and Hongyue Ma conceived the manuscript, Yuyu Zhu, Zijun Ouyang, Haojie Du, Meijing Wang and Jiaojiao Wang wrote the manuscript, Haiyan Sun and Lingdong Kong gave advice and suggestion.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Kong W., Wei J., Abidi P., Lin M., Inaba S., Li C., et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 2.Guo H.H., Feng C.L., Zhang W.X., Luo Z.G., Zhang H.J., Zhang T.T., et al. Liver-target nanotechnology facilitates berberine to ameliorate cardio-metabolic diseases. Nat Commun. 2019;10:1981. doi: 10.1038/s41467-019-09852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao M.M., Lu J., Li S., Wang H., Cao X., Li Q., et al. Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat Commun. 2021;12:5616. doi: 10.1038/s41467-021-25952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Tong Q., Ma S.R., Zhao Z.X., Pan L.B., Cong L., et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson's disease by regulating gut microbiota. Signal Transduct Targeted Ther. 2021;6:77. doi: 10.1038/s41392-020-00456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q.C., Pu Y.L., Bi J., Zhang Y. Protective effects of berberine on senile osteoporosis in mice. J Bone Miner Metab. 2021;39:748–756. doi: 10.1007/s00774-021-01225-2. [DOI] [PubMed] [Google Scholar]
- 6.Han S.Y., Kim Y.K. Berberine suppresses RANKL-induced osteoclast differentiation by inhibiting c-Fos and NFATc1 expression. Am J Chin Med. 2019;47:439–455. doi: 10.1142/S0192415X19500228. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y., Xin Q., Lu J., Miao Y., Lin Q., Cong W., et al. A new therapeutic candidate for cardiovascular diseases: Berberine. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Wei Y., Bai X., Li M., Li H., Wang L., et al. Berberine prevents primary peritoneal adhesion and adhesion reformation by directly inhibiting TIMP-1. Acta Pharm Sin B. 2020;10:812–824. doi: 10.1016/j.apsb.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X., Bian H., Wang L., Sun X., Xu X., Yan H., et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK–SREBP-1c–SCD1 pathway. Free Radic Biol Med. 2019;141:192–204. doi: 10.1016/j.freeradbiomed.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Jia X., Jia L., Mo L., Yuan S., Zheng X., He J., et al. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J Dent Res. 2019;98:107–116. doi: 10.1177/0022034518797275. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Liu X., Zhang N., Yin M., Dong J., Zeng Q., et al. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10:2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y.X., Gao Q.Y., Zou T.H., Wang B.M., Liu S.D., Sheng J.Q., et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5:267–275. doi: 10.1016/S2468-1253(19)30409-1. [DOI] [PubMed] [Google Scholar]
- 13.Wallace M., Pappagallo M. Qutenza®: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15–27. doi: 10.1586/ern.10.182. [DOI] [PubMed] [Google Scholar]
- 14.Abrams R.M.C., Pedowitz E.J., Simpson D.M. A critical review of the capsaicin 8% patch for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet in adults. Expert Rev Neurother. 2021;21:259–266. doi: 10.1080/14737175.2021.1874920. [DOI] [PubMed] [Google Scholar]
- 15.Fattori V., Hohmann M.S.N., Rossaneis A.C., Pinho–Ribeiro F.A., Verri W.A. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21:E844. doi: 10.3390/molecules21070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang D., Luo Z., Ma S., Wong W.T., Ma L., Zhong J., et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010;12:130–141. doi: 10.1016/j.cmet.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J., Li L., Li Y., Liang X., Sun Q., Yu H., et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. 2015;14:22. doi: 10.1186/s12933-015-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L., Zhong J., Zhao Z., Luo Z., Ma S., Sun J., et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc Res. 2011;92:504–513. doi: 10.1093/cvr/cvr245. [DOI] [PubMed] [Google Scholar]
- 19.Hao X., Chen J., Luo Z., He H., Yu H., Ma L., et al. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflugers Arch. 2011;461:345–353. doi: 10.1007/s00424-011-0921-x. [DOI] [PubMed] [Google Scholar]
- 20.Gao F., Liang Y., Wang X., Lu Z., Li L., Zhu S., et al. TRPV1 activation attenuates high-salt diet-induced cardiac hypertrophy and fibrosis through PPAR-δ upregulation. PPAR Res. 2014;2014 doi: 10.1155/2014/491963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang H., Li Q., Yu H., Li P., Lu Z., Xiong S., et al. Activation of TRPV1 attenuates high salt-induced cardiac hypertrophy through improvement of mitochondrial function. Br J Pharmacol. 2015;172:5548–5558. doi: 10.1111/bph.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Zhou Y., Fu J. Advances in antiobesity mechanisms of capsaicin. Curr Opin Pharmacol. 2021;61:1–5. doi: 10.1016/j.coph.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Acharya N., Penukonda S., Shcheglova T., Hagymasi A.T., Basu S., Srivastava P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci U S A. 2017;114:5005–5010. doi: 10.1073/pnas.1612177114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Gerven L., Alpizar Y.A., Steelant B., Callebaut I., Kortekaas Krohn I., Wouters M., et al. Enhanced chemosensory sensitivity in patients with idiopathic rhinitis and its reversal by nasal capsaicin treatment. J Allergy Clin Immunol. 2017;140:437–446. doi: 10.1016/j.jaci.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Xu C., Gulinello M., Frenette P.S. Nociceptors protect sickle cell disease mice from vaso-occlusive episodes and chronic organ damage. J Exp Med. 2021;218 doi: 10.1084/jem.20200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M., Zhang J., Mu Y., Foda M.F., Han H. Activation of TRPV1 by capsaicin-loaded CaCO3 nanoparticle for tumor-specific therapy. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121520. [DOI] [PubMed] [Google Scholar]
- 27.Gao X., Zhang D., Xu C., Li H., Caron K.M., Frenette P.S. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature. 2021;589:591–596. doi: 10.1038/s41586-020-03057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8 doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu M., Pirtskhalava T., Farr J.N., Weigand B.M., Palmer A.K., Weivoda M.M., et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Wang B., Gasek N.S., Zhou Y., Cohn R.L., Martin D.E., et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 2022;34:75–89. doi: 10.1016/j.cmet.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogrodnik M., Miwa S., Tchkonia T., Tiniakos D., Wilson C.L., Lahat A., et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8 doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Zhang J., Lin X., Boyce B.F., Zhang H., Xing L. Age-associated callus senescent cells produce TGF-β1 that inhibits fracture healing in aged mice. J Clin Invest. 2022;132 doi: 10.1172/JCI148073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novais E.J., Tran V.A., Johnston S.N., Darris K.R., Roupas A.J., Sessions G.A., et al. Long-term treatment with senolytic drugs dasatinib and quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12:5213. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou Z., Long X., Zhao Q., Zheng Y., Song M., Ma S., et al. A single-cell transcriptomic atlas of human skin aging. Dev Cell. 2021;56:383–397. doi: 10.1016/j.devcel.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Porras D., Nistal E., Martínez–Flórez S., Olcoz J.L., Jover R., Jorquera F., et al. Functional interactions between gut microbiota transplantation, quercetin, and high-fat diet determine non-alcoholic fatty liver disease development in germ-free mice. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201800930. [DOI] [PubMed] [Google Scholar]
- 37.Xia J., Rong L., Sawakami T., Inagaki Y., Song P., Hasegawa K., et al. Shufeng Jiedu capsule and its active ingredients induce apoptosis, inhibit migration and invasion, and enhances doxorubicin therapeutic efficacy in hepatocellular carcinoma. Biomed Pharmacother. 2018;99:921–930. doi: 10.1016/j.biopha.2018.01.163. [DOI] [PubMed] [Google Scholar]
- 38.Hu K., Miao L., Goodwin T.J., Li J., Liu Q., Huang L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano. 2017;11:4916–4925. doi: 10.1021/acsnano.7b01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K., Liu R., Li J., Mao J., Lei Y., Wu J., et al. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt–mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy. 2011;7:966–978. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Shen L., Li X., Song W., Liu Y., Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13:12511–12524. doi: 10.1021/acsnano.9b02875. [DOI] [PubMed] [Google Scholar]
- 41.Zhang F., Li Z.L., Xu X.M., Hu Y., Yao J.H., Xu W., et al. Protective effects of icariin-mediated SIRT1/FOXO3 signaling pathway on intestinal ischemia/reperfusion-induced acute lung injury. Mol Med Rep. 2015;11:269–276. doi: 10.3892/mmr.2014.2679. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Shen C., Chu J., Zhang R., Li Y., Li L. Icariin decreases the expression of APP and BACE-1 and reduces the β-amyloid burden in an APP transgenic mouse model of Alzheimer's disease. Int J Biol Sci. 2014;10:181–191. doi: 10.7150/ijbs.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su B., Ye H., You X., Ni H., Chen X., Li L. Icariin alleviates murine lupus nephritis via inhibiting NF-κB activation pathway and NLRP3 inflammasome. Life Sci. 2018;208:26–32. doi: 10.1016/j.lfs.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Qin S.K., Li Q., Ming Xu J., Liang J., Cheng Y., Fan Y., et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: immunodynamic biomarkers and overall survival. Cancer Sci. 2020;111:4218–4231. doi: 10.1111/cas.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong Y.K., Xu C., Kalesh K.A., He Y., Lin Q., Wong W.S.F., et al. Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action. Med Res Rev. 2017;37:1492–1517. doi: 10.1002/med.21446. [DOI] [PubMed] [Google Scholar]
- 46.Abba M.L., Patil N., Leupold J.H., Saeed M.E.M., Efferth T., Allgayer H. Prevention of carcinogenesis and metastasis by artemisinin-type drugs. Cancer Lett. 2018;429:11–18. doi: 10.1016/j.canlet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Hou J., Wang D., Zhang R., Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14:5519–5530. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X., Ba Q., Gu Z., Guo D., Zhou Y., Xu Y., et al. Fluorescent coumarin–artemisinin conjugates as mitochondria-targeting theranostic probes for enhanced anticancer activities. Chemistry. 2015;21:17415–17421. doi: 10.1002/chem.201502543. [DOI] [PubMed] [Google Scholar]
