Abstract
To develop small-diameter (<6 mm) scaffolds capable of accelerating rapid endothelialization and improving long-term patency rate, we created acellular vascular scaffolds preloaded with heparin and hepatocyte growth factor (HGF). Heparin was conjugated to suppress thrombogenic responses, and HGF was immobilized to induce endothelial cells (ECs) proliferation and migration. The scaffolds immobilized with heparin exhibited highly effective localization and sustained release of HGF for 30 days in vitro. We implanted this modified scaffold into the carotid artery of a rabbit model to investigate the efficacy in vivo. The acellular vascular scaffold with heparin only was used as control. After transplantation, the patency of this modified scaffold was 91.67% at 1, 3, 6, and 12 months, while the patency rate in the group with grafted heparin only was 83.33% at 1, 3, 6, and 12 months. This modified scaffold significantly stimulated ECs proliferation and the endothelium aligned in the direction of flow after 12 months. In addition, intimal hyperplasia was significantly reduced in the grafts coated with HGF compared with the control grafts. The small-diameter vascular grafts with an inner diameter of 2.5 mm preloaded with heparin and HGF may be a substitute for autologous blood vessels in clinic.
Keywords: acellular vascular scaffold, heparin, hepatocyte growth factor, endothelialization
Introduction
Cardiovascular disease is the leading cause of death worldwide, and its incidence is rapidly increasing1. To restore blood supply to ischemic tissues, coronary artery bypass grafting (CABG) is the most successful treatment. Data indicated that a total of 159,869 procedures involved isolated CABG in the United States in 20162. Autologous blood vessels such as the great saphenous vein and internal mammary artery are the first choice for use as bypass grafts. Unfortunately, up to 40% of patients do not have feasible autologous grafts due to coexisting diseases, operation history, and inappropriate size3. Small-diameter vascular grafts (SDVGs) are urgently needed as a substitute for autologous blood vessels. Although many experimental studies and preclinical trials have been carried out, there are still no satisfactory SDVGs at present, with disadvantages such as thrombosis and intimal hyperplasia, mainly due to the failure of rapid endothelialization and vascular remodeling. Incomplete endothelialization can lead to thrombosis and intimal hyperplasia, while poor vascular remodeling can lead to calcification and even aneurysms. Weinberg and Bell first attempted to solve these problems by creating tissue engineering vascular grafts (TEVGs)4. TEVGs pre-seeded with endothelial cells (ECs) could improve the patency rate5. However, the pretreatment required weeks to months before implantation, making it unsuitable in emergency surgery. Therefore, it is of clinical significance to produce non-seeded TEVGs loaded with anticoagulant drugs and growth factors, achieve rapid endothelialization and vascular remodeling through the differentiation and proliferation of various stem cells and progenitor cells in the blood and surrounding tissues in vivo.
Acellular vascular matrix (AVM) has excellent adhesion to ECs and similar biomechanical and hemodynamic properties to autologous vessels, which could be an ideal material for the preparation of TEVGs6. However, AVM will be damaged to a certain extent during the acellular process, and its mechanical properties would be reduced. Besides, the acellular lumen after the removal of ECs carries a significant risk of thrombosis when exposed to the blood directly7. Heparin is widely used to inhibit thrombosis in clinical practice. Studies have found that heparin can be bound to the TEVGs by forming amine covalent bonds with cross-linking agents8. Hepatocyte growth factor (HGF) is a pluripotent cytokine with extensive biological activity. HGF can induce ECs proliferation and migration, and improve endothelial function without affecting vascular smooth muscle cells (SMCs) proliferation9,10. Growth factors can be bound to heparin by amino acid sequences11. TEVGs are first coated with heparin and then combined with the growth factor, which is currently the most popular strategy for the local release of growth factors12. Binding to heparin can slow down the diffusion of growth factors, prevent the loss of biological activity caused by proteolysis, extend their half-life in vivo, and enhance the affinity of the receptors13–15.
This study was designed to explore a promising TEVG with good mechanical properties, continuous anticoagulation, and a sustained local release of HGF to improve the patency rate. As far as we know, we are the first to use detergents Triton X-100 and sodium dodecyl sulfate (SDS) to decellularize porcine carotid arteries and then coated with heparin and HGF to prepare the TEVG. We determined the coating and release actions of HGF quantitatively and implanted this modified scaffold into the carotid artery of a rabbit model to investigate the efficacy.
Materials and Methods
Preparation and Characterization of Acellular Vascular Scaffolds (AVSs)
Fresh porcine carotid arteries with an inner diameter of 2.5 mm were obtained from a nearby slaughterhouse and dissected carefully. The decellularization was performed using the detergents method as previously described16. Native porcine arteries were immersed into 1% Triton X-100 solution (Labest Biological Technology, Beijing, China) on an orbital shaker (TS-100, Qilinbeier Instrument Equipment Manufacturing, Haimen, China) (100 r/min) for 24 h. Then immersed into 0.3% SDS solution (Hanran Biological Technology, Shanghai, China) and continuous oscillating for 72 h. After extensive washing with phosphate-buffered saline (PBS), we obtained the acellular vascular scaffolds.
