Abstract
Objective
The immunosuppressant tacrolimus is a major cause of new-onset diabetes after transplantation. The aim of this study was to evaluate whether a low dose of the histone-deacetylase inhibitor (vorinostat) might ameliorate tacrolimus-induced new-onset diabetes.
Methods
Thirty 8-week-old male Wistar rats were randomly divided into five groups: a control group, tacrolimus group (1.5 mg/kg intraperitoneally for 28 days), vorinostat group (15 mg/kg orally for 28 days), a group receiving tacrolimus with vorinostat for 28 days; and a group receiving coadministration of tacrolimus for 28 days and vorinostat for 14 days. Diabetes development was assessed on the basis of serum glucose, insulin, HOMA-IR and C-peptide. To investigate the mechanism of vorinostat, we assessed inflammatory markers (tumor necrosis factor-α and interleukin-1β), an antioxidant marker (glutathione), an oxidant marker (nicotinamide adenine dinucleotide phosphate hydrogen oxidase) and an apoptosis marker (caspase-3). Kidney functions (creatinine and blood urea nitrogen) were also assessed.
Results
The administration of tacrolimus for 28 days resulted in significantly increased serum glucose and decreased C-peptide and insulin levels than those in the control group. However, coadministration of vorinostat significantly decreased hyperglycemia and increased C-peptide and insulin levels. Moreover, combined treatment with tacrolimus and vorinostat, compared with tacrolimus treatment alone, resulted in significantly reduced inflammatory and oxidant markers, and increased glutathione. Additionally, vorinostat improved the kidney parameters.
Conclusion
Vorinostat at a low dose (15 mg/kg) induces anti-inflammatory and antioxidative effects that protect the pancreas and kidney against the development of new-onset diabetes due to tacrolimus in rats. This experimental study provides insights supporting further clinical trials to improve the post-kidney transplantation protocol through addition of vorinostat to the immunosuppressive regimen.
Keywords: Anti-inflammatory, Anti-oxidant, Histone deacetylases inhibitors, New-onset diabetes after transplantation, Tacrolimus, Vorinostat
Abbreviations: BUN, blood urea nitrogen; DMSO, dimethyl sulfoxide solution; ELISA, enzyme linked immunosorbent assay; FGT, fast glucose test; GSH, glutathione; HDACi, histone deacetylase inhibitor; HOMA-IR, homeostatic model assessment for insulin resistance index; IP, intraperitoneal; IL-1β, interleukin-1 beta; NADPH-oxidase, nicotinamide adenine dinucleotide phosphate hydrogen oxidase enzyme; NODAT, new-onset diabetes after transplantation; PO, oral gavage; Tacro, tacrolimus; TNF-α, tumor necrosis factor-α; Vorino, vorinostat
الملخص
أهداف البحث
يعتبر مثبط المناعة (التاكروليموس) أهم مسبب للإصابة بنوع السكري الجديد ما بعد زراعة الكلى. تهدف الدراسة لتقييم فعالية الفورينوستات (مثبط الهستون-دياسيتايلاز) في التحكم بالسكري الناتج عن التاكروليموس.
طرق البحث
تم تقسيم ثلاثين ذكر من جرذان ويستر البالغة 8-أسابيع عشوائيا إلى ٥ مجموعات: المجموعة الضابطة، مجموعة التاكروليموس (١.٥ مجم/كجم، داخل الصفاق لمدة ٢٨ يوم)، مجموعة الفورينوستات (١٥ مجم/كجم، عن طريق الفم لمدة ٢٨ يوم). ومجموعتان تم إعطاؤهما الدوائين معا: مجموعة التاكروليموس مع الفورينوستات لمدة ٢٨ يوم، ومجموعة التاكروليموس مع الفورينوستات لمدة ١٤ يوم قبل نهاية التجربة. تم تقييم تطور السكري بقياس نسبة الجلوكوز والأنسولين والببتيد الرابط. وللتحقق من آلية عمل الفورينوستات، تم قياس علامات الالتهاب (عامل نخر الورم-ألفا و انترلوكين١بيتا)، علامات مضادات الأكسدة (الجلوتاثيون) والأكسدة (أكسيد فوسفات ثنائي نيوكلوتيد الأدينين) ومؤشر موت الخلايا المبرمج (كاسباس-٣). أيضاً تم تقييم وظائف الكلى (اليوريا والكرياتينين).
النتائج
أدى العلاج بالتاكروليموس لمدة 28 يوم إلى زيادة ملحوظة في مستوى السكر وانخفاض في مستويات الببتيد الرابط والأنسولين مقارنة بالمجموعة الضابطة. ومع ذلك، فإن تزامن أخذ الدوائين معا قلل بشكل ملحوظ من ارتفاع السكر وزيادة مستويات الببتيد الرابط والأنسولين. كما قلل جمع الدوائين بشكل ملحوظ مستويات علامات الالتهاب والاكسدة وكاسباس-٣ مع زيادة مستوى الجلوتاثيون مقارنة بالمجموعة المعالجة بالتاكروليموس. أيضا، حافظ الفورينوستات على مستويات عوامل الكلى ضمن المعدل الطبيعي.
