Abstract
Study Objectives
To examine the longitudinal association between probable insomnia status and both subjective and objective memory decline in middle-aged and older adults.
Methods
26 363 participants, ≥45 years, completed baseline and follow-up (3 years after baseline) self-reported evaluations of sleep and memory, and neuropsychological testing in the following cognitive domains: memory, executive functions, and psychomotor speed. Participants were categorized as having probable insomnia disorder (PID), insomnia symptoms only (ISO), or no insomnia symptoms (NIS), based on sleep questionnaires. Participants were further grouped based on their sleep change over time. Prospective odds of self-reported memory worsening were assessed using logistic regression, and associations between insomnia and cognitive performance were assessed via linear mixed-effects modeling, adjusted for demographic, lifestyle, and medical factors.
Results
An increased odds (OR 1.70; 95% CI 1.29–2.26) of self-reported memory worsening was observed for NIS participants at baseline who developed PID at follow-up compared to those who developed ISO or remained NIS. Additionally, participants whose sleep worsened from baseline to follow-up (i.e. transitioned from NIS to ISO, ISO to PID, or NIS to PID) displayed increased odds (OR 1.22; 95% CI 1.10–1.34) of subjective memory worsening at follow-up compared to those who remained insomnia-free or improved their sleep. There were no significant associations between the development of PID or worsening sleep and performance on neuropsychological tests.
Conclusions
These findings of an increased odds for subjective memory decline in middle-aged and older adults with insomnia disorder suggest insomnia may be an important target for early interventions addressing age-related cognitive decline.
Keywords: insomnia, cognition, memory, aging, cohort, CLSA
Statement of Significance.
These findings demonstrate an increased odds of experiencing subjective memory decline in middle-aged and older adults who developed insomnia disorder, compared to adults who developed insomnia symptoms alone or no insomnia symptoms. Subjective memory complaints often precede the onset of mild cognitive impairment and dementia; therefore, insomnia disorder may contribute to the early stages of cognitive decline. Clinical management of insomnia in middle-aged and older adults thus may have implications for reducing the risk of cognitive decline.
Introduction
Insomnia disorder is characterized by difficulties initiating or maintaining sleep, or early morning awakenings, accompanied by daytime impairment and poor self-reported quality of sleep [1]. It is one of the most prevalent sleep disorders, accounting for about 10% of the adult population [2, 3]. Moreover, nearly one-third of adults experience insomnia symptoms without impairments of daytime functioning and do not fulfill all criteria for an insomnia disorder diagnosis [2, 3].
Insomnia has been shown to be associated with cognitive impairment across multiple domains [4–10]. Concurrently, cognitive changes occurring with normal aging have also been well documented [11, 12]. Sleep quality also declines with age, and the link between sleep and age-related cognitive decline has been previously established [13]. In our previous cross-sectional study, using a large dataset of a comprehensive cohort study [14], we examined the cognitive performance of middle-aged and older adults with probable insomnia disorder (PID) based on questions aligned with DSM-5 criteria. In that study, we found that PID was associated specifically with poorer memory performance compared to those who experience insomnia symptoms only (ISO) or no insomnia symptoms (NIS), after controlling for age-related demographic, lifestyle, and medical outcomes [15]. Yet, the predictive role of insomnia disorder in relation to subsequent memory decline in later life remains unclear. Several longitudinal studies have shown links between sleep complaints (e.g. sleep duration, self-reported sleep quality, snoring) and the increasing risk of cognitive decline or dementia in middle-aged and older adults [16–22]. However, the longitudinal associations between memory and insomnia disorder in older adults remain to be addressed since earlier studies only evaluated broad sleep measures (e.g. sleep duration, overall self-reported sleep quality) without a probable clinical diagnosis.