A segment from the scaffolds was fixed in 4% paraformaldehyde, embedded in paraffin, and sliced for hematoxylin-eosin (H&E) staining, Elastica Van Gieson (EVG) staining, Masson’s trichrome staining, and DAPI fluorescent staining. The total amount of DNA in native porcine carotid arteries and AVSs were quantified using a DNeasy Blood & Tissue Kit (Qiagen, Venlo, The Netherlands).
Immobilize Heparin
For heparinizing, heparin sodium (200mg) (Beijing Solarbio Science & Technology, China), EDC (108mg) (Sigma), and NHS (67mg) (Sigma) were added to MES (40ml, 30mmol/L) (Sigma) in sequence, and mixed well in an ice bath for 2 hours. Then, an AVS was placed into this solution and incubated at 37°C for 24h. After rinsing with PBS 5 times for 5 min each, the heparinized acellular scaffold (HAS) was obtained.
HGF Coating and Release
The HASs were incubated with Recombinant Human HGF (400 ng/mL) (PeproTech, Cranbury, NJ, USA) in PBS containing bovine serum albumin (1 mg/mL) (Hangzhou Lianke Biotech Co., Ltd, Hangzhou, Zhejiang, China) at 37°C for 90 min. Then, the heparinized acellular scaffolds grafted with HGF (HHASs) were obtained. The amount of coating HGF was obtained by subtracting the residual content from the initial content in the solution. The residual HGF content was measured by a Human HGF Pre-Coated ELISA Kit (PeproTech).
The HHAS was cut into 5 mm × 5 mm, then transferred to 1 mL PBS containing bovine serum albumin (1 mg/mL) at 37°C under continuous shaking, and the PBS was replaced on days 1, 2, 3, 5, 8, 12, 17, 23, and 30. The supernatant was collected and kept at –20°C. The levels of HGF in the supernatant were measured by the Human HGF Pre-Coated ELISA Kit (PeproTech). The percentage of HGF release in each time interval was calculated by the formula (the cumulative HGF release in all PBS in the corresponding time period) / (the amount of coating HGF) ×100%. The release curve was drawn by OriginPro 8.5 (OriginLab Corporation, Northampton, Massachusetts, USA).
Surgical Implantation
All animals received humane care in compliance with the World Medical Association Declaration of Helsinki, and this experiment was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University.
A total of 24 male Japanese white rabbits were used for animal models in vivo, each weighing 3.0 to 4.0 kg. The animals were randomly allocated to either the HHAS group (n = 12, implanted with the HHASs) or HAS group (n = 12, implanted with the HASs). Anesthesia was achieved with 2.5% to 4.0% isoflurane (Hebei Yipin Pharmaceutical Co., Ltd, Shijiazhuang, Hebei, China). Through a longitudinal mid-neck incision, the left common carotid arteries were exposed. Before arterial clamping, heparin (100 U/kg) was administered intravenously. Heparin was then infused intravenously at a rate of 100 U/kg/h throughout the surgery. After excising 2 cm in situ of native carotid, the grafts (2 cm in length) were placed as an end-to-end anastomosis to the left common carotid arteries using a 7-0 suture (Ethicon, Inc., Cincinnati, OH, USA). Following the unclamping of the artery and restoration of normal blood flow, the closure was sutured by layers. All animals received 50 mg aspirin (Bayer) daily after transplantation. The implanted graft patency was monitored by the color Doppler ultrasonography (CX50, Philips, Netherlands) bi-weekly.
Histological Analysis
At 1, 3, 6, and 12 months after implantation, three animals in both groups were randomly selected to be euthanized. Immediately following sacrifice, the grafts were harvested and washed with heparinized saline. The proximal anastomotic sites were fixed in 4% paraformaldehyde for 48 h. After embedded in paraffin, cut them into 5-μm sections, then dewaxed and rehydrated. For the measurement of intimal thickness and cell infiltration, the grafts were stained with standard H&E. The axial cross-section image of each specimen was magnified to 40 times, then using ImageJ software (National Institutes of Health, Bethesda, MD, USA) measured the intima thickness in random eight places, and the mean value was calculated as the intima thickness of the sample. Von Kossa staining was used to evaluate calcifications. For the evaluation of collagen, Masson’s trichrome staining was carried out. Elastin fiber was evidenced by EVG staining.
Immunohistochemistry
The same samples as above were cut into 5-μm sections. After dewaxed and rehydrated, the sections were incubated with 3% H2O2 at room temperature for 15 min and were then rinsed with PBS. Antibody retrieval was achieved by incubation in ethylenediamine tetraacetic acid (EDTA) (pH 8.0) in a microwave oven at 140°C for 3 min. Then 5% normal goat serum was added and incubated at room temperature for 10 min. After blocking, primary antibodies were added and incubated overnight at 4°C. Anti-CD31 antibody (Abcam) was used to identify ECs, and SMCs were identified by anti-alpha smooth muscle actin (α-SMA) antibody (Abcam). After rinsing, the biotin-labeled secondary antibody (Beijing Zhongshan Jinqiao Biological Technology Co., Ltd., China) was added and incubated at 37°C for 60 min. After DAB color development, the sections were observed under a microscope (Olympus, Tokyo, Japan).
