Highlights
-
•
The expression of STEAP1 was up-regulated in osteosarcoma tissues, which positively correlated with the malignant phenotype of osteosarcoma and poor prognosis of patients.
-
•
EFEMP1 could bind to STEAP1 to activate the Wnt/β-catenin and TGF-β/Smad2/3 signaling pathways and induce EMT to promote the progression of osteosarcoma.
Keywords: STEAP1, Osteosarcoma, Wnt/β-catenin signal pathway, TGF-β/Smad2/3 axis, EMT, Invasion, Metastasis
Abstract
Purpose
To investigate the prognostic value and function of six-transmembrane epithelial antigen of prostate 1 (STEAP1) in osteosarcoma and determine whether EFEMP1 mediates its effects.
Methods
IHC (immunohistochemistry)/ICC (immunocytochemistry) in conjunction with RT-qPCR (quantitative real-time polymerase chain reaction) were employed to assess the expression of STEAP1 in paratumoral tissues, osteosarcoma, benign fibrous dysplasia, osteosarcoma cells, normal osteoblastic hFOB cells, as well as various invasive subclones. The association of STEAP1 with outcome was examined with Kaplan–Meier graph among the osteosarcoma population. The effects of the down-regulation and up-regulation of STEAP1 on the biological behavior of osteosarcoma cells were studied through in-vitro and in-vivo functional tests.
Results
Up-regulation of STEAP1 in the osteosarcoma tissues, whose correlations with the malignant osteosarcoma phenotype and the poor patient outcome were positive. In addition, STEAP1 induced the epithelial–mesenchymal transition (EMT) via the Wnt/β-catenin and TGF-β/Smad2/3 pathways and facilitated the osteosarcoma cell infiltration and migration. An increase or decrease in EFEMP1 expression directly promoted or inhibited the expression of STEAP1. In osteosarcoma cells overexpressing EFEMP1, STEAP1 knockdown significantly inhibited cell invasion, EMT process, and increased activity of Wnt/β-catenin and TGF-β/Smad2/3 signaling pathways. Although exogenous EFEMP1 could stimulate the Wnt/β-catenin and TGF-β/Smad2/3 pathways to promote the EMT, it had not effect on osteosarcoma cells with STEAP1 knockdown. Collectively, similar to EFEMP1, STEAP1 acted like an oncogene in the osteosarcoma progression.
Conclusion
EFEMP1 enabled the Wnt/β-catenin and TGF-β/Smad2/3 axises initiation and EMT elicitation by targeting STEAP1, thereby facilitating the osteosarcoma cell infiltration and migration. These results are expected to contribute to the search for new targeted drugs able to effectively inhibit invasion and metastasis and improve prognosis in osteosarcoma.
1. Introduction
Osteosarcoma is a malignant tumor of mesenchymal origin, mainly occurring in children, adolescents, and young adults. It is characterized by early distant metastasis and a poor prognosis [1]. Distant metastasis is found in most patients with osteosarcoma at the time of initial diagnosis, and about 25 % to 35 % of patients without metastasis at diagnosis eventually develop metastasis. The 5-year survival rate in patients with highly metastatic osteosarcoma is extremely low [2], [3]. Therefore, it is necessary to explore the molecular mechanism underlying osteosarcoma occurrence and development and to identify new biomarkers and targeted therapies, with the aim of increasing the range of treatment strategies.
In 1999, Hubert et al. reported that STEAP1 is highly expressed in prostate cancer [4]. STEAP1 belongs to the STEAP protein family, which is currently found to be composed of 5 members, namely STEAP1, STEAP1B, STEAP2, STEAP3 and STEAP4. This family belongs to a class of transmembrane epithelial antigens possessing six transmembrane structures and localized in the cell membrane and cytoplasm. They participate in molecular transport, cell growth, immune responses, and other biological processes. They regulate the growth of tumor cells via intercellular communication and participate in invasion and metastasis in a variety of tumors [5]. Studies have shown that in addition to prostate cancer, STEAP1 is also highly expressed in glioma [6], ovarian cancer [7], gastric cancer [8], and Ewing's sarcoma [9]. Research on STEAP1 is mainly focused on prostate cancer, and antibody drugs targeting STEAP1 have entered clinical trials [10], [11]. However, studies of the relationship between STEAP1 and tumor development in other cancers are still in the initial stages, the differential expression of STEAP1 between normal and cancerous tissues makes it a potential cancer marker and candidate treatment target.
We have previously shown that EFEMP1 facilitated the development of osteosarcoma through the Wnt/β-catenin pathway initiation [12]. Furthermore, an analysis using the Human Epithelial to Mesenchymal Transition (EMT) PCR array has shown that the down-regulation of EFEMP1 by RNA interference significantly inhibits the expression of STEAP1, similar results were obtained by western blotting. Studies of the relationship between STEAP1 and osteosarcoma are lacking. The close relationship between EFEMP1 and STEAP1 prompted us to evaluate whether STEAP1 also promotes the development of osteosarcoma and whether EFEMP1, an extracellular matrix protein, interacts with STEAP1, a membrane protein, and contributes to the osteosarcoma cell infiltration and migration.
2. Materials and methods
2.1. Tissue specimen collection
We selected 88 cases osteosarcoma tissues, 30 cases paratumoral tissues (1–2 cm from the lesion), and 46 cases of bone fibrous dysplasia were selected from the orthopedics department of Shandong University Qilu Hospital. No radiotherapy or chemotherapy was implemented on the patients with osteosarcoma prior to the surgery, and routine follow-up was performed. The research protocol was approved by the Shandong University’s Medical Ethics Committee, and collection of all the tissue specimens was accomplished after receiving the informed consent from patients and their family members.
2.2. Routine cell culture
Human osteosarcoma (HOS, U-2OS) cells were procured from the CAS Cell Bank, along with the normal osteoblastic (hFOB) cells. The subclone HOS-1 with high invasiveness and subclone HOS-29 with minimal invasiveness were screened out from the osteosarcoma cell line HOS by single-cell cloning technology [12]. Cell lines were derived from the same maternal line with high homology and had different invasion and metastasis characteristics. All cell culture operations were performed under aseptic conditions. The cell complete medium was a mixture of 90 % DMEM/F12 and 10 % FBS (fetal bovine serum; Gibco), and double antibodies were added to the medium to prevent contamination at a concentration of 1 %. Cultivation of all the cells was accomplished in a 37 ℃ incubator containing 5 % CO2, and follow-up experiments were conducted when confluence reached 80 %.
2.3. Streptavidin-peroxidase immunohistochemical and immunocytochemical staining
After paraffin tissue sectioning, slices were placed in a baking machine at 65℃ for 1 h to melt the paraffin wax and then placed in xylene for dewaxing twice (15 min each time). The tissue slices were immersed in anhydrous ethanol at a gradient of 90 % and 75 % for hydration. Finally, the slices were placed in 500 mL of 0.01 M citrate buffer for high pressure antigen repair. For the preparation of slides, the logarithmic phase cells were inoculated on coverslips and cultured for 24 h by conventional methods. The coverslips were subjected to careful PBS rinsing and fixation in paraformaldehyde (4 %) for 30 min. Streptavidin-peroxidase (SP) staining procedures for tissue sections and cell slides followed the instructions provided with the SP Staining Kit (ZSGB-BIO, Beijing, China); 3 % H2O2 was added to inactivate endogenous peroxidase, and goat serum working fluid was added to block non-specific binding. The mouse anti-human STEAP1 monoclonal antibody (ab207914; Abcam, Cambridge, UK) was diluted with the dilution solution at a ratio of 1:400 and dropped onto the tissue sections and cell slides. After overnight culture at 4 ℃, a 30-min incubation proceeded at 37 ℃ by dropping the biotin-labeled goat anti-mouse working buffer. After PBS cleaning, diaminobenzidine was added for visualization. When brown particles were observed, tap water terminated the staining reaction and hematoxylin was added to restain nuclei. The slices were dehydrated in ethanol, dried at 25 ℃, and fixed with sealing glue. The staining status of each sample was examined using an optical microscope. Three fields of vision were randomly selected, and scoring and rating of the staining outcomes were accomplished according to the intensity of staining and the ratio of positive cells per field. The staining intensity was assigned a score of 0–3 points indicating completely negative, light brown, brown, and dark brown, in order. The frequency of positive cells was assigned 0 to 3 points, indicating 0 %, 0–25 %, 25–75 %, and ≥ 75 %. The total expression score ranged from 0 to 6, with values<3 indicating low expression and values greater than or equal to 3 indicating high expression. Two pathologists scored the slices separately, and inconsistent results were resolved through discussion.
2.4. RNA extraction, reverse transcription, and RT-qPCR
The logarithmic phase cells were harvested into Eppendorf (EP) tubes, and 1 mL RNAiso PLUS (TaKaRa Biotechnology, China) was added to fully lyse cells for total RNA extraction. Subsequently, a microplate reader was utilized to assess the density and purity of RNA. According to the instructions provided with the reverse transcription kit (TaKaRa Biotechnology), a 20-µl reaction system (2 µl of total RNA solution, 8 µl of DNA removal mixture, and 10 µl of reverse transcription mixture) was prepared and placed in a PCR amplification apparatus to reverse transcribe RNA for 15 min at 37 ℃ and for 5 s at 85 ℃. Design and synthesis of the primers for internal reference (β-actin) and target genes were completed by TaKaRa Bioengineering Co., ltd., and Table 1 details the primer sequences. For PCR amplification, 20 µl of the reaction solution was prepared on ice, Including 10 µl of SYBR® Premix Ex Tap, 7.2 μl of diethyl pyrocarbonate-treated water, 2 µl of cDNA template, and each 0.4 µl of PCR Forward and Reverse Primers. The special 96-well plate was used to added the reaction solution and placed in the real-time fluorescence quantitative detector (ABI Prism SDS 7000, Applied Biosystems Inc). After DNA amplification, fluorescence signals were monitored in real time and Ct values were calculated. Light Cycler 480 System was used to analyze the data and obtain the relative expression levels of genes. The EMT PCR Array (QIAGEN) was exploited for assessing the expressional variations of EMT-associated genes prior to and after EFEMP1 down-regulation.
Table 1.
