Abstract
Background
Myelin oligodendrocyte glycoprotein antibody disease (MOGAD) is a relatively new entity of demyelinating diseases, clinically presenting with optic neuritis, transverse myelitis, or encephalic symptoms. Typical radiological features include demyelinating cerebral and spinal lesions, cortical involvement, leptomeningeal enhancement, or tumefactive lesions. Here we present a rare case of a young patient with extensive brain stem lesion on the MRI while exhibiting nystagmus, singultus and somnolence.
Case presentation
A 30-year-old male patient presented initially with fever and impaired consciousness, but furthermore developed nystagmus, singultus and tetraparesis during the following week. Repeated MRI examinations revealed extensive brain stem edema with notable bilateral affection of the cerebellar peduncles and the pons. Antiviral and antibiotic treatment was changed to intravenous corticosteroids and immunoglobulins as soon as the diagnosis of MOGAD was established by testing serum and cerebrospinal fluid positive for MOG specific antibodies. MRI alterations vanished completely over time with a delayed, nearly complete clinical recovery of our patient.
Conclusion
Brain stem affection in MOGAD is rare. However, in patients presenting with an unclear brain stem encephalitis the possibility of MOGAD should be considered and tested using MOG antibodies. In case of a positive testing treatment with steroids and immunoglobulins seems recommendable.
Keywords: MOG antibody encephalitis, Brainstem encephalitis, Case report
Abbreviations: AB, antibody; ADC, apparent diffusion coefficient; ADEM, acute demyelinating encephalomyelitis; ceT1w, contrast enhanced T1 weighted; CLIPPERS, Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids; CNS, cerebral nervous system; CSF, cerebrospinal fluid; DW, diffusion weighted; IVIG, intravenous immunoglobulin; LETM, longitudinal extensive transverse myelitis; LP, lumbal puncture; MOG, myelin oligodendrocyte glycoprotein; MOGAD, myelin oligodendrocyte glycoprotein antibody disease; MOG-AB, myelin oligodendrocyte glycoprotein antibody; MRI, magnetic resonance imaging; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; OCB, oligoclonal bands; T2w, T2 weighted
Highlights
-
•
A patient presenting with brain stem encephalitis and longitudinal extensive transverse myelitis was diagnosed with MOGAD.
-
•
All lesions resolved after therapy with cortisone and immunoglobulins.
-
•
MOGAD should be considered a differential diagnosis for brainstem encephalitis.
1. Background
Myelin oligodendrocyte glycoprotein (MOG) antibody associated disease (MOGAD) is a differential diagnosis of growing significance for demyelinating lesions in the cerebral nervous system (CNS), and occurs in the presence of MOG antibodies (MOG-AB). Due to considerable overlap between classical demyelinating diseases such as multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD) and acute demyelinating encephalomyelitis (ADEM) regarding clinical and radiological features the diagnosis of MOGAD can be challenging [1].
In this case report we present and discuss a young patient with a rare presentation of MOGAD with initial acute meningitis, developing extensive brain stem encephalitis with only minor correlating symptoms and good clinical recovery.
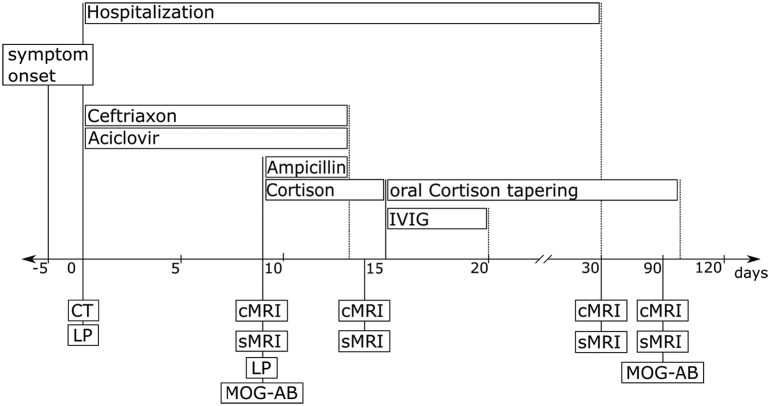
2. Case presentation
A 30-year-old male patient presented with newly occurring headache, fever, nuchal rigidity, and fatigue, increasing over the past five days. His past medical history and family history was unremarkable. Further neurological and general examinations were unremarkable. Following a normal CT scan, cerebrospinal fluid (CSF) analysis revealed pleocytosis (188 cells/μl, dominantly granulocytes), elevated protein (72.1 mg/dL), normal glucose (56.0 mg/dL) and negative oligoclonal bands (OCB). Empiric treatment with aciclovir and ceftriaxone intravenously was initiated under the suspicion of acute meningitis (Fig. 1). After careful consideration antibiotic and antiviral treatment was continued, although general serological workup including PCR and antibody tests showed negative results for common meningitis and encephalitis pathogens. Nine days after admission, the patient showed rapid progression of weakness leading to tetraparesis, predominantly affecting lower extremities, and hypoesthesia below T8 level, also permanent neck stiffness and gaze-evoked nystagmus to both sides. Repeated CSF analysis was comparable to the first, showing lymphatic pleocytosis (138 cells/μl), elevated protein (78.6 mg/dL) and negative OCB. Ampicillin and methylprednisolone were added to the empiric treatment. Additionally, extensive antibody (AB) tests for autoimmune diseases were performed, including MOG-AB, AQP4-AB NMDAR-AB, AMPAR-AB, GABA-AB, LGI1-AB, CASPR2-AB, DPPX-AB, onconeural-AB, anti-glycin-AB, GM1-AB, GQ1b AB.
