Abstract
BACKGROUND
Pregnancy loss is poorly understood, but infection may be a risk factor. Few studies have evaluated pregnancy loss among women living with HIV in the era of potent combination antiretroviral therapy.
OBJECTIVE
We hypothesize that maternal HIV and syphilis infection lead to increased risk of pregnancy loss, including both miscarriage and stillbirth. This study aimed to assess trends and possible predictors of spontaneous miscarriage and stillbirth among women living with HIV in a cohort of nearly 56,000 deliveries at a major referral institution in a city with the highest prevalence of HIV in Brazil.
STUDY DESIGN
Data from hospital records for women delivering from January 1, 2008 to December 31, 2018 were reviewed. Rates of stillbirth, miscarriage, and any pregnancy loss were compared using the Pearson chi-square test. Predictors of pregnancy loss were evaluated by robust univariate log-linear Poisson regression using a generalized estimating equations approach.
RESULTS
A total of 55,844 pregnancies were included in the analysis, with 54,308 pregnancies from 43,502 women without HIV and 1536 pregnancies from 1186 women living with HIV (seroprevalence of maternal HIV: 2.7%). Overall, 1130 stillbirths (2.0%) and 6558 miscarriages (11.7%) occurred. Any pregnancy loss was similar in both groups (13.8% in women without and 14.1% in women with HIV; P=.733). Stillbirth was higher among women living with HIV (3.4%) than among women without HIV (2.0%; P<.001), but there was no difference in overall miscarriage rates (10.7% in women with vs. 11.8% in women without HIV; P=.188). Women living with HIV had higher miscarriage rates between 12 and 20 weeks than women without HIV (34.8% vs 23.7%; P=.001), likely because of syphilis coinfection. Stillbirth rates were higher for women living with HIV from 2008 to 2014; however, a steady plateau was reached from 2014 to 2018, mirroring stillbirth rates in women without HIV. Maternal HIV infection did not increase the risk of miscarriage (relative risk, 0.90; 95% confidence interval, 0.77–1.05) or any pregnancy loss (relative risk, 1.00; 95% confidence interval, 0.88–1.15), but was associated with stillbirth (relative risk, 1.65; 95% confidence interval, 1.23–2.21). Maternal syphilis was associated with any pregnancy loss (relative risk, 1.24; 95% confidence interval, 1.11–1.38) and stillbirth (relative risk, 3.39; 95% confidence interval, 2.77–4.14), but not miscarriage (relative risk, 0.91; 95% confidence interval, 0.80–1.04).
CONCLUSION
In the era of combination antiretroviral therapy, there was no difference in miscarriage rates between women with and without HIV. HIV was associated with stillbirth risk but improved over time. Maternal syphilis was significantly associated with any pregnancy loss and stillbirth in all women. Syphilis is likely the main driver of pregnancy loss in women living with HIV in Brazil.
Key words: adverse pregnancy outcome, coinfection, HIV, miscarriage, pregnancy loss, stillbirth, syphilis
AJOG Global Reports at a Glance.
Why was this study conducted?
Pregnancy loss is poorly understood in women living with HIV (WLH). Syphilis infection, common in WLH and a risk factor for pregnancy loss, may be a potential driver of fetal demise in this population.
Key findings
Miscarriage rates did not differ between women with and without HIV, but WLH had higher late miscarriage and stillbirth rates. Over half of WLH with syphilis miscarried between 12 and 20 weeks.
What does this add to what is known?
Infection and inflammation are key drivers of fetal loss. Despite universal, free, available, and effective antiretroviral therapy in Brazil, syphilis was a key driver of pregnancy loss in WLH, which may require prompt identification and treatment to prevent adverse pregnancy outcomes.
Introduction
Pregnancy loss, including stillbirth and spontaneous miscarriage, is poorly understood and multifactorial in etiology. In >50% of women, risk factors for pregnancy loss are not identified.1,2 Furthermore, gestational age limits used to define pregnancy loss differ by country, rendering studies on risk factors difficult to generalize. It is frequently accepted that miscarriage denotes loss of pregnancy from conception to 20 weeks of gestation. Stillbirth is defined as fetal death at ≥20 weeks of gestation.3 Infection may trigger chronic endometritis and altered immune infiltration into the endometrium, possibly leading to pregnancy loss.4,5 HIV and syphilis are common infections in pregnancy. Although maternal HIV and syphilis infection are known to increase adverse pregnancy outcomes such as preterm birth and low birthweight, few studies have evaluated the risk of pregnancy loss in women living with HIV (WLH) and women coinfected with HIV and syphilis.
Brazil is a country at the forefront of HIV care. As mandated by law since 1996, combination antiretroviral therapy (cART) is free of charge to individuals with HIV, including pregnant women. Porto Alegre, a metropolitan city in southern Brazil, is the epicenter of an ongoing HIV epidemic in pregnancy,6 with an HIV seroprevalence of 20.2 per 1000 births, which is 7 times the national average.6,7 Women are particularly susceptible to HIV acquisition during pregnancy and prone to having undiagnosed, unsuppressed viremia at delivery.8, 9, 10, 11 HIV subtype C, uniquely predominant in southern Brazil, is particularly adapted to HIV mother-to-child transmission.12,13 We hypothesize that the risk of pregnancy loss, both miscarriage and stillbirth, is higher among WLH compared with women without HIV (WWOH); this risk is further elevated among WLH coinfected with syphilis. Using deliveries from nearly 56,000 women, we evaluated rates of spontaneous miscarriage, stillbirth, and any pregnancy loss, and predictors of pregnancy loss among WLH and WWOH at a major HIV-referral, tertiary-level public hospital in Porto Alegre.