Scanning Electron Microscopy (SEM)
The midportion of harvested grafts were fixed in 2.5% glutaraldehyde solution (Beijing Solarbio Science & Technology, China) for 2 h. After washing with PBS, put the samples into 1% osmium tetroxide solution for 2 h and dehydrated in 50%, 70%, 90%, 100% alcohol gradient for 10 min each. After that, put them into hexamethyldisilazane (BASF SE, Ludwigshafen, Germany) for 10 min and dried. In the end, these samples were sputter-coated with gold and examined with the SU-8010 scanning electron microscope (Hitachi, Tokyo, Japan).
Statistical Analysis
SPSS 19.0 (IBM, Armonk, NY, USA) was used for statistical analysis of all experimental data. Numeric data were expressed as mean ± standard deviation. Fisher’s exact test was employed to compare the patency rate between the two groups. Comparisons between the two groups were performed with a Student’s t test for unpaired data. P values <0.05 were considered statistically significant. The bar chart was drawn by GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA).
Results
Assessment of Decellularization
The histological analysis of native arteries and AVSs were shown in Fig. 1. After decellularization, H&E staining showed no residual cells in the AVSs, and DAPI fluorescent staining confirmed the removal of cellular debris. The DNA quantification analysis revealed that most of the DNA content in the AVSs were removed compared with the native arteries (38.11 ± 12.52 ng/mg versus 596.33 ± 15.38 ng/mg, P < 0.001). The EVG staining and Masson’s trichrome staining showed that the collagen (blue) and elastic fibers (black) in AVSs did not suffer significant damage after decellularization.
Figure 1.
H&E staining (A, B), DAPI staining (C, D), EVG staining (E, F), and Masson’s trichrome staining (G, H) of native arteries (A, C, E, G) and AVSs (B, D, F, H). AVSs: acellular vascular scaffolds; EVG: Elastica Van Gieson; H&E: hematoxylin-eosin.
HGF Coating and Release
The coating amount of HGF on the HHASs was 51.23 ± 1.83 ng/cm2 tested by ELISA methods. As shown in Fig. 2, the release of HGF was observed within 30 days in vitro. Within the first 5 days, HGF was released rapidly from the HHASs, with the cumulative release volume reaching 38.21%. After 17 days, the release gradually stabilized, and the cumulative release of HGF reached 93.59% on day 30.
Figure 2.
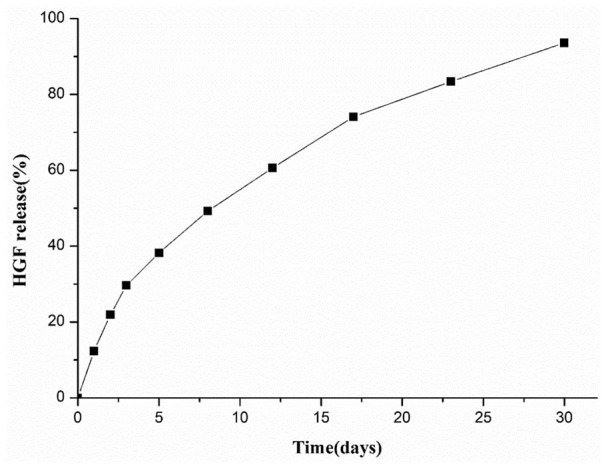
The release curve of HGF on the HHASs in vitro over 30 days. HGF: hepatocyte growth factor; HHASs: heparinized acellular scaffolds grafted with HGF.
Doppler Vascular Ultrasound
As shown in Fig. 3A, rabbit carotid vascular replacement surgery was successful without matrix rupture or deformation. No experimental animals died during the observation period. The grafts were evaluated by Doppler vascular ultrasound at 2-week intervals after implantation (Fig. 3B). Two grafts in the HAS group were observed with thrombotic occlusion within the first 2 weeks after implantation, while only one graft in the HHAS group was occluded. All the other grafts in both groups remained patency for up to 12 months. The patency rate of the HHAS group at 12 months was higher than that of the HAS group without significant difference (91.67% versus 83.33%, P = 1.000). There was no dilatation or aneurysms in both groups at 12 months.
Figure 3.
Implantation of the vascular scaffolds before and after surgery. (A) Surgical implantation of the vascular grafts; (B) Doppler sonographic evaluation of graft patency; (C) harvest of the transplanted vascular grafts.
Histological Analysis
At 1, 3, 6, and 12 months after implantation, the unblocked grafts were retrieved for histologic analyses. As shown in Fig. 3C, the grafts in both groups were surrounded by connective tissue rich in capillaries with no signs of infection.
