Abstract
Background
Clinical utility of universal antigen rapid test (ART) in the pediatric setting is unknown. We aimed to assess the performance and utility of universal ART in hospitalized children (≥5-year-old) to prevent nosocomial COVID-19 transmission.
Methods
Cross-sectional study involving all hospitalized pediatric patients aged ≥5-year-old from 2 periods during Omicron wave. Clinical data, ART and polymerase chain reaction test results were collected.
Results
A total of 444 patients were included from the 2 study periods, and 416 patients (93.7%) had concordant results between ART and polymerase chain reaction. The overall sensitivity and specificity of ART were 83.3% (95% CI: 75.2-89.3) and 97.5% (95% CI: 95.0-98.8), respectively. Negative predictive values of ART between the Omicron emergence and Omicron peak periods for a probable case group were 71.4% and 66.7%, respectively, and for a suspect case group 91.4% and 75.0%, respectively. Negative predictive values for an unlikely case group was >95% in both periods. Positive predictive value of ART was >85% for probable and suspect case groups in both periods. Seventy-five percent of patients (n = 15) who were incorrectly classified as SARS-CoV-2 negative by ART had potentially viable virus. No large nosocomial transmission clusters were detected.
Conclusions
Universal ART screening may limit nosocomial outbreaks in hospitalized children. The performance can be optimized by considering clinical symptoms, exposure and periods within COVID waves.
Key Words: Severe acute respiratory syndrome coronavirus 2, Sensitivity, Specificity, Coronavirus disease 2019
Early and accurate identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection remains an important cornerstone in the control of the Coronavirus Disease 2019 (COVID-19) pandemic. Real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) via nasopharyngeal or mid-turbinate swabs for SARS-CoV-2 is the gold standard diagnostic test for COVID-19.1 However, it is costly and requires skilled health care workers (HCWs) to perform the test, with a risk of transmission of SARS-CoV-2 to HCWs during the procedure. The test usually requires analysis by skilled laboratory technologists and has a turnaround time of at least several hours. Hence, PCR testing as a screening method for hospital admissions is not ideal.
The development of the antigen rapid tests (ART) allowed for rapid identification of COVID-19 cases, which aided in prompt isolation of cases and contact tracing, minimizing the spread of infection in the hospitals and community.2 , 3 ART from mid-turbinate swabs can be done by individuals at home, and a typical ART test result is ready within 15 minutes. The World Health Organization has since issued guidance on the use of COVID-19 ART.4 It was recommended that COVID-19 self-testing can be used for both screening and diagnostics, depending on the epidemiological situation and clinical picture.
Various studies have assessed the utility of ART in clinical settings. Systematic reviews and meta-analyses on the accuracy of ART for SARS-CoV-2 revealed that the overall pooled estimates of sensitivity and specificity were 72%-75% and 98.9%-99.4%, respectively.5 , 6 Specifically, in pediatric patients, a wider range of sensitivity was reported, ranging from 45.4% to 87.9%.7, 8, 9, 10, 11, 12, 13 The diagnostic utility varied among different ART kits and specimen types and was affected by the background COVID-19 risk profiles of the test subjects and symptomology. Moreover, these studies were done where the circulating COVID-19 variants of concern were Alpha, Beta, or Delta.
Since the discovery of the Omicron variant (B.1.1.529) on November 24, 2021, the new variant of concern became the dominant strain in many countries around the globe.14 However, the ART kits were originally designed for detection of the original Wuhan SARS-CoV-2 strain. The emergence of Omicron variants with new mutations poses concern about the diagnostic utility of these ART kits. In addition, no studies had evaluated the applicability of ART in the hospital clinical setting to prevent nosocomial transmission of COVID-19. In Singapore, the first local Omicron case was detected on December 9, 2021.15 In less than 3 months, there was a surge in community COVID-19 cases that peaked in late February 2022, with over 94% of sequenced strains in Singapore belonging to the BA.1 Omicron strain.16
From July 5, 2021, the Ministry of Health, Singapore required all hospitals to conduct ART as well as PCR swabs for admitted patients aged ≥5-year-old, regardless of clinical risk or symptoms. The objective was to identify COVID-19 infections amongst admitted patients, including asymptomatic cases, in order to limit the risk of nosocomial transmission of COVID-19. In this study, we aimed to assess the clinical utility of ART in hospitalized pediatric patients with different clinical likelihood for SARS-CoV-2 infection during one period of Omicron emergence and one peak transmission period. We also evaluated the impact of ART hospital admission screening in preventing nosocomial COVID-19 transmission in a pediatric hospital setting.