- 49.Chen T., Li M., Zhang R., Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med. 2009;13:1358–1370. doi: 10.1111/j.1582-4934.2008.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ba Q., Zhou N., Duan J., Chen T., Hao M., Yang X., et al. Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., Ba Q., Liu Y., Yue Q., Chen P., Li J., et al. Dihydroartemisinin selectively inhibits PDGFRα-positive ovarian cancer growth and metastasis through inducing degradation of PDGFRα protein. Cell Discov. 2017;3 doi: 10.1038/celldisc.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W., Dong Y., Tu Y., Lin Z. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunopharm. 2006;6:1243–1250. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Casteels T., Frogne T., Ingvorsen C., Honoré C., Courtney M., et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell. 2017;168:86–100. doi: 10.1016/j.cell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Y., Du H., Liu X., Fu X., Li X., Cao Q. Artemisinin alleviates atherosclerotic lesion by reducing macrophage inflammation via regulation of AMPK/NF-κB/NLRP3 inflammasomes pathway. J Drug Target. 2020;28:70–79. doi: 10.1080/1061186X.2019.1616296. [DOI] [PubMed] [Google Scholar]
- 55.Dolivo D., Weathers P., Dominko T. Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics. Acta Pharm Sin B. 2021;11:322–339. doi: 10.1016/j.apsb.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Titov D.V., Gilman B., He Q.L., Bhat S., Low W.K., Dang Y., et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7:182–188. doi: 10.1038/nchembio.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y., Zhang Y., Li L., Feng X., Ding S., Zheng W., et al. TAB1: a target of triptolide in macrophages. Chem Biol. 2014;21:246–256. doi: 10.1016/j.chembiol.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 58.Yu D., Liu Y., Zhou Y., Ruiz–Rodado V., Larion M., Xu G., et al. Triptolide suppresses IDH1-mutated malignancy via Nrf 2-driven glutathione metabolism. Proc Natl Acad Sci U S A. 2020;117:9964–9972. doi: 10.1073/pnas.1913633117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ling D., Xia H., Park W., Hackett M.J., Song C., Na K., et al. pH-Sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS Nano. 2014;8:8027–8039. doi: 10.1021/nn502074x. [DOI] [PubMed] [Google Scholar]
- 60.Datan E., Minn I., Xu P., He Q.L., Ahn H.H., Yu B., et al. A glucose–triptolide conjugate selectively targets cancer cells under hypoxia. iScience. 2020;23 doi: 10.1016/j.isci.2020.101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He J., Peng T., Peng Y., Ai L., Deng Z., Wang X.Q., et al. Molecularly engineering triptolide with aptamers for high specificity and cytotoxicity for triple-negative breast cancer. J Am Chem Soc. 2020;142:2699–2703. doi: 10.1021/jacs.9b10510. [DOI] [PubMed] [Google Scholar]
- 62.Liu J., Lee J., Salazar Hernandez M.A., Mazitschek R., Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng X., Guan D., Auen T., Choi J.W., Salazar Hernández M.A., Lee J., et al. IL1R1 is required for celastrol's leptin-sensitization and antiobesity effects. Nat Med. 2019;25:575–582. doi: 10.1038/s41591-019-0358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu M., Luo Q., Alitongbieke G., Chong S., Xu C., Xie L., et al. Celastrol-induced Nur 77 Interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol Cell. 2017;66:141–153. doi: 10.1016/j.molcel.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng S.Z., Chen X.H., Chen S.J., Zhang J., Wang C.Y., Liu W.R., et al. Phase separation of Nur 77 mediates celastrol-induced mitophagy by promoting the liquidity of p62/SQSTM1 condensates. Nat Commun. 2021;12:5989. doi: 10.1038/s41467-021-26295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma X., Xu L., Alberobello A.T., Gavrilova O., Bagattin A., Skarulis M., et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1–PGC1α transcriptional axis. Cell Metabol. 2015;22:695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Hua H., Zhang Y., Zhao F., Chen K., Wu T., Liu Q., et al. Celastrol inhibits intestinal lipid absorption by reprofiling the gut microbiota to attenuate high-fat diet-induced obesity. iScience. 2021;24 doi: 10.1016/j.isci.2021.102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He Z., Lieu L., Dong Y., Afrin S., Chau D., Kabahizi A., et al. PERK in POMC neurons connects celastrol with metabolism. JCI Insight. 2021;6 doi: 10.1172/jci.insight.145306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y., Wan N., Shan X., Deng G., Xu Q., Ye H., et al. Celastrol targets adenylyl cyclase-associated protein 1 to reduce macrophages-mediated inflammation and ameliorates high fat diet-induced metabolic syndrome in mice. Acta Pharm Sin B. 2021;11:1200–1212. doi: 10.1016/j.apsb.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye S., Luo W., Khan Z.A., Wu G., Xuan L., Shan P., et al. Celastrol attenuates angiotensin II-induced cardiac remodeling by targeting STAT3. Circ Res. 2020;126:1007–1023. doi: 10.1161/CIRCRESAHA.119.315861. [DOI] [PubMed] [Google Scholar]
- 71.Chen X., Zhao Y., Luo W., Chen S., Lin F., Zhang X., et al. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics. 2020;10:10290–10308. doi: 10.7150/thno.46728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo P., Liu D., Zhang Q., Yang F., Wong Y.K., Xia F., et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm Sin B. 2022;12:2300–2314. doi: 10.1016/j.apsb.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu K., Feng C., Shao L., Mei L., Cao R. Tanshinone IIA exhibits anti-inflammatory and antioxidative effects in LPS-stimulated bovine endometrial epithelial cells by activating the Nrf 2 signaling pathway. Res Vet Sci. 2021;136:220–226. doi: 10.1016/j.rvsc.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Lu T.C., Wu Y.H., Chen W.Y., Hung Y.C. Targeting oxidative stress and endothelial dysfunction using tanshinone IIA for the treatment of tissue inflammation and fibrosis. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/2811789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robertson A.L., Holmes G.R., Bojarczuk A.N., Burgon J., Loynes C.A., Chimen M., et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med. 2014;6:225ra29. doi: 10.1126/scitranslmed.3007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin K., Gao S., Yang P., Guo R., Li D., Zhang Y., et al. Single-cell RNA sequencing reveals the temporal diversity and dynamics of cardiac immunity after myocardial infarction. Small Methods. 2022;6 doi: 10.1002/smtd.202100752. [DOI] [PubMed] [Google Scholar]
- 77.Cai N., Chen J., Bi D., Gu L., Yao L., Li X., et al. Specific degradation of endogenous tau protein and inhibition of tau fibrillation by tanshinone IIA through the ubiquitin–proteasome pathway. J Agric Food Chem. 2020;68:2054–2062. doi: 10.1021/acs.jafc.9b07022. [DOI] [PubMed] [Google Scholar]
- 78.Ding B., Lin C., Liu Q., He Y., Ruganzu J.B., Jin H., et al. Tanshinone IIA attenuates neuroinflammation via inhibiting RAGE/NF-κB signaling pathway in vivo and in vitro. J Neuroinflammation. 2020;17:302. doi: 10.1186/s12974-020-01981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji K., Zhao Y., Yu T., Wang Z., Gong H., Yang X., et al. Inhibition effects of tanshinone on the aggregation of α-synuclein. Food Funct. 2016;7:409–416. doi: 10.1039/c5fo00664c. [DOI] [PubMed] [Google Scholar]
- 80.Wang S., Jing H., Yang H., Liu Z., Guo H., Chai L., et al. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson's disease. J Ethnopharmacol. 2015;164:247–255. doi: 10.1016/j.jep.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 81.Fang Z.Y., Zhang M., Liu J.N., Zhao X., Zhang Y.Q., Fang L. Tanshinone IIA: a review of its anticancer effects. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.611087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J., Jiang Y.Y., Chen H., Wu Y.C., Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53 doi: 10.1111/cpr.12739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Gao J., Peng S., Shan X., Deng G., Shen L., Sun J., et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019;10:957. doi: 10.1038/s41419-019-2195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng S., Hang N., Liu W., Guo W., Jiang C., Yang X., et al. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharm Sin B. 2016;6:205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo W., Sun Y., Liu W., Wu X., Guo L., Cai P., et al. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy. 2014;10:972–985. doi: 10.4161/auto.28374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo W., Liu W., Chen G., Hong S., Qian C., Xie N., et al. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int Immunopharm. 2012;14:613–619. doi: 10.1016/j.intimp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 87.Wang W., Guo W., Li L., Fu Z., Liu W., Gao J., et al. Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem Pharmacol. 2016;121:8–17. doi: 10.1016/j.bcp.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 88.Geng J., Liu W., Gao J., Jiang C., Fan T., Sun Y., et al. Andrographolide alleviates Parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1. Br J Pharmacol. 2019;176:4574–4591. doi: 10.1111/bph.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geng J., Liu W., Xiong Y., Ding H., Jiang C., Yang X., et al. Andrographolide sulfonate improves Alzheimer-associated phenotypes and mitochondrial dysfunction in APP/PS1 transgenic mice. Biomed Pharmacother. 2018;97:1032–1039. doi: 10.1016/j.biopha.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 90.Shao F., Tan T., Tan Y., Sun Y., Wu X., Xu Q. Andrographolide alleviates imiquimod-induced psoriasis in mice via inducing autophagic proteolysis of MyD88. Biochem Pharmacol. 2016;115:94–103. doi: 10.1016/j.bcp.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Qu J., Liu Q., You G., Ye L., Jin Y., Kong L., et al. Advances in ameliorating inflammatory diseases and cancers by andrographolide: pharmacokinetics, pharmacodynamics, and perspective. Med Res Rev. 2022;42:1147–1178. doi: 10.1002/med.21873. [DOI] [PubMed] [Google Scholar]
- 92.Song Y., Li D., Lu Y., Jiang K., Yang Y., Xu Y., et al. Ferrimagnetic mPEG-b-PHEP copolymer micelles loaded with iron oxide nanocubes and emodin for enhanced magnetic hyperthermia-chemotherapy. Natl Sci Rev. 2020;7:723–736. doi: 10.1093/nsr/nwz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng Y., Huang S., Dou W., Zhang S., Chen J., Shen Y., et al. Emodin, a natural product, selectively inhibits 11 beta-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Br J Pharmacol. 2010;161:113–126. doi: 10.1111/j.1476-5381.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y.J., Huang S.L., Feng Y., Ning M.M., Leng Y. Emodin, an 11β-hydroxysteroid dehydrogenase type 1 inhibitor, regulates adipocyte function in vitro and exerts anti-diabetic effect in ob/ob mice. Acta Pharmacol Sin. 2012;33:1195–1203. doi: 10.1038/aps.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pu X., Ye N., Lin M., Chen Q., Dong L., Xu H., et al. β-1,3-d-Glucan based yeast cell wall system loaded emodin with dual-targeting layers for ulcerative colitis treatment. Carbohydr Polym. 2021;273 doi: 10.1016/j.carbpol.2021.118612. [DOI] [PubMed] [Google Scholar]
