Abstract
Polymorphonuclear leukocytes (PMN) are essential for resolution of infections with Listeria monocytogenes. The present study investigated the role of the listerial exotoxins listeriolysin (LLO) and phosphatidylinositol-specific phospholipase C (PlcA) in human neutrophil activation. Different Listeria strains, mutated in individual virulence genes, as well as purified LLO were used. Coincubation of human neutrophils with wild-type L. monocytogenes provoked PMN activation, occurring independently of phagocytosis events, with concomitant elastase secretion, leukotriene generation, platelet-activating factor (PAF) synthesis, respiratory burst, and enhanced phosphoinositide hydrolysis. Degranulation and leukotriene formation were noted to be solely dependent on LLO expression, as these features were absent when the LLO-defective mutant EGD− and the avirulent strain L. innocua were used. These effects were fully reproduced by a recombinant L. innocua strain expressing LLO (INN+) and by the purified LLO molecule. LLO secretion was also required for PAF synthesis. However, wild-type L. monocytogenes was more potent in eliciting PAF formation than mutants expressing LLO, suggesting the involvement of additional virulence factors. This was even more obvious for phosphoinositide hydrolysis and respiratory burst: these events were provoked not only by INN+ but also by the LLO-defective mutant EGD− and by a recombinant L. innocua strain producing listerial PlcA. We conclude that human neutrophils react to extracellularly provided listerial exotoxins by rapid cell activation. Listeriolysin is centrally involved in triggering degranulation and lipid mediator generation, and further virulence factors such as PlcA apparently contribute to trigger neutrophil phosphoinositide hydrolysis and respiratory burst. In this way, listerial exotoxins may influence the host defense against infections with L. monocytogenes.
The host response to Listeria monocytogenes, a facultative intracellular bacterial pathogen, can be divided into two stages (20, 34). Early nonspecific resistance is thought to be mediated primarily by resident macrophages, in particular Kupffer cells of the liver. A subsequent resistance-specific stage, required for the complete resolution of infection, depends on the generation of specific T-cell-mediated immunity. Several studies have, however, demonstrated that polymorphonuclear neutrophilic granulocytes (PMN) are essential for both stages of host defense. PMN function to lyse Listeria-infected parenchymal cell, thereby exposing the bacteria to professional phagocytes such as the neutrophils themselves, and T-cell-mediated immunity to Listeria organisms is incomplete in the absence of PMN (2, 12, 14, 15, 33, 35).
Neutrophils are well equipped for acute lysis of infected parenchymal cells and destruction of listeriae (25, 38). Their NADPH oxidase system generates superoxide anion and derived oxygen radicals, their granules contain a variety of proteolytic enzymes capable of attacking cellular proteins and connective tissue components, and they release different lipid mediators, such as leukotriene B4 (LTB4) and platelet-activating factor (PAF), known to effect further PMN recruitment. Previous studies addressing PMN-L. monocytogenes interaction in vitro noted that upregulation and secretion of the proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor occurred within hours in the neutrophils in contact with listeriae (3).
This investigation addressed the acute phase of PMN activation by L. monocytogenes, with particular focus on the role of listerial exotoxins. Among the latter, listeriolysin (LLO), a member of sulfhydryl-activated pore-forming toxins and thus far the best-characterized virulence factor of listeriae, and phosphatidylinositol-specific phospholipase C (PlcA) were recently recognized to be potent inductors of endothelial cell signalling events (36, 37) prior to cell invasion. The pathogenic wild-type L. monocytogenes is here reported to provoke oxidative burst, degranulation, generation of leukotrienes and PAF, and enhanced phosphoinositide hydrolysis-related signalling events in human neutrophils within a few minutes, independent of phagocytosis events. Employing genetically engineered strains of L. monocytogenes and L. innocua, used as the host for selective expression of exotoxins, as well as purified exotoxins, we identified listeriolysin as a key agent in human neutrophil activation. Efficacious stimulation of these phagocytes, however, requires additional listerial virulence factors, of which PlcA may play a prominent role.
MATERIALS AND METHODS
Materials.
