Abstract
Aspergillus fumigatus (AF) is an important opportunistic fungal pathogen and causes invasive pulmonary aspergillosis in conditions with compromised innate antifungal immunity, including chronic granulomatous disease, which results from inherited deficiency of the superoxide-generating leukocyte NADPH oxidase 2 (NOX2). Derivative oxidants have both anti-microbial and immunoregulatory activity, and in the context of AF, contribute to both fungal killing and dampening inflammation induced by fungal cell walls. As the relative roles of macrophage vs neutrophil NOX2 in the host response to AF are incompletely understood, we studied mice with conditional deletion of NOX2. When NOX2 was absent in alveolar macrophages (AM) as a result of LysM-Cre-mediated deletion, germination of inhaled AF conidia was increased. Reducing NOX2 activity specifically in neutrophils via S100a8 (MRP8)-Cre also increased fungal burden, which was inversely proportional to the level of neutrophil NOX2 activity. Moreover, diminished NOX2 in neutrophils synergized with corticosteroid immunosuppression to impair lung clearance of AF. Neutrophil-specific reduction in NOX2 activity also enhanced acute inflammation induced by inhaled sterile fungal cell walls. These results advance understanding into cell-specific roles of NOX2 in the host response to AF. We show that alveolar macrophage NOX2 is a non-redundant effector that limits germination of inhaled AF conidia. In contrast, reducing NOX2 activity only in neutrophils is sufficient to enhance inflammation to fungal cell walls as well as to promote invasive AF. This may be relevant in clinical settings with acquired defects in NOX2 activity due to underlying conditions, which overlap risk factors for invasive aspergillosis.
Introduction
Aspergillus fumigatus (AF) conidia are ubiquitous in the environment, and humans inhale up to thousands of AF conidia daily, which are readily cleared in immunocompetent hosts (1). However, in patients with suppressed fungicidal innate immunity, failure to eliminate inhaled conidia and the hyphae that emerge upon germination results in invasive pulmonary aspergillosis (IPA). Mortality remains high despite development of more potent antifungal agents (1, 2). Risk factors include prolonged neutropenia during treatment of myeloid leukemia, impaired leukocyte function from corticosteroid use following allogeneic marrow or solid organ transplant, and primary immunodeficiencies with phagocyte function defects, particularly chronic granulomatous disease (CGD), where NADPH oxidase 2 (NOX2)-dependent reactive oxygen species (ROS) are not produced (1–3). Invasive aspergillosis is increasing in new groups of patients, which include COPD, critically ill patients with underlying co-morbidities or following influenza or SARS-CoV2, and patients with lymphoid malignancies receiving kinase inhibitors (1, 3–5).
Alveolar macrophages and neutrophils are crucial for the host response to inhaled conidia (6–8). Dormant conidia are detected by lung epithelial cells and resident alveolar macrophages (AM) to trigger ingestion and early production of inflammatory mediators, initiating neutrophil recruitment to the lung within hours. In mice, alveolar macrophages are sufficient to eliminate conidia unless their function is compromised, for example, due to corticosteroids (9, 10). However, large inocula exceed the capacity for AM control even in immunocompetent mice (9), and after inhalation of millions of conidia, an influx of neutrophil in the first hours is crucial to prevent IPA (11). Neutrophils ingest and kill conidia(12) as well as eradicate emerging hyphae. Moreover, if functional neutrophils are present, AM are dispensable to prevent IPA (11).
The role of NOX2 in the host response to AF has long been recognized because of its association with CGD, where IPA remains a major clinical problem (13). CGD results from inactivating X-linked or recessive mutations in genes encoding subunits of the NOX2 enzyme complex (14). The absence of NOX2-derived ROS results in recurrent bacterial and fungal infections as well as aberrant inflammation, reflecting their importance not only for killing microbes but their influence on redox-regulated cellular processes that limit inflammation (14, 15). Even sterile fungal cell walls induce neutrophilic hyperinflammation in CGD mice (16–19), and fungal infections in CGD are often accompanied by pyogranulomatous abscesses that can complicate treatment (13, 15).
As a first line of defense, alveolar macrophages and neutrophils possess both non-oxidative and oxidative pathways to eliminate AF. Conidia are ingested and killed within phagosomes. The highly acidic environment of macrophage phagosomes is conidiacidal (20), augmented by NOX2-derived ROS (10, 12, 21). Mitochondria- and copper-derived ROS may also contribute (22, 23). In neutrophils, ingested conidia can be eradicated by non-oxidative mechanisms, including sequestration of iron (24–26); NOX2 can also enhance neutrophil killing of conidia (12, 27). Due to their size, hyphae are killed by extracellular means. This is strongly dependent on neutrophils and NOX2 ROS, which synergizes with MPO and other granule proteins to kill hyphae (26, 28–30).
Despite the importance of NOX2 in the host response to AF, the relative contributions of neutrophil and macrophage NOX2 are not fully resolved. Some suggest effective killing of conidia by murine CGD AM (16, 31, 32), but others found conidiacidal activity was markedly reduced in vitro and/or in vivo (10, 33). Neutrophil NOX2 ROS are essential to kill AF hyphae and prevent progressive infection in mouse cornea or zebrafish (28, 34). However, control of AF in these tissues may not fully reflect the host response in the lung, where neutrophils are rapidly deployed and can eliminate conidia in the absence of NOX2 (12, 35). The impact of reduced but not absent NOX2 activity is also not well understood. Diminished neutrophil NOX2 activity from loss of type 1 interferon signaling or following ethanol exposure increases susceptibility to IPA in mice, but other associated functional defects may also contribute (36, 37). Excessive lung inflammation elicited by fungal cell walls is promoted by inflammatory mediators produced by CGD neutrophils (17, 18) but may also involve other dysregulated leukocytes.
In this study, we took a genetic approach to better define cell-specific roles of NOX2 in responses to pulmonary AF and developed mice with conditional deletion of NOX2 in macrophages and/or neutrophils. We showed that alveolar macrophage NOX2 had a non-redundant role to limit germination of AF conidia in the first 24 hours after inhalation when conidia numbers were not overwhelming. The level of neutrophil NOX2 activity was a key determinant for clearance of larger inocula, and even without complete loss of NOX2, reducing NOX2 activity was sufficient to increase lung AF burden. Deficient neutrophil NOX2 activity also impaired clearance of AF in corticosteroid-immunosuppressed mice. Finally, we showed that neutrophil rather than macrophage NOX2 played a dominant role in limiting excessive lung inflammation to fungal cell walls. That even partial loss of NOX2 activity results in impaired control of AF may have relevance to clinical settings where NOX2 activity is depressed, which could increase the risk of developing invasive or chronic AF infection.
Methods.
Mice
Mice were maintained in specific pathogen-free conditions and all experiments were approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis. Age-matched male and female mice were used in experiments between the ages of 10 to 20 weeks. Wild type (WT) C57BL/6J were purchased from Jackson Laboratory or bred in-house. X-linked CGD (CybbKO) (38) and Ncf2−/− (Ncf2KO) (39) CGD mice were from in-house colonies, as were Ncf2fl/fl mice that have loxP sites flanking Ncf2 exon 3, and Ncf2fl/flLysMWT/Cre mice, referred to as Ncf2LysMCre (40).
We also bred Ncf2fl/fl mice to a neutrophil-directed Cre strain, B6.Cg-Tg(S100A8-cre,-EGFP)1Ilw/J (41) (JAX stock 021614) (Figure S1A). We took advantage of transient embryonic expression of Cre from the S100A8-Cre transgene (42) to identify mice with global deletion of exon 3 to generate Ncf2−Ex3/−Ex3 S100A8-Cre mice. These were crossed with Ncf2fl/fl mice to produce NCF2fl/-Ex3S100A8-Cre mice, denoted as Ncf2S100A8Cre. To generate mice with an X-linked Cybb allele with loxP sites flanking exon 5, C57BL6/J oocytes were fertilized with C57BL/6N-Cybbtm2a(KOMP)Wtsi/H sperm (UKRI-MRC Harwell) and heterozygous female offspring were bred to B6.129S4-Gt(ROSA)26Sortm1(FLP1)Dym/JRainJ mice(43) (JAX stock 009086). These mice were crossed with B6.Cg-Tg(S100A8-cre,-EGFP)1Ilw/J to produce Cybbfl/YS100A8-Cre and Cybbfl/flS100A8-Cre mice (CybbS100A8Cre mice) (Figure S1B). Ncf2fl/fl and Cybbfl/fl mice were crossed to generate Ncf2fl/fl Cybbfl/fl mice. These were bred to B6.Cg-Tg(S100A8-cre,-EGFP)1Ilw/J to produce “double-floxed” Ncf2fl/flCybbfl/flS100A8-Cre mice, referred to as Ncf2CybbS100A8Cre and maintained by crossing Ncf2fl/fl Cybbfl/fl and Ncf2CybbS100A8Cre mice.
Reagents and Cell Isolation
Reagents were from Sigma Aldrich unless indicated. Flow cytometry antibodies were from BD Biosciences unless noted, and data collected on Cytek-modified FACScan instrument and analyzed with FlowJo. Neutrophils were purified from bone marrow cells by non-adherence, and in some experiments, followed by the EasySep Mouse Neutrophil Enrichment Kit (STEMCELL Technologies)(18, 39). Bone marrow monocytes, peripheral blood mononuclear cells, resident peritoneal macrophages, and alveolar macrophages were also isolated as described (39, 40).
NADPH oxidase activity
Neutrophil NADPH oxidase activity was monitored by flow cytometry using dihydrorhodamine 123 (DHR) with phorbol 12-myristate 13-acetate (PMA) stimulation, or by Cytochrome C reduction on a Spectramax 340PC (Molecular Devices) (39, 44). The DHR assay was used to calculate the stimulation index of peripheral blood Ly6C+CD115+CD11b+ monocytes as the ratio of the MFI for PMA-stimulated cells to unstimulated cells (45). In resident alveolar and peritoneal macrophages, NADPH oxidase activity was assessed following stimulation with serum-opsonized zymosan in the presence of nitroblue tetrazolium (NBT) (40).
Western Blots
Cell lysates were electrophoresed for Western blots and probed with antibodies to assess expression of mouse NCF2 (p67phox)(Abcam), mouse CYBB (clone 54.1(46), Santa Cruz Biotechnology) and β-actin (Cell Signaling Technologies), as described (39, 47, 48).
LTB4 production by zymosan-stimulated neutrophils
Purified bone marrow neutrophils at 4×106/ml were stimulated for one hour with zymosan (MOI=1:2) in RPMI1640 (0.4 mM Ca2+) and LTB4 measured by ELISA (Cayman Chemicals) (18).