In older individuals, the presence of self-reported “subjective” memory complaints (SMC) (e.g. a complaint of decreased memory performance) is associated with an elevated risk of eventual progression to mild cognitive impairment (MCI) or dementia (~40.7% over 4-years) [23, 24]. Such conversion to MCI and dementia in individuals with SMC may occur progressively over 10–15 years, depending on factors such as age and the presence of other determined dementia risk factors, including sleep quality [25]. Additionally, memory performance decline assessed through neuropsychological testing, followed by a decline in other cognitive domains has been shown to often precede the clinical manifestations of dementias such as Alzheimer’s disease [26, 27]. Hence, evaluating the association between insomnia disorder and the presence of SMC or objective memory impairments in older adults is important to capture risk factors that may be involved in the earliest stages of cognitive decline.
Therefore, the primary aim of this study was to investigate the longitudinal relationship between (1) the development of PID and the emergence of subjective and objective memory decline in middle-aged and older adults, and (2) the trajectory of insomnia symptoms (i.e. worsening or improving) and the emergence of subjective and objective memory decline. Additionally, an exploratory aim of this study was to establish whether a longitudinal association exists between the progression of no insomnia symptoms to PID and objective deficits across other cognitive domains. We assessed these relationships while controlling for age-related demographic, lifestyle, and medical factors. We hypothesized that we would observe higher proportions of subjective and objective memory decline in adults who developed PID at follow-up, compared to those who remained without insomnia, and that higher proportions of memory decline would also be observed in adults who experienced worsening of their sleep quality (i.e. developing ISO or PID), compared to those whose sleep improved over the same interval.
Methods
Study design and participants
The sample was derived from the 30 097 participants who completed the baseline assessments of the comprehensive cohort from the Canadian Longitudinal Study on Aging (CLSA). The final sample consisted of 26 363 participants who completed both the baseline assessments and the first wave follow-up assessments (mean duration of 3.0 ± 0.3 years after baseline). The CLSA is a national, 20-year, prospective cohort study that started data collection in 2011. The CLSA study design and recruitment process have been comprehensively described and published elsewhere [14]. This large-scale cohort study collects information on the biological, medical, cognitive, psychological, social, lifestyle, and economic aspects of middle-aged and older adults. Briefly, individuals were recruited through either health registration databases or random digit dialing. Eligible participants were aged 45–85 years at the recruitment, lived within 25 or 50 km of 1 of 11 data collection sites (depending on the site), were able to communicate in English or French, and exhibited an absence of cognitive impairment at baseline. Moreover, persons living in the three Canadian territories and remote areas, on federal First Nations reserves and other settlements in the provinces, full-time members of the Canadian Armed Forces, and institutionalized individuals were excluded from the CLSA. For our study, further exclusion criteria included: any prior diagnosis of dementia, stroke, or major head injury resulting in a loss of consciousness >20 min. To assess the relationship between insomnia and self-reported memory decline or objective memory performance, medical and sleep comorbidities were not part of the exclusion criteria.
Study participants who are part of the CLSA comprehensive cohort were asked to provide information through computer-assisted personal interviews, physical examinations, neuropsychological battery, and biological samples—administered per standardized protocols. Computer-assisted personal interviews were administered at home, at baseline, and follow-up. Neuropsychological data were collected during visits to data collection centers or during in-home interviews (when participants were unable to travel to the data collection center), at baseline and follow-up, for most participants (Supplementary Table 1).
Standard protocol approvals, registrations, and patient consents
The Canadian Longitudinal Study on Aging was approved by 13 research ethics boards across Canada, as well as the Canadian Institutes of Health Research (CIHR) Advisory Committee on Ethical, Legal and Social Issues (ELSI) for the CLSA. Written informed consent was obtained from all individual participants included in the study [14].
Sleep and insomnia measures
Measures of sleep habits were collected at baseline and follow-up through structured self-report questions that were designed for this study as part of a larger set of measures to assess physical functioning. The questions were drawn from validated sleep questionnaires [28–30] and captured important aspects of sleep and their relation to health. The questions covered six domains: (1) participants’ satisfaction with the type of sleep they were getting; (2) hours of nighttime sleep; (3) trouble falling asleep or staying asleep; (4) daytime sleepiness; (5) dream enactment behavior; (6) if they experienced, recurrent, uncomfortable feelings or sensations in the legs, or urges to move their legs while sitting or lying down (Supplementary Table 2).