Patients and methods
Setting
The study was conducted in KK Women's and Children's Hospital (KKH), which is the largest pediatric public hospital in Singapore. Our institution houses 48.5% of Singapore's neonatal and pediatric admissions, with approximately 32,000 pediatric admissions per year (data from Ministry of Health, Singapore). During the COVID-19 pandemic, KKH is the main pediatric hospital that admits and manages children with COVID-19 infection.
Study design
This is a retrospective cross-sectional study. For the study period, all hospitalized pediatric patients aged 5-year-old and above with a bilateral mid-turbinate ART and a bilateral nasopharyngeal PCR test for SARS-CoV-2 done on the same calendar day were included in the analysis.
Study period
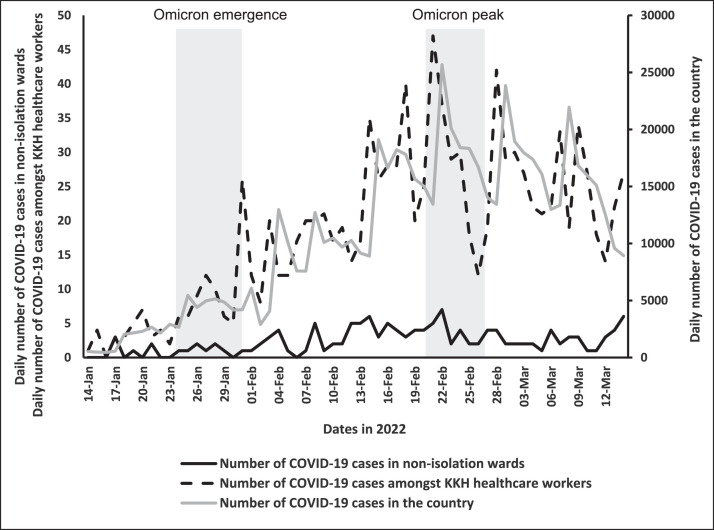
We selected 2 periods: the first period from January 24 to January 30, 2022 (Omicron emergence period) corresponded to the emergence of the Omicron variant, and the second period from February 20 to February 26, 2022 (Omicron peak period) corresponded to the peak of the Omicron wave in Singapore (Fig 1 ). This first period was selected based on 2 key criteria to confirm the start of the Omicron wave: at least 3 consecutive week of increasing community COVID-19 cases and >70% of sequenced cases attributed to the omicron strain.17 , 18 The second period covered the peak period of the Omicron wave where over 94% of sequenced strains in Singapore belonged to the BA.1 Omicron strain.16 , 18 As the diagnostic value of tests can be affected by the prevalence of the disease, the 2 periods provided a comprehensive overview of ART's performance.
Fig 1.
Epi Curve of SARS-CoV-2 in the community, hospital staff and non-isolation wards, from mid-January 2022 to mid-March 2022.
Clinical case definitions for SARS-CoV-2 infection
The clinical case definitions for COVID-19 used in this study were:
-
(a)
Probable case – patients with close COVID-19 contact and acute respiratory tract infective symptoms (cough, runny nose, blocked nose, sore throat, hoarse voice) and/or fever. Close contact refers to mask-off exposure to a COVID-19 infected person within 2 meters distance for a minimum period of 15 minutes, within 2 days from the symptom onset of the infected person.
-
(b)
Suspect case – patients with respiratory tract infective symptoms without significant COVID-19 contact.
-
(c)
Unlikely case – patients without respiratory tract infective symptoms and no COVID-19 contact.
Patient isolation policies
Depending on the presenting symptoms and the ART results, the patients were admitted to different wards based on risk stratification to reduce nosocomial COVID-19 transmission:
-
(1)
Patients with positive ART and/or fulfilled probable case definition were admitted to negative pressure COVID-19 isolation wards with single rooms.
-
(2)
Patients with negative ART and fulfilled suspect case definition were admitted to isolation cohort wards without negative pressure. Isolation cohort wards included beds that were spaced at least 2 meters apart with strict infection control protocols where patients were not allowed to leave or ambulate around the wards.
-
(3)
Patients with negative ART who fulfilled the unlikely case definition were admitted to non-isolation general wards.
