Abstract
Purpose
Recently, the modified apical dissection (MAD) technique in robot-assisted laparoscopic radical prostatectomy (RARP) has shown excellent functional outcomes but has never been rigorously validated at various institutions. This study aimed to evaluate the effect of MAD on early continence and potency compared with the anterior suspension stitch (SS) technique.
Materials and Methods
A total of 100 patients who underwent RARP with SS and 100 who underwent RARP with MAD by a single surgeon were propensity score matched and retrospectively compared for continence and potency recovery at 1 week and 1, 3, 6, 9, and 12 months.
Results
Continence was reached in 20.6%, 33.3%, 67.2%, 74.1%, 81.1%, and 83.0% of patients in the SS group, compared with 49.2%, 73.3%, 86.8%, 96.6%, 100.0%, and 100.0% in the MAD group at postoperative 1 week and 1, 3, 6, 9, and 12 months, respectively. In the SS group, potency rates were 0.0%, 20.0%, 50.0%, 66.7%, 75.0%, and 83.3%; in the MAD group, the rates were 50.0%, 90.0%, 88.9%, 100.0%, 100.0%, and 100.0%. Recovery of continence was higher in the MAD group within the first 6 months (p=0.005, <0.010, 0.041, 0.016 at 1 week, 1, 3, and 6 months). There were no significant differences in potency recovery rates between the two groups (all p≥0.05).
Conclusions
The MAD technique results in earlier recovery of continence compared with the SS technique.
Keywords: Dissection, Neoplasms, Prostatectomy, Robotic surgical procedures, Urinary incontinence
Graphical Abstract
INTRODUCTION
Urinary incontinence is one of the major complications of robot-assisted radical prostatectomy (RARP) that significantly undermines patients’ quality of life [1]. Although continence rates from large robotic centers are around 90% at 1 year after surgery [2], the recovery of early continence remains a challenge, with rates as low as 23% [3] at 1 month postoperatively at some centers.
Therefore, various surgical techniques have been developed to improve early continence after RARP. These techniques include bladder neck reconstruction, posterior reconstruction, anterior suspension stitch, and lateral prostatic fascia preservation [4]. The anterior suspension stitch technique, which was originally described by Walsh in an open radical retropubic prostatectomy series [5], has become one of the most popular techniques for optimizing continence after RARP since Patel et al. [6] first performed it robotically and reported a significant difference in early recovery of continence. However, the necessity of a suspension stitch needs to be reassessed. A recent study by Covas Moschovas et al. [7] in which functional outcomes were compared between a conventional RARP group with the anterior suspension stitch and a modified apical dissection and lateral prostatic fascia preservation group without the stitch revealed that the latter showed faster recovery of continence and potency. Because no other studies have validated Patel’s recent work and because reproducibility is crucial for a novel technique to become more popular, we aimed to prove the superiority of the modified apical dissection technique over the anterior suspension stitch technique by comparing functional outcomes in our center.
MATERIALS AND METHODS
1. Patients
A total of 200 patients who recently underwent RARP, 100 with the placement of a suspension stitch and 100 with the modified apical dissection technique without a suspension stitch, were prospectively collected and retrospectively analyzed. All patients underwent RARP using the modified apical dissection technique since 2020 when we adopted the technique at our center. All operations were performed by a single surgeon who had overcome the RARP learning curve.
