Abstract
Vibrio parahaemolyticus is a marine bacterium known to be the leading cause of seafood gastroenteritis worldwide. A 46-kDa homodimer protein secreted by this microorganism, the thermostable direct hemolysin (TDH), is considered a major virulence factor involved in bacterial pathogenesis since a high percentage of strains of clinical origin are positive for TDH production. TDH is a pore-forming toxin, and its most extensively studied effect is the ability to cause hemolysis of erythrocytes from different mammalian species. Moreover, TDH induces in a variety of cells cytotoxic effects consisting mainly of cell degeneration which often leads to loss of viability. In this work, we examined the cellular changes induced by TDH in monolayers of IEC-6 cells (derived from the rat crypt small intestine), which represent a useful cell model for studying toxins from enteric bacteria. In experimental conditions allowing cell survival, TDH induces a rapid transient increase in intracellular calcium as well as a significant though reversible decreased rate of progression through the cell cycle. The morphological changes seem to be dependent on the organization of the microtubular network, which appears to be the preferential cytoskeletal element involved in the cellular response to the toxin.
Vibrio parahaemolyticus is a marine bacterium known to be one of the major causes of seafood gastroenteritis (15). Although the pathogenic mechanisms of this organism are not well understood, the thermostable direct hemolysin (TDH) secreted by V. parahaemolyticus has been proposed to be a major virulence factor involved in gastrointestinal disorders (12). TDH, the most extensively investigated pathogenic factor produced by the bacterium, is considered very important because of its possible association with the diarrheal disease. In fact, TDH-producing strains were initially distinguished from non-TDH-producing strains by testing for Kanagawa phenomenon, a beta-hemolysis detected by a special blood agar medium (Wagatsuma agar). A high percentage of strains of clinical origin but only 1 to 2% of strains from nonclinical sources resulted positive for this test, indicating the importance of TDH as a virulence factor of V. parahaemolyticus.
TDH is a homodimer protein with a molecular mass of 46 kDa, each peptide being composed of 165 amino acids (12). Among the various biological activities of TDH, hemolysis was one of the first recognized and remains the most extensively studied. As a pore-forming toxin (11), TDH causes a colloidal osmotic lysis (14) of erythrocytes from different mammalian species. The TDH receptor has been reported to be GT1 ganglioside (22), but recent studies produced conflicting results (12). In rabbit ileal mucosa, a role for GT1b as a putative receptor for the toxin has been suggested (18). TDH is cytotoxic to a variety of cells types (10, 21). The effects of the toxin on human amniotic membrane cells (FL cells) have been studied in detail and are characterized by loss of viability and by some morphological changes, such as the disappearance of microvilli from the cell surface, degeneration of the cytoplasm, and disintegration of the nucleus (19). Recently, the induction in a human embryonic cell line (Int407) of a Ca2+-independent cytotoxicity due to the hemolysin was also reported (24). This cytotoxicity is manifested mainly by damage of plasma membrane and lysosomes, as well as cellular degeneration in the form of large transparent blebs. In addition to its hemolytic and cytotoxic effects, TDH elicits lethal activity in small experimental animals (i.e., production of vascular permeability in rabbit skin; cardiotoxicity and enterotoxicity when tested in the rabbit ileal loop model) (20). It was recently shown that the interaction of TDH with the putative receptor in rabbit intestinal mucosa leads to an increased intracellular calcium concentration that in turn induces chloride secretion in a time- and dose-dependent fashion (18).
As suggested by the variety of pathological effects triggered by TDH, the effects of the toxin on nucleated cells probably involve more complex mechanisms than hemolysis. In this work, we examined the cellular changes induced by TDH in noncytotoxic conditions, using rat crypt small intestinal cell (IEC-6) monolayers as target cells. A rapid transient increase in intracellular calcium as well as a reversible decreased rate of progression through the cell cycle were evident upon exposure to TDH. Moreover, the microtubular network integrity and functionality appeared to be prerequisites for the induction of the morphological changes observed in this intestinal cell line. The possible link between the hemolytic activity of the toxin and the above-reported effects on IEC-6 cells is discussed.
MATERIALS AND METHODS
Cell lines.
IEC-6 cells (derived from normal rat small intestine; ATCC CRL 1592) were cultured at 37°C in the appropriate medium supplemented with 10% fetal calf serum (Flow Laboratories, Irvine, United Kingdom), 1% nonessential amino acids, 5 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Medium consisted of Dulbecco’s modified Eagle’s medium plus insulin (10 μg/ml).
Toxins and chemicals.
Kanagawa hemolysin (TDH), Hoechst 33258, propidium iodide, cytochalasin D, vinblastine, and taxol were purchased from Sigma Chemical Co. (St. Louis, Mo); fura-2AM, jasplakinolide, and NBD C6-ceramide were from Molecular Probes (Eugene, Oreg.). According to the manufacturer, TDH was purified as described by Cherwonogrodzky and Clark (4) to a degree of purity above 99%. The purity of TDH was checked on a 10-μl sample (corresponding to 5 hemolytic units [HU] of hemolysin) boiled for 5 min in 2× Laemmli sample buffer prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% acrylamide gel. A single band of about 23 kDa was detected after staining of protein with Coomassie brilliant blue, thus confirming the purity of the toxin (Fig. 1). One HU is defined by Sigma as the amount that causes 5% hemolysis of a 1% erythrocyte suspension in phosphate-buffered saline (PBS) at pH 7.0 after 2 h of incubation at 37°C followed by refrigeration for 12 to 24 h at 4°C (toxin activity, minimum of 400 U per mg of protein).
FIG. 1.
