Abstract
Purpose of Review
Biologics and small molecule inhibitors (SMIs) are a rapidly growing class of highly efficacious therapies in the treatment of chronic immunologic and allergic conditions. With precision targeting of inflammatory signaling molecules, these new agents selectively modulate the immune system to treat a variety of conditions. Dermatologic diseases, including atopic dermatitis and psoriasis, are of particular interest due to the growing number of new biologics and SMIs in recent years. This review serves to summarize and evaluate the recent literature regarding biologics and SMIs.
Recent Findings
Currently approved biologics for AD achieve clear or almost clear skin in less than 40% of patients treated. Several biologics that are still under investigation for AD have shown better efficacy in phase III trials with similar safety profiles. Recently approved SMIs for AD also demonstrate a high degree of efficacy, but safety profiles may limit their use. Psoriasis has several highly efficacious biologics on the market; however, only one SMI is currently available. Additional SMIs for psoriasis have completed phase III trials and demonstrated high efficacy.
Summary
This article evaluates recent literature on biologics and small molecule inhibitors for AD and psoriasis.
Keywords: Atopic dermatitis, Psoriasis, Biologics, Small molecule inhibitors
Introduction
Advances in our understanding of inflammatory cascades have led to a class of highly efficacious drugs known as molecular-targeted therapies. These agents are classified into two representative groups: biologics, which act as antibodies to target cytokines and receptors, and small molecule inhibitors (SMIs), which target intracellular signaling molecules [1]. While biologics are larger in size, produced from living organisms, and typically administered parenterally, SMIs are smaller compounds, manufactured through chemical synthesis, and typically administered orally [1]. Both biologics and SMIs modulate the immune system and have revolutionized treatment of a range of allergic, immunologic, and oncologic disorders. In dermatology, psoriasis and atopic dermatitis (AD) have been a large focus of these drugs. First-generation biologics, which were initially developed and approved over two decades ago, transformed the treatment of psoriasis and established a new standard of care. Not far behind, apremilast became the first SMI approved for psoriasis and in 2017, dupilumab became the first biologic approved for AD. Since this time, a surge in research has led to dozens more molecular-targeted therapies which continue to expand the therapeutic options for both psoriasis and AD. This article serves as a review of the recent literature regarding biologics and SMIs, highlighting the newest approved therapies and selected agents under development.
A PubMed search was conducted in July 2022 using the terms “atopic dermatitis,” “eczema,” “psoriasis,” “allergic skin disease,” “molecular-targeted therapy,” “small molecule inhibitors,” “biologics,” “phase 2,” and “phase 3.” Clinicaltrials.gov was also searched for completed, ongoing, and upcoming clinical trials.
Atopic Dermatitis
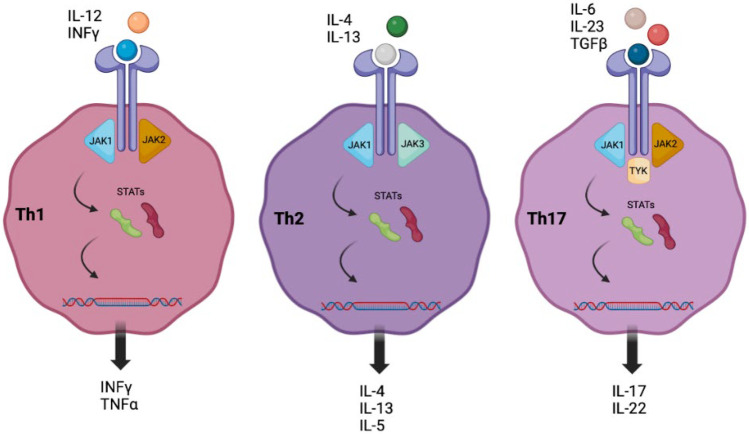
Atopic dermatitis (AD) is a common inflammatory skin condition resulting from a complex interplay of immune dysregulation and skin barrier dysfunction [2]. It occurs in 20–30% of children and perhaps as many as 7–10% of adults and can cause significant distress to those affected [1]. While epidermal barrier dysfunction remains a central component of AD, a variety of cytokines and other cell signaling molecules have been identified as contributors to the dysregulated inflammatory response [3]. Specifically, inflamed AD skin demonstrates an overactivation of T helper (Th) type 2, Th22, and Th17 cells with increased expression of chemokines such as thymus activation-regulated chemokine and cytokines including thymic stromal lymphopoietin (TSLP) and interleukin (IL)-4, IL-5, IL-13, and IL-31 [3, 4]. Many of these signaling molecules activate Janus kinase (JAK) leading to the downstream mobilization of signal transducers and activators of transcription (STAT), facilitating the inflammatory response (Fig. 1) [5]. Given the complex array of inflammatory mediators involved in AD, it is no surprise that much research has been conducted toward the development of biologics and SMIs as therapeutics for this condition. Table 1 serves as an overview of these molecular-targeted therapies.
Fig. 1.
Overview of the inflammatory cascade with regard to extracellular and intracellular signaling molecules. Polarization toward Th1 activation is associated with psoriasis; Th2 activation is associated with AD [5]
Table 1.
Summary of biologics and small molecule inhibitors for AD
| Drug | Target | Route of administration | Status for AD |
|---|---|---|---|
| Dupilumab | IL-4Rα (inhibits binding of IL-4 and IL-13) | Injection | Approved (ages ≥ 6 months) |
| Tralokinumab | IL-13 | Injection | Approved (ages ≥ 18) |
| Lebrikizumab | IL-13 | Injection | Phase III trials completed |
| Nemolizumab | IL-31Rα | Injection | Phase III trials completed |
| Omalizumab | IgE | Injection |
Phase II trials completed No active trials |
| GBR 830 | OX40 | Injection | Phase II trials completed |
| Apremilast | PDE-4 | Oral | Phase II trials completed |
| Crisaborole | PDE-4 | Topical | Approved (ages ≥ 3 months) |
| Upadacitinib | JAK1 | Oral | Approved (ages ≥ 12 years) |
| Abrocitinib | JAK1 | Oral | Approved (ages ≥ 18) |
| Baricitinib | JAK1 and JAK2 | Oral | Phase III trials completed |
| Ruxolitinib | JAK1 and JAK2 | Topical | Approved (ages ≥ 12 years) |
| Tofacitinib | JAK1 and JAK3 | Topical |
Phase II trials complete No active trials |
| Delgocitinib | JAK1, JAK2, and JAK3 | Topical | Phase III trials completed and active |
| Serlopitant | NK-1 | Oral | Phase II trial terminated |
| Tradipitant | NK-1 | Oral | Phase III trials completed |
Biologics for Atopic Dermatitis
Currently Approved
Dupilumab is a monoclonal antibody (mAB) that targets the IL-4 receptor alpha, inhibiting the binding of IL-4 and IL-13 and preventing activation of the JAK1/STAT6 inflammatory cascade. Until recently, dupilumab was the only systemic biologic approved by the Food and Drug Administration (FDA) for treatment of AD, and it is currently the only agent approved in children. It is indicated for moderate-to-severe uncontrolled AD in ages 6 months and older. In the phase III trials SOLO I and II, a significantly greater proportion of patients receiving 300 mg dupilumab injections every other week achieved clear or almost clear skin (Investigator Global Assessment (IGA) score of 0 or 1) compared to placebo (36–38% vs. 8–10%) [6]. Additionally, a 75% reduction from baseline in the eczema area and severity index (EASI-75) at 16 weeks was observed in 44–52% of patients assigned to dupilumab and 12–15% assigned to placebo (p < 0.001). Moreover, dupilumab has demonstrated favorable long-term safety and efficacy in children. Results from an open-label phase IIa study and subsequent phase III extension study showed significant reduction in EASI through 52 weeks of treatment in children ages 6–12 years of age [7]. Most recently, the FDA approved dupilumab for children ages ≥ 6 months based on results from an open-label phase II trial published in 2021 [8•].
Dupilumab has been shown to be generally safe and well tolerated with relatively mild adverse events [9]. The most common adverse effects which occasionally lead to discontinuation of therapy include ocular surface disease and paradoxical head and neck dermatitis [10]. A retrospective chart review of 48 patients found that 14 (29%) endorsed conjunctivitis while on dupilumab leading to discontinuation in 4 of these patients [11]. In a separate retrospective review, Zhu et al. found that 17 of 73 (23%) patients on dupilumab therapy developed new regional dermatoses, including head and neck dermatitis [12]. Of note, dupilumab recently gained approval for the treatment of moderate-to-severe asthma, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis [13, 14].
Tralokinumab
Tralokinumab is an anti-IL-13 mAB approved by the FDA for moderate-to-severe AD in adults (≥ 18 years) [15]. Results from a series of clinical trials known as the ECZTRA (Ecz) studies supported its approval. Ecz I and II evaluated the efficacy and safety of tralokinumab monotherapy in adults with moderate-to-severe AD. At 16 weeks, more patients who received tralokinumab achieved clear or almost clear skin compared to placebo (15.8% vs. 7.1% in Ecz I and 22.2% vs. 10.9% in Ecz II). Additionally, EASI-75 was achieved by 25.0% of subjects treated with tralokinumab compared to 12.7% in the placebo arm in Ecz I and 33.3% and 11.4%, respectively, in Ecz II. Moreover, tralokinumab showed a significant improvement in all key secondary endpoints over placebo [16]. Additional Ecz trials have demonstrated superiority of tralokinumab to placebo in adolescents (Ecz 6) and in patients who failed cyclosporin (Ecz 7) [16, 17]. Lastly, AEs experienced were mild to moderate in severity and were comparable between treatment arms in most Ecz studies [16, 17]. Similar to dupilumab, tralokinumab is associated with an increased incidence of conjunctivitis vs. placebo, but these cases are generally mild and transient [18]. While results suggest that tralokinumab is well tolerated and superior to placebo, IGA and EASI-75 scores indicate a lower efficacy compared to dupilumab (Table 2) [19, 20]. However, given variability of responses and tolerability to all medications, including dupilumab, tralokinumab is an important option to consider for the appropriate patients. Additionally, patients weighing less than 100 kg who achieve clear or almost clear skin at 16 weeks may consider tralokinumab 300 mg every 4 weeks, a more favorable frequency of injections compared to dupilumab [16, 17].
Table 2.
Comparison of endpoints achieved by subjects in two phase III trials at week 16
| Dupilumab | ||||
| SOLO 1 | SOLO 2 | |||
|
300 mg Q2W (n = 224) |
Placebo (n = 224) |
300 mg Q2W (n = 233) |
Placebo (n = 236) |
|
| EASI-75 | 44% | 16% | 52% | 13% |
| vIGA-AD 0/1 | 36% | 12% | 38% | 15% |
| Tralokinumab | ||||
| ECZTRA 1 | ECZTRA 2 | |||
|
300 mg Q2W (n = 603) |
Placebo (n = 199) |
300 mg Q2W (n = 593) |
Placebo (n = 201) |
|
| EASI-75 | 25% | 13% | 33% | 11% |
| vIGA-AD 0/1 | 16% | 7% | 22% | 11% |
Under Investigation
Lebrikizumab
Lebrikizumab is an anti-IL-13 mAB. A phase III trial known as the Adhere study enrolled 228 participants with moderate-to-severe AD and studied the effects of lebrikizumab with TCS use [21]. Among patients receiving lebrikizumab plus TCS, 41.2% achieved clear or almost clear skin (IGA 0 or 1) at 16 weeks compared to 22.1% of those receiving placebo plus TCS [21]. Additionally, 69.5% and 42.2% of subjects in the treatment arm and placebo arm, respectively, achieved an EASI-75 response at 16 weeks. Moreover, patients treated with lebrikizumab plus TCS achieved statistically significant improvement across key secondary endpoints including itching and sleep. Adverse events (AEs) were generally mild and did not lead to treatment discontinuation. Common events included conjunctivitis (4.6%) and headaches (4.6%) [21].
The phase III trials known as Advocate I and II evaluated the efficacy of lebrikizumab as monotherapy and were completed in April 2022. In Advocate I, 43% of patients receiving lebrikizumab achieved clear or almost clear skin at 16 weeks compared to 13% of patients receiving placebo. Additionally, 59% and 16% of those in the treatment and placebo arm, respectively, achieved an EASI-75 response at 16 weeks. Similar results were seen in Advocate II [22]. Both of these studies demonstrated statistically significant improvement across secondary outcomes and showed similar safety profiles. Of note, conjunctivitis occurred in 2.6% of subjects in the treated group, suggesting a decreased association with this AE compared to dupilumab [23]. Although these results have not yet been officially published, this novel biologic appears to be efficacious in the treatment of moderate-to-severe AD.
Nemolizumab
Nemolizumab is an anti-IL-31Rα antibody. IL-31 has been characterized as a key facilitator of the pruritus associated with AD. Relatedly, several phase II trials have demonstrated this drug’s utility in the treatment of pruritus in patients with moderate-to-severe AD. Improvements were noted in the pruritus visual analog scale (VAS) and EASI scores in comparison with the placebo group [24]. In 2021, a 16-week phase III RCT was conducted to further assess the efficacy and safety of nemolizumab. A total of 215 patients were enrolled and the mean percent change in pruritus VAS score was significantly higher in the treatment arm compared to the placebo arm (−42.8% vs. −21.4%, p < 0.001). Ongoing phase III trials will further elucidate the potential utility of nemolizumab in the treatment of AD.
Omalizumab
Omalizumab targets human IgE, limiting mast cell degranulation and inflammatory mediator release [25]. It is currently approved for moderate to severe persistent asthma but its utility in the treatment of AD remains under investigation. While clinical trials completed in the mid-2000s did not demonstrate a clinically significant treatment response with omalizumab [26, 27], a more recent trial published in 2020 found that omalizumab significantly reduced AD severity and improved quality of life (QoL) measures in a pediatric population with atopy and severe eczema [25]. While these results suggest that omalizumab may be an efficacious treatment option for refractory cases of severe eczema in children with atopy, future studies with larger sample sizes and longer durations would clarify the precise role of omalizumab in patients with AD.
GBR 830
GBR 830 is an injectable anti-OX40 monoclonal antibody. After promising results from early clinical trials, a phase 2 trial enrolling 274 patients with moderate-to-severe AD was conducted and completed recently. Official results have not yet been published. However, preliminary reports suggest that this study demonstrated a significant reduction in EASI in treated groups compared to placebo groups. This drug targets the novel OX40L-OX40 axis and, in the future, may become an effective, alternative therapy for AD and other allergic conditions [28].
Small Molecule Inhibitors for Atopic Dermatitis
Phosphodiesterase-4 (PDE) Inhibitors
Inhibition of PDE-4 prevents the cleavage of cyclic adenosine monophosphate, leading to lower levels of downstream metabolites known to stimulate Th1 and Th2 cells. Apremilast is an oral phosphodiesterase-4 (PDE-4) inhibitor FDA approved for psoriatic arthritis and moderate plaque psoriasis. However, due to this dual pathway inhibition, theories regarding its potential use in AD are currently under investigation. In a phase II trial in adults with moderate-to-severe AD, patients receiving 40 mg of apremilast bid were found to have significant improvements in EASI sores compared to placebo (− 31.6 vs. − 11.0, p < 0.04). Adverse events of nausea, diarrhea, headache, and nasopharyngitis were common [29]. As other clinical trials have shown a lack of efficacy for apremilast in the treatment of AD, the overall consensus currently remains that this drug is more efficacious for psoriasis.
Crisaborole is a topical PDE-4 approved for AD in patients 3 months and older, making it a unique medication as it has become the only non-steroidal topical anti-inflammatory indicated for children this young. While studies have demonstrated efficacy in all age groups [30], the most compelling evidence for its use was seen in a study conducted in a pediatric population ages 3–24 months. Results demonstrated that 40% of subjects achieved clear or almost clear skin in only 8 days of treatment [31].
JAK Pathway Inhibitors
JAK inhibitors present an additional immunomodulatory route for SMIs. JAK includes a family of 4 non receptor tyrosine kinases which act on downstream signal transducers and activators of transcription (STATs) to drive the function of a variety of immune cells including the B, T, NK, and mast cell lines. Numerous JAK inhibitors are currently available and many others are the subject of active research for a variety of conditions, including AD.
Approved
Upadacitinib is an oral JAK-1 inhibitor that was approved for AD in patients 12 years and older in January 2022. Additional indications include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Three pivotal phase III trials evaluating the efficacy of upadacitinib in patients with AD demonstrated higher levels of skin clearance and improvement in itch as early as week 1, compared to placebo. In Measure Up I, an EASI-75 was achieved by 80% and 16% of patients receiving upadacitinib 30 mg and placebo, respectively (p < 0.001). Overall adverse events were higher in the treatment group with acne being the most common. In both studies, serious adverse events were similar between all groups and occurred at a rate of 2%, 3%, and 3% in the 15 mg, 30 mg, and placebo groups, respectively [32]. However, all patients are counseled on warnings for increased risk of serious infection, cardiovascular events, cancers, and blood clots which have been previously reported.
Of note, a phase III RCT published in 2021 compared the efficacy of dupilumab and upadacitinib in patients with moderate-to-severe AD. During 16 weeks of treatment, upadacitinib demonstrated superior efficacy compared to dupilumab with no new safety signals [33•].
Abrocitinib is an oral JAK-1 inhibitor approved in January 2022 for AD in adults 18 years and older. Results from 5 clinical trials in the abrocitinib JAK-1 Atopic Dermatitis Efficacy and Safety (JADE) program were included to support FDA approval. Three phase III trials demonstrated improvement in skin clearance and itch compared to placebo. In the JADE MONO-1 trial, 62.7% of participants receiving 200 mg of daily abrocitinib achieved an EASI-75 at 12 weeks compared to 11.8% of placebo (p < 0.001) [34]. In JADE TEEN, 58% of patients receiving 200 mg abrocitinib plus TCS achieved an EASI-75 compared to 27% of patients receiving placebo plus TCS (p < 0.001) [35]. Table 3 serves to compare the efficacies of abrocitinib and upadacitinib. Serious adverse events were reported in 3%, 5%, and 4% in the 100 mg, 200 mg, and placebo group, respectively. Similar to all JAK inhibitors, important safety information includes warning of serious infections, mortality, malignancy, cardiovascular events, and thrombosis.
Table 3.
Comparison of endpoints achieved by subjects in three phase III trials at week 16 for upadacitinib and week 12 for abrocitinib
| Upadacitinib (week 16) | |||||||||
| Measure Up I | Measure Up II | AD Up | |||||||
|
15 mg (n = 281) |
30 mg (n = 285) |
Placebo (n = 281) |
15 mg (n = 276) |
30 mg (n = 282) |
Placebo (n = 278) |
15 mg + TCS (n = 300) |
30 mg + TCS (n = 297) |
Placebo + TCS (n = 304) |
|
| EASI-75 | 70% | 80% | 16% | 60% | 73% | 13% | 65% | 77% | 26% |
| vIGA-AD 0/1 | 48% | 62% | 8% | 39% | 52% | 5% | 40% | 59% | 11% |
| EASI-90 | 53% | 66% | 8% | 42% | 58% | 5% | 43% | 63% | 13% |
| Abrocitinib (week 12) | |||||||||
| JADE MONO I | JADE MONO II | JADE COMPARE | |||||||
|
100 mg (n = 156) |
200 mg (n = 154) |
Placebo (n = 77) |
100 mg (n = 158) |
200 mg (n = 155) |
Placebo (n = 78) |
100 mg + TCS (n = 155) |
200 mg + TCS (n = 155) |
Placebo + TCS (n = 155) |
|
| EASI-75 | 40% | 63% | 12% | 45% | 61% | 10% | 72% | 69% | 42% |
| vIGA-AD 0/1 | 24% | 44% | 8% | 28% | 38% | 9% | 42% | 46% | 25% |
| EASI-90 | 19% | 39% | 5% | 24% | 38% | 4% | 42% | 49% | 18% |
Of note, a phase III trial published in 2022 compared the efficacy of dupilumab and abrocitinib in patients with moderate-to-severe AD. Over 26 weeks, both medications were well tolerated and abrocitinib was found to be more efficacious than dupilumab in reducing clinical signs of AD and itch [34].
Under Investigation
Baricitinib is an oral JAK-1/2 inhibitor that is currently approved for rheumatoid arthritis, alopecia areata, and COVID-19. At doses of 2 and 4 mg, it has been shown to improve AD severity and AD-associated itch and sleep loss. Most recently, a phase III clinical trial known as BREEZE-AD5 evaluated monotherapy of baricitinib in adults with moderate-to-severe AD. A higher proportion of subjects receiving 4 mg of baricitinib daily achieved an EASI-75 at 16 weeks compared to placebo (29.5% vs. 8.2%, respectively p < 0.001). Clinically meaningful improvements in itch were seen as early as week 1 for patients receiving 4 mg baricitinib. Safety findings were similar to previous studies with AEs including nasopharyngitis, diarrhea, and nausea [36]. A pooled safety analysis of 8 baricitinib trials found a higher rate of AEs in baricitinib-treated groups compared to placebo groups with mild-to-moderate treatment-emergent infections being most common [37]. While it is currently approved in the European Union and Japan for AD, according to Lilly pharmaceuticals, ongoing discussions regarding the appropriate indicated population have led to delays in its approval by the FDA [38].
Topical JAK Inhibitors
Approved
Ruxolitinib selectively inhibits JAK-1/2 and was initially approved as an oral medication for myelofibrosis in 2011. While oral ruxolitinib is not currently being evaluated for AD, topical ruxolitinib was approved in September 2021 for mild-to-moderate AD in patients 12 years and older. Two parallel phase III trials compared ruxolitinib 1.5% cream vs. 0.75% cream vs. placebo and both found clinically significant reductions in itch and disease severity compared to placebo at 8 weeks. In TRuE-AD1, an EASI-75 was achieved in 62.1%, 56.0%, and 24.6% of subjects receiving ruxolitinib 1.5%, 0.75%, and placebo, respectively. Similar results were seen in TRuE-AD2 and both studies reported no serious AEs [39]. Of note, in July 2022, ruxolitinib 1.5% cream also gained approval for non-segmental vitiligo [40].
Under Investigation
Tofacitinib selectively targets JAK-1/3 and is currently approved for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and juvenile idiopathic arthritis as an oral medication. Although case series and case reports have demonstrated the efficacy of oral tofacitinib for moderate-to-severe AD, boxed warnings of serious AEs have likely curtailed future clinical trials evaluating oral tofacitinib in patients with AD [41]. However, in a phase IIa trial, 4 weeks of application of tofacitinib 2% ointment resulted in patients achieving an 81% mean reduction in EASI compared to 29.9% in the placebo arm [42]. Despite these promising results, no additional studies are currently active and the future role of topical tofacitinib in the treatment of AD remains unclear [41].
Delgocitinib is a topical JAK-1/2/3 and Tyk2 inhibitor approved in Japan for AD and, according to LEO pharmaceuticals, was granted fast track designation by the FDA for treatment of chronic hand eczema. In a phase III RCT published in 2020, the efficacy of delgocitinib 0.5% bid was evaluated in subjects ages 16 years and older. At 4 weeks, 26.4% and 5.8% of subjects in the delgocitinib 0.5% bid arm and placebo arm, respectively, achieved an EASI-75 (p < 0.001). Additionally, both nighttime and daytime itch showed significant reduction after day 1 and steadily improved over 4 weeks compared to no improvement with placebo. AEs included folliculitis (2.4%) and acne (2.2%) and no serious AEs were reported [43]. Additional phase III trials are currently active in the USA with the first scheduled to be completed in October 2022 (NCT04871711).
NK-1 Inhibitors
Serlopitant targets the NK-1 receptor and was found to be superior to placebo in the management of chronic pruritus in AD patients [44]. However, due to a corporate decision to no longer pursue an indication of treatment for pruritus, the phase II trials of this drug were terminated prematurely (clinicaltrials.gov). Tradipitant is another oral NK-1 inhibitor currently under investigation for its utility in AD. EPIONE, a phase III RCT, did not meet its primary endpoint of average itch VAS. However, it did demonstrate statistically significant improvement in pruritus and sleep for treated subjects with mild AD compared to placebo (p = 0.0457 and p = 0.013, respectively). These results suggest a potential utility for AD patients who, despite having mild lesions, experience significant or refractory pruritus [45].
Psoriasis
Psoriasis is a common inflammatory disorder of the skin and joints that classically presents with well demarcated erythematous scaly plaques. Similar to AD, psoriasis is thought to result from a complex interplay of genetics, environmental triggers, and immune dysregulation. Specifically, inappropriate activation of inflammatory cascades lead to the differentiation of T lymphocytes into Th1, Th17, and Th22 cells. This polarization toward a Th1 response results in excessive levels of cytokines including TNF-α, IL-17A, IL-17F, IL-22, and IL-23 (Fig. 1). The perpetuation of this chronic inflammatory process leads to the proliferation and abnormal differential of epidermal keratinocytes, giving rise to the classic psoriatic plaques. In regard to treatment, an initial wave of first- and second-generation biologics has given rise to novel, highly efficacious classes of biologics and SMIs. Some of these have become commercially available and others are in late stages of development. Table 4 serves as an overview of these molecular-targeted therapies.
Table 4.
Summary of the newest biologics and small molecule inhibitors for psoriasis
| Drug | Target | Route of administration | Status for psoriasis |
|---|---|---|---|
|
Secukinumab Ixekizumab Brodalumab |
IL-17 | Injection |
Approved (ages ≥ 6 years) Approved (ages ≥ 6 years) Approved (ages ≥ 18 years) |
|
Guselkumab Tildrakizumab Risankizumab |
p19 subunit of IL-23 | Injection |
Approved (ages ≥ 18) Approved (ages ≥ 18) Approved (ages ≥ 18) |
| Ustekinumab | p40 subunit of IL-23 and IL-12 | Injection | Approved (ages ≥ 6) |
| Bimekizumab | IL-17A and IL-17F | Injection | Phase III trials completed |
| Apremilast | PDE-4 | Oral | Approved (ages ≥ 18) |
| Deucravacitinib | TYK2 | Oral | Phase III trials completed |
| PF-06826647 | TYK2 | Topical | Phase II trials completed |
| Tofacitinib | JAK1 and JAK3 | Oral |
Phase II trials completed No active trials |
| Tapinarof | Aryl hydrocarbon receptor-modulating agent | Topical | Approved (ages ≥ 18) |
| Roflumilast | PDE-4 inhibitor | Topical | Approved (ages ≥ 12) |
IL-17/23 Axis
The IL-17 inhibitor class consists of secukinumab, ixekizumab, and brodalumab. The IL-23 inhibitor class contains inhibitors of the p19 subunit of IL-23 (guselkumab, tildrakizumab, and risankizumab) and the p40 subunit (ustekinumab) which also binds the p40 subunit IL-12. These agents are generally well tolerated and highly effective as injectable treatment options for moderate-to-severe psoriasis. Since their entrance into the market, a systematic review of 71 trials concluded that, with regard to a 90% reduction from baseline in the psoriasis area and severity index (PASI-90), ixekizumab 80 mg, risankizumab 150 mg, and brodalumab 210 mg had the highest efficacy at 12 weeks while risankizumab had the highest long-term efficacy. Ixekizumab was the most likely to cause one or more adverse events [46•].
Although the IL-17 and IL-23 classes of biologics appear to be very safe, long-term AEs are not fully characterized due to the limited time on the market [46•]. Additionally, limiting factors in their use include price and route of administration (injection), which can be unfavorable to some patients [47].
Under Investigation
Bimekizumab is a novel IL-17A and IL17F mAB currently being reviewed for approval for moderate-to-severe plaque psoriasis. Several phase III trials have demonstrated its efficacy in treatment. A phase III trial directly comparing bimekizumab against secukinumab (BE RADIANT) was completed in 2021. In this study, 61.7% of the bimekizumab arm achieved a PASI-100 compared to 48.9% in the secukinumab arm, demonstrating superiority (p < 0.001). Moreover, 67% of subjects the bimekizumab arm achieved PASI-100 at 48 weeks versus 46.2% in the secukinumab arm. A greater association with oral candidiasis in the bimekizumab arm was noted during the study [48]. In the prior phase III trial focused on safety, the most common side effects for bimekizumab were nasopharyngitis (20.9%), oral candidiasis (16.2%), and upper respiratory infections (URIs) (9.0%). According to a press release in May 2022, the request for FDA approval was denied due to certain pre-approval inspection observations that the company states are likely to be resolved.
Oral SMIs
Approved
Apremilast is an oral PDE-4 inhibitor and became the first FDA-approved SMI for psoriasis in 2014. Many RCTs have demonstrated that apremilast significantly reduces the severity of moderate-to-severe plaque psoriasis and exhibits an acceptable safety profile with a low discontinuation rate [49]. With numerous clinical trials since its existence on the market, a recent meta-analysis published in May 2022 aimed to comprehensively evaluate the efficacy and safety of apremilast monotherapy. Results from an analysis of 8 trials showed that, at 16 weeks, PASI-75 was achieved by 30.81%, 25.19%, and 6.42% of subjects receiving apremilast 30 mg, 20 mg, and placebo, respectively. While significantly more adverse events were reported in those receiving 30 mg compared to 20 mg, most AEs were mild-to-moderate in severity [50]. These results suggest that apremilast remains an effective, safe, and well-tolerated therapeutic alternative for the treatment of psoriasis.
Under Investigation
Tyrosine Kinase Inhibitors
Tyrosine kinase (TYK) inhibitors bind to the active JAK domain, preventing signal transduction and downstream signaling, thus reducing concentrations of IL-12, IL-23, and interferon alpha.
Deucravacitinib is an oral selective TYK2 inhibitor currently under investigation for treatment of plaque psoriasis. In two pivotal phase III trials completed in 2021, deucravacitinib demonstrated superior skin clearance compared with apremilast for key primary and secondary endpoints. At week 16 in POETYK PSO-1, 58.7% of patients receiving deucravacitinib achieved PASI-75 response versus 35.1% receiving apremilast and 12.7% receiving placebo. At week 24, 69.0% of patients receiving deucravacitinib achieved PASI-75 response versus 38.1% receiving apremilast [51, 52].
AEs in all groups were mild-to-moderate in severity. In the deucravacitinib arm, AEs included nasopharyngitis, URIs and low rates of headaches, diarrhea, and nausea. At week 16, 3.8% of patients on placebo, 2.4% of patients on deucravacitinib, and 5.2% of patients on apremilast experienced AEs leading to discontinuation. Results from this study suggest that deucravacitinib may emerge in the near future as a novel, efficacious, oral therapeutic for psoriasis.
PF-06826647 is a (TYK2) inhibitor currently being evaluated for its efficacy in the treatment of plaque psoriasis. In a phase IIb trial completed in November 2021, 179 participants were treated with 50:100:200:400 mg:placebo. PASI-90 scores were reported as a difference in percentage. Results showed that at 16 weeks, 46.5% more of those in the 400 mg treatment arm achieved PASI-90 compared to placebo (p < 0.0001). Significant increases from placebo were observed for all secondary endpoints in subjects receiving 200 mg or 400 mg (p < 0.05). Most AEs were mild-to-moderate in severity and included nasopharyngitis, URIs, and elevated blood pressure [53]. While phase III trials must be conducted to further establish evidence for this drug, results from this study suggest that PF-06826647 is an effective and well tolerated oral option that may emerge as a novel therapy for psoriasis in the future.
JAK Inhibitory Pathway
While tofacitinib is currently approved by the FDA for psoriatic arthritis, its efficacy in the treatment of plaque psoriasis remains under investigation. A recent analysis reviewing 4 phase III trials demonstrated that 59.2–81.1% of subjects receiving 10 mg tofacitinib bid achieved a PASI-75 at weeks 16–24 compared to 5.6–12.5% of subjects receiving placebo (p < 0.001). These studies also showed clinical efficacy in achieving secondary endpoints including QoL, nail psoriasis severity index, and PASI-90 compared to placebo. Tofacitinib was well tolerated overall and AEs included dyslipidemias, elevated creatinine phosphokinase, and decreased hemoglobin and lymphocyte counts [54]. While tofacitinib is the best studied JAK inhibitor for plaque psoriasis, 4 other JAK inhibitors have been evaluated as potential treatments for psoriasis: peficitinib, a pan-JAK inhibitor; solcitinib, a JAK1 inhibitor; baricitinib, a JAK1/2 inhibitor; and itacitinib, a JAK1 inhibitor. The efficacies of these JAK inhibitors were either similar or inferior to that of tofacitinib [55]. While these drugs have undergone phase II trials regarding treatment of plaque psoriasis, plans for further trials are unclear as there are no recent or active trials.
Approved Topical SMIs
Tapinarof
In May 2022, the FDA approved topical tapinarof for the treatment of plaque psoriasis in adults. Tapinarof is an aryl hydrocarbon receptor-modulating agent that modulates the expression of IL-17 and skin-barrier proteins filaggrin and loricrin [56]. In two phase III trials with 510 and 515 patients, tapinarof 1% cream was superior to control in reducing psoriasis severity over 12 weeks. A PASI-75 response was achieved in 36.1% and 47.6% (compared to 10.2% and 6.9% in the control group) in trials 1 and 2, respectively (p < 0.001). Adverse events included folliculitis, nasopharyngitis, contact dermatitis, headache, URI, and pruritus [56].
Roflumilast
In July 2022, the FDA approved topical roflumilast, a PDE-4 inhibitor, for the treatment of plaque psoriasis in adults and children over the age of 12. In two phase III trials known as DERMIS-1 and DERMIS-2 (n = 439 and n = 442, respectively), roflumilast 0.3% cream provided significant, consistent, and sustained improvements in the severity of disease and QoL in patients with plaque psoriasis [57]. IGA success (clear or almost clear skin plus 2 grade improvement from baseline) was achieved in 42.4% and 37.5% in treatment groups compared to 6.1% and 6.9% in placebo groups in DERMIS-1 and DERMIS-2, respectively (p < 0.001). Safety and tolerability of roflumilast cream were similar to vehicle with low rates of application site AEs and discontinuation due to AEs [57].
Conclusion
Advances in our understanding of the pathophysiology of AD and psoriasis have allowed for the development of efficacious and safe biologics and SMIs. In 2017, the biologic dupilumab revolutionized the treatment of AD. Still, clear or almost clear skin is achieved by less than 40% of patients treated. Tralokinumab was approved in December 2021 and other novel biologics, including lebrikizumab, have completed phase III trials. These agents may provide an efficacious alternative to dupilumab. Additionally, the SMIs abrocitinib and upadacitinib are now FDA approved and have demonstrated increased clinical efficacy when compared to dupilumab. Safety profiles for these drugs are continuing to be developed as warnings for serious AEs may limit their use.
In regard to psoriasis, PASI-100 scores of the newest biologics are nearing 50%. While these have higher efficacies compared to biologics for AD, opportunities for continued progress remain, especially in the development of oral SMIs. For example, the newest TYK inhibitor, deucravacitinib, has demonstrated significantly higher clearance rates in phase III trials when compared to apremilast. It is likely that additional TYK and JAK inhibitors will enter the market soon.
Given the multitude of biologics and SMIs currently under investigation, frequent re-evaluation of clinical trials and published studies will be necessary to remain well informed of the latest research. While this review serves to summarize the research through mid-2022, the landscape of therapies for psoriasis and AD will continue to change in the coming years. Broader indications, greater efficacy, better safety profiles, and ease of administration are factors anticipated to be of particular focus in new generations of molecular-targeted therapies.
Compliance with Ethical Standards
Conflict of Interest
Peter A. Lio reports research grants/funding from the National Eczema Association, AOBiome, Regeneron/Sanofi Genzyme, and AbbVie; is on the speaker’s bureau for Regeneron/Sanofi Genzyme, Pfizer, Eli Lilly, LEO, Galderma, and L’Oreal; and reports consulting/advisory boards for Almirall, ASLAN Pharmaceuticals, Dermavant, Regeneron/Sanofi Genzyme, Pfizer, LEO Pharmaceuticals, AbbVie, Eli Lilly, Micreos, L’Oreal, Pierre-Fabre, Johnson & Johnson, Level Ex, Unilever, Menlo Therapeutics, Theraplex, IntraDerm, Exeltis, AOBiome, Realm Therapeutics, Altus Labs (stock options), Galderma, Amyris, Bodewell, and My-Or Diagnostics. Joseph Dodson reports no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Allergic Skin Diseases
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Honma M, Hayashi K. Psoriasis: recent progress in molecular-targeted therapies. J Dermatol. 2021;48:761–777. doi: 10.1111/1346-8138.15727. [DOI] [PubMed] [Google Scholar]
- 2.Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15–26. doi: 10.1080/1744666X.2016.1212660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15:35–50. doi: 10.1038/nrd4624. [DOI] [PubMed] [Google Scholar]
- 5.Howell MD, Fitzsimons C, Smith PA. JAK/STAT inhibitors and other small molecule cytokine antagonists for the treatment of allergic disease. Ann Allergy Asthma Immunol. 2018;120:367–375. doi: 10.1016/j.anai.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 7.Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857–870. doi: 10.1111/bjd.19460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464–475. doi: 10.1111/jdv.16928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albader SS, Alharbi AA, Alenezi RF, Alsaif FM. Dupilumab side effect in a patient with atopic dermatitis: a case report study. Biologics. 2019;13:79–82. doi: 10.2147/BTT.S195512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narla S, Silverberg JI, Simpson EL. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J Am Acad Dermatol. 2022;86:628–636. doi: 10.1016/j.jaad.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Raffi J, Suresh R, Fishman H, Botto N, Murase JE. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD) Int J Womens Dermatol. 2019;5:308–313. doi: 10.1016/j.ijwd.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu GA, Chen JK, Chiou A, Ko J, Honari G. Assessment of the development of new regional dermatoses in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155:850–852. doi: 10.1001/jamadermatol.2019.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maloney NJ, Tegtmeyer K, Zhao J, Worswick S. Dupilumab in dermatology: potential for uses beyond atopic dermatitis. J Drugs Dermatol. 2019;18. [PubMed]
- 14.Office of the Commissioner FDA approves first treatment for eosinophilic esophagitis, a chronic immune disorder. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-eosinophilic-esophagitis-chronic-immune-disorder. Accessed 27 Jul 2022
- 15.Hewett L. LEO Pharma announces FDA approval of Adbry™ (tralokinumab-ldrm) as the first and only treatment specifically targeting IL-13 for adults with moderate-to-severe atopic dermatitis. 2021. https://nationaleczema.org/blog/leo-122821/. Accessed 27 Jul 2022.
- 16.Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184:437–449. doi: 10.1111/bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutermuth J, Pink AE, Worm M, Soldbro L, Bjerregård Øland C, Weidinger S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7) Br J Dermatol. 2022;186:440–452. doi: 10.1111/bjd.20832. [DOI] [PubMed] [Google Scholar]
- 18.Wollenberg A, Beck LA, de Bruin WM, Simpson EL, Imafuku S, Boguniewicz M, Zachariae R, Olsen CK, Thyssen JP. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2022;186:453–465. doi: 10.1111/bjd.20810. [DOI] [PubMed] [Google Scholar]
- 19.Agboola F, Atlas SJ, Brouwer E, Carlson JJ, Hansen RN, Herron-Smith S, Nhan E, Rind DM, Pearson SD. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: effectiveness and value. J Manag Care Spec Pharm. 2022;28:108–114. doi: 10.18553/jmcp.2022.28.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158:523–532. doi: 10.1001/jamadermatol.2022.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safety and efficacy of lebrikizumab (LY3650150) in combination with topical corticosteroid in moderate-to-severe atopic dermatitis. - Study results - ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/results/NCT04250337?id=NCT04250337&draw=2&rank=1&load=cart. Accessed 25 Jul 2022.
- 22.Evaluation of the efficacy and safety of lebrikizumab (LY3650150) in moderate to severe atopic dermatitis (ADvocate1). 2022. https://clinicaltrials.gov/ct2/show/NCT04146363. Accessed 25 Jul 2022.
- 23.Labib A, Ju T, Yosipovitch G. Managing atopic dermatitis with lebrikizumab - the evidence to date. Clin Cosmet Investig Dermatol. 2022;15:1065–1072. doi: 10.2147/CCID.S295672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu C-Y. Treatments for childhood atopic dermatitis: an update on emerging therapies. Clin Rev Allergy Immunol. 2021;61:114–127. doi: 10.1007/s12016-020-08799-1. [DOI] [PubMed] [Google Scholar]
- 25.Chan S, Cornelius V, Cro S, Harper JI, Lack G. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29–37. doi: 10.1001/jamapediatrics.2019.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyengar SR, Hoyte EG, Loza A, Bonaccorso S, Chiang D, Umetsu DT, Nadeau KC. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89–93. doi: 10.1159/000350486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990–998. doi: 10.1111/j.1610-0387.2010.07497.x. [DOI] [PubMed] [Google Scholar]
- 28.Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, Peng X, Wen HC, Govas P, Gudi G, Ca V, Fang H, Salhi Y, Back J, Reddy V, Bissonnette R, Maari C, Grossman F, Wolff G. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144(2):482–493.e7. doi: 10.1016/j.jaci.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 29.Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063–1072. doi: 10.1016/j.jid.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494–503.e6. doi: 10.1016/j.jaad.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Schlessinger J, Shepard JS, Gower R, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1) Am J Clin Dermatol. 2020;21:275–284. doi: 10.1007/s40257-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 33.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400:273–282. doi: 10.1016/S0140-6736(22)01199-0. [DOI] [PubMed] [Google Scholar]
- 35.Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157:1165–1173. doi: 10.1001/jamadermatol.2021.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5) J Am Acad Dermatol. 2021;85:62–70. doi: 10.1016/j.jaad.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Bieber T, Thyssen JP, Reich K, et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35:476–485. doi: 10.1111/jdv.16948. [DOI] [PubMed] [Google Scholar]
- 38.Updates on OLUMIANT® (baricitinib) phase 3 lupus program and FDA review for atopic dermatitis. In: Eli Lilly and Company. 2022. https://investor.lilly.com/news-releases/news-release-details/updates-olumiantr-baricitinib-phase-3-lupus-program-and-fda. Accessed 23 Jul 2022.
- 39.Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863–872. doi: 10.1016/j.jaad.2021.04.085. [DOI] [PubMed] [Google Scholar]
- 40.Center for Drug Evaluation Research FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older. Accessed 10 Aug 2022.
- 41.Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927–940. doi: 10.1016/j.jaci.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175:902–911. doi: 10.1111/bjd.14871. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823–831. doi: 10.1016/j.jaad.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Yosipovitch G, Ständer S, Kerby MB, Larrick JW, Perlman AJ, Schnipper EF, Zhang X, Tang JY, Luger T, Steinhoff M. Serlopitant for the treatment of chronic pruritus: results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J Am Acad Dermatol. 2018;78:882–891.e10. doi: 10.1016/j.jaad.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 45.Welsh SE, Xiao C, Kaden AR, et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: results from EPIONE, a randomized clinical trial. J Eur Acad Dermatol Venereol. 2021;35:e338–e340. doi: 10.1111/jdv.17090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong AW, Soliman AM, Betts KA, Wang Y, Gao Y, Puig L, Augustin M. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther. 2021;11:885–905. doi: 10.1007/s13555-021-00511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nenow J, Balogh E, Feldman S. Emerging treatment regimens in psoriasis: are there advantages over current biologic therapies? EMJ Dermatol. 2021;106–121.
- 48.Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142–152. doi: 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- 49.Van Voorhees AS, Stein Gold L, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96–103. doi: 10.1016/j.jaad.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 50.Aljefri YE, Ghaddaf AA, Alkhunani TA, Alkhamisi TA, Alahmadi RA, Alamri AM, Alraddadi AA. Efficacy and safety of apremilast monotherapy in moderate-to-severe plaque psoriasis: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15544. doi: 10.1111/dth.15544. [DOI] [PubMed] [Google Scholar]
- 51.Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx. Accessed 24 Jul 2022.
- 52.Search of: BMS-986165 - list results - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/results?cond=&term=BMS-986165&cntry=&state=&city=&dist=. Accessed 24 Jul 2022.
- 53.Tehlirian C, Singh RSP, Pradhan V, Roberts ES, Tarabar S, Peeva E, Vincent MS, Gale JD. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333–342. doi: 10.1016/j.jaad.2022.03.059. [DOI] [PubMed] [Google Scholar]
- 54.Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: a review. Dermatol Ther. 2020;10:29–42. doi: 10.1007/s13555-019-00347-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Funk PJ, Perche PO, Singh R, Kelly KA, Feldman SR. Comparing available JAK inhibitors for treating patients with psoriasis. Expert Rev Clin Immunol. 2022;18(3):281–294. doi: 10.1080/1744666X.2022.2039121. [DOI] [PubMed] [Google Scholar]
- 56.Lebwohl MG, Stein Gold L, Strober B, Papp KA, Armstrong AW, Bagel J, Kircik L, Ehst B, Hong HC, Soung J, Fromowitz J, Guenthner S, Piscitelli SC, Rubenstein DS, Brown PM, Tallman AM, Bissonnette R. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385(24):2219–2229. doi: 10.1056/NEJMoa2103629. [DOI] [PubMed] [Google Scholar]
- 57.Lebwohl M, Kircik L, Moore A, Gold LS, Del Rosso J, Draelos Z, Gooderham M, et al. Once-daily roflumilast cream 0.3%, a potent phosphodiesterase-4 inhibitor, provided safe and effective treatment of psoriasis in the DERMIS-1 and DERMIS-2 phase 3 trials. SKIN The Journal of Cutaneous Medicine. 2021;5(6):s42–s42. doi: 10.25251/skin.5.supp.42. [DOI] [Google Scholar]