Materials and Methods
Data source
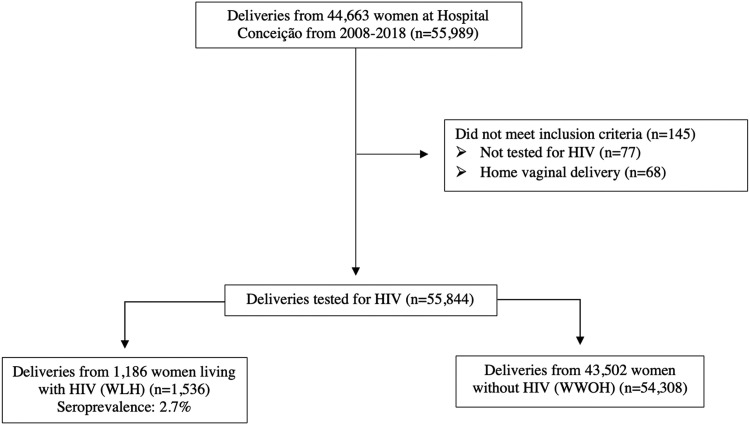
We conducted a retrospective cohort study using institutional hospital records for all women who delivered from January 1, 2008 to December 31, 2018 at Hospital Nossa Senhora da Conceição, a tertiary-level hospital and referral institution for HIV care in Porto Alegre. Data regularly extracted from medical records for government epidemiologic surveillance purposes included sociodemographic factors (eg, age, race and ethnicity, geographic region of residence), obstetrical information (eg, gravidity, twin gestation, gestational age), syphilis coinfection during pregnancy, and type of pregnancy loss, including miscarriage and stillbirth. All women who delivered at this institution during the study period were tested for HIV by rapid antigen-antibody tests (Unified Health System [SUS], Brasília, Brazil).14 Positive results were confirmed by HIV antibody enzyme-linked immunosorbent assays or immunoblot for HIV-1 antigens (p24, gp41, gp120, gp160) and HIV-2 antigen gp36. All women who delivered at our institution were universally screened for maternal syphilis infection during the study period using a reverse sequence algorithm, beginning with a rapid treponemal antibody test. If reactive, reflex testing was conducted with a nontreponemal Venereal Disease Research Laboratory (VDRL) test followed by measurement of nontreponemal antibody titers (all certified by the Brazilian SUS). A confirmed positive diagnosis was made with both a positive rapid treponemal antibody test and a nontreponemal VDRL test. We excluded women who: (1) were not tested for HIV; or (2) had home vaginal births (Figure 1). This study was approved by the Institutional Review Board of Hospital Nossa Senhora da Conceição in Porto Alegre (Protocol #14.124). Because this was a secondary analysis of deidentified data, written informed consent was waived.
Figure 1.
Cohort selection
Yang. Pregnancy loss and HIV in Brazil. Am J Obstet Gynecol Glob Rep 2022.
Covariates and outcome definitions
The primary outcome of the study was any pregnancy loss, and secondary outcomes were miscarriage and stillbirth. In this study, miscarriage is defined as any pregnancy loss before 20 weeks of gestation, and stillbirth is defined as any pregnancy loss at ≥20 weeks of gestation. Recurrent miscarriage is defined as any pregnancy belonging to a woman with a history of ≥1 previous miscarriage. HIV and syphilis infections were categorized as binary variables (positive, negative) in the analysis. In an ancillary analysis of recurrent miscarriage, miscarriage was the primary outcome. Age was defined as: <18, 18 to 24, 25 to 34, and ≥35 years. Geographic region was defined as urban, greater metropolitan region, and rural region or outskirts according to the Porto Alegre City Council. Previous cesarean delivery (CD) was classified as any women having a previous CD between 2008 and 2018. Previous pregnancy loss was classified as any previous pregnancy loss (either stillbirth or miscarriage) between 2008 and 2018; CDs and pregnancy losses before 2008 were not recorded in our database. Race or ethnicity was categorized according to hospital records as White, Black, multiracial (mixed Black/White/Native or Indigenous), Asian, and Native or Indigenous. Our analysis of race should be interpreted within an antiracist framework, with the understanding of race as a social construct and not a biologic marker,15,16 and awareness of the Brazilian context in which maternal morbidity and mortality among cisgender Black women are disproportionately high.17, 18, 19
Statistical analysis
Pearson chi-square tests were used to calculate differences in distribution of categorical variables between subpopulation groups (ie, WLH and WWOH), including stillbirth, miscarriage and any pregnancy loss, sociodemographic characteristics, and obstetrical factors. Median ages with interquartile ranges of WLH and WWOH were calculated using the Mann–Whitney U test. Predictors of any pregnancy loss were evaluated with a robust univariate log-linear Poisson regression model using a generalized estimating equations (GEE) approach, with HIV diagnosis as the main regressor. Given that the same women had multiple gestations within the final dataset used for analysis, GEE was appropriate to account for lack of independence (correlations between observations within a subject).20 Univariate analysis was conducted for each variable, and the categorical outcome, relative risks (RR) with 95% confidence intervals (CIs), and P values were calculated using a 2-sided α<.05. Analyses were conducted in Stata, Version 17.0 (StataCorp LLC, College Station, TX).
Results
A total of 55,844 pregnancies were included: 54,308 pregnancies from 43,502 WWOH and 1536 pregnancies from 1186 WLH (seroprevalence: 2.7%); 145 pregnancies were excluded because they were not tested for HIV or had home vaginal births (Figure 1). Table 1 shows the sociodemographic and obstetrical characteristics of WLH and WWOH. WLH were older than WWOH; >68% of WLH were aged ≥25 years, whereas approximately half of WWOH were in this age group. Regarding race, 41.2% of WLH were non-White, and 27.1% of WWOH were non-White. WLH had almost 4 times the maternal syphilis coinfection frequency (12.4%) relative to that of WWOH (3.3%; P<.001) and were more frequently multiparous (82.9%) compared with WWOH (63.9%).