Arachidonic acid, superoxide dismutase, cytochrome c type IV, bovine serum albumin (BSA), and HEPES were purchased from Sigma (Deisenhofen, Germany). The protein kinase C inhibitor staurosporine was obtained from Calbiochem (Giessen, Germany). The PAF antagonist BN50727 was kindly provided by P. Braquet (Henri Beaufour Institute, Paris, France). S-2484, a substrate for neutrophil elastase, was purchased from Kabi-Vitrum (Stockholm, Sweden). RPMI 1640 medium, Hanks’ balanced salt solution (HBSS), brain heart infusion (BHI), erythromycin, and fetal calf serum were from Gibco Laboratories (Grand Island, N.Y.). Percoll was obtained from Pharmacia Fine Chemicals (Uppsala, Sweden). The leukotrienes LTC4, LTD4, LTE4, LTB4, 20-OH-LTB4, and 20-COOH-LTB4 and the synthetic LTA4 methyl ester were a generous gift from J. Rokach, Merck Frosst (Toronto, Ontario, Canada). Additional leukotrienes were graciously supplied by W. Bartmann, Hoechst AG. 5-, 8-, 9-, 11-, 12-, and 15-hydroxyeicosatetraenoic acid (HETE), 5(S),12(S)-diHETE, 5,15-diHETE, and 12-HHT, as well as the nonenzymatic hydrolysis products of LTA4 (6-trans diasteromeric pair of LTB4 and 5,6-diHETEs), were obtained from Paesel AG (Frankfurt, Germany). Tritiated leukotrienes, used as internal standards, as well as [3H]serotonin were obtained from New England Nuclear (Boston, Mass.). Tritiated inositol phosphates and myo-[2-3H]inositol were purchased from Amersham (Dreieich, Germany). Chromatographic supplies included silica gel 5-μm column packing (Machery Nagel, Düren, Germany), high-pressure liquid chromatography (HPLC)-grade solvents, distilled in glass (Fluka KG, Heidelberg, Germany), and Silica Gel 60 F254 plates (Merck & Co., Darmstadt, Germany). All other biochemicals were obtained from Merck.
Bacterial strains.
Table 1 describes the Listeria strains used in this study. Recombinant strains of L. monocytogenes and L. innocua were obtained as previously described (9). The apathogenic L. innocua strain was used as the host for selective expression of the LLO (hly) or plcA gene. To induce high levels of either protein from the recombinant strain, the hly and plcA genes were cloned onto a plasmid also harboring the prfA regulator. Bacteria were grown in BHI broth at 37°C, and erythromycin (5 μg/ml) was used where appropriate. The hemolysin assay was performed as described previously (30) except that human erythrocytes were used at a final concentration of 0.5%.
TABLE 1.
Listeria strains used in this study
| Strain | Serotype; relevant genotype; hemolytic phenotypea | Abbreviation used |
|---|---|---|
| L. monocytogenes | ||
| EGD | 1/2a; wild type; + | EGD+ |
| EGDdhyly1 | 1/2a; hly1; − | EGD− |
| L. innocua | ||
| 11288/pERL3 | 6a; wild type; − | INN |
| 11288/pERL3 | 6a; prfA hly +; ++ | INN+ |
| 11288/pERL3 | 6a; prfA plcA +; − | INN-PlcA |
Hemolytic phenotypes observed on sheep blood agar plates were scored as follows: ++, strongly hemolytic; +, weakly hemolytic; −, nonhemolytic.
Purification of LLO.
LLO was purified from L. innocua ATCC 11288 harboring plasmid pERL3 (prfA hly), which produces 512-fold more LLO than L. monocytogenes wild-type strain EGD (16). Briefly, supernatant fluids from exponentially growing bacteria were concentrated 20-fold in a Millipore filtration apparatus. The supernatant was first batch absorbed with Q-Sepharose, and the nonabsorbed fraction was recovered by centrifugation. This was then loaded onto a Mono S HR5/5 column and eluted with a linear gradient of 50 to 500 mM NaCl with 40 mM phosphate buffer (pH 5.0). LLO eluted as a sharp peak at 200 to 260 mM NaCl. Following dialysis against phosphate-buffered saline (pH 7.2), LLO was stored at −70°C. Purified LLO migrated as a 58-kDa band in sodium dodecyl sulfate-Coomassie blue-stained gels and was judged to be greater than 95% pure.
Preparation of human granulocytes.
Heparinized blood from healthy donors was centrifuged in a discontinuous Percoll gradient (27) to yield a PMN fraction of approximately 97% purity. Prior to experiments, PMN were kept in RPMI 1640 with 10% fetal calf serum for 30 to 60 min at 37°C. Immediately before stimulus application, cells were washed twice and suspended in HBSS-HEPES buffer to obtain PMN concentrations of 10 × 106 PMN/ml. Cell viability, as assessed by trypan blue exclusion, ranged above 96%, and lactate dehydrogenase (LDH) release was consistently below 3%.
Measurement of leukotrienes.
