Abstract
Dental implants are prosthetic devices that are surgically placed in direct contact with the jawbone to support intra-oral functions and esthetics. Diabetes mellitus may contribute to peri-implant bone loss. During the last few years, there have been attempts to reduce this bone loss and improve the survival rate of implants. Metformin, an anti-diabetic drug known for its osteogenic properties, is thought to prevent peri-implant bone loss in diabetic patients. Although several studies have been conducted to study metformin's effect on diabetic and non-diabetic study models, no systematic review has analyzed and summarized these studies critically. Therefore, the objectives of this systematic review were to summarize the outcomes of these studies and critically appraise them. Seven studies were included in this systematic review. Four studies used only animal models, two used both animal and cell culture models, and one used only cell culture studies. The general characteristics and outcomes of the included studies were summarized, and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines were used to assess the quality of the animal studies. In vitro studies indicate that metformin may induce stem cells to undergo osteoblastic differentiation to produce a higher amount of bone and may also improve osseointegration. Nevertheless, several studies had potential sources of bias. Therefore, it is recommended that emphasis be placed on increasing the quality of future animal studies and human trials to determine the effects of metformin on the osseointegration of dental implants. Future studies are needed with adequate follow-up to evaluate the efficacy of metformin in improving the osseointegration of dental implants.
Keywords: Bioactivity, Dental implants, Diabetes mellitus, Metformin, Osseointegration, Surface coating
الملخص
غرسات الأسنان هي أجهزة توضع جراحياً في اتصال مباشر مع الفكين لدعم التعويضات السنية. قد يساهم داء السكري في فقدان العظام حول الزراعة. خلال السنوات القليلة الماضية ، كانت هناك محاولات لتقليل فقدان العظام وبالتالي تحسين معدل بقاء الغرسات. الميتفورمين ، دواء مضاد لمرض السكري معروف أيضًا بخصائصه المكونة للعظم وقد تم اقتراحه كعامل لمنع فقدان العظام حول غرسات الأسنان لدى مرضى السكري. على الرغم من إجراء العديد من الدراسات لدراسة تأثير الميتفورمين على مرضى السكر وغير المصابين بالسكري .
لم تحاول أي مراجعة منهجية تحليل هذه الدراسات وتلخيصها بشكل نقدي. لذلك ،كان الهدف من هذه المراجعة المنهجية تلخيص نتائج هذه الدراسات وتقييمها بشكل نقدي.
تم تضمين سبع دراسات في هذه المراجعة المنهجية. استخدمت أربع دراسات نماذج حيوانية فقط ، واستخدمت دراستان نماذج حيوانية وزراعة الخلية ودراسة استخدمت فقط دراسات زراعة الخلايا.
تم تلخيص الخصائص والنتائج العامة ، وتم تقييم جودة الدراسات على الحيوانات باستخدام إرشادات (ARRIVE) .
تشير الدراسات الدراسات المختبرية إلى أن الميتفورمين قد يحفز الخلايا الجذعية على الخضوع للتمايز العظمي لإنتاج كمية أكبر من العظام وقد يؤدي أيضًا إلى تحسين عملية الاندماج العظمي ولكن كان لعدد من الدراسات مصادر تحتمل التحيز.
لذا يوصى بإجراء المزيد من الدراسات على الحيوانات والتجارب البشرية للتأكد من تأثير الميتفورمين على الاندماج العظمي لزرعات الأسنان ويجب على أطباء الأسنان تحديد موعد للمتابعة المنتظمة للمرضى الذين تلقوا زرعات الأسنان ويستخدمون الميتفورمين. تركز الأبحاث المستقبلية على إجراء دراسات مع متابعة كافية لتقييم فعالية الميتفورمين في تحسين الاندماج العظمي لزرعات الأسنان.
الكلمات المفتاحية: زراعة الأسنان, النشاط الحيوي, الاندماج العظمي, مرض السكري, الميتفورمين, طلاء السطح
Introduction
To support prosthodontic and orthodontic devices, dental implants are surgically implanted into the mandibular or maxillary bone.1 For a dental implant to be deemed successful, it should have direct contact with the bone, a process known as osseointegration.2 Implants fail if there is a lack of osseointegration.3 Several factors may contribute to implant failure. Systematic disease such as diabetes and osteoporosis may increase implant failure rates.4 Studies have also demonstrated that dental implants may fail at a higher rate in immunocompromised patients compared to immunocompetent individuals.5 Local factors such as parafunctional habits and smoking may further contribute to peri-implant bone loss and lack of osseointegration.1,6 Although various drugs, surface modifications, and growth factors have been proposed to improve osteointegration, preventing peri-implant bone loss remains a significant challenge.7
Diabetes mellitus (DM) is a group of metabolic disorders that result in higher-than-normal blood glucose levels.8 The World Health Organization estimates that approximately 422 million individuals are affected by DM worldwide.9 There are three main types of diabetes mellitus: type 1 diabetes mellitus (DM1), type 2 diabetes mellitus (DM2), and gestational diabetes (GD).9 DM1 results from the autoimmune destruction of beta cells (responsible for producing insulin) in the pancreas. Since insulin controls blood glucose levels, diminished hormone levels result in excessive blood glucose levels.10 DM2, on the other hand, results from cells developing insulin resistance, leading to increased levels of glucose in the blood.11 GD occurs in pregnant women with no previous history of diabetes. Uncontrolled diabetes leads to worsening attachment loss in patients with periodontitis.11,12 High glucose levels trigger the production of advanced glycemic end products, leading to the destruction of periodontal tissues.13 Similarly, uncontrolled diabetes also leads to increased bone loss around dental implants, which may result in implant failure14 due to increased inflammation and impaired periodontal healing.15
Metformin is an oral biguanide antidiabetic drug.16 It is a commonly used medication administered to patients with DM2. Metformin improves periodontal health by controlling blood sugar levels and increases outcomes of periodontal therapy.17 More recent studies have indicated that it may have a direct antiresorptive effect on periodontal bone.18 Metformin inhibits bone resorption by reducing receptor activator of nuclear factor kappa B ligand (RANKL) expression and increasing osteoprotegerin (OPG) expression.19 Studies have also suggested that local delivery of metformin promotes osteoblastic activity and decreases osteoclastogenesis.18
To date, no systematic review has critically analyzed the outcomes and quality of the literature focusing on studying local and systemic metformin to promote or improve osteointegration of dental implants. Therefore, the focus of this review was to summarize the general characteristics, outcomes, and overall quality of the studies to ascertain the current status of metformin as a pro-osteogenic agent that improves the osseointegration of dental implants in diabetic patients and healthy individuals.