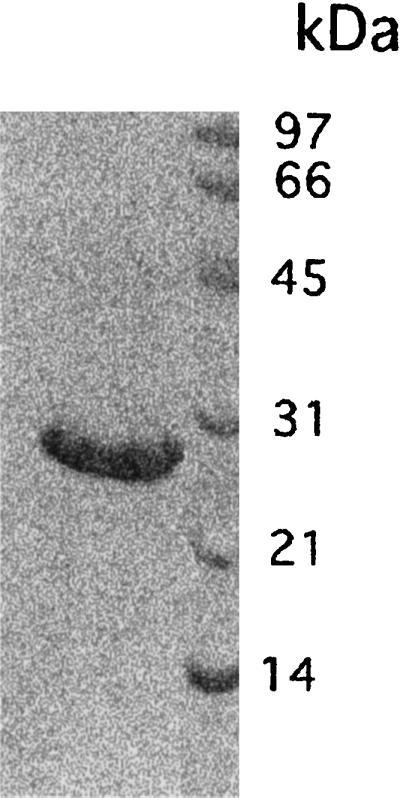
SDS-PAGE of TDH on a 12% acrylamide gel stained with Coomassie brilliant blue. A single band of about 23 kDa is detected, confirming the purity of the toxin used.
Cell treatments.
Twenty-four hours after seeding on glass coverslips in 24-well plates (initial inoculum, 5 × 104 cells/ml), cells were treated with TDH, directly added to the culture medium, for 2, 18, 24, 48, 72, and 96 h. The concentrations used for TDH ranged from 0.32 to 10 HU/ml (twofold dilutions). For all experiments, we used 2.5 HU of the toxin per ml, because this is the lower concentration causing morphological changes (cell shrinkage and filopodium stem) in IEC-6 cells within 18 h. This dose caused no cytotoxicity. Cells were challenged for 30 min before the addition of TDH with drugs perturbing microfilament and microtubule organization: cytochalasin D (60 ng/ml) and jasplakinolide (10 nM) for the microfilament system; taxol (1.5 μM) and vinblastine (1.3 μM) for the microtubular apparatus. To analyze cell viability, trypsinized cells were resuspended in PBS containing the trypan blue dye (1:1). Dead cells were counted in a Neubauer chamber.
Fluorescence microscopy.
Cells were fixed with 3.7% formaldehyde in PBS (pH 7.4) for 10 min at room temperature. After being washed in the same buffer, the cells were permeabilized with 0.5% Triton X-100 (Sigma) in PBS (pH 7.4) for 10 min at room temperature. Cells were stained with Hoechst 33258 or with fluorescein isothiocyanate-phalloidin (both compounds were from Sigma). After 30 min at 37°C, cells were washed and coverslips were mounted with glycerol-PBS (2:1). For detection of microtubules, permeabilized cells were stained with the appropriate primary antibody (antitubulin from Sigma) and, after being washed in PBS, incubated with the secondary fluorescein isothiocyanate-labeled antibody for an additional 30 min. Cells were washed, mounted in glycerol-PBS (2:1), and analyzed with a Nikon Optiphot fluorescence microscope.
For detection of the Golgi apparatus, control cells or cells treated with TDH were incubated in the culture medium with NBD C6-ceramide for 20 min at 37°C. After incubation, cells were fixed in formaldehyde 3.7% in PBS for 10 min. After washing, coverslips were mounted as described above.
SEM.
Cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at room temperature for 20 min. Following postfixation in 1% OsO4 for 30 min, cells were dehydrated through graded ethanols. For scanning electron microscopy (SEM), cells were critical point dried in CO2 and gold coated by sputtering, and the samples were examined with a Cambridge 360 scanning electron microscope.
Cell cycle analysis.
For DNA analysis, performed with propidium iodide (40 μg/ml), the cells were fixed and permeabilized as described by Darzynkiewicz et al. (5). At least 20,000 events for all samples were acquired on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) equipped with a 488-nm argon laser and recorded by a Hewlett-Packard computer using Lysys II software (Becton Dickinson). The percentage of cells each phase of the cell cycle was obtained by CellFT software analysis. The median values were estimated by three different analytical mathematic models: RIFT, SFIT, and SORB.
Intracellular calcium measurement.
IEC-6 cells grown on 35-mm-diameter petri dishes were loaded with 5 μM fura-2AM in Ringer buffer (22 mM CaCl2 · H2O, 4 mM KCl, 147 mM NaCl) for 50 min at room temperature. Fura-2AM was then removed, and all cell measurements were performed in the same buffer. After 5 min of control recording, TDH was directly added to the buffer to obtain a final concentration of 2.5 HU/ml. The intracellular calcium was measured within the first hour of exposure to the toxin. To evaluate the influence of extracellular calcium, the same experiments were performed by adding 1 mM EGTA to the Ringer buffer. Fura-2AM experiments were performed with a Hamamatsu Argus 50 fluorescence-measuring system using 340 and 380 filters with a sampling interval of 12 s.
RESULTS
TDH influences calcium homeostasis in IEC-6 cells.