Leukotrienes and HETEs were extracted from cell supernatants by octadecylsilyl solid-phase extraction columns as described elsewhere (21, 22). Conversion to methyl esters was performed by addition of freshly prepared diazomethane in ice-cold diethyl ether. Reversed-phase HPLC of nonmethylated compounds was carried out on octadecylsilyl columns (Hypersil; 5-μm particles), with a mobile phase of methanol-water-acetic acid (72:28:0.16 [pH 4.9]) (21). In addition to the conventional UV detection at 270 nm (leukotrienes) and 237 nm (HETEs), a photodiode array detector (Waters model 990) was used, which provided full UV spectra (190 to 600 nm) of eluting compounds and allowed checking for peak purity and subtraction of possible coeluting material. Reversed-phase HPLC of methylated compounds was performed isocratically (methanol-water-acetic acid, 66:34:0.16 [pH 4.9]) for 5 min, followed by a linear gradient to 90:10:0.16 over 10 min (Gynkothek gradient former model 250). Straight-phase HPLC of methylated compounds was carried out by a modification of the method of Nadeau et al. (31). The mobile phase consisted of hexane-isopropanol-acetate (86:14:0.1), and the column was eluted isocratically at a flow rate of 1.0 ml/min. All data obtained by the different analytical procedures were corrected for recoveries obtained by the overall analytical procedure and are given as picomoles per milliliter throughout. Recovery was determined by separate recovery experiments using different quantities of the individual compounds in the appropriate concentration range. Factors for recovery were further confirmed by addition of 0.2 μCi of [3H]LTB4 and [3H]5-HETE to buffer medium as internal standards in selected experiments. For quantification of leukotrienes and 5-HETE, correspondence of values calculated from UV absorbancy in two different chromatographic procedures was required (deviation of <10%).
Measurement of PAF.
Neutrophil PAF production was quantified by induction of 3[H]serotonin release from prelabeled rabbit platelets. After termination of PMN incubation in HBSS containing 20 mM HEPES and 0.25% BSA, reactions were stopped by addition of 3 volumes of chloroform-methanol (1:2 [vol/vol]), and extraction was performed by the method of Bligh and Dyer (7). The entire lipid extract was evaporated to dryness, redissolved in 60 μl of mobile phase, and subjected to straight-phase HPLC separation. The column (25 by 0.46 cm) was packed with silica gel (5-μm) particles and eluted isocratically with acetonitrile-methanol-phosphoric acid at a flow rate of 1.8 ml/min. Eluate fractions corresponding to appropriate standard retention times were collected, again lipid extracted for removal of phosphoric acid present in the mobile phase, evaporated to dryness, and redissolved in 50 μl of assay buffer for induction of platelet serotonin release. Preparation of platelets and the protocol of the bioassay were essentially as published by Pinkard et al. (32). [3H]serotonin-labeled platelets (250,000 cells/μl in a total volume of 0.5 ml) were incubated for 60 s. A 200-μl aliquot then was rapidly removed, added to a chilled tube containing 20 μl of 1.5 mM formaldehyde, and centrifuged at 12,000 × g for 2 min. Serotonin secretion into the platelet supernatant was determined by liquid scintillation counting and related to that released from the same volume of platelet suspension after cell lysis with Triton X-100 (final concentration, 0.83% [wt/vol]). Known quantities of PAF were used to establish a calibration curve for the bioassay. Aliquots of each sample were used to ascertain the specificity of platelet secretion by the inhibitory effect of the PAF receptor antagonist BN50727 (1 μM).
Release of granule constituents and LDH.
Elastase was taken as marker for neutrophil degranulation, and enzyme activity in the cell supernatant was measured according to standard procedures (26, 29). LDH, as a marker for overt cytotoxicity, was quantified by a colorimetric technique. Enzyme release was expressed as percentage of total enzyme activity liberated in the presence of 100 μg of melittin per ml.
Superoxide generation.
PMN O2− generation was measured as superoxide dismutase-inhibitable reduction of cytochrome c as described elsewhere (10). Duplicate reaction mixtures containing neutrophils (10 × 106 PMN/ml) and 75 μM ferricytochrome c were incubated at 37°C in the presence or absence of 10 μg of superoxide dismutase per ml.
Phosphoinositide metabolism.
The phosphatidylinositol turnover of stimulated neutrophils was investigated by measuring the accumulation of inositol phosphates as described by Berridge et al. (4). For prelabeling of cellular phospholipid pools, PMN were resuspended to 107 cells/ml with medium 199 containing 2% fetal calf serum plus 40 mM HEPES buffer (pH 7.4). myo-[3H]inositol (50 μCi/ml) was added, and cells were incubated at 37°C for 2 h on a shaking water bath. Before experimental use, cells were washed twice and resuspended in HBSS containing 20 mM HEPES and 10 mM LiCl (107 PMN/ml). At different times after stimulus application, samples were quenched with trichloroacetic acid (final concentration, 7.5%), kept on ice for 15 min, and extracted four times with diethyl ether. The aqueous phase was neutralized with sodium tetraborate to pH 8.0 and processed to separate inositol phosphates on Dowex anion-exchange columns as described by Berridge et al. (4). Under these assay conditions, cyclic inositol monophosphate (cIMP) decomposes quantitatively to generate IP1.
Experimental protocols. (i) Granulocyte-bacterium cocultures.