Materials and Methods
Focused question
Using the PICO procedure (Participants, Intervention, Control, and Outcomes) recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,20 the following focused question was constructed: “Compared to controls, does metformin improve the osseointegration and osteoconductive properties of dental implants in animal models?”
Search strategy
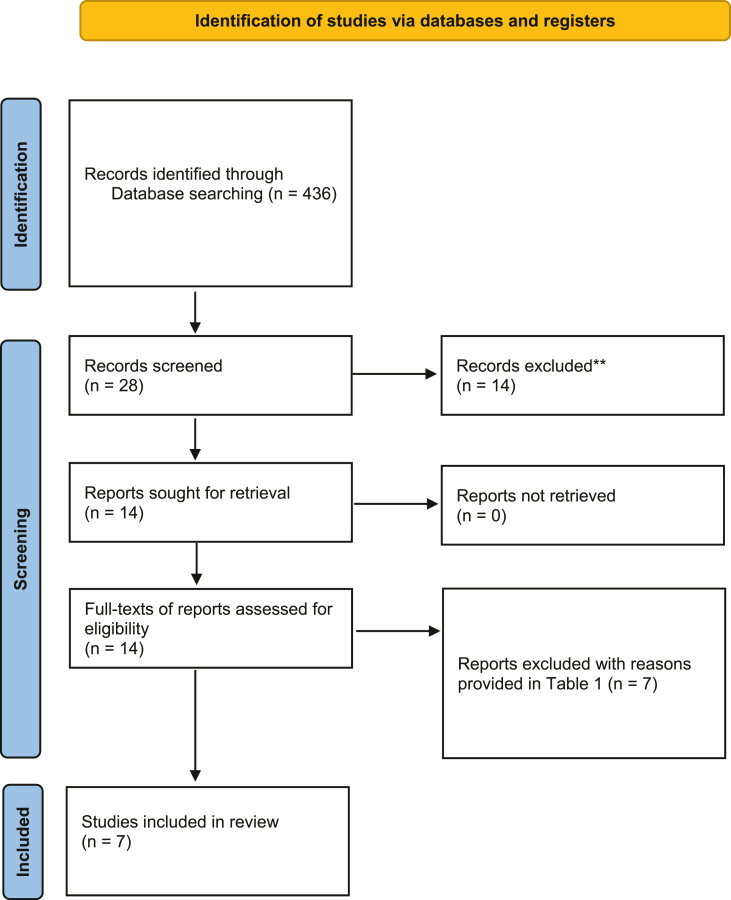
An electronic search was conducted using the Medical Subject Headings phrases (diabetes mellitus) AND ((metformin) AND (dental implant) and (osseointegration)) in PubMed, published from January 2011 to August 2022. A similar search was conducted via Google Scholar, Embase, ISI Web of Knowledge, and Cochrane Central Register of Controlled Trials (CONTROL). A secondary search was done by cross-checking the reference lists of the articles meeting the inclusion criteria for additional studies relevant to this review. Two independent reviewers (VP and SN) looked over the titles and abstracts of all articles found using the search approach, weeding out those that are not relevant. For all potential studies, full publications were assessed by the two reviewers for inclusion. Additionally, the reference lists of the articles included were also read to find any additional reports that may have met our inclusion criteria. The PRISMA flow diagram for the literature search process is illustrated in Figure 1. The Kappa score (inter-examiner reliability score) was also calculated.
Figure 1.
A PRISMA flow diagram for the search methodology employed for this systematic review.
Eligibility criteria
All identified studies were assessed using the following predefined eligibility criteria. Those studies were included that met the following criteria:
-
a)
Prospective clinical studies
-
b)
Animal studies
-
c)
Cell studies
-
d)
Studies reporting effect of metformin on animal or human periodontal cells and osseointegration of dental implants
-
e)
Studies in the English language.
Following studies were excluded from the systematic review:
-
a)
Review articles (narrative and systematic)
-
b)
Short communications
-
c)
Letters to editors
-
d)
Data extraction
The data in the animal studies were extracted by investigators VP, MSKS, and ZK using a predetermined data collection form that comprised of numerous headings: authors and year of study, type of animal model, and number of subjects, duration of study, intervention and main outcomes. The same investigators retrieved data from in vitro experiments and tabulated it according to the technique, cell line employed, metformin concentration used, variables assessed, and overall outcome. Any disagreements were solved by discussion. A third investigator SN and fifth investigator MSZ validated the tables.
Quality assessment of studies
Three investigators, VP, MSKS, and ZK, independently assessed the quality of procedures used in the investigations using the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Any arising disagreements were solved by discussion. The quality of parameters related to title, abstract, introduction, methodology, results, and discussion were assessed to determine the quality of each study. Table 1 shows the details of the items assessed in each experiment.