الاستنتاجات
استخدام الفورينوستات بجرعة صغيرة (15 مجم/كجم) يستحث التأثير المضاد للالتهاب والمضاد للأكسدة والتي تحمي بنكرياس وكلى الجرذان من الاصابة بالسكري الناتج عن التاكروليموس. تفتح هذه الدراسة الطريق لمزيد من التجارب السريرية لتحسين بروتوكول ما بعد زراعة الكلى عن طريق إضافة الفورينوستات في النظام العلاجي لمثبطات المناعة.
الكلمات المفتاحية: مضاد التهاب, مضاد أكسدة, مثبطات هيستون ديسيتيلازس, السكري الجديد بعد الزرع, تاكروليموس, فورينوستات
Introduction
End-stage chronic renal disease has been a substantial problem in KSA, affecting 19,000 people over the past 30 years.1 Although it can be managed through hemodialysis, the optimal treatment for this disease is kidney transplantation.2 According to the most recent report from the Saudi Center for Organ Transplantation, kidney transplantation is the most frequently performed type of organ transplantation (86.9%).1 After transplantation, patients must receive immunosuppressive agents to protect the transplanted kidney from immune system attack and organ rejection.3 However, immunosuppression may cause harmful adverse effects, such as infections, cancer and diabetes.4
Various studies have established a direct relationship between the use of immunosuppressants, particularly corticosteroids and tacrolimus (Tacro), and the development of diabetes.5 New-onset diabetes after transplantation (NODAT) refers to diabetes incidence after organ transplantation in nondiabetic patients; it occurs in 10%–25% of patients taking Tacro.6 However, the pathogenesis of Tacro-induced NODAT remains incompletely understood. Numerous studies have proposed hypotheses regarding its pathogenesis, on the basis of the direct toxicity of Tacro to pancreatic β-cells, and the associated decrease in both insulin synthesis and secretion. Subsequently, hyperglycemia increases oxidative stress, which in turn produces further β-cell apoptosis.7 This cycle leads to the development of NODAT.8 NODAT can generate serious complications that affect many organs,9 and potentially lead to the failure or rejection of transplanted kidneys.8 Although classical hypoglycemic drugs, such as sulfonylureas or biguanides, and insulin may be used to treat NODAT, these drugs have several dangerous adverse effects, such as lactic acidosis,10 in which lactic acid accumulation in the body can lead to failure of the transplanted kidney.11 Thus, further research on new drugs for NODAT treatment that promote the protection of transplanted kidneys is needed.
Over the past 10 years, several studies have focused on histone deacetylase enzymes, which have a pathogenic role in diabetes mellitus.12,13 The development of diabetes is dependent on acetylation reactions that release cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), which induce β-cell death.14 Thus, histone deacetylase inhibitor (HDACi) drugs are promising anti-inflammatory agents.13 Vorinostat (Vorino) is an HDACi that was approved for the treatment of cutaneous T-cell lymphoma in 2006,15,16 and has broad-spectrum anti-inflammatory and antioxidant characteristics.12,17 Previous studies have demonstrated that Vorino has antidiabetic effects in an animal model of type 1 DM.18 These attractive properties of Vorino make it a suitable therapeutic candidate for managing NOD after kidney transplantation.
Because little work has been performed on this topic in KSA, this study aimed to investigate possible novel therapeutic targets for NODAT. We investigated the effects of a low oral dose (15 mg/kg/day) of Vorino,20 which was intended to be an anti-inflammatory dose rather than an anticancer dose, and examined its anti-diabetic mechanism in Tacro-induced NOD in male Wistar rats. Moreover, a literature review focuses on the effectiveness and safety of Vorino in NOD animal models. The results of this study will help improve the protocols and strategies for kidney transplantation regimens.
Materials and Methods
Thirty 8-week-old male Wistar rats weighing between 180 and 200 g were used. They were housed in groups of six in transparent plastic cages. The temperature was controlled, and a 12/12-h light/dark cycle environment was implemented. The rats were provided with tap water and a low-salt diet (0.05% sodium) to avoid another risk factor (hypertension).
Study design
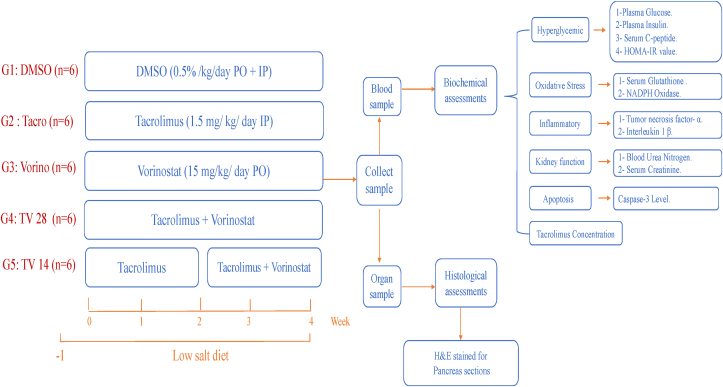
The rats were randomly divided into five groups (n = 6 in each group) (Figure 1):
-
1.
DMSO group (control): received 0.5 ml of 0.5% DMSO (Sigma Aldrich, Darmstadt, Germany) both PO and IP each day for 28 days.
-
2.
Tacro group: received 0.5 ml of 1.5 mg/kg Prograf (Astellas Toyama Co., Tokyo, Japan) IP each day for 28 days19 to induce NOD.
-
3.
