Abstract
Button-like junctions are discontinuous contacts at the border of oak-leaf-shaped endothelial cells of initial lymphatic vessels. These junctions are distinctively different from continuous zipper-like junctions that create the endothelial barrier in collecting lymphatics and blood vessels. Button junctions are point contacts, spaced about 3 µm apart, that border valve-like openings where fluid and immune cells enter lymphatics. In intestinal villi, openings between button junctions in lacteals also serve as entry routes for chylomicrons. Like zipper junctions that join endothelial cells, buttons consist of adherens junction proteins (VE-cadherin) and tight junction proteins (claudin-5, occludin, and others). Buttons in lymphatics form from zipper junctions during embryonic development, can convert into zippers in disease or after experimental genetic or pharmacological manipulation, and can revert back to buttons with treatment. Multiple signaling pathways and local microenvironmental factors have been found to contribute to button junction plasticity and could serve as therapeutic targets in pathological conditions ranging from pulmonary edema to obesity.
Button-like junctions, also called button junctions, are discontinuous intercellular contacts between endothelial cells of the initial region of lymphatic vessels in most organs (Baluk et al. 2007). Button junctions border valve-like openings that serve as routes for interstitial fluid and cells to enter initial lymphatics (lymphatic capillaries). The openings between buttons are positioned upstream to intraluminal valves in collecting lymphatics that prevent retrograde lymph flow. In the intestine, initial lymphatics are the site of entry of chylomicrons that carry dietary lipids to the venous bloodstream. Uniquely located in initial lymphatics, button junctions, and the openings between them, differ from continuous zipper-like junctions (zipper junctions) that seal the endothelium of collecting lymphatics, larger lymphatics, and blood vessels and form the transendothelial permeability barrier (Zhang et al. 2020; Norden and Kume 2021). Button junctions have not been described in other regions of lymphatics or in blood vessels.
This review develops a historical context for what has been learned about the formation, plasticity, and function of button junctions in lymphatics to build on and complement excellent recent reviews on junctions of lymphatic endothelial cells (Zhang et al. 2020; Norden and Kume 2021). The topic has a foundation of solid historical evidence for fluid entering lymphatics through a process coupled to mechanical forces in surrounding tissues. However, the nature of the path taken from interstitium into the lymphatic lumen has been debated over many years.
There is no dispute that lymph flows along a hydrostatic pressure gradient from initial lymphatics to collecting lymphatics, where it is propelled forward by smooth muscle contraction and mechanical forces in surrounding tissues and is prevented from flowing backward by intraluminal valves. The understanding of lymphatic valve development, function, and maintenance has benefitted from advancements described in excellent reviews (Schmid-Schonbein 2003; Muthuchamy and Zawieja 2008; Bazigou et al. 2014; Sabine and Petrova 2014; Vittet 2014; Bernier-Latmani and Petrova 2017; Iyer et al. 2020; Norden and Kume 2020; Petrova and Koh 2020; Francois et al. 2021; González-Loyola and Petrova 2021). By comparison, the process of fluid and cell entry into lymphatics has a more complicated history, where mechanisms have been proposed and adopted, only to be reinterpreted or invalidated by further evidence. The purpose of this review is to consider the evolution of understanding of cellular mechanisms underlying fluid and cell entry into lymphatics and how the discovery of button junctions in initial lymphatics has helped reconcile earlier concepts.
EVOLUTION OF UNDERSTANDING OF LYMPHATIC JUNCTIONS AND VALVES
We begin with historical developments that led to the current understanding of lymphatic junctions and valves. This history is often overlooked in modern texts that lose sight of original observations and concepts that are rediscovered. This history also provides a reminder that discoveries and interpretations reflect the techniques and knowledge of the time and are reinterpreted with continued advances. Throughout the process, scientific controversies and personality conflicts were no less heated historically than today.
Advances in the Seventeenth Century
The discovery of buttons and zipper junctions reflects an evolution built on the work of many. The story of the rediscovery of intestinal lymphatics by Gaspare Aselli in his experiments in 1622 on fed or fasting dogs is well documented (Mayerson 1969; Barrowman and Tso 1989; Suy et al. 2016, 2017). Although it is unclear who first recognized the specialization of initial lymphatics for fluid entry, the discovery of the intraluminal valves is attributed to at least four candidates. Aselli may well have observed lymphatic valves but did not describe them in his famous posthumously published treatise (Aselli 1627). In 1653 in Uppsala, Sweden, Olof Rudbeck clearly described valves (valvula in Latin) in lymphatics of the liver in his treatise entitled Nova Excercitatio Anatomica, translated into English (Nielsen et al. 1942), but Rudbeck depicted lymphatic vessels in his drawings as smooth tubes and did not illustrate valves.
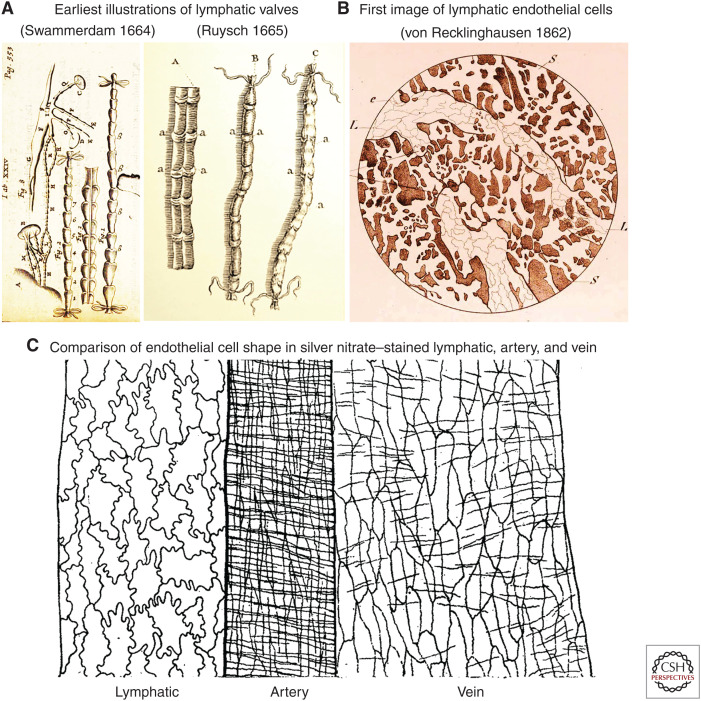
The first accurate drawings of lymphatic valves were made a decade later by two colleagues at the University of Leiden in the Dutch Republic, which was at that time a hothouse of enlightened scientific experimentation. Jan Swammerdam was a 24-year-old medical student when he used fine tubes and ligatures to inflate lymphatics on June 22, 1664, to make the valves visible (Fig. 1A). He described and illustrated the results in a letter to friends, but they were not published until he completed his PhD thesis (Blasius 1666; Suy et al. 2017). Meanwhile, his friend Frederik Ruysch described very similar results in a short treatise in 1665 (Fig. 1B; Ruysch 1665). Multiple fascinating and sometimes conflicting accounts of this early history have been reported (Mayerson 1969; Barrowman and Tso 1989; Ijpma and van Gulik 2013; Suy et al. 2016, 2017; Irschick et al. 2019). The understanding reflected in these early reports has been extensively updated since then through careful study of the development, structure, and function of intraluminal valves (Bazigou et al. 2014; Petrova and Koh 2020; Francois et al. 2021; González-Loyola and Petrova 2021).
Figure 1.
Early studies of lymphatics. (A) The first illustrations of intraluminal (secondary) valves in lymphatics. (Left panel) Dog lymphatics drawn by Jan Swammerdam in June 1664 but only published in 1666 in a commentary on a contemporary textbook of anatomy. (Panel A reprinted from Table 24 in Blasius 1666 without restriction because figure is in the public domain.) (Right panel) Drawings by Frederik Ruysch published 1 year earlier (1665). The lymphatic labeled A has been dissected longitudinally to show the valves (labeled a). (Lymphatic labeled A, right side reprinted from Figure 1 in Ruysch 1665 without restriction because figure is in the public domain.) (B,C) Early drawings of the shape of lymphatic endothelial cells stained by silver nitrate. (B) Faint endothelial cell borders in lymphatics of guinea-pig diaphragm drawn in 1862. (Panel B reprinted from Table 2, Figure 2 in von Recklinghausen (1862) and reprinted without restriction because table and figure are in the public domain.) (C) Endothelial cells in lymphatic, where the cells are oak-leaf shaped, and in artery and vein of cat omentum drawn in 1918. The transverse lines over the artery and veins are outlines of smooth muscle cells. (Panel C is reprinted from Figure 1 in Casparis 1918 without restriction because figure is in the public domain.)
Advances in the Eighteenth and Nineteenth Centuries
In the eighteenth century, William Hunter, using tracer substances to visualize the lymphatics, developed the concept that lacteals and other lymphatics absorb fluid and return it to the blood system (Barrowman and Tso 1989). At the end of the nineteenth century, Ernest Starling and William Bayliss formulated equations of fluid transport from blood capillaries to the interstitial space and uptake by lymphatics, based on transendothelial hydrostatic pressure, osmotic pressure, and permeability (Starling 1894). This was in opposition to the view prevalent at that time that lymph formation involved active secretion (Fine 2014).
The concept of endothelial permeability invokes the barrier function of endothelial cells. Although the concept of intercellular junctions did not become formalized until the advent of transmission electron microscopy (TEM) in the 1950s and 1960s, the size and shape of individual lymphatic endothelial cells was well known in the mid-nineteenth century. Friedrich von Recklinghausen developed an empirical method using silver nitrate to stain endothelial cell borders, designated cement lines (von Recklinghausen 1862; Stadtmüller 1920). This method worked extraordinarily well for lymphatics and for blood vessels and gave results uncannily prescient of modern immunohistochemistry for junctional proteins, which was not developed until a century later. Von Recklinghausen correctly demonstrated that lymphatic vessels are composed of individual endothelial cells—in opposition to the concept of cellular syncytia prevailing at the time. However, he incorrectly concluded that the tips of initial lymphatics are open to the interstitial space. Nonetheless, the peculiar oak-leaf shape of endothelial cells was recognized in initial lymphatics, which were considered specialized for uptake of fluid and cells (Fig. 1C; von Recklinghausen 1862; Casparis 1918), but the significance of the distinctive oak-leaf shape was not linked to focal openings for fluid entry until more than 300 years after the discovery of intraluminal valves.