- 96.Xiao D., Zhang Y., Wang R., Fu Y., Zhou T., Diao H., et al. Emodin alleviates cardiac fibrosis by suppressing activation of cardiac fibroblasts via upregulating metastasis associated protein 3. Acta Pharm Sin B. 2019;9:724–733. doi: 10.1016/j.apsb.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie C., Zhuang X.X., Niu Z., Ai R., Lautrup S., Zheng S., et al. Amelioration of Alzheimer's disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nat Biomed Eng. 2022;6:76–93. doi: 10.1038/s41551-021-00819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chin K.Y. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Dev Ther. 2016;10:3029–3042. doi: 10.2147/DDDT.S117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Tang Q., Duan P., Yang L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol Immunotoxicol. 2018;40:476–482. doi: 10.1080/08923973.2018.1469145. [DOI] [PubMed] [Google Scholar]
- 100.Li X., Zhu R., Jiang H., Yin Q., Gu J., Chen J., et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB–P300–BRD4 axis. Acta Pharm Sin B. 2022;12:2280–2299. doi: 10.1016/j.apsb.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zia A., Farkhondeh T., Pourbagher-Shahri A.M., Samarghandian S. The role of curcumin in aging and senescence: molecular mechanisms. Biomed Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111119. [DOI] [PubMed] [Google Scholar]
- 102.Shen L., Ji H.F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit Rev Food Sci Nutr. 2019;59:2896–2902. doi: 10.1080/10408398.2018.1478388. [DOI] [PubMed] [Google Scholar]
- 103.Banerjee S., Ji C., Mayfield J.E., Goel A., Xiao J., Dixon J.E., et al. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc Natl Acad Sci U S A. 2018;115:8155–8160. doi: 10.1073/pnas.1806797115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramakrishnan P., Loh W.M., Gopinath S.C.B., Bonam S.R., Fareez I.M., Mac Guad R., et al. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm Sin B. 2020;10:399–413. doi: 10.1016/j.apsb.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bao C., Liu B., Li B., Chai J., Zhang L., Jiao L., et al. Enhanced transport of shape and rigidity-tuned α-lactalbumin nanotubes across intestinal mucus and cellular barriers. Nano Lett. 2020;20:1352–1361. doi: 10.1021/acs.nanolett.9b04841. [DOI] [PubMed] [Google Scholar]
- 106.Dai Z., Tang J., Gu Z., Wang Y., Yang Y., Yang Y., et al. Eliciting immunogenic cell death via a unitized nanoinducer. Nano Lett. 2020;20:6246–6254. doi: 10.1021/acs.nanolett.0c00713. [DOI] [PubMed] [Google Scholar]
- 107.Tian J., Geiss C., Zarse K., Madreiter-Sokolowski C.T., Ristow M. Green tea catechins EGCG and ECG enhance the fitness and lifespan of Caenorhabditis elegans by complex I inhibition. Aging. 2021;13:22629–22648. doi: 10.18632/aging.203597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei Q., Ma L., Zhang W., Ma G., Hu Z. EGCG-crosslinked carboxymethyl chitosan-based hydrogels with inherent desired functions for full-thickness skin wound healing. J Mater Chem B. 2022;10:3927–3935. doi: 10.1039/d2tb00074a. [DOI] [PubMed] [Google Scholar]
- 109.Zhang S., Zhu Q., Chen J.Y., OuYang D., Lu J.H. The pharmacological activity of epigallocatechin-3-gallate (EGCG) on Alzheimer's disease animal model: a systematic review. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153316. [DOI] [PubMed] [Google Scholar]
- 110.Singh N.A., Bhardwaj V., Ravi C., Ramesh N., Mandal A.K.A., Khan Z.A. EGCG nanoparticles attenuate aluminum chloride induced neurobehavioral deficits, beta amyloid and tau pathology in a rat model of Alzheimer's disease. Front Aging Neurosci. 2018;10:244. doi: 10.3389/fnagi.2018.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu Z., Huang S., Li T., Li N., Han D., Zhang B., et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9:184. doi: 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fechtner S., Singh A., Chourasia M., Ahmed S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol Appl Pharmacol. 2017;329:112–120. doi: 10.1016/j.taap.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Z.S., Cai H., Xue W., Wang M., Xia T., Li W.J., et al. G3BP1 promotes DNA binding and activation of cGAS. Nat Immunol. 2019;20:18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Q.Y., Zhang L., Yee J.K., Go V.L.W., Lee W.N. Metabolic consequences of LDHA inhibition by epigallocatechin gallate and oxamate in MIA PaCa-2 pancreatic cancer cells. Metabolomics. 2015;11:71–80. doi: 10.1007/s11306-014-0672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei H., Ge Q., Zhang L.Y., Xie J., Gan R.H., Lu Y.G., et al. EGCG inhibits growth of tumoral lesions on lip and tongue of K-Ras transgenic mice through the Notch pathway. J Nutr Biochem. 2022;99 doi: 10.1016/j.jnutbio.2021.108843. [DOI] [PubMed] [Google Scholar]
- 116.Chung J.E., Tan S., Gao S.J., Yongvongsoontorn N., Kim S.H., Lee J.H., et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol. 2014;9:907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J., Blayney A., Liu X., Gandy L., Jin W., Yan L., et al. EGCG binds intrinsically disordered N-terminal domain of p53 and disrupts p53–MDM2 interaction. Nat Commun. 2021;12:986. doi: 10.1038/s41467-021-21258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J., Zhou F., Wu X., Zhang X., Chen Y., Zha B.S., et al. Cellular pharmacokinetic mechanisms of adriamycin resistance and its modulation by 20(S)-ginsenoside Rh2 in MCF-7/Adr cells. Br J Pharmacol. 2012;165:120–134. doi: 10.1111/j.1476-5381.2011.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang L., Zhang X.Y., Li K., Li A.P., Yang W.D., Yang R., et al. Protopanaxadiol inhibits epithelial–mesenchymal transition of hepatocellular carcinoma by targeting STAT3 pathway. Cell Death Dis. 2019;10:630. doi: 10.1038/s41419-019-1733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang J., Liu D., Wang Y., Liu L., Li J., Yuan J., et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71:734–745. doi: 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quan L.H., Zhang C., Dong M., Jiang J., Xu H., Yan C., et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut. 2020;69:1239–1247. doi: 10.1136/gutjnl-2019-319114. [DOI] [PubMed] [Google Scholar]
- 122.Huang M., Lu J.J., Ding J. Natural products in cancer therapy: past, present and future. Nat Prod Bioprospect. 2021;11:5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hesari A., Ghasemi F., Cicero A.F.G., Mohajeri M., Rezaei O., Hayat S.M.G., et al. Berberine: a potential adjunct for the treatment of gastrointestinal cancers?. J Cell Biochem. 2018;119:9655–9663. doi: 10.1002/jcb.27392. [DOI] [PubMed] [Google Scholar]
- 124.Li K., Yao W., Zheng X., Liao K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009;19:1006–1017. doi: 10.1038/cr.2009.76. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Gu Y., Ren H., Wang S., Zhong H., Zhao X., et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study) Nat Commun. 2020;11:5015. doi: 10.1038/s41467-020-18414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu Y., Xie N., Chai Y., Nie Y., Liu K., Liu Y., et al. Apoptosis induction, a sharp edge of berberine to exert anti-cancer effects, focus on breast, lung, and liver cancer. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.803717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y., Hua W., Li Y., Xian X., Zhao Z., Liu C., et al. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem Pharmacol. 2020;174 doi: 10.1016/j.bcp.2019.113776. [DOI] [PubMed] [Google Scholar]
- 128.Tang K., Zhang X., Guo Y. Identification of the dietary supplement capsaicin as an inhibitor of Lassa virus entry. Acta Pharm Sin B. 2020;10:789–798. doi: 10.1016/j.apsb.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Panossian L.A., Garga N.I., Pelletier D. Toxic brainstem encephalopathy after artemisinin treatment for breast cancer. Ann Neurol. 2005;58:812–813. doi: 10.1002/ana.20620. [DOI] [PubMed] [Google Scholar]
- 130.Fan D., Guo Q., Shen J., Zheng K., Lu C., Zhang G., et al. The effect of triptolide in rheumatoid arthritis: from basic research towards clinical translation. Int J Mol Sci. 2018;19:E376. doi: 10.3390/ijms19020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng Y., Kong F., Liu S., Liu X., Pei D., Miao X. Membrane protein-chimeric liposome-mediated delivery of triptolide for targeted hepatocellular carcinoma therapy. Drug Deliv. 2021;28:2033–2043. doi: 10.1080/10717544.2021.1983072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.An L., Li Z., Shi L., Wang L., Wang Y., Jin L., et al. Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-κB and Notch 1 pathways. Nano Lett. 2020;20:7728–7736. doi: 10.1021/acs.nanolett.0c03279. [DOI] [PubMed] [Google Scholar]
- 133.Xi L., Lin Z., Qiu F., Chen S., Li P., Chen X., et al. Enhanced uptake and anti-maturation effect of celastrol-loaded mannosylated liposomes on dendritic cells for psoriasis treatment. Acta Pharm Sin B. 2022;12:339–352. doi: 10.1016/j.apsb.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liang J., He X., Zhou H., Liang P. Effects of Danhong injection on cardiac function and blood lipid in patients with angina pectoris of coronary heart disease: a protocol for randomized, double-blind, placebo-controlled clinical trial. Medicine (Baltim) 2021;100 doi: 10.1097/MD.0000000000027479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu C., He L., Wang J., Wang Q., Sun C., Li Y., et al. Anti-angiogenic effect of shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol. 2020;260 doi: 10.1016/j.jep.2020.113039. [DOI] [PubMed] [Google Scholar]
- 136.Mu Z., Guo J., Zhang D., Xu Y., Zhou M., Guo Y., et al. Therapeutic effects of shikonin on skin diseases: a review. Am J Chin Med. 2021;49:1871–1895. doi: 10.1142/S0192415X21500889. [DOI] [PubMed] [Google Scholar]