The sequence of primer in qRT-PCR.
| Primer name | Sequences |
|---|---|
| STEAP1 | F: 5ʹ‐ACAAGTTGCTAAACTGGGCATATCA‐3ʹ R: 5ʹ‐CAGTATTGCCAATCCCACAATTC‐3ʹ |
| CDH1 | F:5′-GGATTGCAAATTCCTGCCATTC-3′ R:5′-AACGTTGTCCCGGGTGTCA-3′ |
| CDH2 | F:5′-CGAATGGATGAAAGACCCATCC-3′ R:5′-GCCACTGCCTTCATAGTCAAACACT-3′ |
| VIM | F:5′-AACCTGGCCGAGGACATCA-3′ R:5′-TCAAGGTCAAGACGTGCCAGA-3′ |
| SNAIL | F:5′-GCTCCCTCTTCCTCTCCATACC-3′ R: 5′-AAGTCCTGTGGGGCTGATGT-3′ |
| SLUG | F: 5′-GAAGCATTTCAACGCCTCCAA-3′ R: 5′-GTTGTGGTATGACAGGCATGGAGTA-3′ |
| TWIST | F: 5′-CAGCTACGCCTTCTCGGTCT-3′ R: 5′-CTGTCCATTTTCTCCTTCTCTGG-3′ |
| ACTB | F: 5′-TGGCACCCAGCACAATGAA-3′ R: 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
2.5. Protein extraction and western immunoblotting
Logarithmic growth phase cells were collected into EP tubes, and premixed lyse (RIPA: PMSF was 100:1) was added for total protein extraction. Subsequently, bicinchoninic acid kit (BOSTER) was exploited for the protein density quantification. Then, 4 × loading buffer equivalent to 1/3 of the volume of protein was added and mixed, and the protein was denatured in a metal bath at 100 ℃. For electrophoresis, a 10 % separation gel was prepared; according to the estimated protein concentration, a loading amount of 40 μg/lane was used. A marker protein was added to indicate the positions of different molecular weight bands, and electrophoresis was performed at 100 V until the protein sample ran to the gel bottom. This was followed by shifting of the isolated proteins to the PVDF (polyvinylidene fluoride) membrane treated with methanol at a constant flow of 240 mA for 40 min. After membrane transfer, the PVDF membrane was taken out, blocked with 5 % skim milk for 1 h, placed in a primary antibody working solution (EFEMP1 ab256457, STEAP1 ab3679, β-catenin ab32572, GSK3β ab32391, GSK3β-pS9 ab75814, C-myc ab32072, Cyclin D1 ab134175; Abcam; EMT Antibody Sampler Kit #9782, Smad2/3 #8685, p-Smad2/3 #8828, Cell Signaling Technology; Twist 25465–1-AP, Proteintech) diluted following the instructions on the manual, and incubated at 4 ℃ overnight. After washing with TBST (Tris-Buffered Saline with Tween), the membrane was incubated in the secondary antibody working solution at 25 ℃ for 1 h. After TBST cleaning, the protein bands were displayed via enhanced chemiluminescent substrate kit (Millipore), and ImageJ was used to process the gray values.
2.6. Lentivirus transfection, RNA interference, and over-expression assay
Shanghai Genechem Co., ltd was the designer and supplier of the LV-STEAP1-RNAi virus and LV-STEAP1 over-expressed virus used in this experiment. STEAP1-shRNA cells were obtained by knockdown of STEAP1 expression in osteosarcoma subclone HOS-1, and the osteosarcoma subclone HOS-29 was transfected with the STEAP1-over-expressing lentiviral vector to endogenously increase the expression of STEAP1 and obtain EX-STEAP1 cells. Each 3,000–5,000 target cells in good condition were planted per well of 96-well microplates, and the transfection experiments were performed following 24 h of cultivation. The MOI (multiplicity of infection) was set to 50, and the virus volume was figured by the following equation: virus bulk = (MOI × number of cells)/virus titer. After 12 h of infection, fresh culture medium was added in place of the original culture medium containing the virus to attenuate virulence. At approximately 72 h after transfection, as observed microscopically (fluorescence), the transfection efficiency reached about 80 % with good cellular growth, indicating effective transfection. The cell culture was continued, mRNA and protein were extracted, and the down-regulation and up-regulation of STEAP1 were verified by RT-qPCR, western blotting, and immunocytochemistry (ICC).
2.7. Transwell chamber invasion and migration assay
A Transwell chamber invasion test was performed as follows. The fresh complete medium was used to dilute Matrigel at the ratio of 1:5, and 50 μl of diluted solution was evenly added to the upper compartment filter membrane of the Transwell chamber, while the upper chamber was added with 200 μl of cell suspension (about 2 × 105 cells). After 24-h serum-free cultivation of NIH3T3, only the medium was sucked out for filtration, 600 μl of which was dispensed as chemokine into the lower chamber. Cultivation was accomplished at 37 ℃ on the chambers with 5 % CO2 for 24 h and then taken out; after swabbing the cells in the upper chamber that had not penetrated the membrane, a 30-min fixation of the chambers proceeded in paraformaldehyde (4 %), followed by a 15-min crystal violet staining. The chamber membranes were subsequently washed, dried, cut off and sealed by neutral balsam with the bottom facing up. Five randomly-selected fields were subjected to microscopic observation, and the cells crossing the membrane were counted. For the Transwell chamber migration test, the upper chamber filtration membrane was not covered by Matrigel, while the remaining procedures were identical to the transwell invasion assessment. The experiments were repeated 3 times.
2.8. Cell proliferation assay
Differences in cellular multiplication before and after transfection were assessed via the CCK8 kit (Beyotime Institute of Biotechnology, China). The cell suspension was prepared and 2000 cells/well were inoculated into a 96-well culture plate. Every 24 h, 8 wells from each group were supplemented with CCK-8 buffer (10 µl). Two h later, a microplate reader was utilized to evaluate the 450-nm optical density for four consecutive days, and the proliferation curve was drawn based on the detection results. The experiments were repeated 3 times.
2.9. Cell plate clone assay
A cell suspension was prepared, and cells were seeded on 6-well microplates at 1000 cells/well. After mixing gently, the plate was placed in an incubator at 37℃. The culture period was about 14 days, during which the growth status was observed. After that, the medium in the plate was removed. The cells in the microplate were subjected to a 30-min fixation in paraformaldehyde (4 %), and a subsequent 10-min crystal violet staining. The plate was placed on a white background to count the colonies in each well. Three repeated wells were set for each group and the number and size of colonies formed in each group were compared.
2.10. β-Catenin/TCF dual luciferase reporter assay
TOPflash and FOPflash (Upstate Cell Signaling Solutions) are ofen utilized to assess β-catenin/TCF transcriptional activity. The EX-STEAP1-transfected HOS-29 cells and negative control cells were co-transfected with TOPflash or FOPflash plasmids and pRL-TK internal control plasmid (Promega) mediated by Lipofectimine 2000 (Invitrogen). The β-catenin/TCF transcriptional activity was determined through method of relative activity of luciferase. The experiment was carried out in triplicate and corresponding results are expressed relative to activity in controls.
2.11. Tumor transplantation in nude mice
Fifty 3–4 weeks old SPF BALB/C-nu/nu nude mice with no difference in activity status and body weight were assigned to five groups according to the principle of random allocation: HOS-1, HOS-1 STEAP1-shRNA1 and STEAP1-shRNA2 groups, and HOS-29 and HOS-29 EX-STEAP1 groups. Approximately 5.0 × 106 well-grown cells were injected subcutaneously into 5 nude mice in each group to establish the subcutaneous xenograft tumor model, and the other 5 nude mice received an intravenous injection via the lateral tail vein (2.0 × 106 cells/animal) to establish the lung metastasis model. During the normal feeding period, the status of nude mice was observed, and the tumor size was measured with a vernier caliper on a weekly basis. Eight weeks later, all nude mice were sacrificed by carbon dioxide euthanasia, and tumors and lung tissues were made into paraffin embedded sections for immunohistochemistry or hematoxylin eosin (H&E) staining. The computational formula for tumor volume was: tumor volume = length × width2 × 0.5. Thereafter, the tumor growth was described with time (abscissa)-dependent graph of tumor volume (ordinate). All murine experiments were approved by Shandong University’s Institutional Animal Care and Use Committee and conducted following the Declaration of Helsinki principles, as well as the Regulations on the Control of Laboratory Animals.
2.12. Statistical analyses
Every experiment was triplicated (at least), and repeated groups were set up. The counting data was expressed by ratio or constituent ratio, and the measurement data was displayed as means ± SDs (standard deviations). Experimental data analysis was accomplished with the aid of SPSS 26.0. Pairwise comparisons between independent samples were made by Wilcoxon rank sum test, while multiple comparisons between independent samples were achieved through Kruskal-Wallis test. The Chi-square test was employed for inter-rate comparison, while the Kaplan-Meier and Log Rank procedures were employed for survival assessment of cervical cancer patients, and GraphPad Prism 8.0.2 was used for plotting. Differences were regarded as significant when p < 0.05.
3. Results
3.1. EFEMP1 down-expression or over-expression influenced STEAP1 expression
The influence of EFEMP1 on EMT was assessed with EMT PCR array by comparing gene expression differences before and after suppression by RNA interference. In addition to significantly affecting a few crucial EMT effectors (e.g. N-cadherin, E-cadherin and vimentin), the down-regulation of EFEMP1 expression influenced the levels of other genes; for example, FGFBP1, KRT7, and WNT11 were up-regulated, while EGFR, FN1, ILK, and STEAP1 were down-regulated in response to EFEMP1 knockdown. There was a > 5-fold difference in STEAP1 expression between the EFEMP1 knockdown and control groups (Fig. 1A). Western blotting further showed that the expression of STEAP1 decreased after EFEMP1 was knocked down and increased when EFEMP1 was over-expressed (Fig. 1B). In osteosarcoma cells, EFEMP1 knockdown significantly inhibited the expression of STEAP1. We further evaluated whether the extracellular matrix protein EFEMP1 and membrane protein STEAP1 interact to exert biological effects. Immunoprecipitation results showed that EFEMP1 could interact with STEAP1 (Fig. 1C). We have previously found that EFEMP1 could activate EMT to promote the progression of osteosarcoma [12]. STEAP1 might be a key membrane protein that binds to EFEMP1 to facilitate the osteosarcoma cell infiltration and migration.
Fig. 1.
EFEMP1 down-expression or over-expression influenced STEAP1 expression. (A) The influence of EFEMP1 on EMT was assessed with EMT PCR array by comparing gene expression differences before and after RNA interference suppression. (B) By western blotting, the expression of STEAP1 decreased after EFEMP1 was knocked down, while their expression increased when EFEMP1 was over-expressed. (C) According to immunoprecipitation findings, EFEMP1 could mutually combine with STEAP1.