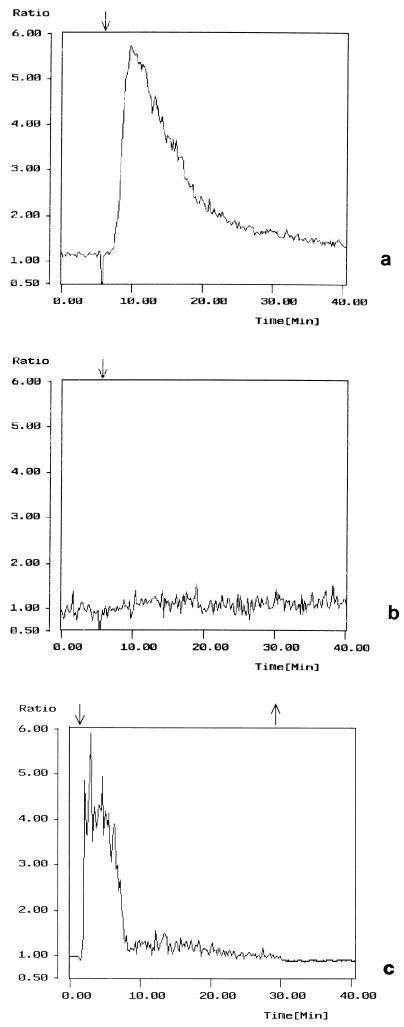
In the various cell models studied so far, the first response to TDH is a relatively fast increase in intracellular calcium (18, 23, 24). To further explore this early response to the hemolysin, we performed experiments using an intestinal cell line (IEC-6) derived from normal crypt cells of the rat small intestine. As already reported (7–9), these cells may represent a useful model for studying toxins produced by enteric bacteria. The intracellular calcium level was measured by the fluorescent probe fura-2AM after exposure to different toxin concentrations. A TDH dose as low as 1 HU/ml had not effect on the cytosolic Ca2+ level (data not shown), whereas a dose of 2.5 HU/ml caused a rapid (within 2 to 5 min) increase in intracellular calcium concentration followed by a decrease to nearly the basal level (Fig. 2a). Higher doses (5 HU/ml or more) provoked an irreversible Ca2+ increase which culminated in cell lysis (data not shown). The TDH-induced calcium response was abolished by preexposing cells for 20 min to the calcium chelator EGTA, implicating the extracellular calcium (and not the cytosolic stores) as crucial for the expression of this effect (Fig. 2b). Interestingly, if cells were washed after the change in calcium concentration due to TDH exposure, the cytosolic Ca2+ level decreased to the initial concentration (Fig. 2c).
FIG. 2.
TDH influences calcium homeostasis in IEC-6 cells. (a) Cells treated with 2.5 HU of TDH per ml; (b) cells exposed to 1 mM EGTA for 20 min before TDH treatment; (c) cells exposed to TDH for 30 min and then washed. Graphs show the first 40 min of the experiments. After the rapid increase in intracellular calcium level, the decrease occurs between 5 and 25 min (a and c). The y axis represents the ratio 340/380. The negative deflection at about 5 min represents an artifact due to the closure of the shutter to expose the dish and check whether the TDH was added correctly. The rise in cytosolic Ca2+ level is transient, totally reversible, and prevented by EGTA. ↓, TDH addition; ↑, washing.
TDH induces time- and dose-dependent changes in IEC-6 cells.
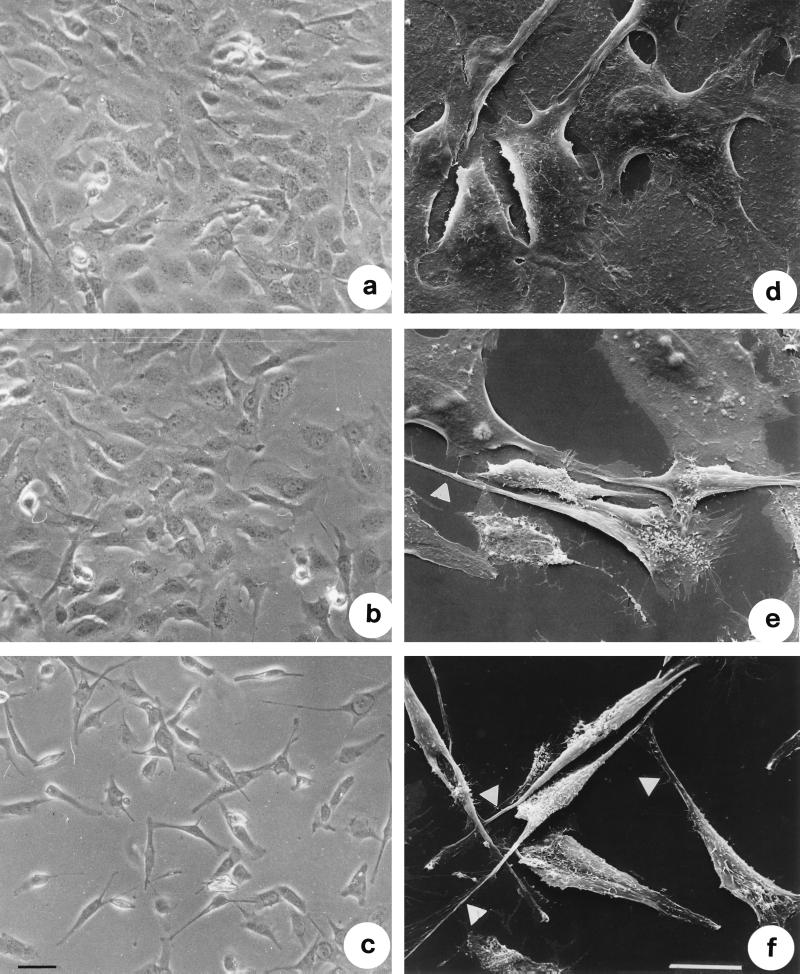
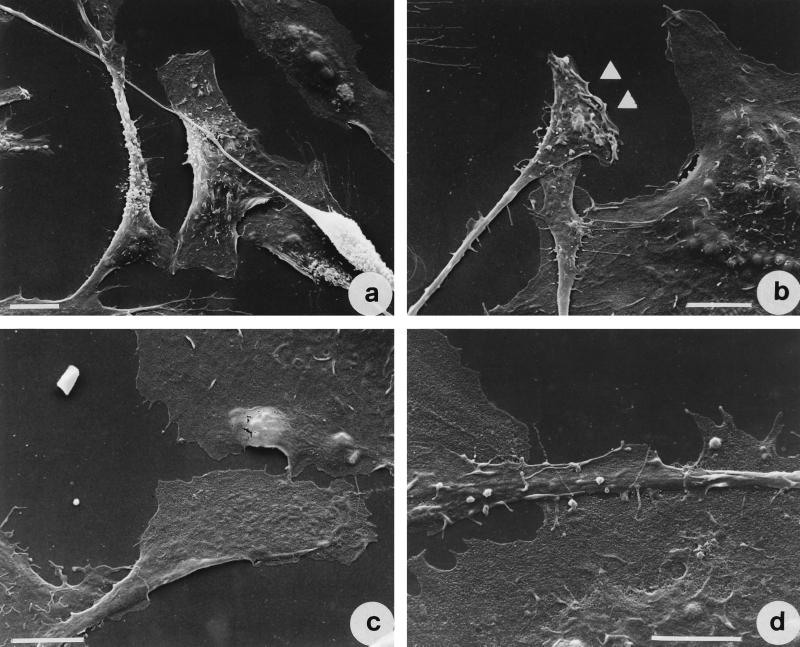