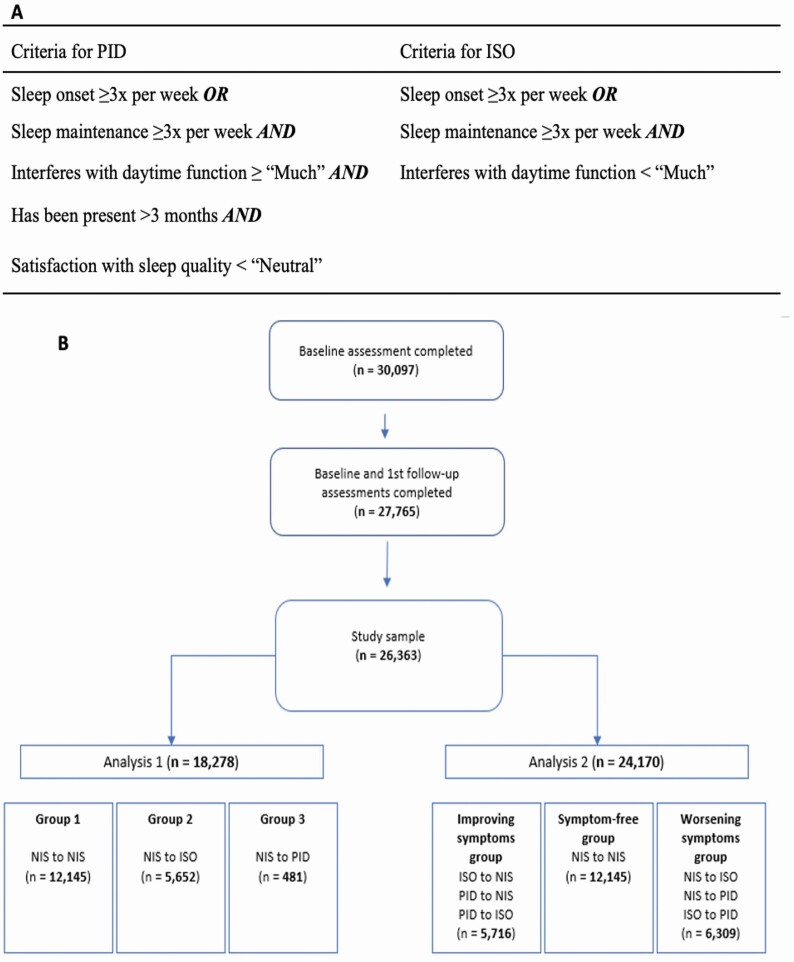
Symptoms related to insomnia were drawn from domains 1 and 3. These questions were utilized to make a probable diagnosis of insomnia disorder based on standard diagnostic criteria from the DSM-5 [1]. For detailed information on the questions used, see the Supplementary Data information. Specifically, only participants who experienced difficulties with sleep onset or maintenance three times or more per week, for longer than 3 months, and stated that it significantly interfered (≥“Much”) with their daily functioning, and additionally were dissatisfied with their sleep pattern (<“Neutral”) were categorized as having PID. Any participant who experienced difficulties with sleep onset or maintenance three times or more per week but did not report any interference (<“Much”) with daytime functioning was categorized as having ISO. All other participants were classified as having no insomnia symptoms (NIS) (Figure 1, A). These criteria for insomnia classification were utilized in our recent cross-sectional study [15].
Figure 1.
(A) Criteria used for categorization into PID and ISO. (B) Study flowchart and group classification for Analysis 1 and Analysis 2. NIS, no insomnia symptoms; ISO, insomnia symptoms only; PID, probable insomnia disorder.
Subjective measures of memory
Subjective measures of memory were obtained through structured self-report questions at follow-up. More specifically, subjective memory decline at follow-up was assessed via the question: “Do you feel like your memory is becoming worse?”. Additionally, as an exploratory measure, subjective memory decline at follow-up was also assessed via the following self-report question: “Has a doctor ever told you that you have a memory problem?”. For this question, participants who reported being diagnosed with a memory problem at baseline were excluded (to evaluate decline in subjective memory from baseline to follow-up). Possible responses for both questions were: yes or no. These questions were utilized as they are considered probable early indicators of cognitive decline and increased risk for dementia (e.g. Alzheimer’s disease) [23, 31–33].
Neuropsychological data
Objective measures of cognitive performances provided by the CLSA were obtained via neuropsychological testing, administered to participants by a trained interviewer at baseline and follow-up. Precisely, declarative memory was assessed with the Rey Auditory Verbal Learning Test (RAVLT), a 15-item word learning test that assesses both learning and retention [34]. The number of words recalled in one immediate recall trial (RAVLT I) and one short delay recall trial (RAVLT II, with a delay of 5 min) were reported [35]. Furthermore, as part of an exploratory analysis, participants additionally completed tests that assessed performance across other cognitive domains such as executive functions and psychomotor speed using well-validated measures. Briefly, measures of executive functions were obtained using the Mental Alternation Test (MAT) and the Stroop Test (Victoria version), whereas psychomotor speed was assessed via the Choice Reaction Time (CRT) Test. For detailed information, see the Supplementary Data information. All tests were scored in a standardized way in collaboration with a CLSA co-investigator who is a clinical neuropsychologist.
Demographic and lifestyle measures
Sociodemographic measures, including age, sex, language, ethnicity, household income, and years of education, were obtained through questionnaire-based interviews at baseline and follow-up. In addition, lifestyle variables, including tobacco consumption (current and former smoking habits), weekly alcohol consumption, and activity level, were also collected at baseline and follow-up. For detailed information on the questions used, see the Supplementary Data information.
Medical and other sleep-related measures
Participants self-reported at baseline and follow-up whether a doctor had ever told them that they had any of a range of chronic conditions, including hypertension, neurological disorders, anxiety, depression, cancer, and chronic pain. Self-reported, clinician-diagnosed, chronic conditions have been shown to have high test–retest reliability in population-based health surveys [14]. Trained CLSA staff also measured the height and weight of participants to calculate body mass index (BMI), as well as depression score using the Center for Epidemiologic Studies Depression (CESD) questionnaire, at baseline and follow-up. Participants also reported whether they were taking any medications that could be prescribed primarily for sleep issues including benzodiazepines, Z-drugs, barbiturates, sedating antidepressants, sedating antipsychotics, sedating antiepileptics, as well as over-the-counter sleep products such as sedating antihistamines and analgesics, at baseline. Participants further reported at baseline and follow-up: how often they found it difficult to stay awake during normal waking hours; whether anyone ever had observed them stop breathing in their sleep (providing a proxy for sleep apnea); whether they reported or had ever been told that they seemed to “act out their dreams” or move while sleeping (providing a proxy for possible REM behavior disorder; pRBD); whether they experienced, recurrent, uncomfortable feelings or sensations in the legs, or urges to move their legs while sitting or lying down (providing a proxy for symptoms of restless leg syndrome; RLS). For detailed information on the questions used, see the Supplementary Data information.
Statistical analysis
The primary outcomes were the associations between both subjective memory decline at follow-up and objective memory decline with PID. Secondary outcomes included self-reported physician-diagnosed memory problem and the associations between PID and other cognitive domains (executive functions and psychomotor speed) with PID. All analyses were conducted with R (R version 4.0.0) [36]. Differences in demographic, lifestyle, and medical characteristics between groups at baseline were assessed using ANOVA with Tukey’s honest significance test, or Pearson’s chi-squared tests. In total, two main analyses were performed (Figure 1, B). Briefly, the first analysis (Analysis 1) examined differences between groups based on participants’ progression from no insomnia symptoms (NIS) at baseline to either NIS, insomnia symptoms only (ISO), or probable insomnia disorder (PID) at follow-up (NIS to NIS; NIS to ISO; or NIS to PID), whereas the second analysis (Analysis 2) was based on whether participants had improving (ISO to NIS, PID to ISO, PID to NIS) or worsening (NIS to ISO, NIS to PID, ISO to PID) insomnia status. The association between the different insomnia groups and self-reported memory decline or self-reported physician-diagnosed memory problem at follow-up was analyzed with logistic regression models (using the default algorithm in R). The association between the different insomnia groups and objective measures of cognitive performance over the following domains: memory, executive functions, and psychomotor speed was assessed with linear mixed-effects models, probing the interaction between change in cognitive performance and the change in insomnia status, from baseline to follow-up. These analyses were performed using the “lmer” function of the “lme4” package in R [36]. All models included participants as a random factor, while group, time, as well as their interaction were set as fixed factors. Pairwise comparisons with false discovery rate (FDR) correction method were performed post-hoc, separately for the group-level comparison of demographic and clinical characteristics, and for each linear mixed model for all cognitive tests. While this approach is less conservative than other methods, type II errors are less likely, and the approach provides better control against errors originating from multiplicity [37]. Results of the associations between the insomnia groups and objective measures of cognitive performance for executive functions and psychomotor speed are shown in the Supplementary Material.
For each cognitive outcome, two models were created with increasing complexity. A minimally adjusted model (model 1) adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up, based on our previous findings from the baseline study [14]. Percentage loss to follow-up was included as a covariate in order to reduce any bias of a difference between groups in this variable. The second model (model 2) additionally adjusted for a range of a priori factors that were considered relevant in the context of cognitive aging. These factors included: BMI, alcohol consumption (≥4 times per week), diagnosis of cancer, anxiety disorder, clinical depression, or hypertension, current level of smoking, presence of chronic pain, activity level, a report of daytime sleepiness, a witness report of breathing interruption during sleep (i.e. sleep apnea), a self-report of RBD (pRBD), a self-report of RLS, a report of using sleep-related medications including hypnotics, antidepressants, antipsychotics, antiepileptics, antihistamines and analgesics, as well as insufficient sleep (<6 h per night). This last variable was chosen based upon a previous finding that only insomnia disorder with <6 h sleep might exhibit cognitive impairment [38]. For both models, these variables were obtained during the baseline assessment. Finally, all primary analyses were repeated to evaluate possible sex differences in memory changes in relation to PID. Sex differences on the association between insomnia and subjective memory were assessed via logistic regression models, while sex differences on the association between insomnia and declarative memory (RAVLT) were assessed via linear mixed-effects modelling.
Results
Overall sample characteristics
The overall sample consisted of 26 363 adults with baseline ages ranging from 45 to 85 with a mean age of 65.5 ± 10.1 years. Approximately half (51.5%, n = 13,579) were females, 25 261 (95.8%) identified as Caucasian and 20 743 (78.7%) spoke English as their primary language, whereas 4997 (19.0%) spoke French primarily. Most participants (85.9%, n = 22 643) had more than 12 years of education and 19 956 (75.7%) participants earned a household income of ≥50 000$ per year.
Participation retention
From the individuals who met the criteria for this study at the baseline assessment, 2122 (7.5%) withdrew from data collection at follow-up. Participants who had withdrawn from the study appeared to be older and a higher proportion reported worse insomnia symptoms at baseline (Supplementary Table 3). In total, 12.1% of individuals who were identified as PID at baseline withdrew, compared to 8.1% of participants who were identified as ISO, and 7.0% of those who were identified as NIS. Causes for withdrawal included: participants developing health-related issues; participants changing locations; participants unable to continue due to cognitive decline; participants entering long-term care; reasons related to study fatigue; and death [39].
Insomnia status conversions over time
Across the entire sample, 12 145 (66.45%) participants who met the criteria for NIS during baseline remained NIS in the follow-up assessment, 5652 (30.92%) transitioned to ISO and 481 (2.63%) to PID. Of the participants who experienced ISO at baseline, 2166 (30.31%) remained ISO at follow-up, while 4,804 (67.22%) converted to NIS and 176 (2.46%) to PID. Finally, of the participants who experienced PID at baseline, only 27 (2.88%) had persisting PID, while 311 (33.12%) reverted to ISO and 601 (64.00%) to NIS.
Analysis 1—NIS at baseline to NIS, ISO, or PID at follow-up
Sample characteristics.
To probe the association between the development of insomnia disorder in middle and later life with changes in cognition, the first analysis included 18 278 adults who initially exhibited NIS during baseline, disaggregated by insomnia status progression. In total, 12 145 (66.4%) participants were classified in group 1 (NIS to NIS), 5652 (30.9%) participants in group 2 (NIS to ISO) and 481 (2.6%) participants in group 3 (NIS to PID). Baseline demographic, lifestyle, and medical characteristics for all groups are shown in Table 1. Most measures were significantly different between group 3 (NIS to PID) and the other groups. Participants who transitioned to PID had a greater prevalence of daytime sleepiness, more smokers, and higher BMI. Moreover, those participants were also more likely to present with various health issues such as witnessed sleep apnea, pRBD, RLS symptoms, reported diagnosis of anxiety disorder, reported diagnosis of depression, and chronic pain. However, the prevalence of cancer or diabetes did not differ across all groups. Additionally, participants who developed PID were more likely to take any medications that have an impact on sleep (including antidepressants and anxiolytics), compared to the other groups. Participants who reported having memory worsening or being diagnosed with a memory problem also demonstrated significant demographic, lifestyle, and medical differences from those with neither subjective memory worsening (Supplementary Table 4) nor self-reported diagnosed memory problem (Supplementary Table 5). Specifically, participants who reported having subjective memory worsening showed greater prevalence of anxiety disorder, depression, higher BMI, cigarette smoking, chronic pain, cancer, daytime sleepiness, use of sleep-related medications, and sleep disturbances compared with those who did not. Similar differences can be observed in participants who described having a diagnosed memory problem from those without. Additionally, participants who regularly slept <6 h per night also exhibited higher proportions of adverse lifestyle and medical outcomes than those who slept ≥6 h (Supplementary Table 6). Finally, significant sex differences can also be observed for demographic, lifestyle, and medical characteristics (Supplementary Table 7).
Table 1.
Baseline demographic, lifestyle, and medical characteristics of participants in Analysis 1
| NIS-NIS (Group 1) n = 12 145 |
NIS-ISO (Group 2) n = 5652 |
NIS-PID (Group 3) n = 481 |
sig. 2 vs. 1 |
sig. 3 vs. 1 |
sig. 3 vs. 2 |
|
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years ± SD | 65.47 (9.99) | 65.75 (10.21) | 62.24 (8.87) | 0.256 | <.0001 | <.0001 |
| Sex, %F | 47.8 | 58.6 | 68.4 | <.0001 | <.0001 | <.0001 |
| English, % | 78.8 | 78.2 | 80.2 | 0.520 | 0.315 | 0.261 |
| Caucasian, % | 95.7 | 96.0 | 94.4 | 0.329 | 0.242 | 0.120 |
| >12 years education, % | 86.9 | 84.1 | 85.4 | <.0001 | .005 | 0.261 |
| Income >50 000$, % | 71.4 | 65.5 | 58.6 | <.0001 | <.0001 | <.0001 |
| Health & lifestyle factors | ||||||
| BMI, kg m2, SD | 27.8 (5.1) | 28.0 (5.4) | 29.0 (6.3) | 0.291 | <.0001 | <.001 |
| Systolic BP, mmHg ± SD | 120.6 (16.3) | 120.9 (16.7) | 119.9 (16.3) | 0.545 | 0.660 | 0.447 |
| Diastolic BP, mmHg ± SD | 73.9 (9.7) | 73.5 (10.0) | 74.2 (10.6) | .023 | 0.837 | 0.316 |
| Smoker, % | 5.6 | 7.3 | 13.3 | .002 | <.0001 | <.001 |