3.2. Expression of STEAP1 in osteosarcoma, osteofibrous dysplasia, and paratumoral tissues
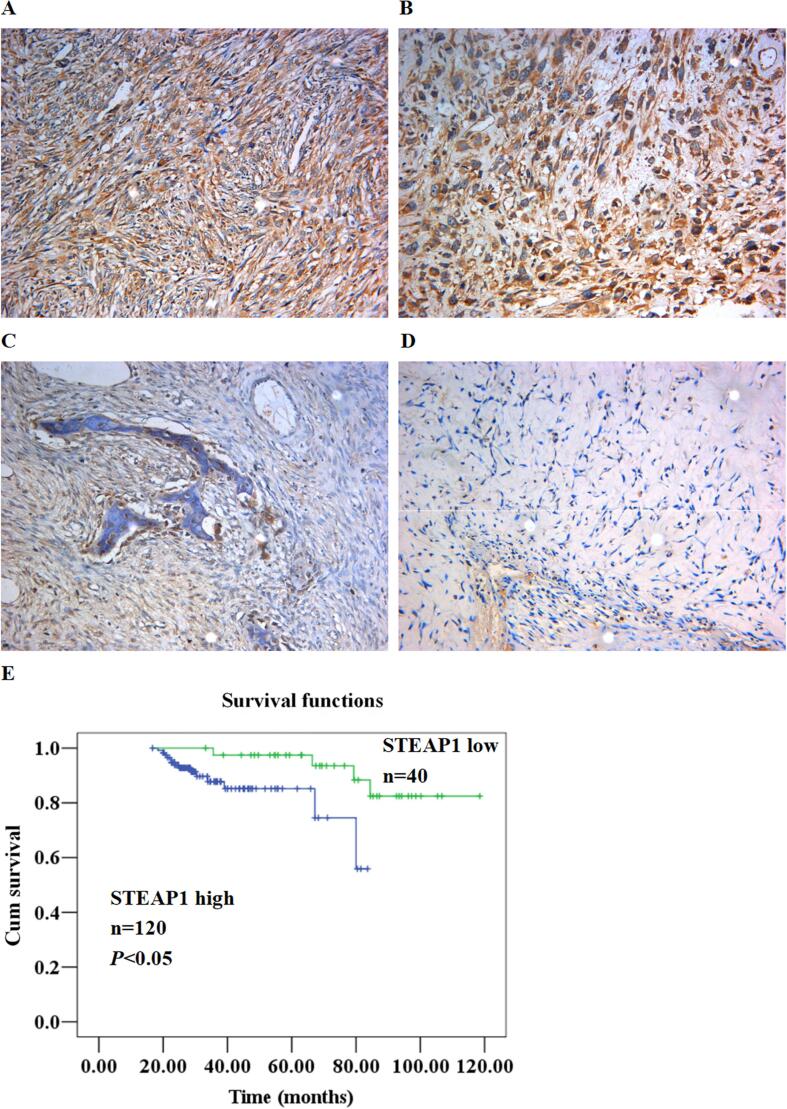
The STEAP1 levels of in osteosarcoma, osteofibrous dysplasia, and paratumoral tissues were determined by an immunohistochemical assay. In the majority of osteosarcoma tissues, the STEAP1 protein level was prominently elevated (Fig. 2AB) but low in osteofibrous dysplasia (Fig. 2C) and paratumoral tissues (Fig. 2D). The strong positive expression of STEAP1 was enriched primarily in the cytoplasm and cellular membrane of osteosarcoma samples. STEAP1 expression showed no difference among pathological types of osteosarcoma. However, high expression of STEAP1 positively correlated with positive lymph node metastasis and low differentiation of osteosarcoma, as shown in Table 2. For the association exploration of STEAP1 with the osteosarcoma patient outcome, the survival assessment was accomplished through the Kaplan-Meier and Log Rank procedures. As revealed by the results, prognosis was remarkably better for the patients expressing STEAP1 protein lowly than those expressing STEAP1 protein highly (Fig. 2E).
Fig. 2.
Expression of STEAP1 in osteosarcoma, osteofibrous dysplasia, and paratumoral tissues. The STEAP1 levels in osteosarcoma (AB), osteofibrous dysplasia (C), and paratumoral tissues (D) were determined with immunohistochemical assay. (E) According to Kaplan-Meier and Log Rank assessments, the outcome of osteosarcoma patients expressing STEAP1 protein lowly (green line) was prominently better than those expressing STEAP1 protein highly (blue line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Protein expression of STEAP1 in human osteosarcoma tissues.
| N | STEAP1 low (-/+) |
STEAP1 high (++/+++) |
X2 | P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Normal tissue | 60 | 52 | 86.7 % | 8 | 13.3 % | 95.1 | <0.01 |
| Fibrous dysplasia | 70 | 55 | 78.6 % | 15 | 21.4 % | ||
| Osteosarcoma | 160 | 40 | 25.0 % | 120 | 75.0 % | ||
| Pathological type | 1.0 | >0.05 | |||||
| Fibroblastic osteosarcoma | 56 | 13 | 23.2 % | 43 | 76.8 % | ||
| Osteoblastic osteosarcoma | 54 | 12 | 22.2 % | 42 | 77.8 % | ||
| Chondroblastic osteosarcoma | 50 | 15 | 30.0 % | 35 | 70.0 % | ||
| Cell differentiation | 24.3 | <0.01 | |||||
| High and intermediate | 82 | 34 | 41.5 % | 48 | 58.5 % | ||
| Low | 78 | 6 | 7.7 % | 72 | 92.3 % | ||
| Nodal status | 23.5 | <0.01 | |||||
| Positive | 96 | 11 | 11.5 % | 85 | 88.5 % | ||
| Negative | 64 | 29 | 45.3 % | 35 | 54.7 % | ||
3.3. Expression of STEAP1 in osteosarcoma cell lines, normal osteoblast cells, and subclones with differences in invasiveness
Using ICC (Fig. 3A), qRT-PCR (Fig. 3B) and western blotting (Fig. 3C), STEAP1 mRNA and protein levels were estimated in osteosarcoma cells U-2OS with low invasive ability and HOS with highly invasive ability [12], as well as normal osteoblastic cells hFOB. STEAP1 expression was weakest in hFOB cells, slightly higher in U-2OS cells than in normal cells, and strongest in the most aggressive HOS cells. Meanwhile, the STEAP1 levels in the subclone HOS-1 with high invasiveness and subclone HOS-29 with minimal invasiveness were also measured by ICC (Fig. 3D), qRT-PCR (Fig. 3E), and western blotting (Fig. 3F). STEAP1 expression was higher in HOS-1 cells than in HOS-29 cells. Consistent with immunohistochemical results for clinical tissue samples, STEAP1 expression was highest in osteosarcoma cells with high invasive ability. To probe deeper into the role of STEAP1 in the osteosarcoma infiltration and metastasis, the STEAP1 expression in the HOS-1 subclone was down-regulated via the RNA interference mediated by lentivirus, while its expression in the HOS-29 subclone was increased through lentivirus-mediated over-expression. Cell proliferation and invasion ability were compared with and without STEAP1 gene knockdown or over-expression by in vivo and in vitro functional experiments.
Fig. 3.
Expression of STEAP1 in osteosarcoma cell lines, normal osteoblast cell line, and subclone cells with different invasiveness. By ICC (A), qRT-PCR (B), as well as western-blot (C), the STEAP1 mRNA and protein levels were estimated in osteosarcoma (U-2OS and HOS) and normal osteoblastic (hFOB) cells. Higher STEAP1 level was detected in U-2OS and HOS cells compared to those in the hFOB cells. Besides, the STEAP1 levels in the highly-invasive HOS-1 and the lowly-invasive HOS-29 subclones were also determined with ICC (D), qRT-PCR (E), as well as western-blot (F). The level of STEAP1 in the HOS-1 subclone was higher than that in lowly invasive subclone HOS-29. *P < 0.05.
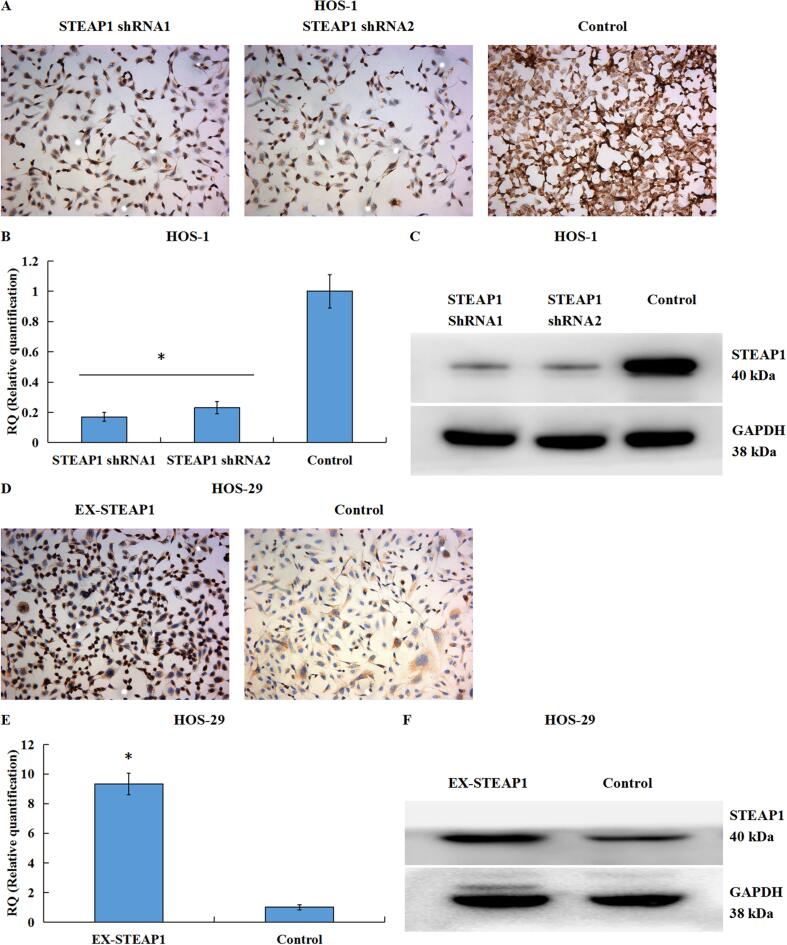
3.4. Validation of STEAP1 downregulation or upregulation in lentivirus knockdown or over-expression transfection systems
HOS-1 and HOS-29 cells were transfected with lentiviral vectors with shRNA1 and shRNA2 targeting STEAP1 and with the EX-STEAP1 over-expression lentiviral vector, respectively. After culturing for 72 h, the cell fluorescence was examined by fluorescent microscopy, and a high transfection efficiency was defined as strong fluorescence in>80 % of cells. The transfected osteosarcoma cell expression of STEAP1 was detected by ICC (Fig. 4A), qRT-PCR (Fig. 4B), and western blotting (Fig. 4C), to verify the effect of RNA interference. The STEAP1 mRNA and protein levels in the HOS-1-shRNA1 and HOS-1-shRNA2 cells were prominently lower than those in the negative controls. Moreover, using ICC (Fig. 4D), qRT-PCR (Fig. 4E), as well as western blotting (Fig. 4F), the STEAP1 mRNA and protein levels in HOS-29-EX-STEAP1 and control cells were also measured to verify the effect of over-expression transfection. STEAP1-over-expressed cells were successfully obtained for subsequent experiments.
Fig. 4.
Identification of down-regulated or up-regulated STEAP1 expression in lentivirus knockdown or over-expression transfection systems. By ICC (A), qRT-PCR (B), as well as western-blot (C), the STEAP1 mRNA and protein expressions in HOS-1-shRNA1 and HOS-1-shRNA2 cells significantly decreased in contrast to those in the negative controls. Moreover, through a combination of ICC (D), qRT-PCR (E) and western-blot (F), the STEAP1 mRNA and protein expressions in HOS-29-EX-STEAP1 and control cells were also measured to verify the effect of over-expression transfection. *P < 0.05.
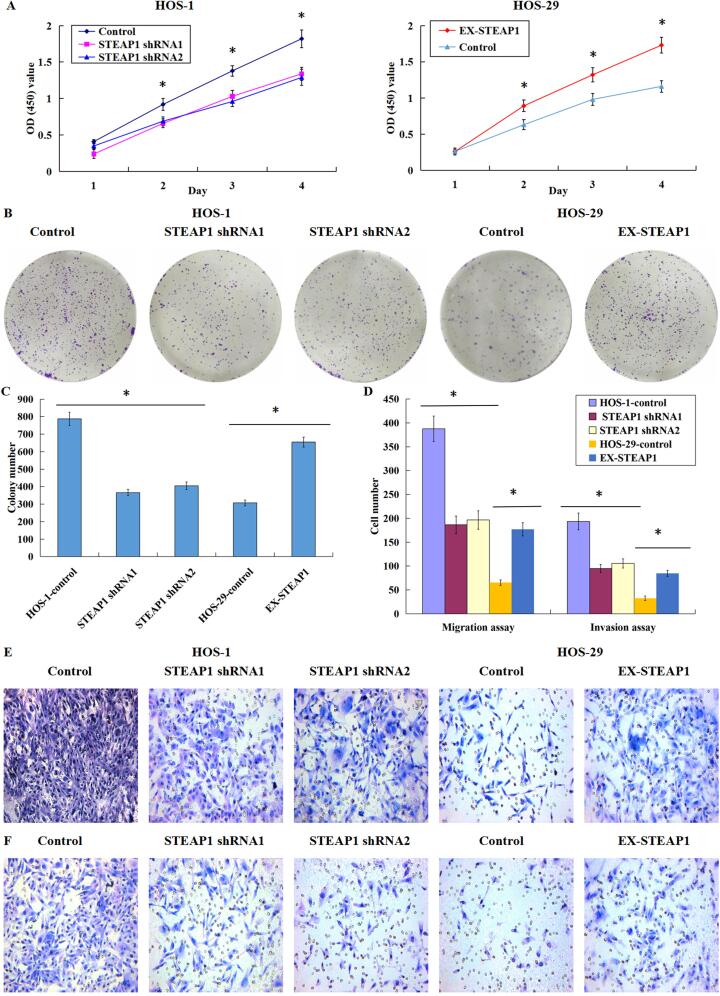
3.5. Effects of STEAP1 down-regulation or over-expression on the proliferation, clonogenicity, migration, and invasion of osteosarcoma cells
The down-regulation of STEAP1 significantly inhibited the proliferation of the subclone HOS-1 with high invasiveness, while the up-regulation of STEAP1 significantly increased the proliferation of the subclone HOS-29 with minimal invasiveness (Fig. 5A). The clonal formation efficiency of STEAP1 down-expressed cells was lower than that of control cells; in contrast, the up-regulation of STEAP1 could improve the colony formation efficiency in the less invasive subclone (Fig. 5BC). Through quantitative comparison of cells migrating or invading microporous membranes before and after the down-expression or over-expression of STEAP1, we found that STEAP1 knockdown inhibited the osteosarcoma cell infiltration and migration, while the STEAP1 overexpression facilitated such infiltration and migration (Fig. 5D). Images of the cell migration experiment (Fig. 5E) and invasion experiment (Fig. 5F) showed that compared to the negative controls, a smaller number of osteosarcoma cells transfected with STEAP1 shRNA1 and shRNA2 invaded through Matrigel or migrated through the PVDF membrane. Moreover, compared to the negative controls, the invading or migrating cells with up-regulated STEAP1 expression were pronouncedly higher in average number. These findings indicate that the knockdown of STEAP1 repressed the proliferation, clonogenicity, infiltration and migration of osteosarcoma cells, while the over-expression of STEAP1 facilitated such cellular events.
Fig. 5.
Effects of down-regulated or over-expressed STEAP1 on the proliferation, clonogenicity, migration, and invasion of osteosarcoma cells. (A) The down-regulation of STEAP1 significantly inhibited the HOS-1 subclone multiplication, whereas the STEAP1 up-regulation facilitated the HOS-29 subclone multiplication prominently. (B) The images of the plate clone formation experiment, the efficiency of colony formation of STEAP1-silenced cells reduced; however, the up-regulation of STEAP1 improved the colony formation efficiency of lowly invasive subclone. (C) The colonies formed in the STEAP1 shRNA1 and shRNA2 groups was lower in quantity pronouncedly than those in the negative controls, while the clones formed in the STEAP1 cDNA group were markedly increased in quantity compared to negative control group. (D) STEAP1 knockdown inhibited the infiltration and migration of osteosarcoma cells, whereas the STEAP1 over-expression facilitated such cellular events. The images of cell migration (E) and invasion (F) experiments, a smaller number of osteosarcoma cells transfected with STEAP1 shRNA1 and shRNA2 invaded through Matrigel or migrated through the PVDF membrane in contrast to the negative control cells. Moreover, the invading or migrating cells with up-regulated STEAP1 expression was pronouncedly higher in average number compared to the negative control cells (Magnification × 200). *P < 0.05.
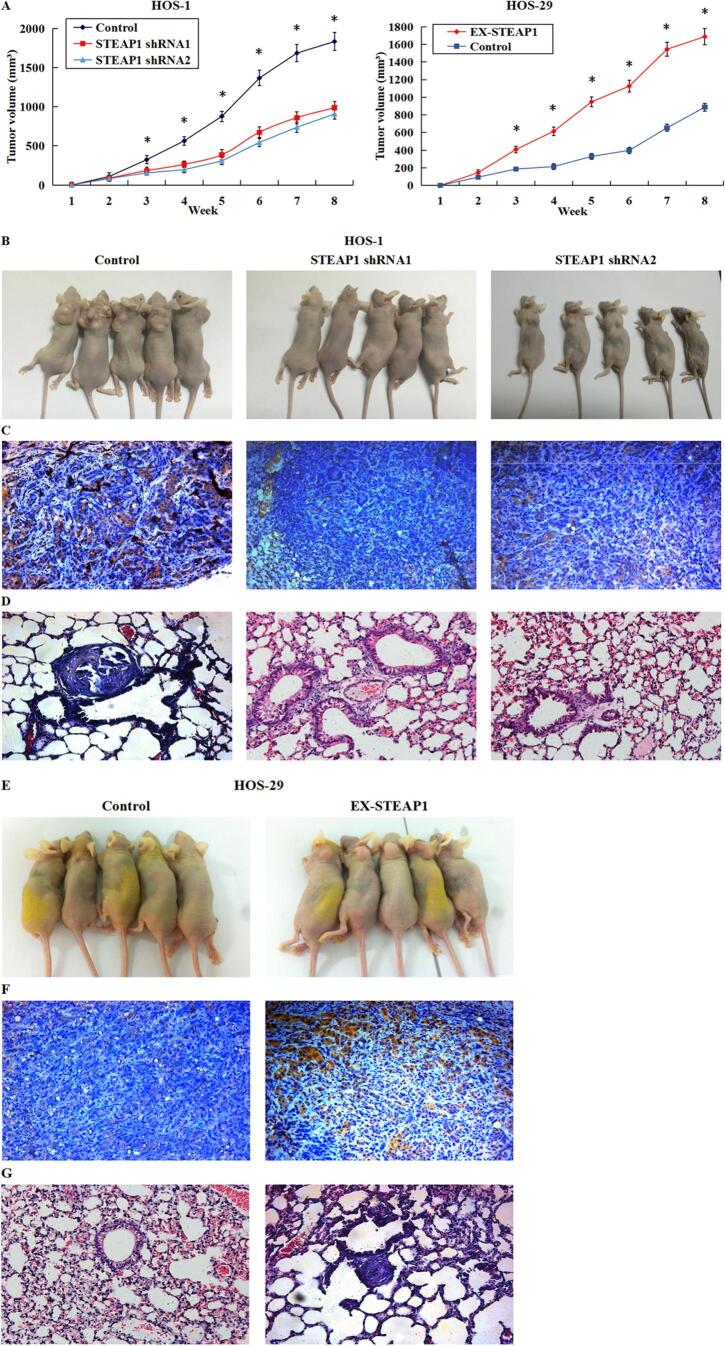
3.6. Effects of STEAP1 knockdown or over-expression on the growth profile and lung metastasis in xenograft tumor models
The effect of STEAP1 on tumor growth and lung metastasis in vivo was further studied by establishing subcutaneous xenograft tumor model and lung metastasis model. Five nude mice were inoculated subcutaneously with HOS-1 cells transfected with STEAP1 shRNA1 and shRNA2, HOS-29 cells transfected with STEAP1 cDNA, as well as corresponding negative control cells, and the other 5 nude mice were injected with the corresponding cells through the tail vein. After 8 weeks of follow-up, tumor growth and size were delayed in the STEAP1 shRNA1- and shRNA2-transfected groups compared with the control group; in contrast, STEAP1 over-expression increased the rate and volume of subcutaneous tumor growth (Fig. 6A). In addition, the STEAP1-shRNA-infected groups exhibited smaller mean tumor volume compared to the control group (Fig. 6B). Moreover, IHC confirmed that lower STEAP1 level in the RNAi group than in the negative control (Fig. 6C), and HE staining results of serial sections of lung tissue showed that lung metastases were found in the control group, while no lung metastases were found in the STEAP1 shRNA1- and shRNA2-transfected groups (Fig. 6D), suggesting that the down-regulation of STEAP1 inhibited tumorigenesis and lung metastasis. In contrast, the mean tumor size of cells transfected with STEAP1 cDNA was much larger compared to the control group (Fig. 6E). Further, in contrast to the control group, the STEAP1 over-expression group exhibited higher STEAP1 level (Fig. 6F), and upregulation of STEAP1 expression increased the risk of lung metastasis (Fig. 6G), suggesting that STEAP1 up-regulation promoted tumor growth and lung metastasis in vivo.
Fig. 6.
Effects of down-regulated or over-expressed STEAP1 on tumor growth and lung metastasis in a xenograft model. (A) After eight weeks’ observation, tumor growth and size reduced in the STEAP1 shRNA1 and shRNA2-transfected groups, and in contrast, STEAP1 over-expression increased the rate and volume of subcutaneous tumor growth. (B) The STEAP1-shRNA-infected groups exhibited smaller mean tumor volume compared to the HOS-1 negative control. (C) By immunohistochemistry, the RNAi group exhibited lower STEAP1 level compared to the control group. (D) HE staining results of serial sections of lung tissue showed that lung metastases were found in the control group, while no lung metastases were found in the STEAP1 shRNA1- and shRNA2-transfected groups. (E) Compared to the control group, the mean tumor size of cells transfected with STEAP1 cDNA was much larger. (F) Immunohistochemistry revealed higher STEAP1 level in the STEAP1 over-expression group in contrast to the control group. (G) Up-regulation of STEAP1 expression increased the risk of lung metastasis. *P < 0.05.
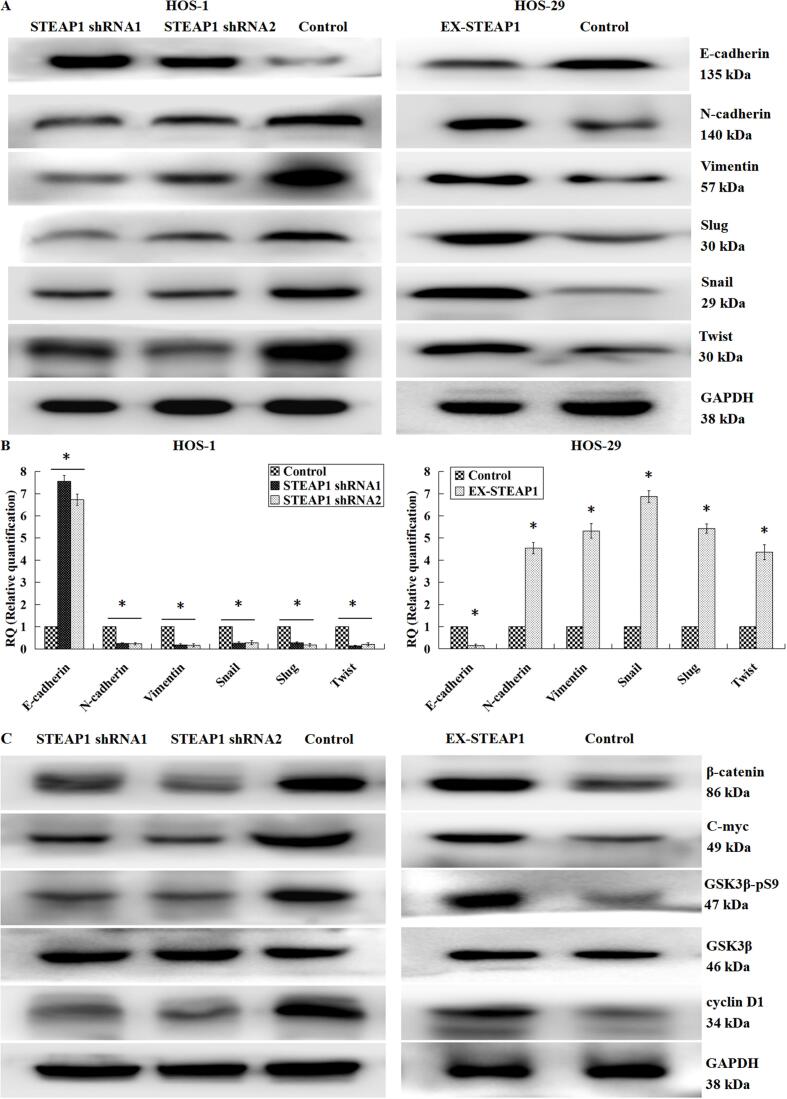
3.7. Effects of STEAP1 knockdown or over-expression on the key EMT hallmarks and the Wnt/β-catenin signal pathway
We demonstrated that STEAP1 plays critical roles in the osteosarcoma cell infiltration and migration. Therefore, we speculated that the knockdown or over-expression of STEAP1 might affect the EMT process. We obtained consistent results by western blotting (Fig. 7A) and RT-qPCR (Fig. 7B), indicating that STEAP1 knockdown significantly increased the epithelial marker E-cadherin level, while down-regulated N-cadherin and vimentin, the interstitial markers. Moreover, the expression of transcriptional factors like Slug, Snail and Twist were also significantly reduced, indicating that down-regulation of STEAP1 inhibited the EMT process. STEAP1 up-regulation induced EMT, showing declined E-cadherin level, as well as elevated levels of N-cadherin, vimentin, Slug, Snail and Twist. Our previous study has shown that EFEMP1 could facilitate the infiltration and migration of osteosarcoma cells through the Wnt/β-catenin axis initiation [12]. In this study, we found that STEAP1 and EFEMP1 could bind to each other and STEAP1 was closely related to the osteosarcoma cell multiplication and infiltration, as well as the EMT event; accordingly, we hypothesized that STEAP1 could also activate the Wnt signal pathway to exert biological effects. A western blotting analysis confirmed this hypothesis (Fig. 7C), i.e., the down-regulation of STEAP1 impaired the phosphorylation of glycogen synthase kinase-3β (GSK3β) pronouncedly, and its kinase activity was initiated to accelerate the decomposition of β-catenin, thereby reducing the expression levels of the c-myc and cyclin-D1 oncogenes downstream of the Wnt signal pathway. The up-regulation of STEAP1 enhanced such phosphorylation pronouncedly, prevented β-catenin decomposition, and thus increased the levels of c-myc and cyclin-D1. Wnt signal pathway stimulator LiCl (final concentration 5, 10, and 20 μmol/L) and restrainer XAV-939 (final concentration 5, 10, and 20 μmol/L) were applied to STEAP1-shRNA-infected cells and STEAP1-cDNA-infected cells for 48 h, respectively. LiCl pronouncedly promoted Wnt/β catenin axis initiation and induction of EMT, which were restrained by the down-regulation of STEAP1 expression. While on the other hand, XAV-939 remarkably impeded Wnt/β catenin axis initiation and induction of EMT, which were initiated by the up-regulation of STEAP1 expression (Fig. 8A). The results of a Transwell chamber invasion assay indicated that LiCl obviously attenuated the inhibitory effect of STEAP1 knockout on osteosarcoma cell invasion (Fig. 8B), and XAV-939 distinctly inhibited the promoting effect of STEAP1 overexpression on osteosarcoma cell invasion (Fig. 8C). Moreover, the effects of LiCl and XAV-939 were quantitatively dependent on and enhanced with the increase in the concentrations (Fig. 8D). A β-catenin/TCF dual luciferase reporter assay revealed that STEAP1 over-expression absolutely increased TCF luciferase activity (Fig. 9A), further verifying that STEAP1 promoted Wnt/β catenin axis initiation.
Fig. 7.
Effects of down-regulated or over-expressed STEAP1 on the key EMT hallmarks and the Wnt/β-catenin pathway. According to western-blot (A) and qRT-PCR (B) outcomes, STEAP1 knockdown significantly increased the epithelial marker E-cadherin level and reduced the levels of N-cadherin and vimentin, the interstitial markers. Besides, the levels of transcriptional factors like Slug, Snail and Twist were also significantly reduced, indicating the EMT event repression by the STEAP1 down-regulation. STEAP1 up-regulation induced EMT, showing the E-cadherin level decline, as well as the elevation of N-cadherin, vimentin, Slug, Snail and Twist levels. (C) According to western-blot outcomes, the STEAP1 down-expression weakened the phosphorylation of GSK3β pronouncedly, and its kinase activity was initiated to accelerate the decomposition of β-catenin, thereby reducing the expression levels of the c-myc and cyclin-D1 oncogenes downstream of the Wnt signal pathway, while the up-regulation of STEAP1 played opposite roles. *P < 0.05.
Fig. 8.
Effects of the Wnt signal pathway stimulator LiCl and restrainer XAV-939 on the osteosarcoma cell invasiveness and the EMT event. STEAP1-shRNA-infected cells and STEAP1-cDNA-infected cells were treated using 5, 10, and 20 μmol/L Wnt signal pathway stimulator LiCl and restrainer XAV-939 for 48 h, respectively. (A)By western blotting, LiCl pronouncedly promoted Wnt/β catenin axis initiation and induction of EMT, which were restrained by the down-regulation of STEAP1 expression. While on the other hand, XAV-939 remarkably impeded Wnt/β catenin axis initiation and induction of EMT, which were initiated by the up-regulation of STEAP1 expression. By transwell chamber invasion assay, (B) LiCl obviously attenuated the inhibitory effect of STEAP1 knockout on osteosarcoma cell invasion; (C) the STEAP1 over-expression-mediated enhancement of the osteosarcoma cell invasive capacities was inhibited by XAV-939. (D) The effects of LiCl and XAV-939 were quantitatively dependent on and enhanced with the increase in the concentrations. *P < 0.05.
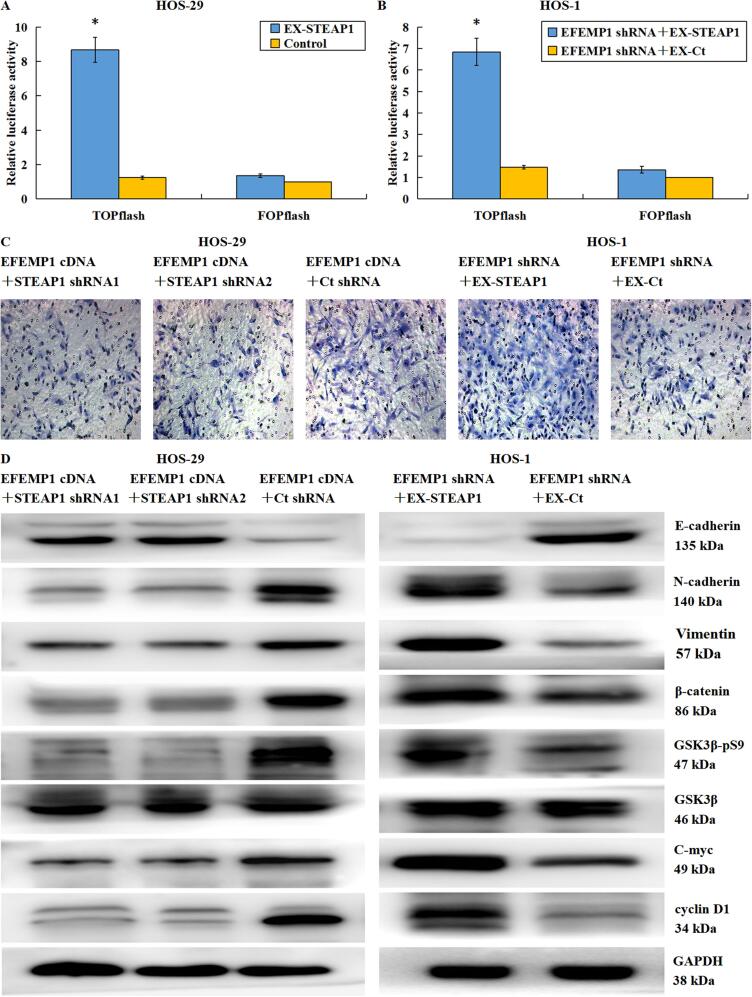
Fig. 9.
EFEMP1 targeted STEAP1 to promote Wnt/β catenin axis initiation and induction of EMT. (A) By β-catenin/TCF dual luciferase reporter assay, STEAP1 over-expression absolutely increased TCF luciferase activity. (B) In osteosarcoma cells co-transfected with STEAP1 cDNA and EFEMP1 shRNA, the overexpression of STEAP1 reversed the inhibition of TCF luciferase activity by EFEMP1 knockdown. (C) Transwell invasion assessment revealed that in osteosarcoma cells co-transfected using EFEMP1 cDNA and STEAP1 shRNA1 or STEAP1 shRNA2, down-regulated expression of STEAP1 significantly inhibited cell invasion enhanced by EFEMP1 over-expression, in contrast, in osteosarcoma cells co-transfected with EFEMP1 shRNA and STEAP1 cDNA, the over-expression of STEAP1 reversed the invasive ability of osteosarcoma cells inhibited by EFEMP1 down-regulation. (D) By western blotting, STEAP1 knockdown suppressed the Wnt/β catenin axis and the EMT event, which were both activated by EFEMP1 over-expression, accompanied by the E-cadherin (epithelial marker) up-expression, as well as the N-cadherin and vimentin (mesenchymal markers) down-expression. The downregulation of STEAP1 decreased the level of phosphorylated GSK3β, accelerated the decomposition of β-catenin, and thus reduced the expression levels of the c-myc and cyclin-D1 oncogenes. However, the exogenous over-expression of STEAP1 significantly enhanced the EMT event and initiated the Wnt/β catenin axis, both of which were repressed via the EFEMP1 down-regulation. *P < 0.05.
3.8. EFEMP1 targeted STEAP1 to promote Wnt/β catenin axis initiation and induction of EMT
We performed additional experiments to verify whether EFEMP1 could target STEAP1 to facilitate the osteosarcoma cell infiltration and migration. EFEMP1 cDNA and STEAP1 shRNA1 or shRNA2 were co-transfected into the minimally invasive subclone cell HOS-29, and the highly invasive subclone cell HOS-1 was co-transfected with EFEMP1 shRNA and STEAP1 cDNA. Cells were evaluated by a β-catenin/TCF dual luciferase reporter assay, Transwell chamber invasion assay, and western blotting. In osteosarcoma cells co-transfected with STEAP1 cDNA and EFEMP1 shRNA, the overexpression of STEAP1 reversed the inhibition of TCF luciferase activity by EFEMP1 knockdown (Fig. 9B). In osteosarcoma cells co-transfected with EFEMP1 cDNA and STEAP1 shRNA1 or STEAP1 shRNA2, the down-regulated of STEAP1 significantly inhibited the increase in cell invasion by EFEMP1 over-expression; in contrast, the over-expression of STEAP1 reversed the inhibition of invasion by EFEMP1 knockdown (Fig. 9C). The knockdown of STEAP1 could inhibit the activation of the EMT process induced by EFEMP1 over-expression, with elevated E-cadherin level, as well as declined levels of N-cadherin and vimentin. The downregulation of STEAP1 decreased the level of phosphorylated GSK3β, accelerated the decomposition of β-catenin, and thus reduced the expression levels of the c-myc and cyclin-D1 oncogenes, ultimately inactivating the Wnt signal pathway. However, the exogenous over-expression of STEAP1 could significantly reverse the inhibition of the EMT process by EFEMP1 knockdown, accompanied by declined E-cadherin level, elevated N-cadherin and Vimentin levels, and ascended levels of phosphorylated GSK3β, which promoted the accumulation of β-catenin and thus reinforced the expression levels of the c-myc and cyclin-D1 oncogenes. Therefore, the Wnt signal pathway was activated (Fig. 9D). On the basis of the aforementioned results, we concluded that EFEMP1 targeted STEAP1 to promote the EMT process and activate the Wnt pathway. In order to illustrate the importance of the Wnt/β-catenin axis to the EFEMP1′s function on the STEAP1′s mediatory role in EMT, β-catenin expression was knocked down in osteosarcoma cells with exogenous overexpression of EFEMP1 or STEAP1. After β-catenin down-regulation, the EMT process induced by EFEMP1 or STEAP1 over-expression was significantly inhibited, while the Wnt signal pathway was inactivated (Fig. 10A). Based on these results, we hypothesized that osteosarcoma cells secreted excessive EFEMP1 into the extracellular matrix, and EFEMP1 interacted with STEAP1 to activate the Wnt signal pathway and facilitate the osteosarcoma cell infiltration and migration by promoting EMT. To verify this hypothesis, we added purified EFEMP1 (100, 200, and 300 ng/mL) to the culture medium for the minimally invasive osteosarcoma subclone HOS-29 for 24 h. With the increase in EFEMP1 concentration, the expression of E-cadherin was declined progressively, while the N-cadherin and vimentin were up-regulated progressively. Besides, EMT was triggered, along with enhanced GSK3β phosphorylation and c-myc and cyclin-D1 expression, and the Wnt signal pathway was activated (Fig. 10B). To elucidate the importance of STEAP1 and the Wnt/β-catenin signal pathway in this process, STEAP1 and/or β-catenin were down-regulated, and 300 ng/mL EFEMP1 was added to the cell culture medium for 24 h. Irrespective of whether STEAP1 was downregulated, β-catenin was downregulated, or both, purified EFEMP1 did not activate EMT and the Wnt signal pathway in these osteosarcoma cells. In other words, the down-regulated expression of STEAP1 or β-catenin blocked EFEMP1 from promoting the EMT process in osteosarcoma cells (Fig. 10C). The extracellular matrix protein EFEMP1 could bind to the membrane protein STEAP1 to initiate the Wnt/β-catenin axis and induce EMT to enhance the migratory and invasive capacities of osteosarcoma cells.
Fig. 10.
The significance of the Wnt/β catenin pathway to the effect of EFEMP1 on the mediating role of STEAP1 in EMT. (A) After β catenin down-regulation, the EMT process induced by EFEMP1 or STEAP1 over-expression was significantly inhibited, while the Wnt signal pathway was inactivated. (B) The lowly invasive osteosarcoma subclone HOS-29 cells were treated with purified EFEMP1 (100, 200, and 300 ng/ml) for 24 h. With the increase in EFEMP1 concentration, the expression of E-cadherin was declined progressively, while the N-cadherin and vimentin levels were elevated progressively. Besides, EMT was triggered, along with enhanced GSK3β phosphorylation and c-myc and cyclin-D1 expression, and the Wnt signal pathway was activated. (C) For osteosarcoma cells with down-regulated STEAP1 and/or β catenin expression, its culture medium was added with 300 ng/mL EFEMP1 for 24 h. With the down-expression of either STEAP1 or β catenin or both, purified EFEMP1 could not activate Wnt/β catenin pathway or EMT in the osteosarcoma cells. In other words, the down-regulated expression of STEAP1 or β catenin blocked EFEMP1 from promoting the EMT process in osteosarcoma cells.