Upon admission to the inpatient wards, a nasopharyngeal PCR swab test was performed on all patients. The turnaround time for the PCR test result was less than 24 hours during the study period. If the PCR returned positive for SARS-CoV-2, the patients were transferred to the negative pressure COVID-19 isolation wards with single rooms.
Nosocomial SARS-CoV-2 cases
Nosocomial SARS-CoV-2 cases were detected via 2 main methods. Firstly, contact tracing would be initiated with all close contacts of any SARS-CoV-2 case that was not isolated at admission. All close contacts of the case would have daily ARTs for the next 5 days. If the ART was positive for the close contacts, PCR test will be done to confirm the diagnosis of COVID-19. Close contacts were individuals within 2 meters for a minimum period of 15 minutes, within 2 days from the symptom onset of the infected person. Secondly, patients who developed new acute respiratory tract symptoms during their inpatient stay would be tested for SARS-CoV-2 using ART and PCR tests. Patients who were positive more than 2 days after admission with no known community SARS-CoV-2 exposures (visitor, care giver, etc) were classified as nosocomial SARS-CoV-2 cases.
HCW SARS-CoV-2 cases
All HCWs who had respiratory symptoms were required to perform self-ART or visit a health care provider for testing. If they were tested positive, the HCWs had to report to their supervisors and contact tracing would also be initiated. Close contacts were required to perform daily self-ART for the 5 days following exposure. Data on all hospital staff SARS-CoV-2 cases during the Omicron wave from January 2022 to March 2022 inclusive of the 2 study periods were extracted for analysis.
SARS-CoV-2 ART
Trained nurses would perform bilateral mid-turbinate swabs for ART according to the manufacturers’ instructions. Test results were read within 15 minutes, and the results were recorded in the medical notes. In cases of discrepant results, another nurse would be asked to read the result. Invalid test results were repeated once. There were 3 types of ART used at KKH: Abbott PanBio COVID-19 Antigen Rapid Test Device (Abbott), SD Biosensor STANDARD Q COVID-19 Ag Test (SD Biosensor) and BD Veritor System for Rapid Detection of SARS-CoV-2 (BD).
SARS-CoV-2 PCR test
SARS-CoV-2 PCR served as the reference standard in this study. Bilateral nasopharyngeal swabs were collected by trained nurses in personal protective equipment using Mini UTM Kits (Copan) with flocked swabs and 1 mL of universal transport medium and transferred to the laboratory for rRT-PCR. PCR was conducted as previously described19 using the primers and probe for the SARS-CoV-2 E gene as published by Corman et al.20
Data collection
The following data were collected from clinical notes: age, ART brand used, ART result, PCR test result, PCR cycle threshold (Ct) if positive, day of illness where PCR and ART were performed and presence of respiratory tract infective symptoms.
Consent
The study was approved by the SingHealth Centralized Institutional Review Board (CIRB 2020/2094). Written informed consent was waived for pandemic public health research.
Statistics
Continuous variables were expressed by their mean (standard deviation [SD]) and median (interquartile range [IQR]) for normal and non-normal distribution, respectively. Categorical variables were presented by the frequencies and proportions. Association between a categorical and a continuous variable was done using the one-way ANOVA method. Spearman‘s rank correlation was used to assess the relationship between Ct values and positive ART. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and prevalence were calculated using the free software website (http://vassarstats.net/). Other statistical analysis was processed using SPSS software v23.0 (IBM Corp.). Statistical significance was defined as P < .05 (2-sided).
Results
A total of 444 patients were included from the 2 study periods: 235 (52.9%) from the week of January 24 to January 30, 2022 and 209 patients (47.1%) from February 20 to February 26, 2022, respectively. There were no duplicate PCR or ART samples from the 444 patients. The clinical characteristics of the cohort are shown in Table 1 . The majority of the patients were tested using Abbot PanBio COVID-19 Antigen Rapid Test Device (n = 376, 84.7%). Children from the Omicron peak period were slightly younger than those tested during the Omicron emergence period [mean age = 10.4 (SD: 3.4) years vs mean age = 11.1 years (SD: 3.4) respectively, P = .035]. There were no significant differences in gender and test kit used in the 2 study periods. One hundred and forty-three patients (32.2%) had acute respiratory tract infective symptoms. For the patients with symptoms (n = 143), the median day of illness where the ART and PCR swabs were done was 2 (IQR: 1-3). The distribution of the COVID-19 clinical case definitions was: 86 (19.4%) probable cases, 82 (18.5%) suspect cases and 276 (62.2%) defined as unlikely.