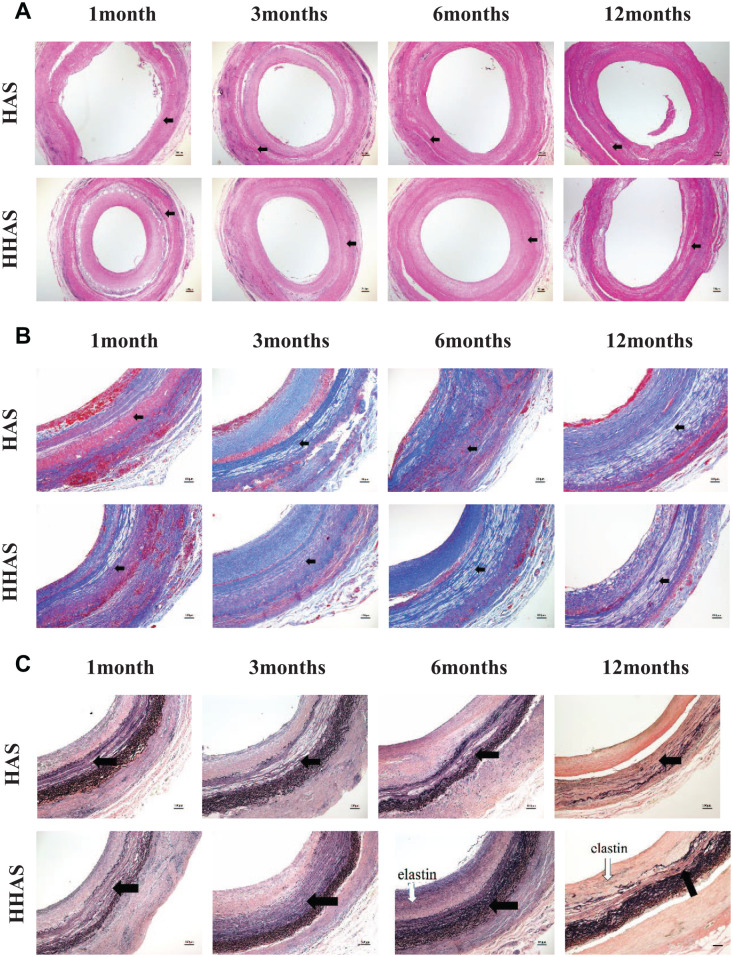
As shown in Fig. 4A, the H&E staining showed that a layer of uniform intimal tissue was attached to the lumen of each graft, and intimal thickness was measured. The intimal thickness of each group increased gradually with the passage of the implantation time (Fig. 4B). At 6 and 12 months postoperatively, the intimal thickness of the HAAS group was significantly smaller than that of the HAS group (P < 0.05). Cells gradually infiltrated into the vessel wall from the endovascular cavity and the outer membrane’ edge with no significant difference in cell number between the two groups. The Von Kossa staining (Fig. 5A) showed no calcification (black) in any of the grafts. The results showed that the neointimal tissues were mainly composed of collagen (Fig. 5B, blue in Masson’s trichrome staining). After 6 months, there was a small number of elastic fibers in the neointimal tissues in the HHAS group (Fig. 5C, black in EVG staining), while no black areas in the HAS group.
Figure 4.
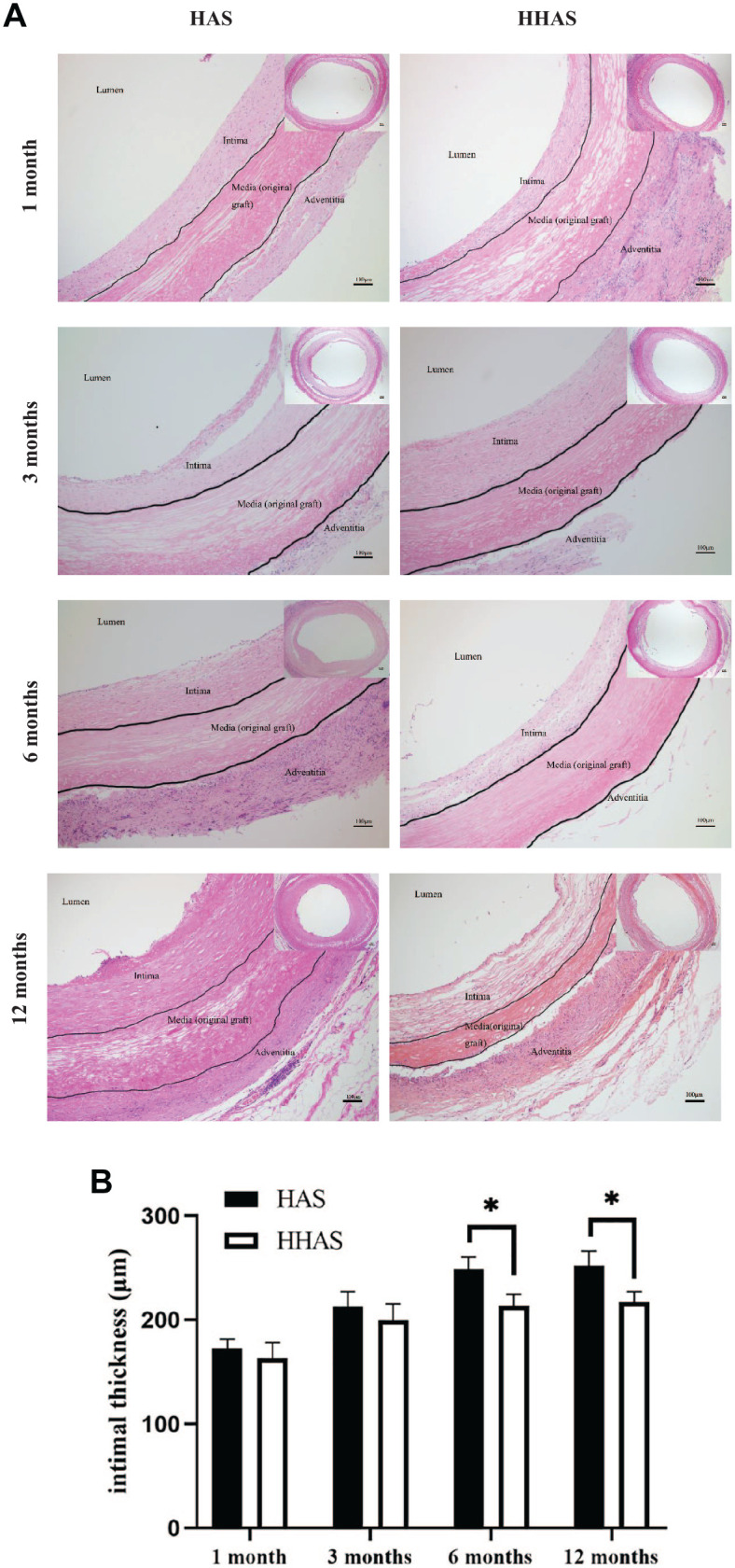
Hematoxylin-eosin (H&E) staining and intimal thickness of the grafted vascular scaffolds. (A) H&E staining of the grafted vascular scaffolds after 1, 3, 6, and 12 months; (B) intimal thickness of the grafted vascular scaffolds after 1, 3, 6, and 12 months. HAS: heparinized acellular scaffold; HHASs: heparinized acellular scaffolds grafted with HGF. Mean ± SD, *P < 0.05.
Figure 5.
Histology of the vascular scaffolds at 1, 3, 6, and 12 months after transplantation. (A) Von Kossa staining indicated no calcium formation; (B) Masson’s trichrome staining of the grafted vascular scaffolds (collagen stained blue); (C) Elastica Van Gieson staining of the grafted vascular scaffolds (elastic fibers stained black). The original acellular graft part was indicated in Figure 5A–C (black arrow). HAS: heparinized acellular scaffold; HHASs: heparinized acellular scaffolds grafted with HGF.