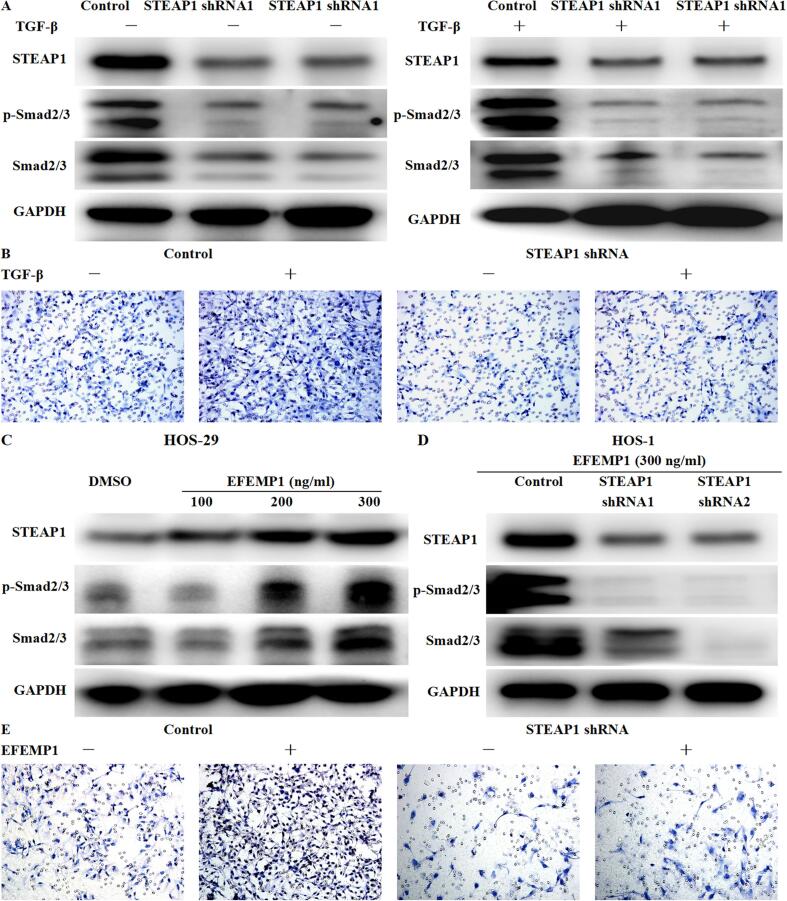
3.9. EFEMP1 targeted STEAP1 to promote TGF-β/Smad2/3 pathway and induce EMT to increase the invasion ability of osteosarcoma cells
Under pathological conditions, overexpression of TGF-β can lead to EMT and promote cancer development, and TGF-β is a well-known activator of the EMT process [13]. Meanwhile, TGF-β can play a pro-tumor role in osteosarcoma by promoting angiogenesis, bone remodeling and cell migration [14]. To determine whether EFEMP1 targeted STEAP1 to promote TGF-β/Smad2/3 signaling pathway, 2 ng/ml human recombinant TGF-β (R&D Systems) was used in the control group and the STEAP1 down-expression group for 24 h. The results revealed that STEAP1 knockdown could significantly inhibit the expression and phosphorylation of Smad2/3. And TGF-β could increase the phosphorylation of Smad2/3 in control cells, but has no obvious effect on STEAP1 down-expression group (Fig. 11A). Furthermore, transwell invasion assay showed that TGF-β significantly increased the invasion ability of cells in the control group, but had no effect on STEAP2 down-expression group (Fig. 11B). STEAP2 knockdown significantly inhibited TGF-β-induced TGF-β/Smad2/3 signaling pathway activation, increased cell invasiveness and EMT process. Given that EFEMP1 could target STEAP1 to promote EMT progression, we next examined whether EFEMP1 could target STEAP1 to activate TGF-β/Smad2/3 signaling. EFEMP1 increased the expression of STEAP1 and promoted the expression and phosphorylation of Smad2/3 in low invasive HOS-29 osteosarcoma cells in a quantity-dependent manner (Fig. 11C). But STEAP1 knockdown could counteract the activation of TGF-β/Smad2/3 signaling pathway by EFEMP1 (Fig. 11D). The results of Transwell invasion assay showed that EFEMP1 significantly increased the invasion ability of cells in the control group, but had no significant impact in the STEAP1 down-regulated group (Fig. 11E). STEAP1 knockdown significantly reversed the activation of TGF-β/Smad2/3 pathway and the promotion of EMT by EFEMP1.
Fig. 11.
EFEMP1 targeted STEAP1 to promote TGF-β/Smad2/3 pathway and induce EMT to increase the invasion ability of osteosarcoma cells. (A) Using 2 ng/ml human recombinant TGF-β in the control group and the STEAP1 down-expression group for 24 h, STEAP1 knockdown significantly inhibited the expression and phosphorylation of Smad2/3, and TGF-β increased the phosphorylation of Smad2/3 in control cells, but has no obvious effect on STEAP1 down-expression group. (B) Transwell invasion assay showed that TGF-β significantly increased the invasion ability of cells in the control group, but had no effect on STEAP2 down-expression group. (C) Exogenous EFEMP1 increased the expression of STEAP1 and promoted the expression and phosphorylation of Smad2/3 in low invasive HOS-29 osteosarcoma cells in a quantity-dependent manner. (D) STEAP1 knockdown inhibited EFEMP1 activation of TGF-β/Smad2/3 signaling pathway. (E) Transwell invasion assay showed that EFEMP1 significantly increased the invasion ability of cells in the control group, but had no significant impact in the STEAP1 down-regulated group.
4. Discussion
We have previously shown that EFEMP1 facilitated the osteosarcoma cell infiltration and migration by activating the Wnt/β-catenin axis. In addition, EFEMP1 could bind to STEAP1, and a decrease or increase in EFEMP1 expression could significantly inhibit or increase the expression of STEAP1. In this study, we found that over-expression of STEAP1 was noted in the osteosarcoma tissues, which was positively linked to the poor outcome among osteosarcoma population. The over-expression of STEAP1 was also found in osteosarcoma cell line and subclone cells with high invasiveness. STEAP1 could induce the EMT via the Wnt/β-catenin and TGF-β/Smad2/3 axises and facilitate the osteosarcoma cell infiltration and migration. Further experimental results showed that EFEMP1 could target STEAP1 to activate the Wnt/β-catenin and TGF-β/Smad2/3 axises and induce EMT, thereby enhancing the migratory and invasive capacities of osteosarcoma cells. The inhibition of STEAP1 gene expression by RNA interference could prevent the increased invasion ability of osteosarcoma cells induced by EFEMP1. These findings clearly indicate that EFEMP1 binds to STEAP1 to promote osteosarcoma progression via the Wnt/β-catenin and TGF-β/Smad2/3 axises.
As suggested by our findings, high STEAP1 level in osteosarcoma tissues is closely related to high histological grade, positive metastasis to lymph nodes and poor patient outcome. In addition, the knockdown of STEAP1 repressed the miltiplicaton, clonogenesis, mobility, and invasion of osteosarcoma cells, while the upregulation of STEAP1 had the opposite effects. Research on the relationship between STEAP1 and tumor characteristics is still in its infancy; however, this relationship has been studied extensively in prostate cancer. As a promising candidate target, STEAP1 has been a recent focus of a number of studies targeting prostate cancer, such as antibody-drug conjugation therapy [15], T cell immunotherapy [16], fusion protein vaccines [17], and the early detection of metastatic prostate cancer [11]. The expression pattern of STEAP1 varies among tumor types, and the role of STEAP1 in different aspects of malignant tumor phenotypes is controversial [5], [18]. High STEAP1 expression in Ewing's sarcoma is a marker of tumor invasiveness and could induce an resultful T cell mediated anti-cancer reaction [19], [20]. In glioma, the over-expression of STEAP1 is closely related to immunosuppression and poor patient outcome [6]. In ovarian cancer, STEAP1 was also highly expressed and was related to the metastasis and growth of cancer cells; its knockdown inhibited EMT progression and MMP2 and MMP9 activities [7]. However, in breast cancer and endometrial cancer, STEAP1 had an anti-tumor effect. Breast cancer patients with high STEAP1 expression had good outcomes, and STEAP1 knockdown enhanced the migratory and invasive capacities of cancer cells and augmented the levels of EMT-related genes MMP2, MMP9, MMP13, VIM, and CDH2 [21], [22]. The expression of STEAP1 was low in endometrial carcinoma, and the downregulation of STEAP1 enhanced the proliferation of cells and promoted cell migration, invasion, and EMT [23]. The expression patterns of EFEMP1 in these tumors were basically consistent with those of STEAP1, and EFEMP1 contributes to the progression of ovarian cancer as a positive regulator of the AKT signal pathway [24]. EFEMP1 is secreted by glioma cells to promote angiogenesis, tumor invasion, and tumor cell survival. The development of blocking antibodies against EFEMP1 or truncation variants and other mutants by gene engineering may more be an effective therapeutic approach for malignant gliomas [25], [26]. However, in breast cancer [27] and endometrial cancer [28], [29], EFEMP1 acts as a tumor suppressor gene, Low EFEMP1 levels predict adverse outcomes, and high EFEMP1 levels inhibit TGF-β-induced EMT, migration, and invasion. Both EFEMP1 and STEAP1 were over-expressed in osteosarcoma, and a change in EFEMP1 expression directly altered the expression of STEAP1. We speculated that EFEMP1 and STEAP1 are closely related, and EFEMP1 could target STEAP1 to activate the Wnt/β-catenin and TGF-β/Smad2/3 signal pathways, thereby facilitating the osteosarcoma cell infiltration and migration.
The mechanisms underlying the effects of EFEMP1, an extracellular matrix protein, are poorly understood. Only a few receptors of EFEMP1 have been confirmed; for example, EFEMP1 binds to epidermal growth factor receptor (EGFR) and plays pivotal roles in the proliferation and invasion of pancreatic cacinoma cells [30]. In a study of glioma, EFEMP1 activated metalloproteinase ADAM17 by competitively binding to its endogenous inhibitor TIMP3, releasing soluble TNFα, and then activating TNF receptor and NF-κB signaling to promote adhesion and invasion [31]. Further studies of glioma cells have shown that EFEMP1 could bind to the Notch autocrine inhibitor DLL3, thereby activating the Notch pathway and promoting cell proliferation and migration in ways that rely on Notch [32]. In our research of osteosarcoma, we found a relationship between changes in EFEMP1 and STEAP1 expression levels. Further immunoprecipitation assays showed that EFEMP1 and STEAP1 interact. The down-regulation of STEAP1 expression significantly inhibited the increases in cell invasion and EMT by EFEMP1 over-expression. In EFEMP1-knockdown osteosarcoma cells, the over-expression of STEAP1 attenuated the inhibition of invasion and EMT. Although endogenous or exogenous EFEMP1 over-expression could stimulate the Wnt/β-catenin and TGF-β/Smad2/3 axises to iuduce EMT, this activation is inhibited by the knockdown of STEAP1 in osteosarcoma cells. Briefly, the extracellular matrix protein EFEMP1 could bind to the membrane protein STEAP1 to stimulate the Wnt/β-catenin and TGF-β/Smad2/3 axises and induce EMT, thereby enhancing the migratory and invasive capacities of osteosarcoma cells.
In short, the expression of STEAP1 was up-regulated in osteosarcoma tissues, exhibiting positive association with the malignant oncocyte phenotype and poor patient outcome. In addition, STEAP1 could induce the EMT via the Wnt/β-catenin and TGF-β/Smad2/3 axises and facilitate the osteosarcoma cell infiltration and migration. EFEMP1 could bind to STEAP1 to promote osteosarcoma progression. The effect of EFEMP1 was inhibited by the inhibition of STEAP1. In view of the interaction between EFEMP1 and STEAP1 in tumor development, further studies of the mechanism by which EFEMP1 and STEAP1 contribute to tumor progression are needed for the development of targeted drugs.