Vorino group: received 0.5 ml of 15 mg/kg Vorino (MedChem Express, NJ, USA) PO each day for 28 days.20
-
4.
Tacro + Vorino 28 group (TV28): received 0.5 ml of Vorino (15 mg/kg) PO and then 0.5 ml of Tacro (1.5 mg/kg) at 2 h after Vorino administration IP daily for 28 days.19
-
5.
Tacro + Vorino 14 group (TV14): received 0.5 ml of Tacro (1.5 mg/kg) alone IP each day for 14 days, and then 0.5 ml of Vorino (15 mg/kg) PO each day and 0.5 ml of Tacro (1.5 mg/kg) at 2 h after Vorino administration on day 15.19
Figure 1.
Experimental design. After a low salt diet for 1 week, animals were divided into five groups (n = 6).
DMSO, control group; PO, oral gavage; IP, intraperitoneal; Tacro, Tacrolimus group; TV28, Tacrolimus with Vorinostat for 28 days; TV14, Tacrolimus for 28 days with Vorinostat for 14 days group; Vorino, Vorinostat group.
All doses were chosen on the basis of previous studies, and the dose of Vorino was selected according to its anti-inflammatory effects in animal models.20 All solutions were prepared daily and administered at 9 a.m. The fast glucose test (FGT) and rat weight were verified weekly. The changes in body weight and FGT results were calculated according to the differences between the mean group weight at the beginning and the end of the experiment.
Finally, the rats were anesthetized with diethyl ether. Blood samples were collected from the heart to measure the following biomarker levels with specific ELISA kits:
-
1.
Hyperglycemic parameters: a glucose ELISA kit (catalog No. MBS7233226), Tacro ELISA kit (catalog No. MBS288390) and CASP3 ELISA kit (catalog No. MBS018987) were obtained from My Bio Source (San Diego, CA); an insulin ELISA kit (catalog No. ERINSX5) was obtained from Thermo Fisher Scientific (Inchinnan, UK); and a C-peptide ELISA kit (catalog No. 90055) was purchased from Crystal Chem (Elk Grove Village, IL).
-
2.
Oxidative stress parameters: a NADPH oxidase ELISA kit (catalog No. MBS2602768) was obtained from My Bio Source, and a glutathione ELISA kit (catalog no. E-EL-0026) was obtained from Elabscience (Houston, TX).
-
3.
Inflammatory parameters: a TNFα ELISA kit (catalog No. MBS355371) and an IL-1β ELISA kit (catalog No. MBS2023030) were obtained from My Bio Source.
-
4.
Kidney parameters: a BUN ELISA kit (catalog No. MBS2600001) and creatinine ELISA kit (catalog No. MBS749827) were obtained from My Bio Source.
Additionally, the homeostatic model assessment of insulin resistance (HOMA-IR) was used to compare insulin resistance according to the equation. Finally, the pancreas was extracted in 10% formalin and stained with H&E for histopathological assessment by light microscopy (Olympus-BX53, Olympus Co., Tokyo, Japan) at 20× magnification.
The experimental protocol was approved by the Unit of Biomedical Ethics of the Research Ethics Committee at King Abdul-Aziz University (reference No. 309-18).
Statistical analysis
Statistical analyses were performed in SPSS, version 21.0. (IBM Inc., NY, USA). All data are presented as the means ± standard deviation (SD), and P values of 0.05 were considered significant. Significant differences were determined with one-way analysis of variance (ANOVA), followed by Bonferroni post hoc comparison.
Results
Effects of Tacro and Vorino on basic parameters
The weights of the animals all groups increased gradually each week (Table 1). However, Tacro, compared with DMSO, resulted in significantly lower weights and higher FGT (P = 0.05).
Table 1.
Effects of tacrolimus (Tacro) and vorinostat (Vorino) on basic parameters.
| Groups (n = 6) | DMSO | Tacro | Vorino | TV28 | TV14 |
|---|---|---|---|---|---|
| ΔBW (g) | 110.84 ± 3.1 | 45 ± 1.62∗ | 112.2 ± 12.4 | 57.5 ± 34.3 | 80 ± 16.1 |
| ΔFGT (mg/dl) | 9.2 ± 9.6 | 46.5 ± 9.6∗ | 27 ± 5.53 | 20.5 ± 3.82† | 29.7 ± 6.34† |
| Tacro level (μg/l) | – | 10.1 ± 1.48 | – | 11.31 ± 3 | 12.03 ± 1.8 |
Values are presented as mean ± SD. n = number of rats.
One-way ANOVA, followed by Bonferroni post hoc comparison test. P = 0.05.
DMSO, control; BW, body weight; FG, fastglucose test;Tacro, Tacrolimus; TV28, Tacrolimus with Vorinostat for 28 days; TV14, Tacrolimus for 28 days with Vorinostat for 14 days; Vorino, Vorinostat group.
P = 0.05 compared with DMSO.
P = 0.05 compared with Tacro.
Remarkably, TV14 significantly increased the Tacro-induced weight loss (P = 0.05), and the FGT values were significantly lower in both coadministration groups at week 3 than in the Tacro group. The Tacro level was measured only for the Tacro, TV28 and TV14 groups (Table 1), and the differences were not significant.
