Abstract
Lentivirus-based transduction systems are widely used in biological science and cancer biology, including cancer immunotherapy. However, in in vivo transplanted tumor model, the immunogenicity of these transduced cells was not appropriately addressed. Here we used empty vector transduced mouse melanoma (B16) and carcinoma (Lewis lung Carcinoma) cells transplanted tumor model to study the immune response due to the transduction processes. We showed that the overall in vivo tumor growth rate gets reduced in transduced cells only in immune-competent mice but not in nude mice. This data indicates the involvement of the immune system in the in vivo tumor growth restriction in the transduced group. Further studies showed that specific activation of CD8+ T cells might be responsible for restricted tumor growth. Mechanistically, transduced tumor cells show the higher activity of type I interferon, which might play an essential role in this activation. Overall, our data indicates the modulation of the immune system by lentiviral vector transduced tumor cells, which required further studies to explore the mechanisms and better understand the biological significance. Our data also indicates the importance of considering the immunogenicity of transduced cells when analyzing in vivo results, especially in studies related to immunotherapy.
Keywords: lentivirus, CD8+ T cells, type I Interferon
Introduction:
Lentiviruses(1) have been extensively utilized as gene delivery vectors in cancer research, due to their high transduction efficiency in dividing and non-dividing cells. Lentivirus-mediated integration of the target gene(s) or different types of RNA (s) into the genome provides a stable expression of a gene(s) or RNA (s) within the cells. 3rd generation Lentiviral vector contains the target element flanked by long terminal repeats (LTRs), which is required for genome integration. In contrast, the other additional accessory genes like a gag, pol, and env are present in trans to help in the packaging(2,3). Other than the target gene, the lentiviral vector contains an exogenous promoter, which allows the expression a transgene and, in general, a drug resistance gene for the selection procedure. This 3rd generation vector constructs design minimized the foreign viral protein in the final transduced cells. With the development of lentiviral-based transduction and stable cell formation, numerous scientific discoveries have been made in diverse research fields, including cancer biology and cancer immunology. One of the interesting aspects of these studies is that, in general, all these studies did not investigate the alteration of immunogenicity of these transduced cells, though it is a valid possibility. The relevant question of immunogenicity of transduced cells becomes significant, particularly when an in vivo model is used to address any therapy or immunotherapy-related questions. In vivo gene therapy by lentivirus showed that immune response can be generated against transgene, promoter element(4,5), and any foreign protein which might be expressed by transduced cells(6-8). However, activation of the immune system due to the anti-viral immune response in transduced cells was not reported. Here we explore the immunogenicity of the lentivirus transduced tumor cells and its effect on the tumor growth. We found that lentivirus transduced tumor cells elicited the immune response and showed reduced tumor growth compared to unmodified parental tumor cells. Our data also indicates that lentiviral-based transduction aid to secrete type I interferon, which might play a key role in stimulating the immune response.
Materials and methods:
Cell line, mice, and Reagents:
Mouse Luise Lung carcinoma (LLC) and B16 cell line were a kind gift from B. Lu’s laboratory, were cultured in DMEM high glucose (Hyclone) with 10 percent heal inactivated FBS (Gibco), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 50 U/penicillin (Invitrogen), streptomycin (50 μg/ml) (Invitrogen). Peripheral blood mononuclear cells (PBMCs) transduced with TCR gp100 and 526-MEL were obtained from Dr. U. Kammula’s laboratory. PBMCs were cultured in complete medium [RPMI-1640, 10% heat-inactivated human AB serum (Gemini Bio-Products, Woodland, CA), 2 mM l-glutamine (Invitrogen, Carlsbad, CA), 50 U/penicillin (Invitrogen), streptomycin (50 μg/ml) (Invitrogen), gentamicin (50 μg/ml) (Invitrogen), 10 mM HEPESs (Invitrogen), and Amphotericin B (250 ng/ml) (Invitrogen). Six-week female C57BL/6j mice and nude mice were purchased from Jackson laboratory. anti-mouse CD45 (clone 30-F11, BD), anti-mouse CD8a (clone 53-6.7, BD), anti-mouse CD4 (clone GK1.5, BD) CD69 (clone H1.2F3, Biolegend), anti-human CD3 (SK7, BioLegend), anti-human CD4 (SK3, BioLegend), anti-human CD8 (SK1, BioLegend), and anti-human 137(4-1BB) (4B4-1, BioLegend).