Table 1.
Sociodemographic and obstetrical characteristics of pregnancies from women living with HIV and women without HIV, 2008–2018 (n=55,844)
| Characteristics | WLH (n=1536) | WWOH (n=54,308) | P value |
|---|---|---|---|
| Median age at delivery (interquartile range) | 28 (23–33)n (%) | 25 (21–31)n (%) | <.001a,b |
| Age at delivery (n=55,837) | <.001b,c | ||
| <18 | 52 (3.4) | 4738 (8.7) | |
| 18–24 | 432 (28.1) | 20,116 (37.1) | |
| 25–34 | 785 (51.1) | 21,713 (40.0) | |
| >35 | 267 (17.4) | 7734 (14.2) | |
| Geographic region | .017b | ||
| Urban | 1041 (67.8) | 35,526 (65.4) | |
| Greater metropolitan region | 440 (28.7) | 16,037 (29.5) | |
| Rural/outskirts | 55 (3.6) | 2745 (5.1) | |
| Race/ethnicity (n=55,825) | <.001b | ||
| White | 900 (58.6) | 39,536 (72.8) | |
| Black | 421 (27.4) | 9066 (16.7) | |
| Multiracial | 212 (13.8) | 5658 (10.4) | |
| Asian | 0 (0.0) | 20 (0.0) | |
| Native/Indigenous | 3 (0.2) | 9 (0.0) | |
| Gravida | <.001b | ||
| Primigravid | 262 (17.1) | 19,594 (36.1) | |
| Multigravid | 1274 (82.9) | 34,714 (63.9) | |
| Fetal sex (n=48,736) | .159 | ||
| Male | 717 (53.1) | 24,246 (51.2) | |
| Female | 633 (46.9) | 23,140 (48.8) | |
| Twin gestation (n=48,765) | 50 (3.7) | 1886 (4.0) | .604 |
| Previous cesarean delivery | 138 (37.7) | 2918 (33.5) | .098 |
| Pregnancy loss | |||
| Any | 216 (14.1) | 7472 (13.8) | .733 |
| Stillbirth | 52 (3.4) | 1078 (2.0) | <.001b |
| Miscarriage | 164 (10.7) | 6394 (11.8) | .188 |
| Previous pregnancy loss (n=11,254) | 93 (20.8) | 2287 (21.2) | .856 |
| Syphilis during pregnancy (n=55,777) | 191 (12.4) | 1814 (3.3) | <.001b |
WLH, women living with HIV; WWOH, women without HIV.
Compared using the Mann–Whitney U test
Statistically significant with P<0.05
Compared using the Pearson chi-square test. Yang. Pregnancy loss and HIV in Brazil. Am J Obstet Gynecol Glob Rep 2022.
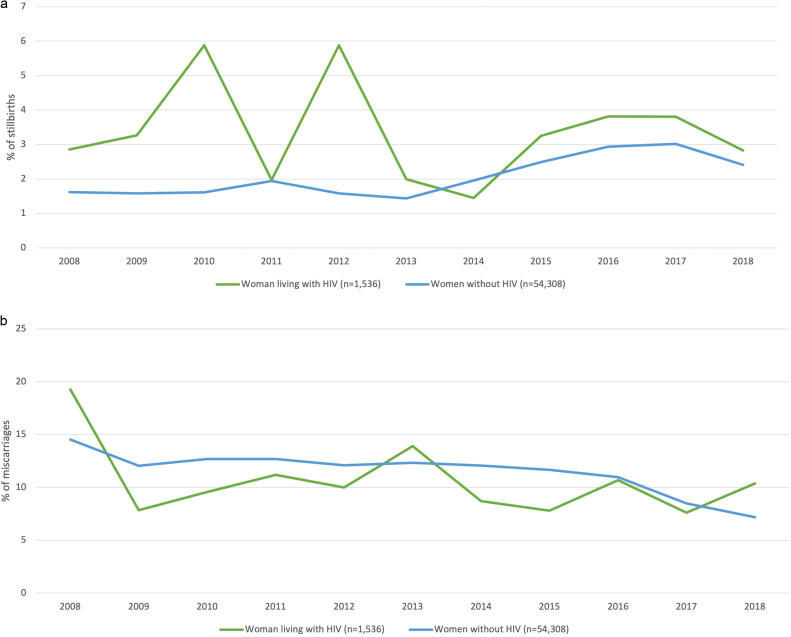
Overall, 1130 stillbirths (2.0%) and 6558 miscarriages (11.7%) occurred. Although the stillbirth rate was higher among WLH (3.4%) than among WWOH (2.0%; P<.001), there was no difference in overall miscarriage rates (10.7% in WLH vs 11.8% in WWOH; P=.188). Any pregnancy loss, either stillbirth or miscarriage, did not differ between WLH and WWOH (14.1% in WLH vs 13.8% in WWOH; P=.733). Trends of stillbirth and miscarriage are shown in Figure 2, A and B. For stillbirth, rates were higher for WLH in the early years of the analysis (2008–2014), reaching a steady plateau below 4% from 2014 to 2018, which mirrored the rate of stillbirths among WWOH. Among 52 stillbirths in WLH, 28 women (53.8%) did not have any data inputted regarding cART use, 18 (34.6%) did not use any cART, and only 6 (11.5%) were registered as initiating cART during pregnancy. Information on cART use was missing or negative for 89% of women with stillbirths, underscoring the absence of consistent HIV treatment in populations with this outcome. Most WLH who miscarried were found to be HIV-infected at the time of admission for miscarriage and had no history of cART use. For these women, cART initiation occurred after pregnancy. Therefore, cART use data were not available in the hospital database nor in the national surveillance registry of records for all WLH in Brazil. Of note, 14 WLH (26.9%) in the group of 52 WLH with stillbirths were coinfected with syphilis, which is more than twice the rate of syphilis coinfection in the overall cohort of WLH regardless of stillbirth status (12.4%).
