Abstract
Compression and irritation at the plantar aspect of the transverse intermetatarsal ligament may lead to a compressive neuropathy called Morton’s neuroma. There are many treatment options for Morton’s neuroma, with the most common surgical option being traction neurectomy. While there has been success in many surgical procedures, up to 35% of patients treated with traction neurectomy have recurrent pain and up to one-third of these patients have a recurrent stump neuroma. These neuromas are caused by abnormal axonal growth during regeneration, leading to an unorganized mass of fibrotic collagenous tissues, Schwann cells, and axons. More recent surgical treatments of neuromas have included nerve capping, which has been proposed to prevent painful neuroma formation by isolating the nerve end from external chemosignaling and reducing disorganized axonal outgrowth. An off-the-shelf, biocompatible porcine small intestine submucosa (pSIS) derived nerve cap with internal chambering has been investigated in a rodent study, which showed less pain sensitivity and less axonal swirling indicative of reduced likelihood of neuroma formation. Furthermore, a recent clinical study indicated that patients experienced a significant reduction in pain 3 months after Morton’s neuroma excision followed by repair using a nerve cap. This article describes the surgical technique of the aforementioned clinical study to mitigate neuroma formation, where a Morton’s neuroma is excised, and the remaining proximal nerve stump is inserted within a nerve cap and buried in the surrounding muscle.
Level of Evidence: Level V: Expert opinion
Keywords: Morton’s neuroma, compressive neuropathy, neurectomy, neuroma, nerve cap
“. . . patients experienced significant pain reduction as early as 3 months after Morton’s neuroma resection with the nerve stump entubulated within a nerve cap.”
Introduction
Morton’s neuroma is a compressive neuropathy related to a perineural fibroma of the common plantar interdigital nerve, which results from compression and constant irritation at the plantar aspect of the transverse intermetatarsal ligament, primarily in the third intermetatarsal space. This compression or irritation may lead to a disruption or injury to the peripheral nerve’s continuity, which microscopically results in Renaut’s bodies, axonal demyelination, axonal loss, and fibrosis.1 Morton’s neuroma pain is most frequently located on the plantar aspect of the forefoot and is usually characterized by a feeling of walking on a pebble, although numbness of the toes and sharp or burning pain radiating proximally toward the leg has been reported.2 Morton’s neuroma is the second most common compressive neuropathy3 and is present in females at a rate of 4 to 15 times that observed in males.4
The treatment algorithm for management of Morton’s neuroma typically involves nonoperative intervention, which may be followed by surgical intervention if necessary.5,6 Nonoperative treatment commonly includes footwear modifications and injections to manage pain.5,7 When these nonoperative treatments do not meet the goals and expectations of patients, surgical options are considered. Although there is no consensus for the optimal treatment of Morton’s neuroma, the most common surgical treatment is a dorsal approach with traction neurectomy,8 where the affected segment of the nerve is excised and removed under tension to ensure that the remaining nerve stump retracts proximal to the weight-bearing zone of the metatarsals.7 This procedure can achieve good results for 70% to 85% of subjects.5,9-12 However, postneurectomy residual pain has been reported in 14% to 35% of patients,9,11 with worsening pain in as many as 8% of patients.11 While the precise etiology is unknown, such recurrent pain may be due to incorrect initial diagnosis, treatment of the wrong interdigital space, inadequate resection, or the formation of a stump neuroma at the terminal end of the nerve stump.5,9,13 It has been found that as many as one-third of patients with recurrent pain after neurectomy may develop a recurrent neuroma on the nerve stump.5
These nerve stump neuromas develop due to an abortive attempt of the nerve to repair an injury. During the regeneration process, the nerve end develops into an unorganized bulbous mass of fibrotic collagenous tissues, Schwann cells, and axons.14 Neuroma pain may be due to a higher ratio of unmyelinated axons in the neuroma, which increases the sensitivity of the nerve fibers to mechanical stimulation such as scar contracture or transdermal compression or tapping.14 Several treatments have been proposed as an alternative to traction neurectomy, which may inhibit the formation of a painful nerve stump neuroma including targeted muscle reinnervation (TMR),15,16 burying of the nerve end into muscle or bone,17,18 and capping the nerve stump.19,20 Results from these procedures are promising; however, the limited space between the metatarsal heads restricts the surgical options after Morton’s neuroma resection to neurectomy, muscle/bone burying, or nerve capping. Studies examining burying the nerve end into muscle or bone have demonstrated improvement in persistent pain; however, the recurrence rate is comparable to neurectomy alone.21,22 More recently, attempts at capping the nerve have been proposed to isolate the nerve end from external signaling and prevent disorganized axonal outgrowth leading to painful neuroma development.