Table 1.
Details of the items assessed in each study to conduct the quality assessment. Adapted from the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.21
| Study characteristics | Item no. | Description |
|---|---|---|
| Title | 1 | Provide as accurate and concise a description of the content of the article as possible |
| Abstract | 2 | Provide an accurate summary of the background, research objectives, including details of the species or strain of animal used, key methods, principal findings and conclusions of the study. |
| Introduction | ||
| Background | 3a | Include sufficient scientific background (including relevant references to previous work) to understand the motivation and context for the study and explain the experimental approach and rationale. |
| 3b | Explain how and why the animal species and model being used can address the scientific objectives and, where appropriate, the study's relevance to human biology. | |
| Objectives | 4 | Clearly describe the primary and any secondary objectives of the study, or specific hypotheses being tested. |
| Methodology | ||
| Ethical statement | 5 | Indicate the nature of the ethical review permissions, relevant licenses (e.g. Animal [Scientific Procedures] Act 1986), and national or institutional guidelines for the care and use of animals, that cover the research. |
| Study design | 6a | The number of experimental and control groups. |
| 6b | Any steps taken to minimise the effects of subjective bias when allocating animals to treatment (e.g. randomisation procedure) and when assessing results (e.g. if done, describe who was blinded and when). | |
| 6c | Any steps taken to minimise the effects of subjective bias when allocating animals to treatment (e.g. randomisation procedure) and when assessing results (e.g. if done, describe who was blinded and when). | |
| Experimental procedures | 7a | How (e.g., drug formulation and dose, site and route of administration, anesthesia and analgesia used [including monitoring], surgical procedure, method of euthanasia). Provide details of any specialist equipment used, including supplier(s). |
| 7b | When (e.g. time of day). | |
| 7c | Where (e.g. home cage, laboratory, water maze). | |
| 7d | Why (e.g. rationale for choice of specific anesthetic, route of administration, drug dose used). | |
| Experimental animals | 8a | Provide details of the animals used, including species, strain, sex, developmental stage (e.g. mean or median age plus age range) and weight (e.g. mean or median weight plus weight range). |
| 8b | Provide further relevant information such as the source of animals, international strain nomenclature, genetic modification status (e.g. knock-out or transgenic), genotype, health/immune status, drug or test naïve, previous procedures, etc. | |
| Housing and husbandry | 9a | Housing (type of facility e.g. specific pathogen free [SPF]; type of cage or housing; bedding material; number of cage companions; tank shape and material etc. for fish). |
| 9b | Husbandry conditions (e.g. breeding program, light/dark cycle, temperature, quality of water etc for fish, type of food, access to food and water, environmental enrichment). | |
| 9c | Welfare-related assessments and interventions that were carried out prior to, during, or after the experiment. | |
| Sample size | 10a | Specify the total number of animals used in each experiment, and the number of animals in each experimental group. |
| 10b | Explain how the number of animals was arrived at. Provide details of any sample size calculation used. | |
| 10c | Indicate the number of independent replications of each experiment, if relevant. | |
| Allocation of animals | 11a | Give full details of how animals were allocated to experimental groups, including randomisation or matching if done. |
| 11b | Describe the order in which the animals in the different experimental groups were treated and assessed. | |
| Experimental outcomes | 12 | Clearly define the primary and secondary experimental outcomes assessed (e.g. cell death, molecular markers, behavioral changes). |
| Statistics | 13 | Appropriate statistics carried out on outcomes of each experimental group (including inter-group comparison if applicable) |
| Results | ||
| Baseline data | 14 | For each experimental group, report relevant characteristics and health status of animals (e.g. weight, microbiological status, and drug or test naïve) prior to treatment or testing. |
| Number analyzed | 15a | Report the number of animals in each group included in each analysis. |
| 15b | If any animals or data were not included in the analysis, explain why. | |
| Outcomes & estimation | 16 | Report the results for each analysis carried out, with a measure of precision (e.g. standard error or confidence interval). |
| Adverse effects | 17a | Give details of all important adverse events in each experimental group. |
| 17b | Describe any modifications to the experimental protocols made to reduce adverse events. | |
| Discussion | ||
| Interpretation/scientific implications | 18a | Interpret the results, taking into account the study objectives and hypotheses, current theory and other relevant studies in the literature. |
| 18b | Comment on the study limitations including any potential sources of bias, any limitations of the animal model, and the imprecision associated with the results. | |
| 18c | Describe any implications of your experimental methods or findings for the replacement, refinement or reduction (the 3Rs) of the use of animals in research. | |
| Generalizability/translation | 19 | Comment on whether, and how, the findings of this study are likely to translate to other species or systems, including any relevance to human biology. |
| Funding | 20 | List all funding sources (including grant number) and the role of the funder(s) in the study |
Results
Results of literature search
There were 436 studies found in the first search. The complete texts of 14 articles were obtained after irrelevant papers were eliminated based on titles and abstracts. Seven studies were eliminated from the analysis.12,22, 23, 24, 25, 26, 27 Table 2 lists the studies that were omitted as well as the grounds for their deletion. After checking the reference lists of the full texts, no new research were found. Therefore, six animal studies and a cell study were included in this review for data synthesis and quality assessment.28, 29, 30, 31, 32, 33, 34 Two studies included both cell cultures and animal models30,33 and four studies included only animal experiments.28,29,31,32 One study only described cell culture (in vitro) experiments.34 The kappa score was calculated as 0.83.
Table 2.
