Abstract
This study aimed to investigate the association of localized periodontitis with proteinuria in 1281 military young adults in Taiwan. Localized periodontitis was classified as Healthy/Stage I (N = 928) or Stage II/III (N = 353). Stage 2 chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) of 60–89 mL/min/1.73 m2. Proteinuria was defined as protein levels of 2+ or 3+ on the dipstick test. Multiple logistic regression analysis with adjustments for age, sex, body mass index, remaining teeth number and other potential covariates were used to determine the association between localized Stage II/III periodontitis and dipstick proteinuria in patients with and without CKD. Localized stage II/III periodontitis was associated with a higher risk of dipstick proteinuria [odds ratio (OR) and 95% confidence interval: 1.89 (1.04–3.42)], but not with stage 2 CKD. However, the association between localized stage II/III periodontitis and dipstick proteinuria was observed only in patients with stage 2 CKD [OR: 3.80 (1.56–9.27)], while the association was null in participants without stage 2 CKD [OR: 1.02 (0.42–2.45)]. Our findings suggest that among young adults, especially those with a mildly impaired eGFR, localized periodontitis might contribute to acute or chronic kidney injury, which manifests as proteinuria.
Subject terms: Diseases, Nephrology, Risk factors
Introduction
Periodontitis is a polymicrobial disease associated with several pathogenic bacteria in the subgingival biofilm and an imbalance in the microbial ecological environment in the mouth1. The effects of periodontitis are not limited to the oral cavity, and may have a negative impact on systemic health. Over the past few decades, substantial evidence has accumulated on the links between periodontitis and many extra-oral systemic disorders, e.g. obesity2, metabolic syndrome3, and hepatitis4. Chronic low-grade inflammation is a common pathogenic denominator of periodontitis and is associated with systemic disorders5.
Chronic kidney disease (CKD), characterized by a reduced estimated glomerular filtration rate (eGFR) and the presence of microalbuminuria or proteinuria, is a public health problem worldwide, with an estimated global prevalence of 13.4%6. Patients with initial-stage CKD are usually asymptomatic for many years and present with typical complications of renal dysfunction only in more advanced stages7. Periodontitis is a novel and potentially modifiable risk factor for advanced CKD8. Periodontal pathogens may migrate systemically via the bloodstream and affect the endothelial function of the nephrons9,10. Additionally, periodontitis can alter systemic homeostasis through the release of endotoxins and inflammatory cytokines11.
A large body of evidence supports the relationship between generalized periodontitis and severe CKD ≥ Stage 3 in middle-aged and older adults12–15. Since the CKD stage and incidence of periodontitis are relatively low in young adults, there have been few studies regarding this association in this population. Therefore, this study aimed to investigate the association between localized periodontitis and acute or chronic kidney injury in young adults with and without initial CKD.
Methods
Study design and population
The study population was selected from participants of the Cardiorespiratory Fitness and Health in Eastern Armed Forces (CHIEF) oral-health study2–4,16,17, which was performed at the Hualien Armed Forces General Hospital in Taiwan, between 2018 and 2021. This study was reviewed and approved by the institutional review board of Mennonite Christian Hospital (No. 16-05-008) in Hualien City, Taiwan, and written informed consent was obtained from all participants. All participants underwent an annual military health examination including oral health screening and other laboratory tests. Each participant completed a self-administered questionnaire evaluating alcohol consumption, smoking status, and educational level. Alcohol intake and tobacco smoking status were categorized as never, former, or current. The degree of education was defined according to the highest school grade (senior high school, college or university, and postgraduate degree). The present study was conducted in accordance with the tenets of the Declaration of Helsinki, 1975, as revised in 2013.
Physical and laboratory measures
Anthropometric parameters, including waist circumference, body height, and body weight, were measured in the standing position. Body mass index was calculated as body weight (kg) divided by the square of body height (m). Blood pressure (BP) was measured on the right upper arm using an automatic oscillometric machine (Parama-Tech Co Ltd, Fukuoka, Japan). Mean BP was calculated as 1/3 x (2 × systolic BP + 1 × diastolic BP). After a 24-h overnight fast, blood samples were collected from the antecubital vein to measure the total cholesterol, high-density lipoprotein cholesterol (HDL), triglycerides, fasting glucose, creatinine, and blood urea nitrogen levels.