2. Surgical technique
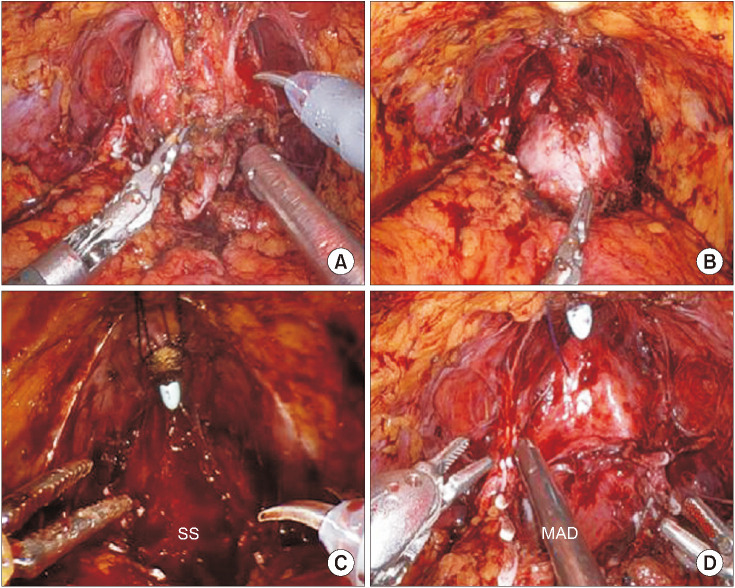
All cases were performed using a transperitoneal six-port approach. In the suspension stitch group, we used the technique described in previous publications [6,8]. In the modified apical dissection group, the endopelvic fascia was opened after bladder dropping, posterior dissection, and retrograde neurovascular bundle (NVB) dissection. By opening the endopelvic fascia closer to the prostate instead of opening it closer to the pelvic side wall, we tried to preserve the apical complex of the puboprostatic ligaments and apical endopelvic fascia. After finishing the NVB dissection, the dorsal vein complex (DVC) was controlled by a running suture (Fig. 1). In both groups, a modified posterior rhabdosphincteric reconstruction and vesicourethral anastomosis were performed. Bilateral standard pelvic lymph node dissection was performed in patients classified as being at high risk based on the D’Amico classification.
Fig. 1. (A) Prostatic apical complex after prostatic arterial pedicles control and before apical dissection in modified apical dissection (MAD). (B) Prostatic apical complex after apical dissection and dorsal venous complex running suture in MAD. Comparison between suspension stitch (SS) (C) and MAD (D) techniques after urethral incision. The apical complex of the puboprostatic ligaments and apical endopelvic fascia are preserved in MAD, resulting in no need for a suspension stitch.
3. Outcome measurement
Patients were followed-up in the clinic postoperatively at 1 week and then at 1, 3, 6, 9, and 12 months. Prostate-specific antigen (PSA) and the recovery of continence and potency were evaluated at all visits. Patients were considered continent if they did not use any pads or if they used one safety pad per day (score 0 or 1 on EPIC-CP questionnaire question 3 [9]). Potency was defined as the ability to achieve and maintain a satisfactory erection firm enough for sexual intercourse in more than 50% of attempts, with or without the use of phosphodiesterase type 5 inhibitors (score ≥4 on Sexual Health Inventory for Men [SHIM] questionnaire questions 2, 3, and 5 [10]).
4. Propensity-score matching
Patients who underwent the suspension stitch technique were matched 1:1 with those who underwent modified apical dissection by propensity-score matching using a logistic regression. We used 10 clinical covariates: age, body mass index, American Society of Anesthesiologists score, SHIM score, prostate volume, PSA level, clinical T stage, biopsy Gleason score, D’Amico risk group, and degree of nerve sparing. After the matching, the two groups were compared by use of the McNemar test and Wilcoxon signed-rank test.
5. Statistical analysis
Continuous variables are reported as mean±standard deviation or as medians and interquartile ranges. Categorical variables are reported as rates. We compared the differences in outcomes between the suspension stitch and modified apical dissection groups using Student’s t-test, Mann–Whitney, chi-squared, and Fisher’s exact tests. We used Kaplan–Meier analyses and log rank tests to compare the times to full continence and potency between the groups. Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as p<0.05.
6. Ethics statement
All analyses were performed in accordance with the Declaration of Helsinki after approval by the Institutional Review Board (approval no. 2022AN0306). Since this was a retrospective study, it was exempt from written informed consent of patients.
RESULTS
1. Demographics and preoperative oncological characteristics
After propensity-score matching, 63 patients were assigned to each group. The demographics and preoperative characteristics of the two groups before and after the matching analysis are depicted in Table 1. The baseline characteristics of the two populations both before and after the matching were not significantly different.
