Summary
Background
Topical on-demand forms for HIV pre-exposure prophylaxis (PrEP) may be a desirable alternative for people that prefer not to use daily PrEP. CONRAD has developed inserts containing tenofovir alafenamide (TAF) and elvitegravir (EVG) for on-demand vaginal or rectal pericoital use. We assessed the pharmacokinetics (PK) and pre-exposure efficacy of rectally applied TAF/EVG inserts in macaques.
Methods
PK was assessed in 12 pigtailed macaques. Tenofovir (TFV) and EVG levels were assayed in rectal biopsies and secretions, and tenofovir-diphosphate (TFV-DP) levels in biopsies and peripheral blood mononuclear cells (PBMC). Drug biodistribution was evaluated in 10 animals at necropsy 4 h post-dosing. For efficacy assessments, one or two TAF/EVG inserts were administered to macaques (n = 6) 4 h before repeated rectal SHIV162p3 challenges.
Findings
One TAF/EVG insert resulted in rapid and high EVG and TFV-DP in rectal tissue 4 h after application. Adding a second insert led to a 10-fold increase in EVG and TFV-DP in rectal tissue. Efficacy of one and two TAF/EVG inserts were 72.6% (CI 24.5%–92.6%) and 93.1% (CI 73.3%–99.2%), respectively.
Interpretation
Although high TFV-DP and EVG levels were observed with one rectal TAF/EVG insert, it only conferred partial protection from rectal SHIV challenges. Adding a second insert led to an increase in TFV and EVG in rectal tissues resulting in higher (>90%) efficacy. These results highlight the high efficacy of TAF/EVG inserts as topical on-demand rectal PrEP, as well as the need for appropriate drug coverage in the deep rectum and colon to achieve high protection.
Funding
The work related to animal studies was funded by CDC intramural funds and an interagency agreement between CDC and USAID (USAID/CDC IAA AID-GH-T-15-00002). The work related to the insert formulation was funded by U.S. PEPFAR through USAID under a Cooperative Agreement (AID-OAA-A-14-00010) with CONRAD/Eastern Virginia Medical School. The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC), USAID, President's Emergency Plan for AIDS Relief (PEPFAR), Eastern Virginia Medical School (EVMS), or the US government.
Keywords: HIV pre-exposure prophylaxis, Macaque models, Topical PrEP, Tenofovir alafenamide, Elvitegravir
Research in context.
Evidence before this study
PrEP with oral tenofovir disoproxil fumarate/emtricitabine, tenofovir alafenamide/emtricitabine, or long-acting cabotegravir (CAB LA) is highly effective in preventing HIV infection among men who have sex with men. However, studies on user preferences and desirability have stressed the need to develop alternative PrEP modalities that can be used topically and on-demand, such as enemas, suppositories, and fast dissolving inserts. Unlike oral formulations, most topical products tested so far have only provided partial protection against rectal transmission in macaque models.
Added value of this study
In a preclinical macaque model, we demonstrate the potential of inserts containing tenofovir alafenamide and elvitegravir (TAF/EVG) for PrEP against rectal HIV infection. We show a favourable pharmacokinetic (PK) profile in rectal tissues that parallels that observed in vaginal tissues. We found that rectal cleansing improves drug biodistribution. However, we noted that, unlike vaginal protection that can be achieved with one insert, high rectal protection requires the addition of a second insert to increase drug coverage in the rectum and colon.
Implications of all the available evidence
TAF/EVG inserts are a promising new HIV prevention modality that provides dual compartment protection against both vaginal and rectal infection. Compared to vaginal, high rectal protection requires a double dose, most likely due to the need to protect a large surface area.
Introduction
New HIV infections are declining annually but not rapidly enough to meet the 90-90-90 goal that the Joint United Nations Program set on HIV and AIDS.1 In the US, the majority of new HIV infections (66%) are associated with receptive anal intercourse (RAI).2 One of the essential interventions aimed at decreasing HIV transmission is pre-exposure prophylaxis (PrEP) with antiretroviral drugs.3 Several PrEP products have shown high effectiveness among gay, bisexual, and other men who have sex with men (collectively referred to as MSM), including daily oral tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC),3, 4, 5, 6 FTC/tenofovir alafenamide (TAF),7 and injectable long-acting cabotegravir (CAB LA).8 Studies on user preference and desirability among MSM have also documented interest in alternative PrEP products that can be used topically, including douches, rectal suppositories, and fast-dissolving inserts.9, 10, 11, 12, 13, 14
Topical inserts are small, tablet-like products that can be individually packaged for discreet use. Initial efforts to develop inserts were primarily focused on combinations containing tenofovir (TFV) or TFV/FTC.15,16 In macaques, TFV/FTC inserts demonstrated favourable pharmacokinetics (PK) with no signs of inflammation in vaginal mucosa and high drug delivery into vaginal tissues.16 However, a study conducted in humans (CONRAD 117)17 documented slow disintegration and leakage, raising some concerns about product acceptability by end-users. To address these issues, CONRAD has developed a new generation of inserts that contain TAF (20 mg) and elvitegravir (EVG) (16 mg) and display an improved disintegration profile.11 TAF is a potent TFV prodrug that improves the uptake of TFV and increases TFV-DP in cells.18 EVG is a potent strand transfer inhibitor that binds to the preintegration complex with a long dissociation half-life.19 EVG blocks HIV integration that occurs in later stages of infection (>6 h after infection), thus expanding the potential of TAF/EVG inserts for pre-and post-exposure use.20 TAF/EVG inserts are intended for dual compartment vaginal and rectal prevention as pre- and post-exposure prophylaxis (PEP).11 In a Phase I clinical trial in women (CONRAD A18-146),11 TAF/EVG inserts were well accepted and demonstrated rapid local exposure with high drug levels in the vaginal mucosa. An ongoing trial among uninfected men and women (MTN-039) is investigating the safety and PK of TAF/EVG inserts administered rectally.21 In preclinical macaque studies, the same inserts provided 91% efficacy against vaginal SHIV infection at 4 h PrEP and 100% efficacy at 4 h PEP, demonstrating a wide protection window when applied vaginally.22,23 The acceptance by end-users and high efficacy in vaginal pre-clinical studies makes this product very promising for dual compartment application if proven effective in preventing rectal infection. Thus, we extended our studies in macaques to test TAF/EVG inserts efficacy against rectal SHIV transmission and define a protective rectal dose.
Non-human primates have been extensively used to identify strategies that can prevent rectal HIV acquisition. Oral TDF/FTC and FTC/TAF provided complete protection against rectal challenge with SHIV and informed clinical trials in humans.24, 25, 26 The first study with a rectal gel containing 1% TFV documented 67% protection from rectal SIV acquisition in macaques.27 Other studies with a rectal 1% TFV low glycerine gel found 83% protection against SHIV infection28 and provided new information on TFV-DP biodistribution up to 30 cm from the anal sphincter. This study also identified correlates of protection by a topical TFV product, defining tissue TFV-DP (1000 fmol/mg) levels associated with high protection against SHIV. A hypo-osmolar enema containing 30 ml of 5.8 mg/ml TFV protected 5/6 macaques at tissue TFV-DP levels ten times higher than with 1% TFV gel.29 However, although highly protective, none of these products achieved complete protection, suggesting that the anatomical and physiological properties of the gastrointestinal tract may complicate the provision of topical PrEP.10,30, 31, 32 Reasons for these observations might include the need to protect a large surface area of a single-layer columnar epithelium that is highly susceptible to micro-trauma and the presence of a lamina propria beneath the epithelium that is populated with a high density of HIV target cells.33 Other potential explanations may include differences in the pattern of biodistribution of microbicides and HIV within the rectosigmoid cavity. In quantitative imaging studies, migration of HIV-sized particles has been observed up to 60 cm after ejaculation,34 while gel distribution was only observed up to 18 cm from the anal sphincter.34,35 Studies with enemas have also noted heterogeneity in the distribution of TFV and TFV-DP within different sections of colorectal tissue.36
In this study, we investigated in macaques the PK and PrEP efficacy of TAF/EVG inserts applied rectally. Our PK approach included precise drug level measurements in tissue sections collected at different depths from the anal sphincter. We further assessed how rectal cleansing impacts drug biodistribution to understand how sexual behaviours influence drug PK. The efficacy of inserts against SHIV infection was investigated using a validated SHIV challenge model that incorporates repeated virus exposures after either one or two TAF/EVG inserts were applied. The results of this study show that rectal cleansing improves TFV-DP and EVG biodistribution, document partial protection with one insert in the absence of rectal cleansing and demonstrate that adding a second insert increases drug distribution and levels and leads to higher (>90%) protection.