After overnight culture in BHI broth, 4 ml of the bacterial suspension was added to 46 ml of fresh BHI (in the presence of 5 μg of erythromycin per ml) and incubated at 37°C until it reached an optical density of 0.45 (photometrically assessed at 600 nm). Then bacteria were spun at 3,000 × g and resuspended in 3.5 ml of HBSS (pH 7.4, absence of erythromycin), and 100 μl of the bacterial suspension was admixed to the 0.9 ml of HBSS buffer (pH 7.4, absence of erythromycin), containing 107 PMN/ml. Thus, approximately 3.5 × 106 bacteria were obtained in the final 1-ml assay volume. After various time periods, reactions were stopped by admixing trichloroacetic acid (inositol phosphates) and chloroform-methanol (1:2 [vol/vol]) (PAF) or placed on ice for 15 min and subsequently spun at 3,000 × g (leukotrienes, elastase secretion, and respiratory burst).
(ii) Incubation of PMN with purified LLO.
LLO was admixed to the HBSS (pH 7.4, absence of erythromycin) buffer containing 107 PMN/ml and incubated for various time periods. Termination of experiments was performed accordingly.
(iii) Leukotriene generation.
All experiments addressing the activation of 5-lipoxygenase were performed in the presence of 10 μM free arachidonic acid. In the absence of bacteria and isolated toxins, the presence of this fatty acid per se did not provoke any substantial leukotriene generation.
(iv) Neutrophil preincubation with botulinum C2 toxin.
Botulinum C2 toxin, composed of a membrane translocation component (C2II) and a component (C2I) effecting ADP-ribosylation of nonmuscle G-actin, thereby acting as a barbed end-capping protein and effecting selective loss of the nonmuscle F-actin content (27), was graciously provided by K. Aktories, Freiburg, Germany. It was provided to the neutrophils at a concentrations of 400 (C2I) and 800 (C2II) ng/ml 30 min before granulocyte-bacterium coincubation. Pilot experiments ascertained that any phagocytosis is fully inhibited in these cells.
Statistics.
For statistical comparison, one-way analysis of variance was performed. A P level of <0.05 was considered significant.
RESULTS
Elastase secretion.
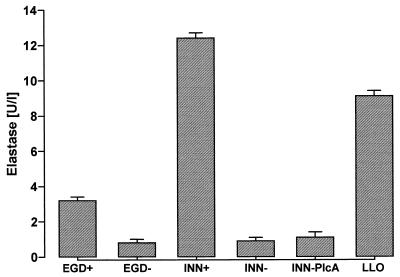
Incubation of human neutrophils with wild-type L. monocytogenes (EGD+) caused a rapid secretion of elastase, plateauing after 10 to 15 min (Fig. 1). In contrast, neither the apathogenic strain L. innocua (INN−), which is nonhemolytic and noninvasive, nor an isogenic strain of L. monocytogenes (EGD−), which produces a truncated nonhemolytic 40-kDa polypeptide, provoked substantial elastase secretion. Protease liberation was reproduced by purified LLO in the absence of bacteria, and the most prominent elastase secretion occurred when neutrophils were incubated with L. innocua engineered to overexpress LLO (INN+). Use of L. innocua as a host to express phospholipase C (INN-PlcA) did not result in significant release of elastase. Microscopic examination of the neutrophil-EGD+ cocultures did not reveal substantial listerial phagocytosis within the 15-min coincubation period (ingestion of bacteria estimated to be <5%). To exclude any impact of phagocytosis events on elastase secretion, additional experiments with botulinum C2 toxin-preincubated neutrophils were performed. Coincubation of these cells with EGD+ resulted in elastase concentrations of 3.3 ± 0.2 U/liter (mean ± standard error of the mean [SEM]; n = 5) within 15 min, consistent with corresponding data for non-C2-toxin-treated PMN (3.2 ± 0.2 U/liter). LDH release in response to EGD+, EGD−, INN-PlcA, INN−, and LLO was less than 5%; release in response to INN+ was less than 15%.
FIG. 1.
Neutrophil elastase secretion evoked by various bacterial strains. PMN (107) were incubated with EGD+, EGD−, INN+, INN−, or INN-PlcA (for each strain, 3.5 × 106 bacteria/ml) or with purified LLO (1 μg/ml) for 10 min. Means ± SEM of five independent experiments each are given.
Lipid mediator generation.
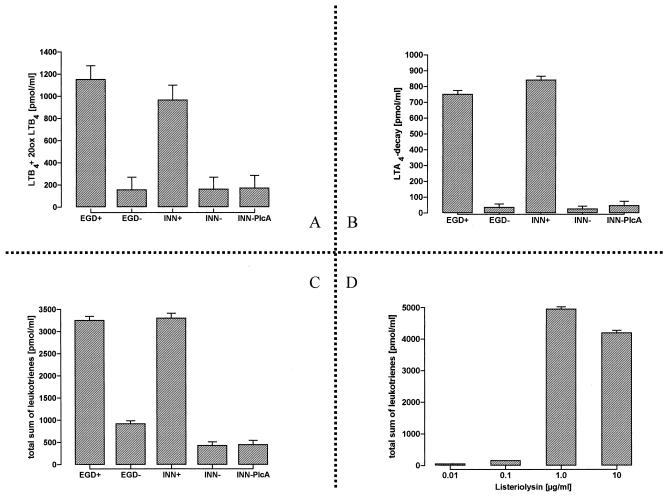
Incubation of PMN with EGD+ induced the synthesis of large amounts of LTB4, ϖ-OH- and -COOH-LTB4, as well as LTA4 decay products (Fig. 2A to C). Product release peaked within 10 min after this challenge. The LLO-negative strains EGD− and INN− failed to activate this metabolic response. Leukotriene formation was fully restored when L. innocua was engineered to express LLO, and it was similarly noted in the presence of purified LLO (Fig. 2D), with an optimum concentration of 1 μg/ml. In contrast, no significant leukotriene synthesis was noted in the presence of INN-PlcA.
FIG. 2.
Generation of leukotrienes in response to various bacterial strains. Neutrophils (107 PMN/ml) were simultaneously exposed to free arachidonic acid (10 μM). Incubation was terminated after 10 min. LTB4, 20-OH-LTB4, and 20-COOH-LTB4 are indicated as LTB4+20ox LTB4 in panel A, nonenzymatic hydrolysis products of LTA4 are summarized as LTA4 decay in panel B, and the sum of data for leukotrienes is shown in panel C. Dose-dependent generation of leukotrienes in response to purified LLO is demonstrated in panel D. Means ± SEM of six independent experiments each are given.
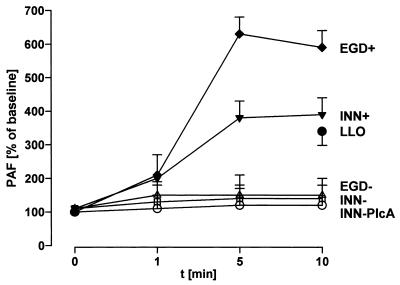
Incubation of PMN with EGD+ provoked marked PAF liberation (Fig. 3), which plateaued after 5 min. In contrast to elastase secretion and leukotriene formation, this effect was only partially reproduced when PMN were incubated with INN+ or purified LLO. EGD− and INN− did not activate PAF synthesis.
FIG. 3.
Time course of PAF generation in response to various bacterial strains. PMN (107) were incubated with strain EGD+, EGD−, INN+, INN−, or INN-PlcA or with purified LLO (10 μg/ml). All bacteria were used at 3 × 106/ml. After various time periods, secreted and cell-bound PAF was lipid extracted, purified by HPLC, and quantified by induction of [3H]serotonin release from prelabeled rabbit platelets. Samples containing the PAF antagonist BN50727 ranged on the level of EGD− (corresponding to control levels; data not shown), thereby indicating that [3H]serotonin secretion was specifically induced by PAF. Means ± SEM of five independent experiments are given.
Respiratory burst.
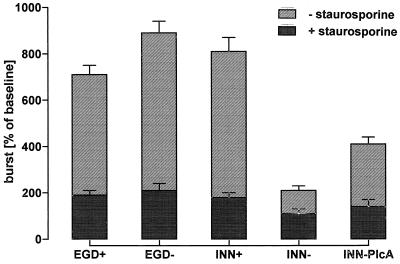
Respiratory burst, measured as O2− release, was induced in PMN incubated with wild-type EGD as well as LLO-expressing L. innocua (Fig. 4). Comparable O2− release did also occur in the presence of EGD−, which failed to express LLO. INN-PlcA, engineered to produce high levels of PlcA, provoked respiratory burst to a minor extent. In contrast, INN− was largely ineffective. In the presence of the protein kinase inhibitor staurosporine, O2− release was suppressed to baseline levels under all experimental conditions used.
FIG. 4.
Neutrophil superoxide production evoked by various bacterial strains. PMN (107) were incubated with EGD+, EGD−, INN+, INN−, or INN-PlcA in the absence or presence of 1 μM staurosporine. Incubation was terminated after 10 min. Means ± SEM of six independent experiments each are given.
Phosphoinositide metabolism.
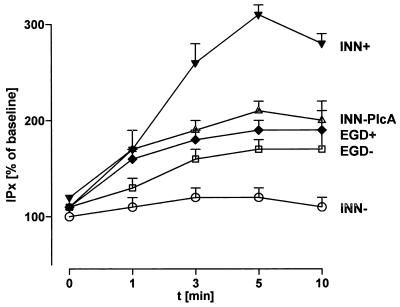
Neutrophil coincubation with EGD+ caused rapid-onset phosphatidylinositol hydrolysis, with a maximal accumulation of inositol phosphates after approximately 5 min (Fig. 5). Incubation of PMN with INN+ induced a strong phosphatidylinositol hydrolysis response, even surpassing that evoked by EGD+. While INN− was unable to activate this pathway in neutrophils, the LLO-negative isogenic strain of L. monocytogenes, EGD−, evoked inositol phosphate accumulation to the same extent as wild-type L. monocytogenes. A corresponding response was provoked by INN-PlcA.
FIG. 5.
Time course of inositol phosphate generation in response to various bacterial strains. PMN were prelabeled with [3H]inositol and subsequently incubated with strains EGD+, EGD−, INN+, INN, and INN-PlcA. After various time periods, inositol phosphates were extracted and separated by anion-exchange chromatography. IP3, IP2, IP1, and cIMP are collectively referred to as IPx. Means ± SEM of five independent experiments each are given.
DISCUSSION
Coincubation of human neutrophils with wild-type L. monocytogenes provoked rapid-onset PMN activation, occurring independently of phagocytosis events, with elastase secretion, leukotriene generation, PAF synthesis, respiratory burst, and enhanced phosphoinositide hydrolysis representing prominent features. The use of recombinant listeriae producing different exotoxins and purified toxin allowed us to dissect the role of listerial exotoxin secretion in PMN stimulation. Thus, the processes of degranulation and leukotriene formation were noted to be exclusively dependent on LLO expression. These features were absent when LLO-defective mutants were used, and they were fully reproduced by an avirulent recombinant expressing LLO and by purified LLO in the absence of bacteria. Respiratory burst and neutrophil phosphoinositide metabolism were induced by overexpression of LLO as the sole exotoxic agent. However, triggering of these events by L. monocytogenes was noted to occur also in the absence of LLO, suggesting the role of additional listerial toxins, particularly PlcA. PAF synthesis was intermediate with respect to LLO dependency.
Incubation of human neutrophils with the virulent wild-type L. monocytogenes provoked rapid degranulation, as assessed by elastase secretion. In parallel, extensive leukotriene generation, indicating activation of the PMN 5-lipoxygenase pathway, was noted, with the appearance of both LTB4 and its omega oxidation products as well as the release of the unstable intermediate LTA4, undergoing decomposition to various decay products in the extracellular space. These events were obviously not triggered by phagocytosis of the bacteria, as (i) hardly any ingestion of listeriae was noted within the short coincubation periods used, in accordance with previous observations on phagocytosis of L. monocytogenes by human neutrophils (15), (ii) L. innocua INN−, devoid of exotoxins, provoked no substantial elastase secretion or leukotriene generation within this time period, and (iii) complete inhibition of neutrophil phagocytic capacity due to blockage of its actin-based cytoskeleton by botulinum C2 toxin did not interfere with the elicited by EGD+ secretory responses. That both PMN elastase liberation and leukotriene synthesis were clearly related to LLO release could be demonstrated in several ways. First, the presence of LLO was noted to be a prerequisite for these neutrophil responses, as EGD− (which produces a truncated nonhemolytic protein instead of the native LLO), the apathogenic INN− (which is nonhemolytic), and an L. innocua mutant expressing PlcA but not LLO were all ineffective. Second, in the presence of LLO as the sole exotoxic agent, both degranulation and leukotriene synthesis were fully reproduced, as evident from the experiments with the L. innocua strain engineered to express LLO and from studies with purified LLO in the absence of bacteria. Third, dose-effect relationships were demonstrated: elastase release was clearly more prominent upon coincubation of neutrophils with the L. innocua strain overexpressing LLO compared to the PMN-EGD+ cocultures, and dose-effect curves with purified LLO showed maximum leukotriene generation at 1 μg of this exotoxin per ml.
This study did not address the question of the PMN signalling events via which both degranulation and 5-lipoxygenase activation occurred in the neutrophils in response to extracellularly provided LLO. As previously suggested for pore-forming toxins, the formation of a transmembrane aqeuous channel might induce metabolic events by enabling an extraintracellular calcium shift (5, 6), and pilot experiments indeed showed marked inhibition of the LLO-induced elastase secretion by complexing extracellular calcium with EGTA (data not shown). Alternatively, as demonstrated for the activation of human neutrophils by the Escherichia coli hemolysin HlyA (23, 24), strong stimulation of the preformed phosphoinositide hydrolysis-related signal transduction pathway may also be due to LLO-induced activation of endogenous phospholipase C (see below), and this pathway is well known to be linked to degranulation and 5-lipoxygenase activation (18, 19). This view is supported by the fact that, concomitant with the different potencies observed in eliciting degranulation, the maximal accumulation of inositol phosphates was noted in the neutrophils treated with the LLO-overexpressing strain INN+. As this mutant is devoid of listerial phospholipase C and other phospholipases, the phosphoinositide hydrolysis in these experiments must derive from an activation of endogenous phospholipase C targeting phosphatidylinositol. The suggestion that LLO-induced neutrophil stimulation employs intracellular phospholipase C activation is also supported by the finding that the high LLO concentration of 10 μg/ml, which effects the maximum membrane perturbation, was less potent in eliciting leukotriene generation than 1 μg/ml. This observation is reminiscent of the bell-shaped dose-effect curves for E. coli HlyA on human neutrophil phosphoinositide hydrolysis (23, 24). Clearly, further studies are required to elucidate the intracellular signalling events in LLO-exposed human neutrophils in more detail.