Assessment of Endothelialization
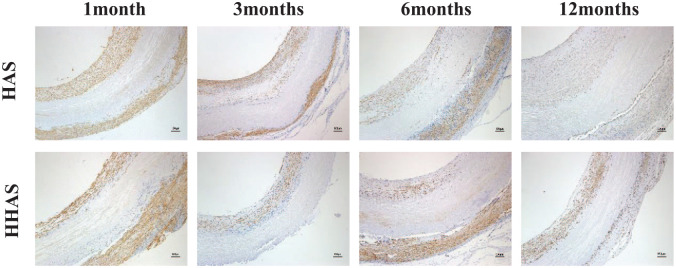
Immunohistochemical staining was used to detect endothelialization, and anti-CD31 antibody was used as ECs markers (Fig. 6A). At 1 month in vivo, ECs appeared on the luminal surface of the grafts in both groups. The percentage of CD31-positive cells covering the lumen surface in the HHAS group was significantly higher than that in the HAS group (95.09 ± 0.23% versus 90.85 ± 2.47%, P < 0.05). The SEM images of the inner surface of grafts were shown in Fig. 6B; there was still collagen exposed between endothelial cells. The collagen area in the HHAS group was significantly smaller than that in the HAS group. At 3 months, endothelium formation further progressed with the complete luminal surface of the grafts in both groups being covered with CD31-positive cells. The SEM images confirmed that the lumen was completely covered by ECs. At 6 months, ECs were more tightly connected than at 3 months, and all line up along the direction of blood flow at 12 months.
Figure 6.
Assessment of endothelialization. (A) Immunohistochemical staining was used to detect grafts’ endothelialization, and anti-CD31 antibody was used as ECs markers (black arrow); (B) the SEM images of the inner surface of grafts (▲collagen exposure).
Vascular Smooth Muscle Regeneration
We used immunohistochemistry staining with anti-α-SMA antibody (Fig. 7) to assess the regeneration of SMCs. The results showed that SMCs were the main cells in the neointimal tissues of the grafts. There was only modest α-SMA-positive cells infiltration in the vessel walls even after 12 months with no difference between the two groups.