We then monitored the fate of IEC-6 cells after TDH exposure for different time intervals. When analyzed by phase-contrast microscopy, cells exposed to 2.5 HU of TDH per ml for 2 h showed a clear shrinkage of the cell body and formation of filopodia (Fig. 3b). After 18 h of treatment both modifications were clearly detectable, every cell showing one or two filopodia (Fig. 3c). As viewed by SEM, while control cells appeared polygonal (Fig. 3d), cells exposed to 2.5 HU of TDH per ml for 2 h (Fig. 3e) up to 18 h (Fig. 3f) showed the shrinkage and the appearance of filopodia which protruded from the cell body via a little ruffle. As viewed in detail in Fig. 4, shrunken cells exhibited one or two filopodia capable of overlapping neighboring cells (Fig. 4a). These protrusions always ended with an enlarged region (Fig. 4 b to d), which is probably responsible for the movement and always proved to be rich in F-actin (see below).
FIG. 3.
TDH induces morphological effects in IEC-6 cells. Shown are phase-contrast images of control cells (a) and cells treated with 2.5 HU of TDH per ml for 2 (b) and 18 (c) h and scanning electron micrographs of control cells (d) and cells exposed to 2.5 HU of TDH per ml for 2 (e) and 18 (f) h all at the same magnification. Note the extrusion of thin filopodia from the cell body of cells exposed to the hemolysin (arrows). Bars represent 10 μm.
FIG. 4.
Scanning electron micrographs of IEC-6 cells treated with 2.5 HU of TDH per ml for 18 h. Note the small lamellipodia at the end of filopodia (arrows). Bar represents 10 μm.
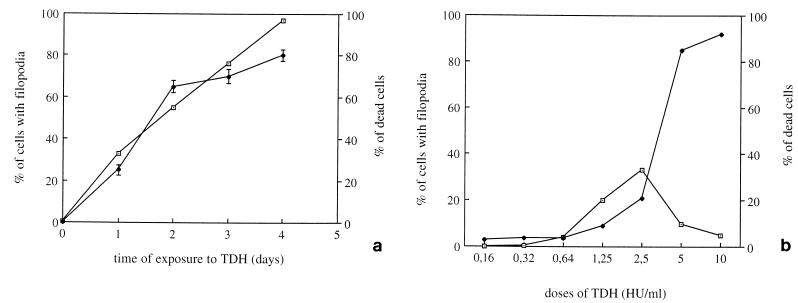
A time course study showed no other morphological modification, even if cells were exposed to the toxin for 96 h, although at this time the percentage of cells exhibiting filopodia increased up to 90% (Fig. 5a). Cytotoxicity, defined as the percentage of dead cells, increased over time of exposure to TDH (Fig. 5a). However, upon overnight challenge with the toxin (the time of treatment used for all experiments), only about 20% of cells were killed (Fig. 5a). TDH-induced effects were also dose dependent since after 18 h of exposure to the hemolysin, concentrations higher than 2.5 HU/ml were toxic to cells and doses lower than 0.32 HU/ml induced only slightly detectable alterations (Fig. 5b).
FIG. 5.
TDH-induced effects in IEC-6 cells are dose and time dependent. (a) Percentages of cells with filopodia and of dead cells after various times of exposure to 2.5 HU of TDH per ml; (b) dose dependence of TDH-induced cellular effects (filopodium formation and cell death), detectable after 18 h of exposure to the toxin. The results reported as percentages (± standard deviations) of cells with filopodia (⊡) or of dead cells (⧫) (as detected by trypan blue) with respect to the total number of cells counted, are from three different experiments in each of which at least 500 cells (randomly chosen) were counted.
TDH reversibly influences growth but does not induce cell cycle arrest in IEC-6 cells.
