Abstract
Mechanisms by which TLR4-based adjuvants enhance immunogenicity are not fully understood. We have taken advantage of a novel knock-in mouse strain that homozygously expresses two single nucleotide polymorphisms (SNPs) that are homologous to human TLR4 (rs4986790 and rs4986791) and have been associated with LPS-hyporesponsiveness in vivo and in vitro. “TLR4-SNP” (D298G/N397I) mice that recapitulate the human phenotype, were compared to wild-type (WT) mice for their hapten-specific antibody responses after immunization with nitrophenyl (NP) NP-Ficoll or NP-ovalbumin (Ova) in the absence or presence of a water-soluble TLR4 analog adjuvant, E6020. IgM and IgG anti-NP responses were comparable in WT and TLR4-SNP mice after immunization with either NP-Ficoll or NP-Ova only. E6020 significantly, yet transiently improved the IgM and IgG anti-NP responses of both WT and TLR4-SNP mice to NP-Ficoll (T-independent), with modestly enhanced antibody production in WT mice. In contrast, T-dependent (NP-Ova), adjuvant-enhanced responses showed sustained elevation of NP-specific antibody titers in WT mice, intermediate responses in TLR4-SNP mice, and negligible enhancement in TLR4−/− mice. E6020-enhanced early humoral responses in WT and TLR4-SNP mice to NP-Ova favored a IgG1 response. After a second immunization, however, the immune responses of TLR4-SNP mice remained IgG1-dominant, while WT mice re-immunized with NP-Ova and E6020 exhibited increased anti-NP IgG2c titers and a sustained increase in the IgG1 and IgG2c production by splenocytes. These findings indicate that E6020 increases and sustains antibody titers, and promotes isotype class switching, as evidenced by reduced titers and IgG1-dominant immune responses in mice with TLR4 insufficiency.
Introduction
Toll-like receptors (TLRs) are expressed on innate immune cells and are well recognized for their role of detecting microbial infection and inducing an immune response(1, 2). TLR4 is the receptor for Gram-negative lipopolysaccharides (LPS) and other pathogen- and danger-associated molecular patterns(2). TLR4 is highly expressed on granulocytes, monocytes, macrophages, and endothelial cells in most organs, while most circulating B cells and some B cells in bone marrow, spleen, and lymph nodes exhibit low levels of TLR4 expression(3, 4). However, the role of TLR4 in regulating adjuvant enhancement of humoral responses has been disputed. Schnare et al. established that during immunization with a T-dependent antigen (Ova), Th1, but not Th2 immune responses, as measured by IgG2c and IgG1, respectively, depended on TLR signaling(5). Pasare and Medzhitov also demonstrated the essential intrinsic role of TLRs on B cells, along with CD4+ T cell help, in generating robust T-dependent, antigen-specific antibody responses(6). In contrast, Gavin et al. showed that mice deficient in both intracellular adapters for TLR4 signaling, myeloid differentiating factor 88 (MyD88) and TIR-domain-containing adapter-inducing IFNβ (TRIF), that are therefore incapable of TLR signaling, were fully capable of mounting an adjuvant-enhanced antibody response when immunized with T-dependent antigens in emulsified adjuvant systems without or with TLR agonist components(7). Finally, Palm and Medzhitov demonstrated TLR4-independent immunostimulatory activity of haptenated proteins(8). However, adjuvant systems used in these early studies likely included multiple components besides the TLR4 agonist, and endotoxin contamination is so prevalent that even control groups could carry low concentrations of unintended immunostimulants. Since these important initial controversial findings were reported, several other groups have studied in detail the role of TLR ligands in mediating an adjuvant-enhanced antibody response to T-dependent antigens. This led to the inclusion of monophosphoryl lipid A (MPL) as a TLR4-based adjuvant in several FDA-approved vaccine formulations, the first of its kind in the new panoply of patten recognition receptor-utilizing adjuvants(reviewed in 9). Besides the Salmonella minnesota R595 lipid A-derived MPL, synthetic TLR4 agonists have been developed as potential vaccine adjuvants(reviewed in 10).
E6020 is a synthetic, water-soluble, relatively non-toxic LPS lipid A analog(11. 12). E6020 is hexa-acylated and has been shown to interact with TLR4/ Myeloid differentiating factor 2 (MD-2) complexes(11). E6020 is attenuated compared to hexaacylated LPS(12), and its adjuvanticity has been described in several animal models(11–19). We previously compared E6020 with synthetic MPL and found that it elicits comparable adjuvanticity to MPL in WT mice in both an Ova immunization model and in a nanoparticle immunization model using antigens from Francisella tularensis, despite marked differences in the degree of MyD88- and TRIF-dependent gene expression in vitro(20).
Human populations are diverse in their responses to both infectious diseases and immunizations. Two single nucleotide polymorphisms (SNPs) in the human TLR4 gene encode point mutations, D299G and T399I, in the extracellular domain of TLR4. Analysis of 25 published studies revealed that the minor allele encoded by each SNP is typically inherited heterozygously in healthy populations of diverse ethnicities with average allele frequencies of 0.06 and 0.03, respectively, and with evidence for co-segregation when both SNPs are inherited in the same individual(reviewed in 21). Interestingly, inheritance of TLR4 D299G and/or T399I differs among ethnic populations, but allele frequencies did not exceed 0.1 for any specific ethnicity(22). Inheritance of these mutations has been reported to diminish responses to LPS in vivo and in vitro and has been associated with differential susceptibility to infectious and inflammatory diseases(reviewed in 23, 24). For example, in two cohorts of pediatric patients with severe Respiratory Syncytical Virus requiring hospitalization, the allele frequencies of the minor alleles were 0.45 and 0.44, respectively (~90% heterozygous for both SNPs with co-segregation in the majority of samples tested), significantly higher than observed in healthy control populations(21). We recently engineered a novel homozygous knock-in mouse strain encoding homologous amino acid substitutions, D298G/N397I (“TLR4-SNP” mice), that recapitulates the human phenotype (i.e., LPS-hyporesponsive and is more susceptible to Respiratory Syncytical Virus and Klebsiella pneumoniae infections). TLR4 expression, internalization, signaling, downstream metabolic responses, and cytokine production are reduced in LPS-stimulated TLR4-SNP macrophages, but can be overcome, in part, by higher concentrations of LPS. In response to weak TLR4 agonists, such as MPL, the differences between the TLR4-SNP and WT mouse macrophages are amplified(24), as was previously reported in human monocytes expressing TLR4 SNPs upon stimulation with weak TLR4 agonists(25). We hypothesize that these mutations in TLR4 may hinder immunization efficiency, especially in the case where the immunogen is formulated with TLR4-based adjuvants.
To address the discrepancies in the field regarding the role of TLRs in antibody responses, we investigated whether well-defined, TLR4-adjuvanted immunogens would heighten antibody responses in the TLR4 signaling-insufficient TLR4-SNP mice. Immunization of WT and TLR4-SNP mice with T-independent and T-dependent antigens alone elicited comparable antibody titers. Inclusion of E6020 in immunizations with T-independent (NP-Ficoll) and T-dependent (NP-Ova) antigens enhanced antibody responses to a greater extent in WT mice than in LPS-hyporesponsive TLR4-SNP mice. In the T-dependent NP-Ova + E6020 immunization model, isotype class switching from IgG1 to IgG2c was sustained in WT mice after the second immunization while being significantly compromised in the TLR4-SNP mice. Collectively, these data support the efficacy of the TLR4 agonist adjuvant, E6020, in WT mice, to increase both antibody titer and isotype switching and suggest that individuals expressing TLR4 SNPs may require additional immunizations to achieve optimal antibody responses.
Materials and Methods
Mice
WT C57BL/6J mice were purchased from the Jackson Laboratory. TLR4−/− (kindly provided by S. Akira; Osaka University, Osaka, Japan) were backcrossed >12 generations onto a C57BL/6J background, and TLR4-SNP mice, generated on a C57BL/6J background using CRISPR/Cas9-based targeting and homology-directed repair(24), were bred in the accredited specific pathogen-free animal facility at the University of Maryland, Baltimore. Both male and female mice were used, n = 4 to 6 mice/treatment group/experiment. All animal experiments were performed with Institutional Animal Care and Use Committee approval from University of Maryland, Baltimore.
Reagents
The synthetic TLR4 agonist, E6020, was provided by Eisai, Inc. (Cambridge, MA) and reconstituted in endotoxin-free saline (E6020)(11). Sterile fluid thioglycollate was purchased from Remel (ThermoFisher, Dover, DE). The hapten 4-Hydroxy-3-nitrophenylacetyl (NP) conjugates, NP28-bovine serum albumin (BSA), NP44-Ficoll, and NP17-Ovalbumin (Ova), were purchased from Biosearch Technologies (Novarto, CA) and reconstituted in endotoxin-free water from Quality Biological (Gaithersburgh, MD). NP-Ficoll and NP-Ova were twice purified over endotoxin removal columns from Pierce Biotechnologies (ThermoFisher, Dover, DE). HRP-conjugated secondary antibodies include goat-anti-mouse IgM, IgG1, IgG2b, IgG2c, and IgG3 that had been purchased as adsorbed against all other mouse immunoglobulin isotypes as well as against human sera to limit cross-reactivity from Southern Biotech (Birmingham, AL).