5. Availability of data and material
All data generated or analyzed during the current study are available from the corresponding author on reasonable request are included in this published article.
Ethical statements
This study was approved by the Institutional Medical Ethics Committee of Shandong University Qilu Hospital. All methods were performed in accordance with the relevant guidelines and regulations. We obtained the written informed consents of the patients involved in the study. All mouse experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of People’s Republic of China.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by Suzhou Science and Technology Development Plan (SYSD2018056). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Longo D.L., Meltzer P.S., Helman L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med.. 2021;385(22):2066–2076. doi: 10.1056/NEJMra2103423. [DOI] [PubMed] [Google Scholar]
- 2.Lilienthal I., Herold N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020;21(18):6885. doi: 10.3390/ijms21186885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattinger C.M., Patrizio M.P., Fantoni L., Casotti C., Riganti C., Serra M. Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers (Basel). 2021;13(12):2878. doi: 10.3390/cancers13122878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubert R.S., Vivanco I., Chen E., Rastegar S., Leong K., Mitchell S.C., Madraswala R., Zhou Y., Kuo J., Raitano A.B., Jakobovits A., Saffran D.C., Afar D.E. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. PNAS. 1999;96(25):14523–14528. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W.J., Wu H.T., Li C.L., Lin Y.K., Fang Z.X., Lin W.T., Liu J. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.752426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Z., Wang Z., Song Z., Wu Y., Jin Q., Zhao Z. Predictive potential of STEAP family for survival, immune microenvironment and therapy response in glioma. Int. Immunopharmacol. 2021;101(Pt A) doi: 10.1016/j.intimp.2021.108183. [DOI] [PubMed] [Google Scholar]
- 7.Jiao Z., Huang L., Sun J., Xie J., Wang T., Yin X., Zhang H., Chen J. Six-transmembrane epithelial antigen of the prostate 1 expression promotes ovarian cancer metastasis by aiding progression of epithelial-to-mesenchymal transition. Histochem. Cell Biol. 2020;154(2):215–230. doi: 10.1007/s00418-020-01877-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z., Hou W.B., Zhang C., Tan Y.E., Zhang D.D., An W., Pan S.W., Wu W.D., Chen Q.C., Xu H.M. A research of STEAP1 regulated gastric cancer cell proliferation, migration and invasion in vitro and in vivos. J. Cell Mol. Med. 2020;24(24):14217–14230. doi: 10.1111/jcmm.16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markey F.B., Romero B., Parashar V., Batish M. Identification of a New Transcriptional Co-Regulator of STEAP1 in Ewing's Sarcoma. Cells. 2021;10(6):1300. doi: 10.3390/cells10061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danila D.C., Szmulewitz R.Z., Vaishampayan U., Higano C.S., Baron A.D., Gilbert H.N., Brunstein F., Milojic-Blair M., Wang B., Kabbarah O., Mamounas M., Fine B.M., Maslyar D.J., Ungewickell A., Scher H.I. Phase I Study of DSTP3086S, an Antibody-Drug Conjugate Targeting Six-Transmembrane Epithelial Antigen of Prostate 1, in Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019;37(36):3518–3527. doi: 10.1200/JCO.19.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrasquillo J.A., Fine B.M., Pandit-Taskar N., Larson S.M., Fleming S.E., Fox J.J., Cheal S.M., O'Donoghue J.A., Ruan S., Ragupathi G., Lyashchenko S.K., Humm J.L., Scher H.I., Gönen M., Williams S.P., Danila D.C., Morris M.J. Imaging Patients with Metastatic Castration-Resistant Prostate Cancer Using 89Zr-DFO-MSTP2109A Anti-STEAP1 Antibody. J. Nucl. Med. 2019;60(11):1517–1523. doi: 10.2967/jnumed.118.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S., Zhang D., Han S., Gao P., Liu C., Li J., Pan X. Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway. Sci. Rep. 2017;7(1):6215. doi: 10.1038/s41598-017-06353-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Peng D., Fu M., Wang M., Wei Y., Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104. doi: 10.1186/s12943-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verrecchia F., Rédini F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay Between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018;8:133. doi: 10.3389/fonc.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosellini M., Santoni M., Mollica V., Rizzo A., Cimadamore A., Scarpelli M., Storti N., Battelli N., Montironi R., Massari F. Treating Prostate Cancer by Antibody-Drug Conjugates. Int. J. Mol. Sci. 2021;22(4):1551. doi: 10.3390/ijms22041551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorff T.B., Narayan V., Forman S.J., Zang P.D., Fraietta J.A., June C.H., Haas N.B., Priceman S.J. Novel Redirected T-Cell Immunotherapies for Advanced Prostate Cancer. Clin. Cancer Res. 2022;28(4):576–584. doi: 10.1158/1078-0432.CCR-21-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo L., Xie H., Zhang Z., Wang Z., Peng S., Niu Y., Shang Z. Fusion Protein Vaccine Based on Ag85B and STEAP1 Induces a Protective Immune Response against Prostate Cancer. Vaccines (Basel). 2021;9(7):786. doi: 10.3390/vaccines9070786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C., Xiong K., Ji Z., Liu F., Li X., Poggi A. The Prognostic Value and Immunological Role of STEAP1 in Pan-Cancer: A Result of Data-Based Analysis. Oxid Med Cell Longev. 2022;2022:1–28. doi: 10.1155/2022/8297011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schober S.J., Thiede M., Gassmann H., Prexler C., Xue B., Schirmer D., Wohlleber D., Stein S., Grünewald T.G.P., Busch D.H., Richter G.H.S., Burdach S.E.G., Thiel U. MHC Class I-Restricted TCR-Transgenic CD4+ T Cells Against STEAP1 Mediate Local Tumor Control of Ewing Sarcoma In Vivo. Cells. 2020;9(7):1581. doi: 10.3390/cells9071581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin T.-Y., Park J.A., Long A., Guo H.-F., Cheung N.-K. Novel potent anti-STEAP1 bispecific antibody to redirect T cells for cancer immunotherapy. J. ImmunoTher. Cancer. 2021;9(9):e003114. doi: 10.1136/jitc-2021-003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H.T., Chen W.J., Xu Y., Shen J.X., Chen W.T., Liu J. The Tumor Suppressive Roles and Prognostic Values of STEAP Family Members in Breast Cancer. Biomed Res. Int. 2020;2020:9578484. doi: 10.1155/2020/9578484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J., Yang Y., Sun J., Jiao Z., Zhang H., Chen J. STEAP1 Inhibits Breast Cancer Metastasis and Is Associated With Epithelial-Mesenchymal Transition Procession. Clin Breast Cancer. 2019;19(1):e195–e207. doi: 10.1016/j.clbc.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Sun J., Ji G., Xie J., Jiao Z., Zhang H., Chen J. Six-transmembrane epithelial antigen of the prostate 1 is associated with tumor invasion and migration in endometrial carcinomas. J. Cell. Biochem. 2019;120(7):11172–11189. doi: 10.1002/jcb.28393. [DOI] [PubMed] [Google Scholar]
- 24.Yin X., Fang S., Wang M., Wang Q., Fang R., Chen J. EFEMP1 promotes ovarian cancer cell growth, invasion and metastasis via activated the AKT pathway. Oncotarget. 2016;7(30):47938–47953. doi: 10.18632/oncotarget.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandhu M.S., Behera P., Bhaskaran V., Longo S.L., Barrera-Arenas L.M., Sengupta S., Rodriguez-Gil D.J., Chiocca E.A., Viapiano M.S. Development of a Function-Blocking Antibody Against Fibulin-3 as a Targeted Reagent for Glioblastoma. Clin. Cancer Res. 2018;24(4):821–833. doi: 10.1158/1078-0432.CCR-17-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke C., Luo J.R., Cen Z.W., Li Y., Cai H.P., Wang J., Chen F.R., Siegel E.R., Le K.N., Winokan J.R., Gibson G.J., McSwain A.E., Afrasiabi K., Linskey M.E., Zhou Y.X., Chen Z.P., Zhou Y.H. Dual antivascular function of human fibulin-3 variant, a potential new drug discovery strategy for glioblastoma. Cancer Sci. 2020;111(3):940–950. doi: 10.1111/cas.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian H., Liu J., Chen J., Gatza M.L., Blobe G.C. Fibulin-3 is a novel TGF-β pathway inhibitor in the breast cancer microenvironment. Oncogene. 2015;34(45):5635–5647. doi: 10.1038/onc.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang T., Qiu H., Bao W., Li B., Lu C., Du G., Luo X., Wang L., Wan X., Sun L.-Z. Epigenetic inactivation of EFEMP1 is associated with tumor suppressive function in endometrial carcinoma. PLoS ONE. 2013 Jun 28;8(6):e67458. doi: 10.1371/journal.pone.0067458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T., Zhang H., Qiu H., Li B., Wang J., Du G., Ren C., Wan X. EFEMP1 is repressed by estrogen and inhibits the epithelial-mesenchymal transition via Wnt/β-catenin signaling in endometrial carcinoma. Oncotarget. 2016;7(18):25712–25725. doi: 10.18632/oncotarget.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camaj P., Seeliger H., Ischenko I., Krebs S., Blum H., De Toni E.N., Faktorova D., Jauch K.W., Bruns C.J. EFEMP1 binds the EGF receptor and activates MAPK and Akt pathways in pancreatic carcinoma cells. Biol. Chem. 2009;390(12):1293–1302. doi: 10.1515/BC.2009.140. [DOI] [PubMed] [Google Scholar]
- 31.Nandhu M.S., Kwiatkowska A., Bhaskaran V., Hayes J., Hu B., Viapiano M.S. Tumor-derived fibulin-3 activates pro-invasive NF-κB signaling in glioblastoma cells and their microenvironment. Oncogene. 2017;36(34):4875–4886. doi: 10.1038/onc.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu B., Nandhu M.S., Sim H., Agudelo-Garcia P.A., Saldivar J.C., Dolan C.E., Mora M.E., Nuovo G.J., Cole S.E., Viapiano M.S. Fibulin-3 promotes glioma growth and resistance through a novel paracrine regulation of Notch signaling. Cancer Res. 2012;72(15):3873–3885. doi: 10.1158/0008-5472.CAN-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the current study are available from the corresponding author on reasonable request are included in this published article.