Advances in the Twentieth Century to Present
In the 1930s, Stephen Hudack and Philip McMaster explored the permeability of lymphatics and introduced the mouse ear and other models still in use today (Hudack and McMaster 1932; McMaster and Hudack 1932). They used vital dyes and particulate tracers to show that, under baseline conditions, initial lymphatics are permeable to diffusible dyes, but not to particulates such as colloidal carbon (India ink) and concluded that the wall of lymphatics acts as a semipermeable membrane. However, they found that lymphatic permeability increased in inflammation resulting from mechanical, thermal, or chemical injury, and that this increase preceded the appearance of edema (McMaster and Hudack 1932). They also reported that lymphatics proliferated in sustained inflammation through a process now called lymphangiogenesis, and that lymphatics were spontaneously contractile in some tissues but not in others (Pullinger and Florey 1937).
Howard Florey, who received the Nobel Prize in Physiology or Medicine in 1945 for his work on penicillin, used TEM to examine cement lines visible after silver nitrate staining and found that the lines were up to 50 times wider than gaps between endothelial cells (Florey et al. 1959). Florey concluded that the lines represent more likely the overlap of adjacent endothelial cells than gaps between cells (Florey et al. 1959). Florey also suggested that the borders of endothelial cells have special cytochemical properties because similar lines were evident after highly negatively charged sodium indigotetrasulfonate, heparin, indigo-carmine, dextran sulfate, or other highly sulfonated dyes, followed by Azure II or other basic dye (Florey et al. 1959). Some years later, using TEM, Leak (1986) found lymphatic endothelial cell borders to have a high density of anionic sites that bind cationized ferritin particles. Subsequently, silver staining was found to be unusually wide at the tip of the scalloped borders of oak-leaf-shaped lymphatic endothelial cells and clearly different from the continuous borders of endothelial cells in collecting lymphatics (Zöltzer 2003).
In the second half of the twentieth century, openings between endothelial cells were described in initial lymphatics examined by TEM (Palay and Karlin 1959a; Casley-Smith 1965; Leak 1971). At that time, the nature of the route into lymphatics was hotly debated, but the striking TEM findings fit the view that intercellular openings were routes for entry of fluid, tracer particles, chylomicrons, and cells.
However, John Casley-Smith presciently considered four separate routes across the endothelial barrier, supported by evidence from TEM studies of ferritin, colloidal carbon, and thorium dioxide used as electron-dense tracers (Casley-Smith 1965). The first route was intercellular, where substances could pass through open junctions between endothelial cells, which were considered the main transit route in inflammation (Casley-Smith 1965). The other three routes were transendothelial pores, transcytosis of cellular organelles, and transporters that actively move substances into and across cells. Each route had its evidence and advocates, then and now, and was considered to subserve different functions (Leak and Burke 1968a; Azzali 2007; Triacca et al. 2017).
Fluid, cells, and chylomicrons were thought to enter lymphatics through openings between endothelial cells, while cholesterol and insulin entered by receptor-mediated transport (Lim et al. 2013; Yazdani et al. 2019). Caveolae and vesicles visible by TEM could participate in transport across lymphatic endothelial cells, but unlike openings at intercellular junctions, the distribution of tracers in vesicles did not change in inflammation, arguing against the involvement of transcytosis (French et al. 1960; Leak 1971; Azzali 2007). TEM studies also revealed that initial lymphatics had a thin or discontinuous basement membrane and lacked smooth muscle cells that could interfere with entry of substances (Palay and Karlin 1959b; French et al. 1960; Casley-Smith and Florey 1961; Leak 1970).
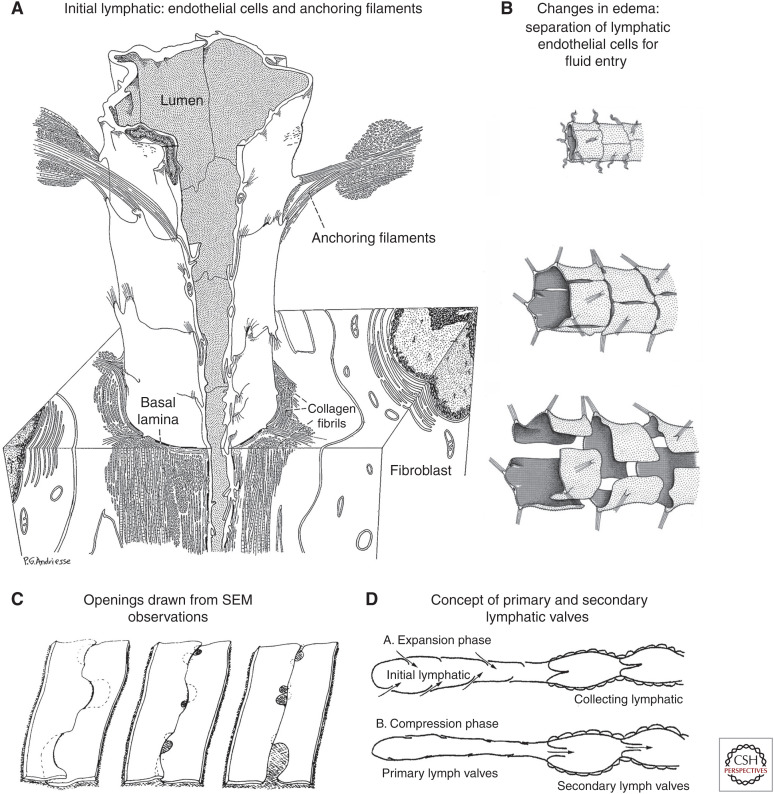
TEM studies by Lee Leak supported the presence of open intercellular junctions in initial lymphatics and led to three-dimensional renderings of lymphatic endothelial cells under baseline conditions and when lymphatic endothelial cells were pulled apart by anchoring filaments tensioned by tissue swelling, enabling fluid entry (Fig. 2A,B; Leak and Burke 1968b; Leak 1970; Majno and Joris 1996). While the diagrams are visually attractive, they raised questions of mechanisms and feasibility of endothelial cells separating completely from one another and then reattaching to restore barrier function.
Figure 2.
Evolving concepts of open junctions between lymphatic endothelial cells. (A) Three-dimensional rendering of initial lymphatic reconstructed from transmission electron micrographs. Anchoring filaments link endothelial cells to the surrounding connective tissue. (Panel A from Figure 25 in Leak and Burke 1968b; reprinted, with permission, from Rockefeller University Press © 1968.) (B) Concept of changes in endothelial cell junctions in initial lymphatic. (Top) Normal condition, junctions are closed. (Middle) Moderate edema, slits form between endothelial cells. (Bottom) Severe edema, endothelial cells are fully detached and widely separated from one another. (Panel B from Figure 12.14 in Majno and Joris 1996; reprinted, with permission, from John Wiley © 1996.) (C) Three-dimensional renderings of lymphatic endothelial cell borders based on scanning electron microscopy (SEM) images of initial lymphatics with interstitial pressure at normal level (left), moderately increased (middle), and greatly increased (right). Focal regions of intercellular junction detachment are shown as shaded openings that enlarge as lymphatics dilate. (Panel C from Figure 16 in Castenholz 1987; reprinted, with permission, from the International Society of Lymphology © 1987.) (D) Concept of “expansion” phase, when primary valves are open, lymph enters the initial lymphatic along the hydrostatic pressure gradient and secondary valves are closed to prevent backflow, and “compression” phase, when primary valves are closed, secondary valves are open, and lymph is pushed through the collecting lymphatic. (Panel D is from Figure 7 in Mendoza and Schmid-Schonbein 2003; reprinted, with permission, from the American Society of Mechanical Engineers © 2003.)
By using scanning electron microscopy (SEM) to observe the luminal surface of lymphatics, Anton Castenholz found that initial lymphatic endothelial cells had open junctions and overlapping endothelial cell borders that could function as valves (Fig. 2C; Castenholz 1984, 1987; Zöltzer 2003; Baluk et al. 2007). Complementary SEM views of the abluminal surface of lymphatics were obtained using a method introduced by Ushiki (1990) for removing extracellular connective tissue by collagenase digestion and alkali hydrolysis, but questions remained about the mechanism of fluid entry.
ANCHORING FILAMENTS OF LYMPHATICS
Anchoring filaments are thought to couple interstitial tension to the lymphatic lumen opening. These filaments were first described in 1935 by Beatrice Pullinger and Howard Florey who reported that lymphatics of the mouse ear were surrounded by a network of collagen and reticular fibers thought to promote lymphatic opening in the presence of edema (Pullinger and Florey 1935). Their work revealed anchoring filaments that attached the abluminal edges of lymphatic endothelial cells to the connective tissue matrix. They concluded that anchoring filaments open lymphatics as interstitial tension increases. Decades later, Lee Leak and John Burke described anchoring filaments viewed by TEM as 10-nm elastin-like filaments that connect regions near lymphatic endothelial cell junctions to the surrounding connective tissue (Fig. 2A,B; Leak and Burke 1968b).
Although further progress has been limited, the main protein component of anchoring filaments was reported to be fibrillin (Gerli et al. 2000). Fibrillin, which is secreted by lymphatic endothelial cells in culture (Weber et al. 2002), attaches to endothelial cells by integrins a2b1, a3b1, and avb3 and is linked to focal adhesion kinase (FAK) (Gerli et al. 2000; Vainionpää et al. 2007). Consistent with other unresolved issues, based on SEM observations, Castenholz questioned the nature of anchoring filaments described by Lee Leak, and reported instead a broader, more diffuse network of connective tissue filaments around initial lymphatics (Castenholz 1987). Casley-Smith maintained that anchoring filaments did not pull the wall of lymphatics open during normal fluid filling but did so in edema (Casley-Smith 1980).
BASEMENT MEMBRANE OF LYMPHATICS
Based on early TEM observations, endothelial cells of initial lymphatics were reported to have little or no basement membrane (basal lamina) (Palay and Karlin 1959b; French et al. 1960; Casley-Smith and Florey 1961; Leak and Burke 1968a). However, this view has been revised in light of immunohistochemical evidence of a thin, discontinuous basement membrane, which—like that of blood vessels—contains type IV collagen, laminins, perlecan, nidogen, among other proteins (Vainionpää et al. 2007; Pflicke and Sixt 2009). The basement membrane is thought to contribute to binding and entry of immune cells into initial lymphatics without limiting fluid entry (Vainionpää et al. 2007; Pflicke and Sixt 2009).
CONCEPT OF PRIMARY AND SECONDARY VALVES
Ultrastructural observations together with functional and bioengineering considerations led Geert Schmid-Schönbein's group to advance the concept of primary and secondary valves in lymphatics (Trzewik et al. 2001; Mendoza and Schmid-Schönbein 2003; Lynch et al. 2007; Murfee et al. 2007). Accordingly, lymph entering through flap-like primary valves in initial lymphatics is propelled forward by smooth muscle contraction and mechanical forces in surrounding tissues and is prevented from flowing backward by secondary valves in collecting lymphatics (Fig. 2D). Microspheres up to 0.8 µm in diameter could enter lymphatics in their experiments (Lynch et al. 2007). Still unresolved, however, was the cellular mechanism underlying primary valves that enabled entry between lymphatic endothelial cells.
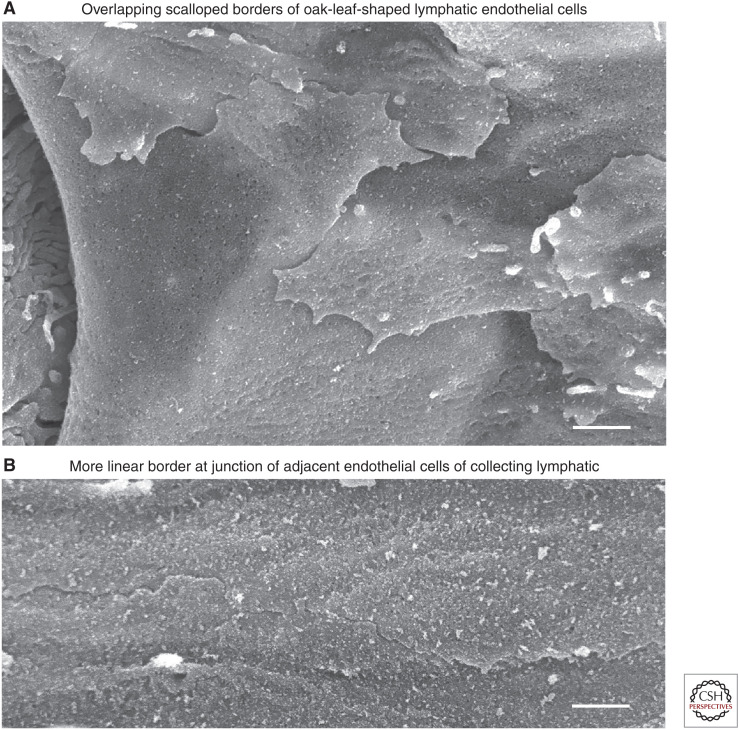
The mystery of primary valve openings began to be solved by findings of loosely overlapping flaps at scalloped endothelial cell borders visible when the abluminal surface of initial lymphatics was viewed in three dimensions by high-resolution field-emission SEM (Fig. 3A,B; Baluk et al. 2007). This finding fits with earlier evidence of oak-leaf-shaped endothelial cells and the presumptive entry route between endothelial cells based on TEM observations. Yet, still missing was a mechanism that reconciled the primary valve concept with the known structure of junctions between lymphatic endothelial cells.
Figure 3.
Abluminal surface of lymphatic endothelial cell borders. Scanning electron microscopy view of abluminal surface of lymphatics after removal of surrounding connective tissue. (A) Initial lymphatic: Loosely apposed, overlapping, scallop-shaped cell borders of endothelial cells. (B) Collecting lymphatic: Tightly apposed linear borders of adjacent endothelial cells. Scale bars, 1 µm. (Panels A and B from Figure 1 and Supplemental Figure 1 in Baluk et al. 2007; reprinted courtesy of Creative Commons License (Attribution–Noncommercial–Share Alike 4.0 Unported license).)
JUNCTIONS BETWEEN LYMPHATIC ENDOTHELIAL CELLS
Endothelial cell junctions were initially identified and characterized by TEM (Brightman and Reese 1969; Simionescu et al. 1975; Pinto da Silva and Kachar 1982), but have acquired much greater functional significance through step-by-step characterization of the proteins of tight junctions and adherens junctions (Tsukita and Tsukita 1989; Lampugnani et al. 1993). Although the identification process began with proteins of desmosomes and then adherens junctions and tight junctions between epithelial cells (Tsukita and Tsukita 1985, 1989; Franke 2009), similar junctional proteins were subsequently found in endothelial cells of blood vessels and then lymphatics (Dejana 2004; Pfeiffer et al. 2008; Dejana et al. 2009; Duong and Vestweber 2020).
In endothelial cells, VE-cadherin is the principal adherens junction protein, and claudin-5, occludin, endothelial selective adhesion molecule (ESAM), and junctional adhesion molecule 1 (JAM1, JAM-A) are the best-studied tight junction proteins, but other claudins and JAMs have also been found (Claesson-Welsh et al. 2021). Gap junction proteins—connexins 37, 40, and 43—mediate electrical and chemical coupling of endothelial cells but do not directly contribute to barrier function (Meens et al. 2017; Okamoto et al. 2019). Endothelial barrier function is governed by the interaction of proteins of tight junctions and adherens junctions with vascular endothelial tyrosine phosphatase (VE-PTP), zonula occludens-1 (ZO1), other cytoplasmic signaling molecules, and links to the actomyosin cytoskeleton (Orsenigo et al. 2012; Arif et al. 2021; Claesson-Welsh et al. 2021; Vestweber 2021).
Technical developments in creating reporter and mutant mice and dissecting the regulation of junctional proteins are advancing the understanding of endothelial junctions and barrier function in lymphatics (Zhang et al. 2020; Geng et al. 2021; Norden and Kume 2021; Stritt et al. 2021). Gene expression assessments of lymphatic endothelial cells by single-cell RNA sequencing have revealed clear differences among initial lymphatics, collecting lymphatics, and other lymphatic phenotypes in the mesentery (González-Loyola et al. 2021), skin (Hernández Vásquez et al. 2021), and lymph nodes (Takeda et al. 2019; Fujimoto et al. 2020; Xiang et al. 2020; Zhang et al. 2020; Norden and Kume 2021; Sibler et al. 2021), but links between gene-expression profiles and formation of regionally specific intercellular junctions remain to be identified.
BUTTON JUNCTIONS OF INITIAL LYMPHATICS
Structure and Composition
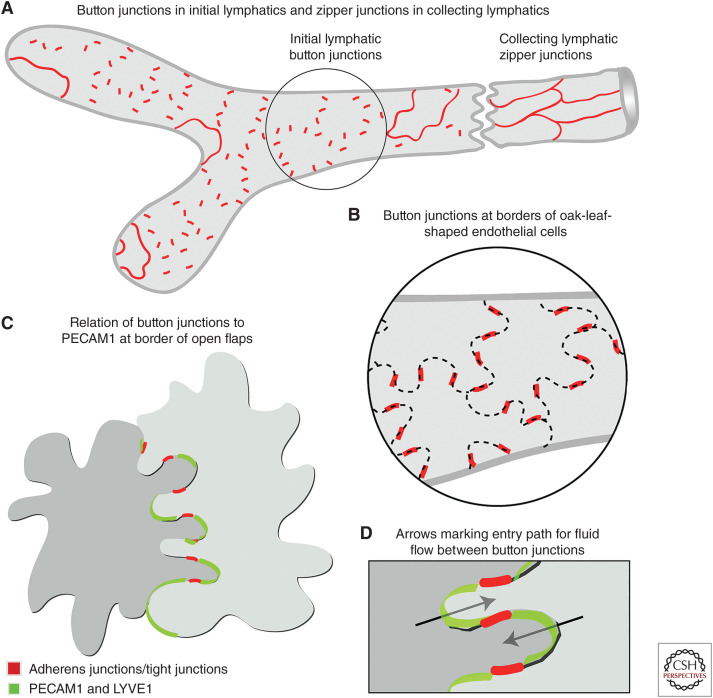
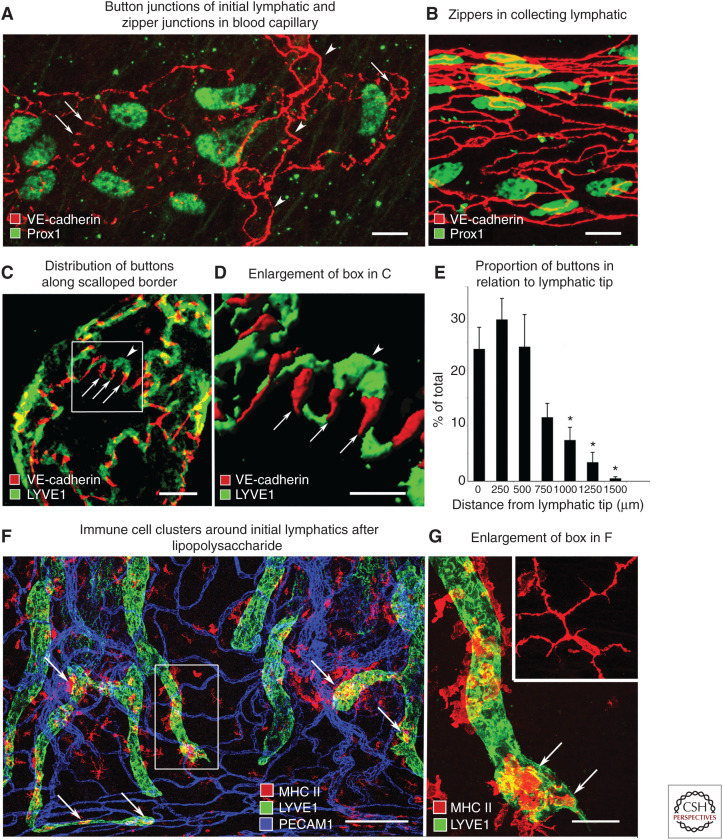
The realization that endothelial cells of initial lymphatics have distinctive intercellular junctions came initially from the discontinuous pattern of VE-cadherin staining in lymphatics of the mouse trachea (Fig. 4A,B; Baluk et al. 2007). The trachea proved well suited for this type of study because blood vessels and lymphatics in tissue whole mounts could be examined in three dimensions by confocal microscopy, which circumvented the limitation of interpreting thin histological sections (Baluk et al. 2005, 2007). Similar discontinuous VE-cadherin staining was found also in initial lymphatics of skin, diaphragm, urinary bladder, and intestinal villi (Baluk et al. 2007; Zheng et al. 2014). Tight junction proteins claudin-5, ESAM, and JAM1 had the same discontinuous distribution as VE-cadherin at the sides of overlapping flaps along the scalloped border of endothelial cells (Fig. 4C). In contrast, PECAM1 (platelet/endothelial cell-adhesion molecule 1, CD31) and LYVE1 were located at the tip of the flaps (Fig. 4C,D; Baluk et al. 2007).
Figure 4.
Diagrams of button and zipper junctions in lymphatics. (A) Drawing showing discontinuous button junctions (red line segments) in endothelium of initial lymphatics and continuous zipper junctions (continuous red lines) in collecting lymphatics. Both types of junctions consist of proteins typical of adherens junctions and tight junctions. (B) More detailed view showing the oak-leaf shape of endothelial cells (dashed lines) of an initial lymphatic. Buttons (red) appear to be oriented perpendicular to the cell border but are in fact parallel to the sides of flaps. (C,D) Enlarged views of buttons along the sides of flaps of adjacent oak-leaf-shaped endothelial cells with overlapping edges. PECAM1 and LYVE1 are located at the tip of flaps. Button-like adherens junctions and tight junctions at the sides of flaps direct fluid entry (arrows) through the junction-free region at the tip. Fluid traverses the basement membrane (not shown) and enters initial lymphatics through these openings without disruption of junctions. (Panels A–D from Figure 7 in Baluk et al. 2007; reprinted courtesy of Creative Commons License (Attribution–Noncommercial–Share Alike 4.0 Unported license).)
The discontinuous pattern of junctional proteins in initial lymphatics was conspicuously different from continuous junctions of collecting lymphatics (Fig. 5A,B; Baluk et al. 2007). Unlike continuous junctions, the junctions in initial lymphatics consisted of roughly parallel linear segments of junctional proteins, about 3 µm in length and 3 µm apart at the sides of flaps along the border of oak-leaf-shaped endothelial cells (Fig. 5C,D). As initial lymphatics joined collecting lymphatics, the junctions transitioned from discontinuous to continuous (Fig. 5E; Baluk et al. 2007).
Figure 5.
Buttons and zippers and cell entry into initial lymphatics. Confocal microscopic images of mouse tracheal lymphatics. (A) Staining for VE-cadherin marks discontinuous buttons (arrows) in an initial lymphatic. Arrowheads point to zipper junctions in a blood capillary. (B) Continuous zipper junctions in a collecting lymphatic. (C,D) Confocal images showing VE-cadherin at buttons (arrows) and LYVE1 between buttons (arrowhead) at the border of oak-leaf-shaped endothelial cells of initial lymphatic. (D) Imaris isosurface rendering of confocal image stack of enlarged boxed region in panel C. (E) Gradient in abundance of buttons shown as a function of distance from the tip of initial lymphatics. Mean ± SEM; *P < 0.05 compared to proportion at the tip. (Panels A–E from Figure 1 in Baluk et al. 2007; reprinted courtesy of Creative Commons License (Attribution–Noncommercial–Share Alike 4.0 Unported license).) (F) Lymphatics (LYVE1, green) and MHC II–positive immune cells (arrows, red) in whole mount of mouse trachea 24 h after intratracheal instillation of lipopolysaccharide. (G) Enlargement of boxed region in F of an initial lymphatic containing MHC II–positive cells (arrows) that are rounded and have fewer processes than a corresponding cell in a pathogen-free mouse (inset). Scale bars, 10 µm (A–C); 5 µm (D); 200 µm (F); 50 µm (G, inset). (Panels F and G from Figure 6 in Baluk et al. 2007; reprinted courtesy of Creative Commons License (Attribution–Noncommercial–Share Alike 4.0 Unported license).)
Discontinuous junctions in initial lymphatics were named “button junctions” because the focal contacts border openings between adjacent cells, analogous to buttons on a shirt. Consistent with the concept of primary valves, the region between buttons had no junctions to retard entry of fluid, cells, or particles the size of chylomicrons (Fig. 4D; Baluk et al. 2007). The presence of button junctions at the border of intercellular openings into initial lymphatics enabled flow to change with local physiological conditions without junction detachment and resealing.
Cell Entry between Button Junctions
Evidence of cell entry into lymphatics came initially from a remarkable series of real-time observations performed during the early twentieth century on living amphibian tail and rabbit ear preparations. Eliot and Eleanor Clark, who described many features of living blood vessels and lymphatics, reported that blood cells could enter or leave through holes in lymphatics (Clark and Clark 1932, 1933, 1935).
Later immunohistochemical studies of lymphatics in mouse tracheas 24 h after lipopolysaccharide exposure revealed MHC II–positive dendritic cells and macrophages near the tip of initial lymphatics with button junctions (Fig. 5F,G; Baluk et al. 2007). Leukocyte entry through transendothelial routes was not excluded (Azzali 2006) until Holger Pflicke and Michael Sixt (2009) used real-time confocal microscopic imaging of fluorescently labeled dendritic cells and lymphatics. Their studies documented dendritic cells entering initial lymphatics in mouse ear through what appeared to be preformed openings in the basement membrane within minutes of a stimulus. The observations have been confirmed and expanded by studies of leukocyte chemotaxis into initial lymphatics in lymphatic-reporter mice with labeled immune cells and other approaches to elucidate mechanisms of chemokine signaling, passage through the endothelium, crawling along the luminal surface, and transit to collecting lymphatics (Tal et al. 2011; Jackson 2019; Arasa et al. 2021a,b; Eichin et al. 2021; Johnson 2021).
While leukocyte entry into initial lymphatics receives greater attention, erythrocytes also can enter lymphatics (Clark and Clark 1926). Howard Florey described the clinical success of blood infusion into the abdomen of newborn infants being due to erythrocyte entry into lymphatics of the diaphragm en route to the bloodstream (Florey and Witts 1928). The process was promoted by increased intra-abdominal pressure, but the route of erythrocyte ingress into lymphatics was not determined (Florey and Witts 1928; Nagy 1992).
BUTTON JUNCTION PLASTICITY
The mechanistic understanding of button junctions has advanced through genetic and pharmacological approaches used to examine how junctions develop and change in disease and with experimental manipulation (Table 1; Zhang et al. 2020; Norden and Kume 2021). Junctional plasticity in initial lymphatics is manifested by transformation of button junctions into zippers or vice versa during development, genetic manipulation, disease, or therapeutic intervention. Button junctions have not been found in collecting lymphatics under normal or pathological conditions.
Table 1.
Effects of mechanistic manipulations on button junctions in lymphatics
| Mechanistic manipulation | Effect on buttons | References |
|---|---|---|
| Angiopoietin-2 (ANGPT2) | Necessary for button formation in initial lymphatics | Zheng et al. 2014 |
| Calcitonin receptor–like receptor (CALCR1) | Adrenomedullin receptor upstream of DLL4; lymphatic endothelial cell-specific Calcr1 mutations result in conversion of buttons to zippers, reduced lipid uptake, and intestinal lymphangiectasia | Davis et al. 2017, 2019 |
| CD36 | Lymphatic endothelial cell-specific Cd36 deletion disrupts button junctions in lacteals and collecting lymphatics in intestine and mesentery and reduced uptake of chylomicrons | Cifarelli et al. 2021 |
| Dexamethasone | Promotes conversion of zippers to buttons during development and in inflammation | Yao et al. 2010 |
| Delta-like4 (DLL4) Notch signaling | Necessary for button formation in lacteals, signaling downstream of VEGFR2 and VEGFR3, supports lacteal regeneration | Bernier-Latmani et al. 2015 |
| EphrinB2/EphB4 signaling | Necessary for stabilization of lymphatic junctions, regulates claudin-5 expression in lymphatic endothelial cells | Frye et al. 2020 |
| Gut microbiome | Promotes VEGF-C production and button formation in lacteals, antibiotic depletion of gut bacteria results in conversion of buttons to zippers in lacteals | Suh et al. 2019 |
| Inflammation | Promotes zipper formation in lymphatic sprouts in mouse airways after infection by Mycoplasma pulmonis | Baluk et al. 2007; Yao et al. 2012 |
| LYVE1 | Lyve1 deletion has no apparent effect on lymphatic junctions or fluid drainage but reduces leukocyte transit and amplifies chronic inflammation | Gale et al. 2007; Vieira et al. 2018 |
| Mechanical stretch | Promotes VEGF-C production and button formation in lacteals | Hong et al. 2020 |
| Neuropilin1 (NRP1) | Deletion of Vegfr1 and Nrp1 together promotes VEGF-A/VEGFR2 signaling and conversion of buttons to zippers in lacteals, reduces chylomicron uptake | Zhang et al. 2018 |
| PECAM1 | Deletion of Pecam1 has no apparent effect on button junctions or leukocyte entry into initial lymphatics | Baluk et al. 2007; Wang et al. 2016 |
| RhoA/ROCK signaling | Necessary for junction formation in lacteals and for secondary valves in collecting lymphatics, ROCK inhibition by Y-27632 promotes conversion of buttons to zippers | Zhang et al. 2018; Norden and Kume 2021 |
| S1P/S1PR1 signaling | S1PR1 regulates lymphatic quiescence by inhibiting VEGF-C signaling induced by shear stress, regulates claudin-5 expression | Pham et al. 2010; Geng et al. 2020 |
| VE-cadherin (cadherin-5, CDH5) | Anti-VE-cadherin antibody promotes rapid dissolution of button junctions, postnatal Cdh5 deletion has pronounced effects on lacteals and limited effect on skin lymphatics | Baluk et al. 2007; Hägerling et al. 2018 |
| VEGF-A/VEGFR2 signaling | VEGF-A signaling through VEGFR2 in lacteals results in button conversion to zippers and reduces chylomicron uptake, inhibition of VEGFR2 by DC101 antibody promotes button formation | Zhang et al. 2018 |
| VEGFR1 | Combined deletion of Vegfr1 and Nrp1 promotes VEGF-A/VEGFR2 signaling and results in button conversion to zipper junctions in lacteals, reduces chylomicron uptake | Zhang et al. 2018 |
| VEGF-C/VEGFR3 signaling | Promotes button formation in lacteals | Nurmi et al. 2015; Hong et al. 2020 |
| YAP/TAZ signaling | Upstream of Vegfc in maintenance of buttons in lacteals, Lats1/2 deletion activates YAP/TAZ signaling and reduces buttons, YAP/TAZ signaling balance needed to maintain button integrity | Cho et al. 2019; Hong et al. 2020 |
Developmental Changes
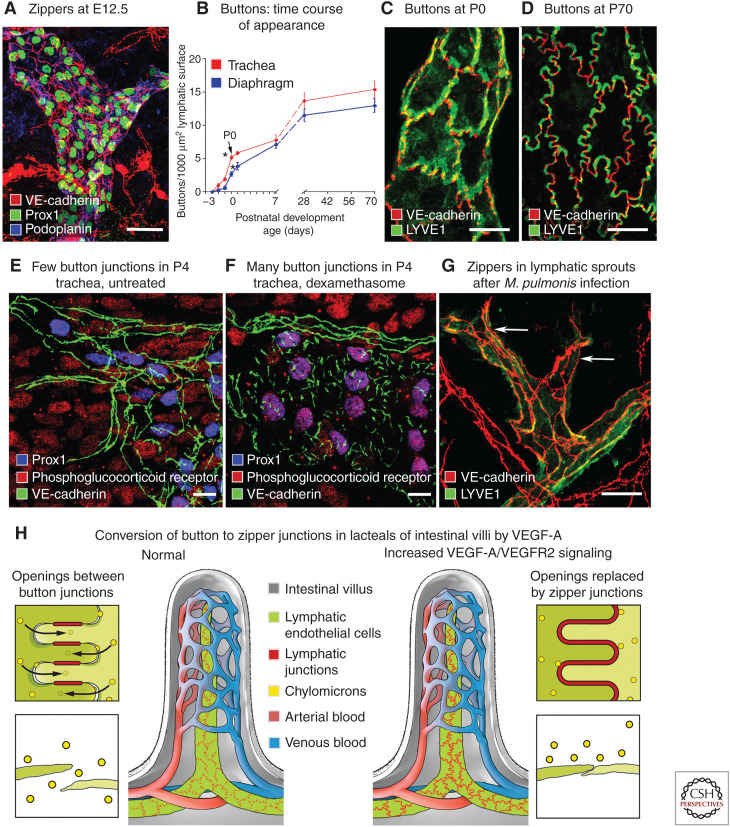
During embryonic development, endothelial cells of lymph sacs at E12.5 and tracheal lymphatics at E16.5 are interconnected by zipper junctions, not buttons (Fig. 6A; Yao et al. 2012). Just before birth, the proportion of buttons in tracheal lymphatics increases from 6% at E17.5 to 12% at E18.5, 35% at birth, 50% at postnatal day P7, 90% at P28, and 100% at 10 wk of age (Fig. 6B–D; Yao et al. 2012). This transformation also occurs in lymphatics in skin and diaphragm but at different times (Zheng et al. 2014).
Figure 6.
Plasticity of button junctions in initial lymphatics. (A) Endothelial cells joined by zipper junctions (arrows) in embryonic lymphatics at E12.5. (B) Time course of development of button junctions in lymphatics of trachea (red) and diaphragm (blue) from E16.5 to P70. Buttons are expressed as number of VE-cadherin-stained junctional segments. Buttons that appear before birth represent only about 35% of the eventual number in adult initial lymphatics in the trachea and 20% in the diaphragm at P70. *P < 0.05, values at P0 (arrow) are significantly different from those at E18.5. (C,D) Confocal microscopic images that illustrate the increasing proportion of buttons in initial lymphatics from P0 to P70. (E,F) Confocal images of tracheal lymphatics of P4 pups, either untreated (E) or treated with dexamethasone from P0 to P4 (F). Without treatment (E), lymphatic endothelial cells (Prox1, blue nuclei) have zipper junctions (VE-cadherin, green) and faint staining for phosphoglucocorticoid receptor (Ser211, red). After dexamethasone (F), lymphatics have button junctions and strong phosphoglucocorticoid receptor staining that colocalizes with Prox1 in lymphatic endothelial cell nuclei (purple, arrows). (G) Zippers in lymphatic endothelium after Mycoplasma pulmonis infection for 14 d. (Panels A–G from Figures 1, 2, 4, and 7 in Yao et al. 2012; reprinted, with permission, from the American Society for Investigative Pathology published by Elsevier © 2012.) (H) Entry of chylomicrons through openings between button junctions in normal intestinal lacteal (left) and lack of entry after conversion of buttons to zippers by activation of VEGF-A/VEGFR2 signaling in lacteal endothelial cells (right). Scale bars, 20 µm (A, E–G); 10 µm (C,D). (Panel H from McDonald 2018; adapted, with permission, from the author.)
Maturation of zippers into buttons can be promoted by the corticosteroid dexamethasone (Yao et al. 2012; Zheng et al. 2014). In the airways of neonates at P4, most junctions in initial lymphatics are intermediate between zippers and buttons (Fig. 6E). But all are buttons after treatment with dexamethasone from P0 to P4 (Fig. 6F; Yao et al. 2012). The junctional change in skin lymphatics can be advanced in utero by treatment of pregnant mice (Zheng et al. 2014). After dexamethasone, lymphatic endothelial cells become oak-leaf in shape, and the distribution of LYVE1 becomes complementary to VE-cadherin, as in the adult (Yao et al. 2012). Consistent with these changes after dexamethasone, nuclear glucocorticoid receptors in lymphatic endothelial cells are phosphorylated at Ser211, unlike in untreated mice (Fig. 6E,F; Yao et al. 2012).
Plasticity Accompanying Sprouting Lymphangiogenesis
Further evidence of button junction plasticity comes from observations that buttons in initial lymphatics are replaced by zippers during sustained inflammation after airway infection by the mouse pathogen Mycoplasma pulmonis (Fig. 6G; Baluk et al. 2007; Yao et al. 2012). Sprouting lymphangiogenesis is widespread in this condition (Baluk et al. 2005), and the sprouts have zippers instead of buttons (Baluk et al. 2007). Conversion of buttons to zippers after infection can be reversed by administration of dexamethasone (Yao et al. 2012).
Altered Button Junction Protein Expression
The adherens junction protein VE-cadherin (cadherin 5, CDH5) is essential for normal lymphatic development and function. Inhibition of VE-cadherin by blocking antibody results in rapid dissolution of button junctions in tracheal lymphatics of adult mice (Baluk et al. 2007). Genetic deletion of Cdh5 during embryonic development impairs lymphatic growth and leads to edema and prenatal death (Hägerling et al. 2018). Deletion of Cdh5 during the neonatal period results in structural abnormalities in lymphatics. Although the distribution of tight junction components of button junctions has not been examined under these conditions, genes encoding claudin-5 (Cldn5), JAM1, and ZO1 are up-regulated in endothelial cells of dermal initial lymphatics after Cdh5 deletion (Hägerling et al. 2018). Deletion of Cldn5 does not abolish the barrier function of lymphatic endothelial cells in vitro, and postnatal conditional deletion of Cldn5 (from P4 to P11) does not eliminate VE-cadherin from lymphatic endothelial cell junctions in vivo (Frye et al. 2020). Although a more complete understanding of the regulation of lymphatic endothelial junctions is clearly needed, depletion of Cldn5 is not singularly essential for maintenance of the endothelial barrier or the integrity of VE-cadherin at adherens junctions in lymphatics (Frye et al. 2020).
EphrinB2/EphB4 signaling regulates claudin-5 at endothelial cell junctions through the Rac1/Rho pathway and is required for stabilization of cell junctions in collecting lymphatics (Frye et al. 2020). Deletion of EphrinB2 disrupts button junctions in initial lymphatics, but the alteration is considered secondary to effects on collecting lymphatics (Frye et al. 2020).
Button junctions are not dependent on PECAM1. Although Pecam1 expression is increased in lymphatic endothelial cells after Cdh5 deletion (Hägerling et al. 2018), initial lymphatics in adult Pecam1-deficient mice have normal buttons and zippers (Baluk et al. 2007). After deletion of Cdh5, PECAM1 staining is discontinuous and located at the tip of endothelial flaps in initial lymphatics but can be broader than normal (Hägerling et al. 2018). Embryos of Pecam1 knockout mice have structurally abnormal mesenteric lymphatics with defective intraluminal valves (Wang et al. 2016), but this occurs at a stage before button junctions form in initial lymphatics.
As a caveat, discontinuous staining for VE-cadherin at lymphatic endothelial cell borders after genetic manipulations can be deceptive and unrelated to button junctions when it instead results from altered junction formation or stability. Discontinuous lymphatic junctions are among many abnormalities in mice with deletion of the zinc-finger transcription factor gene Gata2 or its target miRNA-126, because GATA2 regulates expression of VE-cadherin and claudin-5 in lymphatic endothelial cells (Mahamud et al. 2019). Lymphatic junctions in these mice are jagged, disorganized, irregularly spaced (Mahamud et al. 2019), and unlike button junctions at the border of flaps in initial lymphatics.
RAS-interacting protein 1 (RASIP1), an endothelial-specific regulator of GTPase signaling in endothelial cells, is another factor essential for junction formation. Deletion of Rasip1 in lymphatic endothelial cells results in disorganized junctions in embryos when zippers are normally present (Liu et al. 2018). Although discontinuous, the junctions in these mice lack the organized pattern of button junctions. Similarly disorganized junctions unlike buttons occur in collecting lymphatics after deletion of small GTPase Ras-related protein genes Rap1a and Rap1b (Xu et al. 2018) and have also been described in lymphatic endothelial cells in culture, where the disorganization is amplified by TNF-α exposure (Kakei et al. 2014). Convincing evidence has not yet been obtained for button junction formation in cultured lymphatic endothelial cells despite concerted effort.
Angiopoietin-2 and VEGF Family Members
Unlike the disorganization of junctions that results from disruption of junctional proteins, zippers can form normally but fail to convert into buttons. Angiopoietin-2 (ANGPT2), a partial agonist of Tie2 receptors with potent effects on lymphatic development (Gale et al. 2007; Dellinger et al. 2008), regulates the conversion of zippers to buttons in initial lymphatics during development (Zheng et al. 2014). Angpt2 deletion or inhibition by function-blocking antibody during gestation suppresses the formation of buttons; zippers persist postnatally (Zheng et al. 2014). However, once they have formed, button junctions are not altered by Angpt2 inhibition or overexpression (Zheng et al. 2014).
Manipulation of VEGF family growth factors and receptors has strong but complex effects on the development and maintenance of button junctions in intestinal lacteals (Zhang et al. 2018; Suh et al. 2019). If buttons change into zippers, lacteal uptake of chylomicrons made by intestinal epithelial cells is impaired and absorption of dietary lipid is disturbed (McDonald 2018; Zhang et al. 2018).
An elegant study by Anne Eichmann's group revealed that VEGF-A activation of VEGFR2 signaling induces button to zipper conversion in lacteals and reduces chylomicron uptake (Zhang et al. 2018). VEGFR2 inhibition has the opposite effect. Mechanistic experiments revealed that VEGFR1 expressed on endothelial cells of blood vessels in intestinal villi acts as a decoy receptor that limits the action of VEGF-A on lacteals (Zhang et al. 2018). Neuropilin-1 (NRP1) is a coreceptor for VEGF-A that acts similarly to VEGFR1. Deletion of Nrp1 and Vegfr1 genes together in endothelial cells increases VEGF-A availability, which promotes button conversion to zippers in lacteals and reduces dietary lipid uptake (Zhang et al. 2018). Similarly, inhibition of ROCK/Rho signaling by Y27632 promotes button to zipper conversion in lacteals and reduces uptake of dietary lipids (Zhang et al. 2018).
VEGF-C/VEGFR3 signaling in lacteals is another contributor to button junction maintenance and uptake of dietary lipid (Nurmi et al. 2015; Hong et al. 2020) and is influenced by the gut microbiota (Suh et al. 2019). Deletion of Vegfr3 in lymphatic endothelial cells reduces button junctions in lacteals (Suh et al. 2019). Of potential clinical significance, depletion of gut organisms by antibiotics has a similar effect by reducing macrophage VEGF-C expression, which decreases buttons, increases zippers, and impairs dietary lipid absorption (Suh et al. 2019).
Delta-like4/Notch and YAP/TAZ Signaling
Among other pathways that influence button junctions, Delta-like4 (Dll4)/Notch signaling, which is downstream to VEGFR2 and VEGFR3, promotes continuous, slow regeneration of lacteals and is necessary for button junction formation (Bernier-Latmani et al. 2015). Inactivation of Dll4 expression in lymphatic endothelial cells results in lacteal shortening, conversion of lacteal buttons to zippers, and impaired dietary lipid uptake, although button junctions are still present at the border of oak-leaf-shaped endothelial cells in lymphatics of the intestinal submucosa (Bernier-Latmani et al. 2015).
Activation of YAP/TAZ signaling promotes conversion of buttons to zippers in lacteals, with accompanying reduction of dietary lipid absorption (Hong et al. 2020). YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) are activated by genetic deletion of the upstream inhibitors, Lats1/2 (large tumor suppressor 1 and 2) (Hong et al. 2020). YAP and TAZ are the final transcriptional regulators in the canonical Hippo-YAP/TAZ pathway responsible for many aspects of cell differentiation and are negative regulators of Prox1 expression in lymphatics (Cho et al. 2019). Expression of Yap/Taz in lymphatic endothelial cells is increased by depletion of VE-cadherin (Hägerling et al. 2018). Yap/Taz deletion or hyperactivation can result in abnormal lymphangiogenesis (Cho et al. 2019).
Adrenomedullin, S1P, and CD36
Tamoxifen-induced genetic deletion in lymphatic endothelial cells of calcitonin receptor–like receptor (Calcrl), the receptor for adrenomedullin (ADM), results in intestinal lymphangiectasia and more linear VE-cadherin staining of junctions in intestinal lacteals, with accompanying reduction in dietary lipid uptake (Davis et al. 2017, 2019; Xu et al. 2021), but changes in button junctions have not specifically been examined.
Sphingosine-1-phosphate (S1P), which is essential for lymphocyte egress from lymph nodes, is reduced in lymph—but not in plasma—of mice lacking the S1P synthesis genes sphingosine kinase 1 (Sphk1) and Sphk2 in lymphatic endothelial cells (Pham et al. 2010). Tracheal lymphatics in these mice have fewer and more diffuse button junctions, implicating S1P signaling in the organization of these junctions (Pham et al. 2010). S1P signaling through S1P receptor 1 (S1PR1) regulates claudin-5 expression, promotes intercellular junction formation, and stabilizes lymphatic endothelial cells (Geng et al. 2020). These actions involve Delta-like4, ANGPT2, ADM, and other factors that have effects on lymphatic development and stability (Geng et al. 2020). ADM is expressed by lymphatic endothelial cells and has autocrine effects that stabilize intercellular junctions and the barrier function of the lymphatic endothelium (Klein and Caron 2015).
CD36, also known as scavenger receptor class B member 3, fatty acid translocase, and platelet glycoprotein 4, is a transmembrane protein that binds thrombospondin and long-chain fatty acids, among other ligands, and imports fatty acids into cells. The intensity of CD36 staining in lymphatic endothelial cells increases from intestinal lacteals to collecting lymphatics (Cifarelli et al. 2021). Inducible deletion of Cd36 in lymphatic endothelial cells in adult mice impacts the structure and function of intestinal lacteals: VEGF-C signaling is reduced, intercellular junctions become disorganized, and lacteals are shortened (Cifarelli et al. 2021). Although button and zipper junctions have not been examined in detail in these mice, discontinuous junctions in lacteals have greater separation and are less numerous (Cifarelli et al. 2021).
CONCLUDING REMARKS
The understanding of the process of fluid and cell entry into lymphatics has evolved over many years. Evidence for the involvement of openings between button junctions in initial lymphatics is relatively new but fits with many earlier views and has been documented by diverse contemporary experiments. Developmental, pathophysiologic, and mechanistic studies have revealed the plasticity of button junctions, where buttons can convert to continuous zipper junctions and revert back to buttons, given the appropriate conditions.
Changes in lymphatic junction function can have important functional consequences. Reduction in lipid absorption by intestinal lacteals after button conversion to zippers highlights how junctional alterations in lymphatics of one organ can have systemic effects (Fig. 6H). Elucidation of the effects of button junction plasticity will provide insight into the contributions of lymphatic function to disease pathophysiology of each organ.
Further advances are needed for a complete understanding of button junction formation, maintenance, and plasticity. Single-cell RNA sequencing of gene expression in lymphatic endothelial cells has already revealed heterogeneity not previously appreciated but needs to be extended to address junction formation and the contributions of organ-specific microenvironments in health and disease. Additional studies are also needed to integrate knowledge of growth factors and signaling pathways known to influence button junction plasticity. As in the past, progress will continue step-by-step to uncover new features of lymphatics; and as in the past, interpretations will accompany new findings but will often lag behind the eventual understanding.
ACKNOWLEDGMENTS
We thank Jonas Fuxe, Li-Chin Yao, Hiroya Hashizume, and other former members of the McDonald laboratory and Elisabetta Dejana and other colleagues for their important contributions to the work on button and zipper junctions in lymphatics. Supported in part by NIH Grants R01 HL127402, R01 HL059157, R01 HL143896, and P01 HL024136 from the NIH National Heart, Lung, and Blood Institute and Grant 11CVD03 from the Leducq Foundation.
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.perspectivesinmedicine.org
REFERENCES
- Arasa J, Collado-Diaz V, Halin C. 2021a. Structure and immune function of afferent lymphatics and their mechanistic contribution to dendritic cell and T cell trafficking. Cells 10: 1269. 10.3390/cells10051269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasa J, Collado-Diaz V, Kritikos I, Medina-Sanchez JD, Friess MC, Sigmund EC, Schineis P, Hunter MC, Tacconi C, Paterson N, et al. 2021b. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J Exp Med 218: 1–18. 10.1084/jem.20201413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif N, Zinnhardt M, Nyamay'Antu A, Teber D, Brückner R, Schaefer K, Li YT, Trappmann B, Grashoff C, Vestweber D. 2021. PECAM-1 supports leukocyte diapedesis by tension-dependent dephosphorylation of VE-cadherin. EMBO J 40: e106113. 10.15252/embj.2020106113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aselli G. 1627. De lactibus sive lacteis venis, quarto vasorum mesarai corum genere novo invento. G.B. Bidelli, Milan, Italy. [Google Scholar]
- Azzali G. 2006. On the transendothelial passage of tumor cell from extravasal matrix into the lumen of absorbing lymphatic vessel. Microvasc Res 72: 74–85. 10.1016/j.mvr.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Azzali G. 2007. Tumor cell transendothelial passage in the absorbing lymphatic vessel of transgenic adenocarcinoma mouse prostate. Am J Pathol 170: 334–346. 10.2353/ajpath.2007.060447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. 2005. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 115: 247–257. 10.1172/JCI200522037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. 2007. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204: 2349–2362. 10.1084/jem.20062596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman J, Tso P. 1989. Gastrointestinal lymphatics. In Handbook of physiology, Section 6, Vol. 1, The gastrointestinal system, motility and circulation (ed. Schultz SG, Wood JD), pp. 1733–1777. American Physiological Society, Rockville, MD. [Google Scholar]
- Bazigou E, Wilson JT, Moore JE Jr. 2014. Primary and secondary lymphatic valve development: molecular, functional and mechanical insights. Microvasc Res 96: 38–45. 10.1016/j.mvr.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier-Latmani J, Petrova TV. 2017. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol 14: 510–526. 10.1038/nrgastro.2017.79 [DOI] [PubMed] [Google Scholar]
- Bernier-Latmani J, Cisarovsky C, Demir CS, Bruand M, Jaquet M, Davanture S, Ragusa S, Siegert S, Dormond O, Benedito R, et al. 2015. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J Clin Invest 125: 4572–4586. 10.1172/JCI82045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius G. 1666. Joannis Veslingii syntagma anatomicum commentario atque appendice [Comments on the anatomical constitution of Johannes Veslingius], 2nd ed., Figure XXIV, p. 553. J. van Waesberge, Amsterdam. https://books.google.com/books?id=2edCQwAACAAJ& newbks=0&hl=en&source=newbks_fb [Google Scholar]
- Brightman MW, Reese TS. 1969. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40: 648–677. 10.1083/jcb.40.3.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith JR. 1965. Endothelial permeability. II: The passage of particles through the lymphatic endothelium of normal and injured ears. Br J Exp Pathol 46: 35–49. [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith JR. 1980. Are the initial lymphatics normally pulled open by the anchoring filaments? Lymphology 13: 120–129. [PubMed] [Google Scholar]
- Casley-Smith JR, Florey HW. 1961. The structure of normal small lymphatics. Q J Exp Physiol Cogn Med Sci 46: 101–106. [DOI] [PubMed] [Google Scholar]
- Casparis H. 1918. Lymphatics of the omentum. Anat Rec (Hoboken) 83: 437–447. [Google Scholar]
- Castenholz A. 1984. Morphological characteristics of initial lymphatics in the tongue as shown by scanning electron microscopy. Scan Electron Microsc 1984: 1343–1352. [PubMed] [Google Scholar]
- Castenholz A. 1987. Structural and functional properties of initial lymphatics in the rat tongue: scanning electron microscopic findings. Lymphology 20: 112–125. [PubMed] [Google Scholar]
- Cho H, Kim J, Ahn JH, Hong YK, Mäkinen T, Lim DS, Koh GY. 2019. YAP and TAZ negatively regulate Prox1 during developmental and pathologic lymphangiogenesis. Circ Res 124: 225–242. 10.1161/CIRCRESAHA.118.313707 [DOI] [PubMed] [Google Scholar]
- Cifarelli V, Appak-Baskoy S, Peche VS, Kluzak A, Shew T, Narendran R, Pietka KM, Cella M, Walls CW, Czepielewski R, et al. 2021. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat Commun 12: 1–15. 10.1038/s41467-021-23808-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L, Dejana E, McDonald DM. 2021. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol Med 27: 314–331. 10.1016/j.molmed.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ER, Clark E. 1926. The fate of extruded erythrocytes: their removal by lymphatic capillaries and tissue phagocytes, as seen in living amphibian larvae. Am J Anat 38: 41–70. 10.1002/aja.1000380103 [DOI] [Google Scholar]
- Clark ER, Clark EL. 1932. Observations on the new growth of lymphatic vessels as seen in transparent chambers introduced into the rabbit's ear. Am J Anat 51: 49–87. 10.1002/aja.1000510104 [DOI] [Google Scholar]
- Clark ER, Clark EL. 1933. Further observations on living lymphatic vessels in the transparent chamber in the rabbit's ear—their relation to the tissue spaces. Am J Anat 52: 273–305. 10.1002/aja.1000520204 [DOI] [Google Scholar]
- Clark ER, Clark EL. 1935. Observations on changes in blood vascular endothelium in the living animal. Am J Anat 57: 385–438. 10.1002/aja.1000570303 [DOI] [Google Scholar]
- Davis RB, Kechele DO, Blakeney ES, Pawlak JB, Caron KM. 2017. Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation. JCI Insight 2: e92465. 10.1172/jci.insight.92465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RB, Ding S, Nielsen NR, Pawlak JB, Blakeney ES, Caron KM. 2019. Calcitonin-receptor-like receptor signaling governs intestinal lymphatic innervation and lipid uptake. ACS Pharmacol Transl Sci 2: 114–121. 10.1021/acsptsci.8b00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. 2004. Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol 5: 261–270. 10.1038/nrm1357 [DOI] [PubMed] [Google Scholar]
- Dejana E, Tournier-Lasserve E, Weinstein BM. 2009. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16: 209–221. 10.1016/j.devcel.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, Witte M. 2008. Defective remodeling and maturation of the lymphatic vasculature in angiopoietin-2 deficient mice. Dev Biol 319: 309–320. 10.1016/j.ydbio.2008.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong CN, Vestweber D. 2020. Mechanisms ensuring endothelial junction integrity beyond VE-cadherin. Front Physiol 11: 1–9. 10.3389/fphys.2020.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichin D, Pessia A, Takeda A, Laakkonen J, Bellmann L, Kankainen M, Imhof BA, Stoitzner P, Tang J, Salmi M, et al. 2021. CD73 contributes to anti-inflammatory properties of afferent lymphatic endothelial cells in humans and mice. Eur J Immunol 51: 231–246. 10.1002/eji.201948432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine LG. 2014. Ernest henry starling (1866–1927) on the formation and reabsorption of lymph. Nephron Physiol 126: 9–17. 10.1159/000362620 [DOI] [PubMed] [Google Scholar]
- Florey H, Witts L. 1928. Absorption of blood from the peritoneal cavity. Lancet 211: 1323–1325. 10.1016/S0140-6736(00)81441-5 [DOI] [Google Scholar]
- Florey HW, Poole JC, Meek GA. 1959. Endothelial cells and “cement” lines. J Pathol Bacteriol 77: 625–636. 10.1002/path.1700770234 [DOI] [PubMed] [Google Scholar]
- Francois M, Oszmiana A, Harvey NL. 2021. When form meets function: the cells and signals that shape the lymphatic vasculature during development. Development 148: dev167098. 10.1242/dev.167098 [DOI] [PubMed] [Google Scholar]
- Franke WW. 2009. Discovering the molecular components of intercellular junctions—a historical view. Cold Spring Harb Perspect Biol 1: a003061. 10.1101/cshperspect.a003061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JE, Florey HW, Morris B. 1960. The absorption of particles by the lymphatics of the diaphragm. Q J Exp Physiol Cogn Med Sci 45: 88–103. [DOI] [PubMed] [Google Scholar]
- Frye M, Stritt S, Ortsäter H, Hernandez Vasquez M, Kaakinen M, Vicente A, Wiseman J, Eklund L, Martínez-Torrecuadrada JL, Vestweber D, et al. 2020. EphrinB2-EphB4 signalling provides Rho-mediated homeostatic control of lymphatic endothelial cell junction integrity. eLife 9: e57732. 10.7554/eLife.57732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto N, He Y, D'Addio M, Tacconi C, Detmar M, Dieterich LC. 2020. Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes. PLoS Biol 18: e3000704. 10.1371/journal.pbio.3000704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. 2007. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol 27: 595–604. 10.1128/MCB.01503-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Yanagida K, Akwii RG, Choi D, Chen L, Ho Y, Cha B, Mahamud MR, Berman de Ruiz K, Ichise H, et al. 2020. S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight 5: 1–18. 10.1172/jci.insight.137652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Ho YC, Srinivasan RS. 2021. Biochemical and mechanical signals in the lymphatic vasculature. Cell Mol Life Sci 78: 5903–5923. 10.1007/s00018-021-03886-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli R, Solito R, Weber E, Aglianó M. 2000. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology 33: 148–157. [PubMed] [Google Scholar]
- González-Loyola A, Petrova TV. 2021. Development and aging of the lymphatic vascular system. Adv Drug Deliv Rev 169: 63–78. 10.1016/j.addr.2020.12.005 [DOI] [PubMed] [Google Scholar]
- González-Loyola A, Bovay E, Kim J, Lozano TW, Sabine A, Renevey F, Arroz-Madeira S, Rapin A, Wypych TP, Rota G, et al. 2021. FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure. Sci Adv 7: 1–21. 10.1126/sciadv.abf4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerling R, Hoppe E, Dierkes C, Stehling M, Makinen T, Butz S, Vestweber D, Kiefer F. 2018. Distinct roles of VE-cadherin for development and maintenance of specific lymph vessel beds. EMBO J 37: e98271. 10.15252/embj.201798271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Vásquez MN, Ulvmar MH, González-Loyola A, Kritikos I, Sun Y, He L, Halin C, Petrova TV, Mäkinen T. 2021. Transcription factor FOXP2 is a flow-induced regulator of collecting lymphatic vessels. EMBO J 40: e107192. 10.15252/embj.2020107192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SP, Yang MJ, Cho H, Park I, Bae H, Choe K, Suh SH, Adams RH, Alitalo K, Lim D, et al. 2020. Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi. Nat Commun 11: 4102. 10.1038/s41467-020-17886-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudack S, McMaster PD. 1932. I. The permeability of the wall of the lymphatic capillary. J Exp Med 56: 223–238. 10.1084/jem.56.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijpma FF, van Gulik TM. 2013. “Anatomy lesson of Frederik Ruysch” of 1670: a tribute to Ruysch's contributions to lymphatic anatomy. World J Surg 37: 1996–2001. 10.1007/s00268-013-2013-x [DOI] [PubMed] [Google Scholar]
- Irschick R, Siemon C, Brenner E. 2019. The history of anatomical research of lymphatics—from the ancient times to the end of the European renaissance. Ann Anat 223: 49–69. 10.1016/j.aanat.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Iyer D, Jannaway M, Yang Y, Scallan JP. 2020. Lymphatic valves and lymph flow in cancer-related lymphedema. Cancers (Basel) 12: 2297. 10.3390/cancers12082297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG. 2019. Leucocyte trafficking via the lymphatic vasculature—mechanisms and consequences. Front Immunol 10: 471. 10.3389/fimmu.2019.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA. 2021. In sickness and in health: the immunological roles of the lymphatic system. Int J Mol Sci 22: 4458. 10.3390/ijms22094458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei Y, Akashi M, Shigeta T, Hasegawa T, Komori T. 2014. Alteration of cell–cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymphat Res Biol 12: 136–143. 10.1089/lrb.2013.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein KR, Caron KM. 2015. Adrenomedullin in lymphangiogenesis: from development to disease. Cell Mol Life Sci 72: 3115–3126. 10.1007/s00018-015-1921-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Caveda L, Breviario F, Del Maschio A, Dejana E. 1993. Endothelial cell-to-cell junctions. Structural characteristics and functional role in the regulation of vascular permeability and leukocyte extravasation. Baillieres Clin Haematol 6: 539–558. 10.1016/S0950-3536(05)80187-8 [DOI] [PubMed] [Google Scholar]
- Leak LV. 1970. Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res 2: 361–391. 10.1016/0026-2862(70)90031-2 [DOI] [PubMed] [Google Scholar]
- Leak LV. 1971. Studies on the permeability of lymphatic capillaries. J Cell Biol 50: 300–323. 10.1083/jcb.50.2.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak LV. 1986. Distribution of cell surface charges on mesothelium and lymphatic endothelium. Microvasc Res 31: 18–30. 10.1016/0026-2862(86)90003-8 [DOI] [PubMed] [Google Scholar]
- Leak LV, Burke JF. 1968a. Electron microscopic study of lymphatic capillaries in the removal of connective tissue fluids and particulate substances. Lymphology 1: 39–52. [PubMed] [Google Scholar]
- Leak LV, Burke JF. 1968b. Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol 36: 129–149. 10.1083/jcb.36.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. 2013. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab 17: 671–684. 10.1016/j.cmet.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Liu X, Gu X, Ma W, Oxendine M, Gil HJ, Davis GE, Cleaver O, Oliver G. 2018. Rasip1 controls lymphatic vessel lumen maintenance by regulating endothelial cell junctions. Development 145: dev165092. 10.1242/dev.165092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch PM, Delano FA, Schmid-Schönbein GW. 2007. The primary valves in the initial lymphatics during inflammation. Lymphat Res Biol 5: 3–10. 10.1089/lrb.2007.5102 [DOI] [PubMed] [Google Scholar]
- Mahamud MR, Geng X, Ho YC, Cha B, Kim Y, Ma J, Chen L, Myers G, Camper S, Mustacich D, et al. 2019. GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126. Development 146: dev184218. 10.1242/dev.184218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Joris I. 1996. Cells, tissues, and disease. Blackwell Science, Cambridge, MA. [Google Scholar]
- Mayerson H. 1969. Three centuries of lymphatic history—an outline. Lymphology 2: 143–150.4911483 [Google Scholar]
- McDonald DM. 2018. Tighter lymphatic junctions prevent obesity. Science 361: 551–552. 10.1126/science.aau5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster PD, Hudack S. 1932. Induced alterations in the permeability of the lymphatic capillary. J Exp Med 56: 239–253. 10.1084/jem.56.2.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meens MJ, Kutkut I, Rochemont V, Dubrot J, Kaladji FR, Sabine A, Lyons O, Hendrikx S, Bernier-Latmani J, Kiefer F, et al. 2017. Cx47 fine-tunes the handling of serum lipids but is dispensable for lymphatic vascular function. PLoS ONE 12: e0181476. 10.1371/journal.pone.0181476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza E, Schmid-Schönbein GW. 2003. A model for mechanics of primary lymphatic valves. J Biomech Eng 125: 407–414. 10.1115/1.1568128 [DOI] [PubMed] [Google Scholar]
- Murfee WL, Rappleye JW, Ceballos M, Schmid-Schönbein GW. 2007. Discontinuous expression of endothelial cell adhesion molecules along initial lymphatic vessels in mesentery: the primary valve structure. Lymphat Res Biol 5: 81–90. 10.1089/lrb.2007.1005 [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Zawieja D. 2008. Molecular regulation of lymphatic contractility. Ann NY Acad Sci 1131: 89–99. 10.1196/annals.1413.008 [DOI] [PubMed] [Google Scholar]
- Nagy JA. 1992. Lymphatic and nonlymphatic pathways of peritoneal absorption in mice: physiology versus pathology. Blood Purif 10: 148–162. 10.1159/000170042 [DOI] [PubMed] [Google Scholar]
- Nielsen A, Liljestrand G, Willner D. 1942. A translation of Olof Rudbeck's “Nova Excercitatio Anatomica” announcing the discovery of the lymphatics (1653). Bull Hist Med 11: 304–339. [Google Scholar]
- Norden PR, Kume T. 2020. The role of lymphatic vascular function in metabolic disorders. Front Physiol 11: 404. 10.3389/fphys.2020.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden PR, Kume T. 2021. Molecular mechanisms controlling lymphatic endothelial junction integrity. Front Cell Dev Biol 8: 627647. 10.3389/fcell.2020.627647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. 2015. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med 7: 1418–1425. 10.15252/emmm.201505731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Usuda H, Tanaka T, Wada K, Shimaoka M. 2019. The functional implications of endothelial gap junctions and cellular mechanics in vascular angiogenesis. Cancers (Basel) 11: 237. 10.3390/cancers11020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Young Koh G, Franco D, et al. 2012. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 3: 1208. 10.1038/ncomms2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Karlin LJ. 1959a. An electron microscopic study of the intestinal villus. I: The fasting animal. J Biophys Biochem Cytol 5: 363–371. 10.1083/jcb.5.3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Karlin LJ. 1959b. An electron microscopic study of the intestinal villus. II: The pathway of fat absorption. J Biophys Biochem Cytol 5: 373–384. 10.1083/jcb.5.3.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Koh GY. 2020. Biological functions of lymphatic vessels. Science 369: eaax4063. 10.1126/science.aax4063 [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B. 2008. Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur J Immunol 38: 2142–2155. 10.1002/eji.200838140 [DOI] [PubMed] [Google Scholar]
- Pflicke H, Sixt M. 2009. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 206: 2925–2935. 10.1084/jem.20091739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. 2010. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 207: 17–27. 10.1084/jem.20091619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P, Kachar B. 1982. On tight-junction structure. Cell 28: 441–450. 10.1016/0092-8674(82)90198-2 [DOI] [PubMed] [Google Scholar]
- Pullinger B, Florey H. 1935. Some observations on the structure and functions of lymphatics: their behaviour in local œdema. Br J Exp Pathol 16: 49–61. [Google Scholar]
- Pullinger D, Florey H. 1937. Proliferation of lymphatics in inflammation. J Pathol Bacteriol 45: 157–170. 10.1002/path.1700450115 [DOI] [Google Scholar]
- Ruysch F. 1665. Dilucidatio valvularum in vasis lymphaticis et lacteis [Explanation of the valves in lymphatic and lactic vessels]. Facsimile of the first edition with an introduction by A.M. Luyendijk-Elshout with five plates, 1964, pp. 39–42. B. De Graaf, Nieuwkoop, Netherlands. [Google Scholar]
- Sabine A, Petrova TV. 2014. Interplay of mechanotransduction, FOXC2, connexins, and calcineurin signaling in lymphatic valve formation. Adv Anat Embryol Cell Biol 214: 67–80. 10.1007/978-3-7091-1646-3_6 [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein GW. 2003. The second valve system in lymphatics. Lymphat Res Biol 1: 25–31; discussion 29–31. 10.1089/15396850360495664 [DOI] [PubMed] [Google Scholar]
- Sibler E, He Y, Ducoli L, Keller N, Fujimoto N, Dieterich LC, Detmar M. 2021. Single-cell transcriptional heterogeneity of lymphatic endothelial cells in normal and inflamed murine lymph nodes. Cells 10: 1371. 10.3390/cells10061371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M, Simionescu N, Palade GE. 1975. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol 67: 863–885. 10.1083/jcb.67.3.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmüller F. 1920. Historische darstellung zur deutung des wesens der silbermethode an nicht fixierten objekten und experimentelle studien bezügl. Der behandlung nicht fixierter epithelien und markhaltiger nervenfasern mit Argentum Nitricum [Historical presentation of the interpretation of the nature of the silver method on non-fixed objects and experimental studies regarding. The treatment of non-fixed epithelia and myelinated nerve fibers with Argentum Nitricum]. Anatomische Hefte 59: 77–213. 10.1007/BF02047606 [DOI] [Google Scholar]
- Starling E. 1894. The influence of mechanical factors of lymph production. J Physiol 16: 224–267. 10.1113/jphysiol.1894.sp000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritt S, Koltowska K, Mäkinen T. 2021. Homeostatic maintenance of the lymphatic vasculature. Trends Mol Med 27: 955–970. 10.1016/j.molmed.2021.07.003 [DOI] [PubMed] [Google Scholar]
- Suh SH, Choe K, Hong SP, Jeong SH, Mäkinen T, Kim KS, Alitalo K, Surh CD, Koh GY, Song JH. 2019. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep 20: e46927. 10.15252/embr.201846927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suy R, Thomis S, Fourneau I. 2016. The discovery of the lymphatic system in the seventeenth century. Part II: The discovery of chyle vessels. Acta Chir Belg 116: 329–335. 10.1080/00015458.2016.1195587 [DOI] [PubMed] [Google Scholar]
- Suy R, Thomis S, Fourneau I. 2017. The discovery of the lymphatic system in the seventeenth century. Part IV: The controversy. Acta Chir Belg 117: 270–278. 10.1080/00015458.2017.1326658 [DOI] [PubMed] [Google Scholar]
- Takeda A, Hollmén M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lönnberg T, Boström P, Koskivuo I, Irjala H, et al. 2019. Single-cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity 51: 561–572.e5. 10.1016/j.immuni.2019.06.027 [DOI] [PubMed] [Google Scholar]
- Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. 2011. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 208: 2141–2153. 10.1084/jem.20102392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triacca V, Güç E, Kilarski WW, Pisano M, Swartz MA. 2017. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circ Res 120: 1440–1452. 10.1161/CIRCRESAHA.116.309828 [DOI] [PubMed] [Google Scholar]
- Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schönbein GW. 2001. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J 15: 1711–1717. 10.1096/fj.01-0067com [DOI] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S. 1985. Desmocalmin: a calmodulin-binding high molecular weight protein isolated from desmosomes. J Cell Biol 101: 2070–2080. 10.1083/jcb.101.6.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S. 1989. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol 108: 31–41. 10.1083/jcb.108.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiki T. 1990. The three-dimensional organization and ultrastructure of lymphatics in the rat intestinal mucosa as revealed by scanning electron microscopy after KOH-collagenase treatment. Arch Histol Cytol 53: 127–136. 10.1679/aohc.53.Suppl_127 [DOI] [PubMed] [Google Scholar]
- Vainionpää N, Bützow R, Hukkanen M, Jackson DG, Pihlajaniemi T, Sakai LY, Virtanen I. 2007. Basement membrane protein distribution in LYVE-1-immunoreactive lymphatic vessels of normal tissues and ovarian carcinomas. Cell Tissue Res 328: 317–328. 10.1007/s00441-006-0366-2 [DOI] [PubMed] [Google Scholar]
- Vestweber D. 2021. Vascular endothelial protein tyrosine phosphatase regulates endothelial function. Physiology (Bethesda) 36: 84–93. [DOI] [PubMed] [Google Scholar]
- Vieira JM, Norman S, Villa Del Campo C, Cahill TJ, Barnette DN, Gunadasa-Rohling M, Johnson LA, Greaves DR, Carr CA, Jackson DG, et al. 2018. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J Clin Invest 128: 3402–3412. 10.1172/JCI97192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittet D. 2014. Lymphatic collecting vessel maturation and valve morphogenesis. Microvasc Res 96: 31–37. 10.1016/j.mvr.2014.07.001 [DOI] [PubMed] [Google Scholar]
- von Recklinghausen F. 1862. Die lymphgefässe und ihre beziehung zum bindegewebe [The lymph vessels and their significance in connective tissue]. A. Hirschwald, Berlin. [Google Scholar]
- Wang Y, Baeyens N, Corti F, Tanaka K, Fang JS, Zhang J, Jin Y, Coon B, Hirschi KK, Schwartz MA, et al. 2016. Syndecan 4 controls lymphatic vasculature remodeling during mouse embryonic development. Development 143: 4441–4451. 10.1242/dev.140129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Rossi A, Solito R, Sacchi G, Aglianò M, Gerli R. 2002. Focal adhesion molecules expression and fibrillin deposition by lymphatic and blood vessel endothelial cells in culture. Microvasc Res 64: 47–55. 10.1006/mvre.2002.2397 [DOI] [PubMed] [Google Scholar]
- Xiang M, Grosso RA, Takeda A, Pan J, Bekkhus T, Brulois K, Dermadi D, Nordling S, Vanlandewijck M, Jalkanen S, et al. 2020. A single-cell transcriptional roadmap of the mouse and human lymph node lymphatic vasculature. Front Cardiovasc Med 7: 52. 10.3389/fcvm.2020.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wittchen ES, Hoopes SL, Stefanini L, Burridge K, Caron KM. 2018. Small GTPase Rap1A/B is required for lymphatic development and adrenomedullin-induced stabilization of lymphatic endothelial junctions. Arterioscler Thromb Vasc Biol 38: 2410–2422. 10.1161/ATVBAHA.118.311645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Harris NR, Caron KM. 2021. Lymphatic vasculature: an emerging therapeutic target and drug delivery route. Annu Rev Med 72: 167–182. 10.1146/annurev-med-051419-114417 [DOI] [PubMed] [Google Scholar]
- Yao LC, Baluk P, Feng J, McDonald DM. 2010. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am J Pathol 176: 1525–1541. 10.2353/ajpath.2010.090909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. 2012. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 180: 2561–2575. 10.1016/j.ajpath.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani S, Jaldin-Fincati JR, Pereira RVS, Klip A. 2019. Endothelial cell barriers: transport of molecules between blood and tissues. Traffic 20: 390–403. 10.1111/tra.12645 [DOI] [PubMed] [Google Scholar]
- Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boyé K, Michon P, Künzel SE, et al. 2018. Lacteal junction zippering protects against diet-induced obesity. Science 361: 599–603. 10.1126/science.aap9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zarkada G, Yi S, Eichmann A. 2020. Lymphatic endothelial cell junctions: molecular regulation in physiology and diseases. Front Physiol 11: 509. 10.3389/fphys.2020.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen EA, Orsenigo F, Lohela M, D'Amico G, Holopainen T, et al. 2014. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev 28: 1592–1603. 10.1101/gad.237677.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöltzer H. 2003. Initial lymphatics—morphology and function of the endothelial cells. Lymphology 36: 7–25. [PubMed] [Google Scholar]