The International Diabetes Federation definition18 identified five main cardiometabolic risk factors for vascular diseases and CKD: (1). low serum HDL, (2). high serum triglycerides or with lipid-lowering therapy, (3) hyperglycemia or with antidiabetic therapy, (4) elevated BP or with anti-hypertensive treatment, and (5) central obesity defined as waist circumference ≥ 90 cm for adult males.
Periodontal assessments
A comprehensive periodontal examination was performed to evaluate the severity of the periodontitis. Third molars and impacted teeth were excluded from the present study. Periodontal status was categorized as Healthy/Stage I or Stage II/III periodontitis according to the 2017 workshop of the American Academy of Periodontology (AAP)/European Federation of Periodontology (EFP)19,. As our population included young adults, periodontitis was localized (< 30% of teeth involved)19 in most participants. Periodontal parameters, e.g. probing depth (PD), clinical attachment loss (CAL) and bleeding on probing (BOP) were reported.
Assessment of kidney function and proteinuria
eGFR was calculated using the abbreviated Modification of Diet in Renal Disease formula20 using the following equation: eGFR = 186 × serum creatinine (mg⁄dL)−1.154 × age (years)−0.203. Renal function was graded based on the eGFR level; normal renal function was defined as an eGFR ≥ 90 mL/min/1.73 m2 and Stage 2 CKD was defined as an eGFR of 60–89 mL/min/1.73 m220.
Random urine samples were obtained using the clean-catch technique in a sterile container prior to venipuncture for blood sampling in the morning to avoid stress confounding the interpretation of proteinuria. Urine proteins were graded using AUTION Dipsticks (ARKRAY. Inc., Shiga, Japan) using an AX-4030 urine-chemistry analyzer (ARKRAY Inc, Shiga, Japan). Urine protein concentrations of 30–99 mg/dL, 100–299 mg/dL, 300–999 mg/dL, and ≥ 1000 mg/dL were labeled as “1 + ,” “2 + ,” “3 + ” and “4 + ,” respectively, based on the clinical classifications recommended by the National Kidney Foundation21. The intra-assay coefficient of variation for proteinuria grading was 6.08%.
Statistical analysis
The clinical profiles of participants with normal renal function and those with Stage 2 CKD are presented as mean ± standard deviation (SD) for continuous data and as numbers (%) for categorical data. Continuous variables were compared using one-way analysis of variance, and categorical variables were compared using the X2 test. Multiple logistic regression analysis was used to determine ORs and 95% CIs of localized Stage II/III periodontitis with Stage 2 CKD and dipstick proteinuria, separately. Multiple linear regression analysis for the associations of eGFR and grades of dipstick proteinuria (absence, 1+ , 2+ , 3+ and 4+) with stages of localized periodontitis (healthy, I, II and III), PD and CAL were also performed, separately. In addition, multiple logistic regression analysis was used to determine the association between Stage II/III periodontitis and dipstick proteinuria in participants with and without Stage 2 CKD. Covariates were adjusted stepwise in three models. In Model 1, age, sex, alcohol intake, smoking, and education level were adjusted. In Model 2, Model-1 covariates plus BMI, mean BP, fasting glucose, total cholesterol, and serum triglycerides were adjusted. In Model 3, Model-2 covariates plus the number of remaining teeth were adjusted. Statistical significance was set at p < 0.05. The SPSS statistical software was used for all the statistical analyses (IBM SPSS Statistics for Windows, Version 25.0; IBM Corp., Armonk, NY).
Results
A total of 1583 participants were enrolled in the present study. Of these, 303 participants were excluded, including patients with age > 45 years (N = 281), Type 1 or Type 2 diabetes (N = 5), a history of chemotherapy (N = 1), < 16 teeth (N = 15), and treated and well maintained periodontitis (N = 1). The final sample consisted of 1280 young men and women, aged 18–45 years. The flow diagram for the selection of study participants is shown in Fig. 1.
Figure 1.
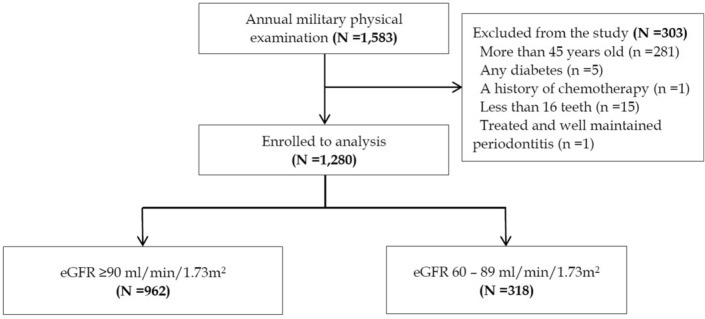
Flow diagram for selection of participants and design of the CHIEF oral health study.
Of the 1280 study participants, 962 (75.2%) had normal renal function, and 318 (24.8%) had Stage 2 CKD, and no one had Stage 3 or Stage 4 CKD (Table 1). Those with Stage 2 CKD were relatively older and had higher levels of several metabolic risk factors, e.g. body mass index, BP, total cholesterol, and serum triglycerides. However, there were no differences in the HDL or fasting glucose levels. Regarding the oral condition, there were relatively fewer remaining teeth in patients with Stage 2 CKD. However, there were no differences in prevalence of bleeding on probing and periodontitis-related indexes (Table 1).
Table 1.
Demographic and clinical characteristics of the study population (N = 1280).
| eGFR ≥ 90 mL/min/1.73 m2 (N = 962) | eGFR 60–89 mL/min/1.73 m2 (N = 318) | p-value | |
|---|---|---|---|
| Age, years | 29.60 ± 6.03 | 32.43 ± 5.17 | < 0.001 |
| Sex, male (%) | 832 (86.5) | 296 (93.1) | 0.001 |
| Smoking, active (%) | 173 (18.0) | 64 (20.1) | 0.39 |
| Alcohol intake, active (%) | 151 (15.7) | 53 (16.7) | 0.68 |
| Education level | |||
| Up to senior high school | 336 (34.9) | 57 (17.9) | < 0.001 |
| College/University degree | 609 (63.3) | 251 (78.9) | |
| Postgraduate degree | 17 (1.8) | 10 (3.1) | |
| Body mass index, kg/m2 | 25.53 ± 3.87 | 26.34 ± 3.39 | 0.001 |
| Systolic blood pressure, mmHg | 121.18 ± 12.65 | 123.50 ± 11.64 | 0.004 |
| Diastolic blood pressure, mmHg | 73.48 ± 10.28 | 75.24 ± 10.11 | 0.008 |
| Mean blood pressure, mmHg | 105.27 ± 11.22 | 107.07 ± 12.11 | 0.01 |
| Total cholesterol, mg/dl | 178.67 ± 32.84 | 191.46 ± 40.48 | < 0.001 |
| High-density lipoprotein, mg/dl | 49.23 ± 10.61 | 48.68 ± 11.27 | 0.42 |
| Serum triglycerides, mg/dl | 124.73 ± 100.80 | 142.47 ± 98.01 | 0.006 |
| Fasting glucose, mg/dl | 92.13 ± 11.08 | 92.88 ± 10.50 | 0.28 |
| Serum creatinine, mg/dl | 0.87 ± 0.11 | 1.08 ± 0.09 | < 0.001 |
| (Range: min–max) | (0.44 – 1.10) | (0.77 – 1.44) | |
| eGFR, ml/min/1.73m2 | 107.61 ± 14.34 | 82.45 ± 5.76 | < 0.001 |
| (Range: min–max) | (90.0 – 186.7) | (60.8 – 89.9) | |
| Blood urea nitrogen, mg/dl | 12.58 ± 2.94 | 13.99 ± 2.95 | < 0.001 |
| Proteinuria (%) | |||
| 1+ | 378 (39.4) | 135 (42.6) | < 0.001 |
| 2+ | 22 (2.3) | 15 (4.7) | |
| 3+ | 6 (0.6) | 10 (3.2) | |
| 4+ | 2 (0.2) | 0 (0.0) | |
| Remaining teeth | 27.25 ± 1.39 | 27.03 ± 1.54 | 0.02 |
| Localized periodontitis, (%) | 477 (49.6) | 191 (60.1) | 0.001 |
| Stage I | 223 (23.2) | 92 (28.9) | 0.03 |
| Stage II | 114 (11.9) | 43 (13.5) | |
| Stage III | 140 (14.6) | 56 (17.6) | |
| BOP (%) | 20.25 ± 9.66 | 27.81 ± 10.64 | 0.10 |
| Probing depth, PD (mm) | 2.54 ± 0.93 | 2.61 ± 0.88 | 0.22 |
| % of teeth with PD ≥ 6 mm | 0.65 ± 0.49 | 0.77 ± 0.15 | 0.95 |
| Clinical attachment loss, CAL (mm) | 2.68 ± 0.91 | 2.67 ± 0.78 | 0.54 |
| % of teeth with CAL ≥ 5 mm | 0.89 ± 0.53 | 1.20 ± 0.47 | 0.98 |
Continuous variables are expressed as mean ± standard deviation (SD), and categorical variables as N (%).
eGFR estimated glomerular filtration rate, BOP bleeding on probing.
Table 2 shows the results of multiple logistic regression analysis for the association of dipstick proteinuria and Stage 2 CKD with localized periodontitis in all participants. There was no association between localized periodontitis and Stage 2 CKD in Models 1–3. In contrast, localized periodontitis was associated with dipstick proteinuria in Model 1 (OR, 1.80; 95% CI, 1.00–3.23), Model 2 (OR, 1.90; 95% CI, 1.05–3.43), and Model 3 (OR, 1.89; 95% CI, 1.04–3.42).
Table 2.
Multivariable logistic regression analysis for stage 2 chronic kidney disease and proteinuria with localized periodontitis in young adults.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| eGFR: 60–89 mL/min/1.73 m2 | 1.11 | 0.83–1.48 | 0.47 | 1.07 | 0.80–1.44 | 0.64 | 1.07 | 0.80–1.44 | 0.64 |
| Dipstick proteinuria 2+ and 3+ | 1.80 | 1.00–3.23 | 0.04 | 1.90 | 1.05–3.43 | 0.03 | 1.89 | 1.04–3.42 | 0.03 |
Data are presented as odds ratios (OR) and 95% confidence intervals (CI) using multiple logistic regression analysis models.
Model 1: age, sex, alcohol intake, smoking and education level adjustments.
Model 2: age, sex, alcohol intake, smoking, education level, BMI, mean blood pressure, fasting glucose, total cholesterol and serum triglycerides.
Model 3: age, sex, alcohol intake, smoking, education level, BMI, mean blood pressure, fasting glucose, total cholesterol and serum triglycerides and remaining teeth.
CKD chronic kidney disease.
Supplementary Table shows the results of multiple linear regression analysis for the associations of eGFR levels and grades of dipstick proteinuria with stages of localized periodontitis, PD and CAL. The results were consistent with Table 2 that there were no associations between levels of eGFR and stages of localized periodontitis in Models 1–3. The associations of grades of dipstick proteinuria with stages of localized periodontitis were marginally significant in Models 1–3 (β = 0.03, p = 0.07, 0.06 and 0.07, respectively). With regard to other periodontal parameters, there were no associations of eGFR and dipstick proteinuria grades with PD and CAL, respectively in models 1–3.
Table 3 shows the results of multiple logistic regression analysis for dipstick proteinuria with localized periodontitis in patients with and without Stage 2 CKD. There was no association between localized periodontitis and dipstick proteinuria in patients without Stage 2 CKD in Models 1–3. However, localized periodontitis was associated with dipstick proteinuria in participants with Stage 2 CKD in Model 1 (OR, 3.56; 95% CI, 1.48–8.60), Model 2 (OR, 3.80; 95% CI, 1.56–9.25), and Model 3 (OR, 3.80; 95% CI, 1.56–9.27).
Table 3.
Multivariable logistic regression analysis for the association of localized periodontitis with proteinuria in young adults with and without early chronic kidney disease.
| eGFR, mL/min/1.73m2 | Prevalence of dipstick | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| proteinuria 2+ or 3+ | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| ≥ 90 | 3.1% (30/962) | 0.99 | 0.42–2.34 | 0.97 | 1.02 | 0.43–2.44 | 0.96 | 1.02 | 0.42–2.45 | 0.96 |
| 60–89 | 7.9% (25/318) | 3.56 | 1.48–8.60 | 0.005 | 3.80 | 1.56–9.25 | 0.003 | 3.80 | 1.56–9.27 | 0.003 |
Data are presented as odds ratios (OR) and 95% confidence intervals (CI) using multiple logistic regression analysis models.
Model 1: age, sex, alcohol intake, smoking and education level adjustments.
Model 2: age, sex, alcohol intake, smoking, education level, BMI, mean blood pressure, fasting glucose, total cholesterol and serum triglycerides.
Model 3: age, sex, alcohol intake, smoking, education level, BMI, mean blood pressure, fasting glucose, total cholesterol and serum triglycerides and remaining teeth.
eGFR estimated glomerular filtration rate.
Discussion
This study is the first to clarify the impact of renal function on the association between localized Stage II/III periodontitis and proteinuria in young adults. The principal finding of this study was that localized periodontitis was associated with a higher risk of proteinuria, a marker of acute or chronic kidney injury, in young adults with Stage 2 CKD, whereas the association was null in participants without Stage 2 CKD.
Previous systematic reviews have revealed an association between kidney health and generalized periodontitis in middle-aged or older adults and indicated that periodontitis might contribute to the development of CKD8,15,22. According to the biological hypothesis of distant pathogenic effects23,24, systemic inflammation is related to generalized periodontitis due to increased exposure time to microorganisms and their products in the blood circulation through persistent bacteremia. Interestingly, elevated levels of IgG directed against periodontal pathogens, such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Treponema denticola, were associated with impaired kidney function25. Long-term exposure to the byproducts of periodontitis and its complications might result in disruption of homeostasis, possibly mediating the deterioration of kidney function. In addition, increased periodontitis-related systemic oxidative stress26, which shares common risk factors with CKD12 is also a possible cause.
Aging affects the progress from localized periodontitis and mildly impaired renal function in young adults to generalized periodontitis and advanced CKD in later life, and this study provides a novel model revealing the association of localized periodontitis with mild CKD in young individuals. The presence of protein molecules in the urine is an early sign of acute kidney injury under stress, for example, surgery27, or represents the progressive deterioration of renal function in middle-aged or older individuals28. Similarly, acute kidney injury can result in dipstick proteinuria29. This study found an association between localized periodontitis and dipstick proteinuria in young adults, specifically in participants with reduced eGFR, highlighting the importance of early treatment of periodontitis in young patients with early CKD. In contrast, the association between periodontitis and proteinuria was null in participants with normal eGFR, probably due to the confounding of exercise-induced proteinuria, which could be considered a benign process in this relatively healthier population21.
The participants in the present study were physically fit military personnel and with greater muscle mass. The findings should be interpreted carefully in this study cohort. Participants with eGFR < 90 mL/min/1.73 m2 had significantly higher creatinine and blood urea nitrogen levels compared with those with eGFR ≥ 90 mL/min/1.73 m2. Some studies had indicated that serum creatinine might not be a good biomarker for measuring kidney function since creatinine is known to be secreted from muscle. In contrast, serum cystatin C is recently reported to be a better biomarker for assessing kidney function instead of serum creatinine30. In addition, a greater BMI level in the military personnel, indicating mild or moderate obesity, may reflect greater muscle mass that could not represent an unhealthy status.
This study had some limitations that need to be addressed. First, although we tried to build various models to clarify the effect of kidney function on the association between periodontitis and proteinuria in young adults, strong evidence of causality could not be obtained due to the cross-sectional design and a presence of any considerable bias. Second, information on serum cystatin C and urine microalbumin levels was lacking. Therefore, we could not perform a sensitivity test for comparison. Third, the results should be highlighted that as the AAP/EFP interpreted the extent a bit differently in 2017 and now. Finally, the findings in the military cohort in the present study may not be applicable to the general population of young adults due to the specificity of the military cohort.
Conclusion
Our findings suggest that among young adults, especially those with a mildly impaired eGFR, localized periodontitis related to systemic inflammation might contribute to acute or chronic kidney injury manifested as proteinuria. The treatment of localized periodontitis in young adults with early CKD might be helpful in preventing acute kidney injury events and deterioration of renal function. Further prospective longitudinal studies are needed for young adults to clarify the association between localized periodontitis, changes in eGFR and the risk of proteinuria.
Supplementary Information
Acknowledgements
This study was supported by the Medical Affairs Bureau, Ministry National Defense and Hualien Armed Forces General Hospital, Taiwan, under the grants MND-MAB-D-11115 and HAFGH-D-111003.
Abbreviations
- CHIEF
Cardiorespiratory fitness and health in armed forces study
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
Author contributions
K.-Z.T. Writing—original draft, Conceptualization, Formal analysis. P.-Y.L. Methodology, Supervision. T.-J.W. Supervision. C.-H.F. Project administration. W.-C.C. Investigation. R.-Y.H. Project administration. G.-M.L. Conceptualization, Investigation, Writing—review & editing, Project administration.
Data availability
As the study materials were obtained from the military personnel in Taiwan, the data were confidential and not allowed to be opened in public. If there are any needs for clarification, the readers can contact Dr. Gen-Min Lin, the corresponding author for sharing the data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-23843-0.
References
- 1.Li L, et al. Periodontitis exacerbates and promotes the progression of chronic kidney disease through oral flora, cytokines and oxidative stress. Front. Microbiol. 2020;12:656372. doi: 10.3389/fmicb.2021.656372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai KZ, et al. Comparisons of various anthropometric indexes with localized stage II/III periodontitis in young adults: The CHIEF oral health study. J. Periodontol. 2021;92:958–967. doi: 10.1002/JPER.20-0275. [DOI] [PubMed] [Google Scholar]
- 3.Tsai KZ, Su FY, Cheng WC, Huang RY, Lin YP, Lin GM. Associations between metabolic biomarkers and localized stage II/III periodontitis in young adults: The CHIEF Oral Health study. J. Clin. Periodontol. 2021;48:1549–1558. doi: 10.1111/jcpe.13555. [DOI] [PubMed] [Google Scholar]
- 4.Tsai KZ, Su FY, Cheng WC, Lin YP, Lin GM. Association between hepatic and systemic inflammation and localized stage II/III periodontitis in young males: The CHIEF oral health study. J. Clin. Periodontol. 2022;49:458–466. doi: 10.1111/jcpe.13556. [DOI] [PubMed] [Google Scholar]
- 5.Holm NR, Holmstrup P, Hansen PR. Comorbidity of periodontitis. Ugeskr. Laeger. 2019;181:V11180758. [PubMed] [Google Scholar]
- 6.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv. Exp. Med. Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 7.Ammirati AL. Chronic kidney disease. Rev. Assoc. Med. Bras. (1992) 2020;66(Suppl 1):S03–S09. doi: 10.1590/1806-9282.66.s1.3. [DOI] [PubMed] [Google Scholar]
- 8.Chambrone L, et al. Periodontitis and chronic kidney disease: A systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J. Clin. Periodontol. 2013;40:443–456. doi: 10.1111/jcpe.12067. [DOI] [PubMed] [Google Scholar]
- 9.Baltch AL, et al. Bacteremia in patients undergoing oral procedures. Study following parenteral antimicrobial prophylaxis as recommended by the American Heart Association, 1977. Arch. Intern. Med. 1988;148:1084–1088. doi: 10.1001/archinte.1988.00380050088015. [DOI] [PubMed] [Google Scholar]