Lentivirus transduction:
293T cells were transfected with empty vector pCDH and pMD2.G and psPAX2 for 48 hours to produce lentivirus. Supernatant-containing lentivirus was collected after 48. LLC and B16 cells were infected by the lentiviruses particles for 24 hours, followed by puromycin selection. Selected cells are considered stable expressing cell lines.
Tumor inoculation and tumor volume measurement:
5- to 6-week-old C57BL/6j mice or athymic nude mice have injected 1 x 106 LLC cells or 5 x 105 B16 cells subcutaneously into the flanks of the mice. Mice were monitored every two days. Tumor volume was measured manually by using a caliper was alculated as: Tumor volume = 0.5 x (width)2 × length mm3. All animal studies were performed in accordance with the institutional guidelines and approved protocol by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh.
Statistical Analysis:
All reported results represent the mean ± SEM Statistical significance was established by unpaired Student t-test (for two groups) or one-way analysis of variance followed by Tukey post-hoc test (for more than two groups) using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). Differences between groups attaining a p-value of 0.05 are considered as significant.
Supplementary methods:
Further information on research methods is available in the supplemented methods section
Results:
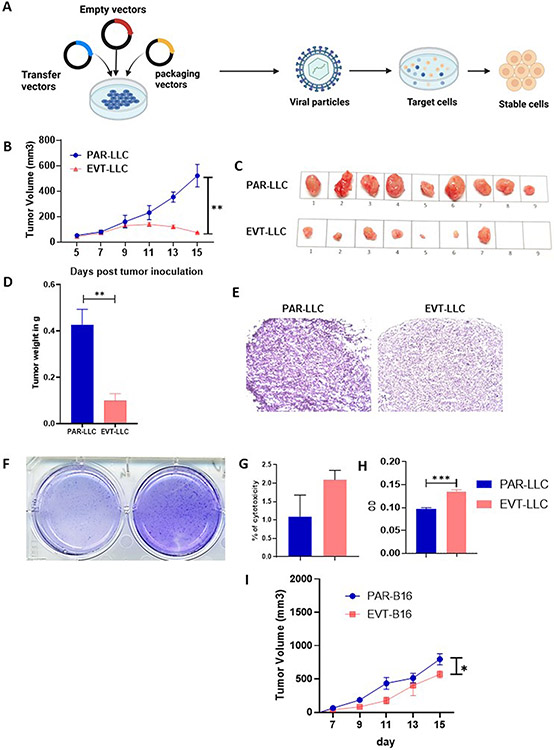
Lentivirus mediated Empty-Vector (pCDH) transduced cancer cell line showed reduced tumor growth in B6 mice.
Lentivirus-mediated transduced cells are frequently used in in vivo and in vitro cancer biology models to establish the importance of specific gene(s) or different types of RNA (s) (protein) in cancer progression(9,10). But whether this transduction itself alters any intrinsic or extrinsic properties of cancer cell lines, which may affect the scientific conclusion where these transduced cells are used, is not demonstrated in current literature. Henceforth, in this report article, we explore whether transduction itself alters any of the properties of the tumor cells. To address this question, we transduced two mouse cancer cell lines (1. Lewis Lung Carcinoma, LLC and 2. melanoma, B16F10) with pCDH empty vector plasmid by utilizing 3rd generation three plasmid-based lentiviral system (Figure 1A). Post selection of stable cell lines, cell lines were maintained in DMEM media without selection pressure. To check whether transduced tumor cells have any altered tumor property, we measure the tumor generation property in these transduced cells in C57BL/6j mice compared to unaltered parental cell lines. We found that both empty-vector transduced LLC (EVT-LLC) have reduced tumor growth (520.1±88.62 PAR-LLC vs 73.73±4.0 EVT-LLC) compared to unaltered parental cell line (PAR-LLC) (figure 1B-C) at the endpoint of experiments. Tumor weight (Figure 1D) and histological analysis (Figure 1E) support this notion. Early-onset necrosis was observed in EVT-LLC tumors (starting as early as day 7 post tumor inoculation in some mice). We also found that the total number of necrotic tumors in the EVT-LLC tumor model was more than its parental LLC tumor (3 Mice in PAR-LLC tumor v/s 6 Mice in EVT-LLC tumor). A similar tumor growth restriction was observed in B16 (EVT-B16) model (Figure 1I). However, the difference in tumor necrosis was not prominent in the B16F10 melanoma tumor model.
Figure 1: Empty vector-induced tumor cell lines showed lower tumor growth.
A. Schematic diagram of vector transduction. B. Tumor volume curve. C. Representative image of tumor growth in PAR-LLC and EVT-LLC groups. D. Bar diagram indicates mean tumor weight ± SEM E. Histological representation of the PAR-LLC and EVT-LLC group tumor sample. F. Representative image of colony formation assay. G. bar diagram indicates the mean % cell death ± SEM. H. Tumor cell proliferation by MTT assay± SEM. I. tumor volume curve for B16 melanoma model*<0.05, **<0.005.
Given that in vivo tumor growth was reduced in the EVT-LLC tumor group, we hypothesized that this observation might occur due to alteration of tumor cell line intrinsic properties such as cell proliferation or cell death. To check this hypothesis, we performed in vitro colony-forming and LDH release assays (for cell death). In contrast to our observation in the mouse model, we found that overall colony formation in the EVT-LLC group was slightly higher than in PAR-LLC cells (figure 1F) and there was no significant alteration of cell death between the two cell lines (figure 1G). Overall data suggested that alteration of in vivo tumor growth was due to the extrinsic factors of the in vivo system rather than alteration of the tumor cells’ proliferation or cell death.
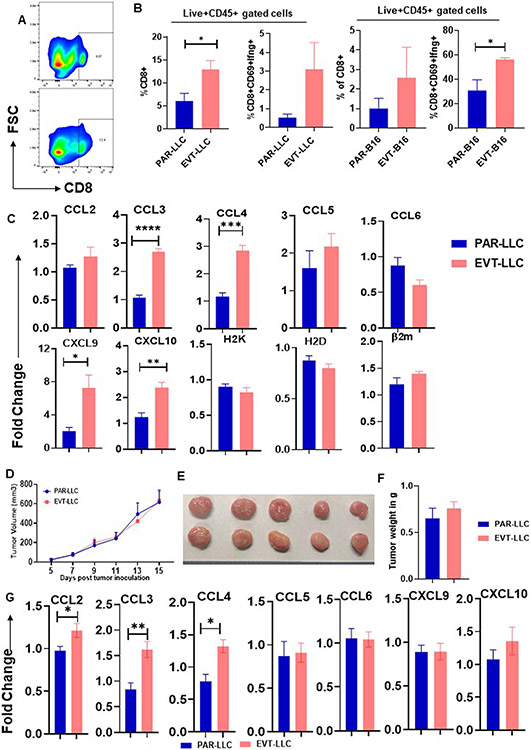
EVT-tumor showed high activated T cells infiltration with tumor stroma.
We previously established that empty vector-induced transduced cells have altered cell growth only in vivo. We hypothesized that the immune system might play a pivotal role in this restriction, as immune-mediated tumor growth restriction is an established fact. To address this hypothesis, we focused on the status of the immune system within the tumor stroma by flow cytometry analysis and found that EVT-LLC tumor showed high CD8+ T cells infiltration along with a CD69hi and IFNγhi phenotypes (Figure 2A-B) compared to the PAR-LLC model. This data indicates that vector transduced cells induced a more potent CD8+ T cell-based immune response. As we found that EVT-LLC tumors have more CD8+ T cells infiltration, next we check the status of different chemokine expressions, which are known to aid in T cells homing within tumor stroma(11), like CCL2 to CCL6, CXCL9 and CXCl10 by qRTPCR. We found that CCL3 and CCL4 expression get significantly upregulated within EVT-LLC tumors (Figure 2C). Overall, our data suggested that EVT-LLC tumor stroma induced higher expression of CCL3 and CCL4 which aid in CD8+ T cells infiltration. And these CD8+ T cells attenuate the tumor growth of EVT-LLC.
Figure 2. Activated CD8 T cells prevent EVT tumor growth.
A. Representative flowcytometry graph. B. Bar diagram indicates the mean percent cell population ± SEM C. Bar diagram indicates mean fold change ± SEM D. Tumor growth curve in nude mice. Graph indicates tumor growth in day dependent manner. Every data point indicates average tumor volume ± SEM E. Representative tumor growth in nude mice. F. Bar diagram indicates mean tumor volume ± SEM in nude mice. G. Bar diagram indicates fold change ± SEM in mRNA expression of different chemokine *<0.05, **<0.005, ***<0.0002, ****<0.0001
To further confirm the contribution of CD8+ T cells in EVT-LLC tumor growth restriction, we check the tumor growth in nude mice (which specifically lack T cells). We found that both the EVT-LLC and PAR-LLC tumors have comparative tumor growth in nude mice (Figure 2D-F). Chemokine analysis by qRT-PCR from nude mice tumors also showed a similar pattern of increased CCL3 and CCL4 expression in the EVT-LLC group. This data indicates that the milieu of EVT-LLC tumors in immune-competent and immune-deficient mice are similar at least in terms of chemokine expression. Overall, our observations suggest that EVT-LLC reduced growth is T cells dependent.
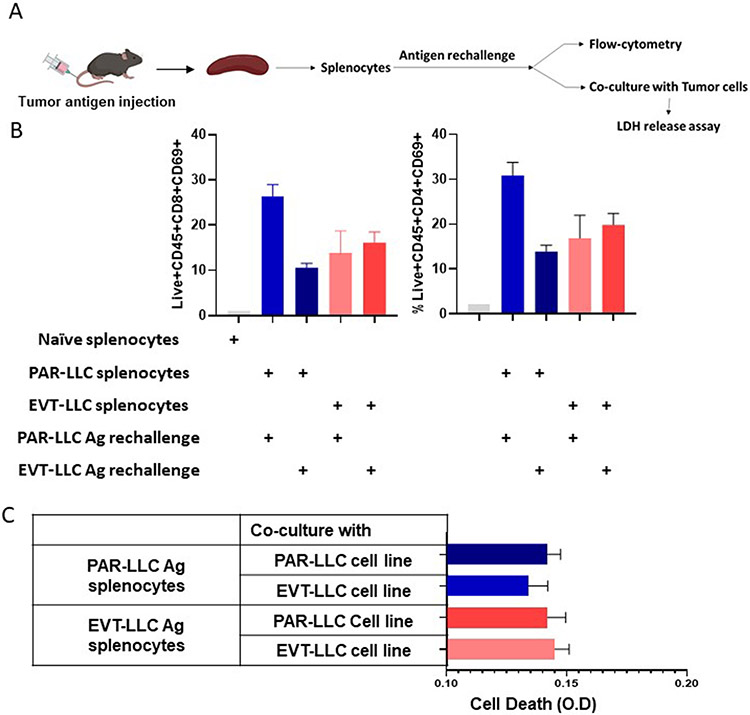
EVT-tumor induced T cells activation through an antigen-independent mechanism.
Next we sought to determine if the antigen-dependent activation of T cells in PAR-LLC and EVT-LLC groups can explain the increase immunogeneicity of lentiviral vector transduced tumor cells because antigen-dependent immune alteration caused by several exogenous proteins in the vectors have been reported by Dubrot et al(12) and Frederick et al(13). To generate antigen-specific response, we first immunized mice with either PAR-LLC or EVT-LLC crude lysate (Figure 3A). Seven days after lysate immunization, spleens and splenocytes were isolated. Splenocytes were restimulated with both antigen separately and early activation marker CD69 was measured. In a separate set of restimulates T cells were co-cultured with tumor cells (1:10 ratio). And cytotoxicity was measured by measuring the LDH release in the media. We found that the same antigen stimulation induced higher CD69 expression, particularly in the PAR-LLC group (Figure 3B). In the EVT-LLC group a very marginal increase in CD69 expression was found when re-stimulated with the same EVT-LLC lysate. However, we found fewer T cells activation in the EVT-LLC lysate treated group compare to the PAR-LLC group. Next, we found that EVT-LLC lysate restimulated splenocytes showed slightly more tumor toxicity against both the PAR-LLC and EVT-LLC tumor cells, and PAR-LLC lysate restimulated T cells showed less toxicity toward EVT-LLC tumor cells (Figure 3B). These data do not provide clear evidence that increased EVT-LLC immunogenicity is mediated by the antigen-dependent activation of T cells.
Figure 3. Effect of the supernatant on splenocytes.
A. Schematic diagram of experimental design. B. Bar diagram indicates average T cells activation ± SEM. C. Bar diagram indicates ave age tumor cell death ± SEM.
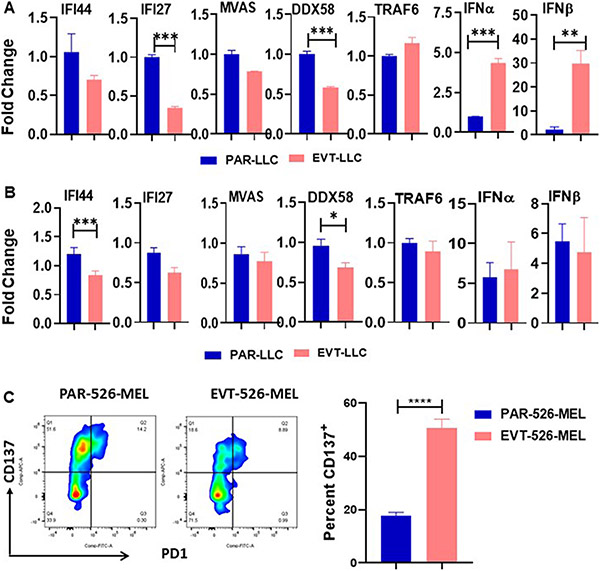
EVT-tumor-induced T cells activation is partially dependent on type I interferon.
Since lentivirus infection can activate cell type I interferon pathway(14), we next sought to determine whether lentivirus-mediated type I interferon alteration in tumor cells might have any effect on T cells activation. To address this at first, we checked alteration in the expression of anti-viral pathway molecules like- RNA virus detecting sensor molecules and type I Interferon and Interferon stimulatory genes in cell lines by quantitative RT-PCR. qRT-PCR data indicate that high expression of IFNa and IFN b in the EVT-LLC cell line (Figure 4 A) compared to PAR-LLC. Next, we check the anti-viral pathway molecules in the tumor sample. However, we do not find any significant alteration in anti-viral pathway molecules. A minor increment of IFNa and IFNb (Figure 4B) was observed in the EVT-LLC tumor. Kranz et al(15) and Kolumam et al(16) and others(17,18) already showed that type I interferon have a positive role in stimulating CD8+ T cells response. Considering all these parameters, we conclude that anti-viral pathways including type I interferon might have some partial role in the CD8+ T cells activation. We used a CD8+ T cell and tumor cell co-culture system to determine to validate this hypothesis. In this co-culture system, peripheral blood mononuclear cells (PBMCs) transduced with TCR gp100 were co-cultured with gp100 expressing PAR-526-MEL and EVT-526-MEL cells. Because the gp100-TCR-CD8+ T cells could only be activated by gp100 antigen and both PAR-526-MEL and EVT-526-MEL express similar level of gp100 antigen, we can use the activation of CD8+ T cell to determine the antigen-independent immunogenicity of two cells. This analysis revealed that EVT-526-MEL cells can induce more T cells activation compared to PAR-526-MEL cells. This data indicates the possibility of cytokine-mediated T cells activation by empty vector transduced tumor cells (Figure 4C).
Figure 4. EVT tumor secret IFNγ.
A. & B. Bar diagram indicates the mean ± SEM fold change of mRNA expression of anti-viral sensor pathway of cell line and tumor respectively. C. Representative Flow cytometry data indicates the CD137 marker. Bar diagram indicates the mean percentage of CD137 ± SEM. *<0.05; **<0.005; ***<0.001.
Discussion:
Lentiviral mediated transduction is a rapid and robust technique to produce stable cell lines expressing the transgene. Hence it is widely used in the diverse biological field to analyze the effect of that specific transgene in biological processes. Previous studies that have utilized the lentiviral-based transduction system did not have reported/studied the antigen-independent immune response when these transduced cells are transplanted into the in vivo system. Here, we present the first study to report that lentiviral vector transduction provides a nonspecific immunogenicity for syngeneic tumor models via type I interferon dependent pathway. Based on the tumor growth quantification and immune populations analysis, we established that lentivirus transduced-tumor cells have a more activated type I interferon pathway, which stimulates the immune response.
In general, earlier studies that have used lentivirus transduced tumor cells did not compare the tumor growth of transduced cells against unmodified parental cells(19-22). In our study, we have shown that transduced cells, even with empty vectors, might contain immuno-stimulatory properties and alter the possible outcome of any in vivo experiments. Here we showed that transduced cells (even with an empty vector) could stimulate an immune response and reduce the overall tumor growth in both LLC and B16 models. Our observation also suggested that lentivirus-mediated transduction does not alter the tumor cells’ intrinsic properties like proliferation and cell death in vitro but stimulates more CD8+ T cells migration within tumor stroma in immuno-competent mice. To further corroborate our hypothesis, we found that in nude mice, the tumor growth rate of both cells was similar and confirmed that the reduced growth rate was due to immune response. One possible explanation of the empty vector transduced cell-mediated immune response is that any foreign protein like – an antibiotic-resistant gene may induce an immune response, as indicated by others(12,13). On the other hand, we hypothesized that lentivirus transduction might stimulate the cell-intrinsic anti-viral immune response (cells response against single-strand RNA, DNA, and double-strand DNA) to mitigate overall in vivo tumor growth. Our hypothesis's rationale is that DNA is transferred into the cytosol during transduction, which is ultimately integrated into the genome to form stably transduced cells. By design of the cellular compartmentalization, DNA is primarily present either in the nucleus or mitochondria. The presence of cytosolic DNA indicates the pathogenic infection or mitochondrial and/or nucleus damage, which is taken care of by innate immune system and anti-viral innate immune response and formation of inflammation. Simultaneously DNA damage and repair systems also stimulate innate immune responses which took place during transduced DNA integration into the DNA. Interestingly, our in vivo experimentation could not establish the antigen-dependent activation of T cells. We found that when we rechallenged the lysate vaccinated splenocytes with the same antigen, only the PAR-LLC lysate showed T cells activation. In contrast, we failed to get similar data from EVT-LLC group stimulation. One of the specific limitations of this particular experiment was that we immunized the mice with lysate. Cell lysate is unknown composition; it may contain various factors to skew the observation, which is a shortcoming of our study. It may require a more refined experiment to overcome the shortcomings of our study and establish the antigen-dependent mechanism observed by others in different experimental settings. Based on this premise, next, we focused on the anti-viral pathway. Though we did not find any activation of the anti-viral pathway as per se, we did observe that lentivirus transduction stimulates type I interferon secretion. Type I interferon gets secreted by cells during the anti-viral immune response. Type I interferon can stimulate CD8+ T cells based on anti-tumor response(23-25), which might play an essential role in our experiment’s in vivo CD8+ T cells activation. To confirm these observations, we utilized a co-culture system of the 526-Mel cell lines which express gp100 and T cells with TCR against gp100. In this system, as both parental and transduced cells express the similar antigen, theoretically, both groups with activated gp100 specific T cells at per the same level. However, our co-culture data indicates that empty vector transduced 526-MEL cells induced more T cells activation. This observation suggests that irrespective of antigens (as both PAR-526-Mel and EVT-526-MEL cells express gp 100), T cells (customized to detect only gp100 antigen) get more activated when co-cultured with EVT-526-MEL cells. This observation indicates that transduction of 526-MEL cells induced T cells stimulation in an antigen-independent manner, which was in line with our hypothesis Overall our data indicate irrespective of the antigen-dependent T cells activation, stably transduced cells can stimulate T cells activation in nonspecific ways.
On the basis of our observation, we believe a future perspective of fine-tuning of plasmid vector sequence will be vital to reducing innate immune activation. Reducing CpG island, altering methylation status, or even incorporating modified based (like the mRNA vaccine) may be incorporated into a plasmid vector to reduce immune response.
Overall, our in vivo data clearly showed that lentivirus transduction reduced tumor growth rate than the unmodified group in both LLC and B16 models. Hence, this data indicates the importance of considering the transduction induced immunogenicity in analyzing in vivo experiments, especially studies exploring cancer immunotherapy. Our data provides a base point for a further in-depth investigation of the immune response generated due to transduction and stable cell generation via type I interferon pathway alteration. Understanding the overall immune alteration by transduced cells will be essential for understanding and analyzing the data more accurately in the future.
Supplementary Material
Acknowledgments
This study was supported by the Shear Family Foundation (to D.Y.), the American Cancer Society Research Scholar Award (132632-RSG-18-179-01-RMC to D.Y.), and National Cancer Institute (1R01CA222274 and R01CA255196 to D.Y.).