Figure 2.
Trends in miscarriages and stillbirth among WLH and WWOH, 2008–2018 (n=55,844)
A, Stillbirth rates. B, Miscarriage rates. WLH, women living with HIV; WWOH, women without HIV. Yang. Pregnancy loss and HIV in Brazil. Am J Obstet Gynecol Glob Rep 2022.
For miscarriage, trends among WLH and WWOH were similar during the entire study period. Over time, miscarriages declined slightly more among WLH, from 19.3% to 10.4%, as opposed to the decline from 14.5% to 7.2% among WWOH. Miscarriages were more frequent in WLH at or beyond 12 to 20 weeks of gestation (34.8%) as compared with WWOH (23.7%; P=.001). WLH coinfected with syphilis had an extraordinarily high percentage of miscarriage at ≥12 weeks of gestation (10/19; 52.6%) relative to that of WLH without syphilis coinfection (46/144; 32.0%; P=.080). In both groups of WLH, the frequency of late miscarriages was extraordinarily high relative to that of WWOH with and without syphilis during the same gestational period (36.9% and 23.3%, respectively; P≤.001). When examining maternal syphilis regardless of HIV status, the rate of late miscarriage was 38.3% in all women with syphilis vs 23.5% in all women without syphilis (P<.001). When examining maternal HIV regardless of syphilis status, the late miscarriage rate was significantly higher among WLH (34.8%) than among WWOH (23.7%; P=.001).
Among 55,844 women in our cohort, HIV infection did not increase the risk of any pregnancy loss nor miscarriage, as seen in Table 2. However, HIV was associated with stillbirth. Maternal syphilis was associated with any pregnancy loss and stillbirth in all women but not associated with overall miscarriage rate. There was an association between HIV and miscarriage, and between syphilis and miscarriage when only the period of late miscarriage was considered (12–20 weeks of gestation).
Table 2.
Predictors of any pregnancy loss, miscarriage, and stillbirth related to HIV or syphilis infection during pregnancy (n=55,844)
| Predictors | RR (95% CI)a | P value |
|---|---|---|
| Maternal HIV | ||
| Any pregnancy loss | 1.00 (0.88–1.15) | .966 |
| Miscarriage | 0.90 (0.77–1.05) | .167 |
| Stillbirth | 1.65 (1.23–2.21) | .001b |
| Maternal syphilis | ||
| Any pregnancy loss | 1.24 (1.11–1.38) | <.001b |
| Miscarriage | 0.91 (0.80–1.04) | .178 |
| Stillbirth | 3.39 (2.77–4.14) | <.001b |
CI, confidence interval; RR, relative risk.
RR and 95% CI were calculated with robust log-linear Poisson regression using a generalized estimating equations approach
Statistically significant with P<0.05 Yang. Pregnancy loss and HIV in Brazil. Am J Obstet Gynecol Glob Rep 2022.
In the HIV-stratified analysis comparing WLH and WWOH (Table 3), age <25 years was protective against any pregnancy loss (miscarriage+stillbirth) for both WLH and WWOH, whereas advanced maternal age (≥35 years) was associated with increased risk of pregnancy loss for both groups of women (Table 3). Among WWOH, age <18 years was also protective against pregnancy loss. Among WLH, coinfection with syphilis did not pose a greater risk for any pregnancy loss; however, maternal syphilis among WWOH was associated with any fetal loss. Fetal sex was not associated with pregnancy loss in either WLH or WWOH (Table 3). Multigravidity was associated with pregnancy loss in WWOH but not in WLH. Previous CD was protective against pregnancy loss for both WLH and WWOH. History of pregnancy loss was a strong predictor of fetal loss for both WLH and WWOH. In an ancillary analysis with miscarriage as the outcome, the risk of recurrent miscarriage was higher among WLH (RR, 4.23; 95% CI, 2.69–6.64; P<.001) than among WWOH (RR, 2.96; 95% CI, 2.65–3.30; P<.001).
Table 3.