The use of autologous vein and epineurium caps have demonstrated promising results for the prevention of neuroma in several studies19,20; however, these autologous tissue techniques are limited as they may not contain axonal sprouting within the cap and have increased surgical time and donor-site morbidity.23,24 An off-the-shelf, biocompatible porcine small intestine submucosa (pSIS)-derived device with internal chambering for containing the cut nerve end as a technique to prevent neuroma formation has been shown in a rodent study to reduce histological evidence of a neuroma and improve the pain behavior exhibited by the animal upon mechanical stimulation.25 A recent clinical study using the surgical approach presented in this article showed that patients experienced significant pain reduction as early as 3 months after Morton’s neuroma resection with the nerve stump entubulated within a nerve cap.26 This article presents a dorsal approach for surgical removal of a Morton’s neuroma with insertion of the proximal nerve stump within a pSIS chambered nerve cap device (Axoguard Nerve Cap, Axogen Inc., Alachua FL, USA) and burying within the local muscle tissue.
Methods
Surgical Procedure
Dorsal exposure of the intermetatarsal nerve
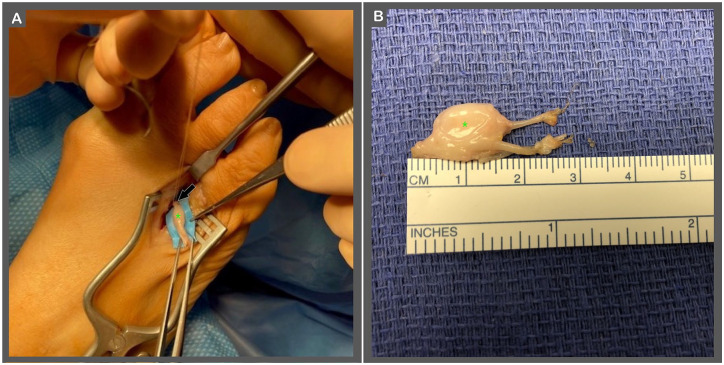
A linear incision is placed equidistant between the metatarsal heads from a dorsal approach (Figure 1). The incision should be made in the metatarsal space with an identified Morton’s neuroma. This incision is deepened down through superficial and subcutaneous tissue being careful to isolate and protect all vital structures with utilization of microsurgical instrumentation and loupe magnification. The identification of the deep structures including the deep transverse metatarsal ligament and the interosseous tendons is completed (Figure 2). From a distal to proximal approach, a Freer elevator is used to separate the deep transverse metatarsal ligament and the dorsal surface of the intermetatarsal nerve. Transection of the deep transverse metatarsal ligament allows for the freeing of the distal aspect of the hypertrophic perineural fibroma within the interspace of the metatarsals. The neuroma can be pushed through the intermetatarsal space by palpating the plantar surface of the foot (Figure 3). The proximal raphae of the tissue is identified and transected proximally to the presentation of the muscle belly of the interosseous within the proximal metatarsal interspace. Prior to neuroma excision, measure the diameter of the nerve stump with a sterile ruler (Figure 4). With meticulous sharp and blunt dissection, the proximal intermetatarsal nerve is identified entering the hypertrophic mass within the interspace. An epineurial anchor suture is placed using an 8-0 or 9-0 nonresorbable monofilament nylon suture with a spatulated or tapered needle (eg, 8-0, 0.4 metric, monofilament nylon suture with a 7 mm 1/2c spatula needle or 9-0, 0.3 metric, nonabsorbable monofilament nylon suture with a side cutting lancet 6.60 mm, 3/8c). The epineurial anchor suture is placed in the epineurium of the proximal native nerve 10 mm proximal to the transected end with the use of a spatulated or tapered needle, ensuring to suture only through the epineural sheath avoiding deeper tissues of the perineurium and nerve fascicles (see Figure 5A). This epineurial suture provides an anchor to the nerve, providing guidance for entubulation of the nerve within the nerve cap. After the epineurial anchor suture is placed, the proximal nerve is transected as distal as possible in the proximal nerve stump. The hypertrophic mass and the distal interdigital sensory nerves are then transected in total (Figure 6).
Figure 1.