Methods
Macaque procedures
Twenty-nine normally cycling female pigtail macaques five to fifteen years of age were utilised throughout the studies. Only female pigtailed macaques were used in the study to compare the results of rectal and vaginal inserts application in the same animal species. Animals were not randomly assigned; however, they were normalised for weight and age. The average ages were 11, 12, and 13 years (p = 0.119, Kruskal–Wallis test) and the average weights were 7.2, 8.3, and 9.2 kg (p = 0.121, Kruskal–Wallis test) for one insert, two inserts, and placebo groups, respectively. The same six macaques were used for one TAF/EVG insert longitudinal PK and one insert efficacy, and another six macaques were used for two TAF/EVG inserts 24 h PK and two inserts efficacy. Four and three placebo control animals were added to the one and two inserts efficacy studies, respectively. An additional ten SHIV-positive macaques were used for all terminal PK studies (Supplemental Figure S1). The group size for virus challenge experiments was chosen based on previously published results and statistical analysis. A minimum of six animals per group are needed to compare differences between treated animals and controls.26,37, 38, 39
Single-dose PK of TAF/EVG inserts
Tenofovir alafenamide (TAF) and elvitegravir (EVG) rectal inserts containing 22.4 mg of tenofovir alafenamide fumarate equivalent to 20 mg of tenofovir alafenamide and 16 mg of EVG were supplied by CONRAD.15
A three-phase study design with six macaques was implemented to allow for biopsy collections at multiple time points for longitudinal PK assessment of a single TAF/EVG insert placed at 4 cm from the anal sphincter to ensure that insert is at the midway point in the macaque's rectum (Supplemental Figure S1a). A two-week wash-out period preceded each subsequent phase, and 0 h samples were collected in each phase to confirm the complete wash-out of the drugs administered in the previous phase. Before procedures, the animals were anesthetised with 1 mg/kg ketamine. Animals were monitored for product leakage. For studies that required rectal biopsy sampling, 4 mg/kg of Telazol was administered to all macaques. Inserts were placed with a 3-cc syringe plunger at 4 cm from the anal sphincter, followed by the collection of blood and rectal secretions at 0 h, 2 h, 24 h, and 120 h (Phase one); 0 h, 4 h, and 72 h (Phase two); and 0 h, 24 h, and 168 h (Phase three) after dosing. Biopsy samples were taken at 2 h and 120 h (phase one), 4 h and 72 h (phase two), and 24 h and 168 h (phase three) and were analysed for TFV-DP, TFV, and EVG levels.40 PBMC separated from whole blood were analysed for TFV-DP only.
PK of double dose TAF/EVG inserts
Two 20 mg/16 mg TAF/EVG inserts were administered at 4 cm from the anal sphincter in six macaques to ensure that inserts were placed at the midway point of the rectum and to compare tissue drug levels after one and two inserts application (Supplemental Figure S1b). Rectal biopsies were collected 4 h after dosing to record the drug levels at the time of challenge, and blood and rectal swabs were collected at 0 h, 4 h, and 24 h post-dosing. All collections were performed using the same procedures described in the single-dose PK. To assess the impact of rectal douching prior to product placement, a rectal enema of 50 ml sodium chloride 0.85% (physiological saline) was repeated until clear discharge was observed (five or six times). The same procedure was used for rectal cleansing in the terminal studies described below.
Terminal PK procedures
Ten SHIV-positive pigtailed macaques from previous PrEP efficacy studies were used for all terminal PK studies (Supplemental Figure S1c). Animals received either one insert at 4 cm (n = 2) or two inserts at 4 cm and 8 cm (n = 2) from the anal sphincter. The second insert was placed deeper than the first one to investigate whether it improves drug distribution in the rectum and adds coverage in the colon. Animals were euthanised 4 h post-insertion of product, and rectum and sigmoid colon (30–40 cm from anal sphincter) was collected and placed on ice for transport.41 The collected tissue was opened along the length and photographed, and loose faeces were removed to collect rectal tissue samples. Rectal tissues were divided into proximal, medial, and distal sections. Samples (n = 8 per location) were taken at each section approximately 4 cm, 8 cm, and 15 cm from the anal sphincter. Colon samples (n = 8) were collected at 25 cm from the anal sphincter.
To assess the effect of rectal cleansing on drug biodistribution, macaques received either a single (n = 3) or two TAF/EVG inserts (n = 3) with prior rectal wash as described above; all animals were also placed on a high-fibre diet at least three days prior to the procedure to minimise loose stool. Blood was collected 4 h post-dosing. All tissue samples were analysed for TFV, EVG, and TFV-DP.
Specimen processing and drug level detection
Whole blood samples were separated for plasma and PBMC. Mucosal secretions were collected with pre-weighed cotton swabs (Fisher Scientific Company, Pittsburgh, PA). The amount of rectal fluid absorbed was determined by weight prior to and after collection. Rectal biopsy samples were collected with Radial Jaw 4 single-use biopsy forceps (Boston Scientific Corporation, Marlborough, MA). Biopsies were weighed and immediately placed into 0.5 ml of ice-cold 80% MeOH buffer for drug extraction. Biopsy extracts were snap-frozen. Samples were stored at −80 °C until further analysis.42
The concentrations of TAF, TFV, and EVG in plasma, rectal fluids, and rectal biopsies were measured by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (Sciex, Foster City, CA, Shimadzu Scientific, Columbus, MD) and drug concentrations were estimated from a standard curve with a range of 0.5–2000 ng/ml using Analyst software.40,43 The detection limit was 10 ng/ml in plasma and rectal fluids and 1 ng/sample in rectal biopsies. Values were expressed as ng per ml of plasma or fluids and ng per g of tissue. Intracellular concentrations of TFV-DP in biopsies and PBMC were measured by LC-MS/MS with a limit of quantification (LOQ) of 100 fmol/sample and limit of detection of 20 fmol/sample as described previously.28,40,44 Values are expressed as fmol per mg of tissue or fmol/106 PBMCs.45
Efficacy of TAF/EVG inserts
Rectally applied TAF/EVG inserts containing 20 mg TAF and 16 mg EVG were assessed for their ability to protect against once-weekly rectal exposures to 50 TCID50 of R5-tropic SHIV162P3 isolate (ARP-6526, HIV Reagent Program, Manassas, VA; GenBank: KF042063). We did not perform a rectal wash during efficacy studies to avoid possible tissue damage and thus to facilitate the pervasion of the virus that would introduce uncontrolled changes in the value of each challenge in the repeat challenge model. Virus challenges occurred 4 h after the application of the inserts. The insert and the virus inoculum were placed at 4 cm from the anal sphincter. The study assessed a single insert efficacy in four female pigtailed macaques used as placebo controls and six treated animals. Animals were scheduled to receive 10 once-weekly exposures. However, interim analyses concluded that the efficacy was much lower than expected, and the study was terminated early to save animal resources (Fig. 4a).