Fig. 1.
Course of disease. _: time point of examination or starting point of therapy, ….: end of therapy, CT: computed Tomography, LP: lumbal puncture, cMRI: cerebral MRI, sMRI: spinal MRI, MOG-AB: MOG Antibody testing, IVIG: Intravenous Immunoglobulin.
Initial magnetic resonance imaging (MRI) depicted T2-weighted (T2w) hyperintense signal alterations with slight swelling in the pons and, more clearly, in both cerebellar peduncles (Fig. 2). Diffusion weighted (DW) MRI with mapping of the apparent diffusion coefficient (ADC) indicated vasogenic edema compatible with brain stem encephalitis (Fig. 3). Additionally, a longitudinal extensive transverse myelitis (LETM) ranging from C3 to C7 was found (Fig. 4). Contrast enhanced T1w imaging of the brain and spine showed no disruption of the blood brain barrier (BBB). After viral and bacterial serological tests showed negative results only methylprednisolone was continued with 2 g per day due to persistent hypoesthesia, slightly worsening para-paresis and a new-onset intermittent singultus.
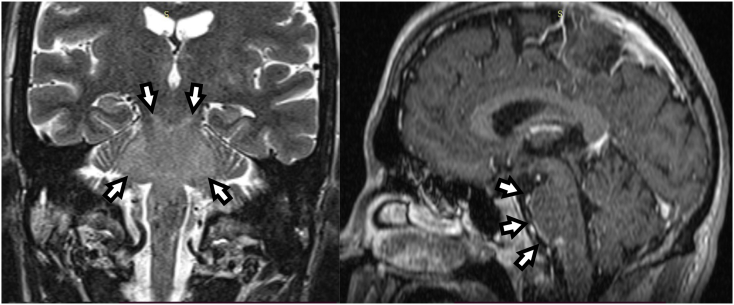
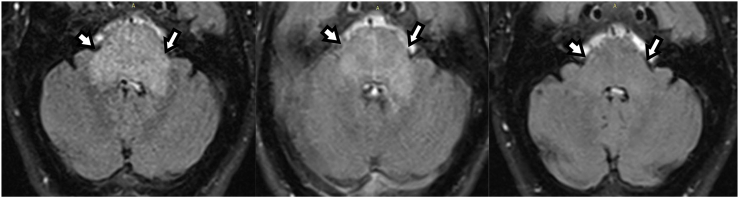
Fig. 2.
MRI performed 9 days after admission showed a signal increase in T2w-imaging of the brain stem (white arrows). Totally, only a slight mass effect was visible.
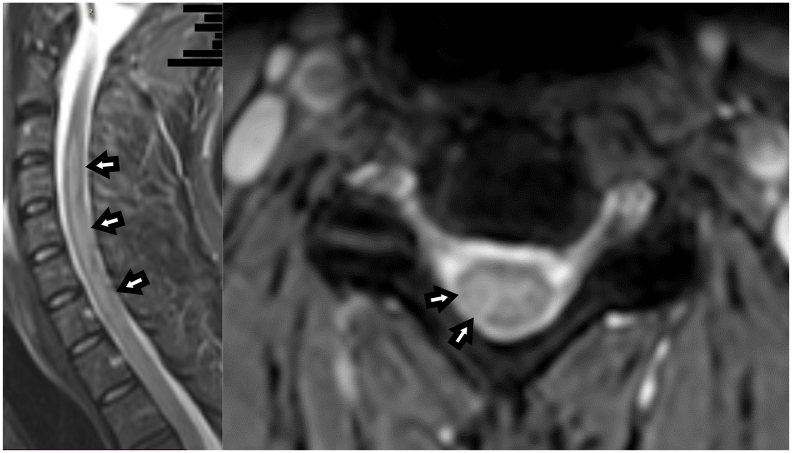
Fig. 3.