Predictors of any pregnancy loss among women living with HIV and women without HIV (n=55,844)
| Predictors | WLH (n=1536) |
WWOH (n=54,308) |
||
|---|---|---|---|---|
| RR (95% CI)a | P value | RR (95% CI) | P value | |
| Age at delivery (n=55,837) | ||||
| <18 | 0.73 (0.32–1.68) | .461 | 0.91 (0.83–0.99) | .032b |
| 18–24 | 0.60 (0.41–0.87) | .006b | 0.90 (0.85–0.95) | <.001b |
| 25–34 | Reference | Reference | ||
| >35 | 1.40 (1.02–1.90) | .035b | 1.62 (1.53–1.72) | <.001b |
| Geographic region | ||||
| Urban | Reference | Reference | ||
| Greater metropolitan region | 0.86 (0.63–1.18) | .338 | 1.04 (0.99–1.09) | .103 |
| Rural/outskirts | 1.17 (0.62–2.21) | .621 | 0.87 (0.78–0.97) | .016 |
| Race/ethnicity (n=55,825) | ||||
| White | Reference | Reference | ||
| Black | 1.05 (0.77–1.41) | .775 | 0.99 (0.93–1.05) | .642 |
| Multiracial | 0.93 (0.61–1.40) | .723 | 0.89 (0.82–0.96) | .003b |
| Syphilis during pregnancy (n=55,777) | 1.37 (0.95–1.98) | .088 | 1.29 (1.17–1.43) | <.001b |
| Gravida | ||||
| Primigravid | Reference | Reference | ||
| Multigravid | 1.31 (0.90–1.90) | .157 | 1.22 (1.17–1.28) | <.001b |
| Fetal sex (n=48,736) | ||||
| Male | 0.66 (0.32–1.36) | .261 | 1.06 (0.89–1.25) | .516 |
| Female | Reference | Reference | ||
| Twin gestation (n=48,765) | 1.80 (0.47–6.95) | .392 | 2.20 (1.56–3.11) | <.001b |
| Previous cesarean delivery | 0.39 (0.18–0.82) | .013b | 0.73 (0.64–0.83) | <.001b |
| Previous pregnancy loss (n=11,254) | 2.55 (1.64–3.97) | <.001b | 2.62 (2.39–2.87) | <.001b |
CI, confidence interval; RR, relative risk; WLH, women living with HIV; WWOH, women without HIV.
RR and 95% CI were calculated with robust log-linear Poisson regression using a generalized estimating equations approach
Statistically significant with P<0.05. Yang. Pregnancy loss and HIV in Brazil. Am J Obstet Gynecol Glob Rep 2022.
Comment
Principal findings
In nearly 56,000 pregnancies followed over 11 years in a city with the highest HIV prevalence in Brazil, the overall pregnancy loss rate did not differ between WLH and WWOH. WLH had a higher stillbirth rate, particularly in the early years of analysis (2008–2012). Both maternal HIV and syphilis were independently associated with increased risk of stillbirth, but not with spontaneous miscarriage. Late miscarriages were more prevalent in WLH, particularly those coinfected with syphilis.
Strengths and limitations
The most notable strength of our study was the robust sample size with nearly 56,000 deliveries across an 11-year period; >1500 deliveries occurred in WLH. Another strength was our study period which coincided with the implementation of integrase strand inhibitors for pregnant women in public health systems in Brazil. One study limitation was that in WLH, we could not evaluate viral load at the moment of fetal loss because these events were often what brought these women to the hospital, triggering subsequent HIV diagnosis, with virus loads not routinely measured. Thus, we were unable to explore associations between virus load and pregnancy loss. Given that most women were not treated at the time of fetal demise, likely the vast majority had detectable HIV viremia. Among women with stillbirths, only 11% were confirmed to be using cART. Similarly, HIV treatment information was not available for most women who miscarried, often because they had not yet been identified as having HIV beforehand. For this reason, we were unable to explore associations between cART use and pregnancy loss.
Risk factors and pregnancy loss among women living with HIV and women without HIV
Our study confirms established risk factors for pregnancy loss in the general population. Although the etiology of spontaneous miscarriage is often unknown, it is thought that chromosomal abnormalities and genetic factors account for 50% to 60% of recurrent miscarriages, with the rest attributed to systemic disease including endocrine, infectious, autoimmune, and hematologic factors.2 In our study, advanced maternal age (≥35 years)21, 22, 23 and previous pregnancy loss were predictors of pregnancy loss, consistent with the existing literature.24,25 Very young age <15 years was previously reported to be associated with pregnancy loss.26 Our finding that younger WWOH were protected from pregnancy loss likely reflects that most women in our youngest age cohort were aged >15 but <18 years. The relationship between previous CD and pregnancy loss is unclear: some studies have reported increased risk,27,28 whereas others did not find statistical significance.29, 30, 31 The finding that previous CD was protective against pregnancy loss may have been influenced by a far greater number of miscarriages than that of stillbirths. Male fetal sex has previously been reported to be associated with approximately 10% higher risk of stillbirth32; however, this finding was not replicated in our cohort. Twin gestation and multigravida status were more frequently associated with pregnancy loss in the general population; such associations were previously noted.33, 34, 35
In women with HIV, pregnancy loss is a known adverse outcome associated with unsuppressed viremia; viral load may be a key determinant of this risk in a dose-dependent manner.36, 37, 38, 39 A study in the United States found that viral load during pregnancy, as measured cross-sectionally by plasma HIV RNA levels, predicted loss of pregnancy better than longitudinal and cumulative viral load over a lifetime.40 Although the etiology of pregnancy loss in WLH is multifactorial, altered immune activation and chronic inflammation induced by HIV infection are thought to play a role by disrupting maintenance of the decidua and contributing to placental dysfunction.41, 42, 43, 44 At our institution, testing guidelines implement rapid testing for HIV and syphilis on admission for pregnancy loss. In this way, many women are diagnosed with HIV at the time of miscarriage or stillbirth, thus not having a history of cART use, most likely representing a vulnerable population with high viral loads. However, in a recent study that evaluated miscarriage and stillbirth in women with HIV on cART, risk of fetal loss remained significantly elevated (7.1%) compared with WWOH (2.3%; P=.002) despite controlled viremia.45 This finding warrants further investigation into the interplay of infection and other maternal factors contributing to fetal demise and suggests that insult to the fetus cannot be explained by infection alone.