In vitro macrophage gene expression
Thioglycollate-elicited macrophages were harvested by peritoneal lavage in sterile saline and pooled from 2–4 mice per strain per experiment as previously described(26). Cells were stimulated with E6020 at concentrations ranging from 0.1 ng/ml – 100 ng/ml for 2 hours after which macrophages were harvested in TriPure reagent (Roche, San Francisco, CA) and frozen at −80° C. RNA was extracted per the manufacturer’s protocol and cDNA was reverse transcribed from 1 μg RNA per sample using Qscript kits (QuantaBio, Beverly, MA)(26). qRT-PCR was performed on an ABI 7900HT instrument (Applied Biosystems, Waltham, MA) using Power SYBR Green PCR master mix (Applied Biosystems) with primers (Sigma-Aldridge, Inc., St. Louis, MO) for Hprt, Tnf, Il1b, Ifnb1, and Cxcl10(27). Levels of mRNA for specific genes are reported and statistical analyses performed on negative delta cycle threshold (CT) values, as normal distributions are preserved in this method. Data are displayed as arithmetic mean ± SEM. Conversion to relative gene expression normalized to that of WT unstimulated macrophages (“fold induction”)(28) are also shown on the right y-axis (Figure 1). The housekeeping gene encoding hypoxanthine-guanine phosphoribosyltransferase (Hprt) was used for normalization of RNA levels within each sample.
Figure 1: Effect of E6020 in activating macrophages from TLR4-SNP mice.
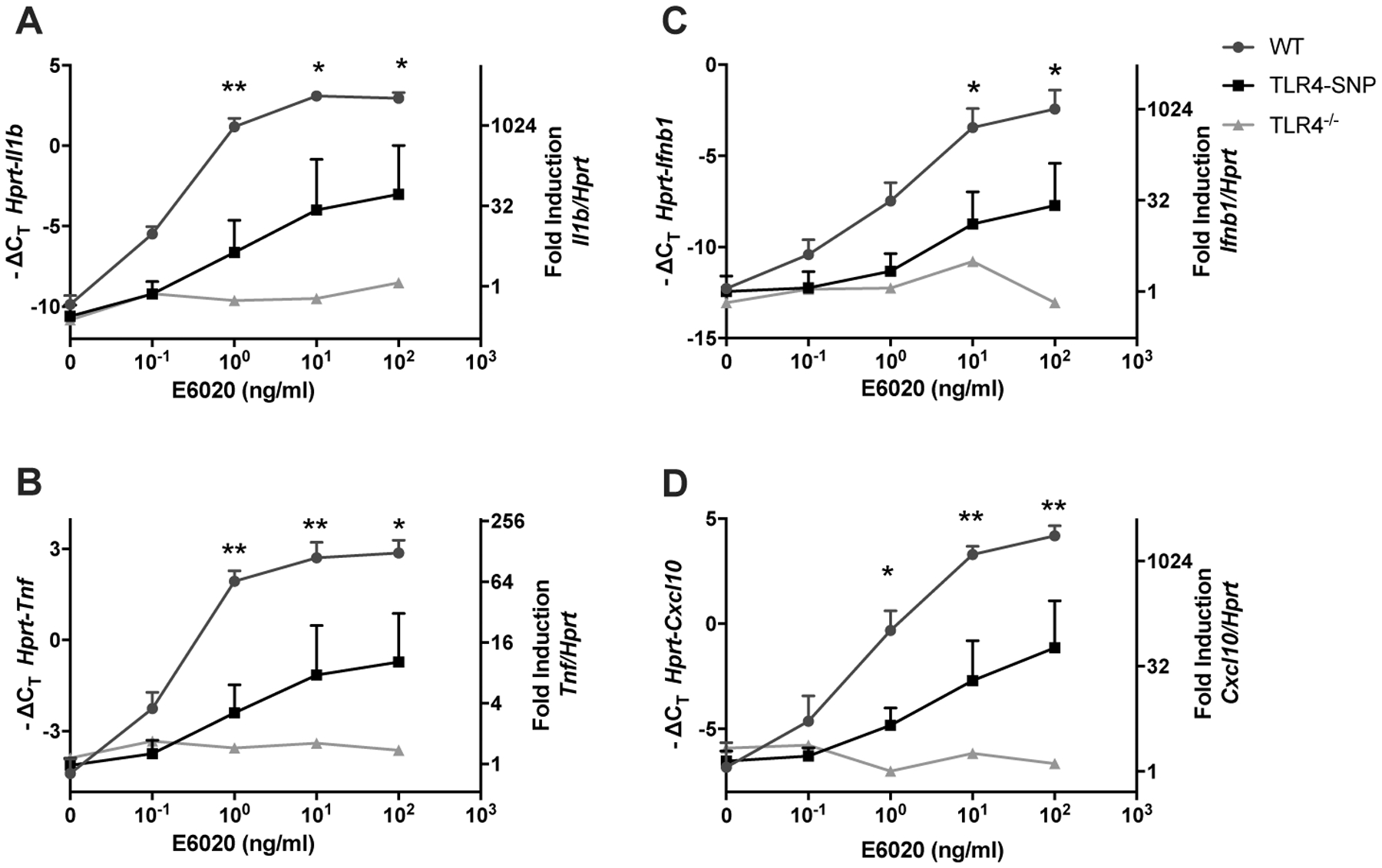
Thioglycollate-elicited peritoneal macrophages were harvested from age-matched C57BL/6J, TLR4-SNP, and TLR4−/− mice and stimulated with increasing concentrations of E6020 (0.1 ng/ml-100 ng/ml) for 2 hours, and RNA was processed as described in Methods. Gene expression of Il1b (A), Tnf (B), Ifnb1 (C) and Cxcl10 (D) were determined. Data were pooled from 4 independent experiments with cells pooled from 3 mice/genotype/experiment and presented as -ΔCT (left y-axis) and fold induction (2^-ΔΔ CT value; right y-axis), mean +/− SEM. Statistical analysis (Repeated measures 2-Way ANOVA with Sidak’s post-hoc comparison) was performed on the average -ΔCT values of WT and TLR4-SNP macrophage responses from each experiment.
Immunizations
Age- (6–8 weeks) and sex-matched WT, TLR4−/−, and TLR4-SNP mice were used in the experiments. Briefly, mice were immunized intraperitoneally on Day 0 (prime) and Day 21 (boost) with 1 mL sterile saline containing 50 μg/mouse of the hapten-conjugate NP-Ficoll, to elicit T-cell independent responses, or NP-Ova, to elicit T-cell dependent responses, without or with 50 μg/mouse of the TLR4 agonist adjuvant E6020(20). Mice were bled at the indicated time points to collect sera for ELISA.
Antigen-specific antibody ELISA
NP-specific antibodies were detected by ELISA as previously described(29). Briefly, high-binding 96-well microplates (Greiner Bio-one, Monroe, NC) were coated with 50 μl/well of 1 μg/ml NP28-BSA in coating buffer (50 mM NaHCO3, pH 9.6) and incubated at 4° C overnight. Plates were blocked at 37° C for 2 hours in PBS + 0.1% Tween 20 (PBST) containing 1% FBS and 0.3% non-fat dry milk (Carnation/Nestlé, Vevey, Switzerland). Five-point serial dilutions (3-fold for IgM or 6-fold for IgG titers) of serum from individual mice were added and incubated at 4° C overnight. Naïve sera (pre-bleed) were interspersed among the samples of the post-immunization timepoints, and samples from each genotype and immunization group were included on the same plate to minimize bias. The following day, plates were incubated at 37° C for 2 hours with 100 μl/well of HRP-conjugated secondary antibodies (either a 1:5,000 dilution of goat-anti-mouse IgM or a cocktail of goat-anti-mouse IgG1, IgG2b, IgG2c, and IgG3 (1:10,000 each; Southern Biotech)). Plates were washed 3–6 times with PBST and PBS between each step. Finally, plates were developed with 50 μl/ well of 2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt in phosphate-citrate buffer with 0.03% H2O2 (all from Sigma) for either 10 min (IgG) or 60 min (IgM). The reaction was stopped with 0.1% SDS. Optical density was measured using a microplate reader at 405 nm (Bio-Tek/Agilent, Santa Clara, CA). Titers were determined by sigmoidal curve fitting (4PL, X is log(conc.)) in Prism, Version 7 (Graphpad, San Diego, CA). Prism calculates log titers, which were then exponentiated (10 raised to the power of the log titer) to obtain titers; however, statistics were performed on the log titers to preserve a normal distribution. For analysis of individual antibody isotypes, log titers of IgG1 and IgG2c were analyzed separately, and the Log titer IgG2c:Log titer IgG1 ratio was calculated. Titers were graphed on a log scale with the geometric mean and 95% confidence interval (Figures 2, 3, 4).
Figure 2: Effect of E6020 adjuvant in the antibody responses to T-independent (NP-Ficoll) immunization.