Spinal MRI of our patient 9 days after admission. Sagittal T2w-MRI (left image) of the cervical spine showed a signal increase from segment C3-C7 (white arrows). Corresponding axial T2w imaging (right image) revealed confluating excentric medullary lesions from C3-C7.
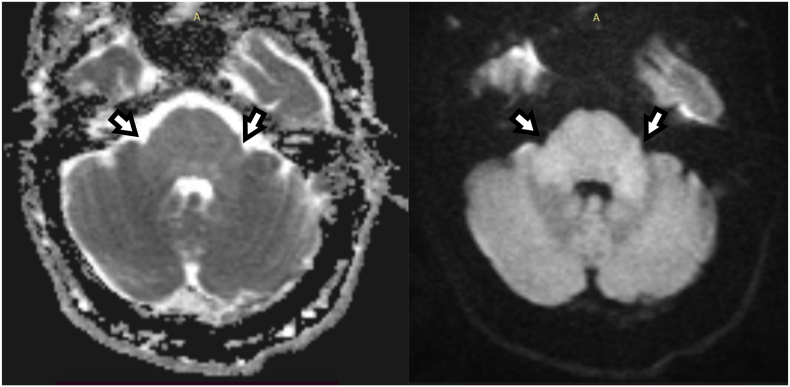
Fig. 4.
MRI performed 9 days after admission. Diffusion weighted (DW) MRI (left side: apparent diffusion coefficient (ADC) image; right side: corresponding DW-MRI with b = 1000 s/mm2) of the posterior fossa at admission. In the pons and, especially, in the cerebellar peduncles a signal increase was detected in DWI related to an increase of the correlated ADC-values indicating brain stem encephalitis with vasogenic edema (white arrows).
Edema in the pons and cerebellar peduncles persisted in follow-up MRI of the brain performed at day 14 (Fig. 5). In spinal MRI a new T2w hyperintense lesion became visible at the level Th 11/12 at this time, while the preexisting LETM between levels C3 to C7 remained widely unchanged. Still, neither in brain nor the spine, a BBB-disruption was found. Due to the spinal progression, IVIG therapy was established for 5 days whereby a clear regression of clinical symptoms was achieved. Prednisolone therapy was tapered subsequently. Preceding blood and CSF tests for autoantibodies showed positive MOG-AB titer of 1:1280 in serum and 1:16 in CSF. The combination of LETM, brainstem encephalitis and positive MOG antibodies, in both serum and CSF, lead us to the conclusion of a MOG antibody associated disease (MOGAD) [2,3].
Fig. 5.
Axial T2w-fluid attenuated MRI of the pontine affections at days 9 (left image), 14 (middle image) and 30 (right image). While the inflammatory edematous alterations (white arrows) reached a maximum at day 9, these findings continuously declined over time (day 14) and had vanished completely at day 30. In comparison, clinical recovery of the patient was rather delayed to the imaging findings.
The patient was discharged home with only residual symptoms, including neurogenic bladder dysfunction and mild hypoesthesia below the T11 level, thirty days after admission. Brain- and spinal-MRI controls were already inconspicuous at this time (Fig. 5). Follow-up MRI of the brain and the spinal cord, after two months, remained normal and residual hypoesthesia persisted. Repeated MOG-AB testing showed persistent positive results with MOG-AB titer of 1:640. During follow-ups he was treated with long term cortisone treatment with tapering over the course of six months, finally resulting in azathioprine therapy.
3. Discussion and conclusion
Typical MOGAD presentation differs depending on age and can include ADEM-like presentation, focal neurologic deficits, optic neuritis, transverse myelitis or encephalitic presentation [4]. Less common symptoms include cortical encephalitis or seizures [1,5]. Initial symptom onset of MOGAD with aseptic meningitis without leptomeningeal enhancement, like in our case, seems to be a rare [2,6]. Leptomeningeal enhancement in MRI is also only described in 6% of MOG patients [5]. While fever at disease onset is described in 9 to 60% of patients, depending on the cohort [7].
Neuroradiological findings in adult MOGAD patients describe relatively few, typically poorly demarcated or tumefactive lesions with variable contrast enhancement. Spinal manifestations ranged from transverse myelitis to LETM, while in the brain affections of the deep grey and white matter as well as the cortex were frequently detected [5,8].
Brainstem lesions are reported in up to 30% of all patients, although isolated affection of the brainstem remains a rare finding (5%). In these cases, the lesions exhibited a poor delineation and were mainly located in the pons, the medulla, the cerebellar peduncles or lay adjacent to the fourth ventricle. [1,5,9] Brain stem lesions may be accompanied by transverse myelitis, ADEM, supratentorial brain lesions or optic neuritis [8,10]. In one patient the MRI resembled CLIPPERS (Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) at disease onset [11]. Only 60% of patients with brain stem lesions were symptomatic presenting with ataxia, diplopia, nausea, vomiting, cranial nerve palsy or vertigo [8].