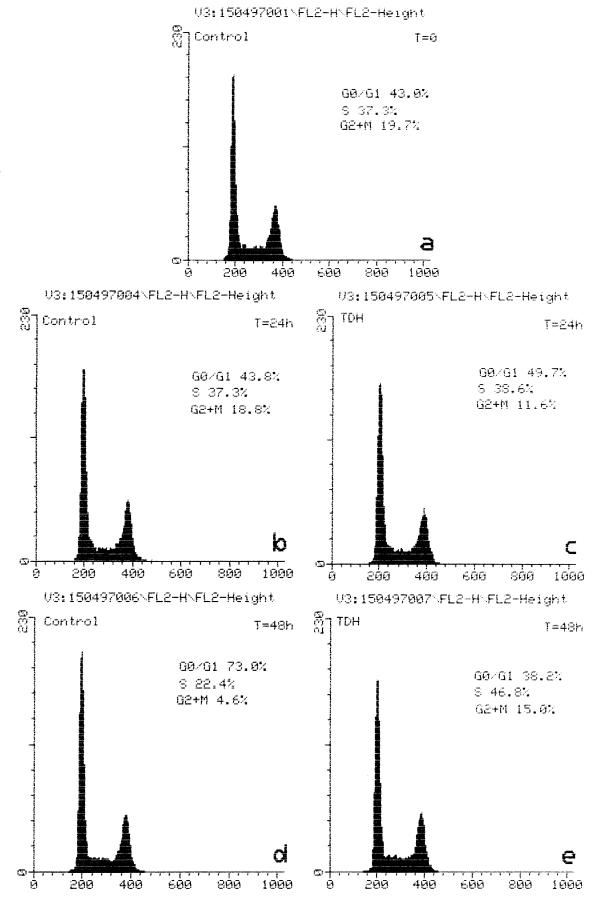
Another typical feature of the TDH-induced effect was the ability to lower the rate of progression through the cell cycle. When IEC-6 cells (7.5 × 105/ml) were treated with the hemolysin for 24 h, their number was only slightly less than in the control culture: (5.8 ± 0.872) × 105/ml versus (9.86 ± 0.153) × 105/ml. After prolonged (48-h) exposure to TDH, the number of treated cells decreased to (3.9 ± 0.513) × 105/ml (probably because of the decreased rate of progression through the cell cycle), whereas control cells reached confluence ([15.3 ± 0.40] × 105/ml). Cell cycle distribution was been determined by measuring the DNA content by flow cytometry. Cells exposed to 2.5 HU of the toxin per ml for 24 h (Fig. 6c) exhibited a G2/M:G1 ratio comparable to that of untreated cells (Fig. 6b). By contrast, after 48 h (Fig. 6d and e), the percentage of cells in S phase was considerably higher in TDH-treated (Fig. 6e) than control (Fig. 6d) cells. At this time, in fact, only small percentages of untreated cells were in S (22.4%) and G2 + M (4.6%) phases, most of them being in G0/G1 phase (73.0%). This arrest of growth in untreated cells was probably due to the contact inhibition caused by the confluent status of the cells, which were in plateau after 48 h of culture. On the other hand, cells exposed to TDH were mostly in S phase (Fig. 6e), this toxin inducing a significant though reversible slowdown of the entry in G2/M phase.
FIG. 6.
TDH influences the cell cycle of IEC-6 cells. Histograms show DNA content of control cells (a, b, and d) and cells treated with 2.5 HU of TDH per ml (c and e) for the time periods indicated. Note the augmented percentage of cells in S phase after 2 days of exposure to TDH.
All of the above-described morphological and functional effects induced by TDH could be completely reversed independently of the length of treatment tested. In fact, after washing and incubation in fresh medium for at least 18 h, cells lose filopodia and begin to divide, thus becoming able to reach confluence (data not shown).
TDH-induced cytoskeletal changes in IEC-6 cells.
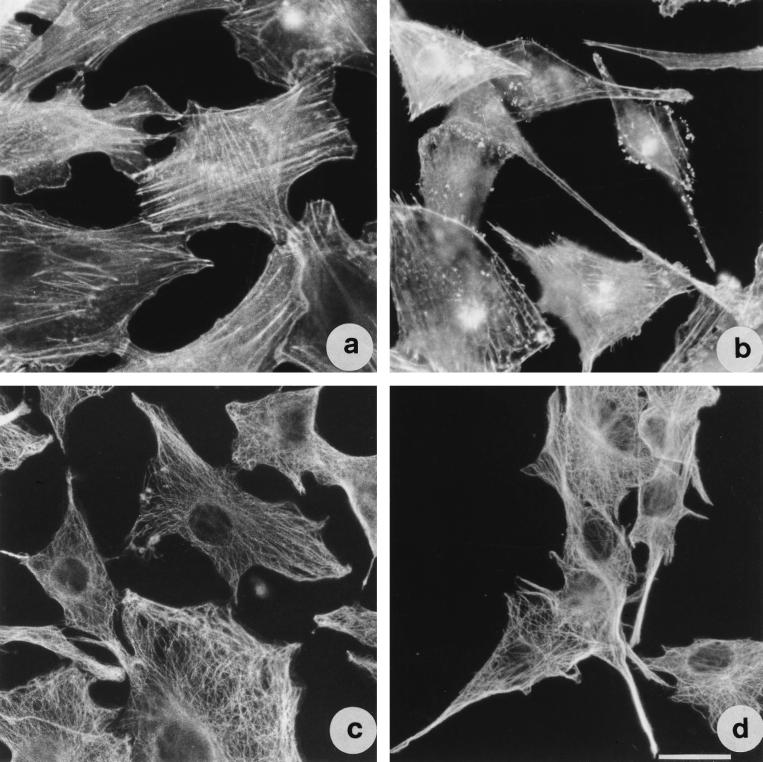
Observed by fluorescence microscopy, control IEC-6 cells stained with phalloidin for F-actin detection presented well-organized stress fibers (Fig. 7a). In cells treated with TDH for 2 h, the organization of the microfilament system was not detectable although the general morphology of the cell remained unaffected (data not shown). The organization of this system was then recovered, and after 18 h of TDH exposure, cells exhibited long, thin filopodia sustained by actin filaments (Fig. 7b). In the terminal part of these protrusions, actin-rich ruffles were evident. With respect to the microtubular apparatus (Fig. 7c and d), no difference in the dynamic of TDH affection was detected with respect to the microfilament system, cells first losing tubulin organization and then recovering it after 18 h of treatment. Noteworthy was the presence of microtubules within the TDH-induced filopodia (Fig. 7d).