- 137.Wang X., Zhang F., Wu X.R. Inhibition of pyruvate kinase M2 markedly reduces chemoresistance of advanced bladder cancer to cisplatin. Sci Rep. 2017;7 doi: 10.1038/srep45983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.James A.D., Richardson D.A., Oh I.W., Sritangos P., Attard T., Barrett L., et al. Cutting off the fuel supply to calcium pumps in pancreatic cancer cells: role of pyruvate kinase-M2 (PKM2) Br J Cancer. 2020;122:266–278. doi: 10.1038/s41416-019-0675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang H., Wang D., Li M., Plecitá-Hlavatá L., D'Alessandro A., Tauber J., et al. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation. 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ning X., Wiraja C., Chew W.T.S., Fan C., Xu C. Transdermal delivery of Chinese herbal medicine extract using dissolvable microneedles for hypertrophic scar treatment. Acta Pharm Sin B. 2021;11:2937–2944. doi: 10.1016/j.apsb.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li G., Zhao M., Zhao L. Lysine-mediated hydroxyethyl starch-10-hydroxy camptothecin micelles for the treatment of liver cancer. Drug Deliv. 2020;27:519–529. doi: 10.1080/10717544.2020.1745329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun M., Jiang H., Liu T., Tan X., Jiang Q., Sun B., et al. Structurally defined tandem-responsive nanoassemblies composed of dipeptide-based photosensitive derivatives and hypoxia-activated camptothecin prodrugs against primary and metastatic breast tumors. Acta Pharm Sin B. 2022;12:952–966. doi: 10.1016/j.apsb.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kotoh S., Naito S., Yokomizo A., Kumazawa J., Asakuno K., Kohno K., et al. Increased expression of DNA topoisomerase I gene and collateral sensitivity to camptothecin in human cisplatin-resistant bladder cancer cells. Cancer Res. 1994;54:3248–3252. [PubMed] [Google Scholar]
- 144.Chan J.K., Brady M.F., Penson R.T., Huang H., Birrer M.J., Walker J.L., et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016;374:738–748. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lou X., Zhang D., Ling H., He Z., Sun J., Sun M., et al. Pure redox-sensitive paclitaxel–maleimide prodrug nanoparticles: endogenous albumin-induced size switching and improved antitumor efficiency. Acta Pharm Sin B. 2021;11:2048–2058. doi: 10.1016/j.apsb.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Quoix E., Zalcman G., Oster J.P., Westeel V., Pichon E., Lavolé A., et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 147.Wei W.L., Hou J.J., Wang X., Yu Y., Li H.J., Li Z.W., et al. Venenum bufonis: an overview of its traditional use, natural product chemistry, pharmacology, pharmacokinetics and toxicology. J Ethnopharmacol. 2019;237:215–235. doi: 10.1016/j.jep.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 148.Yu Z., Guo W., Ma X., Zhang B., Dong P., Huang L., et al. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells. Mol Cancer. 2014;13:203. doi: 10.1186/1476-4598-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xiang R.F., Wang Y., Zhang N., Xu W.B., Cao Y., Tong J., et al. MK2206 enhances the cytocidal effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR pathway. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cao Y., Yu L., Dai G., Zhang S., Zhang Z., Gao T., et al. Cinobufagin induces apoptosis of osteosarcoma cells through inactivation of Notch signaling. Eur J Pharmacol. 2017;794:77–84. doi: 10.1016/j.ejphar.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 151.Xie C.M., Liu X.Y., Yu S., Cheng C.H.K. Cardiac glycosides block cancer growth through HIF-1α- and NF-κB-mediated Plk1. Carcinogenesis. 2013;34:1870–1880. doi: 10.1093/carcin/bgt136. [DOI] [PubMed] [Google Scholar]
- 152.Yin J.Q., Wen L., Wu L.C., Gao Z.H., Huang G., Wang J., et al. The glycogen synthase kinase-3β/nuclear factor-kappa B pathway is involved in cinobufagin-induced apoptosis in cultured osteosarcoma cells. Toxicol Lett. 2013;218:129–136. doi: 10.1016/j.toxlet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 153.Li C., Hashimi S.M., Cao S., Qi J., Good D., Duan W., et al. Chansu inhibits the expression of cortactin in colon cancer cell lines in vitro and in vivo. BMC Compl Alternative Med. 2015;15:207. doi: 10.1186/s12906-015-0723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Han Q., Ma Y., Wang H., Dai Y., Chen C., Liu Y., et al. Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. J Transl Med. 2018;16:201. doi: 10.1186/s12967-018-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yuan Z., Shi X., Qiu Y., Jia T., Yuan X., Zou Y., et al. Reversal of P-gp-mediated multidrug resistance in colon cancer by cinobufagin. Oncol Rep. 2017;37:1815–1825. doi: 10.3892/or.2017.5410. [DOI] [PubMed] [Google Scholar]
- 156.Laursen M., Gregersen J.L., Yatime L., Nissen P., Fedosova N.U. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc Natl Acad Sci U S A. 2015;112:1755–1760. doi: 10.1073/pnas.1422997112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Menger L., Vacchelli E., Kepp O., Eggermont A., Tartour E., Zitvogel L., et al. Trial watch: cardiac glycosides and cancer therapy. OncoImmunology. 2013;2 doi: 10.4161/onci.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gu C., Wang Y., Zhang L., Qiao L., Sun S., Shao M., et al. AHSA1 is a promising therapeutic target for cellular proliferation and proteasome inhibitor resistance in multiple myeloma. J Exp Clin Cancer Res. 2022;41:11. doi: 10.1186/s13046-021-02220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang H., Liu Y., Zhao M.M., Guo Q., Zheng X.K., Liu D., et al. Therapeutic potential of targeting membrane-spanning proteoglycan SDC4 in hepatocellular carcinoma. Cell Death Dis. 2021;12:492. doi: 10.1038/s41419-021-03780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wen L., Huang Y., Xie X., Huang W., Yin J., Lin W., et al. Anti-inflammatory and antinociceptive activities of bufalin in rodents. Mediat Inflamm. 2014;2014 doi: 10.1155/2014/171839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu S.C., Yi P.F., Guo X., Zhang L.Y., Xu D.X., Fu Y.X., et al. Cinobufagin enhances the protective efficacy of formalin-inactivated Salmonella typhimurium vaccine through Th1 immune response. Microb Pathog. 2016;99:264–270. doi: 10.1016/j.micpath.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 162.Hu Y., Chen J., Hu G., Yu J., Zhu X., Lin Y., et al. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar Drugs. 2015;13:202–221. doi: 10.3390/md13010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sagar S., Kaur M., Minneman K.P. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619–2638. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fiume L., Cerenzia M.R., Bonino F., Busi C., Mattioli A., Brunetto M.R., et al. Inhibition of hepatitis B virus replication by vidarabine monophosphate conjugated with lactosaminated serum albumin. Lancet. 1988;2:13–15. doi: 10.1016/s0140-6736(88)92946-7. [DOI] [PubMed] [Google Scholar]
- 165.Salehi B., Selamoglu Z., Mileski K S., Pezzani R., Redaelli M., Cho W.C., et al. Liposomal cytarabine as cancer therapy: from chemistry to medicine. Biomolecules. 2019;9:E773. doi: 10.3390/biom9120773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kremer W.B. Drugs five years later: cytarabine. Ann Intern Med. 1975;82:684–688. doi: 10.7326/0003-4819-82-5-684. [DOI] [PubMed] [Google Scholar]
- 167.Jiménez C. Marine natural products in medicinal chemistry. ACS Med Chem Lett. 2018;9:959–961. doi: 10.1021/acsmedchemlett.8b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pope J.E., Deer T.R., Amirdelfan K., McRoberts W.P., Azeem N. The pharmacology of spinal opioids and ziconotide for the treatment of non-cancer pain. Curr Neuropharmacol. 2017;15:206–216. doi: 10.2174/1570159X14666160210142339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dybdal–Hargreaves N.F., Risinger A.L., Mooberry S.L. Eribulin mesylate: mechanism of action of a unique microtubule-targeting agent. Clin Cancer Res. 2015;21:2445–2452. doi: 10.1158/1078-0432.CCR-14-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gajula P.K., Asthana J., Panda D., Chakraborty T.K. A synthetic dolastatin 10 analogue suppresses microtubule dynamics, inhibits cell proliferation, and induces apoptotic cell death. J Med Chem. 2013;56:2235–2245. doi: 10.1021/jm3009629. [DOI] [PubMed] [Google Scholar]
- 172.Barreca M., Stathis A., Barraja P., Bertoni F. An overview on anti-tubulin agents for the treatment of lymphoma patients. Pharmacol Ther. 2020;211 doi: 10.1016/j.pharmthera.2020.107552. [DOI] [PubMed] [Google Scholar]
- 173.Singh S.B. Discovery and development of dolastatin 10-derived dntibody drug conjugate anticancer drugs. J Nat Prod. 2022;85:666–687. doi: 10.1021/acs.jnatprod.1c01135. [DOI] [PubMed] [Google Scholar]
- 174.Gao G., Wang Y., Hua H., Li D., Tang C. Marine antitumor peptide dolastatin 10: biological activity, structural modification and synthetic chemistry. Mar Drugs. 2021;19:363. doi: 10.3390/md19070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Younes A., Yasothan U., Kirkpatrick P. Brentuximab vedotin. Nat Rev Drug Discov. 2012;11:19–20. doi: 10.1038/nrd3629. [DOI] [PubMed] [Google Scholar]
- 176.van de Donk N.W.C.J., Dhimolea E. Brentuximab vedotin. mAbs. 2012;4:458–465. doi: 10.4161/mabs.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Silber J., Kramer A., Labes A., Tasdemir D. From discovery to production: biotechnology of marine fungi for the production of new antibiotics. Mar Drugs. 2016;14:E137. doi: 10.3390/md14070137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abraham E.P., Newton G.G.F., Crawford K., Burton H.S., Hale C.W., Cephalosporin N. A new type of penicillin. Nature. 1953;171:343. doi: 10.1038/171343a0. [DOI] [PubMed] [Google Scholar]
- 179.Djuricic I., Calder P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. 2021;13:2421. doi: 10.3390/nu13072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Y.F., Yan J.W., Wu Q., Shi J.Z., Liu J., Shi J.S. Realgar- and cinnabar-containing an-gong-niu-huang wan (AGNH) is much less acutely toxic than sodium arsenite and mercuric chloride. Chem Biol Interact. 2011;189:134–140. doi: 10.1016/j.cbi.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 181.Zhu H.H., Wu D.P., Jin J., Li J.Y., Ma J., Wang J.X., et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31:4215–4221. doi: 10.1200/JCO.2013.48.8312. [DOI] [PubMed] [Google Scholar]
- 182.Wu J., Shao Y., Liu J., Chen G., Ho P.C. The medicinal use of realgar (As₄S₄) and its recent development as an anticancer agent. J Ethnopharmacol. 2011;135:595–602. doi: 10.1016/j.jep.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 183.Zhang L., Yang X.M., Chen J., Hu L., Yang F., Zhou Y., et al. Population pharmacokinetics and safety of oral tetra-arsenic tetra-sulfide formula in pediatric acute promyelocytic leukemia. Drug Des Dev Ther. 2021;15:1633–1640. doi: 10.2147/DDDT.S305244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang M.H., Wan W.Q., Luo J.S., Zheng M.C., Huang K., Yang L.H., et al. Multicenter randomized trial of arsenic trioxide and Realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: interim results of the SCCLG-APL clinical study. Am J Hematol. 2018;93:1467–1473. doi: 10.1002/ajh.25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu H.H., Hu J., Lo-Coco F., Jin J. The simpler, the better: oral arsenic for acute promyelocytic leukemia. Blood. 2019;134:597–605. doi: 10.1182/blood.2019000760. [DOI] [PubMed] [Google Scholar]