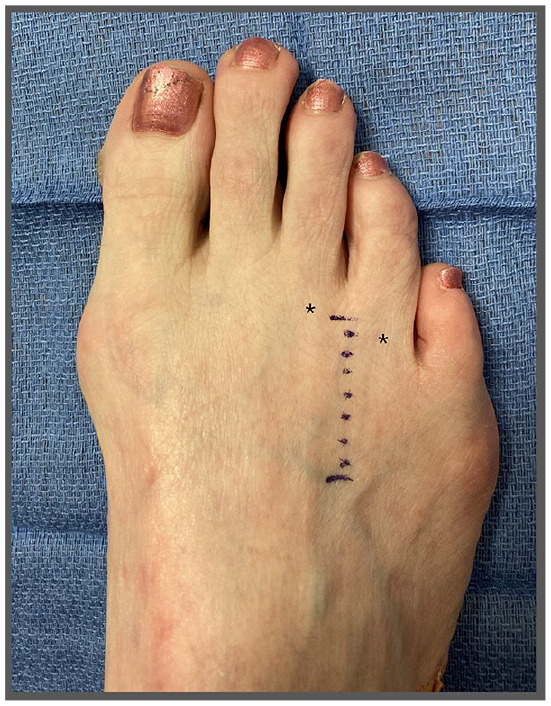
With the dorsal approach, a linear incision (indicated by surgical marker) should be placed equidistant between the metatarsal heads (indicated by black asterisks).
Figure 2.
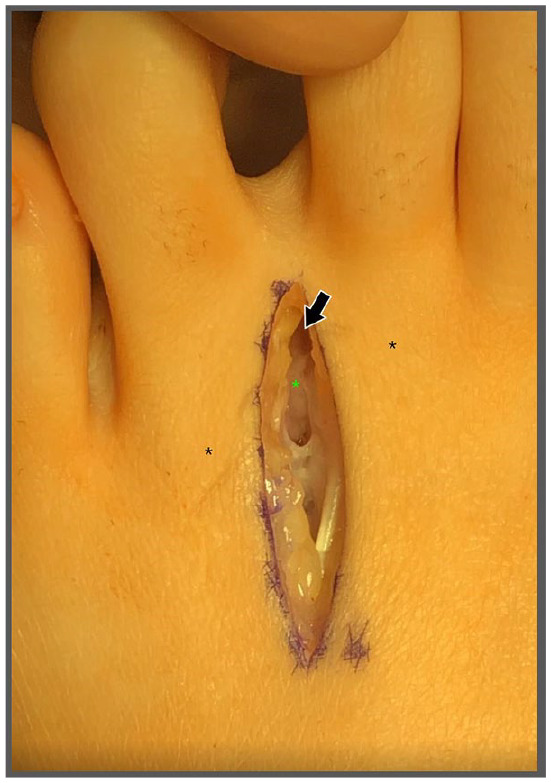
Microsurgical instruments and loupe magnification are used to deepen the incision between the metatarsal heads (indicated by black asterisk) through the superficial and subcutaneous tissues to ensure that all vital structures are isolated. A Freer elevator may be used to separate and identify the deep transverse metatarsal ligament (indicated by green asterisk) and the intermetatarsal nerve. The Freer elevator insert is at the distal aspect of the incision (indicated by black arrow).
Figure 3.
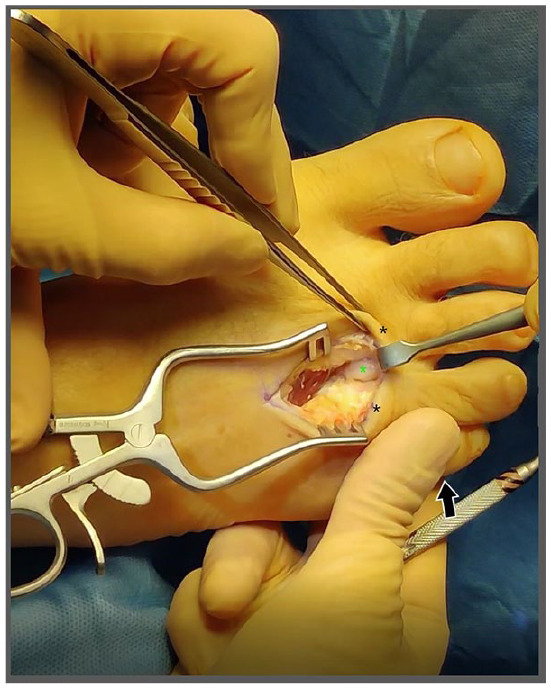
The distal aspect of the hypertrophic perineurial fibroma may be freed through the interspace of the metatarsals (metatarsal heads indicated by black asterisks). The fibroma (green asterisk) is elevated through the intermetatarsal space by palpation through the plantar surface of the foot (black arrow).