Figure 7.
Immunohistochemistry staining with anti-α-SMA antibody of the vascular scaffolds at 1, 3, 6, and 12 months after transplantation.
Discussion
Endothelialization is critical for vascular grafts because ECs play an important role in maintaining vascular hemostasis and patency by releasing regulatory molecules including nitric oxide, prostaglandin, and plasmin17. These molecules can inhibit the proliferation and migration of SMCs. It has been widely recognized that in situ endothelialization is more effective than in vitro endothelialization18. Ideal in situ endothelialization requires more attention to biomaterial types, surface modifications, and releasing factors to regulate cell performance, including enhanced adhesion, orientation, proliferation, and activation of ECs and endothelial progenitor cells (EPCs) on the graft surface19.
Naturally derived acellular vessels have good mechanical properties and molecular proteins that support host cell migration and tissue remodeling, which may have the potential to replace artificial vessels and help solve the problems of donor vessels shortage and recipient immune response20. In our study, the histological analysis showed that 1% Triton X-100 combined with 0.3% SDS could remove the cellular components completely, and preserve the AVM well. AVM can support the adhesion and growth of ECs and SMCs, and maintain the differentiation and phenotype21,22. Nagaoka et al. constructed the AVSs from rats’ abdominal aorta (1 cm in length, 1.3 mm in diameter) and performed carotid artery replacement in rats. The patency rate was 83% after 4 weeks, and endothelial-like cells could be seen on the lumen surface23. Although acellular vessels are effective in animal models, long-term patency rates were not satisfactory, and cell infiltration and vascular remodeling occurred only around the anastomotic sites24. It is necessary to modify acellular vessels to improve their biological properties.
To avoid aneurysms, cross-linking agents are widely used to enhance the mechanical properties of AVSs25–27. Cross-linking agents could make the binding between proteins more stable and enhance the resistance to enzyme-mediated degradation of collagen and glycosaminoglycan28,29. In this study, there was no aneurysm formation, calcification, or wall damage occurred in the grafts after 12 months, indicating that the mechanical properties of AVSs after cross-linking meet the requirements of ideal TEVGs and can effectively reduce the incidence of aneurysms. However, the pore size of AVSs decreased after cross-linking, which caused a physical barrier to cell infiltration. Even after 12 months, the number of infiltrated cells was still low, indicating that cross-linked AVSs may affect the regeneration of SMCs.
Heparin has excellent antithrombotic properties and has been widely used as a coating drug for TEVGs. After acellular porcine femoral artery combined with heparin, Bergmeister et al.30 performed the abdominal aortic replacement in rats, the patency rate was 97.3% at 6 months, and ECs and myofibroblast could be detected within 1 month. HASs can endothelialize spontaneously after implantation, but the time required to restore endothelial function seems too long to prevent the early critical events that lead to SMCs activation and neointimal hyperplasia31,32. Once blood flow in TEVGs is established, endothelialization should be completed as soon as possible because any exposed collagen will activate the coagulation cascade. A drug that can promote the growth of ECs and inhibit the proliferation and migration of SMCs is of great value in preventing intimal hyperplasia after transplantation of TEVGs.
HGF action on ECs can stimulate cell migration, proliferation, and differentiation. HGF can also enhance the biological function of EPCs, promote the migration of EPCs to the damaged intima of arteries, and repair the ECs33. Furthermore, HGF is an anti-fibrosis and anti-inflammatory factor and can be used to treat chronic inflammatory diseases such as atherosclerosis, chronic kidney disease, and heart failure34,35. The mechanism is that HGF can reduce angiotensin-induced oxidative stress and regulate the stimulation of endothelin-1, endotoxin, and transforming growth factor-β by downregulating the epithelial growth factor receptor in a ligand-dependent manner36,37. Harada et al.38 injected HGF intravenously after the replacement of ePTFE artificial vessels in the carotid artery of Japanese white rabbits. The results showed that HGF could inhibit intimal thickness and improve the patency rate. Our study showed that the proportion of ECs covered on the lumen surface in the HAAS group was significantly higher than that in the HAS group within 1 month, indicating that HGF can significantly accelerate endothelialization. At 6 and 12 months, the intimal thickness of the HHAS group was lower than that of the HAS group, indicating that HGF can inhibit intimal hyperplasia at the anastomotic sites. In the study, the patency rate of the grafts in the HHAS group was higher than that of the HAS group, but there was no statistical difference. It may not make sense because of the small sample size.