Effects of Tacro and Vorino on hyperglycemic parameters
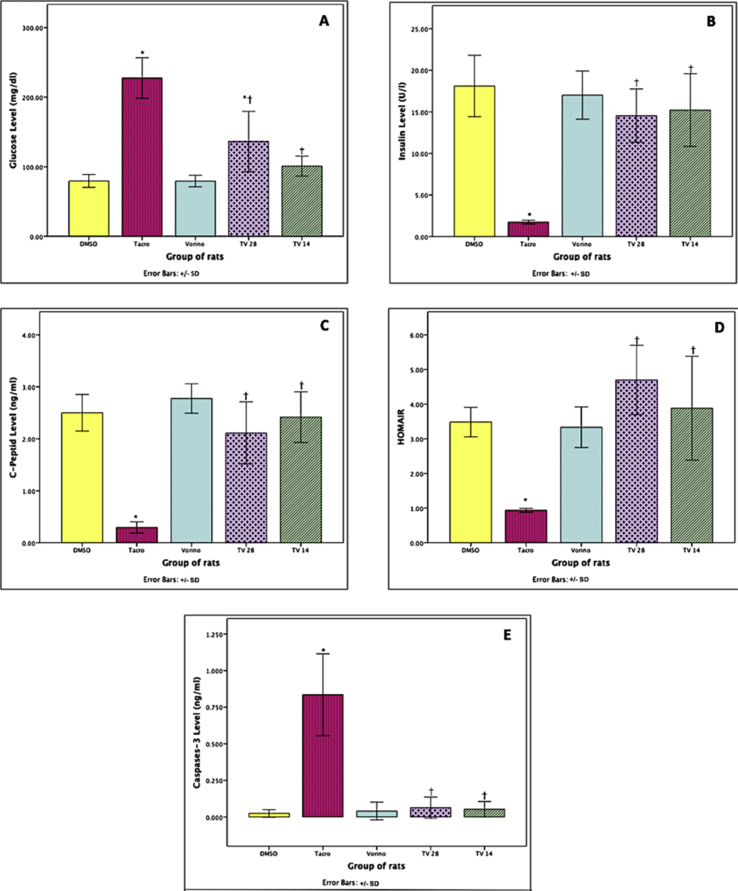
Figure 2 shows that Tacro, compared with DMSO, caused an approximately 3-fold increase in glucose levels and a significant decrease in serum insulin of approximately 90% (P = 0.05). Additionally, compared with DMSO, TV28 significantly increased the glucose level by approximately 2-fold (P = 0.05), whereas TV14 significantly decreased the glucose level to nondiabetic level. Both coadministration groups showed insulin levels comparable to those in the DMSO group.
Figure 2.
Effects of tacrolimus (Tacro) and vorinostat (Vorino) on hyperglycemic parameters.
(A) Glucose serum concentration, (B) insulin serum concentration, (C) C-peptide serum concentration, (D) HOMA-IR value, (E) caspase-3 homogenized concentration.
Values are presented as mean ± SD.
One-way ANOVA, followed by Bonferroni post hoc comparison test.
DMSO, control; Tacro, Tacrolimus; TV28, Tacrolimus with Vorinostat for 28 days; TV14, Tacrolimus for 28 days with Vorinostat for 14 days; Vorino, Vorinostat.
∗P=0.05 compared with DMSO;†P=0.05 compared with Tacro.
In addition, the serum C-peptide concentration was measured to evaluate whether Tacro had a similar effect to that observed in type 1 DM. Tacro, compared with DMSO, significantly decreased the serum C-peptide by 88% (P = 0.05). Both TV28 and TV14 exhibited values that were not significantly different from those with DMSO.
Additionally, Tacro caused glucose resistance, a feature similar to type 2 DM. The HOMA-IR value was significantly lower by 73% in the Tacro group than the DMSO group (P = 0.05). Notably, the values in the other treatment groups increased and were similar to those in the DMSO group.
Finally, Tacro caused a significant 40-fold increase (P = 0.05) in caspase-3 in the homogenized pancreas, whereas the other treatment groups showed a decrease, and the values did not significantly differ from those in the DMSO group.
Effects of Tacro and Vorino on oxidative stress parameters
The antioxidative effects of Vorino on Tacro-induced oxidative stress were assessed on the basis of the glutathione (GSH) and NADPH oxidase levels (Figure 3). In the Tacro group, compared with the DMSO group, GSH was significantly lower by more than 80%, while NADPH-oxidase was more than 4-fold higher. Interestingly, no significant differences were observed between the other treatment groups and DMSO.
Figure 3.
Effects of tacrolimus (Tacro) and vorinostat (Vorino) on oxidative stress, inflammatory and kidney parameters.
(A) Glutathione serum concentration, (B) NADPH-oxidase serum concentration, (C) TNF-α serum concentration, (D) IL-1β serum concentration, (E) BUN serum concentration, (F) creatinine serum concentration.
Values are presented as mean ± SD.
One-way ANOVA, followed by Bonferroni post hoc comparison test.
DMSO, control; Tacro, Tacrolimus; TV28, Tacrolimus with Vorinostat for 28 days; TV14, Tacrolimus for 28 days with Vorinostat for 14 days; Vorino, Vorinostat.
∗P=0.05 compared with DMSO;†P=0.05 compared with Tacro.
Effects of Tacro and Vorino on inflammatory parameters
Figure 3 shows that Tacro, compared with DMSO, significantly increased (P = 0.05) TNF-α, by more than 67%, and IL-1β, by more than 24%. Interestingly, no significant differences were observed between the other treatment groups and DMSO.