Syphilis during pregnancy predisposes to stillbirth.46 We found a 3.4-fold higher risk of stillbirth and 1.24-fold risk of any pregnancy loss in the presence of syphilis. Although we did not observe an association between any pregnancy loss and syphilis in women with HIV, the association between syphilis and fetal loss was observed in the general population. An association between HIV and stillbirth was also noted. When pregnancy loss was stratified into specific gestational periods, the frequency of late miscarriage in women with syphilis and HIV was 53%, whereas women with HIV alone also had high rates of late miscarriage (32%). The association between pregnancy loss, HIV, and syphilis in our study may not have been apparent because late pregnancy loss (late miscarriage and stillbirth) was also frequent in women with HIV without syphilis. Potentially, women with HIV did not present to care as frequently with early pregnancy loss, which could also explain the current findings. Two recent large cohort studies in Brazil, including one analyzing this same obstetrical cohort, suggested that maternal syphilis is undertreated in women with low syphilis titers, that penicillin treatment might not be frequently optimized for treatment of syphilis in pregnancy, and that partner treatment is often lacking.47,48 With 4-fold higher rates of syphilis in women with HIV in our cohort, untreated syphilis is likely the main driver of pregnancy loss among WLH. We did note, however, high frequencies of late pregnancy loss in women with HIV without syphilis. HIV mother-to-child transmission, when occurring before the third trimester of pregnancy, is often associated with pregnancy loss,36 whereas untreated pregnant women with HIV have higher rates of miscarriage and stillbirth.49 In this way, lack of early cART (which would prevent HIV mother-to-child transmission) may also increase the chances of pregnancy loss in women with HIV. Nevertheless, other known factors that contribute to fetal demise should be explored, such as preeclampsia, placental abruption, gestational diabetes mellitus, growth restriction, chromosomal abnormalities, and other congenital infections including toxoplasmosis or cytomegalovirus.
Clinical and research implications
In the general obstetrical population, most miscarriages and early pregnancy losses are thought to occur in the first trimester: it is estimated that 50% of all gestations are lost in early stages because of implantation failure or biochemical loss before 5 weeks of gestation. An additional 9% to 20% are miscarried during 5 to 12 weeks of gestation, with the incidence of early pregnancy loss after 12 weeks dropping to 1%.5,50 We found that WLH had higher rates of miscarriage after 12 weeks, suggesting that the mechanisms underlying miscarriage lay outside of the window of expected human reproduction and are more likely because of extrinsic factors such as coinfections.
Integrase strand inhibitors, most notably dolutegravir and raltegravir, are particularly useful in achieving rapid viral load reduction during pregnancy.51, 52, 53 We speculate that their widespread adoption may have improved stillbirth rates among WLH over time. We noted a steady fall in stillbirth rates from 2015 to 2018 among WLH, which may be owing to better cART. However, this decline in fetal loss was also observed in WWOH and could reflect better obstetrical care, unrelated to HIV status. The stillbirth rate was still significantly higher in WLH; this may be attributable to social and structural determinants of health such as homelessness and substance abuse. Multiple studies have shown that maternal illicit drug use including cocaine, methamphetamine, tobacco, and alcohol may lead to placental abruption and stillbirth.54, 55, 56, 57 Our study suggests that improvement and access to prenatal care with identification of comorbidities, coinfection, and linkage to treatment are paramount for prevention of fetal demise in WLH.
Conclusions
In a setting of universal, free cART, rates of any pregnancy loss over 11 years were similar between women with and without HIV. Late miscarriages and stillbirth, however, were more frequently observed in women with HIV, who also had a 4-fold risk of syphilis. Over time, the risk of stillbirth equalized between WLH and WWOH, likely because of more potent cART. Although syphilis may explain the higher rates of stillbirth in WLH, other factors such as coinfections and lack of previous HIV diagnosis and prompt cART initiation may contribute to late fetal demise.
Footnotes
The authors report no conflict of interest.
This research is supported by the University of California, Los Angeles (UCLA) South American Program in HIV Prevention and R25 grant MH08722 from the National Institute of Health/National Institute of Mental Health; principal investigator: Jesse Clark.
Prevention Research (National Institutes of Health/National Institute of Mental Health grant R25 MH08722; principal investigator: Jesse Clark). The funders had no role in the study design, data collection, or analysis.
This study was approved by the Institutional Review Board (Protocol #14.124) of Hospital Nossa Senhora da Conceição (Porto Alegre, Brazil).
Patient consent was not required because no personal information or details were included.
This study was presented as an oral presentation at the 32nd European Congress of Clinical Microbiology and Infectious Diseases, Lisbon, Portugal, April 23–26, 2022.
Cite this article as: Yang L, Cambou MC, Segura ER, et al. Patterns of pregnancy loss among women living with and without HIV in Brazil, 2008–2018. Am J Obstet Gynecol Glob Rep 2022;XX:x.ex–x.ex.
References
- 1.van Dijk MM, Kolte AM, Limpens J, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update. 2020;26:356–367. doi: 10.1093/humupd/dmz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alijotas-Reig J, Garrido-Gimenez C. Current concepts and new trends in the diagnosis and management of recurrent miscarriage. Obstet Gynecol Surv. 2013;68:445–466. doi: 10.1097/OGX.0b013e31828aca19. [DOI] [PubMed] [Google Scholar]
- 3.Baker CC, Creinin MD. Management of Stillbirth. Obstetric Care Consensus No, 10. Obstet Gynecol. 2020;135:e110–e132. doi: 10.1097/AOG.0000000000003719. [DOI] [PubMed] [Google Scholar]
- 4.Cicinelli E, Matteo M, Tinelli R, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. 2014;21:640–647. doi: 10.1177/1933719113508817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020;6:98. doi: 10.1038/s41572-020-00228-z. [DOI] [PubMed] [Google Scholar]
- 6.Cambou MC, Saad E, McBride K, Fuller T, Swayze E, Nielsen-Saines K. Maternal HIV and syphilis are not syndemic in Brazil: hot spot analysis of the two epidemics. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministério da Saúde. Boletim Epidemiológico Especial de HIV/AIDS 2019.https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2019/boletim-epidemiologico-especial-hiv-aids-2019/view.