As found for elastase and leukotriene secretion, neutrophil PAF synthesis was provoked by purified LLO and by the L. innocua strain expressing LLO and was absent in studies with the LLO-defective mutant EGD−. However, the maximum PAF response to the challenges using only LLO (INN+, purified toxin) was clearly inferior to the response provoked by the wild-type L. monocytogenes, suggesting that additional virulence factors of the wild-type strain cooperate with LLO. The role of such additional factors was even more obvious for the respiratory burst and for neutrophil inositol phosphate accumulation, as both features were provoked by the LLO-defective mutant EGD− to nearly the same extent as by the wild-type L. monocytogenes strain. Interestingly, in contrast to degranulation, leukotriene generation, and PAF synthesis, the L. innocua strain engineered to express the listerial phospholipase PlcA as the sole exotoxic agent displayed some intermediate potency to provoke both neutrophil inositol phosphate accumulation and respiratory burst. The close relationship between phosphoinositide hydrolysis and superoxide anion generation is also supported by the fact that under all experimental conditions investigated, the respiratory burst was inhibited by the protein kinase C inhibitor staurosporine, suggesting the well-known sequence of phosphoinositide hydrolysis, diacylglycerol formation, protein kinase C activation, and subsequent assembling of the multienzyme complex NADPH oxidase as the underlying sequence of events. Additional studies are required to determine to what extent the appearance of inositol phosphates is due to direct activity of the listerial PlcA activity and to what extent it is caused by stimulation of an endogenous phospholipase C pathway in neutrophils exposed to both LLO and listerial PlcA and possibly to other listerial virulence factors. The listerial PlcA is known to catalyze predominantly the scission of phosphatidylinositol into diacylglycerol and cIMP (8); however, the latter is detected as IP1 by the analytical technique used in this study. We recently (37) presented evidence that for endothelial cells, LLO-induced pore formation may facilitate access of the listerial PlcA to the phosphatidylinositol moieties located predominantly or even exclusively in the inner leaflet of the eukaryotic plasma membranes (17). In the present investigation, however, inositol phosphate accumulation by neutrophils was also noted upon challenge with the INN+ mutant expressing listerial PlcA in the absence of LLO. Finally, listerial virulence factors in addition to LLO and PlcA may contribute to the induction of phosphoinositide metabolism and respiratory burst in human neutrophils in contact with L. monocytogenes.
In conclusion, our results suggest a hitherto unappreciated role for listerial exotoxins, the provocation of strong and rapid PMN stimulation independent of phagocytosis events. The spectrum of neutrophil metabolic events includes degranulation, the formation of inflammatory lipid mediators such as leukotrienes and PAF, the release of reactive oxygen species, and phosphoinositide hydrolysis. Listeriolysin was noted to be a prerequisite for degranulation and lipid mediator synthesis, but the induction of phosphatidylinositol hydrolysis with the subsequent appearance of inositol phosphates and diacylglycerol and the related respiratory burst is evidently linked to additional listerial virulence factors, among which the PlcA may be of major importance. While the production of exotoxins is important for intracellular survival and cell-to-cell spreading of L. monocytogenes (8, 11, 13, 28), the ability of human neutrophils to promptly react with these toxins when extracellularly offered might be important for the host defense response to systemic listerial infection.
ACKNOWLEDGMENT
This work was supported by Deutsche Forschungsgemeinschaft grant SFB 249/TP A13 to T.C.
REFERENCES
- 1.Aktories K, Bärmann M, Ohisi I, Tsukayama S, Jakobs K H, Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg R, Castro A G, Silva M T. Neutrophils as effector cells of T-cell-mediated, acquired immunity in murine listeriosis. Immunology. 1994;83:302–307. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R, Scheffer J, König B, König W. Effects of Listeria monocytogenes and Yersinia enterocolitica on cytokine gene expression and release from human polymorphonuclear granulocytes and epithelial (Hep-2) cells. Infect Immun. 1993;61:2545–2552. doi: 10.1128/iai.61.6.2545-2552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge M J, Dawson R M C, Downes C P, Heslop J P, Irvine R F. Changes in the level of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysisns. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 6.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:753–757. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Camilli A, Goldfine H, Portnoy D A. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med. 1991;173:751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty T, Leimeister-Wächter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermens S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen H J, Chovaniec M E. Superoxide generation by digitonin-stimulated guinea-pig granulocytes. J Clin Investig. 1978;61:1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlan J W, North R. Roles of Listeria monocytogenes virulence factors in survival: virulence factors distinct from listeriolysin are needed for the organism to survive an early neutrophil-mediated host defense mechanism. Infect Immun. 1992;60:951–957. doi: 10.1128/iai.60.3.951-957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–286. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossart P M, Vicente F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czuprynski C J, Brown J F, Maroushek N, Wagner R D, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1863–1846. [PubMed] [Google Scholar]
- 15.Czuprynski C J, Brown J F, Wagner R D, Steinberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–5163. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darji A, Chakraborty T, Niebuhr K, Tsonis N, Wehland J, Weiss S. Hyperexpression of listeriolysin in the nonpathogenic species Listeria innocua and high yield purification. J Biotechnol. 1995;43:205–212. doi: 10.1016/0168-1656(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 17.Devaux-PF Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty R W, Godfrey P P, Hoyle P C, Putney J W, Jr, Freer R J. Secretagogue-induced phosphoinositide metabolism in human neutrophils. Biochem J. 1984;222:307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downey G P, Fukushima T, Fialkow L, Waddell T K. Intracellular signalling in neutrophil priming and activation. Semin Cell Biol. 1995;6:345–356. doi: 10.1016/s1043-4682(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 20.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimminger F, Becker G, Seeger W. High yield enzymatic conversion of intravascular leukotriene A4 in blood-free perfused lungs. J Immunol. 1988;141:2431–2436. [PubMed] [Google Scholar]
- 22.Grimminger F, Menger M, Becker G, Seeger W. Potentiation of leukotriene production following sequestration of neutrophils in isolated lungs: indirect evidence for intercellular leukotriene A4 transfer. Blood. 1988;72:1687–1692. [PubMed] [Google Scholar]
- 23.Grimminger F, Scholz C, Bhakdi S, Seeger W. Subhemolytic doses of Escherichia coli hemolysin evoke large quantities of lipoxygenase products in human neutrophils. J Biol Chem. 1990;226:14262–14269. [PubMed] [Google Scholar]
- 24.Grimminger F, Sibelius U, Bhakdi S, Suttorp N, Seeger W. Escherichia coli hemolysin is a potent inductor of phosphoinositide hydrolysis and related metabolic responses in human neutrophils. J Clin Investig. 1991;88:1531–1539. doi: 10.1172/JCI115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasslett C, Savill J S, Meagher L. The neutrophil. Curr Opin Immunol. 1989;2:10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 26.Henson P M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to non-phagocytosable surfaces. J Immunol. 1971;107:1547–1552. [PubMed] [Google Scholar]
- 27.Hjorth R, Jonson A-K, Vretblad P. A rapid method for purification of human granulocytes using Percoll: a comparison with dextran sedimentation. J Immunol Methods. 1981;43:95–101. doi: 10.1016/0022-1759(81)90040-5. [DOI] [PubMed] [Google Scholar]
- 28.Kathariou S, Metz P, Hoff H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramps J A. l-Pyroglutamyl-l-propyl-l-valine-p-nitro-anilide, a highly specific substrate for granulocyte elastase. Scand J Clin Lab Investig. 1983;43:427–432. [PubMed] [Google Scholar]
- 30.Leimeister-Wächter M, Chakraborty T. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect Immun. 1989;57:2350–2357. doi: 10.1128/iai.57.8.2350-2357.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadeau M, Fruteau de Laclos B, Picard S, Braquet P, Corey E J, Borgeat P. Studies on leukotriene B4− oxidation in human leukocytes. Can J Biochem Cell Biol. 1984;62:1321–1326. doi: 10.1139/o84-168. [DOI] [PubMed] [Google Scholar]
- 32.Pinckard R N, Farr R S, Hanahan D J. Physio-chemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE-sensitized basophils. J Immunol. 1979;123:1847–1854. [PubMed] [Google Scholar]
- 33.Rakhmilevich A. Neutrophils are essential for resolution of primary and secondary infection with Listeria monocytogenes. J Leukoc Biol. 1995;57:827–831. doi: 10.1002/jlb.57.6.827. [DOI] [PubMed] [Google Scholar]
- 34.Riesenberg D. Listeriosis. JAMA. 1989;261:9. [Google Scholar]
- 35.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibelius U, Rose F, Chakraborty T, Darji A, Wehland J, Weiss S, Seeger W, Grimminger F. Listeriolysin is a potent inductor of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–676. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibelius U, Chakraborty T, Krögel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 38.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]