Effects of Tacro and Vorino on kidney parameters
Tacro, compared with DMSO, caused a significant increase (P = 0.05), by more than 3-fold, in both BUN and creatinine (Figure 3). Interestingly, the coadministration groups showed significantly (P = 0.05) lower BUN and creatinine, by more than 55%, for TV28 vs. Tacro and by more than 62% for TV14 vs. Tacro; however, significant differences were not observed relative to DMSO.
Effects of Tacro and Vorino on pancreatic sections
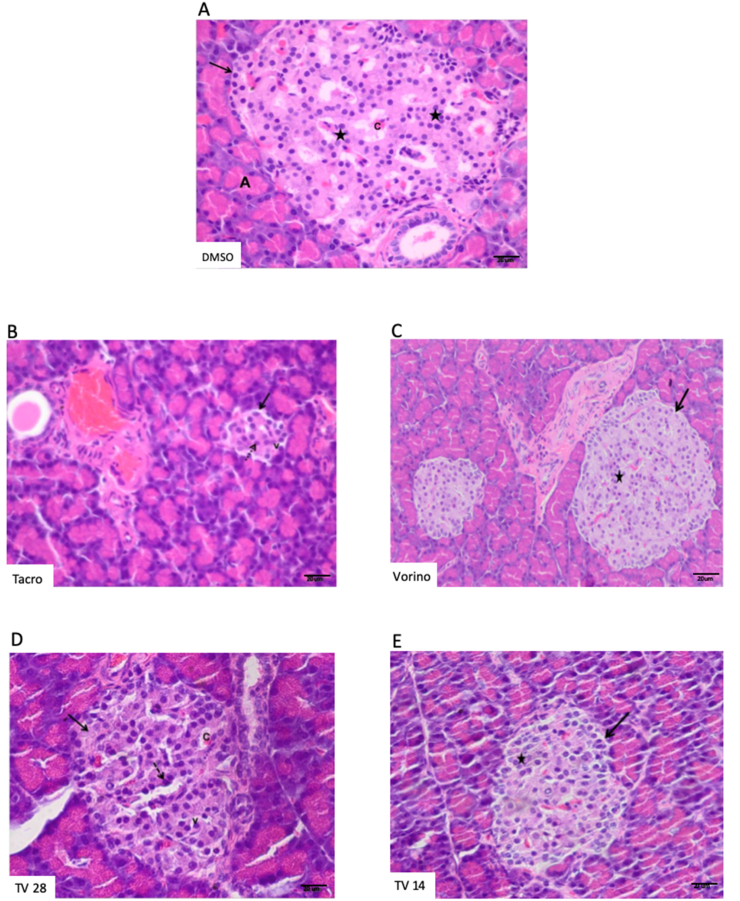
The H&E pancreatic sections of DMSO and Vorino showed large regular pale staining areas that represented the endocrine portion of the pancreatic lobules (islets of Langerhans). They appeared scattered between the exocrine parenchyma, as pale stained areas arranged in anastomosing branching cords, with blood capillaries between them. Each islet consisted of clusters of polygonal cells with central rounded pale nuclei and acidophilic cytoplasm (Figure 4A, C). Sections in the Tacro group showed few ill-defined islets with marked histological structural changes in the cells. Shrunken distorted islets with a marked loss of cells were observed. Many islet cells showed marked cytoplasmic vacuolation and small deeply stained nuclei (Figure 4B). The sections in the TV28 group showed partial improvement in the morphological structure of the islets and acidophilic cytoplasm, with pale rounded nuclei. However, some cells still had vacuolated cytoplasm with pyknotic nuclei (Figure 4D). Unexpectedly, the sections in the TV14 group appeared more or less normal and showed an increase in size. The islet cells were improved, and most of them had vesicular round nuclei and acidophilic cytoplasm (Figure 4E).
Figure 4.
Effects of tacrolimus (Tacro) and vorinostat (Vorino) on pancreatic sections stained with H&E; scale bar 20 μm.
(A) DMSO group: large well-defined pancreatic islets (arrows) and acidophilic cells with rounded vesicular nuclei (stars). (B) Tacro group: distorted shrunken islets with loss of cellular cords (arrows), small pyknotic nuclei (dotted arrow) and vacuolated cytoplasm (v). (C) Vorino group: large well-defined islets (arrow), with acidophilic cells with rounded vesicular nuclei (star). (D) Tacrolimus and vorinostats for 28 days: Increase in the islets size with more or less normal cellular cord arrangement (arrows); some cells show vacuolated cytoplasm (v), and others show pyknotic nuclei (dotted arrow). (E) Tacrolimus and vorinostats for 14 days: Increase in islet size (arrows), with more or less normal cells have acidophilic cytoplasm and pale round nuclei (stars). The letter A represents pancreatic acinus, and the letter C represents blood capillaries.