- 186.Wang G., Zhang T., Sun W., Wang H., Yin F., Wang Z., et al. Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma. Free Radic Biol Med. 2017;106:24–37. doi: 10.1016/j.freeradbiomed.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 187.Yang F.R., Zhao Y.F., Hu X.W., Liu Z.K., Yu X.D., Li C.Y., et al. Nano-realgar suppresses lung cancer stem cell growth by repressing metabolic reprogramming. Gene. 2021;788 doi: 10.1016/j.gene.2021.145666. [DOI] [PubMed] [Google Scholar]
- 188.Wang S., Liu X., Wang S., Ouyang L., Li H., Ding J., et al. Imatinib co-loaded targeted realgar nanocrystal for synergistic therapy of chronic myeloid leukemia. J Control Release. 2021;338:190–200. doi: 10.1016/j.jconrel.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 189.Wang J., Ding L., Zhou J., Ma H., Wu Y., Wang J., et al. Target lipidomics approach to reveal the resolution of inflammation induced by Chinese medicine combination in Liu-Shen-Wan against realgar overexposure to rats. J Ethnopharmacol. 2020;249 doi: 10.1016/j.jep.2019.112171. [DOI] [PubMed] [Google Scholar]
- 190.Wang J., Xu D., Ni Z., Yu C., Wang J., Wu Q., et al. Analyzing liver protein-bound DMAV by using size exclusion and ion exchange HPLC combined with ICP-MS and MRM mode in rats exposed to AS4S4. Talanta. 2021;234 doi: 10.1016/j.talanta.2021.122714. [DOI] [PubMed] [Google Scholar]
- 191.Zhou J., Ma H., Wu Y., Lv X., Wang J., Liu S., et al. Lipidomic profiling of subchronic As4S4 exposure identifies inflammatory mediators as sensitive biomarkers in rats. Metallomics. 2019;11:576–585. doi: 10.1039/c8mt00181b. [DOI] [PubMed] [Google Scholar]
- 192.Hu J., Hu W.X. Targeting signaling pathways in multiple myeloma: pathogenesis and implication for treatments. Cancer Lett. 2018;414:214–221. doi: 10.1016/j.canlet.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 193.Kist M., Vucic D. Cell death pathways: intricate connections and disease implications. EMBO J. 2021;40 doi: 10.15252/embj.2020106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 195.Peng Q.L., Huang M. Effects of compound Salvia miltiorrhiza injection on coagulation and renal function in preeclampsia rats. Modern Medical Health. 2021;37:3989–3992. [Google Scholar]
- 196.Liu P., Yang S., Wang Z., Dai H., Wang C. Feasibility and mechanism analysis of Shenfu injection in the treatment of idiopathic pulmonary fibrosis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.670146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhong C., Jiang C., Ni S., Wang Q., Cheng L., Wang H., et al. Identification of bioactive anti-angiogenic components targeting tumor endothelial cells in Shenmai injection using multidimensional pharmacokinetics. Acta Pharm Sin B. 2020;10:1694–1708. doi: 10.1016/j.apsb.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sun C.Q., Xie Y.M., Hou H.Y. Clinical characteristics and drug combination of Pulse-activating injection in treatment of coronary heart disease angina pectoris. World Chinese Medicine. 2022;17:123–128. [Google Scholar]
- 199.Zhang H., Chen Z., Zhong Z., Gong W., Li J. Total saponins from the leaves of Panax notoginseng inhibit depression on mouse chronic unpredictable mild stress model by regulating circRNA expression. Brain Behav. 2018;8 doi: 10.1002/brb3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu L., Gao Y., Zhang S., Fang Z. The effects of Breviscapine injection on hypertension in hypertension-induced renal damage patients: a systematic review and a meta-analysis. Front Pharmacol. 2019;10:118. doi: 10.3389/fphar.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu Y., Hua G., Xi Y. Clinical study of erviscapus injection combined with uricline in the treatment of acute cerebral infarction. Modern Medicine and Clinical. 2021;36:901–905. [Google Scholar]
- 202.Chen A., Ding S., Kong L., Xu J., He F., Ru C., et al. Safflower injection inhibits pulmonary arterial remodeling in a monocrotaline-induced pulmonary arterial hypertension rat model. Z Naturforsch, C: J Biosci. 2021;76:27–34. doi: 10.1515/znc-2020-0004. [DOI] [PubMed] [Google Scholar]
- 203.Wang L.T., Huang H., Chang Y.H., Wang Y.Q., Wang J.D., Cai Z.H., et al. Biflavonoids from Ginkgo biloba leaves as a novel anti-atherosclerotic candidate: inhibition potency and mechanistic analysis. Phytomedicine. 2022;102 doi: 10.1016/j.phymed.2022.154053. [DOI] [PubMed] [Google Scholar]
- 204.Yu D.D., Xie Y.M., Zhang Y.L., Liao X., Zhi Y.J., Zhao H. Bitter dish injection in treatment of acute cerebral infarction is effective and safety studies: systematic review of randomized controlled trials and meta-analysis. China J Tradit Chin Med. 2019;44:372–380. doi: 10.19540/j.cnki.cjcmm.2019.0008. [DOI] [PubMed] [Google Scholar]
- 205.Sun Z.Y., Wang F.J., Guo H., Chen L., Chai L.J., Li R.L., et al. Shuxuetong injection protects cerebral microvascular endothelial cells against oxygen–glucose deprivation reperfusion. Neural Regen Res. 2019;14:783–793. doi: 10.4103/1673-5374.249226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang W., Shi Z., Yang H.Q., Teng J., Zhao J., Xiang G. Mailuoning for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;1:CD007028. doi: 10.1002/14651858.CD007028.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xu M., Su W., Xu Q., Huang W. Effect of Xingnaojing injection on cerebral edema and blood–brain barrier in rats following traumatic brain injury. Chin J Traumatol. 2010;13:158–162. [PubMed] [Google Scholar]
- 208.Zheng D., Chu Y., Li S., Zhou S., Li W., Xie Y., et al. Enhancing effect of borneol on pharmacokinetics of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 in healthy volunteers after oral administration of compound Danshen dropping pills. Biomed Chromatogr. 2022;36 doi: 10.1002/bmc.5311. [DOI] [PubMed] [Google Scholar]
- 209.Ma J., Yin C., Ma S., Qiu H., Zheng C., Chen Q., et al. Shensong Yangxin capsule reduces atrial fibrillation susceptibility by inhibiting atrial fibrosis in rats with post-myocardial infarction heart failure. Drug Des Dev Ther. 2018;12:3407–3418. doi: 10.2147/DDDT.S182834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.He Y., Su W., He X., Chen T., Zeng X., Yan Z., et al. Pharmacokinetics and biotransformation investigation in beagle dog of active compounds from naoxintong capsule. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110940. [DOI] [PubMed] [Google Scholar]
- 211.Wang H., Qu S.C., Yu X.F., Zhang H., Sui D.Y. Effects of Xueshuoxinmaining tablet on platelet function and blood rheology in acute blood stasis model rats. Chin J Gerontol. 2010;30:3335–3337. [Google Scholar]
- 212.Fu M.W., Li H.Z., Teng Y.O., Han Y.Z., Zhao X., Chen H.W., Wang J. Meta-analysis of the efficacy and safety of combined use of Yixinshu capsule for ventricular premature contraction. J Vascular Dis. 2022;20:197–206. [Google Scholar]
- 213.Jiang B., Wu R.M., Zhang C., Zhong X.Z., Hong Y., Shen X.Y. Effects of Yangxin's tablets on depressed rats after myocardial infarction. Chin Patent Med. 2020;42:2868–2875. [Google Scholar]
- 214.Liu Q., Zhang Y., Liu K.C., Wang X.X., Zhang C.Q., Zhou H.L., Xia Q. Study on the active components of Xinkeshu tablets to promote angiogenesis based on zebrafish model and molecular docking technology. Chin Herbal Med. 2022;53:1418–1433. [Google Scholar]
- 215.Li C.M., Ding H. Clinical study of Xinyuan capsule in the treatment of thoracic arthralgia, heart–kidney yin deficiency and blood stasis yin syndrome. Chin Med Pharmacol Clin. 2002;18:43–45. [Google Scholar]
- 216.Guo J., Qin Z., He Q., Fong T.L., Lau N.C., Cho W.C.S., et al. Shexiang Baoxin pill for acute myocardial infarction: clinical evidence and molecular mechanism of antioxidative stress. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/7644648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang L., Wang Y., Yu L., Liu L., Qu H., Wang Y., et al. QI-SHEN-YI-QI accelerates angiogenesis after myocardial infarction in rats. Int J Cardiol. 2010;143:105–109. doi: 10.1016/j.ijcard.2008.11.210. [DOI] [PubMed] [Google Scholar]
- 218.Xie X., Meng T., Li Ti, Yang Q., Li M., Gao Y. Net meta-analysis of Panax notoginseng oral preparation in the treatment of acute cerebral infarction. Chin J Tradit Chin Med. 2021;52:4277–4288. [Google Scholar]
- 219.Wu Z., Zhao Z., Chen Y., Huang J., Liu Z., Jiang X., Ke L., Song Y., Wang L. A randomized controlled study of Yindanxin Naotong Soft capsule in treatment of blood stasis and collateral obstruction syndrome in convalescent period of cerebral infarction. Chin J Integr Tradit West Med. 2014;34:1310–1314. [PubMed] [Google Scholar]
- 220.Zhao J.N., Zhang Y., Lan X., Chen Y., Li J., Zhang P., et al. Efficacy and safety of Xinnaoning capsule in treating chronic stable angina (qi stagnation and blood stasis syndrome): study protocol for a multicenter, randomized, double-blind, placebo-controlled trial. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000016539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Duan X., Zhou L., Wu T., Liu G., Qiao J., Wei J., et al. Chinese herbal medicine suxiao jiuxin wan for angina pectoris. Cochrane Database Syst Rev. 2008;2008:CD004473. doi: 10.1002/14651858.CD004473.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang J.H., Yu L.J., Yang H., Hui Z., Jiang S., Chen L., et al. Huatuo Zaizao pill ameliorates cognitive impairment of APP/PS1 transgenic mice by improving synaptic plasticity and reducing Aβ deposition. BMC Compl Alternative Med. 2018;18:167. doi: 10.1186/s12906-018-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jiang Y., Sui D., Li M., Xu H., Yu X., Liu J., et al. Ginsenoside Re improves inflammation and fibrosis in hepatic tissue by uupregulating PPARγ expression and inhibiting oxidative stress in db/db mice. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/9003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang S., Qiu X.J. The efficacy of Xue Fu Zhu Yu prescription for hyperlipidemia: a meta-analysis of randomized controlled trials. Compl Ther Med. 2019;43:218–226. doi: 10.1016/j.ctim.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 225.Yao Y., Liu Y., Zeng Z., Zhao Y., Li T., Chen R., et al. Identification of target genes of antiarrhythmic traditional Chinese medicine Wenxin Keli. Cardiovasc Ther. 2020;2020 doi: 10.1155/2020/3480276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu L., Chen Y.M., Zhou Y.J. The effect and mechanism of Songling Xuemaikang capsule on atherosclerosis in rats. Sci Technol Eng. 2017;17:185–188. [Google Scholar]