Figure 4.
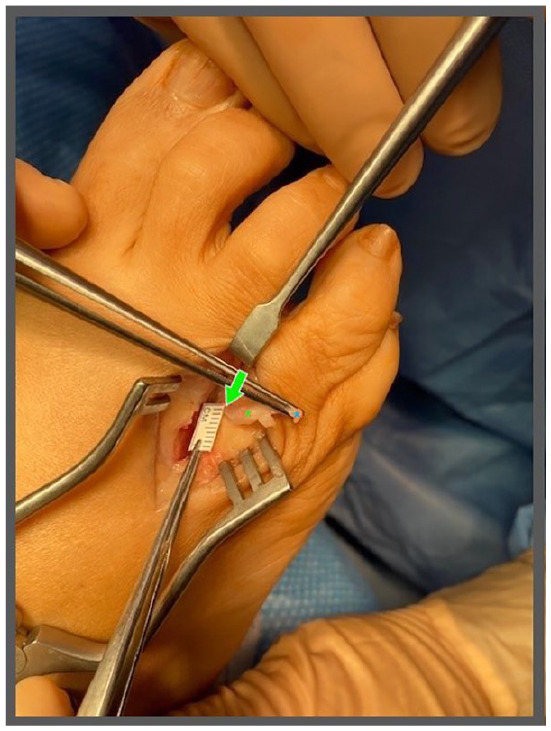
Using a modified sterile ruler, measure the diameter of the unaffected, healthy nerve (indicated by green arrow) proximal to the fibroma (indicated by green asterisk) to approximate the proper sizing for the nerve cap. This nerve stump should be measured proximal to the fibroma (in healthy nerve tissue), to ensure that an appropriately sized nerve cap is selected for implant. The nerve can be secured by the digital nerve (indicated by blue asterisk) distal to the fibroma.
Figure 5.
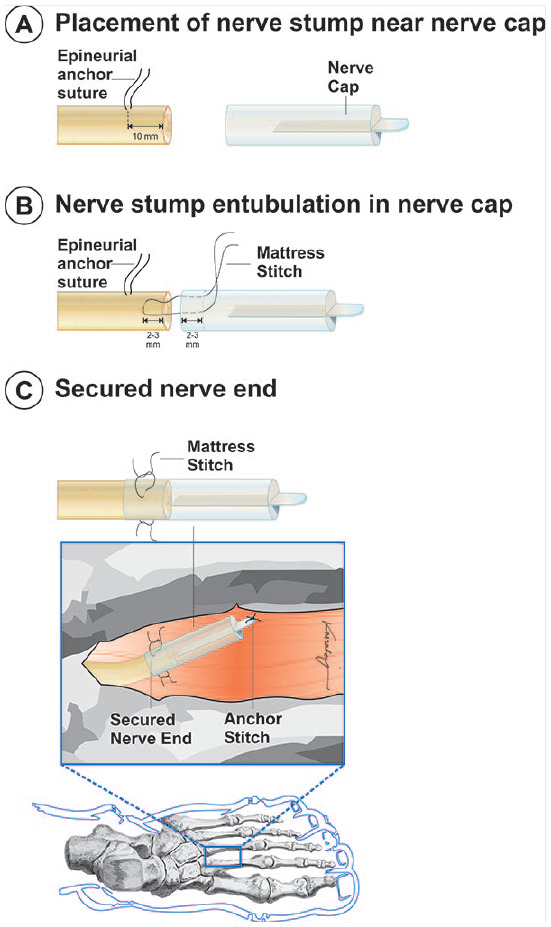
Placement and securing the nerve cap include the following: (A) place an epineurial anchor suture, placed approximately 10 mm proximal to the transected nerve end. (B) Secure the nerve stump within the nerve cap with the use of a mattress stitch. The mattress stitch is placed from outside to inside, 2 to 3 mm from the open end of the nerve cap. The suture is then placed transversely through the epineurium of the native nerve stump approximately 2 to 3 mm proximal to the distal end of the nerve stump. The suture is then passed back through the nerve cap and secured. The epineurial anchor suture can be removed upon completing the mattress suture. A second suture should be added 180° from the first mattress suture. (C) A suture may be placed through the distal end of the nerve cap tab to assist with burying the nerve cap within the muscle. The subcutaneous, intradermal, or subcuticular tissue should be closed with monofilament suture. The skin should be closed using the surgeon’s preferred method.
Figure 6.
Removal of the perineural fibroma is performed by: (A) identifying the proximal raphae of the tissue, which is then transected proximally to the presentation of the muscle belly of the interosseous within the proximal intermetatarsal interspace. The proximal intermetatarsal nerve should be identified entering the hypertrophic mass. An epineurial anchor suture may then be placed in the proximal nerve stump (indicated by black arrow), which an assistant can hold to stabilize the nerve stump. This epineurial suture should be placed proximal to the fibroma (indicated by green asterisk) to prevent nerve retraction into the deep tissues when the fibroma is excised. A blue background may be placed under the nerve to improve visualization. (B) The interdigital nerve is transected proximal and distal to the fibroma (indicated by green asterisk), with the proximal nerve being transected as distal as possible. The fibroma is then removed.
Placement of nerve cap
Axoguard Nerve Cap (Axogen Inc., Alachua, FL) is soaked for approximately 2 minutes immediately before application. This soak time can range between 30 seconds and 20 minutes, according to the nerve cap instructions for use. As the nerve cap soaks, the material softens. It is recommended to use the nerve cap when the nerve cap is supple, but still firm enough to handle and suture. Suturing the material when it is not hydrated or under-hydrated may result in bending or breaking the needle. The nerve cap consists of a 15 mm long tube, 10 mm internal bifurcation that separates the tube into 2 chambers, and 3 mm tab on the distal end of the cap (see Figure 7). The bifurcated chambers and the enclosed tube provide physical separation of the sentinel exiting axons and serves to protect and isolate them from the extra-neural soft tissue environment. The nerve cap is composed of a semi-translucent material, which allows for visualization of the nerve stump. A mattress stitch is placed approximately 2 to 3 mm from the open end of the nerve cap, suturing from outside to inside using an 8-0 or 9-0 monofilament nylon suture (see Figures 5B and 8). A mattress suture allows for entubulation of the nerve within the cap and secures the nerve to the cap. The suture is then placed transversely through the epineurium of the native nerve matching the distance of the provisional mattress stitch (ie, 2-3 mm from the end of the proximal nerve stump), which is then returned through the lumen of the nerve cap. This completes the mattress stitch. To guide the nerve into the cap, an assistant may hold the previously placed epineurial anchor suture above the plane of the surgical field as the native nerve is placed within the nerve cap, ensuring that the nerve stump does not encroach on the partition. The epineurial anchor suture is then removed and the mattress stitch is tied to the dorsal surface of the cap. A second suture should be placed 180° from the first suture, which secures the edge of the cap to the epineurium of the nerve stump. At the surgeon’s discretion, a 5-0 monofilament absorbable suture may be placed to act as an anchoring suture through the distal tab of the nerve cap to allow for burying in the muscle, where the cap is inserted within the interosseous muscle belly within the intermetatarsal space (Figure 5C). Subsequent closure of the subcutaneous, intradermal, or subcuticular tissues should be performed with monofilament absorbable suture. Skin closure should be performed using the surgeon’s preferred method (Figure 9). If multiple Morton’s neuromas have been identified, multiple neuromas may be excised simultaneously; however, adequate space between the incisions should be left to ensure that there is adequate blood supply to prevent tissue necrosis.
Figure 7.
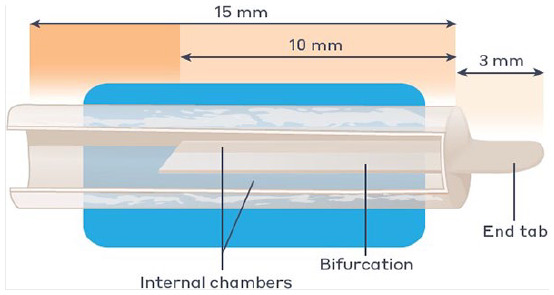
The nerve cap is made of small intestine submucosa. The tube is separated by internal bifurcation into 2 chambers with tab at the closed end of the tube. This tab provides surface area for handling and suturing to the muscle.
Figure 8.
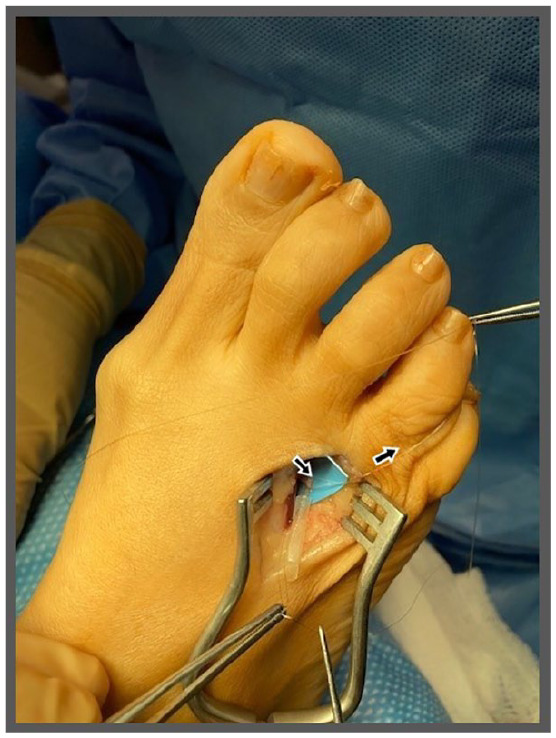
The epineurial suture is continued to be used to ensure the nerve stump does not retract into the deep tissues by placing slight tension on the suture (indicated by black arrows), which is secured by forceps or equivalent. A mattress stitch is placed through the nerve cap and the epineurium of the nerve. The epineurial anchor suture may be removed as the nerve is entubulated within the nerve cap. The mattress stitch should be tied to the dorsal surface of the cap, with a second suture placed 180° from the first suture to secure the edge of the nerve cap.
Figure 9.
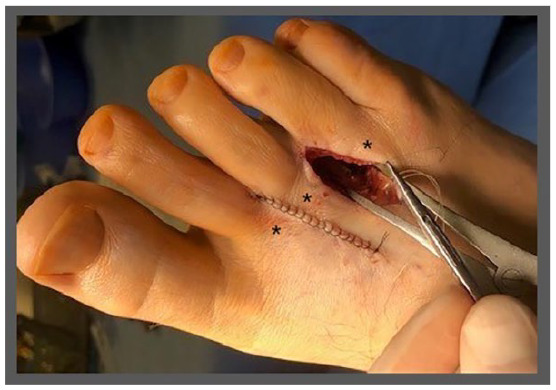
Wound closure of the subcutaneous, intradermal, or subcuticular tissues should be performed using monofilament absorbable suture. Neuroma excision may be performed between metatarsal spaces with identified fibromas. If multiple neuromas are present in adjacent metatarsal spaces, incisions should be placed with sufficient distance to prevent dermal necrosis. In this example, the incisions were shifted medial or lateral to increase the dermal flap surface area between the incisions while ensuring the incisions could be used to access the deep tissues between the metatarsal heads (indicated by black asterisks).
Discussion
Following disruption of a nerve end, whether due to traumatic injury, iatrogenic injury, or planned surgical excision, a symptomatic neuroma often forms. In particular, following Morton’s neuroma surgery, recurrent pain in the foot has been reported between 14% and 35% of patients.9,11 This article describes a dorsal approach to secure a chambered nerve cap to the proximal nerve stump after Morton’s neuroma excision with subsequent burying of the nerve stump and cap intermetatarsal space. The plantar approach to Morton’s neuroma excision is not described in this article, as there are notable comorbidities associated with the plantar surgical approach including wound complications and scar sensitivity.6 Therefore, the preferred surgical approach is dorsal; however, visualization of the surgical field in this procedure is challenging for several reasons. First, the incision is small, and the nerve is located deep to the metatarsal heads. Furthermore, the procedure must be performed in a hole, with limited ability to retract the surrounding tissues.
This surgical technique offers 2 main benefits. First, dorsal incisions provide the patient an opportunity for a faster return to normal activities. Second, entubulating the terminated nerve end within the chambered nerve cap helps to prevent disorganized axonal sprouting and may help to limit residual neuroma pain after surgery. The pSIS material used for the nerve cap has been used clinically for over 20 years in various indications.27-32 The pSIS material serves as a biologic scaffold that revascularizes and remodels into a new permanent soft tissue layer,33 which may reduce neurotrophic signaling from the surrounding environment while also providing permanent protection of the nerve end from mechanical stimulation.
This nerve cap has been successfully used in both preclinical and clinical studies. In a preclinical study, the nerve cap exhibited less sensitivity to mechanical stimulation and reduced likelihood of a neuroma compared to an untreated nerve stump in a rat tibial nerve model up to 16 weeks postoperative.25 Furthermore, results of a recent clinical study that utilized the procedure described in this article showed that patients reported a significant reduction in pain as soon as 3 months postoperative, with a sustained reduction in pain throughout the 12-month follow-up.26 These studies indicated that with correct use, the nerve cap can be successfully used to reduce the likelihood of neuroma recurrence after excision and repair with a nerve cap.
Successful use of the nerve cap can be dependent on several factors including, proper hydration, using appropriate tools for microsurgery, care when suturing the nerve and the nerve cap, and ensuring the nerve and nerve cap are not under tension prior to closure. The handling of any extracellular matrix requires proper saturation to improve tissue handling with microsurgical instrumentation and microsurgical suture. The use of a suture as an epineurial anchor provides guidance for the entubulation of the nerve while in the intermetatarsal space. Furthermore, experience in microsurgical technique and microsurgical instrumentation is a prerequisite to doing these procedures. As the surgeon becomes more comfortable with the surgical technique and materials, the surgical exposure can be reduced to approximately 5 cm. The size of the surgical incision is highly dependent on ability to access the nerve. It is important to suture the nerve to the muscle belly without tension; therefore, additional neurolysis may be needed to free the nerve from surrounding soft tissue before securing the nerve in the muscle belly. This will allow the nerve to glide within the intermetatarsal space after wound closure. The foot can be flexed and extended to evaluate the tension on the nerve and nerve cap prior to closure.
Conclusions
In patients with painful Morton’s neuroma recalcitrant to conservative treatment, surgery is an effective method for relieving pain. Isolating and protecting the distal nerve end after removal of the perineural fibroma in these patients may help prevent recurrent neuroma formation and the return of pain. This article describes a microsurgical technique using a pSIS chambered nerve cap to entubulate the proximal nerve stump. The use of pSIS chambered nerve cap has been demonstrated to result in less pain sensitivity and less axonal swirling in a rodent model25 and reduced pain after Morton’s neuroma resection clinically.26 This technique provides a method to isolate and protect the nerve within a nerve cap and may improve outcomes compared with neurectomy alone.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author serves as a consultant for Axogen Corporation.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
References
- 1. Wu KK. Morton’s interdigital neuroma: a clinical review of its etiology, treatment, and results. J Foot Ankle Surg. 1996;35(2):112-119. doi: 10.1016/S1067-2516(96)80027-5. [DOI] [PubMed] [Google Scholar]
- 2. Munir U, Tafti D, Morgan S. Morton Neuroma. In: Statpearls. Statpearls Publishing; 2021. http://www.ncbi.nlm.nih.gov/books/NBK470249/. Accessed March 31, 2021. [PubMed]
- 3. Latinovic R. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265. doi: 10.1136/jnnp.2005.066696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters PG, Adams SB, Schon LC. Interdigital neuralgia. Foot Ankle Clin. 2011;16(2):305-315. doi: 10.1016/j.fcl.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5. Di Caprio F, Meringolo R, Shehab Eddine M, Ponziani L. Morton’s interdigital neuroma of the foot. Foot Ankle Surg. 2018;24(2):92-98. doi: 10.1016/j.fas.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 6. Bhatia M, Thomson L. Morton’s neuroma—current concepts review. J Clin Orthop Trauma. 2020;11(3):406-409. doi: 10.1016/j.jcot.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gougoulias N, Lampridis V, Sakellariou A. Morton’s interdigital neuroma: instructional review. EFORT Open Rev. 2019;4(1):14-24. doi: 10.1302/2058-5241.4.180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valisena S, Petri GJ, Ferrero A. Treatment of Morton’s neuroma: a systematic review. Foot Ankle Surg. 2018;24(4):271-281. doi: 10.1016/j.fas.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 9. Coughlin MJ, Pinsonneault T. Operative treatment of interdigital neuroma. A long-term follow-up study. J Bone Joint Surg Am. 2001;83(9):1321-1328. [PubMed] [Google Scholar]
- 10. Kasparek M, Schneider W. Surgical treatment of Morton’s neuroma: clinical results after open excision. Int Orthop. 2013;37(9):1857-1861. doi: 10.1007/s00264-013-2002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bucknall V, Rutherford D, MacDonald D, Shalaby H, McKinley J, Breusch SJ. Outcomes following excision of Morton’s interdigital neuroma. Bone Joint J. 2016;98-B(10):1376-1381. doi: 10.1302/0301-620X.98B10.37610. [DOI] [PubMed] [Google Scholar]