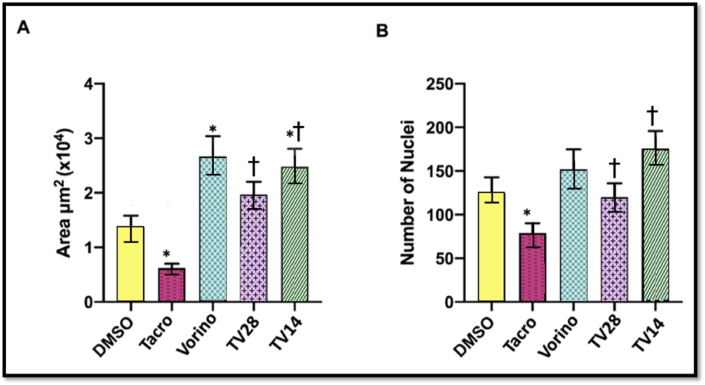
As shown in Figure 5, Tacro, compared with DMSO, resulted in a significantly decreased total area and number of nuclei in the islets of Langerhans cells (P = 0.05). Remarkably, the area size was significantly increased (P = 0.05) in the Vorino and TV14 groups than the DMSO group (Figure 5A). In addition, as shown in Figure 5B, the nuclei counts were significantly increased in both coadministration groups than the Tacro group (P = 0.05), although no significant differences were observed with respect to DMSO. These findings suggested that the two treatments affected the area and number of nuclei differently.
Figure 5.
Effects of tacrolimus (Tacro) and vorinostat (Vorino) on the total area of islets and the number of nuclei.
(A) Total area of islets, (B) Number of nuclei in islets.
Values are presented as mean ± SD.
DMSO, control; Tacro, Tacrolimus; TV28, Tacrolimus with Vorinostat for 28 days; TV14, Tacrolimus for 28 days with Vorinostat for 14 days; Vorino, Vorinostat.
∗P=0.05 compared with DMSO;†P=0.05 compared with Tacro.
Discussion
To our knowledge, this is the first study to examine the possible therapeutic and preventive effects of Vorino on NOD induced by Tacro. The results clearly confirmed that Vorino, through its anti-inflammatory and antioxidant effects, ameliorates both the hyperglycemia and the renal function disturbance induced by Tacro. This finding may provide a rationale for the use of Vorino in renal transplants patients with Tacro-induced NOD.
Weight loss is a common symptom of type 1 DM. The present study revealed that Tacro led to significant weight loss, as confirmed by several previous experiments.7,19,21,22 Simultaneously, Tacro caused a gradual increase in FGT, as also previously reported.23 To confirm these results, we determined random blood glucose levels, and found that the Tacro group showed diabetic glucose levels, in agreement with findings from previous studies.6,7,19,21,24 Hyperglycemia was associated with a decrease in insulin and C-peptide, thus suggesting that Tacro induces type 1 DM, as reported in previous studies.7,19,28 The histopathology results confirmed that the Tacro-induced decrease in insulin was caused by a decrease in islet size rather than insulin secretory dysfunction, in agreement with previous findings.25,26 The current work provides evidence that caspase-3 in the homogenized pancreas significantly increases after Tacro treatment and enhances islet cell death. Previous studies have confirmed this finding by measuring TUNEL and caspase-3.6,7,27 Additionally, Tacro significantly induced HOMA-IR, as previously reported.6,23,25,27 Together, these results suggest that Tacro predominantly induces type 1 DM with some features of type 2 DM.
Several lines of evidence indicate that Vorino has antidiabetic effects.13,18 Indeed, Vorino has been found to increase β-cell area in a mouse model of type1 DM.18 Therefore, Vorino is an attractive candidate for the management of Tacro-NOD. The current study indicated that the preventive coadministration of Vorino with Tacro (TV28) as well as the therapeutic administration of Vorino after the development of Tacro-induced NOD (TV14) effectively normalized the FGT and random glucose concentration. Moreover, these treatments significantly ameliorated the Tacro-induced pancreatic dysfunction and the impairments in insulin secretion and resistance. Vorino alone was administered to normal rats to investigate its mechanisms in glucose hemostasis; however, it did not affect all hyperglycemia parameters.
Unexpectedly, Vorino significantly increased the total islet area, as demonstrated by H&E staining. This effect might be explained by the nonsignificant increase in the number of nuclei counted in the sections. Because this observation was not associated with increased insulin levels, the increase in islet area was unlikely to have been due to an increase in the number of β-cells. However, this speculation has not been confirmed by any previous studies. Next, the Tacro concentration was evaluated to investigate whether the preventive effect of Vorino on Tacro-NOD is related to the pharmacokinetic drug–drug interactions between them. Administration of Vorino for the same duration as Tacro or after the development of NOD did not affect Tacro concentration. These results suggested that Vorino does not interfere with Tacro pharmacokinetics. However, this speculation has not been confirmed by any previous studies.
The results of this study indicated that Tacro induced apoptosis. Oxidative stress plays a key role in apoptosis29 and diabetes development.30 Therefore, the antioxidant properties of Vorino in the treatment of Tacro-induced oxidative stress were evaluated. In the present study, Vorino ameliorated NADPH oxidase elevation and restored the GSH depletion caused by Tacro, in agreement with previously reported findings.12 However, Vorino alone, compared with the control, did not alter the GSH nor NADPH oxidase levels, thus suggesting that it is effective against oxidative stress induced by hyperglycemia but not under normal glucose conditions.