Footnotes
Conflict of Interested:
None
Reference:
- 1.Vecchio C Del, Calistri A, Parolin C, Mucignat-caretta C. Lentiviral Vectors as Tools for the Study and Treatment of Glioblastoma. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durand S, Cimarelli A. The Inside out of Lentiviral Vectors. Viruses. 2011;3:132–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dull TOM, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J Virol. 1998;72:8463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16:741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune Responses to Viral Gene Therapy Vectors. Mol Ther [Internet]. Elsevier Ltd.; 2020;28:709–22. Available from: 10.1016/j.ymthe.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresser K, Dijkgraaf FE, Pritchard CEJ, Huijbers IJ, Song JY, Rohr JC, et al. A mouse model that is immunologically tolerant to reporter and modifier proteins. Commun Biol [Internet]. Springer US; 2020;3:6–11. Available from: 10.1038/s42003-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergwerf I, De Vocht N, Tambuyzer B, Verschueren J, Reekmans K, Daans J, et al. Reporter gene-expressing bone marrow-derived stromal cells are immune-tolerated following implantation in the central nervous system of syngeneic immunocompetent mice. BMC Biotechnol. 2009;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiotto MT, Reth MA, Schreiber H. Genetic changes occurring in established tumors rapidly stimulate new antibody responses. Proc Natl Acad Sci U S A. 2003;100:5425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. 2007;104:15735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullock BL, Kimball AK, Poczobutt JM, Neuwelt AJ, Li HY, Johnson AM, et al. Tumor-intrinsic response to IFN γ shapes the tumor microenvironment and anti – PD-1 response in NSCLC. 2019;2:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sackstein R, Schatton T, Barthel SR. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Investig [Internet]. Nature Publishing Group; 2017;97:669–97. Available from: 10.1038/labinvest.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubrot J, Lane-reticker SK, Kessler EA, Haining WN, Yates KB, Manguso RT, et al. Resource In vivo screens using a selective CRISPR antigen removal lentiviral vector system reveal immune dependencies in renal cell carcinoma In vivo screens using a selective CRISPR antigen removal lentiviral vector system reveal immune dependencies in r. Immunity [Internet]. Elsevier Inc.; 2021;54:571–585.e6. Available from: 10.1016/j.immuni.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Differentiation CDTC, Frederick MA, Li B, Johnston RJ, Xiao N, Chen R, et al. Resource In Vivo RNA Interference Screens Identify Regulators of Antiviral. 2014;325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Jin T, et al. PNAS Plus: From the Cover: IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci [Internet]. 2013;110:E4571–80. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1311669110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. antiviral defence for cancer immunotherapy. Nature [Internet]. Nature Publishing Group; 2016;534:396–401. Available from: 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- 16.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. 2005;202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 Interferons and Antiviral CD8 T-Cell Responses. 2012;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings RN, Grayson JM, Barton ES. Type I Interferon Signaling Enhances CD8 ؉ T Cell Effector Function and Differentiation during Murine Gammaherpesvirus 68 Infection. 2014;88:14040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Jiang H, Zhang H. EBioMedicine In situ administration of cytokine combinations induces tumor regression in mice. EBioMedicine [Internet]. The Authors; 2018;37:38–46. Available from: 10.1016/j.ebiom.2018.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang CH, Yue J, Pfeffer SR, Handorf CR, Pfeffer LM. MicroRNA miR-21 Regulates the Metastatic Behavior of. J Biol Chem [Internet]. © 2011 ASBMB. Currently published by Elsevier Inc; originally published by American Society for Biochemistry and Molecular Biology.; 2011;286:39172–8. Available from: 10.1074/jbc.M111.285098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Duan Y, Cheng S, Chen Y, Hu Y, Zhang L, et al. EBV-encoded RNA via TLR3 nasopharyngeal carcinoma induces inflammation in. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, et al. Report VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. 2012;191–9. [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Klement JD, Ibrahim ML, Xiao W, Redd PS. Type I interferon suppresses tumor growth through activating the STAT3-granzyme B pathway in tumor-infiltrating cytotoxic T lymphocytes. Journal for ImmunoTherapy of Cancer; 2019;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benci JL, Johnson LR, Choa R, Wolchok JD, Kambayashi T, Minn AJ, et al. Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Article Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade. Cell [Internet]. Elsevier Inc.; 2019;178:933–948.e14. Available from: 10.1016/j.cell.2019.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuertes MB, Woo S, Burnett B, Fu Y, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol [Internet]. Elsevier Ltd; 2013;34:67–73. Available from: 10.1016/j.it.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.