| Alcohol ≥ 4 times per week, % | 27.0 | 26.5 | 18.1 | .015 | <.0001 | <.001 |
| Cancer, % | 14.5 | 15.5 | 15.6 | 0.106 | 0.545 | 0.960 |
| Diabetes mellitus, % | 7.8 | 9.4 | 13.9 | 0.199 | 0.763 | 0.937 |
| Current anxiety disorder, % | 7.3 | 9.6 | 23.1 | <.0001 | <.0001 | <.0001 |
| Current or history of depression, % | 14.0 | 18.2 | 41.2 | <.0001 | <.0001 | <.0001 |
| CESD score ± SD | 4.3 (4.8) | 6.3 (6.0) | 10.3 (6.3) | <.0001 | <.0001 | <.0001 |
| Chronic pain, % | 31.9 | 41.9 | 62.0 | <.0001 | <.0001 | <.0001 |
| Other sleep-related issues | ||||||
| Daytime sleepiness, % | 35.7 | 41.2 | 62.2 | <.0001 | <.0001 | <.0001 |
| Breathing stops in sleep, % | 14.1 | 14.1 | 21.4 | 0.905 | <.0001 | <.0001 |
| Acting out on dreams while asleep, % | 10.3 | 11.9 | 17.5 | .008 | <.0001 | <.001 |
| Uncomfortable feelings or sensations in the legs, or urges to move their legs while sitting or lying down, % | 14.3 | 19.6 | 28.3 | <.0001 | <.0001 | <.0001 |
| Medications | ||||||
| Taking sleep-related medication, % | 6.8 | 9.3 | 18.5 | <.0001 | <.0001 | <.0001 |
| Taking hypnotic medication, % | 1.5 | 2.2 | 3.1 | <.001 | <.006 | 0.256 |
| Taking antidepressant medication, % | 1.0 | 1.3 | 2.1 | 0.107 | .024 | 0.175 |
| Taking antipsychotic medication, % | 0.4 | 0.3 | 1.7 | 0.685 | <.0001 | <.0001 |
| Taking antiepileptic medication, % | 1.7 | 2.2 | 4.6 | .024 | <.0001 | <.0001 |
| Taking antihistamine medication, % | 0.5 | 0.6 | 1.2 | 0.364 | .026 | 0.110 |
| Taking analgesic medication, % | 3.1 | 4.4 | 9.4 | <.0001 | <.0001 | <.0001 |
CESD, Center for Epidemiologic Studies Depression Scale; BMI, body mass index; BP, blood pressure.
Tukey’s honest significance test or χ 2 test, as appropriate. All analyses are FDR adjusted to correct for multiple comparisons.
Bold and italicized text refers to values that pass the FDR adjusted threshold for statistical significance at p < .05.
Associations between insomnia and subjective memory decline.
Results of the logistic regression analysis for both models are reported in Table 2. For the first dichotomous dependent variable—subjective memory worsening (Yes/No), 57.6% of participants across all groups described having memory worsening at follow-up. After adjusting for age, sex, income, education, ethnicity, language, time to follow-up, and percentage loss to follow-up (model 1), the odds of having memory worsening at follow-up increased by 100% (OR 2.00; 95% CI 1.57–2.55) for NIS participants who converted to PID (group 3) compared to participants who remained NIS (group 1) and the odds were 24.1% higher (OR 1.24; 95% CI 1.15–1.34) for NIS participants who converted to ISO (group 2) compared to participants who remained NIS (group 1). Moreover, the odds were 64.1% higher (OR 1.64; 95% CI 1.28–2.10) for NIS participants who converted to PID (group 3) compared to NIS participants who changed for ISO (group 2). Additionally, after adjusting for selected comorbidities factors (model 2), the odds of having memory worsening at follow-up were 70.4% higher (OR 1.70; 95% CI 1.29–2.26) for NIS participants who converted to PID (group 3) compared to NIS participants who remained NIS (group 1) and 19.4% higher (OR 1.19; 95% CI 1.10–1.30) for NIS participants who changed to ISO (group 2) compared to NIS participants who remained NIS (group 1). Furthermore, the odds were 45.3% higher (OR 1.45; 95% CI 1.09–1.94) for NIS participants converting to PID (group 3) compared to ISO (group 2).