Another proposed mechanism of Vorino in Tacro-NOD may involve anti-inflammatory effects. Administration of Vorino, either before or after the development of NOD, significantly ameliorated the increase in TNF-α and IL-1β levels caused by Tacro. These results are consistent with findings from previous studies.17,31, 32, 33
In the current study, Tacro increased the creatinine and BUN levels, as confirmed by previous studies.19,23,27 The most important finding was that Vorino had protective effect on the increase in creatinine and BUN caused by Tacro through a glucose-independent mechanism. This preventive effect of Vorino has been investigated previously.12
This study has several limitations. The results from this preclinical study need to be translated to clinical practice with caution, because patients receiving kidney transplants usually take other diabetogenic drugs, such as corticosteroids. Furthermore, this study did not focus on the mechanism underlying the preventive effects of Vorino against Tacro-induced nephrotoxicity.
The unexpected observation that late administration of Vorino (TV14) was more effective than early administration of Vorino (TV28) needs to be explained by doing further experiments that out of the scope of this study.
Conclusion
This study provides pharmacological evidence of the efficacy of Vorino (15 mg/kg/PO) in decreasing the hyperglycemia induced by Tacro. Its antihyperglycemic effect may occur through its antioxidant and anti-inflammatory effects. Remarkably, the administration of Vorino protected renal function in a rat model of Tacro-NOD. Therefore, Vorino may be a promising therapeutic agent for Tacro-NOD in patients receiving renal transplantation, because it does not pose a risk to the transplanted kidneys.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All experimental protocols were approved by the Unit of Biomedical Ethics of the Research Ethics Committee at King Abdul-Aziz University (reference No.309-18). In addition, all procedures performed in this study were in accordance with ethical guidelines for animal studies.
Authors contributions
FAB conceived and designed the study, conducted research, provided research materials, collected and organized data, and wrote the initial draft of the article. HSA and RMM supervised the research, provided logistic support and reviewed the final draft. EAE analyzed and interpreted data on histopathological assessment of the pancreas, and ASA analyzed and interpreted data on morphometric assessment of the pancreas. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgements
We thank Dr. Ebtessam Essa from the Pharmaceutical Technology Department at Tanta University, Dr. Abdulrazaq Mallitye from the Pathology Department at King Faisal Specialist Hospital and Dr. Bashir Alsiddig Yousef from the Pharmacology Department at Khartoum University.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Saudi Center for Organ Transplantation Organ transplantation in Saudi Arabia - 2017. Saudi J Kidney Dis Transpl. 2018;29(6):1523–1536. [Google Scholar]
- 2.Almutairi F., Al-Duais M., Shalaby K., Sakran M. Analysis of patients with end-stage renal disease on dialysis in Tabuk City, Saudi Arabia: a single-center, three-year retrospective study. Saudi J Kidney Dis Transpl. 2017;28(1):349–354. doi: 10.4103/1319-2442.202769. [DOI] [PubMed] [Google Scholar]
- 3.Pratschke J., Dragun D., Hauser I., Horn S., Mueller T., Schemme P. Immunological risk assessment: the key to individualized immunosuppression after kidney transplantation. Transplant Rev. 2016;30(2):77–84. doi: 10.1016/j.trre.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Katzung B., Trevor A. 14th ed. McGraw-Hill Medical. Lange Medical publisher; 2018. Basic & clinical pharmacology. [Google Scholar]
- 5.Palepu S., Prasad G. New-onset diabetes mellitus after kidney transplantation: current status and future directions. World J Diabetes. 2015;6(3):445. doi: 10.4239/wjd.v6.i3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L., Lim S., Doh K., Piao S., Jin J., Heo S. Dipeptidyl peptidase IV inhibitor MK-0626 attenuates pancreatic islet injury in tacrolimus-induced diabetic rats. PLoS One. 2014;9(6):1–10. doi: 10.1371/journal.pone.0100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S., Jin L., Jin J., Yang C. Effect of exendin-4 on autophagy clearance in beta cell of rats with tacrolimus-induced diabetes mellitus. Sci Rep. 2016;6 doi: 10.1038/srep2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivaswamy V., Boerner B., Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37(1):37–61. doi: 10.1210/er.2015-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo Clinic . Mayo Foundation for Medical Education and Research; 2014. Diabetes.https://www.mayoclinic.org/diseases-conditions/diabetes/symptoms-causes/syc-20371444 [Google Scholar]
- 10.Khong M., Chong C. Prevention and management of new-onset diabetes mellitus in kidney transplantation. Neth J Med. 2014;72(3):127–134. [PubMed] [Google Scholar]