- 227.Du Y.J., Zhao Y.T., Kong L.J., Wang Y.M. Protective effect of Hedan tablets on vascular endothelium in atherosclerotic rats. Genom Appl Biol. 2020;39:890–894. [Google Scholar]
- 228.Cheng W., Wang L., Yang T., Wu A., Wang B., Li T., et al. Qiliqiangxin capsules optimize cardiac metabolism flexibility in rats with heart failure after myocardial infarction. Front Physiol. 2020;11:805. doi: 10.3389/fphys.2020.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tan Y., Hu J., Zhang Y., Wu Q., Ni Q. Qizhijiangtang capsule for the treatment of diabetic kidney disease: a protocol for systematic review and meta-analysis. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gao X., Wang Y., Li Y., Wang Y., Yan M., Sun H., et al. Huganpian, a traditional Chinese medicine, inhibits liver cancer growth in vitro and in vivo by inducing autophagy and cell cycle arrest. Biomed Pharmacother. 2019;120 doi: 10.1016/j.biopha.2019.109469. [DOI] [PubMed] [Google Scholar]
- 231.Li H., Tang Y., Wei W., Yin C., Tang F. Effects of Xiaochaihu decoction on the expression of cytochrome P450s in rats. Exp Ther Med. 2021;21:588. doi: 10.3892/etm.2021.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang L., Hu Z., Zhu J., Fei B. Effects of Weifuchun tablet for chronic atrophic gastritis: a protocol for systematic review and meta-analysis. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000020374. [DOI] [PubMed] [Google Scholar]
- 233.Cai G.X., Liu B.Y., Yi J., Chen X.M., Liu F.L. Simotang enhances gastrointestinal motility, motilin and cholecystokinin expression in chronically stressed mice. World J Gastroenterol. 2011;17:1594–1599. doi: 10.3748/wjg.v17.i12.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li X., Feng M., Yuan G. Clinical efficacy of Weisu granule combined with Weifuchun tablet in the treatment of chronic atrophic gastritis and its effect on serum G-17, PG I and PG II levels. Am J Transl Res. 2022;14:275–284. [PMC free article] [PubMed] [Google Scholar]
- 235.Wan J.H., Zhan Y., Yang N., Hu X., Li Y.M., Liu W.J. Long term toxicity of Jianwei Xiaoshi tablet on rats of different sexes. China J Mod Med. 2020;22:25–29. [Google Scholar]
- 236.Sun J., Huang S., Qin Y., Zhang P., Li Z., Zhang L., et al. Anti-allergic actions of a Chinese patent medicine, huoxiangzhengqi oral liquid, in RBL-2H3 cells and in mice. Pharm Biol. 2021;59:672–682. doi: 10.1080/13880209.2021.1928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang W.D., Yao Y.L. Repair effect of Sanjiu Weitai Granule on acute gastric mucosal injury in rats. Chin J Integr Tradit Chin Western Med Digest. 2002;10:148–150. [Google Scholar]
- 238.Chen H., Luo Y., Tsoi B., Gu B., Qi S., Shen J. Angong Niuhuang Wan reduces hemorrhagic transformation and mortality in ischemic stroke rats with delayed thrombolysis: involvement of peroxynitrite-mediated MMP-9 activation. Chin Med. 2022;17:51. doi: 10.1186/s13020-022-00595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guo J., Xiong X., Cao Y., Ding Y., He Q. Safety and clinical efficacy of Yangxue Qingnao Granules in the treatment of chronic cerebral circulation insufficiency: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/8484263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dongjie S., Rajendran R.S., Xia Q., She G., Tu P., Zhang Y., et al. Neuroprotective effects of Tongtian oral liquid, a traditional Chinese medicine in the Parkinson's disease-induced zebrafish model. Biomed Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112706. [DOI] [PubMed] [Google Scholar]
- 241.Yang L., Xu B., Yuan C., Dai Z., Wang Y., Li Q., et al. The antioxidative action of ZTP by increasing Nrf 2/ARE signal pathway. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/5421528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xia W., Zhu M., Zhang Z., Kong D., Xiao W., Jia L., et al. Effect of Tianshu capsule in treatment of migraine: a meta-analysis of randomized control trials. J Tradit Chin Med. 2013;33:9–14. doi: 10.1016/s0254-6272(13)60093-x. [DOI] [PubMed] [Google Scholar]
- 243.Qian L., Yan S., Li Y., Wu L., Zheng Y., Wang Y., et al. The effects of gastrodin injection on hypertension: a systematic review and meta-analysis. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000020936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wei Y.C., Zhang H.F., Wang L., Chen C., Li H., Li Q., Huo H.L., Bai M. Study on clinical efficacy of zhennaoning capsules in treatment of cerebral arteriosclerosis and analysis on its economic benefits. China J Chin Mater Med. 2013;38:1247–1250. [PubMed] [Google Scholar]
- 245.Shen Z.M., Zhu M.L., Zhao A.Q. Comparative observation on efficacy of jieyu pill and maprotiline in treating depression. Chin J Integr Tradit Chin West Med. 2004;24:415–417. [PubMed] [Google Scholar]
- 246.Yi Y., Li C.Y., Zhao Y., Tian J.Z., Wang L.M., Pan C., Liang A.H. Study on synergistic effect of Qingkailing injection and Shengmai injection on organ injury in endotoxemia rats. China J Chin Mater Med. 2021;46:4193–4200. doi: 10.19540/j.cnki.cjcmm.20210524.404. [DOI] [PubMed] [Google Scholar]
- 247.Zhang G., Li Y., Chen T., Gao Y., Sun J., Yang W., et al. Comparative study of the efficacy and pharmacokinetics of reduning injection and atomization inhalation. Biomed Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109226. [DOI] [PubMed] [Google Scholar]
- 248.Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., et al. Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm Sin B. 2020;10:1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shi Y., Xu J., Qiao Y., Zhang W., Liu D., Qin M., et al. Effects of Shuanghuanglian injection on the activities of CYP1A2, 2C11, 2D1 and 3A1/2 in rats in vivo and in vitro. Xenobiotica. 2019;4:905–911. doi: 10.1080/00498254.2018.1523487. [DOI] [PubMed] [Google Scholar]
- 250.Li C., Liu S., Luo G., Wang G., Zhang B., Nie Q. Comparison of plasma pharmacokinetics of Tanreqing solution between intratracheal aerosolization and intravenous injection in rats. Biomed Chromatogr. 2018;32:3843–3857. doi: 10.1002/bmc.4116. [DOI] [PubMed] [Google Scholar]
- 251.Zhang X.Y., Lv L., Zhou Y.L., Xie L.D., Xu Q., Zou X.F., et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: a multicenter, prospective, open-label and randomized controlled trial. Phytother Res. 2021;35:4401–4410. doi: 10.1002/ptr.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lu Y.R., Liu Q.Q., Zhao G.Z., Chen Y.S., He L.Y., Wang Y.G., Wen Z.H., Sun Z.T., Li Q., Zhang H.Y. Expert consensus on Antiviral Oral Liquid in treatment of influenza in clinical practice. China J Chin Mater Med. 2021;46:2304–2308. doi: 10.19540/j.cnki.cjcmm.20200927.501. [DOI] [PubMed] [Google Scholar]
- 253.Tao Z., Meng X., Han Y.Q., Xue M.M., Wu S., Wu P., et al. Therapeutic mechanistic studies of ShuFengJieDu capsule in an acute lung injury animal model using quantitative proteomics technology. J Proteome Res. 2017;16:4009–4019. doi: 10.1021/acs.jproteome.7b00409. [DOI] [PubMed] [Google Scholar]
- 254.Yang J. Observation of the effects of the Suhuang Zhike capsule on acute bronchitis. J Biol Regul Homeost Agents. 2017;31:453–457. [PubMed] [Google Scholar]
- 255.Luo L.P., Suo P., Ren L.L., Liu H.J., Zhang Y., Zhao Y.Y. Shenkang injection and its three anthraquinones ameliorates renal fibrosis by simultaneous targeting IκB/NF-κB and Keap 1/Nrf 2 signaling pathways. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huang Y.R., Wei Q.X., Wan Y.G., Sun W., Mao Z.M., Chen H.L., et al. Ureic clearance granule, alleviates renal dysfunction and tubulointerstitial fibrosis by promoting extracellular matrix degradation in renal failure rats, compared with enalapril. J Ethnopharmacol. 2014;155:1541–1552. doi: 10.1016/j.jep.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 257.Zhang W.T., Miao R.P., Zhao Q.H., Sun Y., Han S.J., Yang H.W., et al. Clinical and fundamental research Yinhua Miyanling tablets in treating urinary tract infection. China J Chin Mater Med. 2019;44:2403–2410. doi: 10.19540/j.cnki.cjcmm.20190117.004. [DOI] [PubMed] [Google Scholar]
- 258.Wang H., Mu W., Zhai J., Xing D., Miao S., Wang J., et al. The key role of Shenyan Kangfu tablets, a Chinese patent medicine for diabetic nephropathy: study protocol for a randomized, double-blind and placebo-controlled clinical trial. Trials. 2013;14:165. doi: 10.1186/1745-6215-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zang L., Tian F., Yao Y., Chen Y., Shen Y., Han M., et al. Qianliexin capsule exerts anti-inflammatory activity in chronic non-bacterial prostatitis and benign prostatic hyperplasia via NF-κB and inflammasome. J Cell Mol Med. 2021;25:5753–5768. doi: 10.1111/jcmm.16599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li X., Zhang Y., Hong Z., Gong S., Liu W., Zhou X., et al. Transcriptome profiling analysis reveals the potential mechanisms of three bioactive ingredients of Fufang E’jiao Jiang during chemotherapy-induced myelosuppression in mice. Front Pharmacol. 2018;9:616. doi: 10.3389/fphar.2018.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cheng Z., Wu J.L., Chen J.F. Clinical observation on the treatment of male neoplastic anemia with Yixuesheng capsule combined with recombination human erythropoietin. Chin J Integr Med. 2009;15:63–65. doi: 10.1007/s11655-009-0063-3. [DOI] [PubMed] [Google Scholar]
- 262.Shu S., Zhang Y., Wang Q., Tao P., Li Z., Xu Z., et al. Liuwei Dihuang pill attenuates diabetic nephropathy by inhibiting renal fibrosis via TGF-β/Smad 2/3 pathway. Comput Math Methods Med. 2022;2022 doi: 10.1155/2022/5063636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 263.Law P.C., Auyeung K.K., Chan L.Y., Ko J.K. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Compl Alternative Med. 2012;12:160. doi: 10.1186/1472-6882-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Li J., Wang J.C., Ma B., Gao W., Chen P., Sun R., et al. Shenqi Fuzheng injection for advanced gastric cancer: a systematic review of randomized controlled trials. Chin J Integr Med. 2015;21:71–79. doi: 10.1007/s11655-014-1768-8. [DOI] [PubMed] [Google Scholar]
- 265.Yang Y., Sun M., Yao W., Wang F., Li X., Wang W., et al. Compound Kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lu Y., Wang Y., He Y., Pan J., Jin Y., Zheng L., et al. Aidi injection altered the activity of CYP2D4, CYP1A2, CYP2C19, CYP3A2, CYP2E1 and CYP2C11 in normal and diethylnitrosamine-induced hepatocellular carcinoma in rats. J Ethnopharmacol. 2022;286 doi: 10.1016/j.jep.2021.114930. [DOI] [PubMed] [Google Scholar]