FIG. 7.
TDH provokes changes in actin and tubulin cytoskeletal networks in IEC-6 cells. Shown are fluorescence micrographs of cells stained for detection of F-actin (a and b) and tubulin (c and d). (a and c) Control cells; (b and d) cells exposed to 2.5 HU of TDH per ml for 18 h. Both cytoskeletal elements are present in the filopodia. Bar represents 10 μm.
TDH-induced changes are controlled by the microtubular apparatus.
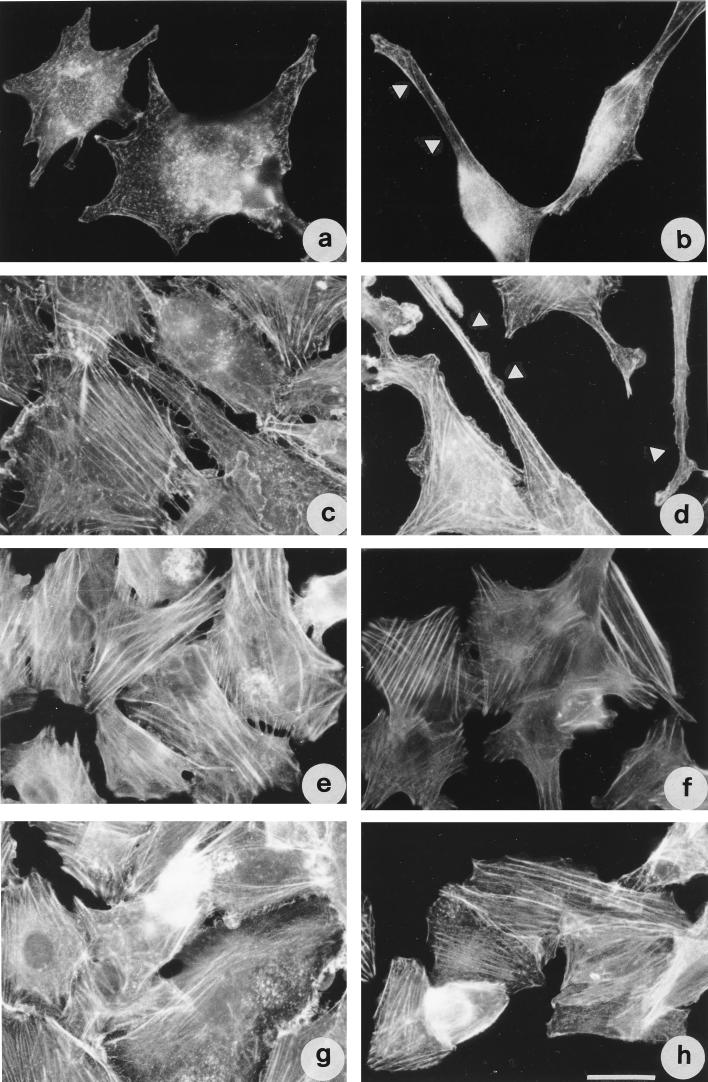
Since TDH caused the rearrangement of both microfilaments and microtubules, we wondered if a perturbation of these cytoskeletal systems could alter the cell response to the toxin. We therefore used agents known to either depolymerize or stabilize such elements, thus allowing study of the role played by these systems in response to TDH. When IEC-6 cells were pretreated with cytochalasin D (Fig. 8a) at a dose which, although disrupting the microfilament system, allows the maintenance of cell shape, or with jasplakinolide (Fig. 8c), which stabilize the microfilament system (3), addition of TDH still provoked the formation of some protrusions upon 18 h of exposure (Fig. 8b and d). By contrast, when cells were first exposed to either vinblastine or taxol (Fig. 8e and g), an agent known to depolymerize or stabilize microtubules, respectively, and subsequently to TDH for 18 h, no filopodia were detected (Fig. 8f and h), cells maintaining the shape of untreated monolayers.
FIG. 8.
Microtubule perturbants inhibit TDH-induced morphological changes in IEC-6 cells as viewed by fluorescence detection of F-actin. Cells were treated with cytochalasin D (a and b), jasplakinolide (c and d), vinblastine (e and f), or taxol (g and h). Cells in panel b, d, f, and h were subsequently exposed to TDH for 18 h. Note that only the microtubule-perturbing drugs are able to inhibit the formation of filopodia. Arrows indicate filopodia. Bar represents 10 μm.
Nuclear shape and Golgi apparatus positioning, both microtubule dependent, are altered in TDH-treated cells.