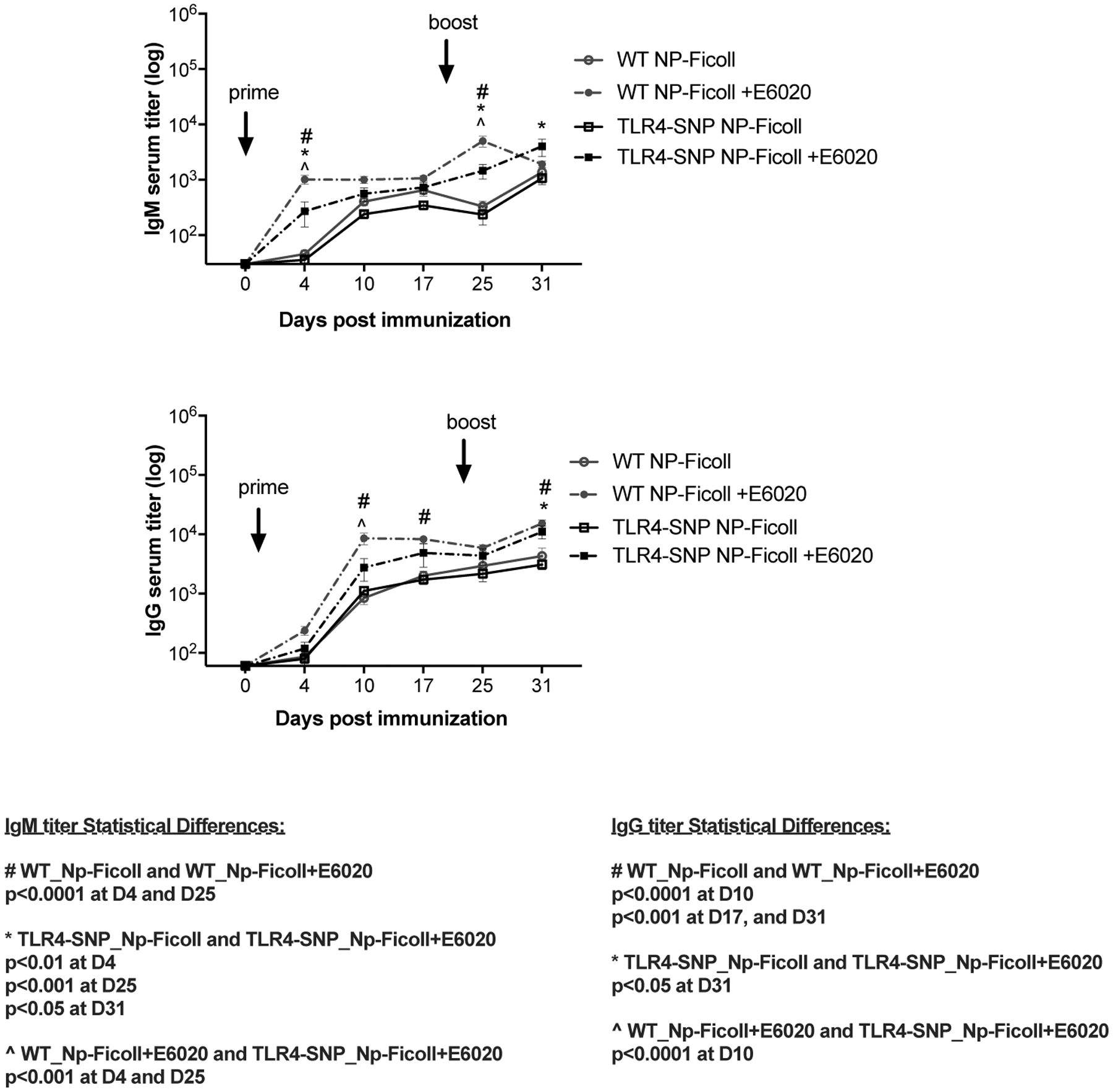
Age matched C57BL/6J WT (circles) and TLR4-SNP (squares) mice were immunized on days 0 and 21 with NP-Ficoll (Antigen alone; open symbols, solid lines) or with NP-Ficoll + E6020 (Antigen + Adjuvant; filled symbols, dashed lines). Serum was harvested from individual mice on days 0, 4, 10, 17, 25, and 31 post-prime. NP-specific IgM (top panel) and IgG (bottom panel) titers were measured by ELISA. Data were pooled from two independent experiments with 3–5 mice/treatment group/experiment. Statistical analysis (Repeated Measures 2-Way ANOVA with Sidak’s post-hoc comparison) was performed on the log transformed data.
Figure 3: Effect of E6020 adjuvant in the antibody responses to T-dependent (NP-Ova) immunization.
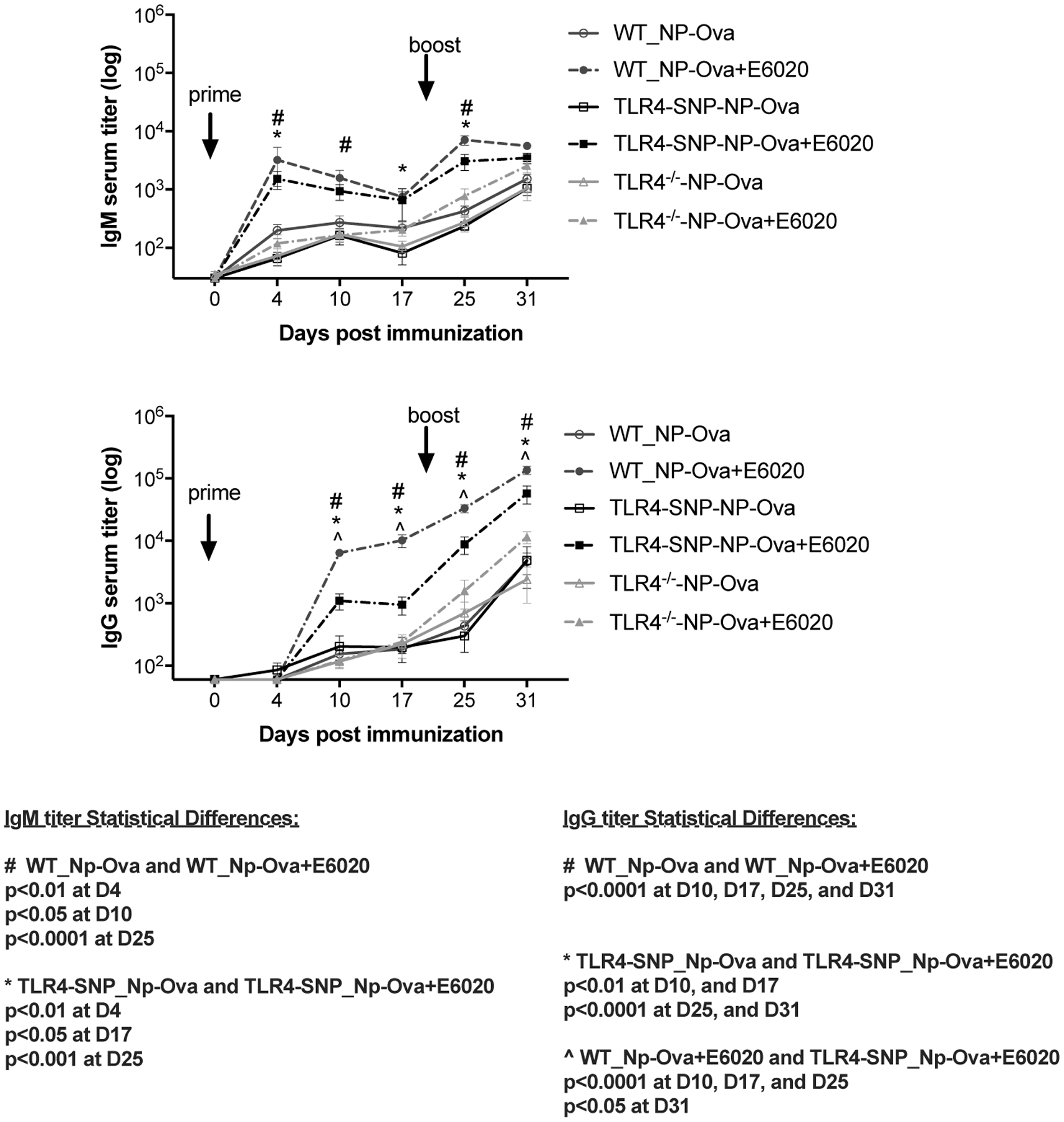
Age-matched C57BL/6J WT (circles), TLR4-SNP (squares), and TLR4−/− (triangles) mice were immunized using NP-Ova as a T-dependent antigen and bled as described for Figure 2. Serum IgM (top panel) and IgG (bottom panel) titers were measured by ELISA. Data were pooled from two independent experiments with 4–6 mice/treatment group/experiment. Statistical analysis (Repeated Measures 2-Way ANOVA with Sidak’s post-hoc comparison) was performed on the log transformed data.
Figure 4: Effect of E6020 adjuvant in the IgG2c and IgG1 antibody responses to T-dependent (NP-Ova) immunization.