- 267.Pan L., Zhang T., Sun H., Liu G. Ginsenoside Rg3 (Shenyi capsule) combined with chemotherapy for digestive system cancer in China: a meta-analysis and systematic review. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/2417418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sun C., Dong F., Xiao T., Gao W. Efficacy and safety of Chinese patent medicine (Kang-ai injection) as an adjuvant in the treatment of patients with hepatocellular carcinoma: a meta-analysis. Pharm Biol. 2021;59:472–483. doi: 10.1080/13880209.2021.1915340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yu Y.L., Lu Y., Tang X., Cui F.D. Formulation, preparation and evaluation of an intravenous emulsion containing Brucea javanica oil and Coix Seed oil for anti-tumor application. Biol Pharm Bull. 2008;31:673–680. doi: 10.1248/bpb.31.673. [DOI] [PubMed] [Google Scholar]
- 270.Yang T., Shi R., Chang L., Tang K., Chen K., Yu G., et al. Huachansu suppresses human bladder cancer cell growth through the Fas/Fasl and TNF-alpha/TNFR1 pathway in vitro and in vivo. J Exp Clin Cancer Res. 2015;34:21. doi: 10.1186/s13046-015-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Xu H.H., Wang M.X., Tan H.L., Wang Y.G., Tang X.L., Xiao C.R., et al. Effect of clinical doses of Realgar–Indigo Naturalis formula and large-dose of realgar on CYP450s of rat liver. China J Chin Mater Med. 2017;42:593–599. doi: 10.19540/j.cnki.cjcmm.20170103.019. [DOI] [PubMed] [Google Scholar]
- 272.Wei Y., Wang Y., Zeng M. Meta-analysis of Pingxiao capsules (tablets) combined with platinum-based chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Tradit Pat Med. 2017;39:2216–2221. [Google Scholar]
- 273.Zhang Q., Ma X., Qiu J., Jia Z. Main pharmacodynamics and acute toxicity of Duyiwei total iridoid glycoside capsules. Chin Tradit Pat Med. 2018;40:2048–2051. [Google Scholar]
- 274.Yang S.H., Zhang Y., Lin X.F., Wen J.M., Bai X., Fang S.N., et al. Systematic review on clinical efficacy and safety of Cheezheng Pain Relieving Plaster for soft tissue injury. China J Chin Mater Med. 2020;45:1167–1173. doi: 10.19540/j.cnki.cjcmm.20190807.501. [DOI] [PubMed] [Google Scholar]
- 275.Gu C., Xiong W., Li L., Pang L. Comparative study on transdermal permeability of Tongluo Qutong patches in vitro based on volatile components in different matrixes. Guid J Tradit Chin Med Pharm. 2018;24:45–48. [Google Scholar]
- 276.Zhu Y. Comparative study of effects of Shangke Jiegu tablet and Jianbu Huqian pill on postoperative rehabiilitation of patients with lisfranc joint injury. Chin J Exp Tradit Med Form. 2014;20:199–202. [Google Scholar]
- 277.Cheng Y., Dong X., Wang P. Influence of Huoxue Zhitong Plaster on histology and expression of IGF mRNA after acute contusion of rabbit skeletal muscle. Chin Arch Tradit Chin Med. 2012;30:2699–2703. [Google Scholar]
- 278.Shen W., Guan Y.Y., Wu R.M., Liu L.X., Li H.D., Bao W.L., et al. Protective effects of Wang-Bi tablet on bone destruction in collagen-induced arthritis by regulating osteoclast–osteoblast functions. J Ethnopharmacol. 2019;238 doi: 10.1016/j.jep.2019.111861. [DOI] [PubMed] [Google Scholar]
- 279.Wang F., Shi L., Zhang Y., Wang K., Pei F., Zhu H., et al. A traditional herbal formula Xianlinggubao for pain control and function improvement in patients with knee and hand osteoarthritis: a multicenter, randomized, open-label, controlled trial. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/1827528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tang Z.L., Wang X., Yi B., Li Z.L., Liang C., Wang X.X. Effects of the preoperative administration of Yunnan Baiyao capsules on intraoperative blood loss in bimaxillary orthognathic surgery: a prospective, randomized, double-blind, placebo-controlled study. Int J Oral Maxillofac Surg. 2009;38:261–266. doi: 10.1016/j.ijom.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 281.Niu D. Effect of Ma Yinglong Shexiang hemorrhoids cream combined with pearl powder on the pain and complications of severe pressure ulcer patients. Medicine (Baltim) 2021;100 doi: 10.1097/MD.0000000000026767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang Q. Analysis of the effect of musk hemorrhoid suppository combined with traditional Chinese medicine fumigation and washing on the recovery of hemorrhoids after. Chin J Mod Drug Appl. 2020;14:200–202. [Google Scholar]
- 283.Feng Y. 43 cases of keloid treated with Xiaotongzui Qingliangyou mixture. Chin J Ethnic Med. 1999;1:101. [Google Scholar]
- 284.Liu Y., Li Y., Du Y., Huang T., Zhu C. Multicenter clinical trials analyzing efficacy and safety of topical cortex phellodendri compound fluid in treatment of diabetic foot ulcers. Med Sci Monit. 2020;26 doi: 10.12659/MSM.923424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu J.P., Yang H., Xia Y., Cardini F. Herbal preparations for uterine fibroids. Cochrane Database Syst Rev. 2013;4:CD005292. doi: 10.1002/14651858.CD005292.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wang Y.Q., Guo X., Shan T. Clinical effect of Xianling Gubao capsule combined with baofukang suppository in treating atrophic vaginitis. Chin J Integr Tradit Chin West Med. 2010;30:766–768. [PubMed] [Google Scholar]
- 287.Wu W.X., Liu Z.H., Liao Q.P., Dong Y. Clinical study of kangfute suppository in the treatment of senile vaginitis. Chin J Obstet Gynecol. 2005;6:181–184. [Google Scholar]
- 288.Zhang Y., Li W., Zou L., Gong Y., Zhang P., Xing S., et al. Metabonomic study of the protective effect of Fukeqianjin formula on multi-pathogen induced pelvic inflammatory disease in rats. Chin Med. 2018;13:61. doi: 10.1186/s13020-018-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Guo Q.Y., Wu Q.H., Cao H.Y., Ru L., Liu C., Wu J., et al. Experimental study of Wuji Baifeng pill in the treatment of primary dysmenorrhea. Northwest Pharm J. 2016;31:482–485. [Google Scholar]
- 290.Chen Z., Wang J.L., Zhuang P.W., Liu D., Cui G.Z., Tong Y.L., et al. Experimental study on the treatment of oral ulcers in rats with Qingyan Dropping pills. Chin Herbal Med. 2016;47:106–109. [Google Scholar]
- 291.Mei Q.X., Hu Y. Research progress on the pharmacological effects and clinical application of Zongjiefeng. Shizhen Chin Med Chin Med. 2011;22:230–232. [Google Scholar]
- 292.Jin G.F., Zhang W.J., Tan Y.Z. Biyankang tablet anti-inflammatory analgesic effect research. Chin Herbal Med. 2009;32:1108–1111. [Google Scholar]
- 293.Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wu M., Shen A., Chen Y., Liu L., Li L., Sankararaman S., et al. Xinhuang tablets improve intestinal barrier function via regulating epithelial tight junctions in dextran sulfate sodium-induced ulcerative colitis mice. J Med Food. 2021;24:33–39. doi: 10.1089/jmf.2020.0008. [DOI] [PubMed] [Google Scholar]
- 295.Huang Z., Zhou X., Zhang X., Huang L., Sun Y., Cheng Z., et al. Pien-Tze-Huang, a Chinese patent formula, attenuates NLRP3 inflammasome-related neuroinflammation by enhancing autophagy via the AMPK/mTOR/ULK1 signaling pathway. Biomed Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111814. [DOI] [PubMed] [Google Scholar]
- 296.Tong H., Gong L.Z., Shi N. Clinical observation of Yiqing capsule combined with minocycline capsule in the treatment of moderate acne vulgaris. Chin Patent Med. 2018;40:245–246. [Google Scholar]
- 297.Chen B., Li F.Y., Li F.Y., Chen B.Y. Effects of Wanshi Niuhuang Qingxin pills with and without cinnabar on febrile convulsion rat model. Chin herbal med. 2011;42:2502–2506. [Google Scholar]
- 298.Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z., et al. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol Res. 2020;160 doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gordi T. Artesunate for malaria. N Engl J Med. 2008;359:314–315. [PubMed] [Google Scholar]
- 300.Fang Y., Yang C., Yu Z., Li X., Mu Q., Liao G., et al. Natural products as LSD1 inhibitors for cancer therapy. Acta Pharm Sin B. 2020;11:621–631. doi: 10.1016/j.apsb.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhang H.W., Lv C., Zhang L.J., Guo X., Shen Y.W., Nagle D.G., et al. Application of omics- and multi-omics-based techniques for natural product target discovery. Biomed Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111833. [DOI] [PubMed] [Google Scholar]
- 302.Liu L., Hua Y., Wang D., Shan L., Zhang Y., Zhu J., et al. A sesquiterpene lactone from a medicinal herb inhibits proinflammatory activity of TNF-α by inhibiting ubiquitin-conjugating enzyme UbcH5. Chem Biol. 2014;21:1341–1350. doi: 10.1016/j.chembiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 303.Dong T., Li C., Wang X., Dian L., Zhang X., Li L., et al. Ainsliadimer A selectively inhibits IKKα/β by covalently binding a conserved cysteine. Nat Commun. 2015;6:6522. doi: 10.1038/ncomms7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Liu C.X., Yin Q.Q., Zhou H.C., Wu Y.L., Pu J.X., Xia L., et al. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat Chem Biol. 2012;8:486–493. doi: 10.1038/nchembio.935. [DOI] [PubMed] [Google Scholar]
- 305.Liao L.X., Song X.M., Wang L.C., Lv H.N., Chen J.F., Liu D., et al. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc Natl Acad Sci U S A. 2017;114:E5986–E5994. doi: 10.1073/pnas.1706778114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang J., Tan X.F., Nguyen V.S., Yang P., Zhou J., Gao M., et al. A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis. Mol Cell Proteomics. 2014;13:876–886. doi: 10.1074/mcp.M113.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Dai J., Liang K., Zhao S., Jia W., Liu Y., Wu H., et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115:E5896–E5905. doi: 10.1073/pnas.1801745115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wang D., Cao Y., Zheng L., Lv D., Chen L., Xing X., et al. Identification of Annexin A2 as a target protein for plant alkaloid matrine. Chem Commun. 2017;53:5020–5023. doi: 10.1039/c7cc02227a. [DOI] [PubMed] [Google Scholar]