A list of studies excluded and reasons for exclusion after reading the full texts.
| Study | Reason(s) for exclusion |
|---|---|
| Shi et al. 202112 | No dental implants used; retrospective study. |
| Khajuria et al. 201822 | No dental implants used |
| Ma et al. 200923 | No dental implants used |
| Kim et al. 201724 | No dental implants used |
| Liang et al. 202025 | No dental implants used |
| Nagakawa et al. 202126 | No dental implants used |
| Nicolaev et al. 202127 | Systematic review |
General characteristics of studies
Animal studies
The general characteristics of the animal experiments conducted in the included studies are presented in Table 3. All studies assessed the impact of metformin on osseointegration in rats.28, 29, 30, 31, 32, 33 Two studies tested the effect of metformin on implants placed in diabetic rats.31,32 In one study, a genetic type 2 diabetic rat model (Goto-Kakizaki rats) was used32 while in one study, diabetes was induced by means of streptozotocin injections.31 In all other studies, healthy rats were used.28, 29, 30,33 Among the studies using healthy rats, two studies used Wistar rats29,30 and two studies used Sprague-Dawley rats.28,33 Two studies compared the effect of metformin on the osteointegration in diabetic rats not receiving metformin and those receiving metformin with healthy rats not receiving any treatment.31,32 In three studies, metformin's effect on osseointegration was compared to dental implants placed without any treatment among healthy rats.28, 29, 30 In one of these studies, the effect of metformin at doses of 50 and 100 mg/kg/day were also evaluated.30 In one study, the effect of metformin on osseointegration in rats with osteoporosis (induced by ovariectomy) was compared to osseointegration in rats that had undergone sham surgeries and osteoporotic rats that had received no treatment.33 Among the two studies in which diabetic rats had been used, only one study mentioned the inclusion criteria for diabetic rats which was >300 mg/dl.31 In all studies, titanium implants were used with lengths ranging from 3 to 4 mm and diameters ranging from 1 to 2.5 mm.28, 29, 30, 31, 32, 33
Table 3.
General characteristics and outcomes of the included animal studies. DM, diabetes mellitus; BV, bone volume; BF, bone fill, BIC, bone-implant contact; BA, bone area; TV trabecular volume; TT, trabecular thickness; and immunohistochemistry.
| Study | Animal model (n) | Groups |
DM inclusion criteria | Implant details | Duration of study | Method of metformin administration | Metformin dose | Method of osseointegration assessment | Other variables assessed | DM outcomes | Osteointegration outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test (n) | Control (n) | |||||||||||
| Inouye et al. 201432 | Wistar-Kyoto rats (non-DM) (n = 12) Goto-Kakizaki DM rats (24) |
DM + MET (n = 12) DM (n = 12) |
Non-DM, no treatment (n = 12) | Not defined | 3 × 1 mm, Ti; replacing right maxillary molars | 4 weeks | Fed in water | 100 mg/kg/day | Micro-CT: BIC, BV, TN, BMD at weeks 1 and 4 | Blood glucose, HbA1c, and pyridinoline |
MET reduced glucose levels in diabetic mice. | No significant effect of MET observed on BIC and TN in diabetic rats. MET improved BV in diabetic rats. |
| Bastos et al. 201729 | Wistar rats (n = 20) | MET (n = 10) | No treatment (n = 10) | N/A | 4.0 × 2.2 mm, Ti; placed in tibia | 30 days | Gavage | 40 mg/kg/day | Histo: BIC, BA | IHC: RANKL-and OPG-positive cells | Not measured | MET reduced BIC and BA, and increased number of RANKL+ cells. |
| Serrão et al. 201731 | Diabetic rats (n = 30) | DM (n = 10) DM + MET (n = 10) |
Non-diabetic, no treatment (n = 10) | >300 mg/dL | 4.0 × 2.2 mm, Ti; placed in tibia | 30 days | Gavage | 100 mg/kg/day | Histo: BIC, BA | IHC: RANKL-and OPG-positive cells | Not measured | MET did not have significant effect on BIC and BA but increased OPG+ cells, decreasing RANKL/OPG ratio. |
| Lin et al. 202033 | Sprague Dawley rats (n = 30) | Sham surgery (n = 10) OVX + MET (n = 10) OVX only (n = 10) |
Not specified | None | 3 × 1.5 mm, Ti; replacing right maxillary molars | 14 days | Injected into implant site (in PBS) | 20 mg/ml/day | Micro-CT: BV/TV %, TT, TS, TN | IHC: Calcified bone matrix | Not measured | MET accelerated osseointegration and bone formation in osteoporotic rat model. |
| Yıldırım et al. 202028 | Sprague Dawley rats (n = 20) | MET (n = 10) | No treatment (n = 10) | N/A | 4 × 2.5 mm, Ti; placed in tibia | 28 days | Gavage | 40 mg/kg/day | Histological: BF ratios | None | Not measured | MET induced higher periimplant BF ratios. |
| Sun et al. 202130 | Wistar rats (n = 18) | MET-50 (n = 6) MET-100 (n = 6) |
No treatment (n = 6) | N/A | 3 × 2 mm, Ti; placed in femur | 4 weeks | Gavage | MET-50: 50 mg/kg/day MET-100: 100 mg/kg/day |
Histo: BIC | None | Not measured | MET increased BIC. 100 mg/kg/day Met had higher impact on BIC than 50 mg/kg/day. |
The duration of the studies ranged from 14 to 30 days.28, 29, 30, 31, 32, 33 In four studies, metformin was administered via gavage.28, 29, 30, 31 In one study, metformin was locally administered at implant sites,33 while in one study, metformin was fed to the rats in water.32 In five studies, doses of metformin ranged from 20 to 100 mg/kg/day,28, 29, 30, 31, 32 and in one study, a dose of 20 mg/ml/day was administered.33 Two studies assessed osteointegration via microcomputed tomography (micro-CT).32,33 In one of these studies, micro-CT was used to measure bone implant contact (BIC), bone volume (BV), trabecular number, and bone mineralization density at weeks 1 and 4.32 In the other study, micro-CT was used to assess BV/trabecular volume percentage (TV%), trabecular thickness, trabecular separation, and trabecular number.33 In four studies, only histological examination was used to assess osteointegration.28, 29, 30, 31 Two studies assessed BIC and bone area (BA) histologically29,31 and one study only assessed BIC.30 One study assessed only assessed bone fill via histology.28 Finally, only one study assessed effect of metformin on indicators of diabetes (hemoglobin A1c and blood glucose levels).32
Cell studies
Two studies described the effect of metformin on bone marrow-derived stem cells (BMSCs) obtained from rats.33,34 One study used BMSCs obtained from DM2 and healthy patients.30 In one study, healthy rat BMSCs were cultured on titanium samples that were exposed to 50 μM metformin, and the cellular activity and growth were compared with titanium samples not treated with metformin33. It was observed that metformin exerted osteogenic, anti-aging, antioxidative, and pro-autophagic effects on the cells.33 In another study, titanium was modified by means of anodization and chitosan coating to produce metformin-releasing surfaces to study its osteogenic effect on rat BMSCs.34 The general characteristics of the cell experiments are presented in Table 4.