Cells infiltration is a crucial step in the reconstruction of TEVGs, and the regeneration and function of SMCs in the vascular wall are essential to maintain the mechanical properties and biological effects of TEVGs in the later stage. The ideal TEVGs should be the gradual infiltration of SMCs accompanied by the gradual degradation of TEVGs, finally entirely replaced by host cells and extracellular matrix and the in situ formation of autogenous new arteries with physiological activity and function39. In this study, SMCs gradually infiltrated into the vascular wall, but the number of cells remained low at 12 months. The reason may be that the pore size after cross-linking limits its migration. The cross-linking agent delayed HHAS degradation and avoided aneurysm formation, but inhibited SMCs migration, which was a balance. It is necessary to observe the degradation of the HHASs and the regeneration of SMCs for a longer time.
In conclusion, the small-diameter TEVGs constructed by acellular porcine carotid artery preloaded with heparin and HGF have good anticoagulant and mechanical properties that can inhibit thrombosis and aneurysm formation effectively. HGF can promote rapid endothelialization and inhibit the proliferation of neointimal tissues. As a result, the HHASs have a promising clinical application prospect. In the future, the degradation of the HHASs and the infiltration of SMCs need to be further studied. Besides, it is also necessary to increase the sample size.
Footnotes
Ethical Approval: This study was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University (Approval number: AEEI-2017-141).
Statement of Human and Animal Rights: All experimental procedures involving animals were conducted in accordance with the Committee on the Ethics of Animal Experiments of Capital Medical University approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key Research and Development Program of China (Grant 2017YFC1104100) and the Capital Health Research and Development of Special, Beijing, China (Grant 2016-1-2012).
ORCID iD: Yongquan Gu  https://orcid.org/0000-0003-1497-3287
https://orcid.org/0000-0003-1497-3287
References
- 1. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–45. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, et al. Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528. [DOI] [PubMed] [Google Scholar]
- 3. Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, Edwards A, Carson RJ. The mechanical behavior of vascular grafts: a review. J Biomater Appl. 2001; 15(3):241–78. [DOI] [PubMed] [Google Scholar]
- 4. Weinberg C, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736): 397–400. [DOI] [PubMed] [Google Scholar]
- 5. Amiel GE, Komura M, Shapira O, Yoo JJ, Yazdani S, Berry J, Kaushal S, Bischoff J, Atala A, Soker S. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006;12(8):2355–65. [DOI] [PubMed] [Google Scholar]
- 6. Roy S, Silacci P, Stergiopulos N. Biomechanical properties of decellularized porcine common carotid arteries. Am J Physiol Heart Circ Physiol. 2005;289(4):H1567–76. [DOI] [PubMed] [Google Scholar]
- 7. Wang XN, Chen CZ, Yang M, Gu YJ. Implantation of decellularized small-caliber vascular xenografts with and without surface heparin treatment. Artif Organs. 2010;31(2):99–104. [DOI] [PubMed] [Google Scholar]
- 8. Xu XF, Guo HP, Gong D, Ma JH, Xu ZW, Wan JY, Wang ZG, Zhou ZF, Li WB, Xin Y. Decellularized porcine pulmonary arteries cross-linked by carbodiimide. Int J Clin Exp Med. 2013;6(7):524–31. [PMC free article] [PubMed] [Google Scholar]
- 9. Ma H, Calderon TM, Fallon JT, Berman JW. Hepatocyte growth factor is a survival factor for endothelial cells and is expressed in human atherosclerotic plaques. Atherosclerosis. 2002;164(1):79–87. [DOI] [PubMed] [Google Scholar]
- 10. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25. [DOI] [PubMed] [Google Scholar]
- 11. Joung YK, Bae JW, Park KD. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin Drug Deliv. 2008;5(11):1173–84. [DOI] [PubMed] [Google Scholar]
- 12. Tan Q, Tang H, Hu J, Hu Y, Zhou X, Tao Y, Wu Z. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int J Nanomedicine. 2011;6:929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2–3):121–36. [DOI] [PubMed] [Google Scholar]
- 14. Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roghani M, Mansukhani A, Dell'Era P, Bellosta P, Moscatelli D. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. J Biol Chem. 1994;269(6):3976–84. [PubMed] [Google Scholar]
- 16. Cai Z, Gu Y, Cheng J, Li J, Xu Z, Xing Y, Wang C, Wang Z. Decellularization, cross-linking and heparin immobilization of porcine carotid arteries for tissue engineering vascular grafts. Cell Tissue Bank. 2019;20(4):569–78. [DOI] [PubMed] [Google Scholar]
- 17. Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9(8):439–53. [DOI] [PubMed] [Google Scholar]