- 309.Lomenick B., Olsen R.W., Huang J. Identification of direct protein targets of small molecules. ACS Chem Biol. 2011;6:34–46. doi: 10.1021/cb100294v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lomenick B., Hao R., Jonai N., Chin R.M., Aghajan M., Warburton S., et al. Target identification using drug affinity responsive target stability (DARTS) Proc Natl Acad Sci U S A. 2009;106:21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Lomenick B., Jung G., Wohlschlegel J.A., Huang J. Target identification using drug affinity responsive target stability (DARTS) Curr Protoc Chem Biol. 2011;3:163–180. doi: 10.1002/9780470559277.ch110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Pai M.Y., Lomenick B., Hwang H., Schiestl R., McBride W., Loo J.A., et al. Drug affinity responsive target stability (DARTS) for small-molecule target identification. Methods Mol Biol. 2015;1263:287–298. doi: 10.1007/978-1-4939-2269-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhou X., Zhao S., Liu T., Yao L., Zhao M., Ye X., et al. Schisandrol A protects AGEs-induced neuronal cells death by allosterically targeting ATP6V0d1 subunit of V-ATPase. Acta Pharm Sin B. 2022;12:3843–3860. doi: 10.1016/j.apsb.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Martinez Molina D., Jafari R., Ignatushchenko M., Seki T., Larsson E.A., Dan C., et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 315.Jafari R., Almqvist H., Axelsson H., Ignatushchenko M., Lundbäck T., Nordlund P., et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–2122. doi: 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 316.Lv Q., Xing Y., Liu J., Dong D., Liu Y., Qiao H., et al. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm Sin B. 2021;11:2880–2899. doi: 10.1016/j.apsb.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wang Y.S., Li H., Li Y., Zhu H., Jin Y.H. Identification of natural compounds targeting Annexin A2 with an anti-cancer effect. Protein Cell. 2018;9:568–579. doi: 10.1007/s13238-018-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Jin Y., Yoon Y.J., Jeon Y.J., Choi J., Lee Y.J., Lee J., et al. Geranylnaringenin (CG902) inhibits constitutive and inducible STAT3 activation through the activation of SHP-2 tyrosine phosphatase. Biochem Pharmacol. 2017;142:46–57. doi: 10.1016/j.bcp.2017.06.131. [DOI] [PubMed] [Google Scholar]
- 319.Tian Y., Wan N., Ding M., Shao C., Wang N., Bao Q., et al. Chemoproteomics maps glycolytic targetome in cancer cells. bioRxiv. 2020 doi: 10.1101/2020.11.18.387670. Available from: [DOI] [PubMed] [Google Scholar]
- 320.Chan J.N.Y., Vuckovic D., Sleno L., Olsen J.B., Pogoutse O., Havugimana P., et al. Target identification by chromatographic co-elution: monitoring of drug–protein interactions without immobilization or chemical derivatization. Mol Cell Proteomics. 2012;11:M111. doi: 10.1074/mcp.M111.016642. 016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 322.Chung C.W., Coste H., White J.H., Mirguet O., Wilde J., Gosmini R.L., et al. Discovery and characterization of small molecule inhibitors of the BET family bromodomains. J Med Chem. 2011;54:3827–3838. doi: 10.1021/jm200108t. [DOI] [PubMed] [Google Scholar]
- 323.Dückert H., Pries V., Khedkar V., Menninger S., Bruss H., Bird A.W., et al. Natural product-inspired cascade synthesis yields modulators of centrosome integrity. Nat Chem Biol. 2011;8:179–184. doi: 10.1038/nchembio.758. [DOI] [PubMed] [Google Scholar]
- 324.Mathis G. Probing molecular interactions with homogeneous techniques based on rare earth cryptates and fluorescence energy transfer. Clin Chem. 1995;41:1391–1397. [PubMed] [Google Scholar]
- 325.Seidel S.A.I., Wienken C.J., Geissler S., Jerabek-Willemsen M., Duhr S., Reiter A., et al. Label-free microscale thermophoresis discriminates sites and affinity of protein–ligand binding. Angew Chem Int Ed Engl. 2012;51:10656–10659. doi: 10.1002/anie.201204268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Han C., Li Y., Zhang Y., Wang Y., Cui D., Luo T., et al. Targeted inhibition of GRK2 kinase domain by CP-25 to reverse fibroblast-like synoviocytes dysfunction and improve collagen-induced arthritis in rats. Acta Pharm Sin B. 2021;11:1835–1852. doi: 10.1016/j.apsb.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wang K., Chen Y., Gao S., Wang M., Ge M., Yang Q., et al. Norlichexanthone purified from plant endophyte prevents postmenopausal osteoporosis by targeting ER α to inhibit RANKL signaling. Acta Pharm Sin B. 2021;11:442–455. doi: 10.1016/j.apsb.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Li Q., Yi D., Lei X., Zhao J., Zhang Y., Cui X., et al. Corilagin inhibits SARS-CoV-2 replication by targeting viral RNA-dependent RNA polymerase. Acta Pharm Sin B. 2021;11:1555–1567. doi: 10.1016/j.apsb.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schier A.F. Single-cell biology: beyond the sum of its parts. Nat Methods. 2020;17:17–20. doi: 10.1038/s41592-019-0693-3. [DOI] [PubMed] [Google Scholar]
- 330.Reynolds G., Vegh P., Fletcher J., Poyner E.F.M., Stephenson E., Goh I., et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. 2021;371 doi: 10.1126/science.aba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Han X., Wang R., Zhou Y., Fei L., Sun H., Lai S., et al. Mapping the mouse cell atlas by microwell-seq. Cell. 2018;172:1091–1107.e17. doi: 10.1016/j.cell.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 332.Liu Y., Chen X., Zhang Y., Liu J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. Analyst. 2019;144:846–858. doi: 10.1039/c8an01503a. [DOI] [PubMed] [Google Scholar]
- 333.Rosenberg A.B., Roco C.M., Muscat R.A., Kuchina A., Sample P., Yao Z., et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360:176–182. doi: 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hagemann-Jensen M., Ziegenhain C., Chen P., Ramsköld D., Hendriks G.-J., Larsson A.J.M., et al. Single-cell RNA counting at allele and isoform resolution using Smart-seq 3. Nat Biotechnol. 2020;38:708–714. doi: 10.1038/s41587-020-0497-0. [DOI] [PubMed] [Google Scholar]
- 335.Hashimshony T., Senderovich N., Avital G., Klochendler A., de Leeuw Y., Anavy L., et al. CEL-Seq 2: sensitive highly-multiplexed single-cell RNA-seq. Genome Biol. 2016;17:77. doi: 10.1186/s13059-016-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zheng G.X.Y., Terry J.M., Belgrader P., Ryvkin P., Bent Z.W., Wilson R., et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8 doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang B., Wang Y., Sun X., Deng G., Huang W., Wu X., et al. CXCR6 is required for antitumor efficacy of intratumoral CD8+ T cell. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhu Y., Wu Z., Yan W., Shao F., Ke B., Jiang X., et al. Allosteric inhibition of SHP2 uncovers aberrant TLR7 trafficking in aggravating psoriasis. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Cao J., Spielmann M., Qiu X., Huang X., Ibrahim D.M., Hill A.J., et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502. doi: 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Trapnell C., Cacchiarelli D., Grimsby J., Pokharel P., Li S., Morse M., et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kumar M.P., Du J., Lagoudas G., Jiao Y., Sawyer A., Drummond D.C., et al. Analysis of single-cell RNA-seq identifies cell–cell communication associated with tumor characteristics. Cell Rep. 2018;25:1458–1468. doi: 10.1016/j.celrep.2018.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Method of the year 2019: single-cell multimodal omics. Nat Methods. 2020;17:1. doi: 10.1038/s41592-019-0703-5. [DOI] [PubMed] [Google Scholar]
- 343.Wang Z., Wu W., Guan X., Guo S., Li C., Niu R., et al. 20(S)-Protopanaxatriol promotes the binding of P53 and DNA to regulate the antitumor network via multiomic analysis. Acta Pharm Sin B. 2020;10:1020–1035. doi: 10.1016/j.apsb.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zhang S., Zhang J., An Y., Zeng X., Qin Z., Zhao Y., et al. Multi-omics approaches identify SF3B3 and SIRT3 as candidate autophagic regulators and druggable targets in invasive breast carcinoma. Acta Pharm Sin B. 2021;11:1227–1245. doi: 10.1016/j.apsb.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Li X., Ge J., Li Y., Cai Y., Zheng Q., Huang N., et al. Integrative lipidomic and transcriptomic study unravels the therapeutic effects of saikosaponins A and D on non-alcoholic fatty liver disease. Acta Pharm Sin B. 2021;11:3527–3541. doi: 10.1016/j.apsb.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lee J., Hyeon D.Y., Hwang D. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med. 2020;52:1428–1442. doi: 10.1038/s12276-020-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Chen S., Lake B.B., Zhang K. High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat Biotechnol. 2019;37:1452–1457. doi: 10.1038/s41587-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ma S., Zhang B., LaFave L.M., Earl A.S., Chiang Z., Hu Y., et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell. 2020;183:1103–1116. doi: 10.1016/j.cell.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhang Y., Chen H., Mo H., Hu X., Gao R., Zhao Y., et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39:1578–1593. doi: 10.1016/j.ccell.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 350.Vistain L.F., Tay S. Single-cell proteomics. Trends Biochem Sci. 2021;46:661–672. doi: 10.1016/j.tibs.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Xu H., Zhang Y., Wang P., Zhang J., Chen H., Zhang L., et al. A comprehensive review of integrative pharmacology-based investigation: a paradigm shift in traditional Chinese medicine. Acta Pharm Sin B. 2021;11:1379–1399. doi: 10.1016/j.apsb.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nogales C., Mamdouh Z.M., List M., Kiel C., Casas A.I., Schmidt H.H.H.W. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43:136–150. doi: 10.1016/j.tips.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 354.Li T., Zhang W., Hu E., Sun Z., Li P., Yu Z., et al. Integrated metabolomics and network pharmacology to reveal the mechanisms of hydroxysafflor yellow A against acute traumatic brain injury. Comput Struct Biotechnol J. 2021;19:1002–1013. doi: 10.1016/j.csbj.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chan H.C.S., Shan H., Dahoun T., Vogel H., Yuan S. Advancing drug discovery via artificial intelligence. Trends Pharmacol Sci. 2019;40:592–604. doi: 10.1016/j.tips.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 356.Zhong F., Wu X., Yang R., Li X., Wang D., Fu Z., et al. Drug target inference by mining transcriptional data using a novel graph convolutional network framework. Protein Cell. 2022;13:281–301. doi: 10.1007/s13238-021-00885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Cravens A., Payne J., Smolke C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat Commun. 2019;10:2142. doi: 10.1038/s41467-019-09848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hou M., Wang R., Zhao S., Wang Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm Sin B. 2021;11:1813–1834. doi: 10.1016/j.apsb.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Tao H., Lauterbach L., Bian G., Chen R., Hou A., Mori T., et al. Discovery of non-squalene triterpenes. Nature. 2022;606:414–419. doi: 10.1038/s41586-022-04773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Shen Z.F. Research progress of total alkaloids of mulberry branch in the treatment of diabetes. Chin J Pharmacol Toxicol. 2021;35:1. [Google Scholar]