- 12. Pace A, Scammell B, Dhar S. The outcome of Morton’s neurectomy in the treatment of metatarsalgia. Int Orthop. 2010;34(4):511-515. doi: 10.1007/s00264-009-0812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pet MA, Ko JH, Friedly JL, Smith DG. Traction neurectomy for treatment of painful residual limb neuroma in lower extremity amputees. J Orthop Trauma. 2015;29(9):e321. doi: 10.1097/BOT.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 14. Lu C, Sun X, Wang C, Wang Y, Peng J. Mechanisms and treatment of painful neuromas. Rev Neurosci. 2018;29(5):557-566. doi: 10.1515/revneuro-2017-0077. [DOI] [PubMed] [Google Scholar]
- 15. Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop. 2014;472(10):2984-2990. doi: 10.1007/s11999-014-3528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pet MA, Ko JH, Friedly JL, Mourad PD, Smith DG. Does targeted nerve implantation reduce neuroma pain in amputees? Clin Orthop. 2014;472(10):2991-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg. 1984;74(2):182-185. doi: 10.1097/00006534-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 18. Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77(3):427-438. doi: 10.1097/00006534-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 19. Koch H, Haas F, Hubmer M, Rappl T, Scharnagl E. Treatment of painful neuroma by resection and nerve stump transplantation into a vein. Ann Plast Surg. 2003;51(1):45-50. doi: 10.1097/01.SAP.0000054187.72439.57. [DOI] [PubMed] [Google Scholar]
- 20. Galeano M, Manasseri B, Risitano G, et al. A free vein graft cap influences neuroma formation after nerve transection. Microsurgery. 2009;29(7):568-572. doi: 10.1002/micr.20652. [DOI] [PubMed] [Google Scholar]
- 21. Rungprai C, Cychosz CC, Phruetthiphat O, Femino JE, Amendola A, Phisitkul P. Simple neurectomy versus neurectomy with intramuscular implantation for interdigital neuroma: a comparative study. Foot Ankle Int. 2015;36(12):1412-1424. doi: 10.1177/1071100715596741. [DOI] [PubMed] [Google Scholar]
- 22. Stokvis A, van der Avoort DJJC, van Neck JW, Hovius SER, Coert HJ. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869. doi: 10.1016/j.pain.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 23. Tupper JW, Booth DM. Treatment of painful neuromas of sensory nerves in the hand: a comparison of traditional and newer methods. J Hand Surg. 1976;1(2):144-151. [DOI] [PubMed] [Google Scholar]
- 24. Wu J, Chiu DT. Painful neuromas: a review of treatment modalities. Ann Plast Surg. 1999;43(6):661-667. [PubMed] [Google Scholar]
- 25. Tork S, Faleris J, Engemann A, Deister C, DeVinney E, Valerio IL. Application of a porcine small intestine submucosa nerve cap for prevention of neuromas and associated pain. Tissue Eng Part A. 2020;26(9-10):503-511. doi: 10.1089/ten.TEA.2019.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereira R, Dauphinee D, Frania S, et al. Clinical evaluation of an innovative nerve termination cap for treatment and prevention of stump neuroma pain: results from a prospective pilot clinical study. Foot Ankle Surg Tech Rep Cases. 2022;2(2):100179. doi: 10.1016/j.fastrc.2022.100179. [DOI] [Google Scholar]
- 27. Cook WA, Hiles MC, Kozma TG, Patel UH. Cook Biotech, Inc. (West Lafayette, IN), MED Institute Inc. (West Lafayette, IN). https://www.cookbiotech.com/. Published online 2001. Accessed February 6, 2021. [Google Scholar]
- 28. D’Eredità R. Porcine small intestinal submucosa (SIS) myringoplasty in children: a randomized controlled study. Int J Pediatr Otorhinolaryngol. 2015;79(7):1085-1089. doi: 10.1016/j.ijporl.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 29. Guest JF, Weidlich D, Singh H, et al. Cost-effectiveness of using adjunctive porcine small intestine submucosa tri-layer matrix compared with standard care in managing diabetic foot ulcers in the US. J Wound Care. 2017;26(suppl 1):S12-S24. doi: 10.12968/jowc.2017.26.Sup1.S12. [DOI] [PubMed] [Google Scholar]
- 30. Mosala Nezhad Z, Poncelet A, de Kerchove L, Gianello P, Fervaille C, El Khoury G. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2016;22(6):839-850. doi: 10.1093/icvts/ivw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238-1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 32. Neumayer L, Giobbie-Hurder A, Jonasson O, et al. Open mesh versus laparoscopic mesh repair of inguinal hernia. N Engl J Med. 2004;350(18):1819-1827. doi: 10.1056/NEJMoa040093. [DOI] [PubMed] [Google Scholar]
- 33. Kokkalis ZT, Pu C, Small GA, Weiser RW, Venouziou AI, Sotereanos DG. Assessment of processed porcine extracellular matrix as a protective barrier in a rabbit nerve wrap model. J Reconstr Microsurg. 2011;27(1):19-28. doi: 10.1055/s-0030-1267379. [DOI] [PubMed] [Google Scholar]