Table 4.
General characteristics and the outcomes of the cell (in vitro) studies included in this review. DM, diabetes mellitus; BMSCs, bone marrow derived stem cells; RT-PCR, real-time polymerase chain reaction; BMP, bone morphogenic protein; ALP, alkaline phosphatase; histo, histology; UV, ultraviolet; Ti, titanium.
| Study | Methodology | Cell line used | Metformin concentration | Variables assessed | General outcomes |
|---|---|---|---|---|---|
| Lin et al 2020 [33] | Cells from animal models on Ti samples for in vitro experiments | Rat BMSC | 50 µM | Autophagy markers (LC3), ROS, production, senescence proteins (P16, P21, and P53), and osteogenic differentiation markers (Runx2, OCN, and ALP) | MET had osteogenic, anti-aging, antioxidative and pro-autophagic effects. |
| Hashemi et al 2020 [34] | Ti implants coated with TiO2 nanotubes coated with chitosan releasing MET. Cells cultured on untreated Ti, anodized Ti and anodized Ti coated with MET-chitosan samples. | Rat BMSC | N/A | Metformin release (UV spectrometry), ALP activity and expression, type I collagen expression | MET-chitosan coated implants had a sustained release of MET. MET promoted pro-osteogenic effect. |
| Sun et al 2021 [30] | BMSCs derived from the alveolar bone particles acquired from the implant bed of normal and DM2 patients. | Human BMSC from diabetic and healthy patients | DM group: 0, 100, 200, 300, 400, and 500 µM Healthy group: (0, 50, and 100 µM |
ALP detection, alizarin red staining, RT-PCR and Western blotting (anti-p-AMPK, AMPK, BMP-2, Smad1, Runx-2 | MET had a dose dependent positive effect on osteogenesis when <200 µM but decreased osteogenesis when >200 µM. MET had pro-osteogenic effect on BMSCs from normal and DM2 individuals. |
Main outcomes
In three of the six included animal studies, micro-CT and histological assessment indicated that metformin improved osteointegration in diabetic or healthy rats.28,30,32 In one study, metformin negatively affected osseointegration in healthy rats and increased the number of RANKL+ positive cells.29 In one study, metformin did not have a significant impact on diabetic rats.31 Finally, in one study, metformin improved osteointegration in osteoporotic rats.33 In the vitro experiments, metformin showed osteogenic, anti-aging, antioxidative, and pro-autophagic effects on rat BMSCs,33 and another study showed dose-dependent pro-osteogenic effects on human BMSCs isolated from healthy and diabetic subjects.30 Similarly, in one study metformin released from surface-modified dental implant promoted osteogenesis in rat BMSCs.34
Results of quality assessment
Overall, three studies were deemed medium quality29, 30, 31 and three studies received a low-quality grading.28,32,33 None of the studies received a high rating. The details of the scores received by each in each category are provided in Table 5.
Table 5.
Results of the quality assessment of the included studies. The details of the items are provided in Table 2.
| Study characteristics | Item no. | Inouye et al. 201432 | Bastos et al. 201729 | Serrão et al. 201731 | Lin et al. 202033 | Yıldırım et al. 202028 | Sun et al. 202130 |
|---|---|---|---|---|---|---|---|
| Title | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Abstract | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Introduction | |||||||
| Background | 3a | 1 | 1 | 1 | 1 | 1 | 1 |
| 3b | 0 | 0 | 0 | 0 | 0 | 0 | |
| Objectives | 4 | 1 | 1 | 1 | 1 | 1 | 1 |
| Methodology | |||||||
| Ethical statement | 5 | 1 | 1 | 1 | 1 | 1 | 1 |
| Study design | 6a | 1 | 1 | 1 | 1 | 1 | 1 |
| 6b | 0 | 1 | 1 | 1 | 1 | 1 | |
| 6c | 1 | 1 | 1 | 0 | 1 | 1 | |
| Experimental procedures | 7a | 1 | 1 | 1 | 1 | 1 | 1 |
| 7b | 0 | 0 | 0 | 0 | 0 | 0 | |
| 7c | 0 | 0 | 0 | 0 | 0 | 0 | |
| 7d | 0 | 0 | 0 | 0 | 0 | 0 | |
| Experimental animals | 8a | 1 | 1 | 1 | 1 | 1 | 1 |
| 8b | 1 | 1 | 0 | 0 | 0 | 0 | |
| Housing and husbandry | 9a | 0 | 1 | 1 | 0 | 0 | 1 |
| 9b | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9c | 0 | 0 | 1 | 0 | 0 | 0 | |
| Sample size | 10a | 1 | 1 | 1 | 1 | 1 | 1 |
| 10b | 0 | 1 | 1 | 0 | 0 | 0 | |
| 10c | 0 | 0 | 0 | 0 | 0 | 0 | |
| Allocation of animals | 11a | 1 | 1 | 1 | 1 | 1 | 1 |
| 11b | 0 | 0 | 0 | 0 | 0 | 0 | |
| Experimental outcomes | 12 | 1 | 1 | 1 | 1 | 1 | 1 |
| Statistics | 13 | 1 | 1 | 1 | 1 | 1 | 1 |
| Results | |||||||
| Baseline data | 14 | 0 | 0 | 1 | 0 | 1 | 0 |
| Number analyzed | 15a | 1 | 1 | 1 | 1 | 1 | 1 |
| 15b | 1 | 1 | 1 | 0 | 0 | 0 | |
| Outcomes & estimation | 16 | 1 | 1 | 1 | 1 | 1 | 1 |
| Adverse effects | 17a | 0 | 0 | 0 | 0 | 0 | 0 |
| 17b | 0 | 0 | 0 | 0 | 0 | 0 | |
| Discussion | |||||||
| Interpretation/scientific implications | 18a | 1 | 1 | 1 | 1 | 1 | 1 |
| 18b | 0 | 1 | 1 | 0 | 0 | 0 | |
| 18c | 0 | 1 | 0 | 0 | 0 | 0 | |
| Generalizability/translation | 19 | 1 | 1 | 1 | 1 | 1 | 1 |
| Funding | 20 | 1 | 1 | 1 | 1 | 1 | 1 |
| Overall quality of study | Low | Medium | Medium | Low | Low | Medium | |
Discussion
The purpose of this systematic review was to examine the literature with a focus on the effect of metformin on the osteogenic qualities of titanium and its osteointegration in the jaws. It can be concluded that, overall, metformin may have a favorable impact on the osteogenic properties of stem cells or the osseointegration of dental implants.28,30,32,33 Furthermore, the data from these studies also provide insights into the mechanism of action of metformin in affecting the osseointegration of dental implants. Serrão et al.31 suggested that although metformin does not have a significant impact on the osteoclastic activity, it promotes osteoblast activity as indicated by the upregulation of OPG in the peri-implant tissue of diabetic and non-diabetic rats.
Metformin is a drug that is used for the management of DM2. It acts mainly by reducing blood glucose levels by opposing the signaling of glucagon in the liver.16 In diabetes-associated periodontitis, metformin may reduce the severity of the disease by lowering the glucose-mediated inflammatory cascades in the periodontal tissues. Reduction of blood glucose would help improve periodontal health in patients with the DM2. Indeed, studies focusing on the local application of the drug as an adjunct to surgical and non-surgical periodontal therapy have suggested that it may directly affect periodontal tissues.18 More recent studies indicate that metformin has anti-inflammatory and antioxidative effects on periodontal tissues and may have a similar impact on peri-implant tissues.33 Moreover, metformin may also counter the effects of lipopolysaccharides, an endotoxin produced by Porphyromonas gingivalis, a major periodontal pathogen.35,36 It is not surprising that metformin was observed to improve the bone implant in some studies included in this review.
The direct impact of metformin on peri-implant bone may be ascertained by the results obtained by Lin et al.,33 who observed that metformin might improve bone formation around dental implants in rats that had undergone ovariectomy to induce osteoporosis. This opens an exciting avenue of using metformin in osteoporotic patients to adjunct conventional osteoporotic treatment in patients receiving dental implants.
When a dental implant is placed in living tissue, it triggers a cascade of inflammatory and foreign body reactions.5 Studies have also revealed that dental implants may induce a higher production of reactive oxidative species, contributing to peri-implantitis and early implant failure.37 Since in vitro experiments conducted on rat BMSCs reveal that metformin also has anti-aging, antioxidative and pro-autophagic effects in addition to osteogenic properties, metformin may have a protective effect on the implant-bone interface.33 Nevertheless, to date, very few studies have been conducted that have evaluated the effect of metformin on implants placed in diabetic mice or on cells isolated from diabetic mice.29,30,32 Therefore, to date, it is unknown if intake or administration of metformin has any benefits on osteointegration in diabetic patients. Indeed, in studies that included diabetic animal models to compare osseointegration in diabetes with healthy models,31,32 metformin had no significant impact on osseointegration. Additionally, in one study, metformin had a negative effect on bone-implant contact,29 which could potentially affect the drug on dental implants. Therefore, more studies on the dose-dependent effect of metformin on the outcomes of dental implant placement are necessary. Furthermore, it is unknown if the age of the dental implants and/or time of implant placement is a factor that may impact the interaction of dental implants with the peri-implant tissues.
The studies included in this systematic review had several limitations. First, none of the studies used additional methods of evaluating osseointegration, such as resonance frequency analysis. Using only histological examination and micro-CT scanning are insufficient to deduce the bone-implant interface. None of the studies measured implant stability by resonance frequency analysis.38 Furthermore, the duration of the studies was limited. Complete bone regeneration or healing can take up to 6 months.39 Hence, there is a lack of knowledge regarding the long-term impact of metformin on peri-implant bone regeneration.
The quality assessment of the studies revealed numerous sources of bias and deficiencies in methodologies. None of the studies justified using the selected animal models. Indeed, since metformin is an antihyperglycemic agent, it is imperative to use diabetic animal or human subjects if testing its effect. Therefore, future studies should focus on diabetic subjects. None of the studies adequately described the experimental procedures according to the ARRIVE guideline, leading to underreported outcomes.
Furthermore, none of the studies reported the sequence of the treatment groups, which may have led to allocation bias. Only two studies described attempts to use a statistically calculated predetermined sample size.29,31 No study stated the experimental replicate, which may have contributed to unreported or biased outcomes. Moreover, no studies described any non-experimental adverse effects observed in the animal subjects during the experiments.
This systematic review had some limitations. First, no clinical studies were found in the literature search relevant to our focused question. Furthermore, due to the heterogeneity of the included papers, meta-analysis was not possible, and only a qualitative assessment was possible. As a result, the magnitude of metformin's overall effect on osseointegration and bioactivity of dental implants remains uncertain.
Conclusion
Within the limitations of this review, it may be concluded that metformin improves osseointegration by reducing blood sugar levels and exerting a direct osteogenic effect on the peri-implant tissues. However, due to several sources' bias and deficiencies in the methodology of the studies, more long-term animal and human trials are required to ascertain the overall impact of the drug on osteointegration dental implants. Therefore, dentists should schedule regular follow-up of patients who have received dental implants and are using metformin.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not Applicable.
Authors contributions
V.P., M.S.S., and S.N. conceptualized the study; V.P., M.S.S., S.N., and Z.K. conducted the methodology; V.P., M.S.S., and S.N. validated the study; S.N. and Z.K. conducted the formal analysis; V.P., M.S.S., and S.N. Z.K. curated the data; K.S.A. conceived the study; M.S.Z and A.H. supervised the study; M.S.Z and A.H. administered the project; All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Ali D., Kunzel C. Diabetes mellitus: update and relevance for dentistry. Dent Today. 2011;30(12):45–46. [PubMed] [Google Scholar]
- 2.de Araújo Aurigena Antunes, Pereira Aline de Sousa Barbosa Freitas, de Medeiros Caroline Addison Carvalho Xavier, de Castro Brito Gerly Anne, de Carvalho Leitão Renata Ferreira, de Souza Araújo Lorena, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS One. 2017;12(8):e0183506. doi: 10.1371/journal.pone.0183506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baseri M., Radmand F., Hamedi R., Yousefi M., Kafil H.S. Immunological aspects of dental implant rejection. BioMed Res Int. 2020 doi: 10.1155/2020/7279509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastos M.F., Serrão C.R., Miranda T.S., Cruz D.F., de Souza Malta F., Duarte P.M. Effects of metformin on bone healing around titanium implants inserted in non-diabetic rats. Clin Oral Implants Res. Oct 2017;28(10):e146–e150. doi: 10.1111/clr.12960. [DOI] [PubMed] [Google Scholar]
- 5.Chrcanovic B.R., Albrektsson T., Wennerberg A. Diabetes and oral implant failure: a systematic review. J Dent Res. 2014;93(9):859–867. doi: 10.1177/0022034514538820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorellini J.P., Chen P.K., Nevins M., Nevins M.L. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. Aug 2000;20(4):366–373. [PubMed] [Google Scholar]
- 7.Flory J., Lipska K. Metformin in 2019. Jama. 2019;321(19):1926–1927. doi: 10.1001/jama.2019.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashemi A., Ezati M., Mohammadnejad J., Houshmand B., Faghihi S. Chitosan coating of TiO2 nanotube Arrays for improved metformin release and osteoblast differentiation. Int J Nanomed. 2020;15:4471–4481. doi: 10.2147/IJN.S248927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inouye K.A., Bisch F.C., Elsalanty M.E., Zakhary I., Khashaba R.M., Borke J.L. Effect of metformin on periimplant wound healing in a rat model of type 2 diabetes. Implant Dent. Jun 2014;23(3):319–327. doi: 10.1097/id.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 10.Kang W., Wang T., Hu Z., Liu F., Sun Y., Ge S. Metformin inhibits Porphyromonas gingivalis lipopolysaccharide-influenced inflammatory response in human gingival fibroblasts via regulating activating transcription factor-3 expression. J Periodontol. Oct 2017;88(10):e169–e178. doi: 10.1902/jop.2017.170168. [DOI] [PubMed] [Google Scholar]
- 11.Khajuria D.K., Patil O.N., Karasik D., Razdan R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch Oral Biol. Jan 2018;85:120–129. doi: 10.1016/j.archoralbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. Jun 29 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H.K., Park Y.S. Plasma concentration of metformin and dexamethasone after administration through Osseogate. Drug Deliv. Nov 2017;24(1):437–442. doi: 10.1080/10717544.2016.1261380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kittur N., Oak R., Dekate D., Jadhav S., Dhatrak P. Dental implant stability and its measurements to improve osseointegration at the bone-implant interface: a review. Mater Today Proc. 2021;43:1064–1070. [Google Scholar]
- 15.Klinge B. Peri-implant marginal bone loss: an academic controversy or a clinical challenge. Eur J Oral Implantol. 2012;5(Suppl):S13–S19. [PubMed] [Google Scholar]