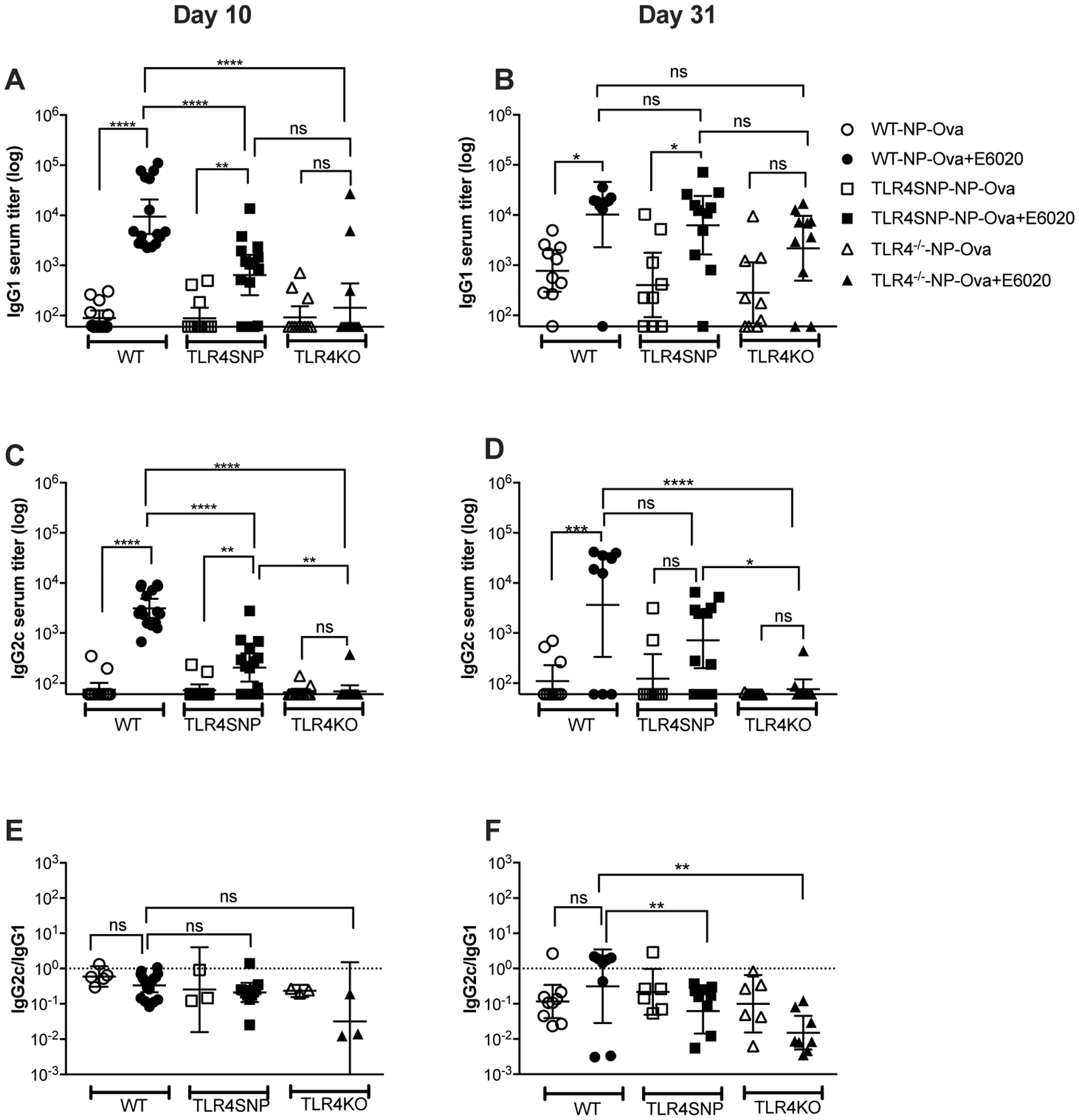
Age-matched C57BL/6J WT, TLR4-SNP, and TLR4−/− mice were immunized on days 0 and 21. Sera were harvested from blood collected from individual mice on day 10 (A, C, E) and on day 31 (B, D, F) post-prime. Serum IgG1 (A, B) and IgG2c (C, D) titers were measured by ELISA. Ratios of IgG2c/IgG1 were plotted for each mouse, excluding sera with IgG1 titers below the detection threshold (E, F). Data were pooled from three (A, C, E) and two independent experiments (B, D, F) with 4–6 mice/treatment group/experiment. Statistical analysis A-D: Ordinary 1-Way ANOVA with Tukey’s post-hoc comparison was performed on the log transformed data; E, F: Ordinary 1-Way ANOVA with Tukey’s post-hoc comparison was performed on the ratios.
ELISpot analysis
NP-specific ELISpot assays were modified from Wahid et al.(30) and Richard et al.(29). Briefly, nitrocellulose plates (MAHAN, Millipore, Billerica, MA) were coated with 10 μg/ml NP28-BSA (Biosearch Technologies) or donkey anti-mouse IgG+IgM Ab (Jackson ImmunoResearch Laboratories, West Grove, PA) overnight at 4° C and blocked with 10% FBS in RPMI 1640 (Corning, Manassas, VA) for 2 hours at 37° C. Splenocytes were serially diluted into wells and incubated overnight at 37° C, 5% CO2. Plates were washed with PBS and PBS containing 0.05% Tween 20. Spot-forming cells (SFC; representing antibody-secreting cells) were detected with HRP-labeled goat anti-mouse IgG1 or IgG2c antibodies (Southern Biotech, Birmingham, AL), and visualized by HRP substrate 3-Amino-9 ethyl-carbazole C (AEC substrate; Calbiochem, La Jolla, CA) at room temperature in the dark for 8–10 min. Spots on fully dried plates were counted in an automated ELISpot reader (Immunospot®, Cellular Technology Ltd., Cleveland, OH) and analyzed using the Immunospot® version 5.0 software followed by semi-automatic quality control. Results are expressed as the frequency of antigen-specific SFC per 106 splenocytes.
Surface expression of TLR4
Splenocytes were stained with Invitrogen LIVE/DEAD™ Fixable Yellow Dead Cell Stain Kit (Thermo Fischer Scientific) per manufacturer’s instructions, washed twice with ice-cold FACS buffer, blocked with anti-CD16/32 (clone 93, Biolegend, San Diego, CA) and stained with antibodies targeting CD284 (TLR4) (clone SA15–21, PE-conjugated, Biolegend), CD45 (clone 30F11, FITC, Biolegend), CD19 (clone 1D3, Alexa Fluor 700, BD Biosciences, Franklin Lakes, NJ), CD45R (B220) (clone RA3–6B2, eFluor450, Invitrogen/Thermo Fischer), and either CD3 (clone17A2, APC, Biolegend) or F4/80 (clone BM8, APC, Biolegend), using their respective isotype controls and FMOs as negative controls. After 30 min of staining, samples were washed twice in FACS buffer, once in PBS, and fixed with 1% paraformaldehyde. Data were acquired the following day on a Cytek Aurora instrument (Cytek Biosciences, Fremont, CA). Spectral unmixing was performed with Cytek’s SpectroFlo software based on single-color stained and unstained splenocytes. Proper unmixing/compensation was verified and TLR4 staining levels were analyzed in FCS Express 7 (De Novo Software, Pasadena, CA) with the following gating strategy: 1. Forward scatter signal height vs. area to select singlets, 2. Forward vs. Side scatter signals to select lymphocytes, 3. CD45 vs. Live/Dead to select live lymphocytes, 4. CD3 vs. CD19 to select B cells, 5. confirm that CD19+CD3- B cells are also B220+F4/80-, 6. Analyze TLR4 expression level on single-color histogram.
Statistics
For statistical analyses, Repeated Measures 2-Way ANOVA with Sidak’s multiple comparison or Ordinary 1-Way ANOVA with Tukey’s multiple comparison were calculated in Prism Software Version 7.0c (La Jolla, CA). All p-values are adjusted for multiple comparisons. The antibody responses of male and female mice were initially analyzed separately and, upon finding no significant sex differences, data were analyzed together for the final figures.
Results
TLR4-SNP macrophages are hyporesponsive to the TLR4 agonist adjuvant, E6020, in vitro
E2020 is a synthetic TLR4 agonist that has been used as an adjuvant in vaccine formulations in animal models(11–20). We initially examined the ability of E6020 to induce expression of MyD88-dependent (Figure 1 A, B) and TRIF-dependent (Figure 1 C, D) genes in peritoneal macrophages derived from age-matched WT, TLR4-SNP, and TLR4−/− mice. Consistent with our previously published data(20), macrophages from C57BL/6J mice (WT) demonstrate dose-dependent gene expression in response to stimulation by E6020 (Figure 1). TLR4−/− macrophages were included as negative control and were unresponsive to stimulation by E6020. TLR4-SNP macrophages, which maintain surface expression of TLR4 at ~50% the level expressed by WT macrophages(24), exhibit an intermediate dose-dependent response to E6020 that was significantly reduced compared to the response of WT macrophages, for both MyD88- and TRIF-dependent gene expression. These data demonstrate that TLR4 D298G/N397I-expressing macrophages respond to E6020, albeit to a lower extent than WT macrophages.
E6020-enhanced antibody responses to a T-independent antigen
Initial experiments compared the antibody responses of WT and TLR4-SNP mice immunized with the T-independent antigen, NP-Ficoll in the absence or presence of the TLR4 adjuvant, E6020. Since both the antigen and adjuvant are amenable to aqueous formulations, this limited TLR4-independent adjuvant effects elicited by oil-in-water emulsions(31, 32). Mice were immunized on days 0 and 21 (prime/boost), and blood (serum) was collected at the indicated time points. NP-specific IgM and IgG titers were measured by ELISA on NP-BSA-coated plates which detect moderate to high avidity NP-specific antibodies(29).
The response to NP-Ficoll in the absence of adjuvant was not statistically different in WT and TLR4-SNP mice for both IgM and IgG isotypes (Figure 2, solid lines). In the presence of E6020, both WT and TLR4-SNP mice accelerated induction of NP-specific IgM responses by approximately one week, showing significantly increased IgM titers at 4 days post-prime and 4 days post-boost, compared to unadjuvanted immunization groups that showed similar IgM titers 10 days post-prime and 10 days post-boost (Figure 2, top panel). However, NP-specific IgM titers were significantly lower in the TLR4-SNP mice than in WT mice when immunized with NP-Ficoll in the presence of E6020 (Figure 2, top panel).
In WT mice, the NP-specific IgG antibody titers were also enhanced by the inclusion of E6020 with antigen 10 days post-prime and titers remained significantly higher compared to the unadjuvanted immunization groups. However, in the TLR4-SNP mice, the E6020-enhanced increase in NP-specific IgG titers were significantly lower than in WT mice on day 10, but they increased significantly to the level of WT mice after the second immunization (Figure 2, bottom panel, dashed lines). These findings indicate that the T-independent antibody responses are enhanced by TLR4 adjuvant stimulation, but to a lesser extent in TLR4-SNP than in WT mice after primary immunization.
Adjuvant-enhanced IgG responses to a T-dependent antigen are decreased in TLR4-SNP mice
The dependence on pattern recognition receptor stimulation for full activation of T-independent B cell responses is uncontested(7, 33–38), and is supported by our data in Figure 2. However, the role of TLR stimulation for antibody responses to T-dependent antigens remains controversial(6–8). Therefore, WT, TLR4-SNP, and TLR4−/− mice were immunized with the T-dependent antigen, NP-Ova, in the absence or presence of E6020 as described for Figure 2.
In the absence of E6020, NP-Ova elicited comparable IgM and IgG responses for all three strains of mice (Figure 3, solid lines). This indicates that unadjuvanted responses to this T-dependent hapten-carrier antigen are not affected by the lack of expression or mutations within TLR4. Inclusion of E6020 significantly increased anti-NP IgM titers in both WT and TLR4-SNP mice, but not the TLR4−/− mice as early as 4 days post-immunization (day 4 and day 25; Figure 3, top panel). In contrast, adjuvant-mediated enhancement of the NP-specific IgG response was significantly greater in WT mice than in TLR4-SNP mice, and absent in TLR4−/− mice (Figure 3, bottom panel). Together, the data in Figures 2 and 3 show that TLR4-SNP mice are partially deficient in humoral response amplitude and kinetics to a TLR4-based adjuvant in both the T-independent and T-dependent immunizations.
E6020 drives isotype switching from IgG1 (Th2) to IgG2c (Th1) and is dependent on TLR4 signaling sufficiency
Immunoglobulin isotype switching is inducible by TLR adjuvants(34, 36, 37). Therefore, we sought to determine if the observed decrease in the IgG response in TLR4-SNP mice reflected, in part, a shift in the Th1:Th2 balance of the response to NP-Ova, measured by proxy by comparing IgG2c versus IgG1 titers in day 10 and day 31 serum samples.
In the absence of E6020, ≤50% of individual mice of all three genotypes mounted a measurable IgG1 or IgG2c response (titer >60) 10 days after primary immunization with NP-Ova (“responders;” Figure 4A, C), and a booster immunization with antigen only increased the frequency of IgG1 responders to 90% in WT mice, 75% in TLR4-SNP mice, and 65% in the TLR4−/− mice (Figure 4B). However, the number of IgG2c responders remained <50% in all three genotypes in the absence of E6020 following the second immunization (Figure 4D).
The inclusion of E6020 in the antigen formulation significantly altered the relative proportion of mice responding with IgG1 vs. IgG2c anti-NP production. Specifically, primary immunization with NP-Ova + E6020 increased the proportion of WT responders to 100% and TLR4-SNP mice to ~70% producing measurable titers of NP-specific IgG1 and IgG2c with WT mice producing ~10-fold higher titers of each isotype (Figure 4A, C). However, responses of WT mice to the NP-Ova + E6020 booster dose showed a dichotomy, with ~67% of mice increasing NP-specific IgG2c titers to match or exceed IgG1 titers, with the remaining ~33% of WT mice failing to produce detectable IgG2c titers (one mouse also lost detectable NP-specific IgG1) (Figure 4B, D). TLR4-SNP IgG1 responders increased from ~70% to ~90% after the NP-Ova + E6020 booster dose, with titers approaching those of WT mice (Figure 4B); however, IgG1 responses in TLR4−/− mice immunized with NP-Ova + E6020 were not significantly different from the unadjuvanted responses of all mouse strains, again indicating that TLR4 was not required for non-adjuvanted responses (Figure 4A, B). Importantly, the frequency of IgG2c responders remained unchanged in TLR4-SNP mice, despite the fact that the NP-specific IgG2c serum titer increased in ~40% of these mice, titers remained ~10-fold lower than the adjuvanted WT responders and were not significantly different from the non-adjuvanted TLR4-SNP group (Figure 4D). As expected, E6020 did not significantly affect the frequency of immunization-responders or serum titers in the TLR4−/− mice (Figure 4, A–D). To summarize, E6020 effectively increased the number of IgG responders in both the WT and TLR4-SNP mice to primary immunization. Notably, E6020-enhanced IgG1 titers of TLR4-SNP mice caught up to WT responses upon a second immunization, but the isotype switch to IgG2c titers remained lower in the TLR4-SNP mice.
Analysis of the ratios of IgG2c:IgG1 titers (for sera with detectable IgG1 titers) further support the conclusion that the balance of a Th1:Th2 milieu was differentially altered over time by the presence of E6020 adjuvant. At 10 days post-prime, all groups of mice, in the absence and presence of E6020, exhibited a predominantly Th2 milieu, as evidenced by IgG1 > IgG2c titers (Figure 4E). These ratios are more reliable in the adjuvanted immunization groups, as antibody titers in the non-adjuvanted immunization groups were near the limit of detection at 10 days after the primary immunization (Figure 4A, B). At 10 days post-boost (day 31), WT mice immunized with NP-Ova alone still produced IgG1 predominantly (median IgG2c:IgG1 ratio = 0.1). However, a trend was observed in WT mice immunized with NP-Ova formulated with E6020 towards exhibiting a shift towards a Th1:Th2-balanced state after the second immunization, with the median IgG2c:IgG1 ratio increasing from 0.4 (day 10) to 1.6 (day 31; IgG2c titer > IgG1 titer). In TLR4-SNP mice, the Th2-dominance of the responses remained unchanged after the second dose, regardless of immunization without or with E6020, with the median IgG2c:IgG1 ratio of ~0.2 (Figure 4E, F). Similarly, in TLR4−/− mice, the Th2-dominance did not change from primary to boosted response (median IgG2c:IgG1 ratio <0.2 (without or with E6020; Figure 4E, F). Together, these findings suggest that inclusion of the TLR4 agonist adjuvant enhances isotype class switching to a Th1 phenotype in TLR4-sufficient mice.
A separate group of mice were immunized (primary, i.p.) as described above and euthanized at day 10 to quantify the frequency of NP-specific IgG secreting cells in spleens by ELISpot analysis. Total SFC were visualized on wells coated with donkey-anti-mouse IgG+IgM capture antibody. NP-specific SFC (NP28-BSA coated wells) were only detectable in splenocytes from WT mice that received both NP-Ova antigen and E6020 adjuvant, with average frequencies of 37 ± 23 IgG1 SFC/106 splenocytes and 9.8 ± 5.5 IgG2c SFC/106 splenocytes for IgG1 and IgG2c, respectively (p < 0.01 compared to undetectable frequencies (SFC ≤ 1/106 splenocytes) in WT mice immunized with NP-Ova alone and in TLR4-SNP mice). These SFC responses exhibited a median IgG2c:IgG1 ratio of 0.3 (range 0.03 – 0.8), which is comparable to the ratio derived from the ELISA data (Figure 4E). While considerably less sensitive than the ELISA assay, the results of the ELISpot experiments support our findings that WT antibody responses to immunization with NP-Ova + E6020 are significantly stronger and more Th1-skewed than TLR4-SNP responses. Finally, TLR4-SNP splenic B cells (live CD45+ CD3− F4/80− B220+ CD19+) expressed 58% ± 2% of surface TLR4 (antibody clone SA15–21; WT = 100%, TLR4−/− = 0%), consistent with our previous report showing that peritoneal macrophages from TLR4-SNP mice express approximately half as much surface TLR4/MD-2 as WT macrophages(24). Collectively, these results demonstrate that the T-independent and T-dependent responses to hapten-carrier protein adjuvants is TLR4-independent and that E6020 is a potent TLR4-dependent adjuvant that results in both increased amplitude and maturation of antibody responses in response to immunization with a T-dependent hapten-carrier antigen.
Lastly, we sought to determine whether the E6020-induced differential IgG response in NP-Ova immunized WT and TLR4-SNP mice was sustained. Six weeks following the boost of NP-Ova +/− E6020, sera and spleens were harvested from WT and TLR4-SNP mice. Sera were used to determine NP-specific IgG1 and IgG2c antibody titers by ELISA. Significant titers of both were sustained in WT and TLR4-SNP mice immunized with NP-Ova + E6020; however, IgG1 (Th2) was the dominant circulating isotype sustained by both genotypes of mice (levels comparable to mice euthanized on day 31) and IgG2c responses had contracted more rapidly in TLR4-SNP mice than in the WT (Supplementary Figure 1A). Single cell suspension of splenocytes were plated for an ELISpot assay to determine the number and intensity of NP-specific IgG1- and IgG2c-producing SFC (representing plasmacytes). WT mice immunized with NP-Ova + E6020 showed the highest sustained SFCs with average frequencies of 54 ± 6.4 IgG1 SFC/106 splenocytes and 23 ± 4.2 IgG2c SFC/106 splenocytes for IgG1 and IgG2c, respectively (p < 0.01 for IgG1 and p = 0.057 for IgG2c compared to low sustained SFCs of WT mice immunized with NP-Ova alone). In contrast, TLR4-SNP mice sustained similar frequencies of SFCs whether they were immunized with NP-Ova alone or NP-Ova + E6020 (Supplementary Figure 1A). Importantly, E6020 significantly enhanced the spot size of IgG1- and IgG2c-producing SFC in only the WT mice and not TLR4-SNP mice as indicated in size distribution histograms (Supplementary Figure 1B). This shift in the size of the spots induced by inclusion of E6020 in the initial prime-boost immunization shows that the majority of WT SFCs (plasmacytes) are qualitatively better at secreting NP-specific antibodies than their TLR4-SNP counterparts.
Discussion
We previously reported that the “TLR4-SNP” mice exhibit significantly reduced responses to LPS both in vitro and in vivo(24). Given that the homologous TLR4 SNPs in humans, encoding D299G/T399I in the extracellular domain of TLR4, also confer LPS-hyporesponsiveness in a significant subpopulation of individuals(reviewed in 23, 24), we hypothesized that the use of TLR4-based adjuvants in vaccines might not elicit an optimal antibody response in individuals bearing these SNPs. The findings presented in this report indicate that the TLR4-SNP mice respond equivalently to WT mice in response to immunization with T-independent and T-dependent antigens in the absence of adjuvant, as do TLR4−/− control mice immunized with T-dependent antigen; however, enhanced responses to immunization in the presence of E6020, including elevated immunoglobulin titers and isotype class switching to IgG2c, were significantly impaired in TLR4-SNP mice, but not completely absent as observed in TLR4−/− mice. Reduced antibody titers in sera may derive from either a low level of antibody secretion and/or reduced proliferation of antigen-responsive B cells. ELISpot analysis suggests that after primary immunization (Day 10), reduced frequencies of antibody secreting cells account for the lower antibody titers observed in sera of TLR4-SNP mice. Our previous report revealed that decreased TLR4 signaling in thioglycollate-elicited peritoneal macrophages from TLR4-SNP mice was associated with reduced expression of TLR4, as had been reported for human epithelial cells derived from people expressing the human TLR4 SNPs(24, 39, 40). TLR4 expression was similarly decreased in splenic B cells of TLR4-SNP vs. WT mice as determined by flow cytometry. Cytokine responses by TLR4-SNP peritoneal macrophages to E6020 in vitro also were significantly lower than those seen in WT macrophages, but not lacking as observed in the TLR4−/− mice (Figure 1).
Whether or not adjuvant effects are B cell-intrinsic has been a longstanding debate. Early studies used an emulsified immunogen and there is no indication that contaminating endotoxin was removed from these products. Incomplete Freund’s Adjuvant (IFA) is an emulsion of mineral oil and mannide monooleate in water which is associated with significant side effects, probable release of endogenous danger-associated molecules in tissues at the injection site(reviewed in 41), as well as stimulating inflammatory responses through nucleotide-binding oligomerization domain protein 2 (NOD2)(42). Ribi Adjuvant is another oil in water emulsion containing bacterial-derived monophosphoryl lipid A (MPL, a lipid A analog adjuvant)(reviewed in 43) as well as the C-type lectin agonist trehalose dimycolate(44). Palm and Medzhitov attributed the early differences observed in the role of TLR signaling in B cell responses to the immunogenicity of the antigens themselves: haptenated protein conjugates, such as NP-Ova, were found to be more immunogenic than native proteins (each administered in IFA) and, thus, were proposed to bypass the requirement for TLR signaling in eliciting antibody responses(8). Another possible explanation is that an increased dependency on TLR signaling for B cell antibody responses arises when B cell receptor (BCR) stimulation and/or T cell help is limiting(45). However, even in the original study by Gavin et al., responses of TLR signaling-deficient mice to haptenated proteins supplemented with Ribi adjuvant exhibited almost 10-fold lower IgG2c and IgG2b responses(7), similar to our own observations. In contrast to the earlier studies, we further purified the haptenated immunogens by applying them to endotoxin removal columns and used a synthetic TLR4 adjuvant that is water-soluble, so that the immunizations could be carried out in endotoxin-free normal saline. Responses to T-independent (NP-Ficoll) and T-dependent (NP-Ova) antigens alone were indeed TLR4-independent, as anti-NP antibody responses in unadjuvanted, immunized mice across all three genotypes yielded similar titers with similar kinetics (Figures 2 and 3). By immunizing mice in an otherwise “clean” system, inclusion of the TLR4 agonist adjuvant yielded significantly higher titers in the WT control mice.
There are, however, other studies that contradict our findings. In Meyer-Bahlburg et al. study of C57BL/6 and MyD88−/− B cells adoptively transferred to μMT mice, B-cell intrinsic TLR4 signaling by LPS amplified IgM and IgG2c responses, but MyD88-dependent signaling was not required to enhance IgG1 responses to NP-conjugated T-dependent antigen adjuvanted with LPS(46). Other cell types capable of interacting with B cells would have been MyD88- (and TLR-signaling)-sufficient in this study, whereas our mice exhibit the TLR4-SNP mutations in all cell types. In addition to activating traditional antigen presenting cells, TLR4 stimulation is thought to activate B cells to present antigen to T cells and secrete cytokines, creating a positive feedback loop for adaptive immune activation(reviewed in 47). In addition, the MyD88 signaling-deficient B cells in the Meyer-Bahlburg et al. study may respond to paracrine and cytokine signals elicited by TLR-responsive innate immune cells.
We recognize that there are limitations to our study, particularly the identification of the cell type(s) mediating the adjuvant effect in humoral responses, that would require analysis of responses of mice harboring TLR4-deficiency limited to either B cells, T-helper cells, or antigen-presenting cells. Ideally, conditional TLR4-deficient mice in B cells (CD19-Cre or mb1-Cre(48, 49)), T-helper cells (CD4-Cre(50)), dendritic cells (CD11c-Cre(51, 52)), and myeloid cells (LysM-Cre(53, 54)) would be required, which is beyond the scope of this study. Adoptive transfer of sorted cells into B6.129S2-Ighmtm1Cgn/J (μMT) mice is an alternative approach for questions of B cell intrinsic responses; however, this would require sorting of B cells without activating them, and then transfer of purified cells into neonatal mice to elicit development of proper lymphoid architecture(55, 56). Kreuk et al. showed adoptive transfer of TLR2−/−TLR4−/−Unc93b13d/3d CD19+ cells into 2-day old μMT mice led to a decreased B-1a-derived serum IgM compared to μMT mice that received WT CD19+ B cells, despite comparable frequencies of CD19+ donor B cells in the recipient mice, and other responses were intact(55). In contrast, μMT mice reconstituted with B cells later in life are highly dependent on TLR4 for IgG responses(57). B cell-derived cytokines were also found to be essential in studies of mixed bone marrow chimeric mice for development of both innate and adaptive immune responses (reviewed in 56).
Additional cell types that are potentially involved in mediating E6020’s adjuvant effect include macrophages, and particularly subcapsular macrophages, that are exquisitely sensitive to TLR4 stimulation and enhance humoral immune responses through IL-18 secretion(32). In addition, dendritic cells are very responsive to LPS stimulation through TLR4/MD-2 along with CD11b to prime T cells efficiently(58). T-helper cells are instrumental in full activation of T-dependent humoral responses and are affected by the activation status of dendritic cells and the cytokine milieu. Follicular dendritic cells have only recently been shown to respond to TLR4, markedly upregulating their transfer of antigen to cognate B cells(59), a process that may be suboptimal in TLR4-SNP mice. In Heester et al., B cells did not increase uptake of antigen in response to MPL stimulation directly(59); thus, we would not expect antigen uptake to differ in WT and TLR4-SNP B cells in response to E6020.
Most B cell subsets express TLR4 and can be activated directly as well. Pone et al. noted no differences in ex vivo B cell expansion and class switch recombination to IgG1 whether they started with purified B cells or total splenocytes (red blood cell depleted) in their recent experiments showing synergistic B cell activation between TLR4 and BCR stimulation (anti-IgM)(60). The more innate-like B-1a and marginal zone subsets are known for their rapid activation in response to TLR adjuvants and, in principle, can undergo immunoglobulin class switching in response to T-dependent antigen, though this is a rare event(61–64). IgM-responses in our experiments follow patterns of accelerated kinetics previously observed in marginal zone B cell responses(65). Marginal zone and follicular B cells, especially during germinal center reaction, respond to ex vivo TLR4 stimulation(46, 65). IgG2c class switching and not total IgG titers has previously been shown to have a B-cell intrinsic MyD88-dependence(46, 57).
Stimulation of TLR4 is also a primary activator of regulatory B cells, leading to production of IL-10(reviewed in 66). E6020-induced regulatory B cell-derived IL-10 may account for increased isotype switching (IgG1 to IgG2c) following immunization with NP-Ova in WT mice. Th2 responses (IgG1) are exquisitely sensitive to IL-10-mediated suppression(67). In our study using a T-dependent antigen, the presence of E6020 increased the Th1 response (IgG2c) in WT mice to a greater extent than in TLR4-SNP mice. Indeed, TLR4-SNP mice also exhibit enhanced allergic responses to house dust mites, though they were less susceptible to inhaled Ova + LPS-induced allergic inflammation(68). Regulatory B cell-derived IL-10 may also be involved in the isotype switching following immunization with NP-Ova + E6020 in the TLR4-sufficient mice, particularly in the subset of mice that lose IgG2c after booster immunization (Figure 4).
E6020 is a strictly TLR4-dependent adjuvant(11). Compared to LPS stimulation, TLR4 responses to E6020 are somewhat attenuated for the MyD88-dependent pathway than for the TRIF-dependent pathway, and yet E6020 induces a considerably more potent TRIF-dependent response than MPL(20). The TRIF pathway is thought to be especially important for B cell division and class switch recombination(69). Indeed, we see the result of class switch recombination in WT mice (with adjuvant), but in TLR4-SNP mice, class switching to IgG2c lags behind (Figure 4). The capacity of E6020 to promote a sustained memory response in WT vs. TLR4-SNP mice is also indicative of the role of TLR4 signaling in this process. Notably, the responses were sustained in the absence of additional exogenous antigen stimulation(70, 71), indicating that early B cell dynamics(72) may have long-ranging effects.
Our data suggests that for the immunization of individuals expressing the TLR4 D299G/T399I mutations, TLR4-based adjuvants may not provide the same degree of enhanced humoral immune responses that would be expected to be induced in fully TLR4-responsive individuals. Nonetheless, since the inheritance of the TLR4 SNPs in humans is most frequently heterozygous(21–23), coupled with the observation that expression of TLR4 in individual B cells is monoallelically expressed(73), a significant degree of adjuvanticity elicited by E6020 is still expected in humans expressing these SNPs. Future studies in humans will be required to determine if inheritance of these SNPs affects the efficacy of TLR4-based adjuvants.
Supplementary Material
Key Points.
WT and TLR4-SNP mice respond comparably to T-dependent & -independent antigens alone.
TLR4-SNP mice exhibit reduced antibody titers to NP-Ova and TLR4 adjuvant (E6020).
TLR4-SNP mice exhibit reduced isotype class switching to NP-Ova and E6020.
Acknowledgements
Flow cytometry experiments were performed at the Flow Cytometry and Mass Cytometry Core Facility of the University of Maryland School of Medicine Center for Innovative Biomedical Resources (CIBR), Baltimore, Maryland (RRID:SCR_018835).
Grant support:
This study was supported in part by NIH grants AI125215 and AI123371 (to SNV) and U19-AI082655 (to MBS). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CT
cycle threshold (RT-PCR)
- MD-2
myeloid differentiating factor 2
- MPL
monophosphoryl lipid A
- NP
nitrophenyl (hapten)
- SFC
spot-forming cell (ELISpot)
- SNP
single nucleotide polymorphism
- TLR4-SNP
mice co-expressing mutations D298G/N397I in TLR4
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- WT
wild type (C57/BL6J)
References
- 1.Akira S, Hoshino K, and Kaisho T. 2000. The role of Toll-like receptors and MyD88 in innate immune responses. J. Endotoxin Res 6(5):383–387. [PubMed] [Google Scholar]
- 2.Fitzgerald KA, and Kagan JC. 2020. Toll-like Receptors and the Control of Immunity. Cell. 180(6):1044–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R 2008. Origin and physiological roles of inflammation. Nature. 454(7203):428–435. [DOI] [PubMed] [Google Scholar]
- 4.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S, and Hartmann G. 2002. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol 168(9):4531–4537. [DOI] [PubMed] [Google Scholar]
- 5.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, and Medzhitov R. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol 2(10):947–950. [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, and Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature.438(7066):364–368. [DOI] [PubMed] [Google Scholar]
- 7.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, and Nemazee D. 2006. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 314(5807):1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palm NW, and Medzhitov R. 2009. Immunostimulatory activity of haptenated proteins. Proc. Natl. Acad. Sci. U. S. A 106(12):4782–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JX, Tseng JC, Yu GY, Luo Y, Huang CF, Hong YR, and Chuang TH. 2022. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics. 14(2):423: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowen WS, Gandhapudi SK, Kolb JP, and Mitchell TC. 2013. Immunopharmacology of lipid A mimetics. Adv. Pharmacol 66:81–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizaka ST and Hawkins LD. 2007. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev. Vaccines 6(5):773–784. [DOI] [PubMed] [Google Scholar]
- 12.Haensler J, Probeck P, Su J, Piras F, Dalençon F, Cotte JF, Chambon V, Iqbal SM, Hawkins L, and Burdin N. 2015. Design and preclinical characterization of a novel vaccine adjuvant formulation consisting of a synthetic TLR4 agonist in a thermoreversible squalene emulsion. Int. J. Pharm 486(1–2):99–111. [DOI] [PubMed] [Google Scholar]
- 13.Morefield GL, Hawkins LD, Ishizaka ST, Kissner TL, and Ulrich RG. 2007. Synthetic Toll-like receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome. Clin. Vaccine Immunol 14(11):1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baudner BC, Ronconi V, Casini D, Tortoli M, Kazzaz J, Singh M, Hawkins LD, Wack A, and O’Hagan DT. 2009. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm. Res 26(6):1477–1485. [DOI] [PubMed] [Google Scholar]
- 15.Singh M, Kazzaz J, Ugozzoli M, Baudner B, Pizza M, Giuliani M, Hawkins LD, Otten G, and O’Hagan DT. 2012. MF59 oil-in-water emulsion in combination with a synthetic TLR4 agonist (E6020) is a potent adjuvant for a combination Meningococcus vaccine. Hum. Vaccin. Immunother 8(4):486–490. [DOI] [PubMed] [Google Scholar]
- 16.Visan L, Sanchez V, Kania M, de Montfort A, de la Maza LM, and Ausar SF. 2016. Phosphate substitution in an AlOOH – TLR4 adjuvant system (SPA08) modulates the immunogenicity of Serovar E MOMP from Chlamydia trachomatis. Hum. Vaccin. Immunother 12(9):2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seid CA, Jones KM, Pollet J, Keegan B, Hudspeth E, Hammond M, Wei J, McAtee CP, Versteeg L, Gutierrez A, Liu Z, Zhan B, Respress JL, Strych U, Bottazzi ME, and Hotez PJ. 2017. Cysteine mutagenesis improves the production without abrogating antigenicity of a recombinant protein vaccine candidate for human chagas disease. Hum. Vaccin. Immunother 13(3):621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones K, Versteeg L, Damania A, Keegan B, Kendricks A, Pollet J, Cruz-Chan JV, Gusovsky F, Hotez PJ, and Bottazzi ME. 2018. Vaccine-Linked Chemotherapy Improves Benznidazole Efficacy for Acute Chagas Disease. Infect. Immun 86(4):e00876–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora R, Haile CN, Kosten TA, Wu Y, Ramakrishnan M, Hawkins LD, Orson FM, and Kosten TR TR. 2019. Preclinical efficacy of an anti-methamphetamine vaccine using E6020 adjuvant. Am. J. Addict 28(2):119–126. [DOI] [PubMed] [Google Scholar]
- 20.Richard K, Perkins DJ, Harberts EM, Song Y, Gopalakrishnan A, Shirey KA, Lai W, Vlk A, Mahurkar A, Nallar S, Hawkins LD, Ernst RK, and Vogel SN. 2020. Dissociation of TRIF bias and adjuvanticity. Vaccine. 38(27):4298–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, Hemming VG, Blanco JC, and Vogel SN. 2007. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J. Immunol 179(5):3171–3177. [DOI] [PubMed] [Google Scholar]
- 22.Ferwerda B, McCall MB, Alonso S, Giamarellos-Bourboulis EJ, Mouktaroudi M, Izagirre N, Syafruddin D, Kibiki G, Cristea T, Hijmans A, Hamann L, Israel S, ElGhazali G, Troye-Blomberg M, Kumpf O, Maiga B, Dolo A, Doumbo O, Hermsen CC, Stalenhoef AF, van Crevel R, Brunner HG, Oh DY, Schumann RR, de la Rúa C, Sauerwein R, Kullberg BJ, van der Ven AJ, van der Meer JW, and Netea MG. 2007. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. U S A 104(42):16645–16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schröder NW and Schumann RR. 2005. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect. Dis 5(3):156–164. [DOI] [PubMed] [Google Scholar]
- 24.Richard K, Piepenbrink KH, Shirey KA, Gopalakrishnan A, Nallar S, Prantner DJ, Perkins DJ, Lai W, Vlk A, Toshchakov VY, Feng C, Fanaroff R, Medvedev AE, Blanco JCG, and Vogel SN. 2021. A mouse model of human TLR4 D299G/T399I SNPs reveals mechanisms of altered LPS and pathogen responses. J. Exp. Med 18(2):e20200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rallabhandi P, Awomoyi A, Thomas KE, Phalipon A, Fujimoto Y, Fukase K, Kusumoto S, Qureshi N, Sztein MB, and Vogel SN. 2008. Differential activation of human TLR4 by Escherichia coli and Shigella flexneri 2a lipopolysaccharide: combined effects of lipid A acylation state and TLR4 polymorphisms on signaling. J. Immunol 180(2):1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirey KA, Cole LE, Keegan AD, and Vogel SN. 2008. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol 181(6):4159–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, Cuesta N, and Vogel SN. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol 176(11):6888–6899. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ and Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 29.Richard K, Pierce SK, and Song W. 2008. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J. Immunol 181(3):1746–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahid R, Kotloff KL, Levine MM, and Sztein MB. 2019. Cell mediated immune responses elicited in volunteers following immunization with candidate live oral Salmonella enterica serovar Paratyphi A attenuated vaccine strain CVD 1902. Clin. Immunol 201:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox CB, Moutaftsi M, Vergara J, Desbien AL, Nana GI, Vedvick TS, Coler RN, and Reed SG. 2013. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine. 31(49):5848–5955. [DOI] [PubMed] [Google Scholar]
- 32.Desbien AL, Reed SJ, Bailor HR, Dubois Cauwelaert N, Laurance JD, Orr MT, Fox CB, Carter D, Reed SG, and Duthie MS. 2015. Squalene emulsion potentiates the adjuvant activity of the TLR4 agonist, GLA, via inflammatory caspases, IL-18, and IFN-γ. Eur. J. Immunol 45(2):407–417. [DOI] [PubMed] [Google Scholar]
- 33.Fields ML, Metzgar MH, Hondowicz BD, Kang SA, Alexander ST, Hazard KD, Hsu AC, Du YZ, Prak EL, Monestier M, and Erikson J. 2006. Exogenous and endogenous TLR ligands activate anti-chromatin and polyreactive B cells. J. Immunol 176(11):6491–6502. [DOI] [PubMed] [Google Scholar]
- 34.Ueda Y, Liao D, Yang K, Patel A, and Kelsoe G. 2007. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J. Immunol 178(6):3593–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber JR 2012. Role of Toll like receptors in the antibody response to encapsulated bacteria. Front. Biosci 4(7):2638–2646. [DOI] [PubMed] [Google Scholar]
- 36.Pone EJ, Xu Z, White CA, Zan H, and Casali P. 2012. B cell TLRs and induction of immunoglobulin class-switch DNA recombination. Front. Biosci 17(7):2594–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pone EJ, Lou Z, Lam T, Greenberg ML, Wang R, Xu Z, Casali P. 2015. B cell TLR1/2, TLR4, TLR7 and TLR9 interact in induction of class switch DNA recombination: modulation by BCR and CD40, and relevance to T-independent antibody responses. Autoimmunity. 48(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maglione PJ, Simchoni N, Black S, Radigan L, Overbey JR, Bagiella E, Bussel JB, Bossuyt X, Casanova JL, Meyts I, Cerutti A, Picard C, and Cunningham-Rundles C. 2014. IRAK-4 and MyD88 deficiencies impair IgM responses against T-independent bacterial antigens. Blood. 124(24):3561–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tulic MK, Hurrelbrink RJ, Prêle CM, Laing IA, Upham JW, Le Souef P, Sly PD, and Holt PG. 2007. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J. Immunol 179(1):132–140. [DOI] [PubMed] [Google Scholar]
- 40.Hajjar AM, Ernst RK, Yi J, Yam CS, and Miller SI. 2017. Expression level of human TLR4 rather than sequence is the key determinant of LPS responsiveness. PLoS One. 12(10):e0186308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindblad EB 2000. Freund’s Adjuvants. In: Methods in Molecular Medicine, Vol. 42: Vaccine Adjuvants: Preparation Methods and Research Protocols. O’Hagan DT, ed. Humana Press, Inc., Totowa, NJ. p. 49–63. [Google Scholar]
- 42.Moreira LO, Smith AM, DeFreitas AA, Qualls JE, El Kasmi KC, and Murray PJ. 2008. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2. Vaccine. 26(46):5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casella CR and Mitchell TC. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol. Life Sci 65(20):3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, and Yamasaki S. 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med 206(13):2879–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzavecchia A and Sallusto F. 2007. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr. Opin. Immunol 19(3):268–274. [DOI] [PubMed] [Google Scholar]
- 46.Meyer-Bahlburg A, Khim S, and Rawlings DJ. 2007. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J. Exp. Med 204(13):3095–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng S, Wang H, and Zhou H. 2016. The role of TLR4 on B Cell activation and anti-beta 2GPI antibody production in the antiphospholipid syndrome. J. Immunol. Res 2016:1719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rickert RC, Roes J, and Rajewsky K. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25(6):1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, and Reth M. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. U. S. A 103(37):13789–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Pérez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, and Wilson CB. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 15(5): 763–774. [DOI] [PubMed] [Google Scholar]
- 51.Caton ML, Smith-Raska MR, and Reizis B. 2007. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med 204(7):1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou B, Reizis B, and DeFranco AL. 2008. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 29(2):272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, and Akira S. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 10(1):39–49. [DOI] [PubMed] [Google Scholar]
- 54.Clausen BE, Burkhardt C, Reith W, Renkawitz R, and Förster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8(4):265–277. [DOI] [PubMed] [Google Scholar]
- 55.Kreuk LS, Koch MA, Slayden LC, Lind NA, Chu S, Savage HP, Kantor AB, Baumgarth N, and Barton GM. 2019. B cell receptor and Toll-like receptor signaling coordinate to control distinct B-1 responses to both self and the microbiota. Elife. 8:e47015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeFranco AL, Rookhuizen DC, and Hou B. 2012. Contribution of Toll-like receptor signaling to germinal center antibody responses. Immunol. Rev 247(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, and Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 470(7335):543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, Scott D, Franzoso G, Cook HT, and Botto M. 2014. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat. Commun 5:3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heesters BA, van Megesen K, Tomris I, de Vries RP, Magri G, and Spits H. 2021. Characterization of human FDCs reveals regulation of T cells and antigen presentation to B cells. J. Exp. Med 218(10):e20210790: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pone EJ, Hernandez-Davies JE, Jan S, Silzel E, Felgner PL, and Davies DH. 2022. Multimericity Amplifies the Synergy of BCR and TLR4 for B Cell Activation and Antibody Class Switching. Front. Immunol 13:882502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendricks J, Visser A, Dammers PM, Burgerhof JG, Bos NA, and Kroese FG. 2011. Class-switched marginal zone B cells in spleen have relatively low numbers of somatic mutations. Mol. Immunol 48(6–7):874–882. [DOI] [PubMed] [Google Scholar]
- 62.Phan TG, Gardam S, Basten A, and Brink R. 2005. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. J. Immunol 174(8):4567–4578. [DOI] [PubMed] [Google Scholar]
- 63.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, and Baumgarth N. 2017. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J. Exp. Med 214(9):2777–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch MA, Reiner GL, Lugo KA, Kreuk LS, Stanbery AG, Ansaldo E, Seher TD, Ludington WB, and Barton GM. 2016. Maternal IgG and IgA Antibodies Dampen Mucosal T Helper Cell Responses in Early Life. Cell. 165(4):827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubtsov AV, Swanson CL, Troy S S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008. Mar 15;180(6):3882–8. doi: 10.4049/jimmunol.180.6.3882. [DOI] [PubMed] [Google Scholar]
- 66.Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, and van de Veen W. 2021. Regulatory B cells, A to Z. Allergy. 76(9):2699–2715. [DOI] [PubMed] [Google Scholar]
- 67.Brosseau C, Durand M, Colas L, Durand E, Foureau A, Cheminant MA, Bouchaud G, Castan L, Klein M, Magnan A, and Brouard S. 2018. CD9+ Regulatory B Cells Induce T Cell Apoptosis via IL-10 and Are Reduced in Severe Asthmatic Patients. Front. Immunol 9:3034: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fink MY, Qi X, Shirey KA, Fanaroff R, Chapoval S, Viscardi RM, Vogel SN, and Keegan AD. 2022. Mice Expressing Cosegregating Single Nucleotide Polymorphisms (D298G and N397I) in TLR4 Have Enhanced Responses to House Dust Mite Allergen. J. Immunol 208(9):2085–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanagibashi T, Nagai Y, Watanabe Y, Ikutani M, Hirai Y, and Takatsu K. 2015. Differential requirements of MyD88 and TRIF pathways in TLR4-mediated immune responses in murine B cells. Immunol. Lett 163(1):22–31. [DOI] [PubMed] [Google Scholar]
- 70.Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L, Jin H, and Woo J. 2012. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS One. 7(12):e51618: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, and Garçon N. 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol 183(10):6186–6197. [DOI] [PubMed] [Google Scholar]
- 72.Barrio L, Saez de Guinoa J, and Carrasco YR. 2013. TLR4 signaling shapes B cell dynamics via MyD88-dependent pathways and Rac GTPases. J. Immunol 191(7):3867–3875. [DOI] [PubMed] [Google Scholar]
- 73.Pereira JP, Girard R, Chaby R, Cumano A, and Vieira P. 2003. Monoallelic expression of the murine gene encoding Toll-like receptor 4. Nat. Immunol 4(5):464–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