Following differential diagnosis, among others, should be taken in close consideration when seeing a patient with brainstem encephalitis: NMOSD, Behçet disease, Bickerstaff encephalitis, Listeria encephalitis and CLIPPERS [12]. The combination of brainstem encephalitis with LETM in our patient, lead towards the differential diagnosis of NMOSD and MOGAD.
LETM lead to tetraparesis and hypoesthesia in our patient and brain stem affection caused nystagmus, impaired consciousness and singultus. No other demyelinating lesions, contrast enhancing lesions or opticus neuritis were seen in our case. The lesions resolved without residual signs in MRI, consistent with complete regression in more than half of the patients in the literature [5,8].
Typically, cerebrospinal fluid shows pleocytosis in 44–85% of patients and negative OCB in the majority of patients, consistent with our patient [13]. The persistent positive MOG-AB titer are an indicator for an relapsing disease course [3]. Therapy of acute MOGAD consists of corticosteroids, intravenous immunoglobulins or plasma exchange. In patients with a high risk for relapses, long term immune modulating therapies include intravenous immunoglobulins, rituximab, azathioprine, and mycophenolate mofetil [2,13,14].
This case report highlights the importance of MOGAD as a differential diagnosis in patients with brain stem encephalitis. In addition, it should be considered in atypical lesions and unusual clinical presentations, such as aseptic meningitis or encephalopathic symptoms.
Ethics approval and consent to participate
All data were handled following the current WMA-recommendations of ethical principles for medical research involving human subjects. (Ethics Approval 1086/2021, Commission for Scientific Integrity and Ethics of the Karl Landsteiner University).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated.
Funding
We acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria.
Authors' contributions
EA, CB, NA, CN, WS contributed to the writing of manuscript. CN analyzed and choose MRI images. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
The authors want to appreciate the contribution of NÖ Landesgesundheitsagentur, legal entity of University Hospitals in Lower Austria, for providing the organizational framework to conduct this research. They also would like to acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria.
References
- 1.Jurynczyk M., Jacob A., Fujihara K., et al. Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease: practical considerations. Pract. Neurol. 2019;19:187–195. doi: 10.1136/practneurol-2017-001787. [DOI] [PubMed] [Google Scholar]
- 2.Marignier R., Hacohen Y., Cobo-Calvo A., et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20:762–772. doi: 10.1016/s1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Chiriboga A.S., Majed M., Fryer J., et al. Association of MOG-IgG Serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018;75:1355–1363. doi: 10.1001/jamaneurol.2018.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurynczyk M., Messina S., Woodhall M.R., et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–3138. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 5.Cobo-Calvo A., Ruiz A., Maillart E., et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90:e1858–e1869. doi: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 6.Gombolay G.Y., Gadde J.A. Aseptic meningitis and leptomeningeal enhancement associated with anti-MOG antibodies: A review. J. Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577653. [DOI] [PubMed] [Google Scholar]
- 7.Lampros A., De Broucker T., Bonnan M. Fever is a common onset feature of MOG-IgG associated disorders (MOGAD) Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102748. [DOI] [PubMed] [Google Scholar]
- 8.Banks S.A., Morris P.P., Chen J.J., et al. Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-325121. 2020/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurynczyk M., Geraldes R., Probert F., et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain J. Neurol. 2017;140:617–627. doi: 10.1093/brain/aww350. [DOI] [PubMed] [Google Scholar]
- 10.Spadaro M., Gerdes L.A., Krumbholz M., et al. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016;3 doi: 10.1212/nxi.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symmonds M., Waters P.J., Küker W., et al. Anti-MOG antibodies with longitudinally extensive transverse myelitis preceded by CLIPPERS. Neurology. 2015;84:1177–1179. doi: 10.1212/wnl.0000000000001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotoudeh H., Razaei A., Saadatpour Z., et al. Brainstem encephalitis. the role of imaging in diagnosis. Curr. Probl. Diagn. Radiol. 2021;50:946–960. doi: 10.1067/j.cpradiol.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Wynford-Thomas R., Jacob A., Tomassini V. Neurological update: MOG antibody disease. J. Neurol. 2019;266:1280–1286. doi: 10.1007/s00415-018-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittam D.H., Karthikeayan V., Gibbons E., et al. Treatment of MOG antibody associated disorders: results of an international survey. J. Neurol. 2020;267:3565–3577. doi: 10.1007/s00415-020-10026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated.