Abstract
Introduction
Long-onset COVID syndrome has been described in patients with COVID-19 infection with persistence of symptoms or development of sequelae beyond 4 weeks after the onset of acute symptoms, a medium- and long-term consequence of COVID-19. This syndrome can affect up to 32% of affected individuals, with symptoms of fatigue, dyspnea, chest pain, cognitive disorders, insomnia, and psychiatric disorders. The present study aimed to characterize and evaluate the prevalence of sleep symptoms in patients with long COVID syndrome.
Methodology
A total of 207 patients with post-COVID symptoms were evaluated through clinical evaluation with a neurologist and specific exams in the subgroup complaining of excessive sleepiness.
Results
Among 189 patients included in the long COVID sample, 48 (25.3%) had sleep-related symptoms. Insomnia was reported by 42 patients (22.2%), and excessive sleepiness (ES) was reported by 6 patients (3.17%). Four patients with ES were evaluated with polysomnography and test, multiple sleep latencies test, and actigraphic data. Two patients had a diagnosis of central hypersomnia, and one had narcolepsy. A history of steroid use was related to sleep complaints (insomnia and excessive sleepiness), whereas depression was related to excessive sleepiness. We observed a high prevalence of cognitive complaints in these patients.
Conclusion
Complaints related to sleep, such as insomnia and excessive sleepiness, seem to be part of the clinical post-acute syndrome (long COVID syndrome), composing part of its clinical spectrum, relating to some clinical data.
Keywords: Long-COVID, Sleep, Hypersomnia, Narcolepsy
Introduction
The new coronavirus SARS-CoV-2 rapidly spread around the world, turning into a pandemic [1, 2]. SARS-CoV-2 enters cells through interaction with angiotensin converting enzyme-2 (ACE2) receptors present in many cell types, including nasal mucosa, lungs, heart, liver, kidneys and brain, as well as arterial and venous endothelial cells [2]. Once internalized, the virus replicates and matures, leading to a widespread inflammatory response with massive cytokine release [3].
The World Health Organization (WHO) defines “post-COVID-19” as a symptom complex with typical symptoms such as fatigue, shortness of breath and cognitive disorders, which occurs within 3 months after infection, lasts at least 2 months, can fluctuate and for which there is no explanatory explanation there is not attributed to alternative diagnoses. The English NICE (National Institute for Health and Care Excellence) guidelines distinguish between acute COVID-19 illness (< 4 weeks after infection), “persistent COVID-19 symptoms” (4–12 weeks) and post-COVID Syndrome (> 12 weeks), with the last two stages commonly grouped together as “Long-COVID” [4–7].
Long-term effects of COVID-19 occur in up to 32% of infected patients. Persistence of symptoms after active infection has also been reported in diseases caused by other coronaviruses, such as the SARS epidemic of 2003 and the 2012 MERS epidemic [8].
While at the beginning of the pandemic a large number of investigations focused on the psychosocial effects of the COVID-19 pandemic on sleep [9], there is now a wealth of data on sleep disorders as a result of SARS-CoV-2 infection. A systematic review and meta-analysis of 39 cohort studies on persistent symptoms in the long-term course after COVID-19 reports a prevalence of sleep disorders of 36% for the first 3 months and 33% for the period from 3 months to just over half a year, particularly insomnia [10–14].
The objective of this study was to assess the prevalence and characteristics of sleep-related symptoms in long COVID-19 patients in a Brazilian population, specifically analyzing the causes of excessive sleepiness.
Methods
This was a prospective cohort study. We evaluated patients with post-COVID symptoms for the presence of sleep disorders and conducted a more extensive analysis in those with excessive sleepiness.
Study population
A total of 207 consecutive patients with post-COVID symptoms were evaluated at a neurology outpatient clinic in Hospital Universitário Walter Cantídio – Universidade Federal do Ceará in the state of Ceará, northeast Brazil, from August 2020 to September 2021. The recruitment of patients was carried out by invitation on all local news and radio stations, as well as on the hospital’s Instagram. A number for contacting and making an appointment was provided. Patients who contacted this number were screened for enrollment in the study.
Considering an estimated prevalence of insomnia in the general population of 10%, the sample size was calculated to detect statistically significant differences with the R software (R version 4.2.1, Free Software Foundation’s GNU General Public License), using the code samplingbook::sample.size.prop(e = 0.05,P = 0.10,N = Inf, level = 0.95). This resulted in a total of N = 139 patients. After this step, we stipulated a dropout of 25% to compose the final sample, totaling a minimum sample size of 186 participants [15].
Inclusion criteria were previous infection by SARS-CoV-2 documented with RT–PCR assay (Allplex™ SARS-CoV-2 Assay - N/RdRP/S genes – Seegene Brazil), persistence of symptoms for longer than four weeks and, in patients with sleep disorders, new onset of sleep-related symptoms after COVID infection. Patients who did not fulfill the inclusion criteria or who did not agree to participate in the study were excluded.
Patient data were collected using a semi structured questionnaire. Sociodemographic data, COVID infection severity, defined by hypoxemia, systemic involvement and/or need for hospital admission, neurologic symptoms, cognitive performance and psychiatric symptoms were assessed. Psychiatric disorders were evaluated through a structured psychiatric interview conducted by a neurologist. Participants underwent application of the Geriatric Depression Scale (GDS) to assess mood, or the Beck Inventory, depending on patient age. We used a cutoff point of 3 on the GDS and 10 on the Beck inventory for the diagnosis of depression [16, 17]. Psychiatric disorders were classified according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Participants were submitted to the Addenbrooke’s Cognitive Examination–Revised (ACE-R) and Mini-Mental State Examination (MMSE). Cutoff values of 58, 76 and 83 points were used for patients with < 4, 4–8 and > 8 years of education, respectively, in the ACE-R [18, 19]. For the MMSE, cutoffs of 19 for illiterate patients and 24 for literate patients were used [20, 21]. Cognitive diagnosis was established according to scores on the MMSE and ACE-R.
Subgroup analysis of excessive sleepiness (ES) patients
Excessive sleepiness is the cardinal feature of the central disorders of hypersomnolence. ES is defined as the “inability to stay awake and alert during major waking episodes of the day, resulting in periods of irrepressible need for sleep or unintended lapses into drowsiness or sleep.” Though brief periods of sleepiness can be normal, it is problematic when it interferes with daily activities and quality of life, manifesting as inappropriate periods of drowsiness. In patients with ES, sleep analysis included polysomnography, Multiple Sleep Latency Test (MSLT) and actigraphy. Diagnoses were established according to the international classification of sleep disorders-third edition (ICSD-3) (Table 1) [22].
Table 1.
International Classification of Sleep Disorders, Third Edition, Diagnostic Criteria
| Narcolepsy Type 1 Criteria A and B |
Narcolepsy Type 2 All Criteria A-E |
Central Hypersomnia due to medical disorder All Criteria A-F |
|---|---|---|
| A. Daily periods of irrepressible need to sleep or daytime lapses into sleep, present for at least 3 mo | A. Daily periods of irrepressible need to sleep or daytime lapses into sleep, present for at least 3 mo | A. Daily periods of irrepressible need to sleep or daytime lapses into sleep, present for at least 3 mo |
| B. Either 1 or 2 or both | B. Mean sleep latency ≤ 8 min and two or more SOREMPs on MSLT. REM within 15 min of sleep onset on the preceding nocturnal polysomnogram may replace one of the SOREMPs. | B. Daytime occurs as consequence of a medical condition. |
| 1. Cataplexy and mean sleep latency ≤ 8 min and two or more SOREMPs on MSLT. REM within 15 min of sleep onset on the preceding nocturnal polysomnogram may replace one of the SOREMPs. | C. No cataplexy | C. The mean latency is ≤ 8 min), and fewer than two SOREMPs on MSLT. |
| 2. Low CSF hypocretin-1 concentration (< 110 pg/mL or less than one-third of control values) | D. CSF hypocretin-1 concentration has not been measured or CSF hypocretin-1 concentration is ≥ 110 pg/mL or greater than one-third of control values. |
D. Either 1 or 2 or both:. 1.Mean sleep latency ≤ 8 min on MSLT. 2. Total 24-h sleep time ≥ 660 min on 24-h polysomnographic monitoring or wrist actigraphy (averaged over ≥ 7 d) |
| E. The hypersomnolence and/or MSLT findings are not better explained by other causes. | E. Insufficient sleep syndrome is ruled out. | |
| F. The hypersomnolence and/or MSLT findings are not better explained by other sleep disorder or medications. |
CSF cerebral spinal fluid, MSLT multiple sleep latency test, REM rapid eye movement, SOREMP sleep-onset rapid eye movement period
PSG was performed with a digital polygraph. Data were collected using an electroencephalogram (EEG) (according to the International 10–20 System), bilateral electrooculogram (E1-M2, E2-M1), electrocardiogram (modified V2 lead), and surface electromyography of the mental and submental muscles. Surface electrodes were placed on both anterior tibialis muscles, masseters, and extensors of fingers. Digital video was recorded with an infrared camera (Sony Ipela., CA) and then synchronized with the PSG data. Respiration was monitored as follows: airflow was measured by a nasal pressure transducer system (AcSleep 119, Biolink Medical, São Paulo, Brazil) and nasal and mouth thermocouple airflow sensor (Pro-Tech Services Inc., Mukilteo, WA); chest and abdominal efforts were measured by respiratory inductive plethysmographic belts (Pro-Tech zRIP module, Pro-Tech Services Inc.); arterial SaO2 was measured by pulse oximetry (Netlink Headbox, Natus Biologic Systems Inc.); snoring sounds were measured using a snoring microphone; body position was determined using a sensor (Netlink Body Sensor Position, Natus Biologic Systems Inc.). All of the technical parameters used were performed in accordance with the AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification (2007, [23]). The multiple sleep latency test (MSLT) was performed on the day after PSG, with a 5-nap protocol, according to the American Academy of Sleep Medicine guidelines [24].
Actitgraph (Actitrust, São Paulo, SP, Brazil) accelerometers were worn on the nondominant wrist continuously for 15 days prior to PSG/MSLT, only taken out during baths, to assess sleep-wakefulness patterns in these patients. Data were stored in the bracelet and transferred by a USB cable to software for analysis. Accelerometer data were stored in 1-minute samples and analyzed for circadian rhythm pattern, total sleep time, sleep latency and number of awakenings.
We performed brain image acquisition using a Siemens Somatom Vision 1.5-T Magnetic Resonance Imaging (MRI) scanner. The device was in regular activity at the University Hospital of Universidade Federal do Ceará under normal operating conditions.
Laboratory tests, including complete blood count (CBC), TSH, T4, blood iron, ferritin, transferrin saturation, liver enzymes, creatinine and electrolytes, were performed in these patients. All patients with excessive sleepiness underwent brain magnetic resonance imaging.
Subgroup analysis of chronic insomnia
The term chronic insomnia will be used as a disorder with the following diagnostic criteria: (1) difficulty falling asleep, staying asleep or nonrestorative sleep; (2) this difficulty is present despite adequate opportunity and circumstance to sleep; (3) this impairment in sleep is associated with daytime impairment or distress; and (4) this sleep difficulty occurs at least 3 times per week and has been a problem for at least 3 months after COVID infection [22].
Statistical analysis
Categorical variables were described as absolute frequencies and percentages, while numerical variables were described as averages and standard deviations. Association analyses were performed using Pearson’s chi-square test for categorical variables. Two-tailed p values of < 0.05 were considered statistically significant. Statistical analyses were performed with Jamovi®, version 1.0 (The Jamovi Project, n.d.).
Ethical aspects
The study project was approved by the Research Ethics Committee of Hospital Universitário Walter Cantídio under number 4.092.933. HUWC Research Ethics Committee (CEP) – Rua Coronel Nunes de Melo, 1142 - Rodolfo Teófilo, phone: 3366–8589 (opening hours: 7:00–12:00 h and from 13:00–15:30 h)– E-mail: cephuwc@huwc.ufc.br.
This study complies with the ethical principles of the Declaration of Helsinki. The free and informed consent form was applied. The document was read and informed and agreed by the patient or legal representative, with registration, before the beginning of the protocol, ensuring respect for the patient’s freedom to refuse to participate or to withdraw their consent, at any stage of the research, without no penalty and without prejudice to their care, with, in addition, a guarantee of secrecy to ensure their privacy regarding the confidential data involved in the research. All patients signed an informed consent form, with the right to secrecy and confidentiality of the information obtained, in addition to freedom of refusing to participate in the proposed activities and questions.
Results
The initial evaluation consisted of 207 patients. Eight patients were excluded for lack of RT–PCR confirmation of COVID infection, eight patients were excluded for not having persistent symptoms, and 3 other patients were excluded for preexisting sleep complaints (Fig. 1).
Fig. 1.
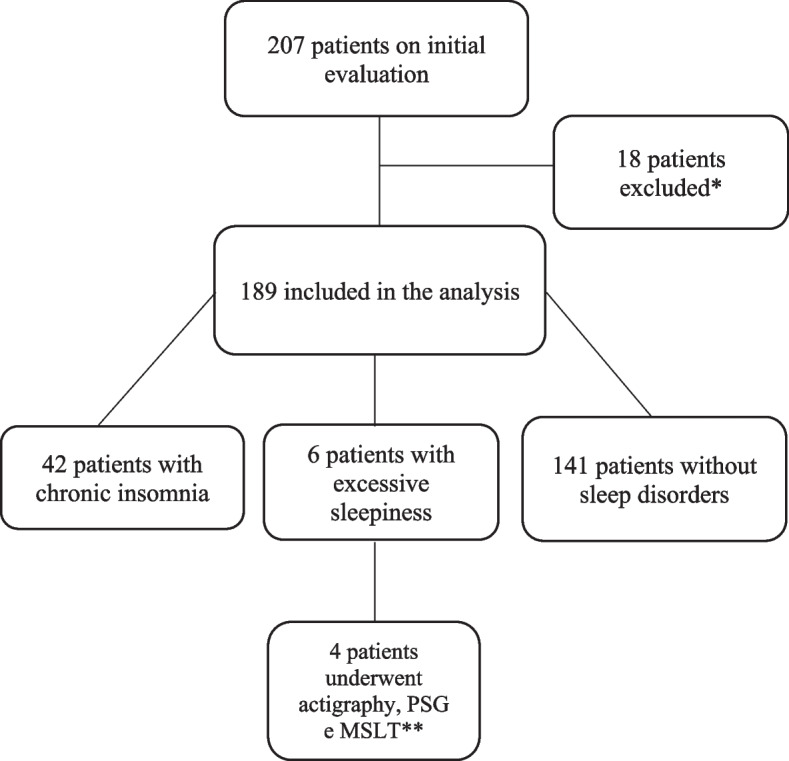
Flowchart of evaluated patients * Eight patients were excluded for lack of RT–PCR confirmation of COVID infection, 8 patients for not having persistent symptoms and 2 for preexisting sleep complaints. **2 patients refused to undergo actigraphy, PSG and MSLT. PSG – polysomnographic; MSLT - multiple sleep latency test
Among 189 patients included in the long-COVID sample (Table 2), 48 (25.3%) had sleep-related symptoms. Age, sex and COVID severity were similar between patients with sleep disorders and those without. Depression, corticosteroid use and subjective cognitive decline were significantly more common in patients with sleep disorders.
Table 2.
Sociodemographic and clinical characteristics of patients with long COVID-19, according to sleep symptoms
| No sleep disorder (n = 138) | Chronic Insomnia (n = 42) | Excessive Sleepiness (n = 6) | p | |
|---|---|---|---|---|
| Age (sd) | 44.9(± 46) | 49.7(± 28) | 41(± 30) | 0.350 |
| Males, n(%) | 50 (39) | 14(33) | 1(13) | 0.503 |
| Alcoholism, n(%) | 14(11) | 9(22) | 4(50) | 0.068 |
| COVID hospitalization, n(%) | 35(28) | 13(32) | 2(25) | 0.856 |
| Corticosteroid use, n(%) | 12(9) | 17(40) | 5(63) | <.001 |
| Headache, n(%) | 37(29) | 15(36) | 4(50) | 0.413 |
| Hypo/anosmia, n(%) | 37(29) | 20(48) | 1(13) | 0.064 |
| Depression, n(%) | 12(9) | 12(29) | 4(50) | <.001 |
| Anxiety, n(%) | 28(22) | 12(29) | 2(25) | 0.565 |
| Cognitve impairment, n(%) | ||||
| Dementia, n(%) | 10(7.8) | 0 | 0 | – |
| Subjective Cognitive | ||||
| Decline, n(%) | 63(48.8) | 33(78.6) | 5(83.3) | 0.005 |
Insomnia was reported by 42 patients (22.2%), and excessive sleepiness (ES) was reported by 6 patients (3.17%). Two of these patients with ES refused to undergo PSG and MSLT. The final subgroup of ES consisted of four patients.
Clinical and PSG characteristics of patients with ES
Of a total of 4 patients with ES, 3 fulfilled the clinical criteria for central hypersomnia, and one reported symptoms of ES that resolved spontaneously after 6 months without intervention (Table 3) before her first evaluation. Patients with ES also complained of fatigue, inattention and persistent hyposmia.
Table 3.
Clinical characteristics of four patients with excessive sleepiness after COVID-19 infection
| Case one | Case two | Case three | Case four | |
|---|---|---|---|---|
| Sex | F | M | F | F |
| Age (years) | 35 | 42 | 32 | 55 |
| Time from COVID-19 infection (months) | 18 | 18 | 13 | 18 |
| Need for hospitalization | N | N | N | N |
| Cognitive complaint | Y | Y | Y | Y |
| Time from ES onset (months) | 17 | 17 | 12 | 17 |
| Epworth scale | 22 | 16 | 21 | 4 |
| Snoring | N | N | N | N |
| Fatigue | Y | N | Y | N |
| Restorative sleep | N | N | N | N |
| Cataplexy | N | N | N | N |
| RLS symptoms | N | N | N | N |
| Treatment | Modafinil 200 mg/d + Methylphenidate 10 mg/d | Modafinil 200 mg/d | Lisdexamphetamine 35 mg/d | – |
| Improvement of ES | Y | Y | Y | Y |
For the majority of patients, symptoms persisted for up to 18.6 weeks (+ 72/−6 mean) after acute infection. The symptoms varied in their prevalence over time.
Magnetic resonance imaging (MRI) revealed olfactory bulb atrophy in one patient (Fig. 2), while it was normal in the other three. Laboratory results did not reveal abnormalities in any of the patients.
Fig. 2.
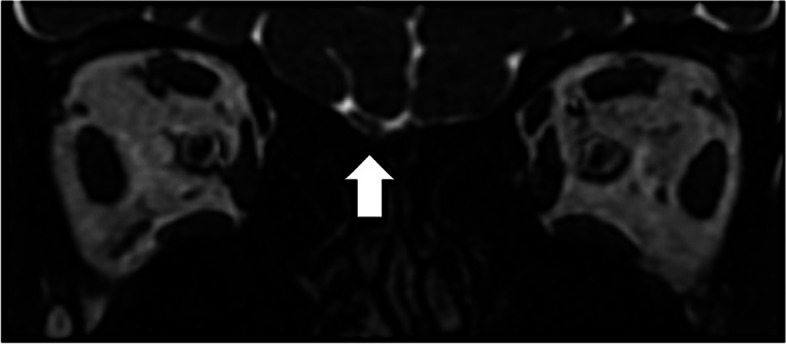
Olfactory bulb atrophy in MRI
Polysomnographic, MSLT and actigraphic data are described in Table 4. We found two patients with central hypersomnia and one patient with narcolepsy.
Table 4.
Polysomnographic, MSLT and actigraphic data of four patients with excessive sleepiness after COVID-19 infection
| Case one | Case two | Case three | Case four | |
|---|---|---|---|---|
| Polysomnographic data | ||||
| Total sleep time (h) | 350 | 380 | 488 | 462 |
| Sleep eficiency (%) | 87.9 | 77 | 94.5 | 94 |
| WASO (%) | 60 | 44.5 | 59.3 | 20 |
| Total microarousal index (number/h) | 21.9 | 11.7 | 21.4 | 8.4 |
| Total microarousal | 128 | 97 | 153 | 81 |
| N1 latency (min)/% of sleep | 0.2/13.7 | 66/0.8 | 5.9/0.4 | 0/6.4 |
| N2 latency (min)/% of sleep | 17.7/68.3 | 88/35.3 | 7.4/49.6 | 2/57.5 |
| N3 latency (min)/% of sleep | 38/5 | 102.5/36.7 | 20.9/25.3 | 27/53 |
| AHI total (events/h) | 7.7 | 4.9 | 9.9 | 8.7 |
| PLMI total (events/h) | 0.8 | 0 | 0.1 | 0.1 |
| Atonia in REM sleep | Y | Y | Y | Y |
| REM latency (min)/% of sleep | 240/13 | 81/27.2 | 58/24.7 | 164/21 |
| MSLT data | ||||
| SOREMPs (number) | 0 | 0 | 2 | 0 |
| Mean latency (min) | 1.5 | 5.9 | 6 | – |
| Actigraphic data | ||||
| Mean total daily sleep time (hours) | 11.6 | 13.2 | 7 | 8.4 |
PSG polysomnographic, RLS restless legs syndrome, WASO wake after sleep onset, N1 NREM 1, N2 NREM 2, N3 NREM 3, AHI apnea hypopnea index, PLMI periodic leg movement index, REM rapid eye movement, MSLT multiple sleep latency test, SOREMP sleep onset REM period, Y yes, N no
After diagnosis, all patients with persistent ES were treated with wakefulness promoters (modafinil in the first case, modafinil and methylphenidate in the second, and lisdexamfetamine in the third). All patients reported improvement with a decrease in Epworth scores and functional improvement.
Discussion
This study demonstrated a high prevalence of sleep symptoms in a cohort of long-term COVID-19 patients with persistent sleep disorders up to 18 months after infection. Insomnia was the most frequent sleep disorder in these patients. To the best of our knowledge, we have reported the first cases of central hypersomnia after COVID. One of our patients fulfilled clinical and polysomnographic criteria for narcolepsy. All patients with ES responded to wakefulness-promoting agents.
The neuropathological mechanisms in COVID-19 can be related to direct viral invasion, systemic inflammation, neuroinflammation, microvascular thrombosis and neurodegeneration [6, 25]. Autopsy series have demonstrated abnormalities in brain parenchyma and blood vessels possibly related to blood–brain barrier dysfunction leading to neuronal inflammation after hematogenous dissemination [26, 27]. Another possible pathway for the virus to reach the central nervous system (CNS) is viral invasion of the olfactory epithelium and propagation toward the brain through axonal transport [28, 29].
Prolonged latent inflammation, memory T-cell accumulation and decreased ability to respond to new antigens (markers of immunosenescence) might play a role in persistent dysfunction after COVID-19 [26]. Other proposed mechanisms for long COVID include glymphatic system impairment, with glymphatic congestion and reduced clearance of toxins and inflammatory mediators from the CNS [30].
A possible explanation for persistent sleep disorders after COVID-19 might be related to prolonged dysfunction of brainstem nuclei [31]. This dysfunction could be explained by a high concentration of ACE2 receptors (which are used by SARS-CoV-2 to enter cells) in the brainstem [31]. Some of these brainstem nuclei are involved in sleep-wakefulness regulation, such as the dorsal raphe nucleus, pedunculus-pontine nucleus, periaqueductal gray, and laterodorsally tegmental nucleus [31].
Chronic insomnia was the most common sleep disorder after COVID-19 infection, in line with other studies of long COVID-19 patients [32]. Social isolation, stress, anxiety, persistent inflammatory response, and corticosteroid use might justify the high prevalence in our sample. Corticosteroid use was significantly associated with sleep disorders in our cohort. It is possible that changes associated with the acute infection might have precipitated dysfunctional habits, such as excessive caffeine consumption or poor sleep hygiene, which may also contribute to insomnia in a multifactorial mechanism [12].
Anosmia is a common symptom in long COVID-19 patients. A possible mechanism for this association could be invasion of the olfactory epithelium and olfactory nerve with retrograde axonal transport to the hypothalamus via the stria terminalis. One of our patients with excessive sleepiness had olfactory bulb atrophy on MRI, reinforcing a possible association of olfactory nerve invasion and hypothalamic dysfunction resulting in sleep disorders.
Persistent cognitive symptoms were also more common in patients with sleep disturbances in our sample. It is possible that widespread central nervous system damage could result in cognitive dysfunction and sleep disturbances. Neuroinflammation, endothelial dysfunction, systemic inflammation, hypoxia, cerebrovascular disease and the presence of an APOE4 polymorphism might contribute to the coexistence of cognitive and sleep disturbances [32, 33]. Patients with ES could also suffer from impairment of attention, which could impact performance in other cognitive domains.
The association of excessive sleepiness and depression has been previously documented [34]. Depressive symptoms after COVID infection have been reported in up to 22% of patients [12]. In our cohort, depression was more common in patients with sleep disorders, reaching 50% in patients with ES. Depression in COVID patients might be associated with social isolation, uncertainties about the future, systemic inflammatory response and disability associated with long-term COVID symptoms and sequelae. The relationship between ES and depression is probably bidirectional, with poor sleep quality negatively affecting mood state and poor sleep quality and ES as a possible result of depression [12, 32].
We have reported three patients with documented central hypersomnia after COVID-19. One of them fulfilled the criteria for narcolepsy. All 3 patients were young and had no sleep disorders prior to COVID infection, suggesting a strong association with SARS-CoV-2 infection. To our knowledge, this is the first report of this association in the literature. Similar to other neurotropic viruses, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be the culprit for instigating the relapse of Klein Levin Syndrome, in a young patient, with no other precipitating factors [35].
During the H1N1 influenza pandemic of 2009, an increased incidence of narcolepsy was reported in China and Europe [36]. Large-scale vaccination for H1N1 was also associated with an increased risk of narcolepsy in children and young adults, particularly the Pandemrix vaccine (GlaxoSmithKline, Dresden, Germany) [37]. Narcolepsy was hypothesized to be the result of molecular mimicry, with CD4+ T cells cross-reactive to hypocretin fragments leading to hypothalamic damage [38].
In addition to the possibility of direct viral infection, hypersomnia after COVID might be related to immune and inflammatory hyperactivation resulting in hypothalamic infiltration by CD4+ and CD8+ T cells leading to neuronal damage [29]. There is also the possibility of molecular mimicry between viral antigens and hypocretin fragments leading to an immune response against hypocretinergic cells, similar to what has been described with H1N1 influenza infections [38].
Responses to treatment with wakefulness-promoting agents were observed in all cases, suggesting a similar response profile to other patients with central hypersomnia. On the other hand, 2 patients (50%) had self-limited symptoms that resolved after 6 months in one case and 15 months in another, implying reversible dysfunction of the sleep-wakefulness cycle in some cases.
The present study has some limitations. An initial selection bias resulted from a higher probability of patients with more severe symptoms participating in our study. Unfortunately, we were not able to perform PSG and MSLT and dose CSF hypocretin levels in all patients with sleep-related symptoms due to financial limitations. A few patients with ES did not undergo PSG and MSLT due to lack of consent. Nevertheless, we believe these limitations do not impact the clinical findings that expand central hypersomnia as a possible spectrum of long-onset COVID syndrome.
Conclusion
Sleep disorders are common in patients with long COVID syndrome, particularly insomnia. Chronic insomnia and central hypersomnia may expand the spectrum of post-COVID sleep disturbances. These patients seem to respond to the wakefulness promoter and may have a self-limited course in some cases. This study underscores the importance of sleep disorders in post-COVID patients. Further studies should address the possible pathologic mechanisms and clinical course of these conditions to understand their prognosis and guide their management.
Acknowledgements
The authors are grateful to the Brazilian National Council for Scientific and Technological Development (CNPq) for the funding of the productivity shcolarship of the author Pedro Braga Neto. We also would like to thank the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) for the funding of the author Pedro Braga Neto (88881.505364/2020-01).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Financial disclosure
We have nothing to disclose.
Ethical statement
Patients signed an informed consent form and allowed publication of these data.
Abbreviations
- AASM
American Academy of Sleep Medicine
- ACE2
Angiotensin Converting Enzyme 2
- AHI
Apnea hypopnea index
- APOE4
Apolipoprotein E4
- CA
California
- Cognitive Examination–Revised
Cognitive Examination–Revised
- CBC
Complete blood count
- TCD4
Thymus-derived lymphocytes CD4
- TCD8
Thymus-derived lymphocytes CD8
- CNS Central
Central nervous system
- CSF
Cerebrospinal fluid
- COVID-19
Corona Virus Disease 2019 caused by SARS-CoV-2
- DSM-V
Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition)
- EEG
Electroencephalogram
- ES
Excessive sleepiness
- H1N1
Influenza A
- MERS
Middle East Respiratory Syndrome
- MMSE
Mini-Mental State Examination
- MRI
Magnetic resonance imaging
- MSLT
Multiple sleep latency test
- NREM
Non–rapid eye movement
- PLMI
Periodic leg movement index
- PSG
Polysomnography
- REM
Rapid eye movement
- RLS
Restless legs syndrome
- RT-PCR
Reverse transcription polymerase chain reaction (this is the test used to detect a specific RNA)
- SARS
Severe Acute Respiratory Syndrome
- SARS-COV-2
Severe Acute Respiratory Syndrome coronavirus 2
- SOREMP
Sleep-onset rapid eye movement period.
- T4
Thyroxine
- TSH
Thyroid Stimulating Hormon
- WA
Washington
- WASO
Wake after sleep onset
Authors’ contributions
Conception and design of work: AEFM, PB-N and MAS-N; Acquisition, analysis, or interpretation of data and work: AEFM, DNO, DMT and JWLT; Drafting the work: AEFM, PRN, PB-N and MAS-N. All authors were involved in the critical review of the manuscript for important intellectual content. The author(s) read and approved the final manuscript.
Funding
The research was carried out with funding from the authors themselves. No contribution from other funds.
Availability of data and materials
All of the material is owned by the authors and no permissions are required.
All of the material are available for publication.
To consult and check the data of this study, contact alisamoura@gmail.com or pbraganeto@ufc.br
Declarations
Ethics approval and consent to participate
I inform that the research Central hypersomnia and chronic insomnia: expanding the spectrum of sleep disorders in long COVID syndrome - a prospective cohort study, was designed in accordance with what governs resolution n°466/2012 of the National Health Council (BRASIL, 2012).
All experimental protocols were approved by the Ethics Committee of the University Hospital Walter Cantídio (HUWC), with registration number 4,092,933. HUWC Research Ethics Committee is located on Coronel Nunes de Melo street, 1142 - Rodolfo Teófilo, phone: + 55–85- 3366-8589 (opening hours: 7:00–12:00 a.m. and from 13:00–15:30 p.m)– contact: cephuwc@huwc.ufc.br.
This study complies with the ethical principles of the Declaration of Helsinki. The free and informed consent form was applied.
The document was read and informed and agreed by the patient or legal representative, with registration, before the beginning of the protocol, ensuring respect for the patient’s freedom to refuse to participate or to withdraw their consent, at any stage of the research, without no penalty and without prejudice to their care, with, in addition, a guarantee of secrecy to ensure their privacy regarding the confidential data involved in the research.
Consent for publication
I grant publication of the article to BMC journal.
I confirm that I understand BMC Neurology is an open access journal that levies an article processing charge per articles accepted for publication.
Competing interests
I declare that the all authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alissa Elen Formiga Moura, Danilo Nunes Oliveira both authors have similar contributions to the article.
References
- 1.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE. https://www.nice.org.uk/guidance/ng188. Accessed 27 Sep 2022.
- 5.Koczulla AR, Ankermann T, Behrends U, Berlit P, Böing S, Brinkmann F, et al. S1 guideline post-COVID/long-COVID. Pneumol Stuttg Ger. 2021;75:869–900. doi: 10.1055/a-1551-9734. [DOI] [PubMed] [Google Scholar]
- 6.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, Nyirenda T, Friedman T, Gupta A, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15:e0243882. doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AM, Wong JGWS, McAlonan GM, Cheung V, Cheung C, Sham PC, et al. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry Rev Can Psychiatr. 2007;52:233–240. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- 9.Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2021;17:299–313. doi: 10.5664/jcsm.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Pérez O, Merino E, Leon-Ramirez J-M, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Inf Secur. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennings G, Monaghan A, Xue F, Mockler D, Romero-Ortuño R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;10:5913. doi: 10.3390/jcm10245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombardo MDM, Foppiani A, Peretti GM, Mangiavini L, Battezzati A, Bertoli S, et al. Long-term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open forum. Infect Dis. 2021;8:ofab384. doi: 10.1093/ofid/ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2022;17(2):173–191. doi: 10.1016/j.jsmc.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Gomes-Oliveira MH, Gorenstein C, Lotufo Neto F, Andrade LH, Wang YP. Validation of the Brazilian Portuguese version of the Beck depression inventory-II in a community sample. Braz J Psychiatry. 2012;34:389–394. doi: 10.1016/j.rbp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP, Almeida SA. Reliability of the Brazilian version of the geriatric depression scale (GDS) short form. Arq Neuropsiquiatr. 1999;57:421–426. doi: 10.1590/S0004-282X1999000300013. [DOI] [PubMed] [Google Scholar]
- 18.Nitrini R, Caramelli P, Bottino CM de C, Damasceno BP, Brucki SMD, Anghinah R. Diagnóstico de doença de Alzheimer no Brasil: avaliação cognitiva e funcional. Recomendações do Departamento Científico de Neurologia Cognitiva e do Envelhecimento da Academia Brasileira de Neurologia. Arq Neuropsiquiatr. 2005;63:720–727. doi: 10.1590/S0004-282X2005000400034. [DOI] [PubMed] [Google Scholar]
- 19.César KG, Yassuda MS, Porto FHG, Brucki SMD, Nitrini R. Addenbrooke’s cognitive examination-revised: normative and accuracy data for seniors with heterogeneous educational level in Brazil. Int Psychogeriatr. 2017;29:1345–1353. doi: 10.1017/S1041610217000734. [DOI] [PubMed] [Google Scholar]
- 20.Herrera E, Caramelli P, Silveira ASB, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16:103–108. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Brucki SMD, Nitrini R, Caramelli P, Bertolucci PHF, Okamoto IH. Sugestões para o uso do mini-exame do estado mental no Brasil. Arq Neuropsiquiatr. 2003;61:777–781. doi: 10.1590/S0004-282X2003000500014. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep med JCSM off Publ am Acad. Sleep Med. 2017;13:665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C, American Academy of Sleep Medicine . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 25.Muccioli L, Pensato U, Cani I, Guarino M, Cortelli P, Bisulli F. COVID-19-associated encephalopathy and cytokine-mediated Neuroinflammation. Ann Neurol. 2020;88:860–861. doi: 10.1002/ana.25855. [DOI] [PubMed] [Google Scholar]
- 26.Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordvig AS, Fong KT, Willey JZ, Thakur KT, Boehme AK, Vargas WS, et al. Potential neurologic manifestations of COVID-19. Neurol Clin Pract. 2021;11:e135–e146. doi: 10.1212/CPJ.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol (Berl) 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021;146:110469. doi: 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12:573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- 32.Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12:69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020;12:170. doi: 10.1186/s13195-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plante DT, Finn LA, Hagen EW, Mignot E, Peppard PE. Longitudinal associations of hypersomnolence and depression in the Wisconsin Sleep cohort study. J Affect Disord. 2017;207:197–202. doi: 10.1016/j.jad.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasrullah A, Javed A, Ashraf O, Malik K. Possible role of COVID-19 in the relapse of Klein-Levin syndrome. Respir Med Case Rep. 2021;33:101445. doi: 10.1016/j.rmcr.2021.101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecendreux M, Berthier J, Corny J, Bourdon O, Dossier C, Delclaux C. Intravenous immunoglobulin therapy in pediatric narcolepsy: a nonrandomized, open-label, controlled, longitudinal observational study. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2017;13:441–453. doi: 10.5664/jcsm.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mignot E, Black S. Narcolepsy risk and COVID-19. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2020;16:1831–1833. doi: 10.5664/jcsm.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latorre D, Kallweit U, Armentani E, Foglierini M, Mele F, Cassotta A, et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature. 2018;562:63–68. doi: 10.1038/s41586-018-0540-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the material is owned by the authors and no permissions are required.
All of the material are available for publication.
To consult and check the data of this study, contact alisamoura@gmail.com or pbraganeto@ufc.br