Table 1. Comparison of demographic and preoperative oncological characteristics between SS and MAD groups before and after propensity matching.
| Parameter | Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|---|
| SS group (n=100) | MAD group (n=100) | p-value | SS group (n=63) | MAD group (n=63) | p-value | ||
| Age (y) | 68 (62–72.75) | 59.25 (67–74) | 0.470 | 68 (61–72) | 70 (65–74) | 0.098 | |
| Body mass index (kg/m2) | 24.19±2.85 | 24.49±2.77 | 0.451 | 24.14±2.68 | 24.42±2.68 | 0.492 | |
| ASA score | 2 (1–2) | 2 (1–2) | 0.409 | 2 (1–2) | 2 (1–2) | 0.669 | |
| Preoperative SHIM score | 13 (6–17) | 11.50 (3–16) | 0.124 | 13 (5.50–17) | 12 (5–15) | 0.457 | |
| Prostate volume (mL) | 28.80 (25.00–39.32) | 30.61 (25.57–39.89) | 0.588 | 28.40 (23.31–39.26) | 30.53 (25.43–37.97) | 0.907 | |
| Preoperative PSA (ng/mL) | 7.88 (5.70–12.79) | 6.73 (5.22–11.04) | 0.144 | 7.78 (5.50–12.17) | 6.57 (5.25–10.07) | 0.649 | |
| Clinical tumor stage | 0.059 | 0.534 | |||||
| cT1 | 28 (28.0) | 24 (24.0) | 18 (28.6) | 18 (28.6) | |||
| cT2 | 40 (40.0) | 56 (56.0) | 27 (42.9) | 32 (50.8) | |||
| cT3 | 32 (32.0) | 20 (20.0) | 18 (28.6) | 13 (20.6) | |||
| Biopsy Gleason score | 0.051 | 0.552 | |||||
| 3+3 | 41 (41.0) | 34 (34.0) | 25 (39.7) | 26 (41.3) | |||
| 3+4 | 17 (17.0) | 29 (29.0) | 14 (22.2) | 17 (27.0) | |||
| 4+3 | 6 (6.0) | 12 (12.0) | 6 (9.5) | 5 (7.9) | |||
| ≥4+4 | 36 (36.0) | 25 (25.0) | 18 (28.6) | 15 (23.8) | |||
| D'Amico risk group | 0.068 | 0.959 | |||||
| Low | 18 (18.0) | 12 (12.0) | 12 (19.0) | 9 (14.3) | |||
| Intermediate | 32 (32.0) | 48 (48.0) | 23 (36.5) | 30 (47.6) | |||
| High | 50 (50.0) | 40 (40.0) | 28 (44.4) | 24 (38.1) | |||
| Nerve sparing degree | 0.194 | 0.645 | |||||
| Bilateral none | 4 (4.0) | 1 (1.0) | 1 (1.6) | 1 (1.6) | |||
| Unilateral partial | 7 (7.0) | 3 (3.0) | 3 (4.8) | 3 (4.8) | |||
| Unilateral full | 8 (8.0) | 4 (4.0) | 2 (3.2) | 3 (4.8) | |||
| Bilateral partial | 5 (5.0) | 9 (9.0) | 3 (4.8) | 4 (6.3) | |||
| Unilateral full+contralateral partial | 17 (17.0) | 25 (25.0) | 12 (19.0) | 13 (20.6) | |||
| Bilateral full | 59 (59.0) | 58 (58.0) | 42 (66.7) | 39 (61.9) | |||
Values are presented as median (interquartile range), mean±standard deviation or number (%).
SS, suspension stitch; MAD, modified apical dissection; ASA, American Society of Anesthesiologists; SHIM, Sexual Health Inventory for Men; PSA, prostate-specific antigen.
2. Perioperative characteristics
The suspension stitch group had similar perioperative outcomes to the modified apical dissection group as listed in Table 2. The mean operative time and console time were similar between the two groups (121.98 vs. 129.22 min and 87.51 vs. 95.08 min; p=0.128 and 0.095, respectively). The lengths of hospital stay were not significantly different (8 [8-8] vs. 8 [8-8] days, p=0.527). The overall complication rates were comparable (15.9% vs. 9.5%, p=0.537), and all the complications were Clavien I or II. In the suspension stitch group, the complications were elevation of liver function tests (n=3 [4.8%]), drug fever (n=1 [1.6%]), hyponatremia (n=1 [1.6%]), urinary tract infection (n=1 [1.6%]), acute urinary retention (n=3 [4.8%]), and pneumonia (n=1 [1.6%]). In the modified apical dissection group, the complications were wound infection (n=1 [1.6%]), liver function test elevation (n=1 [1.6%]), and acute urinary retention (n=4 [6.3%]).
Table 2. Comparison of perioperative characteristics, and pathological and oncological outcomes, between SS and MAD groups.
| Parameter | SS group (n=63) | MAD group (n=63) | p-value | |
|---|---|---|---|---|
| Total operative time (min) | 121.98±26.67 | 129.22±23.46 | 0.128 | |
| Total console time (min) | 87.51±26.57 | 95.08±23.01 | 0.095 | |
| Length of hospital stay (d) | 8 (8–8) | 8 (8–8) | 0.527 | |
| Postoperative complications | 10 (15.9) | 6 (9.5) | 0.537 | |
| None | 53 (84.1) | 57 (90.5) | ||
| Clavien I | 5 (7.9) | 2 (3.2) | ||
| Clavien II | 5 (7.9) | 4 (6.3) | ||
| Clavien III | 0 (0.0) | 0 (0.0) | ||
| Clavien IV | 0 (0.0) | 0 (0.0) | ||
| Clavien V | 0 (0.0) | 0 (0.0) | ||
| Type of complications | NA | |||
| Wound infection | 0 (0.0) | 1 (1.6) | ||
| Liver function test elevation | 3 (4.8) | 1 (1.6) | ||
| Drug fever | 1 (1.6) | 0 (0.0) | ||
| Hyponatremia | 1 (1.6) | 0 (0.0) | ||
| Urinary tract infection | 1 (1.6) | 0 (0.0) | ||
| Acute urinary retention | 3 (4.8) | 4 (6.3) | ||
| Pneumonia | 1 (1.6) | 0 (0.0) | ||
| Pathological stage | 0.785 | |||
| ≤pT2c | 44 (69.8) | 48 (76.2) | ||
| pT3a | 14 (22.2) | 8 (12.7) | ||
| pT3b | 5 (7.9) | 7 (11.1) | ||
| pT4 | 0 (0.0) | 0 (0.0) | ||
| Nodal stage | 0.285 | |||
| pN0 | 28 (44.4) | 22 (34.9) | ||
| pN1 | 1 (1.6) | 2 (3.2) | ||
| pNx | 34 (54.0) | 39 (61.9) | ||
| Pathological Gleason score | 0.850 | |||
| 3+3 | 7 (11.1) | 7 (11.1) | ||
| 3+4 | 34 (54.0) | 34 (54.0) | ||
| 4+3 | 18 (28.6) | 17 (27.0) | ||
| ≥4+4 | 4 (6.3) | 4 (6.3) | ||
| Positive surgical margin | 15 (23.8) | 14 (22.2) | >0.999 | |
| Positive surgical margin by stage | ||||
| ≤pT2c | 9 (20.5) | 8 (16.7) | >0.999 | |
| pT3a | 5 (35.7) | 4 (50.0) | >0.999 | |
| pT3b | 1 (20.0) | 2 (28.6) | >0.999 | |
| pT4 | 0 (0.0) | 0 (0.0) | NA | |
| Positive surgical margin site | 0.535 | |||
| Apical | 3 (4.8) | 6 (9.5) | 0.508 | |
| Anterior | 2 (3.2) | 2 (3.2) | ||
| Posterolateral | 4 (6.3) | 1 (1.6) | ||
| Base-bladder neck | 2 (3.2) | 1 (1.6) | ||
| Multifocal | 4 (6.3) | 4 (6.3) | ||
| BCR at postoperative 3 mo | 0.289 | |||
| No | 57 (90.5) | 61 (96.8) | ||
| Yes | 6 (9.5) | 2 (3.2) | ||
Values are presented as mean±standard deviation, median (interquartile range) or number (%).
SS, suspension stitch; MAD, modified apical dissection; NA, not applicable; BCR, biochemical recurrence.
3. Pathological and oncological outcomes
There were no significant differences between the two groups in terms of pathological and nodal stage (p=0.785 and 0.285, respectively), pathological Gleason score (p=0.850), and positive surgical margin (PSM) rates (p>0.999). The PSM rates were 22.2% in the modified apical dissection group and 23.8% in the suspension stitch group. The sites of PSM were also similar between the groups (p=0.535). Apical margin rates were 9.5% in the modified apical dissection group and 4.8% in the suspension stitch group (p=0.508). The rates of biochemical recurrence at 3 months of follow-up postoperatively were similar between the two groups (3.2% in modified apical dissection vs. 9.5% in suspension stitch, p=0.289).
4. Functional outcomes
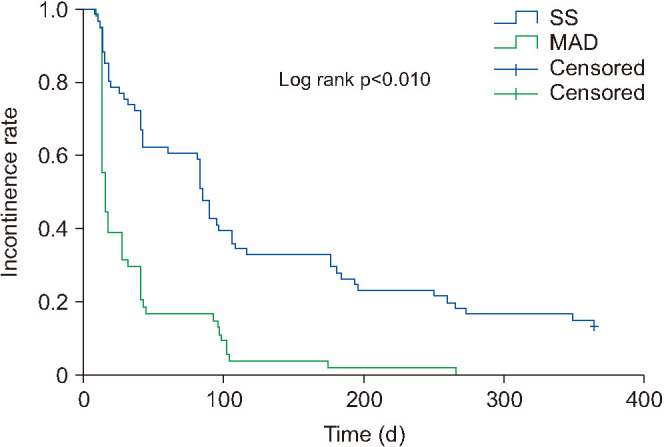
Continence rates were higher for the modified apical dissection group than for the suspension stitch group at 1 week and at 1, 3, and 6 months postoperatively: 49.2% vs. 20.6% (p=0.005), 73.3% vs. 33.3% (p<0.010), 86.8% vs. 67.2% (p=0.041), and 96.6% vs. 74.1% (p=0.016), respectively (Table 3). However, there were no significant differences in 9-month and 12-month continence rates (p=0.250 and >0.999). The modified apical dissection group showed faster recovery of continence than did the suspension stitch group (15 [13-41] vs. 85 [30.50–195] days, p<0.010). Kaplan–Meier analysis showed a significantly higher probability of continence recovery in the modified apical dissection group (p<0.010; Fig. 2).
Table 3. Comparison of functional outcomes between SS and MAD groups.
| Parameter | SS group (n=63) | MAD group (n=63) | p-value | |
|---|---|---|---|---|
| Follow-up period (d) | 997.50 (361.25–1,387.50) | 97 (27–183) | <0.010 | |
| Time to continence (d) | 85 (30.50–195) | 15 (13–41) | <0.010 | |
| Postoperative continence rate/No. of patients with follow-up | ||||
| At 1 wk | 13/63 (20.6) | 31/63 (49.2) | 0.005 | |
| At 1 mo | 21/63 (33.3) | 44/60 (73.3) | <0.010 | |
| At 3 mo | 41/61 (67.2) | 46/53 (86.8) | 0.041 | |
| At 6 mo | 40/54 (74.1) | 28/29 (96.6) | 0.016 | |
| At 9 mo | 43/53 (81.1) | 16/16 (100.0) | 0.250 | |
| At 12 mo | 44/53 (83.0) | 4/4 (100.0) | >0.999 | |
| Time to potency (d) | 95 (61–317.5) | 19 (15–33) | 0.028 | |
| Postoperative potency rate/No. of patients with follow-up | ||||
| At 1 wk | 0/15 (0.0) | 6/12 (50.0) | 0.500 | |
| At 1 mo | 3/15 (20.0) | 9/10 (90.0) | 0.250 | |
| At 3 mo | 7/14 (50.0) | 8/9 (88.9) | >0.999 | |
| At 6 mo | 8/12 (66.7) | 4/4 (100.0) | >0.999 | |
| At 9 mo | 9/12 (75.0) | 2/2 (100.0) | NA | |
| At 12 mo | 10/12 (83.3) | 2/2 (100.0) | NA | |
Values are presented as median (interquartile range) or number (%).
SS, suspension stitch; MAD, modified apical dissection; NA, not applicable.
Fig. 2. Kaplan–Meier curve showing incontinence reduction rate over time. SS, suspension stitch; MAD, modified apical dissection.
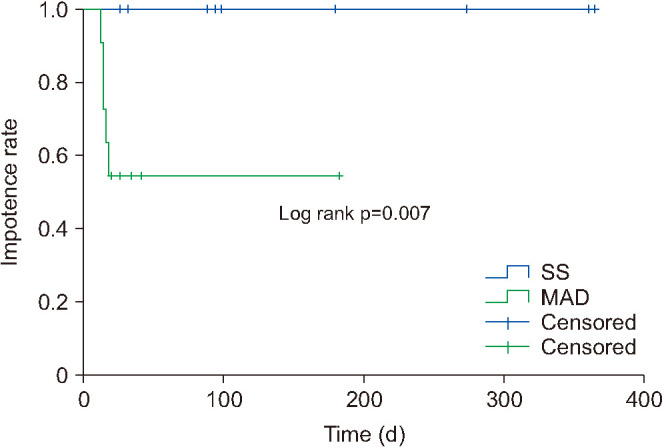
In terms of potency recovery, there were no significant differences between the modified apical dissection group and the suspension stitch group at 1 week and at 1, 3, and 6 months. The 9-month and 12-month potency outcomes could not be compared because of an insufficient number of patients. The time to recovery of potency was significantly shorter in the modified apical dissection group (19 [15-33] days) than in the suspension stitch group (95 [61–317.5] days; p=0.028). Kaplan–Meier analysis showed a significantly higher probability of potency recovery in the modified apical dissection group (p=0.007; Fig. 3).
Fig. 3. Kaplan–Meier curve showing impotence reduction rate over time. SS, suspension stitch; MAD, modified apical dissection.
DISCUSSION
Various techniques have been modified and refined to improve continence after RARP. Some techniques described recently emphasize the importance of preserving the apical complex for early recovery of continence. These techniques include the “Hood” technique by Wagaskar et al. [11], in which the continence rates at 1, 2, 4, 6, 12, 24, and 48 weeks after catheter removal were 21%, 36%, 83%, 88%, 91%, 94%, and 95%, respectively, and the “Collar” technique by Bianchi et al. [12], in which the continence rates were 46.3%, 70.4%, 94.4%, and 99.1% at 1, 3, 6, and 12 months after catheter removal, respectively. Porpiglia et al. [13] recently described the totally anatomic reconstruction technique, which includes meticulous anatomical dissection of the prostatic apex and led to high continence rates of 71.8%, 77.8%, 89.3%, 94.4%, and 98.0% immediately after catheter removal and at 1, 4, 12, and 24 weeks after RARP, respectively.
Our modified apical dissection technique also focused on preserving the apical complex by avoiding excessive dissection of the prostatic apex. In fact, this technique was first described in a study by Covas Moschovas et al. [7], in which they reported significantly improved early continence rates of 36.9%, 78.4%, and 92% at 1 week, 6 week, and 3 months, respectively. Our results also demonstrated that the modified apical dissection technique was associated with higher earlier recovery of continence compared with the conventional suspension stitch technique. Postoperative 1-week rates increased from 20.6% to 49.2%, 1-month rates from 33.3% to 73.3%, 3-month rates from 67.2% to 86.8%, and 6-month rates from 74.1% to 96.6%. This was probably due to better preservation of the prostatic apical support structures. For example, levator ani muscle fibers, the contraction of which compresses the urethra when abdominal pressure is raised [14], are well preserved in the modified apical dissection technique. Moreover, because some of the key nerve branches that contribute to passive urethral closure enter the urethra from the anterolateral aspects of the lateral fascia, relatively better preservation of the lateral prostatic fascia in the modified apical dissection technique compared with the suspension stitch technique may contribute to higher continence rates [15]. Finally, by avoiding a suspension stitch, the modified apical dissection technique leads to less devascularization of the sphincter complex [7] and minimizes tissue disruption lateral to the membranous urethra [16].
There also have been efforts to increase the rate of potency after RARP. With early retrograde release of the NVBs without opening the endopelvic fascia and without ligating the DVC, de Carvalho et al. [17] reported potency recovery rates of 53.1% and 86.7% at 1 month and 1 year postoperatively. Kang et al. [4] described NVB preservation that was performed in a retrograde manner using the method of toggling. Using these techniques, inadvertent clipping can be prevented and a good plane between the prostate fascia and the NVB can be achieved, avoiding thermal damage and mechanical trauma to the NVB. Our center adopted the techniques of retrograde NVB releasing and toggling and reported in a previous study that the potency recovery rates were higher in the toggling group (82%) than in the group without toggling (75%) at the 1-year follow-up [18].