- 8.Yeganeh N, Kreitchmann R, Leng M, Nielsen-Saines K, Gorbach PM, Klausner J. High prevalence of sexually transmitted infections in pregnant women living in Southern Brazil. Sex Transm Dis. 2021;48:128–133. doi: 10.1097/OLQ.0000000000001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeganeh N, Varella I, Santos BR, et al. Risk-taking behavior for HIV acquisition during pregnancy in Porto Alegre. Brazil. Infect Dis Obstet Gynecol. 2012;2012 doi: 10.1155/2012/490686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Melo MG, Varella I, Gorbach PM, et al. Antiretroviral adherence and virologic suppression in partnered and unpartnered HIV-positive individuals in southern Brazil. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo MG, Santos BR, De Cassia, Lira R, et al. Sexual transmission of HIV-1 among serodiscordant couples in Porto Alegre, southern Brazil. Sex Transm Dis. 2008;35:912–915. doi: 10.1097/OLQ.0b013e31817e2491. [DOI] [PubMed] [Google Scholar]
- 12.Gräf T, Bello G, Andrade P, et al. HIV-1 molecular diversity in Brazil unveiled by 10 years of sampling by the national genotyping network. Sci Rep. 2021;11:15842. doi: 10.1038/s41598-021-94542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souto B, Triunfante V, Santos-Pereira A, Martins J, Araújo PMM, Osório NS. Evolutionary dynamics of HIV-1 subtype. C in Brazil. Sci Rep. 2021;11:23060. doi: 10.1038/s41598-021-02428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro MC, Massuda A, Almeida G, et al. Brazil's unified health system: the first 30 years and prospects for the future. Lancet. 2019;394:345–356. doi: 10.1016/S0140-6736(19)31243-7. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler SM, Bryant AS, Bonney EA, Howell EA. Society for Maternal-Fetal Medicine Special Statement: race in maternal-fetal medicine research- dispelling myths and taking an accurate, antiracist approach. Am J Obstet Gynecol. 2022;226:B13–B22. doi: 10.1016/j.ajog.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Evans MK, Graves JL, Shim RS, Tishkoff SA, Williams WW. Race in medicine – genetic variation, social categories, and paths to health equity. N Engl J Med. 2021;385:e45. doi: 10.1056/NEJMp2113749. [DOI] [PubMed] [Google Scholar]
- 17.Siqueira TS, de Souza EKG, Martins-Filho PR, et al. Clinical characteristics and risk factors for maternal deaths due to COVID-19 in Brazil: a nationwide population-based cohort study. J Travel Med. 2022;29:taab199. doi: 10.1093/jtm/taab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almino MAFB, Maia MAG, Feitosa PWG, et al. High maternal mortality rates: racial, geographic, and socioeconomic disparities in Brazil. J Pediatr Nurs. 2022;65:e3–e4. doi: 10.1016/j.pedn.2021.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Small MJ, Allen TK, Brown HL. Global disparities in maternal morbidity and mortality. Semin Perinatol. 2017;41:318–322. doi: 10.1053/j.semperi.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7:127–150. [Google Scholar]
- 21.Fretts RC, Schmittdiel J, McLean FH, Usher RH, Goldman MB. Increased maternal age and the risk of fetal death. N Engl J Med. 1995;333:953–957. doi: 10.1056/NEJM199510123331501. [DOI] [PubMed] [Google Scholar]
- 22.Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol. 2006;195:764–770. doi: 10.1016/j.ajog.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts RC. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215–221. doi: 10.1016/s0029-7844(99)00536-0. [DOI] [PubMed] [Google Scholar]
- 24.Lamont K, Scott NW, Jones GT, Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta-analysis. BMJ. 2015;350:h3080. doi: 10.1136/bmj.h3080. [DOI] [PubMed] [Google Scholar]
- 25.Lamont K, Scott NW, Gissler M, Gatt M, Bhattacharya S. Risk of recurrent stillbirth in subsequent pregnancies. Obstet Gynecol. 2022;139:31–40. doi: 10.1097/AOG.0000000000004626. [DOI] [PubMed] [Google Scholar]
- 26.Balayla J, Azoulay L, Assayag J, Benjamin A, Abenhaim HA. Effect of maternal age on the risk of stillbirth: a population-based cohort study on 37 million births in the United States. Am J Perinatol. 2011;28:643–650. doi: 10.1055/s-0031-1276739. [DOI] [PubMed] [Google Scholar]
- 27.Gray R, Quigley MA, Hockley C, Kurinczuk JJ, Goldacre M, Brocklehurst P. Caesarean delivery and risk of stillbirth in subsequent pregnancy: a retrospective cohort study in an English population. BJOG. 2007;114:264–270. doi: 10.1111/j.1471-0528.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith GC, Shah I, White IR, Pell JP, Dobbie R. Previous preeclampsia, preterm delivery, and delivery of a small for gestational age infant and the risk of unexplained stillbirth in the second pregnancy: a retrospective cohort study, Scotland, 1992-2001. Am J Epidemiol. 2007;165:194–202. doi: 10.1093/aje/kwj354. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill SM, Agerbo E, Kenny LC, et al. Cesarean section and rate of subsequent stillbirth, miscarriage, and ectopic pregnancy: a Danish register-based cohort study. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351:2581–2589. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 31.Bahtiyar MO, Julien S, Robinson JN, et al. Prior cesarean delivery is not associated with an increased risk of stillbirth in a subsequent pregnancy: analysis of U.S. perinatal mortality data, 1995-1997. Am J Obstet Gynecol. 2006;195:1373–1378. doi: 10.1016/j.ajog.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 32.Mondal D, Galloway TS, Bailey TC, Mathews F. Elevated risk of stillbirth in males: systematic review and meta-analysis of more than 30 million births. BMC Med. 2014;12:220. doi: 10.1186/s12916-014-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaacson A, Diseko M, Mayondi G, et al. Prevalence and outcomes of twin pregnancies in Botswana: a national birth outcomes surveillance study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji W, Hou B, Li W, Guo F, He P, Zheng J. Associations between first-trimester intrauterine hematoma and twin pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2021;21:46. doi: 10.1186/s12884-020-03528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altijani N, Carson C, Choudhury SS, et al. Stillbirth among women in nine states in India: rate and risk factors in study of 886,505 women from the annual health survey. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moodley Y, Tomita A, de Oliveira T, Tanser F. HIV viral load and pregnancy loss: results from a population-based cohort study in rural KwaZulu-Natal, South Africa. AIDS. 2021;35:829–833. doi: 10.1097/QAD.0000000000002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst JE, Villar J, Victora CG, et al. The antepartum stillbirth syndrome: risk factors and pregnancy conditions identified from the INTERGROWTH-21st Project. BJOG. 2018;125:1145–1153. doi: 10.1111/1471-0528.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. 2016;3:e33–e48. doi: 10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- 39.Kim HY, Kasonde P, Mwiya M, et al. Pregnancy loss and role of infant HIV status on perinatal mortality among HIV-infected women. BMC Pediatr. 2012;12:138. doi: 10.1186/1471-2431-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cates JE, Westreich D, Edmonds A, et al. The effects of viral load burden on pregnancy loss among HIV-infected women in the United States. Infect Dis Obstet Gynecol. 2015;2015 doi: 10.1155/2015/362357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, Li J, Huang SY, Sun X. Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. J Reprod Immunol. 2015;110:81–88. doi: 10.1016/j.jri.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Clark DA, Croitoru K. TH1/TH2,3 imbalance due to cytokine-producing NK, gammadelta T and NK-gammadelta T cells in murine pregnancy decidua in success or failure of pregnancy. Am J Reprod Immunol. 2001;45:257–265. doi: 10.1111/j.8755-8920.2001.450501.x. [DOI] [PubMed] [Google Scholar]
- 43.Richardson K, Weinberg A. Dynamics of regulatory T-cells during pregnancy: effect of HIV infection and correlations with other immune parameters. PLoS One. 2011;6:e28172. doi: 10.1371/journal.pone.0028172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolte L, Gaardbo JC, Karlsson I, et al. Dysregulation of CD4+CD25+CD127lowFOXP3+ regulatory T cells in HIV-infected pregnant women. Blood. 2011;117:1861–1868. doi: 10.1182/blood-2010-07-298992. [DOI] [PubMed] [Google Scholar]
- 45.Tukei VJ, Hoffman HJ, Greenberg L, et al. Adverse pregnancy outcomes among HIV-positive women in the era of universal antiretroviral therapy remain elevated compared With HIV-negative women. Pediatr Infect Dis J. 2021;40:821–826. doi: 10.1097/INF.0000000000003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217–226. doi: 10.2471/BLT.12.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swayze EJ, Nielsen-Saines K, Segura ER, et al. Failure to recognize Low non-treponemal titer syphilis infections in pregnancy May lead to widespread under-treatment. Int J Infect Dis. 2021;104:27–33. doi: 10.1016/j.ijid.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swayze EJ, Cambou MC, Melo M, et al. Ineffective penicillin treatment and absence of partner treatment may drive the congenital syphilis epidemic in Brazil. AJOG Glob Rep. 2022;2 doi: 10.1016/j.xagr.2022.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marazzi MC, Palombi L, Nielsen-Saines K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS. 2011;25:1611–1618. doi: 10.1097/QAD.0b013e3283493ed0. [DOI] [PubMed] [Google Scholar]
- 50.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 51.Lockman S, Brummel SS, Ziemba L, et al. Efficacy and safety of dolutegravir with Emtricitabine and Tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/Vested): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2021;397:1276–1292. doi: 10.1016/S0140-6736(21)00314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kintu K, Malaba TR, Nakibuka J, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. 2020;7:e332–e339. doi: 10.1016/S2352-3018(20)30050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.João EC, Morrison RL, Shapiro DE, et al. Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial. Lancet HIV. 2020;7:e322–e331. doi: 10.1016/S2352-3018(20)30038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varner MW, Silver RM, Rowland Hogue CJ, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. 2014;123:113–125. doi: 10.1097/AOG.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorman MC, Orme KS, Nguyen NT, Kent EJ, 3rd, Caughey AB. Outcomes in pregnancies complicated by methamphetamine use. Am J Obstet Gynecol. 2014;211:429. doi: 10.1016/j.ajog.2014.06.005. e1–7. [DOI] [PubMed] [Google Scholar]
- 56.Hulse GK, Milne E, English DR, Holman CD. Assessing the relationship between maternal cocaine use and abruptio placentae. Addiction. 1997;92:1547–1551. [PubMed] [Google Scholar]
- 57.Hoskins IA, Friedman DM, Frieden FJ, Ordorica SA, Young BK. Relationship between antepartum cocaine abuse, abnormal umbilical artery Doppler velocimetry, and placental abruption. Obstet Gynecol. 1991;78:279–282. [PubMed] [Google Scholar]