- 11.Angioi A., Cabiddu G., Conti M., Pili G., Atzeni A., Matta V., et al. Metformin associated lactic acidosis: a case series of 28 patients treated with sustained low-efficiency dialysis (SLED) and long-term follow-up. BMC Nephrol. 2018;19(1):1–7. doi: 10.1186/s12882-018-0875-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadden J., Advani A. Histone deacetylase inhibitors and diabetic kidney disease. Int J Mol Sci. 2018;19(9):2630. doi: 10.3390/ijms19092630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S., Taliyan R. Histone deacetylase inhibitors: future therapeutics for insulin resistance and type 2 diabetes. Pharmacol Res. 2016;113:320–326. doi: 10.1016/j.phrs.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Lohman R., Lyer A., Fairlie T., Cotterell A., Gupta P., Reid R. Differential anti-inflammatory activity of HDAC inhibitors in human macrophages and rat arthritis. JPET. 2016;356(2):387–396. doi: 10.1124/jpet.115.229328. [DOI] [PubMed] [Google Scholar]
- 15.Suraweera A., O'Byrne K., Richard D. Combination therapy with Histone Deacetylase Inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front Oncol. 2018;8:92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canadian Institutes of Health Research . 2019. Vorinostat.https://www.drugbank.ca/drugs/DB02546 [Google Scholar]
- 17.Choi S., Gatza E., Hou G., Sun Y., Whitfield J., Song Y. Histone deacetylase inhibition regulates inflammation and enhances Tregs after allogeneic hematopoietic cell transplantation in humans. Blood Adv. 2015;125(5):815–819. doi: 10.1182/blood-2014-10-605238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera S., Colvin S., Tersey S., Maier B., Nadler J., Mirmira R. Effects of combination therapy with dipeptidyl peptidase-IV and histone deacetylase inhibitors in the non-obese diabetic mouse model of type 1 diabetes. J Clin Exp Immunol. 2013;172(3):375–382. doi: 10.1111/cei.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin J., Lim S., Jin L., Yu J., Kim H., Chung B., Yang C. Effects of metformin on hyperglycemia in an experimental model of tacrolimus-and sirolimus-induced diabetic rats. Korean J Intern Med. 2017;32(2):314. doi: 10.3904/kjim.2015.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao S., Zhang X., Shi M., Xiao Y., Zhang Y., Wang Y. Suberoylanilide hydroxamic acid attenuates paraquat-induced pulmonary fibrosis by preventing Smad7 from deacetylation in rats. J Thorac Dis. 2016;8(9):2485–2494. doi: 10.21037/jtd.2016.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim S., Jin L., Luo K., Jin J., Yang C. Ginseng extract reduces tacrolimus-induced oxidative stress by modulating autophagy in pancreatic beta cells. Lab Invest. 2017;97:1271–1281. doi: 10.1038/labinvest.2017.75. [DOI] [PubMed] [Google Scholar]
- 22.Love S., Mudasir M., Bhardwaj S., Singh G., Tasduq S. Long-term administration of tacrolimus and everolimus prevents high cholesterol-high fructose-induced steatosis in C57BL/6J mice by inhibiting de-novo lipogenesis. Oncotarget. 2017;8(69):113403–113417. doi: 10.18632/oncotarget.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma R., Liu L., Jiang W., Yu Y., Song H. FK506 ameliorates podocyte injury in type 2 diabetic nephropathy by down-regulating TRPC6 and NFAT expression. Int J Clin Exp Pathol. 2015;8(11):14063–14074. [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Sun F., Zhang Y., Chen H., He N., Chen H. Tacrolimus induces insulin resistance and increases the glucose absorption in the jejunum: a potential mechanism of the diabetogenic effects. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conte C., Secchi A. Post-transplantation diabetes in kidney transplant recipients: an update on management and prevention. Acta Diabetol. 2018;55(8):763–779. doi: 10.1007/s00592-018-1137-8. . [DOI] [PubMed] [Google Scholar]
- 26.Jin J., Jin L., Luo K., Lim W., Chung B., Yang C. Effect of empagliflozin on tacrolimus induced pancreas islet dysfunction and renal injury. Am J Transplant. 2017;17:2601–2616. doi: 10.1111/ajt.14316. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Rodriguez A., Triñanes J., Velazquez-Garcia S., Porrini E., Vega Prieto M., Diez Fuentes M. The higher diabetogenic risk of tacrolimus depends on pre-existing insulin resistance. A Study in obese and lean zucker rats. Am J Transplant. 2013;13(7):1665. doi: 10.1111/ajt.12236. [DOI] [PubMed] [Google Scholar]
- 28.Chakkera H., Kudva Y., Kaplan B. Calcineurin inhibitors: pharmacologic mechanisms impacting both insulin resistance and insulin secretion leading to glucose dysregulation and diabetes mellitus. Clin Pharmacol Ther. 2017;101(1):114–120. doi: 10.1002/cpt.546. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar T., Sheikh N., Shan T., Ghazanfar R. Tacrolimus induced nephrotoxicity and pulmonary toxicity in Wistar rats. J Biol Regul Homeost Agents. 2017;31(4):1061–1066. [PubMed] [Google Scholar]
- 30.Ma X., Chen Z., Wang L., Wang G., Wang Z., Dong X. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: its inhibition by berberine. Front Pharmacol. 2018;9:782. doi: 10.3389/fphar.2018.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun P. Therapeutic effects of histone deacetylase inhibitors on kidney disease. Arch Pharm Res. 2018;41:162–183. doi: 10.1007/s12272-017-0998-7. [DOI] [PubMed] [Google Scholar]
- 32.Bagchi R., Weeks K. Histone deacetylases in cardiovascular and metabolic diseases. J Mol Cell Cardiol. 2019;130:151–159. doi: 10.1016/j.yjmcc.2019.04.003. . [DOI] [PubMed] [Google Scholar]
- 33.Fang S., Meng X., Zhang Z., Wang Y., Liu Y., You C., Yan H. Vorinostat modulates the imbalance of T cell subsets, suppresses macrophage activity, and ameliorates experimental autoimmune uveoretinitis. NeuroMolecular Med. 2016;18(1):134–145. doi: 10.1007/s12017-016-8383-0. [DOI] [PubMed] [Google Scholar]