- 10.Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. Am. J. Kidney Dis. 2005;45:650–657. doi: 10.1053/j.ajkd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Bui FQ, et al. Association between periodontal pathogens and systemic disease. Biomed. J. 2019;42:27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deschamps-Lenhardt S, Martin-Cabezas R, Hannedouche T, Huck O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019;25:385–402. doi: 10.1111/odi.12834. [DOI] [PubMed] [Google Scholar]
- 13.Parsegian K, Randall D, Curtis M, Ioannidou E. Association between periodontitis and chronic kidney disease. Periodontol. 2022;2000(89):114–124. doi: 10.1111/prd.12431. [DOI] [PubMed] [Google Scholar]
- 14.Serni L, et al. Association between chronic kidney disease and periodontitis. A systematic review and metanalysis. Oral Dis. 2021 doi: 10.1111/odi.14062. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D, Khawaja AT, Jin L, Li KY, Tonetti M, Pelekos G. The directional and non-directional associations of periodontitis with chronic kidney disease: A systematic review and meta-analysis of observational studies. J. Periodontal Res. 2018;53:682–704. doi: 10.1111/jre.12565. [DOI] [PubMed] [Google Scholar]
- 16.Lin GM, et al. Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in Eastern Taiwan. World J. Cardiol. 2016;8:464–471. doi: 10.4330/wjc.v8.i8.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai KZ, Su FY, Cheng WC, Huang RY, Lin YP, Lin GM. Associations of decayed and filled teeth with localized stage II/III periodontitis in young adults: The CHIEF oral health study. J. Dent. Sci. 2022;17:1018–1023. doi: 10.1016/j.jds.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet P, Shaw J, Group IDFETFC The metabolic syndrome–A new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 19.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 21.Fan CH, et al. Association of single measurement of dipstick proteinuria with physical performance of military males: The CHIEF study. BMC Nephrol. 2020;21:287. doi: 10.1186/s12882-020-01948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapellas K, Singh A, Bertotti M, Nascimento GG, Jamieson LM. Perio-CKD collaboration. Periodontal and chronic kidney disease association: A systematic review and meta-analysis. Nephrology. 2019;24:202–212. doi: 10.1111/nep.13225. [DOI] [PubMed] [Google Scholar]
- 23.Miller W. The human mouth as a focus of infection. Lancet. 1891;138:340–342. doi: 10.1016/S0140-6736(02)01387-9. [DOI] [Google Scholar]
- 24.Scannapieco FA. Position paper of the American Academy of Periodontology: Periodontal disease as a potential risk factor for systemic diseases. J. Periodontol. 1998;69:841–850. [PubMed] [Google Scholar]
- 25.Kshirsagar AV, Offenbacher S, Moss KL, Barros SP, Beck JD. Antibodies to periodontal organisms are associated with decreased kidney function. The dental atherosclerosis risk in communities study. Blood Purif. 2007;25:125–132. doi: 10.1159/000096411. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, et al. Oxidative stress links periodontal inflammation and renal function. J. Clin. Periodontol. 2021;48:357–367. doi: 10.1111/jcpe.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahl TS, et al. Association between preoperative proteinuria and postoperative acute kidney injury and readmission. JAMA Surg. 2018;153:e182009. doi: 10.1001/jamasurg.2018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark WF, et al. Dipstick proteinuria as a screening strategy to identify rapid renal decline. J. Am. Soc. Nephrol. 2011;22:1729–1736. doi: 10.1681/ASN.2010111217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parr SK, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int. 2018;93:460–469. doi: 10.1016/j.kint.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu-Tokiwa A, et al. Serum cystatin C is a more sensitive marker of glomerular function than serum creatinine. Nephron. 2002;92:224–226. doi: 10.1159/000064453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As the study materials were obtained from the military personnel in Taiwan, the data were confidential and not allowed to be opened in public. If there are any needs for clarification, the readers can contact Dr. Gen-Min Lin, the corresponding author for sharing the data.