The modified apical dissection technique in our study further promotes potency by avoiding excessive dissection of the lateral prostatic fascia in addition to the retrograde release of the NVB and toggling. Because more medial incisions are made with lateral endopelvic fascia, the NVBs are protected underneath, leading to less trauma to the NVB. In addition, preservation of lateral endopelvic fascia results in greater preservation of the anterolateral nerve fibers, which some studies have proved leads to better erectile function [19,20]. However, there were no significant differences between the suspension stitch and the modified apical dissection groups in terms of potency recovery rates in our study. This was probably attributable to the small sample sizes because of missed data and the relatively short follow-up periods in the modified apical dissection group and needs further study with a larger population and longer follow-up.
In our study, there were no significant differences in overall PSM rates (23.8% in suspension stitch vs. 22.2% in modified apical dissection, p>0.999) and PSM locations (p=0.535). The apical margin rates, which could be a concern in performing modified apical dissection, were also similar between the two groups (4.8% in suspension stitch vs 9.5% in modified apical dissection, p=0.508).
Although the biochemical recurrence rates at the 3-month follow-up were not significantly different between the suspension stitch and modified apical dissection groups (9.5% vs. 3.2%, p=0.289), a longer follow-up period and a larger number of patients are needed to draw conclusions in terms of the oncological outcomes between the modified apical dissection and the suspension stitch groups.
This study has several limitations: (1) The study was conducted by a single surgeon within a single institution, and therefore the results may not be generalizable to other surgeons. (2) This was a retrospective study, so the results need to be validated in a randomized clinical trial. (3) The length of hospital stay was longer relative to many other countries [21,22]. This is presumably to allow for catheter removal before discharge because many patients are reluctant to keep Foley catheters at home. (4) Because this study lacks long-term follow-up data regarding oncological outcomes, a future study with a larger population and longer follow-up is needed.
CONCLUSIONS
In conclusion, the modified apical dissection technique enables earlier recovery of continence compared with the conventional suspension stitch technique. We have confirmed that increased early continence rates are reproducible among surgeons using the modified apical dissection technique and believe that this novel technique might be now considered “conventional” RARP.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING: This research was supported by the Korea University College of Medicine.
- Research conception and design: Ha Eun Kang and Sung Gu Kang.
- Data acquisition: all authors.
- Drafting of the manuscript: Ha Eun Kang.
- Critical revision of the manuscript: Ha Eun Kang and Sung Gu Kang.
- Obtaining funding: Sung Gu Kang.
- Supervision: Sung Gu Kang.
- Approval of the final manuscript: all authors.
References
- 1.Carrier J, Edwards D, Harden J. Men's perceptions of the impact of the physical consequences of a radical prostatectomy on their quality of life: a qualitative systematic review. JBI Database System Rev Implement Rep. 2018;16:892–972. doi: 10.11124/JBISRIR-2017-003566. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–417. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 4.Kang SG, Shim JS, Onol F, Bhat KRS, Patel VR. Lessons learned from 12,000 robotic radical prostatectomies: is the journey as important as the outcome? Investig Clin Urol. 2020;61:1–10. doi: 10.4111/icu.2020.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418–2424. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 6.Patel VR, Coelho RF, Palmer KJ, Rocco B. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol. 2009;56:472–478. doi: 10.1016/j.eururo.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Covas Moschovas M, Bhat S, Onol FF, Rogers T, Roof S, Mazzone E, et al. Modified apical dissection and lateral prostatic fascia preservation improves early postoperative functional recovery in robotic-assisted laparoscopic radical prostatectomy: results from a propensity score-matched analysis. Eur Urol. 2020;78:875–884. doi: 10.1016/j.eururo.2020.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Patel VR, Shah KK, Thaly RK, Lavery H. Robotic-assisted laparoscopic radical prostatectomy: the Ohio State University technique. J Robot Surg. 2007;1:51–59. doi: 10.1007/s11701-007-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang P, Szymanski KM, Dunn RL, Chipman JJ, Litwin MS, Nguyen PL, et al. Expanded prostate cancer index composite for clinical practice: development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol. 2011;186:865–872. doi: 10.1016/j.juro.2011.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel VR, Sivaraman A, Coelho RF, Chauhan S, Palmer KJ, Orvieto MA, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011;59:702–707. doi: 10.1016/j.eururo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Wagaskar VG, Mittal A, Sobotka S, Ratnani P, Lantz A, Falagario UG, et al. Hood technique for robotic radical prostatectomy-preserving periurethral anatomical structures in the space of Retzius and sparing the pouch of Douglas, enabling early return of continence without compromising surgical margin rates. Eur Urol. 2021;80:213–221. doi: 10.1016/j.eururo.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi L, Turri FM, Larcher A, De Groote R, De Bruyne P, De Coninck V, et al. A novel approach for apical dissection during robot-assisted radical prostatectomy: the "collar" technique. Eur Urol Focus. 2018;4:677–685. doi: 10.1016/j.euf.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Porpiglia F, Bertolo R, Manfredi M, De Luca S, Checcucci E, Morra I, et al. Total anatomical reconstruction during robot-assisted radical prostatectomy: implications on early recovery of urinary continence. Eur Urol. 2016;69:485–495. doi: 10.1016/j.eururo.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai S, Muraki O, Yoshimura Y. Evaluation of the effect of levator ani muscle contraction on post-prostatectomy urinary incontinence using cine MRI. Neurourol Urodyn. 2022;41:616–625. doi: 10.1002/nau.24861. [DOI] [PubMed] [Google Scholar]
- 15.Karam I, Droupy S, Abd-Alsamad I, Korbage A, Uhl JF, Benoît G, et al. The precise location and nature of the nerves to the male human urethra: histological and immunohistochemical studies with three-dimensional reconstruction. Eur Urol. 2005;48:858–864. doi: 10.1016/j.eururo.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JJ, Kim BY, Uchio EM. Improving urinary continence after radical prostatectomy: review of surgical modifications. Korean J Urol. 2009;50:935–941. [Google Scholar]
- 17.de Carvalho PA, Barbosa JABA, Guglielmetti GB, Cordeiro MD, Rocco B, Nahas WC, et al. Retrograde release of the neurovascular bundle with preservation of dorsal venous complex during robot-assisted radical prostatectomy: optimizing functional outcomes. Eur Urol. 2020;77:628–635. doi: 10.1016/j.eururo.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Shim JS, Tae JH, Noh TI, Kang SH, Cheon J, Lee JG, et al. Toggling technique allows retrograde early release to facilitate neurovascular bundle sparing during robot-assisted radical prostatectomy: a propensity score-matching study. J Korean Med Sci. 2022;37:e6. doi: 10.3346/jkms.2022.37.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basourakos SP, Kowalczyk K, Moschovas MC, Dudley V, Hung AJ, Shoag JE, et al. Robot-assisted radical prostatectomy maneuvers to attenuate erectile dysfunction: technical description and video compilation. J Endourol. 2021;35:1601–1609. doi: 10.1089/end.2021.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen ME, Schaeffer EM, Marschke P, Walsh PC. High anterior release of the levator fascia improves sexual function following open radical retropubic prostatectomy. J Urol. 2008;180:2557–2564. doi: 10.1016/j.juro.2008.08.047. discussion 2564. [DOI] [PubMed] [Google Scholar]
- 21.Strother MC, Michel KF, Xia L, McWilliams K, Guzzo TJ, Lee DJ, et al. Prolonged length of stay after robotic prostatectomy: causes and risk factors. Ann Surg Oncol. 2020;27:1560–1567. doi: 10.1245/s10434-020-08266-3. [DOI] [PubMed] [Google Scholar]
- 22.Hajj AE, Labban M, Ploussard G, Zarka J, Abou Heidar N, Mailhac A, et al. Patient characteristics predicting prolonged length of hospital stay following robotic-assisted radical prostatectomy. Ther Adv Urol. 2022;14:17562872221080737. doi: 10.1177/17562872221080737. [DOI] [PMC free article] [PubMed] [Google Scholar]