- 16.Kuang Y., Hu B., Feng G., Xiang M., Deng Y., Tan M., et al. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology. 2020;21(1):13–27. doi: 10.1007/s10522-019-09838-x. [DOI] [PubMed] [Google Scholar]
- 17.Le Guéhennec L., Soueidan A., Layrolle P., Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Liang C., Sun R., Xu Y., Geng W., Li J. Effect of the abnormal expression of BMP-4 in the blood of diabetic patients on the osteogenic differentiation potential of alveolar BMSCs and the rescue effect of metformin: a bioinformatics-based study. BioMed Res Int. 2020;2020 doi: 10.1155/2020/7626215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J., Xu R., Shen X., Jiang H., Du S. Metformin promotes the osseointegration of titanium implants under osteoporotic conditions by regulating BMSCs autophagy, and osteogenic differentiation. Biochem Biophys Res Commun. Oct 15 2020;531(2):228–235. doi: 10.1016/j.bbrc.2020.06.146. [DOI] [PubMed] [Google Scholar]
- 20.Ma L., Wu X., Ling-Ling E., Wang D.S., Liu H.C. The transmembrane transport of metformin by osteoblasts from rat mandible. Arch Oral Biol. Oct 2009;54(10):951–962. doi: 10.1016/j.archoralbio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Moraschini V., Barboza E.S.P., Peixoto G.A. The impact of diabetes on dental implant failure: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45(10):1237–1245. doi: 10.1016/j.ijom.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Moy P.K., Medina D., Shetty V., Aghaloo T.L. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20(4) [PubMed] [Google Scholar]
- 24.Mozzati M., Arata V., Gallesio G., Mussano F., Carossa S. Immediate postextractive dental implant placement with immediate loading on four implants for mandibular-full-arch rehabilitation: a retrospective analysis. Clin Implant Dent Relat Res. 2013;15(3):332–340. doi: 10.1111/j.1708-8208.2011.00412.x. [DOI] [PubMed] [Google Scholar]
- 25.Najeeb Shariq, Zafar Muhammad Sohail, Khurshid Zohaib, Zohaib Sana, Madathil Sreenath Arekunnath, Mali Maria, et al. Efficacy of metformin in the management of periodontitis: a systematic review and meta-analysis. Saudi Pharm J. 2018;26(5):634–642. doi: 10.1016/j.jsps.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa T., Tsuka S., Aonuma F., Nodai T., Munemasa T., Tamura A., et al. Effects of metformin on the prevention of bisphosphonate-related osteonecrosis of the jaw-like lesions in rats. J Prosthodont Res. Jun 30 2021;65(2):219–224. doi: 10.2186/jpr.JPOR_2019_629. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaev N., Romanos G.E., Malmstrom H., Elad S. Commonly used systemic drugs interfering with bone remodeling: a case report and literature review. Quintessence Int. Oct 19 2021;52(10):880–886. doi: 10.3290/j.qi.b2053577. [DOI] [PubMed] [Google Scholar]
- 28.Pietropaoli D., Ortu E., Severino M., Ciarrocchi I., Gatto R., Monaco A. Glycation and oxidative stress in the failure of dental implants: a case series. BMC Res Notes. 2013;6(1):1–6. doi: 10.1186/1756-0500-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preshaw P.M., Alba A.L., Herrera D., Jepsen S., Konstantinidis A., Makrilakis K., et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emerging Risk Factors Collaboration. Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. Jun 26 2010;375(9733):2215–2222. doi: 10.1016/s0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serrão C.R., Bastos M.F., Cruz D.F., de Souza Malta F., Vallim P.C., Duarte P.M. Role of metformin in reversing the negative impact of hyperglycemia on bone healing around implants inserted in type 2 diabetic rats. Int J Oral Maxillofac Implants. May/Jun 2017;32(3):547–554. doi: 10.11607/jomi.5754. [DOI] [PubMed] [Google Scholar]
- 32.Shi S., Ding F., Liu X., Wang L., Wang X., Zhang S., et al. Clinical and radiographic variables related to implants with simultaneous grafts among type 2 diabetic patients treated with different hypoglycemic medications: a retrospective study. BMC Oral Health. Apr 27 2021;21(1):214. doi: 10.1186/s12903-021-01583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steigenga J.T., Al-Shammari K.F., Nociti F.H., Misch C.E., Wang H.-L. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003;12(4):306–317. doi: 10.1097/01.id.0000091140.76130.a1. [DOI] [PubMed] [Google Scholar]
- 34.Sun R., Liang C., Sun Y., Xu Y., Geng W., Li J. Effects of metformin on the osteogenesis of alveolar BMSCs from diabetic patients and implant osseointegration in rats. Oral Dis. Feb 19 2021 doi: 10.1111/odi.13808. [DOI] [PubMed] [Google Scholar]
- 35.Wang H.W., Lai E.H., Yang C.N., Lin S.K., Hong C.Y., Yang H., et al. Intracanal metformin promotes healing of apical periodontitis via suppressing inducible nitric oxide synthase expression and monocyte recruitment. J Endod. Jan 2020;46(1):65–73. doi: 10.1016/j.joen.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y.-Y., Xiao E., Graves D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015;7(2):63–72. doi: 10.1038/ijos.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yıldırım T.T., Dündar S., Bozoğlan A., Karaman T., Kahraman O.E., Özcan E.C. The effects of metformin on the bone filling ration around of TiAl(6)Va(4) implants in non diabetic rats. J Oral Biol Craniofac Res. Oct-Dec 2020;10(4):474–477. doi: 10.1016/j.jobcr.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y., Gao J., Luo L., Wang Y. Does bruxism contribute to dental implant failure? A systematic review and meta-analysis. Clin Implant Dent Relat Res. Apr 2016;18(2):410–420. doi: 10.1111/cid.12300. [DOI] [PubMed] [Google Scholar]
- 39.Zimmet P.Z., Magliano D.J., Herman W.H., Shaw J.E. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]