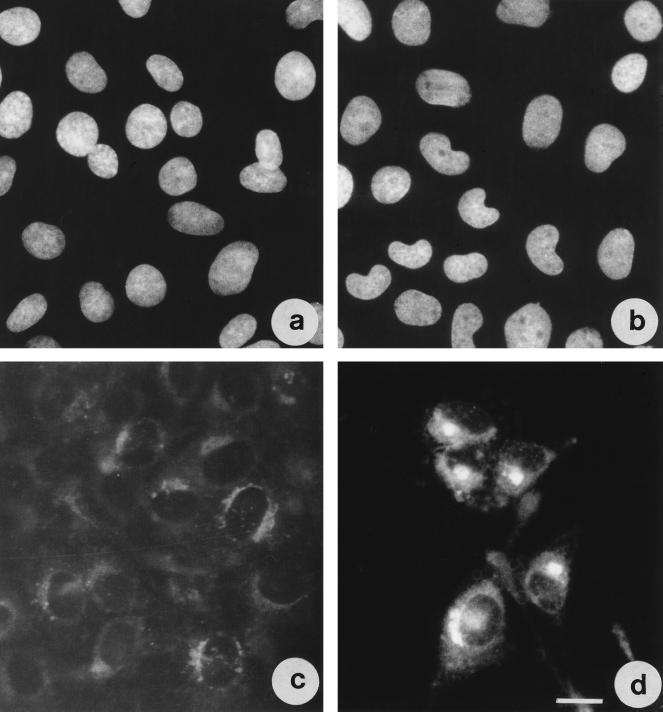
As viewed by Hoechst staining in TDH-treated cells, although chromatin distribution was unaffected, the nuclei showed a bean-like appearance (Fig. 9a and b). The Golgi apparatus appeared localized around the nucleus in control cells (Fig. 9c), whereas overnight TDH-treated cells exhibited a clear shrinkage and condensation (Fig. 9d), with the Golgi apparatus localized mainly in the nuclear cleft. After IEC-6 cells were washed, the Golgi structure reverted to that of control cells (data not shown).
FIG. 9.
Nuclear shape and Golgi apparatus positioning are altered in TDH-treated IEC-6 cells. Shown are fluorescence micrographs of cells stained with Hoechst 33258 (a and b) or with NBD C6-ceramide (c and d) for Golgi detection. (a and c) Control cells; (b and d) cells exposed to TDH for 18 h. Bar represents 10 μm.
DISCUSSION
In this work, we investigated the effect of TDH on IEC-6 cells, a normal cell line derived from rat crypt intestine. Our results showed that in this cell line, the first response to the hemolysin was an increase of intracellular calcium within 2 to 5 min after the addition of TDH in the extracellular medium. EGTA was capable of inhibiting this cellular response, indicating the extracellular calcium, not the cytosolic stores, as a source of calcium ions. This permeabilization to calcium ions could result from the formation of pores on the cell membrane. In erythrocytes, TDH has been reported to acts as a pore-forming toxin (11, 13, 23) causing membrane permeabilization and colloidal osmotic lysis. However, although TDH has been reported to induce calcium influx in cells other than erythrocytes (10, 18, 24), formation of pores in such cell lines has not been reported. Thus, we cannot rule out the possibility that in our cell model, TDH acts extracellularly, binding to a membrane receptor and transducing a signal which, in turn, leads to the opening of calcium channels. We may speculate that in IEC-6 cells, TDH acts in a different way depending on the toxin concentration. In particular, it could cause cell lysis at high concentrations, acting as a pore-forming toxin, but simply bind to a membrane receptor at low concentrations, thus having less deleterious effect on cells.
To our knowledge, all of the morphological changes due to the hemolysin reported so far for cells other than erythrocytes were represented by degeneration of the cells which, in most cases, resulted in loss of viability (19, 24). By contrast, IEC-6 cells responded to TDH initially with an increase in cytosolic calcium; when exposed to the hemolysin for a longer period of time, they underwent reversible morphological alterations consisting of mainly filopodium formation. Reversibility has also been reported to occur in mouse myocardial and melanoma cells (10), but only if cells were washed and incubated in TDH-free medium not later than after 2.5 min of hemolysin exposure. In contrast, in our model it was possible to revert all observed effects irrespective of the length of treatment.
The microfilament system is known to be a preferential target of bacterial protein toxins (2), while the tubulin has been reported to be modified by only a few bacterial toxins and always as a secondary target (6). While both systems, microtubules and microfilaments, were present in TDH-induced filopodia, our results showed that the process of filopodium formation required the integrity of the microtubule network only. Interestingly, Abrami and coworkers (1) reported that the aerolysin produced by Aeromonas hydrophila, a toxin which forms pores on cell membranes (for a review, see reference 17), induces vacuolation of the endoplasmic reticulum in a tubulin-dependent fashion. TDH shares certain properties with the aerolysin other than the tubulin-dependent morphological alteration in mammalian nucleated cells: hemolytic activity, formation of pores, secretion of the protein as a dimer, and molecular mass (46 kDa for TDH and 51 kDa for the aerolysin). These characteristics may suggest that TDH and aerolysin belong to the same group of toxins.
Another feature characteristic of IEC-6 cells exposed to TDH was a decreased rate of progression through the cell cycle. While control cells actively divided and grew in number, TDH-treated cells showed no increase in cell number with respect to the initial seeding. This is in accordance with the observation that the hemolysin, although it allows progression of cells through the cell cycle, delays entry into the G2/M phase. Moreover, we never observed in IEC-6 cells exposed to TDH the extensive Golgi fragmentation which aids the partitioning process occurring as cells enter M phase (16, 25); the Golgi apparatus always appeared well compacted and localized in the nuclear cleft. As for morphological changes, the involvement of tubulin may be the common feature which influences the cellular activities perturbed by TDH.
As a conclusion taking into account all of the above-reported findings, we tentatively propose the following chain of events. The first cellular response evoked by TDH in IEC-6 cells is a transient raise in calcium level, which accounts for the hemolytic activity of the toxin. Since a permanent increase in cytosolic calcium is not compatible with survival, presumably cells are able to actively and rapidly extrude ions. If TDH remains in the medium, cells need energy to continuously pump out calcium, which would probably induce cell stress phenomena. Signs of such a cellular response are the extrusion of filopodia, typical of starved cells deprived of sufficient nutrients, and the marked decreased rate of progression through the cell cycle. Once TDH is removed from the medium, cells rapidly recover their growth characteristics and morphology, exhibiting no apparent damage to the original cell shape and physiology. Speculatively, the self-limiting diarrheal form of disease caused by V. parahaemolyticus may be reconducted to the above-reported cellular response observed in cultured intestinal cells.
ACKNOWLEDGMENT
This work was partly funded by grant 97.01187.PF49 from the Italian National Research Council Targeted Project “Biotechnology,” Subproject 2.
REFERENCES
- 1.Abrami L, Fivaz M, Glauser P E, Parton R G, van der Goot F G. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boquet P, Munro P, Fiorentini C, Just I. Toxins from anaerobic bacteria: specificity and molecular mechanisms of action. Curr Opin Microbiol. 1998;1:66–74. doi: 10.1016/s1369-5274(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 3.Budd M, Senderowicz A, Sausville E, Duncan K, Korn E. Jasplakinolide, a cytotoxic natural product, induces action polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 4.Cherwonogrodzky J W, Clark A G. The purification of the Kanagawa haemolysin from Vibrio parahaemolyticus. FEMS Microbiol Lett. 1982;15:175. . (Abstract.) [Google Scholar]
- 5.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 6.Donelli G, Fiorentini C. Bacterial protein toxins acting on the cell cytoskeleton. Microbiologica. 1994;17:345–362. [PubMed] [Google Scholar]
- 7.Fasano A, Fiorentini C, Donelli G, Kaper J B, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum S E. Zonula occludens toxin (ZOT) modulates tight junctions through protein kinase C-dependent actin reorganization in vitro. J Clin Investig. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorentini C, Donelli G, Nicotera P, Thelestam M. Clostridium difficile toxin A elicits Ca2+-independent cytotoxic effects in cultured normal rat intestinal crypt cells. Infect Immun. 1993;61:3988–3993. doi: 10.1128/iai.61.9.3988-3993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorentini C, Fabbri A, Falzano L, Fattorossi A, Matarrese P, Rivabene R, Donelli G. Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect Immun. 1998;66:2660–2665. doi: 10.1128/iai.66.6.2660-2665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goshima K, Owaribe K, Yamanaka H, Yoshino S. Requirement of calcium ions for cell degeneration with a toxin (vibriolysin) from Vibrio parahaemolyticus. Infect Immun. 1978;22:821–832. doi: 10.1128/iai.22.3.821-832.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda T, Ni Y, Miwatani T, Adachi T, Kim J. The thermostable direct hemolysin of Vibrio parahaemolyticus is a pore forming toxin. Can J Microbiol. 1992;38:1175–1180. doi: 10.1139/m92-192. [DOI] [PubMed] [Google Scholar]
- 12.Honda T, Iida T. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct hemolysin and related hemolysins. Rev Med Microbiol. 1993;4:106–113. [Google Scholar]
- 13.Huntley J S, Hall A C, Sathyamoorthy V, Hall R H. Cation flux studies of the lesion induced in human erythrocyte membranes by the thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1993;61:4326–4332. doi: 10.1128/iai.61.10.4326-4332.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huntley J S, Hall A C. Aspects of the haemolytic reaction induced by Kanagawa haemolysin of Vibrio parahaemolyticus. Toxicon. 1994;32:1397–1412. doi: 10.1016/0041-0101(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 15.Janda J M, Powers C, Bryant R G, Abbott S L. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp Clin. Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucocq J M, Pryde J G, Berger E G, Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J Cell Biol. 1987;104:865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker M W, van der Goot F G, Buckley J T. Aerolysin—the ins and outs of a model channel-forming toxin. Mol Microbiol. 1996;19:205–212. doi: 10.1046/j.1365-2958.1996.355887.x. [DOI] [PubMed] [Google Scholar]
- 18.Raimondi F, Kao J P, Kaper J B, Guandalini S, Fasano A. Calcium-dependent intestinal chloride secretion by Vibrio parahaemolyticus thermostable direct hemolysin in a rabbit model. Gastroenterology. 1995;109:381–386. doi: 10.1016/0016-5085(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai J, Honda T, Jinguji Y, Arita M, Miwatani T. Cytotoxic effect of the thermostable direct hemolysin produced by Vibrio parahaemolyticus on FL cells. Infect Immun. 1976;13:876–883. doi: 10.1128/iai.13.3.876-883.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Methods Enzymol. 1988;165:189–193. doi: 10.1016/s0076-6879(88)65029-4. [DOI] [PubMed] [Google Scholar]
- 21.Takeda Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol Ther. 1983;19:123–146. doi: 10.1016/0163-7258(82)90044-4. [DOI] [PubMed] [Google Scholar]
- 22.Takeda Y, Takeda T, Honda T, Miwatani T. Inactivation of the biological activities of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside GT1. Infect Immun. 1976;14:1–5. doi: 10.1128/iai.14.1.1-5.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang G Q, Iida T, Yamamoto K, Honda T. A mutant toxin of Vibrio parahaemolyticus thermostable direct hemolysin which has lost hemolytic activity but retains ability to bind to erythrocytes. Infect Immun. 1994;62:3299–3304. doi: 10.1128/iai.62.8.3299-3304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang G Q, Iida T, Yamamoto K, Honda T. Ca+2-independent cytotoxicity of Vibrio parahaemolyticus thermostable direct hemolysin (TDH) on Intestine 407, a cell line derived from human embryonic intestine. FEMS Microbiol Lett. 1995;134:233–238. doi: 10.1111/j.1574-6968.1995.tb07943.x. [DOI] [PubMed] [Google Scholar]
- 25.Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]