Sterile inflammation
Zymosan (20 mcg) was administered by intranasal (IN) instillation and mice euthanized after 18hours for analysis of BAL cell counts, leukocyte differential on cytospins, and lung histology (18). Sterile peritoneal inflammation was induced by intraperitoneal (IP) injection of 1 mL of 5mM sodium meta-periodate (48) and mice euthanized after 72 hours. Peritoneal cavities were lavaged to determine cell counts and leukocyte differential by cytospin. For IL-1α neutralization experiments, mice received 0.5mg of anti-IL-1α or isotype control antibody (BioXCell) retro-orbitally one hour prior to induction of peritonitis (48).
A. fumigatus pneumonia
A. fumigatus strains AF10 (clinical isolate 90240; ATCC) or AF293 (MYA-4609, ATCC; obtained from Nancy Keller, University of Wisconsin) were grown on minimal glucose media (MGM) plates using an overlay method for 3 days at 37°C (22) and conidia were harvested in PBS by gentle scraping with a cell spatula, passed through two layers of sterile Miracloth and a 40μM strainer (39). Conidia were enumerated using a hemocytometer and numbers subsequently verified by plate culture for CFU. Mice were anesthetized by IP ketamine and challenged IN with indicated doses of conidia in 25μl of sterile PBS. In some experiments, mice received 200mg/kg freshly prepared cortisone acetate IP on day −3, 0 (day of challenge) and day 4. Mice were euthanized at the indicated times and 1mL BAL collected for cell counts, cytospins for leukocyte differential and colony forming unit (CFU). The left lung was processed for histology and staining with H&E and with Gomori methamine silver (GMS) (39). Images were captured with a NanoZoomer digital slice scanner and NDP.view2 software (Hamamatsu Photonics, Japan). The right lung was homogenized in 500 μl PBS. For fungal burden, aliquots of BAL and lung homogenates were each plated on MGM for CFU analysis. ELISAs on BAL samples were performed using ProcartaPlex panels from Thermo Fisher Scientific.
Analysis of in vivo germination of A. fumigatus conidia
Mice were challenged with 5×104 AF293 conidia by IN administration. Mice were treated by IP injection with either a double-antibody-based strategy using 200 μg anti-Ly6G (day −2 and just before challenge on D0) and 100 μg anti-rat-kappa immunoglobulin (day −1) to produce neutropenia or with 100 μg isotype control on Day −2 and D0 (clones 1A8, MAR18.5, or clone 2A3, respectively; BioXcell) (18, 49, 50). Peripheral blood was obtained by retro-orbital puncture at time of challenge (day 0) and at 24 hpi to determine the absolute neutrophil count (ANC), based on the complete blood count determined by a Hemavet 950S (Drew Scientific) and the percentage of neutrophils on Wright-Giemsa-stained blood smears. At 24 hpi, 1mL BAL was collected for cell counts, Wright-Giemsa-stained cytospins for leukocyte differential and analysis of hyphae, and CFU. In some experiments, the right lung was homogenized and also analyzed for CFU. BAL cytospins were photographed with a Zeiss AxioSkope (Carl Zeiss), and hyphae lengths determined using the Zen Black software (Carl Zeiss) measurement tool. To assess hyphae number in BAL samples, 700uL from each sample was centrifuged in an Eppendorf tube, resuspended in 900ul PBS with two drops of ORTHO BSA (ORTHO Clinical Diagnostics), and equal volumes cytospun onto 3 slides per animal. Hyphae numbers were hand-counted on each slide under 40X magnification, and the total number of hyphae per mouse from 1 ml BAL fluid calculated.
Phagocytosis of A. fumigatus conidia
Mice were challenged IN with either saline or 5×106 conidia prepared from the YFP-expressing AF293.1 strain TBK1.1 (51) (obtained from Nancy Keller). After 2 hours, mice were euthanized and BAL was obtained by five sequential one ml lavage with ice cold PBS with 2mM EDTA and 2% FBS. AM were identified as CD45+(BV 450, clone: 30-F11) SiglecF+ (PE, clone: E50–2440) and CD11c+(PE Cy™7clone: HL3). To quench any extracellular conidia adherent to AM, 0.1% Trypan blue was added, and AM gated for YFP to assess the percentage of AM with ingested conidia.
Statistics
Statistical analyses used GraphPad Prism 9.0 (GraphPad Software). A P value of <0.05 was considered statically significant. For normally distributed data, either the Student’s t-test to compare two groups or one-way ANOVA with Tukey’s multiple comparisons test were used. Frequency testing used the Chi-squared test. Non-parametric data was evaluated using the Kolmogorov-Smirnov test and Mann-Whitney U test.
Results
Development of mice with macrophage and/ or neutrophil conditional deletion of NOX2 subunits
To investigate the impact of cell-type-specific NOX2 deficiency, we developed mice with conditional deletion of NOX2 subunit genes Ncf2 and/or Cybb. We previously produced Ncf2LysMCre mice by crossing mice with a conditional loss-of-function Ncf2 allele (Ncf2fl/fl) to LyzM (LysM)-Cre(52), which deletes Ncf2 and NOX2 activity in ≈95% of Ncf2LysMCre resident alveolar and peritoneal macrophages (40). Ncf2 deletion in Ncf2LysMCre neutrophils is less efficient, with only a ≈two-fold reduction in expression and NOX2 activity (40). To target NOX2 expression specifically in neutrophils, we crossed mice with a S100A8-Cre (MRP8-Cre) transgene (41) to Ncf2fl/fl (Figure S1A) or Cybbfl/fl mice (Figure S1B). Ncf2S100A8Cre neutrophils retained partial NOX2 activity by dihydrorhodamine (DHR) fluorescence, although more reduced compared to Ncf2LysMCre (Figure 1A). Reflecting these results, superoxide production measured by cytochrome c assay averaged 50% and 20% of WT for Ncf2LysMCre and Ncf2S100A8Cre neutrophils, respectively (Figure 1B). CybbS100A8Cre neutrophils also had residual NOX2 activity (Figure 1A). We therefore developed “double-floxed” Ncf2CybbS100A8Cre mice that harbored floxed alleles of both Ncf2 and Cybb. DHR fluorescence was further reduced in Ncf2CybbS100A8Cre neutrophils (Figure 1A), and by cytochrome c assay, superoxide production was only ≈10% of WT (Figure 1C). These results correlated with reduced NCF2 and CYBB levels on Western blots (Figure S1C, D). As anticipated, resident macrophages had intact NOX2 activity and subunit expression in S100A8-Cre mice, in contrast to Ncf2LysMCre (Figure 1D, Figure S1E). Finally, Ncf2S100A8Cre and Ncf2CybbS100A8Cre monocytes had normal NOX2 activity (Figure 1E) and expression of NCF2 and CYBB (Figure S1F), respectively, similar to Ncf2LysMCre monocytes (40).
Figure 1. Conditional deletion of Ncf2 and Cybb NOX2 subunits and characterization of expression and NOX2 activity in phagocytes.
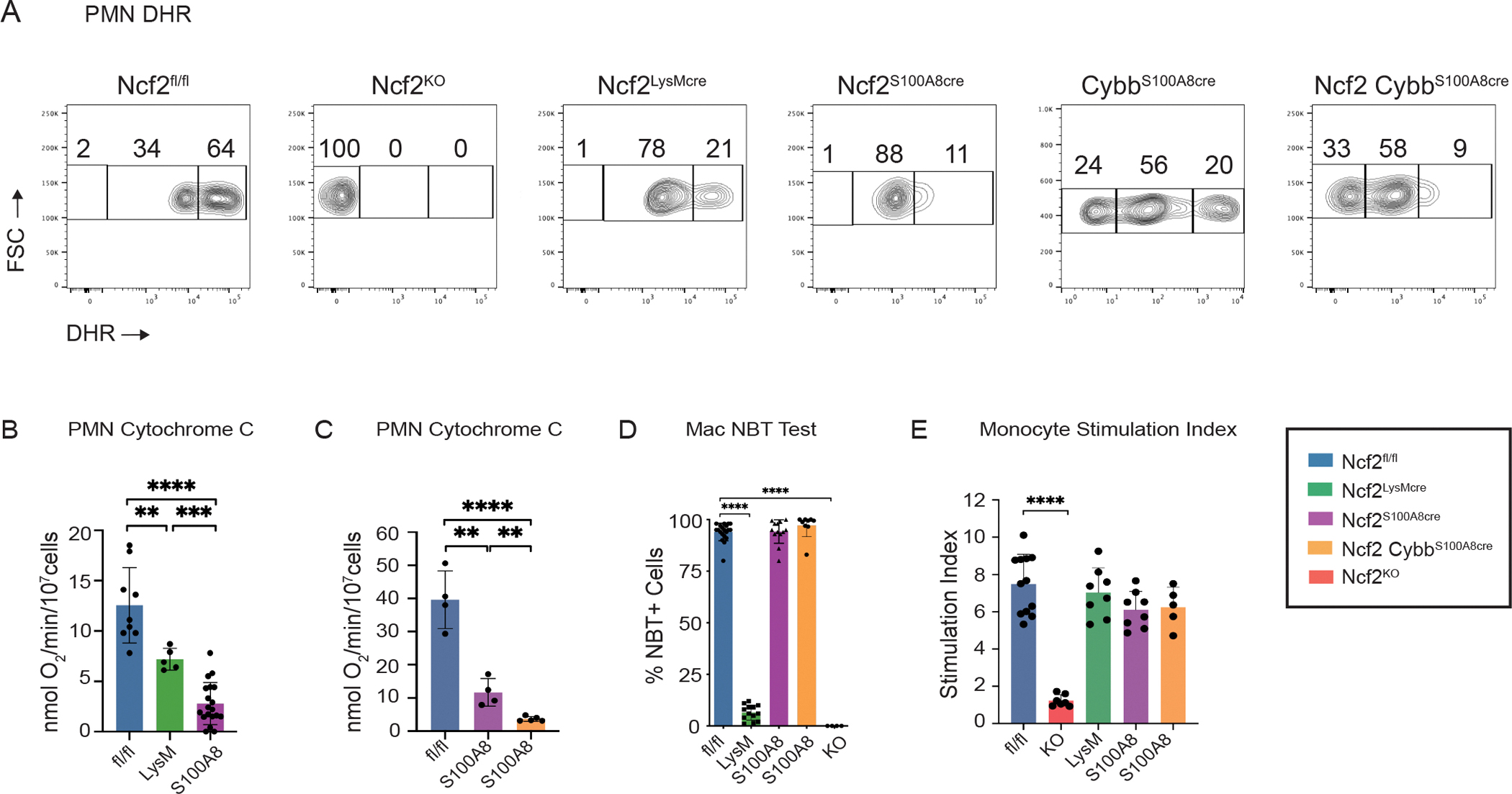
(A) Dihydrorhodamine (DHR) assay for NOX2 activity in peripheral blood PMN. Percent of DHR-high, low DHR-low and DHR-negative cells as indicated in Ncf2fl/fl, Ncf2KO, Ncf2LysMcre, Ncf2S100A8Cre, CybbS100A8Cre and Ncf2CybbS100A8Cre mice. Representative samples shown. (B, C) Superoxide production by cytochrome C assay of marrow neutrophils from Ncf2fl/fl, Ncf2LysMcre and Ncf2S100A8Cre mice (B) and Ncf2fl/fl, Ncf2S100A8Cre and Ncf2CybbS100A8Cre mice (C). (D) Percent of oxidase positive (NBT-positive) RPM and AM in the indicated mice. 200 cells scored per mouse. (E) Monocyte stimulation index calculated from DHR+ bone marrow monocytes in the indicated mice. Each data point represents one mouse with n≥4 per group. Bar graph data shown as mean ± SD. Student’s ‘t’ test was performed for comparisons between 2 groups and **P<0.01, ***P<0.001 ****P< 0.0001 were considered as significant. Data represents at least 2 independent sets of experiments.
Although we succeeded in producing mice with absent NOX2 activity in resident macrophages, we were unable to entirely eliminate NOX2 specifically in neutrophils. Floxed alleles in self-renewing resident macrophages are deleted by LysM-Cre during fetal development. In neutrophils, Ncf2 and Cybb are expressed early in their differentiation (53), and some could be produced before S100A8- or LysM-Cre-mediated gene deletion is complete. Nevertheless, these mice allowed us to interrogate how reduced but not absent NOX2 neutrophil activity impacts host responses. Table 1 summarizes NOX2 activity in among the different mouse genotypes. The majority of our studies compared Ncf2LysMCre and Ncf2S100A8Cre mice, but we also used double-floxed Ncf2CybbS100A8Cre mice in some experiments.
Table 1.
Summary of mouse genotypes and NOX2 activity*
| Genotype | Resident Macrophage NOX2 | Neutrophil NOX2 | Monocyte NOX2 |
|---|---|---|---|
|
| |||
| Ncf2 fl/fl | WT | WT | WT |
| Ncf2 LysMCre | 5% | 50% | WT |
| Ncf2 S100A8Cre | WT | 20% | WT |
| Ncf2Cybb S100A8Cre | WT | 10% | WT |
| Ncf2 KO | 0 | 0 | 0 |
For resident alveolar and peritoneal macrophages, the percentage of macrophages that were nitroblue tetrazolium-positive following stimulation with serum-opsonized zymosan is shown. Wild-type (WT) corresponds to ≥95% nitroblue tetrazolium-positive. For neutrophils, the percent reduction of NOX2 activity compared to wild-type neutrophils is shown, as determined using the cytochrome C assay. Monocyte NOX2 activity was determined by flow cytometry and was at WT levels, expect for Ncf2KO
Responses to infectious A. fumigatus conidia in mice with conditional NOX2 deletion
As NOX2 is an essential effector in the host response to AF, we examined whether conditional deletion of NOX2 activity would differentially impair control of inhaled conidia. CGD mice show markedly increased susceptibility compared to immunocompetent mice, with progressive neutrophilic lung infiltrates containing hyphae (16, 38, 54, 55). Since Ncf2LysMCre, Ncf2S100A8Cre and Ncf2CybbS100A8Cre mice all have residual neutrophil NOX2 activity, we reasoned that the control of AF would display more modest defects compared to CGD mice. As using mortality as an endpoint could require large numbers of mice to achieve significant differences, we instead compared responses in the first few days following conidia challenge.
Variation among AF strains can influence the host response in both wild-type and immunosuppressed mice (56–59). The widely used AF293 strain is considered less virulent because of slower germination and reduced induction of pro-inflammatory cytokines compared to CEA10, another commonly used strain (56, 59, 60). Our prior studies in CGD mice used AF10, which resulted in high mortality, even with administration of only a few hundred conidia into the lung (16, 55). AF10 is reported to be similar or even identical to CEA10 (61, 62). We compared AF10 and AF293 in Ncf2KO CGD mice. At 48 hours following challenge with 1-million conidia of either strain, Ncf2KO mice had multiple lung foci containing scattered hyphae (Figure S2A); hyphae were seen in almost all AF10 foci but in only ≈ 50% of foci in AF293-challenged mice. AF10 also induced greater inflammation, with significantly higher bronchoalveolar lavage (BAL) neutrophils and CXCL2, TNF-α, IL-1α and IL-1β levels (Figure S2B, C). Although both strains produced IPA in CGD mice, the results are consistent with differential virulence attributes, and thus we studied both AF10 and AF293 in selected experiments.
Early control of A. fumigatus following inhalation of conidia requires alveolar macrophage NOX2 activity
As sentinels of the airways, alveolar macrophages play a major role in early elimination of inhaled AF conidia, especially when inocula do not exceed AM numbers, estimated to be ≈5 × 105 in mice (63). We investigated whether early control of inhaled AF conidia in vivo was defective in Ncf2LysMCre mice, which lack NOX2 in 95% of AM. We challenged Ncf2LysMCre mice with 5 × 104 AF293 conidia, along with mice with WT NOX2 activity, global knockout of NOX2, or neutrophil-specific reduction in NOX2 for comparison (Figure 2A). As recruited neutrophils can ingest and kill conidia, we also examined the impact of anti-Ly6G-mediated neutrophil depletion (18, 49) initiated 24 hours prior to challenge, which significantly reduced peripheral blood neutrophils at the time of challenge (Figure 2B).
Figure 2. A. fumigatus germination at 24 hours following inhalation of conidia is increased in absence of alveolar macrophage NOX2, especially with neutropenia.
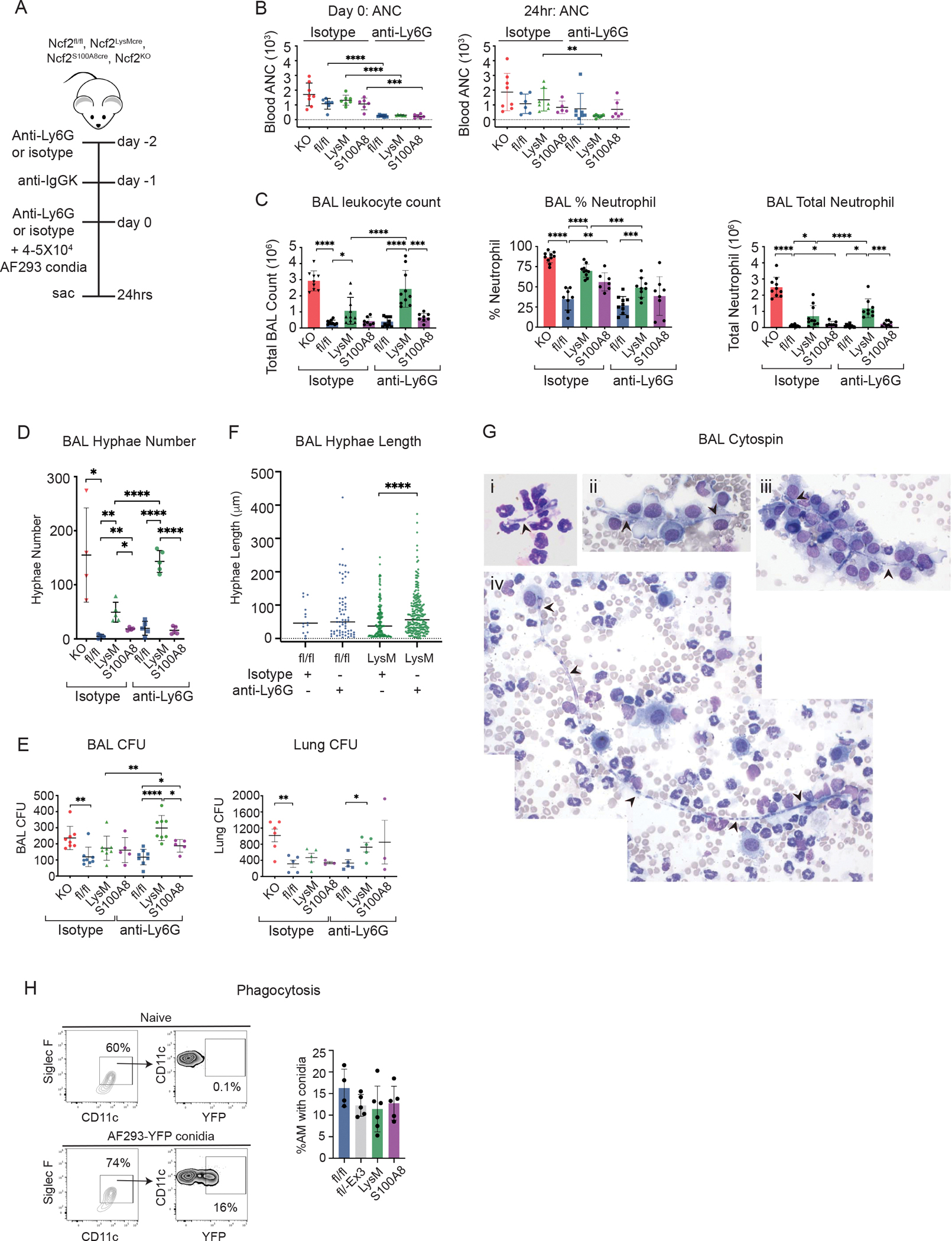
(A) Schema to assess germination into hyphae of inhaled AF293 conidia at 24 hpi without or with antibody-mediated neutrophil depletion. (B) Peripheral blood obtained by retro-orbital puncture was used to determine the absolute neutrophil count (ANC) at time of challenge (day 0) and at 24 hpi. (C) Total leukocyte counts from 1 ml BAL fluid. The percentage of neutrophils were identified by cytospin and used to calculate total BAL neutrophils. (D) Enumeration of hyphae in 1 ml BAL fluid. Each point represents an individual mouse. (E) Aspergillus CFU in 1 ml BAL fluid and in homogenate of right lung for the indicated mice. (F) Hyphae length measured on BAL cytospins from indicated groups, combined data from at least three mice per group. (G) Photomicrographs of hyphae (indicated with black arrowheads). i. 28 μm hypha from Ncf2LysMCre mouse treated with isotype (63X). ii. 44 μm hypha from Ncf2LysMCre mouse treated with anti-Ly6G (100X). iii. 66 μm hypha from Ncf2fl/fl mouse treated with anti-Ly6G (100X); iv. tiled photomicrographs showing 244 μm hypha from Ncf2LysMCre mouse treated with isotype (40 X). (H) Phagocytosis of YFP-labeled AF293 conidia by AM from indicated Ncf2 genotypes at two hours after inhalation. Ncf2fl/−Ex3 mice are heterozygous for a floxed and an Exon 3-deleted Ncf2 allele. CD45+ AM were identified as SiglecF+CD11c+ and gated for YFP using BFL1 channel to identify AM with ingested conidia. Data represents at least 2 independent sets of experiments with n≥4 per group. Graphical data shown as mean ± SD. Student’s ‘t’ test was performed for comparisons between 2 groups and *P<0.05, **P<0.01, ***P<0.001 ****P< 0.0001 were considered as significant. For (F), distribution of hyphae length is non-normal per the Kolmogorov-Smirnov test and statistical comparisons between 2 groups used the Mann-Whitney test.
To assess responses, we analyzed BAL fluid at 24 hours-post-infection (hpi). Notably, BAL samples from isotype-treated Ncf2LysMCre mice lacking AM NOX2 had significantly higher numbers of neutrophils and hyphae compared to Ncf2fl/fl or to Ncf2S100A8Cre mice, where AM NOX2 activity is intact (Figure 2C, D). This supports the importance of AM NOX2 to eradicate conidia before germination in vivo. BAL hyphae numbers were further increased in neutropenic Ncf2LysMCre mice (Figure 2D), showing that reducing neutrophil assistance further unmasks the conidiacidal defect in NOX2-deleted AM. Neutropenia in Ncf2LysMCre mice also led to a significant increase in BAL CFU compared to Ncf2fl/fl or to Ncf2S100A8Cre mice and lung CFU compared to Ncf2fl/fl (Figure 2E). Longer hyphae were present in neutropenic WT and Ncf2LysMCre mice compared to isotype-treated mice, a difference that was statistically significant for Ncf2LysMCre (Figure 2F). This result is consistent with the importance of neutrophils to damage and kill hyphae. Representative examples of hyphae with different lengths in BAL cytospins from mice treated with either isotype or anti-Ly6G are shown in Figure 2G. Finally, to compare phagocytosis of conidia by AM among different mouse genotypes, we challenged mice with YFP-AF293, but ingestion by Ncf2LysMCre mice was similar to others (Figure 2H). Taken together, these findings establish that alveolar macrophage NOX2 activity has a non-redundant role in vivo to prevent germination of inhaled conidia in the first 24 hours, which becomes even more important in the setting of neutropenia.
Impaired control of A. fumigatus following inhalation of larger numbers of conidia when NOX2 activity is reduced only in neutrophils
We also challenged mice with conditional deletion of NOX2 with much higher numbers (5 – 8 million) of either AF293 or AF10 conidia, a range where neutrophils are required to prevent IPA in WT mice and AM are dispensable (11). Mice were euthanized at 72 hpi, and appeared mildly ill, but weights and activity scores (39) were not significantly different among the study groups for either AF strain (not shown). For comparison, CGD mice challenged with 5 million AF10 conidia were very ill by 72 hpi, with lungs showing extensive inflammatory infiltrates containing hyphae (Figure S2D).
We first compared inflammation and fungal burden in response to AF293 (Figure 3A). Ncf2S100A8Cre mice, where NOX2 activity is reduced 5-fold specifically in neutrophils, had significantly higher numbers of neutrophils and CFU in BAL and lung homogenates (Figure 3B–D) compared to Ncf2fl/fl mice with WT NOX2 or to Ncf2LysMCre mice with only a two-fold reduction in neutrophil NOX2 activity. Pulmonary histology (Figure 3E) showed scattered peribronchial and alveolar infiltrates that were more prominent in Ncf2S100A8Cre mice, and neutrophil-filled bronchi were seen in two of the seven mice in this group. AF hyphae by GMS-staining were not seen in Ncf2fl/fl or Ncf2LysMCre samples, but were present within a neutrophil-filled airway in a Ncf2S100A8Cre mouse (Figure 3E). Taken together, these results show increased inflammation with higher fungal burden in Ncf2S100A8Cre mice at 72 hpi, consistent with delayed AF293 clearance by mice with reduced neutrophil NOX2 activity.
Figure 3. Reduced NOX2 activity in neutrophils increases A. fumigatus burden following challenge with AF293.
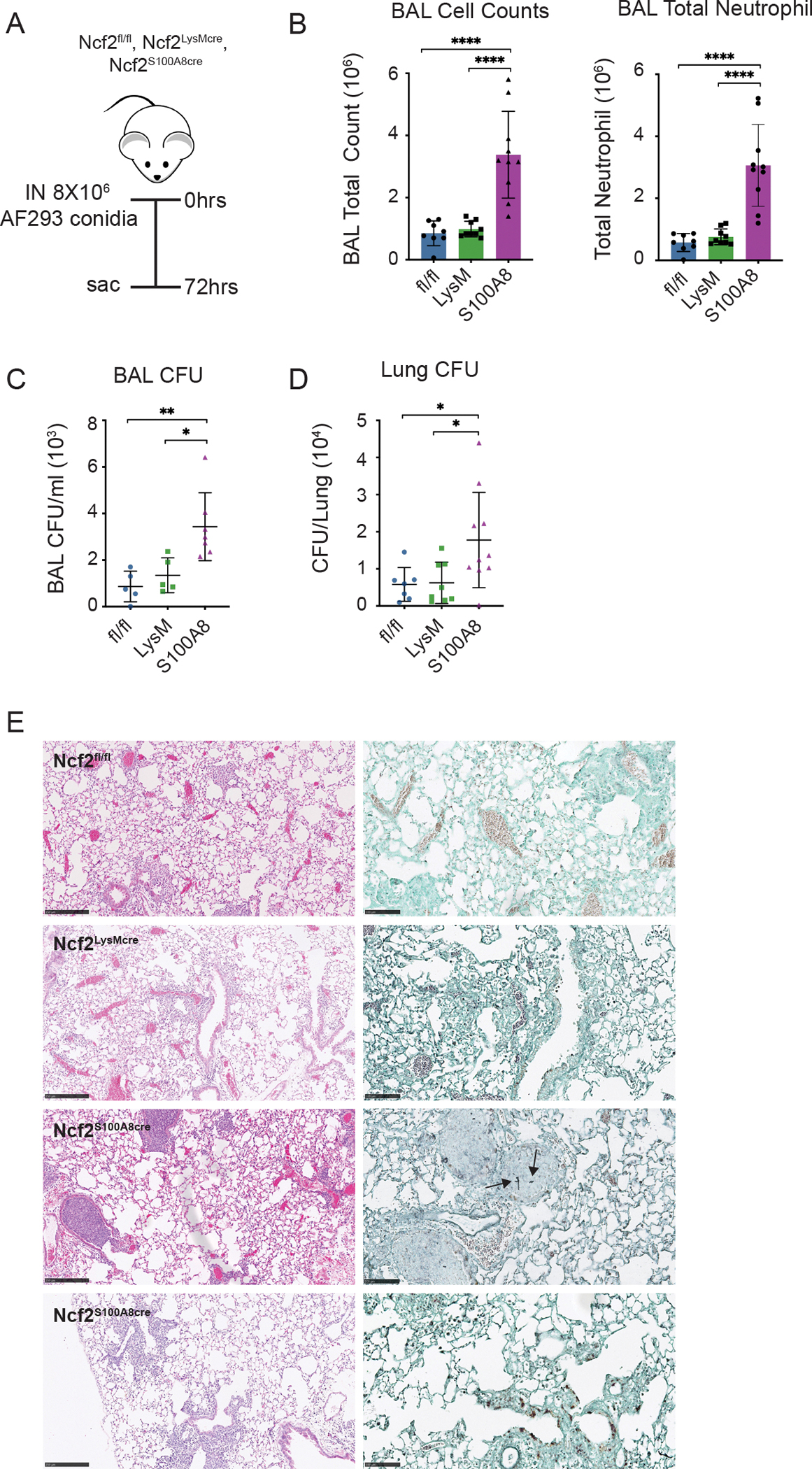
(A) Schema for AF293 challenge in indicated mice, with analysis at 72 hpi. (B) Total leukocyte and neutrophil counts from 1 ml BAL fluid. (C, D) Aspergillus CFU in 1 ml BAL fluid (C) and in homogenate of right lung (D) for the indicated mice. (E). Representative histology of lung sections for the indicated genotypes. From left to right, panels show H&E (bar 250 μm), and GMS (bar 100 μm). Arrows indicate hyphae. Data represents three independent sets of experiments with n≥5 per group. Graphical data shown as mean ± SD. Student’s ‘t’ test was performed for comparisons between 2 groups and *P<0.05, **P<0.01, ***P<0.001 ****P< 0.0001 were considered as significant.
Next, we studied the response to AF10 (Figure 4A). We compared Ncf2fl/fl, Ncf2LysMCre, Ncf2S100A8Cre mice, as well as double-floxed Ncf2CybbS100A8Cre mice, which have ten-fold less NOX2 activity than WT. Ncf2LysMCre mice were similar to Ncf2fl/fl mice, except for a modest but a significant increase in lung CFU. The S100A8Cre cohorts, with more greatly reduced neutrophil NOX2 activity, had significantly higher numbers of BAL neutrophils as well as BAL and lung CFU compared to Ncf2fl/fl mice (Figure 4B–D). Notably, inflammation and fungal burden were the highest in the Ncf2CybbS100A8Cre mice. BAL and lung CFU in the double-floxed S100A8Cre mice were also significantly greater than for the single-floxed S100A8Cre mice. Lung histology for all four genotypes showed scattered peribronchial and alveolar infiltrates (Figure 4E). Hyphae were not found in Ncf2fl/fl or Ncf2LysMCre mice, but one Ncf2S100A8Cre mouse had a few hyphae detected (Figure 4E). In the double-floxed Ncf2CybbS100A8Cre mice, lung infiltrates were the most prominent and hyphae were seen in all mice (Figure 4E). The presence of hyphae among Ncf2CybbS100A8Cre mice (7 of 7 mice) was significantly greater than for Ncf2S100A8Cre (1 of 8 mice) (p = 0.0014). As for AF293, these results show that reduced NOX2 activity specifically in neutrophils impairs clearance of AF10 in proportion to NOX2 activity. The impact of neutrophil NOX2 may be greater following AF10 challenge, as lung CFU in Ncf2S100A8Cre mice were ≈40-fold higher than WT compared to only ≈2-fold higher with AF293 challenge. Finally, Ncf2CybbS100A8Cre mice, which have the lowest neutrophil NOX2 activity, had the most extensive disease at 72 hpi, with hyphal forms in lung sections and significantly greater CFU.
Figure 4. Reduced NOX2 activity in neutrophils increases A. fumigatus burden following challenge with AF10 conidia.
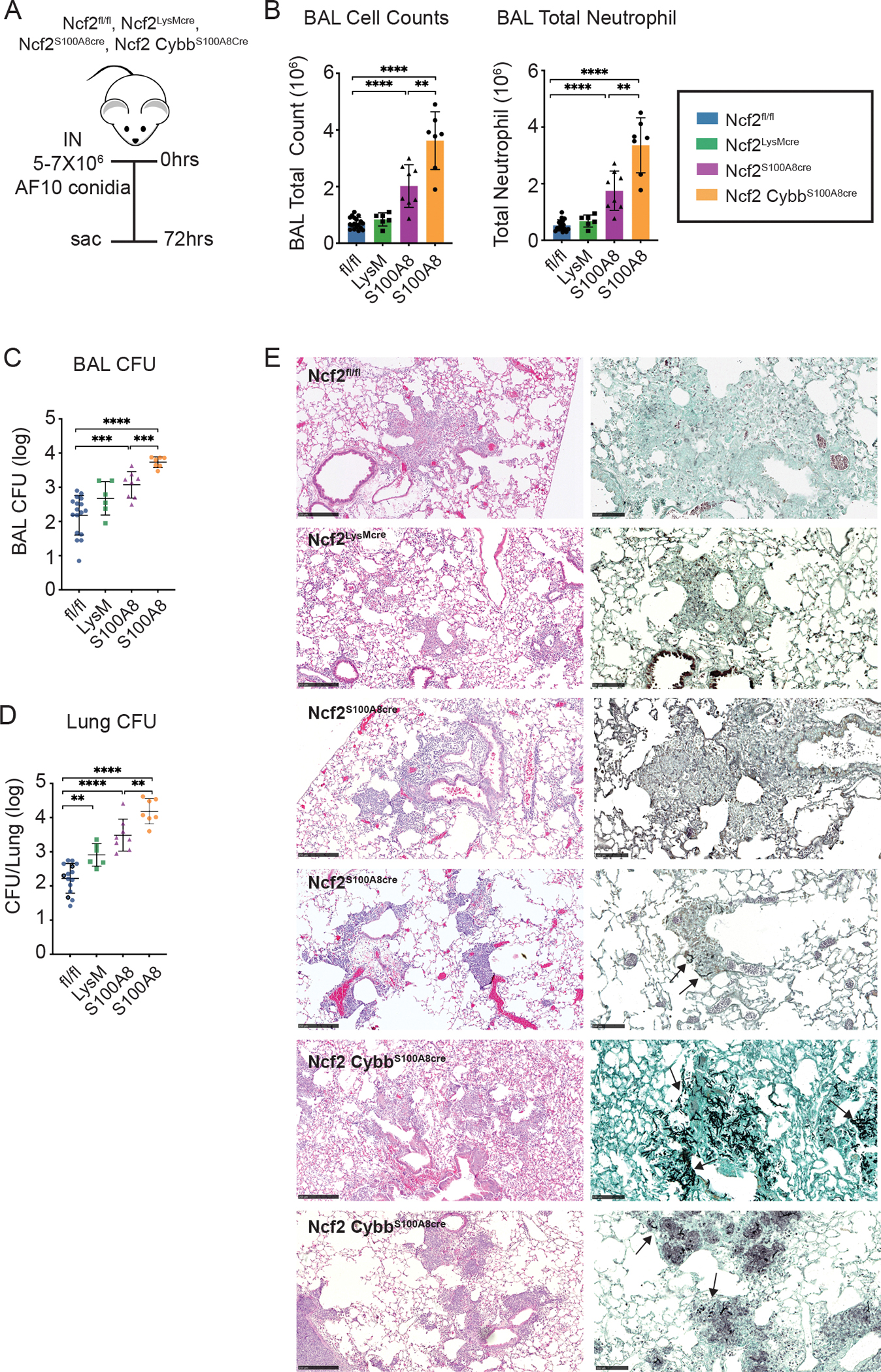
a) Schema for AF10 challenge in Ncf2fl/fl, Ncf2LysMCre, Ncf2S100A8Cre and Ncf2CybbS100A8Cre mice, with analysis at 72 hpi. B) Total leukocyte and neutrophil counts from 1 ml BAL fluid. (C, D) Aspergillus CFU in 1 ml BAL fluid (C) and in homogenate of right lung (D) for the indicated mice. (E). Representative histology of lung sections for the indicated genotypes. From left to right, panels show H&E (bar 250 μm), and GMS (bar 100 μm). Arrows indicate hyphae. Data represents 2 – 4 independent sets of experiments with n≥4 per group. Graphical data shown as mean ± SD. Student’s ‘t’ test was performed for comparisons between 2 groups and *P<0.05, **P<0.01, ***P<0.001 ****P< 0.0001 were considered as significant.
Reduced neutrophil NOX2 activity further impairs control of A. fumigatus in mice immunosuppressed by cortisone acetate
Immunosuppression by corticosteroids is a well-recognized risk factor for developing invasive AF. In addition to suppressing transcription of pro-inflammatory cytokines that activate immune cells, corticosteroids compromise alveolar macrophage function and reduce their conidiacidal activity, in part by suppressing NOX2 ROS and LC3-mediated phagosome maturation (9, 10, 64–66). Neutrophil-mediated damage to AF hyphae and neutrophil NOX2 activity are also depressed by corticosteroid treatment (67, 68). Cortisone acetate-treated mice challenged with inhaled AF develop invasive infection characterized by accumulation of dysfunctional phagocytes surrounding only a few hyphae (65).
We investigated the impact of NOX2 conditional deletion in mice immunocompromised by cortisone acetate and challenged with AF293 conidia (Figure 5A). We used small numbers (5000–8000) of conidia, which produced little disease in the majority of corticosteroid-treated Ncf2fl/fl mice with WT NOX2 activity. We analyzed the mice seven days after inhalation of conidia (Figure 5B–D). In the cortisone acetate-treated Ncf2fl/fl cohort, 5 of 6 mice had normal lung histology, with few BAL neutrophils or AF cultured from the lung. One cortisone acetate-treated Ncf2fl/fl mouse developed a parenchymal abscess with numerous hyphae, accompanied by increased BAL neutrophils and lung CFU. In comparison, cortisone acetate-treated Ncf2LysMCre and Ncf2S100A8Cre cohorts (8 and 7 mice, respectively) had significantly higher numbers of BAL neutrophils and lung CFU (Figure 5B, C). AF was cultured from the lungs of all 13 conditional deletion mice for which CFU were available. Abscesses were present in three Ncf2LysMCre and three Ncf2S100A8Cre mice, and focal infiltrates in others (Figure 5D). One Ncf2S100A8Cre mouse died at day 4, and one Ncf2LysMCre mouse died at day 7 where post-mortem analysis revealed extensive Aspergillus pneumonia (not shown). We conclude that a reduction in neutrophil NOX2 activity is sufficient to synergize with corticosteroid-mediated immunosuppression and significantly further impair the eradication of inhaled AF. In the Ncf2LysMCre mice, absence of AM NOX2 may also enhance susceptibility.
Figure 5. Reduced neutrophil NOX2 activity impairs control of A. fumigatus in corticosteroid-treated mice.
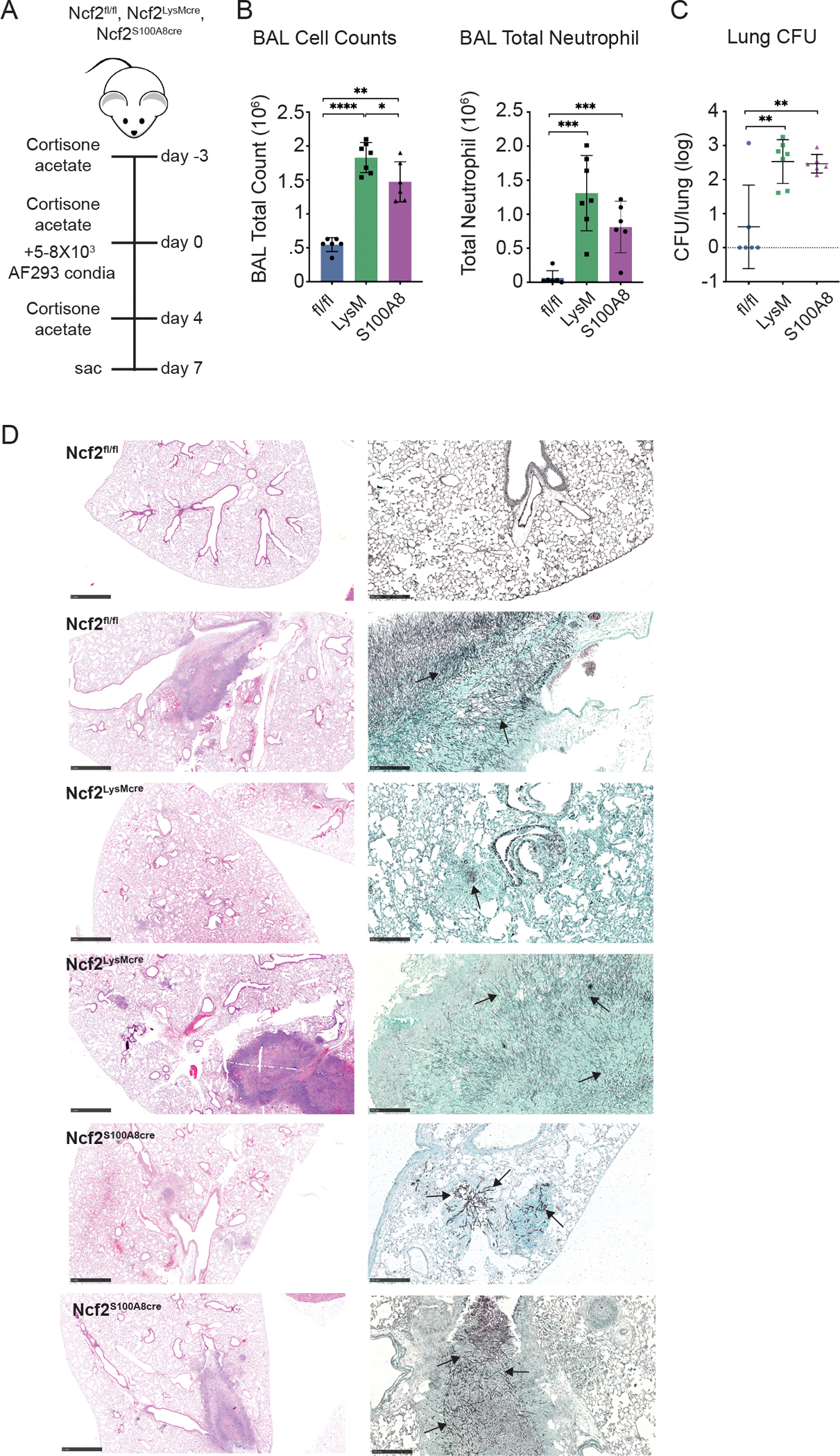
a) Schema for AF293 challenge of cortisone acetate-treated Ncf2fl/fl, Ncf2LysMCre, and Ncf2S100A8Cre mice, with analysis at 7 days after challenge. B) Total leukocyte and neutrophil counts from 1 ml BAL fluid. (C) Aspergillus CFU in homogenate of right lung for the indicated mice. (D) Representative histology of lung sections for the indicated genotypes. From left to right, panels show H&E (bar 1 mm), and GMS (bar 100 μm). Arrows indicate hyphae.
Data represents 3 independent sets of experiments with n≥5 per group. Graphical data shown as mean ± SD. Student’s ‘t’ test was performed for comparisons between 2 groups and *P<0.05, **P<0.01, ***P<0.001 ****P< 0.0001 were considered as significant.
Conditional deletion of NOX2 in macrophages and/or neutrophils differentially impacts responses to sterile inflammation
Increased neutrophilic inflammation even in the absence of infection is a hallmark of CGD (14), and NOX2-deficient macrophages and neutrophils may each contribute to dysregulated responses (18, 48). To separate the effects of immunoregulatory ROS from infection, we studied two different models of sterile inflammation to determine whether conditional NOX2 deletion could reveal cell-type specific roles.
Inhalation of the fungal particle zymosan produces increased acute lung inflammation in CGD mice, and is initiated by increased LTB4 production by CGD neutrophils (18). We therefore compared this response in mice with conditional deletion of NOX2 to CGD (Ncf2KO) mice and mice with WT NOX2 (Ncf2fl/fl). Mice were challenged with 20 μg zymosan (≈1–2 million particles) and analyzed after eighteen hours (Figure 6A). CGD (Ncf2KO) mice had the highest numbers of inflammatory neutrophils in BAL (Figure 6B). Ncf2LysMCre mice lacking alveolar macrophage NOX2 along with a partial reduction in neutrophil NOX2 activity had a small but significant increase in BAL neutrophils compared to Ncf2fl/fl mice (Figure 6B). Ncf2S100A8Cre and Ncf2CybbS100A8Cre mice, which had a greater reduction in neutrophil NOX2 activity, had even higher numbers of BAL neutrophils, which were similarly elevated and more than two-fold higher compared to Ncf2fl/fl mice (Figure 6B). By histology (Figure 6C), Ncf2fl/fl mice had minimal lung inflammation and, rarely, very small neutrophil foci were seen in alveoli of Ncf2LysMCre mice. However, S100A8Cre cohorts had scattered alveolar neutrophil infiltrates, albeit smaller in size than in Ncf2KO mice. Ncf2S100A8Cre neutrophils stimulated with LTB4 in vitro also produced significantly higher amounts of LTB4 compared to WT neutrophils, although not as elevated as Ncf2KO neutrophils (Figure 6D). We conclude that reducing NOX2 activity specifically in neutrophils is sufficient to enhance acute zymosan-induced lung inflammation, and that even a partial loss of NOX2 activity suffices to increase production of the key inflammatory mediator LTB4.
Figure 6. Sterile inflammation is increased in conditional NOX2-deleted mice and differentially dependent on neutrophil or macrophage NOX2 activity.
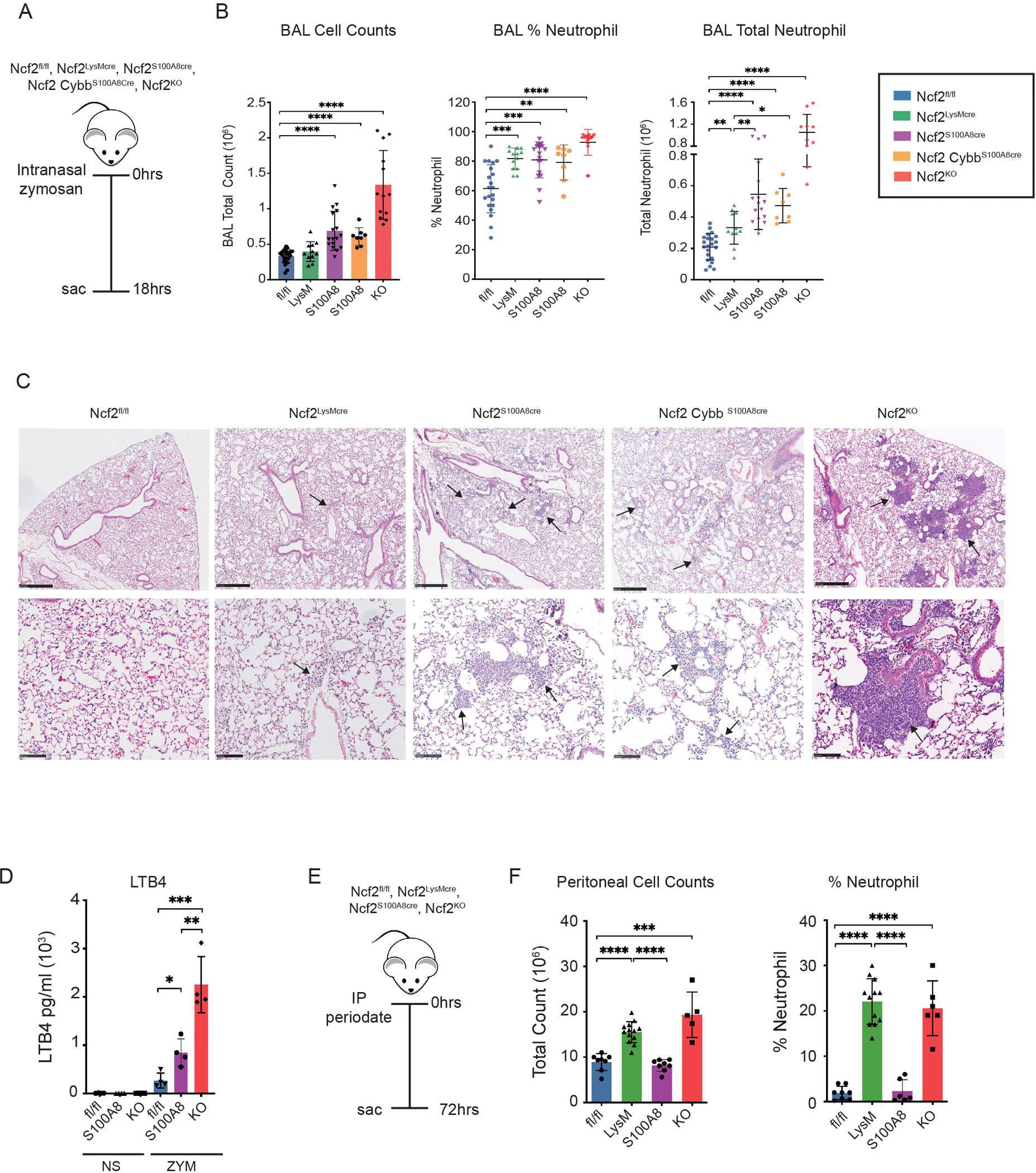
(A) Schema for zymosan-induced lung inflammation in indicated mice. Mice were challenged with 20 μg zymosan IN and studied after 18 hours. (B) Total leukocyte counts from 3 ml BAL fluid. The percentage of neutrophils were identified by cytospin and used to calculate total BAL neutrophils. (C) Representative histology of H&E-stained lung sections for the indicated genotypes. Scale bar for top row of panels is 500 μm and 100 μm for the lower panels. Arrows indicate neutrophil foci. (D) LTB4 ELISA from supernatants obtained from purified bone marrow neutrophils incubated for one hour either with no stimulus (NS) or with zymosan (1 neutrophil:2 zymosan particles). Ncf2fl/fl, Ncf2S100A8Cre and Ncf2KO mice were studied as indicated. (E) Schema for periodate-induced peritonitis in indicated mice and (F) peritoneal lavage cell counts and % neutrophils at 72 hours following IP injection of periodate. Data represents 3 independent sets of experiments with n≥4 per group. Graphical data shown as mean ± SD. Student’s ‘t’ test was performed for comparisons between 2 groups and *P<0.05, **P<0.01, ***P<0.001 ****P< 0.0001 were considered as significant.
CGD mice also have increased acute inflammation in DAMP (damage-associated molecular pattern)-induced tissue injury, as studied following intraperitoneal injection of periodate, a mild oxidizing agent (48). CGD macrophages release higher amounts of IL-1α in response to necrotic cells and are implicated in driving this augmented response. We therefore evaluated this model in mice with conditional NOX2 deletions. Of note, peritoneal inflammation induced by zymosan is not increased in Ncf2LysMCre mice, which lack NOX2 in resident macrophages (40). However, at 72 hours following periodate-induced injury, Ncf2LysMCre mice, similar to Ncf2KO mice, displayed significantly increased leukocyte numbers in the peritoneal lavage and the fraction that were neutrophils, compared to mice with WT NOX2 activity or with neutrophil-specific reduction of NOX2 (Figure 6E, F). Moreover, administration of an antibody blocking IL-1α significantly reduced the neutrophilic peritoneal inflammation in Ncf2fl/fl, Ncf2KO mice and Ncf2LysMCre mice (Figure S3), consistent with importance of IL-1α as a key mediator of DAMP-induced inflammation. While we cannot rule out that the partial reduction in neutrophil NOX2 activity contributes to increased DAMP-induced inflammation in Ncf2LysMCre mice, these results strongly implicate NOX2 activity in resident macrophages as critical for regulating the response to tissue injury.
Discussion
These results advance understanding into cell-specific functions of NOX2 in two different innate immune cells that are key effectors in the host response to inhaled AF. Analysis of mice with conditional genetic deficiency of NOX2 highlight distinct but complementary roles in alveolar macrophages and neutrophils. Prior studies on the importance of alveolar macrophage NOX2 for killing ingested conidia yielded conflicting results, likely related to different endpoints and methodologies (1). Here, we show that AM NOX2 has a non-redundant role to reduce germination of inhaled conidia in vivo and the early emergence of hyphae when conidia numbers are not overwhelming. Hyphae numbers in BAL at 24 hpi were significantly increased in Ncf2LysMCre mice that lack AM NOX2 activity. Numbers of germinated hyphae increased still further in Ncf2LysMCre mice made neutropenic, which illustrates the importance of cooperation between these two cell types for early control of inhaled AF conidia. Hyphae numbers at 24 hpi were little affected in Ncf2S100A8Cre mice, where neutrophil NOX2 activity is even more impaired than in Ncf2LysMCre mice. This is congruent with recent study showing that early non-oxidative killing of conidia by CGD neutrophils could protect CGD mice from IPA when additional neutrophils were pre-recruited to the airways by stimulation of Type I interferon (35). Our results also show that even with intact NOX2 in monocytes and macrophages, robust neutrophil NOX2 activity is critical to limit AF progression following inhalation of larger numbers of conidia that overload the capacity of AM or to smaller inocula when there is additional immunosuppression from corticosteroids. This is consistent with the recognized importance of neutrophil NOX2 activity to kill emerging hyphae (26, 28–30, 34). Impaired control of AF as a consequence of depressed neutrophil NOX2 activity was observed for two AF strains of differing relative virulence. Moreover, our results demonstrate experimentally for the first time that there is a “dose dependence” of neutrophil NOX2 activity to eliminate AF, which was defective even when neutrophil NOX2 activity was not completely absent, with fungal burden inversely proportional to the level of NOX2 activity.
Our study also identified non-overlapping roles of NOX2 in neutrophils and macrophages for limiting acute inflammation in response to fungal cell walls and to DAMPs, respectively. The results reinforce the importance of NOX2 activity to modulate immune responses, and show that in this context, NOX2 activity can act in a cell-specific manner and depend on the inciting agent. That acute inflammation elicited by fungal cell walls is regulated by neutrophil NOX2 activity is consistent with prior work demonstrating that increased production of LTB4 by CGD neutrophils instigates an over-amplified feed-forward loop to recruit neutrophils into the lungs following zymosan challenge. (18). An important result was that even a partial reduction in NOX2 activity promoted lung inflammation induced by zymosan and increased neutrophil production of LTB4. While augmented recruitment of neutrophils, which are able to kill AF conidia by non-oxidative means, might be beneficial in NOX2-deficient hosts for controlling infection, ultimately, overexuberant inflammation can be detrimental and worsen disease outcomes.
In the clinical setting, there are many variables that can interact and lead to failed eradication of AF, especially for those patients that are not severely immunocompromised. As evidenced in the current study, NOX2 activity in macrophages and neutrophils are each important for the innate response to AF and if either is impaired, could contribute to a “net state of immunosuppression“ (3) that increases the risk of invasive or chronic aspergillosis. Corticosteroids are well-known to impair both neutrophil and macrophage NOX2 activity (9, 10, 64–68). Neutrophil NOX2 activity can also be depressed by intercurrent co-morbidities, including diabetes, liver disease, chronic alcohol use, chronic obstructive pulmonary disease, post-influenza infection, and with aging, which overlap risk factors for invasive aspergillosis (36, 69–73). Invasive aspergillosis was also discovered to be an unexpected complication in lymphoma patients being treated with the Bruton tyrosine kinase (BTK) inhibitor ibrutinib (3). While the importance of BTK in lymphocytes is well understood, little is known about its role in innate immune cells. However, a recent study found that human neutrophils treated with ibrutinib show impaired killing of AF hyphae in vitro (74). Influenza infection is another risk factor for IPA, and reduced neutrophil ROS post-influenza is reported in mice (70). Other than corticosteroids, little is known regarding how drugs or co-morbidities associated with invasive AF impact NOX2 function in alveolar macrophages, but may warrant investigation. Regardless, recognizing impaired NOX2 activity as a predisposing factor for aspergillosis may help in developing mitigating strategies for at-risk patients.
Supplementary Material
Key Points.
Alveolar macrophage NOX2 limits germination of inhaled A. fumigatus conidia in mice
Reduced neutrophil NOX2 activity promotes invasive AF and enhances inflammation
Acknowledgements
The authors thank Tina McGrath for assistance with manuscript preparation, Hongjie Gu in the Division of Biostatistics at Washington University School of Medicine in St. Louis (WUSM) for assistance with statistical analysis, and Wandy Beatty and the Imaging Core of the Molecular Microbiology Department at WUSM. These studies used the WUSTL Mouse Embryonic Stem Cell Core (affiliated with the Siteman Cancer Center at the time the work was performed) for providing ES cell culture services, the Mouse Genetics Core at WUSM for their support with animal production and care, the Andrew M. and Jane M. Bursky Center for Human Immunology and Immunotherapy Programs (CHiiPs), and the Hope Center Alafi Neuroimaging Laboratory.
Grant Support:
This work was supported by grants from the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (R01HL140837 to M.C.D), NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR072212 to M.C.D.), the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (M.C.D.), National Institute of Allergy and Infectious Diseases (5R01AI150669-01A1 to N.P.K.), National Institute of General Medical Sciences (R35GM118027-01 to AH), and by a NIH Shared Instrumentation grant (S10 RR027552)
Footnotes
Disclosures
The authors have no financial conflicts of interest.
REFERENCES
- 1.Latge JP, and Chamilos G. 2019. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev 33: e00140–00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohl TM 2017. Immune responses to invasive aspergillosis: new understanding and therapeutic opportunities. Curr Opin Infect Dis 30: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamilos G, Lionakis MS, and Kontoyiannis DP. 2018. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin Infect Dis 66: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewi IM, Janssen NA, Rosati D, Bruno M, Netea MG, Brüggemann RJ, Verweij PE, and van de Veerdonk FL. 2021. Invasive pulmonary aspergillosis associated with viral pneumonitis. Curr Opin Microbiol 62: 21–27. [DOI] [PubMed] [Google Scholar]
- 5.Tischler BY, and Hohl TM. 2019. Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense. Journal of molecular biology 431: 4229–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa V, and Rivera A. 2016. First Line of Defense: Innate Cell-Mediated Control of Pulmonary Aspergillosis. Front Microbiol 7: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obar JJ 2020. Sensing the threat posed by Aspergillus infection. Curr Opin Microbiol 58: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagenais TR, and Keller NP. 2009. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev 22: 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffner A, Douglas H, and Braude A. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest 69: 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philippe B, Ibrahim-Granet O, Prevost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, and Latge JP. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infection and immunity 71: 3034–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, and Hohl TM. 2009. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 200: 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, and Hohl TM. 2012. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep 2: 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, Yockey L, Darnell DN, Barnhart L, Daub J, Boris L, Rump AP, Anderson VL, Haney C, Kuhns DB, Rosenzweig SD, Kelly C, Zelazny A, Mason T, DeRavin SS, Kang E, Gallin JI, Malech HL, Olivier KN, Uzel G, Freeman AF, Heller T, Zerbe CS, and Holland SM. 2015. Common severe infections in chronic granulomatous disease. Clin Infect Dis 60: 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinauer MC 2019. Inflammatory consequences of inherited disorders affecting neutrophil function. Blood 133: 2130–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singel KL, and Segal BH. 2016. NOX2-dependent regulation of inflammation. Clin Sci (Lond) 130: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, and Dinauer MC. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med 185: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cagnina RE, Michels KR, Bettina AM, Burdick MD, Scindia Y, Zhang Z, Braciale TJ, and Mehrad B. 2021. Neutrophil-Derived Tumor Necrosis Factor Drives Fungal Acute Lung Injury in Chronic Granulomatous Disease. J Infect Dis 224: 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Z, Huang G, Chiquetto Paracatu L, Grimes D, Gu J, Luke CJ, Clemens RA, and Dinauer MC. 2020. NADPH oxidase controls pulmonary neutrophil infiltration in the response to fungal cell walls by limiting LTB4. Blood 135: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, Dennis CG, Vethanayagam RR, Yull FE, Capitano M, Wallace PK, Minderman H, Christman JW, Sporn MB, Chan J, Vinh DC, Holland SM, Romani LR, Gaffen SL, Freeman ML, and Blackwell TS. 2010. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5: e9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim-Granet O, Philippe B, Boleti H, Boisvieux-Ulrich E, Grenet D, Stern M, and Latge JP. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infection and immunity 71: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, Boumpas D, Muszkieta L, Prevost MC, Kontoyiannis DP, Chavakis T, Netea MG, van de Veerdonk FL, Brakhage AA, El-Benna J, Beauvais A, Latge JP, and Chamilos G. 2016. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 19: 79–90. [DOI] [PubMed] [Google Scholar]
- 22.Wiemann P, Perevitsky A, Lim FY, Shadkchan Y, Knox BP, Landero Figueora JA, Choera T, Niu M, Steinberger AJ, Wuthrich M, Idol RA, Klein BS, Dinauer MC, Huttenlocher A, Osherov N, and Keller NP. 2017. Aspergillus fumigatus Copper Export Machinery and Reactive Oxygen Intermediate Defense Counter Host Copper-Mediated Oxidative Antimicrobial Offense. Cell Rep 19: 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlezinger N, and Hohl TM. 2021. Mitochondrial Reactive Oxygen Species Enhance Alveolar Macrophage Activity against Aspergillus fumigatus but Are Dispensable for Host Protection. mSphere 6: e0026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, and Gallin JI. 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol 178: 6367–6373. [DOI] [PubMed] [Google Scholar]
- 25.Leal SM Jr., Roy S, Vareechon C, Carrion S, Clark H, Lopez-Berges MS, Di Pietro A, Schrettl M, Beckmann N, Redl B, Haas H, and Pearlman E. 2013. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS pathogens 9: e1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazendam RP, van Hamme JL, Tool AT, Hoogenboezem M, van den Berg JM, Prins JM, Vitkov L, van de Veerdonk FL, van den Berg TK, Roos D, and Kuijpers TW. 2016. Human Neutrophils Use Different Mechanisms To Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects. J Immunol 196: 1272–1283. [DOI] [PubMed] [Google Scholar]
- 27.Shlezinger N, Irmer H, Dhingra S, Beattie SR, Cramer RA, Braus GH, Sharon A, and Hohl TM. 2017. Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death. Science 357: 1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leal SM Jr., Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, and Pearlman E. 2012. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest 122: 2482–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rex JH, Bennett JE, Gallin JI, Malech HL, and Melnick DA. 1990. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infect Dis 162: 523–528. [DOI] [PubMed] [Google Scholar]
- 30.Diamond RD, and Clark RA. 1982. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infection and immunity 38: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornish EJ, Hurtgen BJ, McInnerney K, Burritt NL, Taylor RM, Jarvis JN, Wang SY, and Burritt JB. 2008. Reduced nicotinamide adenine dinucleotide phosphate oxidase-independent resistance to Aspergillus fumigatus in alveolar macrophages. J Immunol 180: 6854–6867. [DOI] [PubMed] [Google Scholar]
- 32.Bonnett CR, Cornish EJ, Harmsen AG, and Burritt JB. 2006. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus Conidia. Infection and immunity 74: 6528–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm MJ, Vethanayagam RR, Almyroudis NG, Dennis CG, Khan AN, D’Auria AC, Singel KL, Davidson BA, Knight PR, Blackwell TS, Hohl TM, Mansour MK, Vyas JM, Rohm M, Urban CF, Kelkka T, Holmdahl R, and Segal BH. 2013. Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J Immunol 190: 4175–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen TJ, Rosowski EE, Knox BP, Bennin D, Keller NP, and Huttenlocher A. 2019. Neutrophil phagocyte oxidase activity controls invasive fungal growth and inflammation in zebrafish. J Cell Sci 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seyedmousavi S, Davis MJ, Sugui JA, Pinkhasov T, Moyer S, Salazar AM, Chang YC, and Kwon-Chung KJ. 2018. Exogenous Stimulation of Type I Interferon Protects Mice with Chronic Granulomatous Disease from Aspergillosis through Early Recruitment of Host-Protective Neutrophils into the Lung. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malacco N, Souza JAM, Martins FRB, Rachid MA, Simplicio JA, Tirapelli CR, Sabino AP, Queiroz-Junior CM, Goes GR, Vieira LQ, Souza DG, Pinho V, Teixeira MM, and Soriani FM. 2020. Chronic ethanol consumption compromises neutrophil function in acute pulmonary Aspergillus fumigatus infection. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espinosa V, Dutta O, McElrath C, Du P, Chang YJ, Cicciarelli B, Pitler A, Whitehead I, Obar JJ, Durbin JE, Kotenko SV, and Rivera A. 2017. Type III interferon is a critical regulator of innate antifungal immunity. Sci Immunol 2: eaan5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, and Dinauer MC. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9: 202–209. [DOI] [PubMed] [Google Scholar]
- 39.Jacob CO, Yu N, Yoo DG, Perez-Zapata LJ, Barbu EA, Kaplan MJ, Purmalek M, Pingel JT, Idol RA, and Dinauer MC. 2017. Haploinsufficiency of NADPH Oxidase Subunit Neutrophil Cytosolic Factor 2 Is Sufficient to Accelerate Full-Blown Lupus in NZM 2328 Mice. Arthritis Rheumatol 69: 1647–1660. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya S, Idol RA, Yang W, Rojas Marquez JD, Li Y, Huang G, Beatty WL, Atkinson JJ, Brumell JH, Bagaitkar J, Magee JA, and Dinauer MC. 2022. Macrophage NOX2 NADPH oxidase maintains alveolar homeostasis in mice. Blood 139: 2855–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passegué E, Wagner EF, and Weissman IL. 2004. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 119: 431–443. [DOI] [PubMed] [Google Scholar]
- 42.Baker JR, Jeffery R, May RD, Mathies M, Spencer-Dene B, Poulsom R, and Hogg N. 2011. Distinct roles for S100a8 in early embryo development and in the maternal deciduum. Dev Dyn 240: 2194–2203. [DOI] [PubMed] [Google Scholar]
- 43.Farley FW, Soriano P, Steffen LS, and Dymecki SM. 2000. Widespread recombinase expression using FLPeR (flipper) mice. Genesis (New York, N.Y. : 2000) 28: 106–110. [PubMed] [Google Scholar]
- 44.Dahlgren C, Karlsson A, and Bylund J. 2007. Measurement of respiratory burst products generated by professional phagocytes. Methods Mol Biol 412: 349–363. [DOI] [PubMed] [Google Scholar]
- 45.Vowells SJ, Fleisher TA, Sekhsaria S, Alling DW, Maguire TE, and Malech HL. 1996. Genotype-dependent variability in flow cytometric evaluation of reduced nicotinamide adenine dinucleotide phosphate oxidase function in patients with chronic granulomatous disease. J Pediatr 128: 104–107. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RM, Burritt JB, Baniulis D, Foubert TR, Lord CI, Dinauer MC, Parkos CA, and Jesaitis AJ. 2004. Site-specific inhibitors of NADPH oxidase activity and structural probes of flavocytochrome b: characterization of six monoclonal antibodies to the p22phox subunit. J Immunol 173: 7349–7357. [DOI] [PubMed] [Google Scholar]
- 47.Yoo DG, Paracatu LC, Xu E, Lin X, and Dinauer MC. 2021. NADPH Oxidase Limits Collaborative Pattern-Recognition Receptor Signaling to Regulate Neutrophil Cytokine Production in Response to Fungal Pathogen-Associated Molecular Patterns. J Immunol 207: 923–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bagaitkar J, Pech NK, Ivanov S, Austin A, Zeng MY, Pallat S, Huang G, Randolph GJ, and Dinauer MC. 2015. NADPH oxidase controls neutrophilic response to sterile inflammation in mice by regulating the IL-1alpha/G-CSF axis. Blood 126: 2724–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boivin G, Faget J, Ancey PB, Gkasti A, Mussard J, Engblom C, Pfirschke C, Contat C, Pascual J, Vazquez J, Bendriss-Vermare N, Caux C, Vozenin MC, Pittet MJ, Gunzer M, and Meylan E. 2020. Durable and controlled depletion of neutrophils in mice. Nat Commun 11: 2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faget J, Boivin G, Ancey P-B, Gkasti A, Mussard J, Engblom C, Pfirschke C, Vazquez J, Bendriss-Vermare N, Caux C, Vozenin M-C, Pittet MJ, Gunzer M, and Meylan E. 2018. Efficient and specific Ly6G+ cell depletion: A change in the current practices toward more relevant functional analyses of neutrophils. BioRxiv: 498881. [Google Scholar]
- 51.Knox BP, Deng Q, Rood M, Eickhoff JC, Keller NP, and Huttenlocher A. 2014. Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae. Eukaryot Cell 13: 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clausen BE, Burkhardt C, Reith W, Renkawitz R, and Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8: 265–277. [DOI] [PubMed] [Google Scholar]
- 53.Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, Sieow JL, Penny HL, Ching GC, Devi S, Adrover JM, Li JLY, Liong KH, Tan L, Poon Z, Foo S, Chua JW, Su IH, Balabanian K, Bachelerie F, Biswas SK, Larbi A, Hwang WYK, Madan V, Koeffler HP, Wong SC, Newell EW, Hidalgo A, Ginhoux F, and Ng LG. 2018. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 48: 364–379.e368. [DOI] [PubMed] [Google Scholar]
- 54.Dinauer MC, Gifford MA, Pech N, Li LL, and Emshwiller P. 2001. Variable correction of host defense following gene transfer and bone marrow transplantation in murine X-linked chronic granulomatous disease. Blood 97: 3738–3745. [DOI] [PubMed] [Google Scholar]
- 55.Bjorgvinsdottir H, Ding C, Pech N, Gifford MA, Li LL, and Dinauer MC. 1997. Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease. Blood 89: 41–48. [PubMed] [Google Scholar]
- 56.Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, and Cavalieri D. 2013. Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PLoS One 8: e56651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amarsaikhan N, O’Dea EM, Tsoggerel A, Owegi H, Gillenwater J, and Templeton SP. 2014. Isolate-dependent growth, virulence, and cell wall composition in the human pathogen Aspergillus fumigatus. PLoS One 9: e100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caffrey AK, Beattie SR, Blaseg N, Hilmer KM, Zickovich JM, Cramer RA, and Obar JJ. 2016. Molecular Signatures of Eukaryotic Pathogens: Aspergillus fumigatus strain-specific host response is regulated by glucose sensing. J Immunol 196 (1 Supplement) 205.6. [Google Scholar]
- 59.Kowalski CH, Beattie SR, Fuller KK, McGurk EA, Tang YW, Hohl TM, Obar JJ, and Cramer RA Jr. 2016. Heterogeneity among Isolates Reveals that Fitness in Low Oxygen Correlates with Aspergillus fumigatus Virulence. mBio 7: e01515–01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caffrey-Carr AK, Kowalski CH, Beattie SR, Blaseg NA, Upshaw CR, Thammahong A, Lust HE, Tang YW, Hohl TM, Cramer RA, and Obar JJ. 2017. Interleukin 1alpha Is Critical for Resistance against Highly Virulent Aspergillus fumigatus Isolates. Infection and immunity 85: : e00661–00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Rubio R, Monzon S, Alcazar-Fuoli L, Cuesta I, and Mellado E. 2018. Genome-Wide Comparative Analysis of Aspergillus fumigatus Strains: The Reference Genome as a Matter of Concern. Genes 9: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertuzzi M, van Rhijn N, Krappmann S, Bowyer P, Bromley MJ, and Bignell EM. 2021. On the lineage of Aspergillus fumigatus isolates in common laboratory use. Med Mycol 59: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busch CJ, Favret J, Geirsdottir L, Molawi K, and Sieweke MH. 2019. Isolation and Long-term Cultivation of Mouse Alveolar Macrophages. Bio-protocol 9: e3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P, Boumpas D, Netea MG, van de Veerdonk FL, Kontoyiannis DP, and Chamilos G. 2013. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J Immunol 191: 1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balloy V, Huerre M, Latge JP, and Chignard M. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infection and immunity 73: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duong M, Ouellet N, Simard M, Bergeron Y, Olivier M, and Bergeron MG. 1998. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J Infect Dis 178: 1472–1482. [DOI] [PubMed] [Google Scholar]
- 67.Lewis RE, and Kontoyiannis DP. 2009. Invasive aspergillosis in glucocorticoid-treated patients. Med Mycol 47 Suppl 1: S271–281. [DOI] [PubMed] [Google Scholar]
- 68.Roilides E, Uhlig K, Venzon D, Pizzo PA, and Walsh TJ. 1993. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infection and immunity 61: 4870–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simmons SR, Bhalla M, Herring SE, Tchalla EYI, and Bou Ghanem EN. 2021. Older but Not Wiser: the Age-Driven Changes in Neutrophil Responses during Pulmonary Infections. Infection and immunity 89: e00653–006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun K, and Metzger DW. 2014. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol 192: 3301–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jasper AE, McIver WJ, Sapey E, and Walton GM. 2019. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engelich G, Wright DG, and Hartshorn KL. 2001. Acquired disorders of phagocyte function complicating medical and surgical illnesses. Clin Infect Dis 33: 2040–2048. [DOI] [PubMed] [Google Scholar]
- 73.Dowey R, Iqbal A, Heller SR, Sabroe I, and Prince LR. 2021. A Bittersweet Response to Infection in Diabetes; Targeting Neutrophils to Modify Inflammation and Improve Host Immunity. Frontiers in immunology 12: 678771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blez D, Blaize M, Soussain C, Boissonnas A, Meghraoui-Kheddar A, Menezes N, Portalier A, Combadière C, Leblond V, Ghez D, and Fekkar A. 2020. Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica 105: 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