Table 1.
Clinical characteristics of children aged ≥ 5 years during Omicron emergence (January 24 to January 30, 2022) and Omicron peak (February 20 to February 26, 2022) period
| Omicron emergence period, January 24 to January 30, 2022 (n = 235, 52.9%) | Omicron peak period, February 20 to February 26, 2022 (n = 209, 47.1%) | |
|---|---|---|
| Age in y, mean (SD) | 11.1 (3.4) | 10.4 (3.4) |
| Male, n (%) | 139 (59.1) | 111 (53.1) |
| Weight in kg, mean (SD) | 41.4 (18.4) | 39.6 (18.1) |
| ART brand, n (%) | ||
| Panbio | 192 (81.7) | 184 (88.0) |
| SD Biosensor | 5 (2.1) | 2 (1.0) |
| BD Veritor | 4 (1.7) | 2 (1.0) |
| Unknown | 34 (14.5) | 21 (10.0) |
| Presence of any respiratory tract infective symptoms, n (%) | 84 (35.7) | 59 (28.2) |
| Day of illness when PCR/ART done, median (IQR)* | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) |
| Runny/blocked nose, n (%) | 47 (20.0) | 41 (19.6) |
| Cough, n (%) | 54 (23.0) | 51 (24.4) |
| Sore throat or hoarse voice, n (%) | 29 (12.3) | 28 (13.4) |
| Fever, n (%) | 64 (27.2) | 50 (23.9) |
| COVID-19 risk, n (%) | ||
| High risk | 32 (13.6) | 54 (25.8) |
| Moderate risk | 48 (20.4) | 34 (16.3) |
| Low risk | 155 (66.0) | 121 (57.9) |
Persons without any respiratory tract infective symptoms were excluded.
Diagnostic performance of SARS-CoV-2 ART in hospitalized pediatric patients
A total of 416 patients (93.7%) had concordant results between ART and PCR (positive PCR and ART or negative PCR and ART). The overall sensitivity and specificity values of ART in diagnosing COVID-19 infection were 83.3% (95% CI: 75.2-89.3) and 97.5% (95% CI: 95.0-98.8), respectively (Table 2 ). The PPV and NPV were 92.6% (95% CI: 85.5-96.5) and 94.0% (95% CI: 90.8-96.2), respectively, with a COVID-19 prevalence of 27.0%.
Table 2.
Diagnostic performance of ART for COVID-19 during the Omicron emergence period (January 24 to January 30, 2022) and Omicron peak period (February 20 to February 26, 2022) in hospitalized children aged ≥ 5 years
| Omicron emergence period, January 24 to January 30, 2022 (n = 235, 52.9%) |
Omicron peak period, February 20 to February 26, 2022 (n = 209, 47.1%) |
Total (n = 444) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | |
| ART positive | 38 | 6 | 44 | 62 | 2 | 64 | 100 | 8 | 108 |
| ART negative | 7 | 184 | 191 | 13 | 132 | 145 | 20 | 316 | 336 |
| Sensitivity, % (95% CI) | 84.4 (69.9-93.0) | 82.7 (71.8-90.1) | 83.3 (75.2-89.3) | ||||||
| Specificity, % (95% CI) | 96.8 (92.9-98.7) | 98.5 (94.2-99.7) | 97.5 (95.0-98.8) | ||||||
| PPV, % (95% CI) | 86.4 (72.0-94.3) | 96.9 (88.2-99.5) | 92.6 (85.5-96.5) | ||||||
| NPV, % (95% CI) | 96.3 (92.3-98.4) | 91.0 (84.9-94.9) | 94.0 (90.8-96.2) | ||||||
| Prevalence of COVID-19 in cohort, % | 19.1 | 35.9 | 27.0 | ||||||
| Probable case group* |
Probable case group* |
Probable case group* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | |
| ART positive | 24 | 1 | 25 | 38 | 1 | 39 | 62 | 2 | 64 |
| ART negative | 2 | 5 | 7 | 5 | 10 | 15 | 7 | 15 | 22 |
| Sensitivity, % (95% CI) | 92.3 (73.4-98.7) | 88.4 (74.1-95.6) | 89.9 (79.6-95.5) | ||||||
| Specificity, % (95% CI) | 83.3 (36.5-99.1) | 90.9 (57.1-99.5) | 88.2 (62.3-97.9) | ||||||
| PPV, % (95% CI) | 96.0 (77.7-99.8) | 97.4 (84.9-99.9) | 96.9 (88.2-99.5) | ||||||
| NPV, % (95% CI) | 71.4 (30.3-94.9) | 66.7 (38.7-87.0) | 68.2 (45.1-85.3) | ||||||
| Prevalence of COVID-19 in group, % | 81.3 | 79.6 | 80.2 | ||||||
| Suspect case group† |
Suspect case group† |
Suspect case group† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | |
| ART positive | 12 | 1 | 13 | 21 | 1 | 22 | 33 | 2 | 35 |
| ART negative | 3 | 32 | 35 | 3 | 9 | 12 | 6 | 41 | 47 |
| Sensitivity, % (95% CI) | 80.0 (51.4-94.7) | 87.5 (66.5-96.7) | 84.6 (68.8-93.6) | ||||||
| Specificity, % (95% CI) | 97.0 (82.5-99.8) | 90.0 (54.1-99.5) | 95.3 (82.9-99.2) | ||||||
| PPV, % (95% CI) | 92.3 (62.1-99.6) | 95.5 (75.1-99.8) | 94.3 (79.5-99.0) | ||||||
| NPV, % (95% CI) | 91.4 (75.8-97.8) | 75.0 (42.8-93.3) | 87.2 (73.6-94.7) | ||||||
| Prevalence of COVID-19 in group, % | 31.3 | 70.6 | 47.6 | ||||||
| Unlikely case group‡ |
Unlikely case group‡ |
Unlikely case group‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | PCR positive | PCR negative | Total | |
| ART positive | 2 | 4 | 6 | 3 | 0 | 3 | 5 | 4 | 9 |
| ART negative | 2 | 147 | 149 | 5 | 113 | 118 | 7 | 260 | 267 |
| Sensitivity, % (95% CI) | 50.0 (9.2-90.8) | 37.5 (10.2-74.1) | 41.7 (16.5-71.4) | ||||||
| Specificity, % (95% CI) | 97.4 (92.9-99.1) | 100.0 (95.9-100.0) | 98.5 (95.9-99.5) | ||||||
| PPV, % (95% CI) | 33.3 (6.0-75.9) | 100.0 (31.0-100.0) | 55.6 (22.7-84.7) | ||||||
| NPV, % (95% CI) | 98.7 (94.7-99.8) | 95.8 (89.9-98.4) | 97.4 (94.4-98.8) | ||||||
| Prevalence of COVID-19 in group, % | 2.6 | 6.6 | 4.3 | ||||||
Probable case group - patients with close COVID-19 contact and acute respiratory tract infective symptoms (cough, runny nose, blocked nose, sore throat, hoarse voice) and/or fever. Close contact refers to mask-off exposure to a COVID-19 infected person who was within 2 metres for a minimum period of 15 minutes, within 2 days from the symptom onset of the infected person.
Suspect case group – patients with respiratory tract infective symptoms without significant COVID-19 contact.
Unlikely case group – patients without respiratory tract infective symptoms and no COVID-19 contact.
There was a decrease in NPV performance of ART between the Omicron emergence period and the Omicron peak period for the probable case group (71.4% vs 66.7%) and the suspect case group (91.4% vs 75.0%). The decrease was less for the unlikely case group (98.7% vs 95.8%). The PPV performance of ART between the Omicron emergence period vs the Omicron peak period increased slightly for the probable case group (96.0% vs 97.4%) and the suspect case group (92.3% vs 95.5%). However, there was a large increase in PPV of ART for the unlikely case group between the 2 periods (33.3% vs 100%), although this was based on very small numbers of positives (Table 2).
There were a total of 20 ART-negative but PCR-positive (presumably falsely ART-negative) specimens, of which 15 (75.0%) had PCR Ct values below 30. Within the probable and suspect case groups, there were 13 ART-negative (7 in probable case group and 6 in suspect case group), PCR-positive specimens, of which 12 (92.3%) had Ct values below 30. There were 7 ART-negative, PCR-positive specimens in the unlikely case group, of which 3 (42.9%) had Ct values below 30. On Spearman's rank correlation, there was a positive correlation between low PCR Ct values and positive ART results (r = 0.855, P < .001). The difference in the median Ct values between ART-positive and ART-negative patients in all PCR-positive patients was statistically significant (20 ART-negative [16.7%], median Ct value 22.5 [IQR: 20.3-29.8] vs 100 ART-positive [83.3%], median Ct value 17.3 [IQR: 15.2-19.1], P < .001).
Impact of universal SARS-CoV-2 ART screening on nosocomial SARS-CoV-2 transmission
During the study periods, 7 cases in the unlikely case group were incorrectly diagnosed as being SARS-CoV-2 negative by ART and were initially admitted to the non-isolation general wards. Upon detection of SARS-CoV-2 by PCR, these patients were isolated. Contact tracing did not identify any evidence of nosocomial transmission amongst close contacts. In Figure 1, the epi-curve of COVID-19 amongst hospital staff was similar to that in the community, due to a similar risk of exposure outside the hospital setting. However, the epi curve of COVID-19 amongst patients in non-isolation general wards demonstrated a lower force of infection, with no evidence of large nosocomial transmission clusters.
Discussion
Our findings revealed that the overall sensitivity and specificity of ART for hospitalized pediatric patients were 83.3% and 97.5%, respectively. There was a decrease in NPV of ART in the probable (71.4%-66.6%) and suspect (91.4%-75%) case groups from the Omicron emergence to the Omicron peak period, when there was an increase in the prevalence of COVID-19. Reassuringly, the NPV of ART for unlikely case group during both periods remained high, above 95%. The PPV of ART was above 80% for the probable and suspect case groups during both study periods. A total of 20 presumably false negative ART results were obtained, due to which 75% of associated patients were presumably incorrectly classified as SARS-CoV-2-negative by ART, but had potentially viable virus, with PCR Ct values less than 30. Although 7 cases in the unlikely case group were incorrectly diagnosed as being SARS-CoV-2-negative by ART and were admitted to the non-isolation general wards, there was no evidence of large nosocomial transmission clusters. Lastly, the median PCR Ct values of samples with concordant PCR-ART results were significantly lower than those of samples with discordant PCR-ART results (PCR-positive but ART-negative), thus confirming that positive ART results were associated with higher viral loads. This is consistent with other studies.7 , 8 , 10, 11, 12 , 21
Since the discovery of the Omicron variant (B.1.1.529), various studies found that some ART kits continue to perform well as a point-of-care test for diagnosis of COVID-19, with preserved sensitivity.22, 23, 24, 25, 26 However, there was a lack of pediatric data, and the utility of ART in the control of SARS-CoV-2 transmission in a hospital setting had not been evaluated, especially with the highly transmissible Omicron variant. In our pediatric hospital setting during the Omicron wave, we found the performance of ART to be preserved, with the overall sensitivity and specificity comparable to other published studies in which the majority of patients were adults.22 , 25
The NPV of ART remained high, above 95%, for the unlikely case group in the 2 periods of study, despite a rising prevalence of 2.6%-6.6% in this cohort of patients. During rising community transmission of COVID-19, negative ART results for asymptomatic patients with no reported COVID-19 contact appear sufficient to rule out COVID-19 infection. Although 7 patients in the unlikely case group were incorrectly diagnosed as being SARS-CoV-2 negative by ART and admitted to the non-isolation general wards, contact tracing showed no evidence of significant nosocomial transmission clusters in our institution. Moreover, 4 of the specimens had Ct values above 30, suggesting a lower risk of transmission due to lower viral load correlated with higher Ct values. Conversely, the PPV was 33.3% during the Omicron emergence period and increased to 100% during the Omicron peak period where the community transmission was at its highest. A confirmatory PCR test may still be necessary to confirm the diagnosis of COVID-19 in this group of patients, due to the possibility of a false positive result, especially during periods of low community transmission. False positive test may lead to patients’ anxiety, unnecessary isolation and wastage of medical resources. This observation was also reported in another study which highlighted the impact of false positive ARTs in a low-risk group in the community.27
Based on our data, further deisolation policies can be applied depending on the clinical case definitions and the ART results. During high community transmission of SARS-CoV-2 such as the Omicron peak period in this study, patients under the probable and suspect case groups with positive ART results were highly likely to have COVID-19 infections, as the PPV is high, above 90%. In low-resource clinical settings with limited capabilities to perform PCR tests, these patients could be diagnosed to have COVID-19 based on positive ART tests alone. On the other hand, there was a relatively low NPV of 66.7%-71.4%. There were 13 presumably false-negative ART results, with 62% occurring during the Omicron peak period, and the majority of associated PCR Ct values (92.3%) were below 30. As some studies highlighted the possibility of positive viral cultures when the Ct values were below 30,28 , 29 the patients with presumably false negative ART in this cohort may have been able to transmit SARS-CoV-2 if they had been deisolated without further PCR tests. This suggests that patients under the probable and suspect case groups should undergo confirmatory PCR tests even when they have negative ART results, before deisolation, in order to mitigate the SARS-CoV-2 nosocomial transmission risk.
During periods of rising COVID-19 community transmission, there is a need for a delicate balance of preventing nosocomial transmission and conserving isolation capacity in hospitals. Several other studies highlighted the impact of nosocomial COVID-19 transmission in the vulnerable hospitalized population.30 In our pediatric hospital setting, universal ART testing in combination with PCR testing managed to keep the number of COVID-19 cases detected in non-isolation wards low, with no evidence of substantial nosocomial clusters, despite a surge in COVID-19 cases in the community. There were other infection control practices in place in our general wards, such as universal staff and patient masking, adherence to hand hygiene and limitation on visitor numbers. Therefore, these infection control interventions should be considered together in combination with any form of universal ART screening to prevent nosocomial transmission of SARS-CoV-2.
Our study has a few limitations. This is a single-center study where data were collected for a total of 2 weeks that corresponded to the emergence and peak of the Omicron variant in the country. No SARS-CoV-2 variant typing was done for the patients who had positive PCR tests. However, during the 2 study periods, 70%-94% of SARS-CoV-2 samples sequenced in Singapore corresponded to the Omicron variant.16, 17, 18 Viral culture was also not done, and the amount of virus that led to positive ART cannot be accurately assessed. We were also unable to confirm if the result in this study reflects a particular ART brand's performance, although the majority of the reported ART used in this study were the Abbott PanBio COVID-19 Antigen Rapid Test Device.
Conclusions
In the presence of the highly transmissible COVID-19 Omicron variant, our study suggests that universal ART screening can help to limit nosocomial transmission in hospitalized children. Its performance and utility can be further enhanced by considering the patients’ clinical symptoms, COVID-19 exposure, and prevalence of COVID-19 at the period. Various testing strategies applied together can help with the early isolation and identification of infected individuals, thus decreasing the likelihood of nosocomial SARS-CoV-2 transmission.
Acknowledgments
The authors thank the staff of the Infection Control team and of the Microbiology Section, Department of Pathology and Laboratory Medicine, KK Women's and Children's Hospital, for their dedication and commitment in the challenging work conditions during the COVID-19 pandemic.
Footnotes
Funding/support: This work is partly supported by SingHealth Duke-NUS Academic Medicine COVID-19 Rapid Response Research Grant (AM/COV001/2020).
Conflicts of interest: None to report.
References
- 1.Infectious Diseases Society of America (IDSA). IDSA guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Accessed July 25, 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/
- 2.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity - a strategy for containment. N Engl J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection: interim guidance. Accessed October 27, 2022. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays
- 4.World Health Organization. Use of SARS-CoV-2 antigen-detection rapid diagnostic tests for COVID-19 self-testing. Accessed July 25, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Ag-RDTs-Self_testing-2022.1
- 5.Brümmer LE, Katzenschlager S, McGrath S, et al. Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: an updated systematic review and meta-analysis with meta-regression analyzing influencing factors. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvu V, Gary DS, Mann J, et al. Factors that influence the reported sensitivity of rapid antigen testing for SARS-CoV-2. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.714242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.L'Huillier AG, Lacour M, Sadiku D, et al. Diagnostic accuracy of SARS-CoV-2 rapid antigen detection testing in symptomatic and asymptomatic children in the clinical setting. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00991-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman MC, Freeman TJ, Iagnemma J, et al. Performance of the Sofia SARS-CoV-2 rapid antigen test in symptomatic and asymptomatic pediatric patients. J Pediatric Infect Dis Soc. 2022;11:417–421. doi: 10.1093/jpids/piac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villaverde S, Domínguez-Rodríguez S, Sabrido G, et al. Diagnostic accuracy of the Panbio severe acute respiratory syndrome coronavirus 2 antigen rapid test compared with reverse-transcriptase polymerase chain reaction testing of nasopharyngeal samples in the pediatric population. J Pediatr. 2021;232:287–289. doi: 10.1016/j.jpeds.2021.01.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbonell-Sahuquillo S, Lázaro-Carreño MI, Camacho J, et al. Evaluation of a rapid antigen detection test (Panbio™ COVID-19 Ag Rapid Test Device) as a point-of-care diagnostic tool for COVID-19 in a pediatric emergency department. J Med Virol. 2021;93:6803–6807. doi: 10.1002/jmv.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood N, Shetgiri R, Rodriguez A, et al. Evaluation of the Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection in children: implications for screening in a school setting. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung C, Levy C, Varon E, et al. Diagnostic accuracy of SARS-CoV-2 antigen detection test in children: a real-life study. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.647274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eleftheriou I, Dasoula F, Dimopoulou D, et al. Real-life evaluation of a COVID-19 rapid antigen detection test in hospitalized children. J Med Virol. 2021;93:6040–6044. doi: 10.1002/jmv.27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Accessed July 01, 2022. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 15.The Straits Times. The big questions on Omicron: what we know and what it means for us. Accessed July 01, 2022. https://www.straitstimes.com/singapore/askst-the-big-questions-on-omicron-what-we-know-and-what-it-means-for-us
- 16.GISAID. Tracking of variants: VOC omicron GRA (B.1.1.529+Ba.*) first detected in Botswana/Hong Kong/South Africa. Accessed July 01, 2022. https://www.Gisaid.org/Hcov19-Variants/
- 17.The Straits Times. Singapore likely to see ‘significant’ Omicron wave, measures in place to cut risks. Accessed October 28, 2022. https://www.straitstimes.com/singapore/health/singapore-likely-to-see-significant-omicron-wave-measures-in-place-to-cut-risks
- 18.Ministry of Health Singapore. COVID-19 statistics. Accessed October 28, 2022. https://www.moh.gov.sg/covid-19/statistics
- 19.Kam KQ, Thoon KC, Maiwald M, et al. SARS-CoV-2 viral RNA load dynamics in the nasopharynx of infected children. Epidemiol Infect. 2021;149:e18. doi: 10.1017/S095026882100008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR [published correction appears in Euro Surveill. 2020 Apr;25(14):] [published correction appears in Euro Surveill. 2020 Jul;25(30):] [published correction appears in Euro Surveill. 2021 Feb;26(5):] Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres I., Poujois S., Albert E., Colomina J., Navarro D. Evaluation of a rapid antigen test (PanbioTM COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin Microbiol Infect. 2021;27:636.e1–636.e4. doi: 10.1016/j.cmi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galliez RM, Bomfim L, Mariani D, et al. Evaluation of the Panbio COVID-19 antigen rapid diagnostic test in subjects infected with Omicron using different specimens. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.01250-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak GCK, Lau SSY, Wong KKY, et al. Analytical sensitivity of the rapid antigen test kits for detection of SARS-CoV-2 Omicron variant BA.2 sublineage. J Med Virol. 2022;94:5033–5037. doi: 10.1002/jmv.27908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jungnick S, Hobmaier B, Paravinja N, et al. Analysis of seven SARS-CoV-2 rapid antigen tests in detecting omicron (B.1.1.529) versus delta (B.1.617.2) using cell culture supernatants and clinical specimens. Infection. 2023;51:239–245. doi: 10.1007/s15010-022-01844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Michelena P, Torres I, Ramos-García Á, et al. Real-life performance of a COVID-19 rapid antigen detection test targeting the SARS-CoV-2 nucleoprotein for diagnosis of COVID-19 due to the Omicron variant. J Infect. 2022;84:e64–e66. doi: 10.1016/j.jinf.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raïch-Regué D, Muñoz-Basagoiti J, Perez-Zsolt D, et al. Performance of SARS-CoV-2 antigen-detecting rapid diagnostic tests for Omicron and other variants of concern. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.810576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes DM, Bird SM, Cheyne CP, et al. Rapid antigen testing in COVID-19 management for school-aged children: an observational study in Cheshire and Merseyside, UK. J Public Health (Oxf) Published online February 4, 2022 doi: 10.1093/pubmed/fdac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020 [published correction appears in Euro Surveill. 2021 Feb;26(7):] Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponsford MJ, Ward TJC, Stoneham SM, et al. A systematic review and meta-analysis of inpatient mortality associated with nosocomial and community COVID-19 exposes the Vulnerability of immunosuppressed adults. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.744696. [DOI] [PMC free article] [PubMed] [Google Scholar]