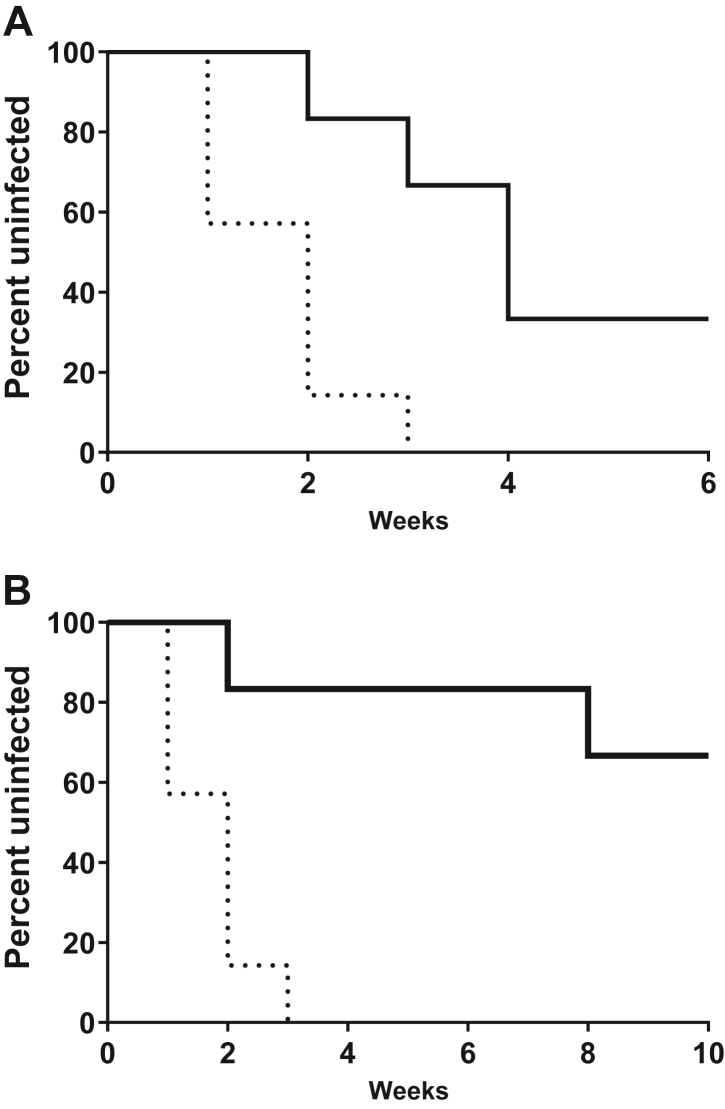
Fig. 4.
Rectal efficacy of TAF/EVG inserts administered 4h before SHIV exposure. Macaques received one or two weekly rectal inserts application and were exposed to SHIV162P3 at 4 h after dosing. The survival curve shows the cumulative percentage of uninfected macaques as a function of the number of weekly rectal SHIV162p3 exposures treated with one (a) and two (b) TAF/EVG inserts. Dashed black and solid black lines represent placebo controls (n = 7) and TAF/EVG insert (n = 6), respectively. Placebo controls were infected after a median of 2 exposures. TAF/EVG 1 insert animals were infected after a median of 4 exposures, and the calculated efficacy was 72.60%, with a 95% exact CI (24.47%, 92.66%); challenges were stopped after six weeks. TAF/EVG 2 inserts animals received 10 challenges. The calculated efficacy was 93.14%, with a 95% exact CI (73.26%, 99.21%). Animals in both arms were followed for an additional 5 weeks to monitor infection.
The second efficacy study was conducted six months later with three additional real-time controls and six treated macaques. All animals received either two placebo or two TAF/EVG inserts placed at 8 cm and 4 cm from the anal sphincter with the virus inoculum introduced at 4 cm. Once weekly exposures to the same virus and dose were administered 4 h after product insertion for a total of ten weeks or until animals were confirmed as virus positive by two consecutive weeks of detectable viral RNA (vRNA) in plasma. All animals in the treatment arm continued to receive inserts for 13 weeks, independent of infection status. Blood was collected at baseline 4 h after dosing to measure drug concentrations. Upon confirmation of SHIV positive status, rectal fluids were collected prior to the blood draw.
Plasma SHIV RNA was monitored by qRT-PCR (Invitrogen SuperScript™ III Platinum™ One-Step qRT-PCR Kit, Fisher Scientific Company, Pittsburgh, PA), as described previously. Briefly, primers amplified the 300-base gag region containing the probe-binding sequence. The vRNA concentration was measured against the standard curve prepared with virus-spiked plasma. The limit of detection for the viral load assay is 50 copies/ml of plasma.46,47 Plasma collections and virus challenges were stopped once the macaque was SHIV RNA positive for two consecutive weeks. Breakthrough infections continued receiving inserts for 8–11 weeks after infection to evaluate risks of TFV resistance due to continued product use during undiagnosed infection. Resistance testing was done by Illumina MiSeq next-generation sequencing (NGS) as previously described.26 Single-nucleotide variants were included at a minimum frequency of 1%.
Statistics
Drug values in rectal tissue were plotted using GraphPad Prism 9.1.2 software, and statistical differences were calculated with the Wilcoxon rank-sum test that compared medians of two groups of independent samples.48 Correlation between drug levels in fluids and tissues were evaluated with a two-tailed Spearman Rank Correlation analysis and plotted with linear regression analysis from GraphPad Prism 9.1.2 software.48 Kruskal–Wallis tests were used to determine if there were any differences in weight (kg) and age (months) between the placebo control, one insert, and two inserts treated arms of the efficacy study. Sample size and power calculation for macaque challenge studies was done by a simulation analysis using the log-rank test (Supplementary Materials). Based on a few simulation scenarios, we determined that the number of animals and the number of challenges used in our study could give us >80% power. Efficacy was defined as the relative decrease in the risk of an adverse event in the exposed group compared to an unexposed group. It can be calculated as 1-RR, where RR is the relative risk computed as the number of infections divided by the total number of challenges. Ninety-five per cent confidence intervals were calculated around efficacy estimates based on the exact method due to the small sample size.49,50 Survival analysis was used to compare time to infection in treated animals relative to the placebo group. Kaplan Meier adjusted survival rates were computed and graphed using the SAS proc lifetest procedure.51 The log-rank test was computed to determine a statistically significant difference in time to infection between (1) the control group that received one insert and the control group that received two inserts and (2) the control and treatment groups. Uninfected macaques at the end of the study were treated as censored observations for survival analysis.52
Ethics
All animal procedures have received prior approval from the Institutional Animal Care and Use Committee (IACUC) of the Centers for Disease Control and Prevention (protocols #3196SMIMONC and #3224SMIMONC) and were conducted in a United States Department of Agriculture (USDA)-registered, Office of Laboratory Animal Welfare (OLAW)-assured, and AAALAC International-accredited animal facility in accordance with the Guide for the Care and Use of Laboratory Animals.53 Euthanasia was performed and confirmed by the attending veterinarian. The animals used for the terminal PK study were anaesthetised with 1 mg/kg ketamine and euthanised using intravenous injection of Pentobarbitol beuthanasia solution (>100 mg/kg). The death was confirmed by monitoring the absence of heart rate and breathing. The procedure was performed in accordance with CDC-Atlanta IACUC Policy 016 on Euthanasia and care guidelines recommended by the American Veterinary Medical Association Guidelines on Euthanasia, 2020.41
Role of funders
The funders of this study had no role in study design, sample collection, and data collection and interpretation, as well as in the writing of the report and decision to submit it for publication.
Results
Rectal PK of single inserts
We investigated the PK of 20 mg/16 mg TAF/EVG inserts following a single rectal dose. Longitudinal analysis of rectal fluids and biopsy samples collected at the same location where the insert was placed (4 cm from the anal sphincter) demonstrated efficient release and bioavailability of TAF and EVG. No leakage of insert content was observed at any time point during the single insert PK study. TAF was rapidly converted into TFV in rectal fluids with peak concentrations observed at the first sample collection (2 h; median 9.1 × 104, range LOQ–6.0 × 105 ng/ml) followed by a half-log decline at 4 h (median 2.8 × 104, range 4.4 × 103–6.1 × 104 ng/ml) and another half-log decline at 24 h. TFV was subsequently undetectable at 72 h and 168 h (Fig. 1a). EVG concentrations in rectal fluids also peaked at 2 h (median 4.6 × 104, range < LOQ–1.0 × 105 ng/ml), remained high at 4 h (median 2.3 × 104, range 6.7 × 103–3.6 × 104 ng/ml) and declined between 24 h and 72 h (Fig. 1b). In rectal tissues, TFV and EVG peaked at 2 h (median 3.9 × 104, range 3.4 × 103–3.9 × 106 and median 1.0 × 104, range 5.0–5.1 × 105 ng/g tissue, respectively) and steadily declined between 4 h and 72 h (Fig. 1c and d). TFV tissue levels at 120 h were unexpectedly high and did not correspond to those in fluids and TFV-DP levels in tissue. TFV and EVG were detectable up to five days after dosing in rectal tissue, although some tissue samples had EVG levels below LOQ.
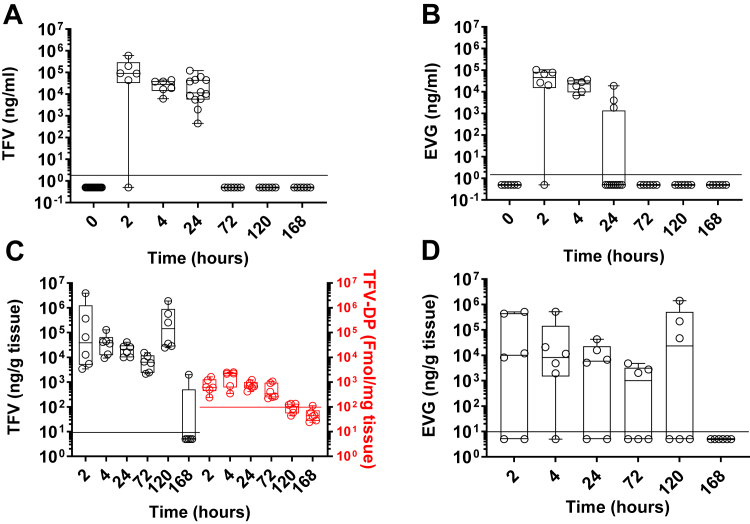
Fig. 1.
Pharmacokinetics of TFV, TFV-DP, and EVG in rectal fluids and tissue after one 20 mg TAF and 16 mg EVG insert dosing. TFV and EVG in rectal fluids (a, b) and tissue (c, d) and TFV-DP in rectal tissue (c right axis) normalised to the weight of the specimen. Each circle represents an individual animal. The horizontal line denotes the median. Values below LOQ (black dotted line for TFV and red dotted line for TFV-DP) were given a value of ½ of the LOQ.
TFV was readily phosphorylated into TFV-DP in rectal tissue (Fig. 1c, red symbols). TFV-DP was detected at the first sample collection at 2 h post-dosing (median 6.1 × 102, range 2.4 × 102–1.6 × 103 fmol/mg tissue) and peaked at 4 h (median 2.2 × 103, range 4.1 × 102–2.5 × 103 fmol/mg tissue). Levels slowly declined for up to three days. They were still above LOQ, although two logs below 4 h levels, seven days after dosing in four of six animals. Levels of TFV-DP in tissue moderately correlated with the presence of TFV in rectal fluids (r 0.5837; p = 0.0002, Spearman Rank correlation) (Supplemental Figure S2a) and strongly correlated with the accumulation of TFV in rectal tissue (r 0.8154; p < 0.0001, Spearman Rank correlation) (Supplemental Figure S2b). Thus, the longitudinal study demonstrated quick absorption and prolonged retention of active metabolites in the tissue at the location where inserts were administered. Overall, 20 mg/16 mg TAF/EVG inserts applied rectally provided levels of EVG in rectal tissue that exceeded the protein adjusted IC9519 by 100-fold and levels of tissue TFV-DP 10 times higher than after 1% TFV gel.28
Although previously tested topical TFV and TDF products did not provide dosing of PBMC,28,54 the detection of TFV-DP in the circulating PBMC was reported after vaginal application of TFV/EVG inserts.23 Thus, we tested whether rectal application of TAF/EVG inserts would provide dosing of circulating PBMC with TFV-DP. We detected TFV-DP in PBMC in time points between 2 h and 120 h post-dosing. However, only at 3 h, 4 h, and 24 h after dosing three or more samples with measurable concentrations were recorded. Still, 30%–50% of samples had levels below LOQ (Supplemental Figure S3). The median level of TFV-DP at 4 h was 98 fmol/106 (range below LOQ–2.9 × 102 fmol/106), comparable to levels observed in macaques between 1 h and 4 h after oral TDF dosing.24
Rectal PK of two inserts
Since rectal protection requires higher tissue drug levels than vaginal protection,28,45 we investigated the rectal PK of two 20 mg/16 mg TAF/EVG inserts applied simultaneously at the same location (4 cm from the anal sphincter) to double the dose (Supplemental Figure S1b). Despite the increase in the amount of placed material, no leakage of the content was detected. Overall, at 4 h, TFV and EVG levels in rectal fluids were ∼1 log higher with two inserts than with one insert (Figs. 1a–c and 2a–c). TFV-DP levels in rectal tissue at 4 h were also one log higher after two inserts dosing (median 6.6 × 104, range 2.8 × 103–5.4 × 105 fmol/mg tissue) compared to tissue with one insert (median 2.2 × 103, range 4.1 × 102–2.5 × 103 fmol/mg) (Figs. 1c and 2c). The detection of EVG in biopsies was inconsistent, with half of the samples having undetectable EVG (Fig. 2d). Overall, EVG tissue levels recorded after two inserts were a half-log higher than one insert and ranged between 1.4 × 104 and 2.7 × 105 ng/g of tissue.
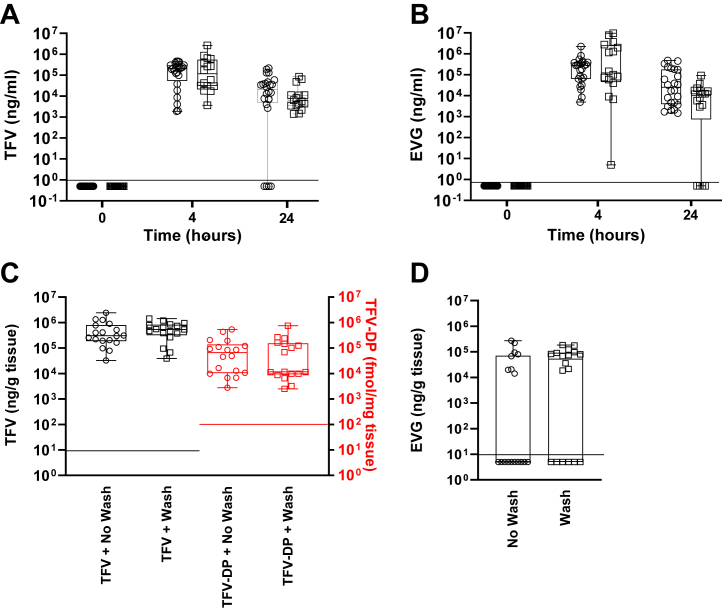
Fig. 2.
Pharmacokinetics of TFV, TFV-DP, and EVG in rectal fluids and tissue after two rectal 20 mg TAF and 16 mg EVG inserts. Median normalised drug levels (horizontal line) in rectal fluids (a, b) and tissue 4 h after dosing (c, d). Circles represent individual samples. Four fluid samples and three tissue samples per animal (n = 6) were plotted. Two rectal inserts were placed at 4 cm deep from the anal sphincter. Circles and squares represent samples after dosing without and with rectal cleansing, respectfully; red symbols are for TFV-DP (d right axis). Values below LOQ (black dotted line for TFV and red dotted line for TFV-DP) were given a value of ½ of the LOQ.
We performed rectal cleansing to assess the influence on PK at 4 h and 24 h when both inserts were placed at the same location. Rectal cleansing did not change median TFV levels in rectal fluids or TFV-DP levels in rectal tissue at 4 h or 24 h post-dosing (Fig. 2a square symbols vs circles and Fig. 2c square symbols vs circles, respectively). Median EVG levels in rectal fluids were not influenced by rectal cleansing (Fig. 2b square symbols vs circles). Notably, median EVG levels in rectal tissue improved, and the number of tissue samples with levels below LOQ decreased (Fig. 2d square symbols vs circles).
TAF/EVG biodistribution in colorectal tissue
Next, we investigated the drug biodistribution in the deeper colorectal tissues following administration of a second insert at 8 cm with and without rectal washing. We analysed EVG, TFV, and TFV-DP concentrations (Fig. 3a–c) in biopsy specimens from tissue sections (4 cm, 8 cm, 12 cm, and 25 cm from the anal sphincter, denoted as R4, R8, R12, and c in Fig. 3) collected at 4 h after dosing (Supplemental Figure S1c) when the peak concentrations of TFV-DP and EVG were recorded during longitudinal PK. With a single insert, median concentrations of EVG did not significantly change with the distance from the drug application site (Fig. 3a, blue symbols). However, the number of samples below LOQ increased at 12 cm and 25 cm sections. TFV values declined with depth with significant differences (p < 0.0001, Wilcoxon rank-sum test) between 4 cm and 25 cm sections (Fig. 3b, blue symbols). TFV-DP in tissue gradually declined with a statistically significant decrease in values between 4 cm and 8 cm sections (p = 0.0330, Wilcoxon rank-sum test) and an insignificant decrease between 8 cm and 12 cm sections (p = 0.2044, Wilcoxon rank-sum test). TFV-DP was undetectable in all colon biopsies collected 25 cm from the anal sphincter (Fig. 3c, blue symbols). Of note, no insert material was noticed in the rectum during necropsy at 4 h after placement.
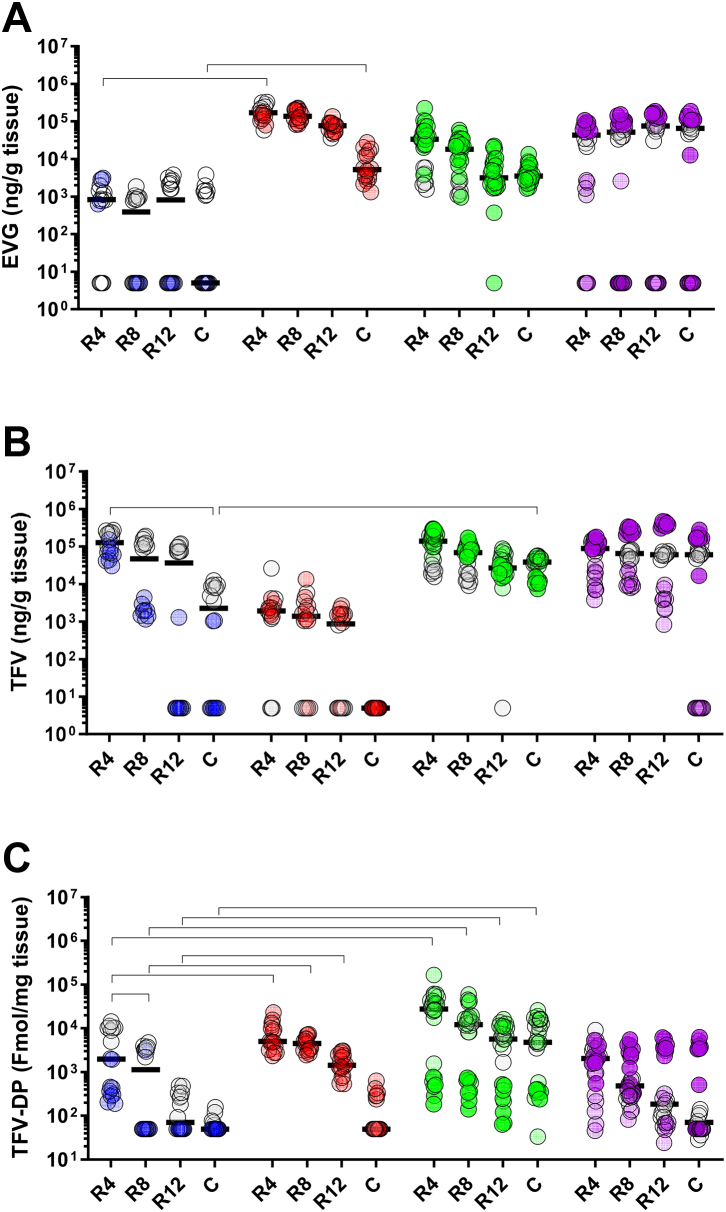
Fig. 3.
Drug distribution in rectal compartment 4 h after TAF/EVG administration and rectal cleansing impact. Rectal biopsies were collected at 4 cm (R4), 8 cm (R8), 12 cm (R12), and 25 cm (c) from the anal sphincter 4 h after dosing. Drug concentrations (n = 8/animal) normalised to biopsy weight are plotted as scattered individual values. Rectal inserts were administered 4 cm deep from the anal sphincter either without prior rectal cleansing (1 insert: n = 2, blue or 2 inserts: n = 2, red) and after rectal cleansing (1 insert: n = 3, green or 2 inserts: purple). The second insert was placed at 8 cm from the anal opening. The horizontal line denotes the median. Zero values were given a value of half of the LOQ. Horizontal lines represent statistically significant differences of p < 0.05 (Wilcoxon rank-sum test).
Since we detected a decline in drug levels with increased depth from the anal sphincter, we investigated if introducing a second insert deeper at 8 cm from the anal sphincter further improved biodistribution in colorectal tissue. Although adding a second insert increased the dosing drug amount only by two times, median concentrations of EVG were significantly increased across all tissue sections by one log (Fig. 3a, red symbols). The median TFV levels were not increased after adding the second insert (Fig. 3b, red symbols). Still, TFV-DP levels after adding the second insert were increased by one log at 4 cm, 8 cm, and 12 cm (p < 0.001, Wilcoxon rank-sum test) sections (Fig. 3c, red symbols). Of note, one of the two animals in the two inserts group had undetectable levels of EVG in the majority of samples and undetectable TFV and TFV-DP in the colon. Further analysis of clinical charts revealed an episode of diarrhoea in this animal.
We then investigated whether rectal cleansing before dosing could improve tissue drug absorption and biodistribution, especially in the deeper sections (Fig. 3, green and purple symbols). When rectal cleansing was administered before one insert application, median levels of EVG were increased by one log in all rectal tissue sections and the colon section from undetected to 3.5 × 103 ng/g compared to animals with no cleansing (Fig. 3a). Rectal cleansing did not change the accumulation of TFV in the rectum but increased in the colon from a median of 2.3 × 103 to 3.9 × 104 ng/g tissue (Fig. 3b). In contrast, levels of TFV-DP increased after rectal cleansing in all rectal tissue sections and from undetectable to 4.8 × 103 fmol/mg in the colon (Fig. 3c). Imaging of the tissue during tissue dissection supported the improvement of tissue dosing after rectal cleansing. Images from two animals that received no rectal cleansing revealed extensive faeces packed in the rectum (Supplemental Figure S4a and b). Rectal cleansing removed most faeces, presumably facilitating drug distribution (Supplemental Figure S4c and d).
Rectal cleansing before two insert applications did not improve drug coverage across rectal tissue except at 25 cm deep into the colon, where tissue concentrations of EVG increased by a log (Fig. 3a). Imaging the tissue during tissue dissection demonstrated that rectal cleansing did not empty the gut completely, as faeces were detected 4 h after cleansing and dosing (Supplemental Figure S4e–h). Altogether, our findings showed that removal of faecal mass with rectal cleansing resulted in better drug dissemination and tissue levels of TFV, TFV-DP, and EVG through the rectum and colon up to 25 cm at 4 h post-dosing.
Rectal inserts protect against rectal SHIV infection
The results of our PK study demonstrated that concentrations of EVG and TFV-DP in rectal tissue were in the range of those associated with protection against vaginal SHIV infection with a once-weekly application of a single insert 4 h prior to virus exposure.23 Therefore, we investigated if the same regimen applied rectally could be protective against rectal SHIV infection. Pigtailed macaques were used instead of rhesus macaques to allow a direct species and virus challenge stock comparison with vaginal efficacy studies with the same inserts. We administered one 20 mg/16 mg TAF/EVG insert at 4 cm to six macaques 4 h before the challenge with SHIV162p3 without rectal cleansing to match the same study design used for the vaginal prevention study.22 Four additional animals received a placebo insert 4 h before the virus challenge. The four placebo controls became infected at one, one, two, and two exposures, and four of the six TAF/EVG animals were infected at two, three, four, and four exposures (Fig. 4a). We analysed TFV-DP levels in PBMC at the time of each challenge and found detectable levels in two out of six animals (median of 75 and 222 fmol/106 cells, Supplemental Figure S3b). Although the study was designed for ten virus exposures, it was terminated after the sixth virus exposure due to the inability to protect macaques.
We then investigated whether adding a second insert would improve biological efficacy. The PK studies conducted with two inserts placed apart have demonstrated increased tissue coverage and absolute levels of TFV-DP and EVG. Thus, six additional macaques received two 20 mg/16 mg TAF/EVG inserts, and three controls received placebo inserts. Inserts were applied at 4 cm and 8 cm depth from the anal sphincter 4 h before the challenge with the same virus dose as the single insert study. The three placebo animals were infected at challenges one, two, and three. In contrast, only two of the six treated animals were infected after ten virus exposures. The two infected animals become SHIV positive from challenges two and eight. We also analysed TFV-DP levels in PBMC at the time of each challenge and found detectable levels in all six animals (median between all animals 373.4 (range 173–1234) fmol/106 cells, Supplemental Figure S3c). The calculated efficacy of one rectal TAF/EVG inserts against rectal infection was 72.6%, with a 95% exact CI of 24.5%, 92.7%.55 The calculated efficacy of two rectal TAF/EVG inserts against rectal infection was 93.1%, with a 95% exact CI of 73.3%, 99.2%. In a repeated low dose model, the efficacy calculation incorporates time as the number of exposures to infection into the formula resulting in higher efficacy than would be obtained if a single high dose exposure model was used.
An analysis of time to infection between the four controls receiving one insert and the three controls receiving two inserts showed no statistically significant difference in time to infection between these two control groups of animals (p = 0.3610, Log-rank test). The median time to infection for the control group with one and two inserts was one and a half and two challenges, respectively. Therefore, we combined the seven placebo-treated animals from the two control groups for statistical evaluations. Infection of animals treated with one insert was delayed compared to placebo controls (p = 0.0046; Log-rank test). In a separate analysis, time to infection was also delayed in animals receiving two inserts relative to placebo controls (p = 0.0022, Log-rank test).
To evaluate whether rectal insert application impacted virus production, we compared viral loads between treated and control groups of infected macaques. Median peak SHIV RNA levels in SHIV-infected animals that received one and two rectal TAF/EVG inserts were 5.4 × 106 (range 2.4 × 105–2.2 × 108) and 1.8 × 106 (range 1.0 × 105–3.6 × 106) copies/ml of plasma, respectively, and were within the range of those seen in animals receiving placebo (median 4.1 × 107, range 8.1 × 105–1.2 × 108 copies/ml) (Supplemental Figure S5). No difference in the plasma viral load set point was noted between treated and placebo animals. Since insert application resulted in measurable TFV-DP in PBMCs (Supplementary Figure S3), we monitored for the emergence of TFV resistance in the breakthrough infections. None of the animals selected for K65R or K70E mutations.
Discussion
On-demand topical modalities for rectal application are an attractive and desirable alternative to daily oral and long-acting injectable PrEP, and studies have shown that some people prefer using inserts to oral dosage forms if their efficacy is demonstrated.12,56 The co-formulation of TAF and EVG in the inserts improves the target product profile and increases the barrier to viral resistance and the window of activity, potentially providing PrEP and PEP protection. Vaginal inserts containing different drug combinations are currently in development for both PrEP and PEP use.11 This work aimed to assess the PK and PrEP efficacy of TAF/EVG inserts applied rectally. To our knowledge, a rectal suppository and an enema, but not topical inserts, were investigated for rectal efficacy. Additional studies may be needed to assess other aspects of rectal insert application, including the PEP option.
Topical interventions rely on the rapid and uniform distribution of antiretroviral drugs into the mucosal tissue. Topical PrEP also requires a high concentration of active drug metabolites in the local mucosa to prevent virus replication in tissue-resident and trafficking target cells and thus prevent virus dissemination and establish a systemic infection. We show here that rectal TAF/EVG inserts readily disintegrate, resulting in EVG and TFV-DP accumulation in the rectal tissue within hours after dosing. We also noted that tissue TFV-DP levels achieved with one insert were two times higher than those achieved with the same TAF/EVG insert in vaginal tissues,22 although rectal EVG levels were ∼10 times lower. Although this PK profile was associated with some rectal protection, applying a second insert 8 cm from the anal sphincter increased EVG and TFV-DP levels and was important to achieve higher (>90%) protection. Two inserts resulted in higher concentrations of EVG and TFV-DP, which reached farther into the rectosigmoid compartment. Together these findings highlight the need to cover a large surface area with effective concentrations of drugs to achieve a high level of rectal protection against HIV.
Our results also illustrate the challenges faced with the rectal application of topical products. The moderate protection observed with one insert was in sharp contrast with the high protection achieved by the same TAF/EVG insert applied vaginally.22 The reasons for the lower than expected rectal efficacy are not fully understood but may include poor drug biodistribution over a large surface area and the presence of faeces that may act as a physical barrier for efficient drug biodistribution and a source for protein binding, particularly for EVG.57 Similar difficulties in achieving a high level of rectal protection were also documented with 1% TFV gel.58 In contrast, rectal TFV enemas used higher amounts of TFV and large volumes leading to better overall drug coverage, flushing of faeces, and high SHIV protection in a repeat dose rectal challenge model.29,59 At this time, however, the TFV enema needs to be prepared right before use.60,61 We noted in our study that TFV-DP levels declined below protective levels in the colon, and tissue EVG levels were not consistently detected. The finding of undetectable TFV-DP in some biopsies collected from the colon, even in the presence of EVG, points to the possible existence of partially protected or unprotected areas in the colon and is in line with imaging data showing uneven biodistribution of TFV-DP in some regions of colorectal tissue.36
The content of the rectosigmoid cavity may also significantly impact the biodistribution of the drugs from inserts. Gao et al. modelled the delivery of TFV from rectal enemas.31 They predicted that faeces would impede the biodistribution of the drugs and that the application of rectal cleansing before rectal medical enema should facilitate drug biodistribution. CONRAD fast-disintegrating inserts are formulated to provide disintegration and drug release by undergoing surface erosion due to water absorption,11 which would be slower than distribution from a liquid-based enema modality. Although rectal douching is common before RAI,60, 61, 62 the impact of rectal cleansing on drug biodistribution has not been thoroughly evaluated in macaques. We showed that rectal cleansing before one insert administration was associated with extended biodistribution of TFV-DP and EVG and increased tissue drug levels in the colon by one log. Rectal cleansing before the application of two inserts also improved EVG levels in the colon by one log. We thus posit that rectal cleansing before insert administration may improve drug coverage and rectal protection. With virus exposures 4 h post product insertion investigated here, however, the benefits of cleansing may not be fully realised in this macaque model. As stated above, rectal cleansing is behaviourally congruent with practices commonly implemented before receptive anal intercourse.60, 61, 62 Thus, future studies to address the impact of rectal cleansing on rectal transmission in the macaque model and the role of time between cleansing, treatment, and virus exposure may be very beneficial.
One limitation of our study is that we did not evaluate whether repeated insert application could lead to some degree of inflammation or irritation in rectal tissues. In some studies with subdermal TAF implants, prolonged exposure to TAF during several months was associated with local adverse events.63,64 These studies, however, involved the continuous and sustained release of TAF within a tight space of subdermal connective tissue, which, unlike vaginal and rectal inserts and oral tablets, concentrates any possible untoward effect on the surrounding tissues. If a shorter duration of TAF (and EVG) exposure associated with intermittent TAF/EVG insert rectal use will also result in some degree of local toxicity is not yet known. The lack of inflammatory changes in the rectal mucosa of rabbits after 14 daily doses in a rabbit rectal irritation model is, however, reassuring. We also didn't evaluate if the efficacy of one insert could be improved by applying a second insert within 24 h after the SHIV challenge. Additional macaque studies can address this question and if a single PEP dose could also provide rectal protection. We also caution that our study did not test a weekly regimen since virus challenges were only done 4 h after product application, which aligns with on-demand PrEP use. Testing a weekly regimen in the macaque model would require a different design incorporating virus challenges done, for instance, 4–5 days after product application. Finally, while residual TFV-DP levels in PBMCs and tissues at day 7 with one insert were at or below the limit of quantification, we did not address if these levels would have increased with the application of two inserts. It is therefore unknown if some of the protection seen in our study might have originated from some residual drug originating from inserts applied the week before. This scenario could also occur if people use on-demand products weekly or more often.
In conclusion, we showed that high concentrations of TFV-DP and EVG from one insert did not result in high rectal protection as was previously observed with vaginal inserts. Adding a second insert, however, improved drug biodistribution and increased efficacy. The inability to fully protect rectally may be associated with uneven tissue biodistribution of TFV-DP and EVG. We further document improved drug coverage with rectal cleansing before application, especially in the colon. Our data highlight the dual-compartment use potential of the TAF/EVG insert, inform dose selection for rectal protection, and support rectal douching before use to enhance drug biodistribution and protection. A clinical safety and PK study of these inserts applied rectally has recently been completed and is currently under analysis (ClinicalTrials.gov: NCT04047420).
Contributors
N.M., W.H., J.G.G-L., M.R.C., J.M.S., and G.F.D: study design, data acquisition and analysis, interpretation of the results, manuscript preparation, and verification of the data, T.S. data acquisition and analysis, and manuscript preparation, A.H., C.D.: data acquisition and analysis, M.M.P.: study design, drug product supplies and coordination, interpretation of the results, V.A.: drug product supplies, interpretation of results, manuscript review, and manuscript preparation, J.M.: animal tech procedures, M.M., Y.P.: statistical analysis and manuscript preparation. All authors read and approved the final version of the manuscript.
Data sharing statement
All data supporting the findings of this study are available within the paper and from the corresponding author upon reasonable request.
Declaration of interests
J.G.G-L and W.H. are named in the US. Government (USG) patents on “Inhibition of HIV infection through chemoprophylaxis” and “HIV post-exposure prophylaxis” and a patent application on “HIV pre-exposure prophylaxis”. W. H. and J. G. G.-L. report royalties or licenses from Mylan, Laurus Generics, TAD Pharma, and CIPLA Limited. M.M.P., V.A., G.F.D., and M.R.C. are named in patent applications on “Pharmaceutical compositions and methods of making on-demand solid dosage formulations,” inventions that were developed under US Agency for International Development (USAID)-funded cooperative agreement. N.M., T.S., J. M., A.H., C.D., M.M., Y.P., and J.M.S., declare no competing interest. The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC), USAID, President's Emergency Plan for AIDS Relief (PEPFAR), Eastern Virginia Medical School (EVMS), or the USG.
Acknowledgements
The authors thank Kristen Kelley, Ryan Johnson, Frank Deyounks, Kamerin Harvey, and Shanon Ellis for animal tech procedures during animal studies and David Garber for animal-related procedures and documentation management. The authors also thank Jonathan Lipscomb for monitoring the emergence of TFV resistance in breakthrough infections. The authors thank Gilead Sciences for the gracious provision of drug substances for CONRAD's development of the topical inserts used in these studies. The work related to animal studies was funded by CDC intramural funds and an interagency agreement between CDC and USAID (USAID/CDC IAA AID-GH-T-15-00002). The work related to the insert formulation was funded by U.S. PEPFAR through USAID under a Cooperative Agreement (AID-OAA-A-14-00010) with CONRAD/Eastern Virginia Medical School.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104338.
Appendix A. Supplementary data
References
- 1.Sidibé M., Loures L., Samb B. The UNAIDS 90-90-90 target: a clear choice for ending AIDS and for sustainable health and development. J Int AIDS Soc. 2016;19(1):21133. doi: 10.7448/IAS.19.1.21133. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Centers for Disease Control and Prevention . Vol. 32. 2021. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (HIV surveillance report, 2019). Available from: [Google Scholar]
- 3.Riddell Jt, Amico K.R., Mayer K.H. HIV preexposure prophylaxis: a review. JAMA. 2018;319(12):1261–1268. doi: 10.1001/jama.2018.1917. [DOI] [PubMed] [Google Scholar]
- 4.Grant R.M., Lama J.R., Anderson P.L., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant R.M., Anderson P.L., McMahan V., et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack S., Dunn D.T., Desai M., et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogbuagu O., Ruane P.J., Podzamczer D., et al. Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet HIV. 2021;8(7):e397–e407. doi: 10.1016/S2352-3018(21)00071-0. [DOI] [PubMed] [Google Scholar]
- 8.Landovitz R.J., Donnell D., Clement M.E., et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carballo-Diéguez A., Giguere R., Dolezal C., et al. Preference of oral tenofovir disoproxil fumarate/emtricitabine versus rectal tenofovir reduced-glycerin 1% gel regimens for HIV prevention among cisgender men and transgender women who engage in receptive anal intercourse with men. AIDS Behav. 2017;21(12):3336–3345. doi: 10.1007/s10461-017-1969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan I. The development of rectal microbicides for HIV prevention. Expert Opin Drug Deliv. 2014;11(1):69–82. doi: 10.1517/17425247.2013.860132. [DOI] [PubMed] [Google Scholar]
- 11.Peet M.M., Agrahari V., Anderson S.M., et al. Topical inserts: a versatile delivery form for HIV prevention. Pharmaceutics. 2019;11(8):374. doi: 10.3390/pharmaceutics11080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauermeister J.A., Downs J.S., Krakower D.S. PrEP product acceptability and dual process decision-making among men who have sex with men. Curr HIV AIDS Rep. 2020;17(3):161–170. doi: 10.1007/s11904-020-00497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauermeister J., editor. Acceptability and choice for 3 placebo products used with receptive anal sex. Conferenceon Retroviruses and Opportunistic Infections (CROI 2021); 2021. Virtual. [Google Scholar]
- 14.MTN . Medica X press; 2021. One size doesn't fit all when it comes to products for preventing HIV from anal sex. [Google Scholar]
- 15.Clark M.R., Peet M.M., Davis S., Doncel G.F., Friend D.R. Evaluation of rapidly disintegrating vaginal tablets of tenofovir, emtricitabine and their combination for HIV-1 prevention. Pharmaceutics. 2014;6(4):616–631. doi: 10.3390/pharmaceutics6040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira L.E., Clark M.R., Friend D.R., et al. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrobial Agents Chemother. 2014;58(5):2665–2674. doi: 10.1128/AAC.02336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauck C.K., Thurman A., Keller M., et al., editors. Pharmacokinetics of tenofovir and emtricitabine delivered by vaginal tablets. Conference on Retroviruses and Opportunistic Infections (CROI 2015); Seattle, WA: 2015. [Google Scholar]
- 18.Ray A.S., Fordyce M.W., Hitchcock M.J. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Adams J.L., Greener B.N., Kashuba A.D. Pharmacology of HIV integrase inhibitors. Curr Opin HIV AIDS. 2012;7(5):390–400. doi: 10.1097/COH.0b013e328356e91c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobard C., Sharma S., Parikh U.M., et al. Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med. 2014;6(227):227ra235. doi: 10.1126/scitranslmed.3007701. [DOI] [PubMed] [Google Scholar]
- 21.MTN . Medica X press; 2021. A phase 1 open label safety and pharmacokinetic study of single dose rectal administration of a tenofovir alafenamide/elvitegravir insert at two dose levels. [Google Scholar]
- 22.Dobard C., Peet M.M., Nishiura K., et al., editors. Protection against vaginal SHIV infection with an insert containing TAF and EVG. Conference on Retroviruses and Opportunistic Infections (CROI 2019); Seattle, Washington: 2019. [Google Scholar]
- 23.Dobard C.W., Peet M., Nishiura K., et al., editors. On-demand HIV postexposure prophylaxis by TAF/EVG vaginal inserts in macaques. Conference on Retroviruses and Opportunistic Infections (CROI 2020); Boston, Massachusetts: 2020. [Google Scholar]
- 24.Garcia-Lerma J.G., Otten R.A., Qari S.H., et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cong M.E., Youngpairoj A.S., Zheng Q., et al. Protection against rectal transmission of an emtricitabine-resistant simian/human immunodeficiency virus SHIV162p3M184V mutant by intermittent prophylaxis with Truvada. J Virol. 2011;85C(15):7933–7936. doi: 10.1128/JVI.00843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massud I., Cong M.E., Ruone S., et al. Efficacy of oral tenofovir alafenamide/emtricitabine combination or single-agent tenofovir alafenamide against vaginal simian human immunodeficiency virus infection in macaques. J Infect Dis. 2019;220(11):1826–1833. doi: 10.1093/infdis/jiz383. [DOI] [PubMed] [Google Scholar]
- 27.Cranage M., Sharpe S., Herrera C., et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5(8):e157. doi: 10.1371/journal.pmed.0050157. discussion e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobard C.W., Sharma S., Cong M.E., et al. Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques. Retrovirology. 2015;12:69. doi: 10.1186/s12977-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao P., Gumber S., Marzinke M.A., et al. Hypo-osmolar formulation of tenofovir (TFV) enema promotes uptake and metabolism of TFV in tissues, leading to prevention of SHIV/SIV infection. Antimicrobial Agents Chemother. 2018;62(1) doi: 10.1128/AAC.01644-17. e01644-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anton P.A., Cranston R.D., Kashuba A., et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28(11):1412–1421. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y., Katz D.F. Multicompartmental pharmacokinetic model of tenofovir delivery to the rectal mucosa by an enema. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0167696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purohit T.J., Hanning S.M., Wu Z. Advances in rectal drug delivery systems. Pharm Dev Technol. 2018;23(10):942–952. doi: 10.1080/10837450.2018.1484766. [DOI] [PubMed] [Google Scholar]
- 33.McGowan I. Microbicides: a new frontier in HIV prevention. Biologicals. 2006;34(4):241–255. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Hendrix C.W., Fuchs E.J., Macura K.J., et al. Quantitative imaging and sigmoidoscopy to assess distribution of rectal microbicide surrogates. Clin Pharmacol Ther. 2008;83(1):97–105. doi: 10.1038/sj.clpt.6100236. [DOI] [PubMed] [Google Scholar]
- 35.Weld E.D., Hiruy H., Guthrie K.M., et al. A comparative pre-phase I study of the impact of gel vehicle volume on distal colon distribution, user experience, and acceptability. AIDS Res Hum Retroviruses. 2017;33(5):440–447. doi: 10.1089/aid.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seneviratne H.K., Hendrix C.W., Fuchs E.J., Bumpus N.N. MALDI mass spectrometry imaging reveals heterogeneous distribution of tenofovir and tenofovir diphosphate in colorectal tissue of Subjects receiving a tenofovir-containing enema. J Pharmacol Exp Ther. 2018;367(1):40–48. doi: 10.1124/jpet.118.250357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otten R.A., Adams D.R., Kim C.N., et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191(2):164–173. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 38.Massud I., Ruone S., Zlotorzynska M., et al. Single oral dose for HIV pre or post-exposure prophylaxis: user desirability and biological efficacy in macaques. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Lerma J.G., Cong M.E., Mitchell J., et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2(14):14ra14. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 40.Jhunjhunwala K., Dobard C.W., Sharma S., et al. Development, characterization and in vivo pharmacokinetic assessment of rectal suppositories containing combination antiretroviral drugs for HIV prevention. Pharmaceutics. 2021;13(8):1110. doi: 10.3390/pharmaceutics13081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NIH . National Institute of Health office of Laboratory Animals Welfare; 2020. AVMA guidelines for the euthanasia of animals: 2020 edition. [Google Scholar]
- 42.Pereira L.E., Singletary T., Martin A., et al. Effects of gel volume on pharmacokinetics for vaginal and rectal applications of combination DuoGel-IQB4012, a dual chamber-dual drug HIV microbicide gel, in pigtailed macaques. Drug Deliv Transl Res. 2018;8(5):1180–1190. doi: 10.1007/s13346-018-0538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massud I., Mitchell J., Babusis D., et al. Chemoprophylaxis with oral emtricitabine and tenofovir alafenamide combination protects macaques from rectal simian/human immunodeficiency virus infection. J Infect Dis. 2016;214(7):1058–1062. doi: 10.1093/infdis/jiw312. [DOI] [PubMed] [Google Scholar]
- 44.Kuklenyik Z., Martin A., Pau C.P., et al. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(29):3659–3666. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Dobard C., Sharma S., Martin A., et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86(2):718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim C.N., Adams D.R., Bashirian S., Butera S., Folks T.M., Otten R.A. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. J Med Primatol. 2006;35(4–5):210–216. doi: 10.1111/j.1600-0684.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 47.Henning T., Fakile Y., Phillips C., et al. Development of a pigtail macaque model of sexually transmitted infection/HIV coinfection using Chlamydia trachomatis, Trichomonas vaginalis, and SHIV(SF162P3) J Med Primatol. 2011;40(4):214–223. doi: 10.1111/j.1600-0684.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.GraphPad . In: Prism G., editor. GraphPad Software; San Diego, California USA: 2020. Kolmogorov-Smirnov unpared nonparametric test and liner regression analysis. (GraphPad prism for windows. 9.1.2 ed). [Google Scholar]
- 49.Qijun Li K., Rice K. Improved inference for fixed-effects meta-analysis of 2 × 2 tables. Res Synth Methods. 2020;11(3):387–396. doi: 10.1002/jrsm.1401. [DOI] [PubMed] [Google Scholar]
- 50.Agresti A., Gottard A. Nonconservative exact small-sample inference for discrete data. Comput Stat Data Anal. 2007;51(12):6447–6458. [Google Scholar]
- 51.SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2013. Base SAS® 9.4 utilities. [Google Scholar]
- 52.Klein J.P., Moeschberger M.L. Springer-Verlag Publishing; New York: 1997. Survival analysis: techniques for censored and truncated data. [Google Scholar]
- 53.National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A . National Academies Press (US); Washington (DC): 2011. The national academies collection: reports funded by National Institutes of Health. Guide for the care and use of laboratory animals. [Google Scholar]
- 54.Dobard C.W., Makarova N., West-Deadwyler R., et al. Efficacy of vaginally administered gel containing emtricitabine and tenofovir against repeated rectal simian human immunodeficiency virus exposures in macaques. J Infect Dis. 2018;218(8):1284–1290. doi: 10.1093/infdis/jiy301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agresti A. A survey of exact inference for contingency tables. Stat Sci. 1992;7(1):131–153. [Google Scholar]
- 56.Traore Y.L., Chen Y., Ho E.A. Current state of microbicide development. Clin Pharmacol Ther. 2018;104(6):1074–1081. doi: 10.1002/cpt.1212. [DOI] [PubMed] [Google Scholar]
- 57.Massud I., Martin A., Dinh C., et al. Pharmacokinetic profile of raltegravir, elvitegravir and dolutegravir in plasma and mucosal secretions in rhesus macaques. J Antimicrob Chemother. 2015;70(5):1473–1481. doi: 10.1093/jac/dku556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobard C.W., Taylor A., Sharma S., et al. Protection against rectal chimeric simian/human immunodeficiency virus transmission in macaques by rectal-specific gel formulations of maraviroc and tenofovir. J Infect Dis. 2015;212(12):1988–1995. doi: 10.1093/infdis/jiv334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng X., Gumber S., Marzinke M.A., et al., editors. Hypo-osmolar rectal enema TFV formulation prevents SHIV acquisition. Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MS: 2018. [Google Scholar]
- 60.Hoang T., Date A.A., Ortiz J.O., et al. Development of rectal enema as microbicide (DREAM): preclinical progressive selection of a tenofovir prodrug enema. Eur J Pharm Biopharm. 2019;138:23–29. doi: 10.1016/j.ejpb.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carballo-Dieguez A., Giguere R., Lentz C., Dolezal C., Fuchs E.J., Hendrix C.W. Rectal douching practices associated with anal intercourse: implications for the development of a behaviorally congruent HIV-prevention rectal microbicide douche. AIDS Behav. 2019;23(6):1484–1493. doi: 10.1007/s10461-018-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carballo-Diéguez A., Lentz C., Giguere R., Fuchs E.J., Hendrix C.W. Rectal douching associated with receptive anal intercourse: a literature review. AIDS Behav. 2018;22(4):1288–1294. doi: 10.1007/s10461-017-1959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatto G.J., Krovi A., Li L., et al. Comparative pharmacokinetics and local tolerance of tenofovir alafenamide (TAF) from subcutaneous implant in rabbits, dogs, and macaques. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.923954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romano J.W., Baum M.M., Demkovich Z.R., et al. Tenofovir alafenamide for HIV prevention: review of the proceedings from the Gates Foundation long-acting TAF product development meeting. AIDS Res Hum Retroviruses. 2021;37(6):409–420. doi: 10.1089/aid.2021.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.