- 18. Avci-Adali M, Ziemer G, Wendel HP. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization—a review of current strategies. Biotechnol Adv. 2010;28(1):119–29. [DOI] [PubMed] [Google Scholar]
- 19. Zhuang Y, Zhang C, Cheng M, Huang J, Liu Q, Yuan G, Lin K, Yu H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact Mater. 2021;6(6):1791–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215–31. [DOI] [PubMed] [Google Scholar]
- 21. Xiong Y, Chan WY, Chua AW, Feng J, Gopal P, Ong YS, Song C. Decellularized porcine saphenous artery for small-diameter tissue-engineered conduit graft. Artif Organs. 2013;37(6):E74–87. [DOI] [PubMed] [Google Scholar]
- 22. Schneider KH, Aigner P, Holnthoner W, Monforte X, Nürnberger S, Rünzler D, Redl H, Teuschl AH. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater. 2016;29:125–34. [DOI] [PubMed] [Google Scholar]
- 23. Nagaoka Y, Yamada H, Kimura T, Kishida A, Fujisato T, Takakuda K. Reconstruction of small diameter arteries using decellularized vascular scaffolds. J Med Dental Sci. 2014;61: 33–40. [PubMed] [Google Scholar]
- 24. Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3(68):68ra9. [DOI] [PubMed] [Google Scholar]
- 25. Pennel T, Fercana G, Bezuidenhout D, Simionescu A, Chuang TH, Zilla P, Simionescu D. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. Biomaterials. 2014;35(24):6311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang Y, Hsu CK, Wei HJ, Chen SC, Liang HC, Lai PH, Sung HW. Cell-free xenogenic vascular grafts fixed with glutaraldehyde or genipin: in vitro and in vivo studies. J Biotechnol. 2005;120(2):207–19. [DOI] [PubMed] [Google Scholar]
- 27. Zhao Y, Zhang Z, Wang J, Yin P, Wang Y, Yin Z, Zhou J, Xu G, Liu Y, Deng Z, Zhen M, et al. Preparation of decellularized and crosslinked artery patch for vascular tissue-engineering application. J Mater Sci Mater Med. 2011;22(6):1407–17. [DOI] [PubMed] [Google Scholar]
- 28. Mercuri JJ, Lovekamp JJ, Simionescu DT, Vyavahare NR. Glycosaminoglycan-targeted fixation for improved bioprosthetic heart valve stabilization. Biomaterials. 2007;28(3):496–503. [DOI] [PubMed] [Google Scholar]
- 29. Raghavan D, Simionescu DT, Vyavahare NR. Neomycin prevents enzyme-mediated glycosaminoglycan degradation in bioprosthetic heart valves. Biomaterials. 2007;28(18):2861–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergmeister H, Plasenzotti R, Walter I, Plass C, Bastian F, Rieder E, Sipos W, Kaider A, Losert U, Weigel G. Decellularized, xenogeneic small-diameter arteries: transition from a muscular to an elastic phenotype in vivo. J Biomed Mater Res B Appl Biomater. 2010;87B(1):95–104. [DOI] [PubMed] [Google Scholar]
- 31. Wang XN, Chen CZ, Yang M, Gu YJ. Implantation of decellularized small-caliber vascular xenografts with and without surface heparin treatment. Artif Organs. 2010;31(2):99–104. [DOI] [PubMed] [Google Scholar]
- 32. Liao D, Wang X, Lin PH, Yao Q, Chen C. Covalent linkage of heparin provides a stable anti-coagulation surface of decellularized porcine arteries. J Cell Mol Med. 2009;13(8B):2736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song MB, Yu XJ, Zhu GX, Chen JF, Zhao G, Huang L. Transfection of HGF gene enhances endothelial progenitor cell (EPC) function and improves EPC transplant efficiency for balloon-induced arterial injury in hypercholesterolemic rats. Vascul Pharmacol. 2009;51(2–3):205–13. [DOI] [PubMed] [Google Scholar]
- 34. Vasir B, Reitz P, Xu G, Sharma A, Bonner-Weir S, Weir GC. Effects of diabetes and hypoxia on gene markers of angiogenesis (HGF, cMET, uPA and uPAR, TGF-α, TGF-β, bFGF and Vimentin) in cultured and transplanted rat islets. Diabetologia. 2000;43(6):763–72. [DOI] [PubMed] [Google Scholar]
- 35. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2010;26(8):2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanada F, Taniyama Y, Iekushi K, Azuma J, Okayama K, Kusunoki H, Koibuchi N, Doi T, Aizawa Y, Morishita R. Negative action of hepatocyte growth factor/c-met system on angiotensin II signaling via ligand-dependent epithelial growth factor receptor degradation mechanism in vascular smooth muscle cells. Circ Res. 2009;105(7):667–75. [DOI] [PubMed] [Google Scholar]
- 37. Shimizu K, Taniyama Y, Sanada F, Azuma J, Iwabayashi M, Iekushi K, Rakugi H, Morishita R. Hepatocyte growth factor inhibits lipopolysaccharide-induced oxidative stress via epithelial growth factor receptor degradation. Arterioscler Thromb Vasc Biol. 2012;32(11):2687–93. [DOI] [PubMed] [Google Scholar]
- 38. Harada M, Takenaka H, Ikenaga S, Zhang H, Zempo N, Esato K, Nagano T, Taiji M, Noguchi H. Hepatocyte growth factor prevents intimal hyperplasia in rabbit carotid expanded polytetrafluoroethylene grafting. J Vasc Surg. 2002;35(4):786–91. [DOI] [PubMed] [Google Scholar]
- 39. Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med. 2013;6(1):61–76. [DOI] [PubMed] [Google Scholar]