Table 2.
Associations between insomnia and subjective memory decline at follow-up—Analysis 1
| Subjective memory | |||
|---|---|---|---|
| Group 2 vs. Group 1† | Group 3 vs. Group 1† | Group 3 vs. Group 2† | |
| Self-reported memory worsening | |||
| Model 1 OR [95% CI] | 1.24*** [1.15–1.34] | 2.00*** [1.57–2.55] | 1.64*** [1.28–2.10] |
| Model 2 OR [95% CI] | 1.19*** [1.10–1.30] | 1.70*** [1.29–2.26] | 1.45* [1.09–1.94] |
| Self-reported diagnosed memory problem | |||
| Model 1 OR [95% CI] | 0.94 [0.60–1.44] | 3.92*** [1.86–7.73] | 3.75*** [1.79–7.86] |
| Model 2 OR [95% CI] | 0.70 [0.41–1.21] | 2.80* [1.16–6.77] | 5.55*** [2.02–15.20] |
OR, odds ratio; 95% CI, 95% confidence intervals.
Group 1 = NIS to NIS; Group 2 = NIS to ISO; Group 3 = NIS to PID.
Model 1 = adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up; Model 2 = adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up, BMI, alcohol consumption (≥4 times per week), diagnosis of cancer, anxiety disorder, clinical depression, or hypertension, current level of smoking, presence of chronic pain, activity level, a report of daytime sleepiness, a witness report of breathing interruption during sleep, a self-report of RBD, a self-report of RLS, a report of using sleep-related medications including hypnotics, antidepressants, antipsychotics, antiepileptics, antihistamines, and analgesics, as well as insufficient sleep (<6 h per night).
Bold and italicized text refers to values that pass the threshold for statistical significance.
*p < .05; **p < .01; ***p < .001.
†Reference group.
Finally, for the second dichotomous dependent variable—self-reported diagnosed memory problem (Yes/No), 0.8% of participants across all groups self-reported being diagnosed with a memory problem at follow-up. After adjusting for model 1, the odds of having a diagnosed memory problem at follow-up increased by 292.0% (OR 3.92; 95% CI 1.86–7.73) for NIS participants who converted to PID (group 3) compared to those who remained NIS (group 1) and the odds were 275.0% higher (OR 3.75; 95% CI 1.79–7.86) for NIS participants who converted to PID (group 3) compared to NIS participants who changed to ISO (group 2). Furthermore, after adjusting for model 2, the odds of having a diagnosed memory problem at follow-up were 180.3% higher (OR 2.80; 95% CI 1.16–6.77) for NIS participants converting to PID (group 3) compared to NIS participants staying NIS (group 1) and 454.6.0% higher (OR 5.55; 95% CI 2.02–15.20) for NIS participants converting to PID (group 3) compared to NIS participants who converted to ISO (group 2). No significant difference was found for participants in group 2 compared to group 1 in both models.
Associations between insomnia and objective memory decline.
The association between insomnia and declarative memory (RAVLT) was assessed via linear mixed-effects modeling and results for model 2 are reported in Table 3, while results for model 1 are shown in Supplementary Table 8. On the immediate recall trial (RAVLT I), the minimally adjusted model 1 showed a significant performance improvement over time (β = 0.71 ± 0.02; 95% CI 0.68–0.74), but no significant group effect (i.e. insomnia groups) was found. Furthermore, there was no significant interaction between time and insomnia groups. After adjusting for model 2, there was still a significant positive effect of time (β = 0.73 ± 0.02; 95% CI 0.69–0.76), but no significant insomnia group effect, nor significant interaction between time and insomnia groups. On the delayed recall trial (RAVLT II), there was a significant positive effect of time (β = 0.60 ± 0.02; 95% CI 0.57–0.64) in model 1, but there was no significant insomnia group effect. Moreover, there was no significant association between time and insomnia groups. After adjusting for model 2, there was still a significant positive effect of time (β = 0.61 ± 0.02; 95% CI 0.57–0.65), but no significant insomnia group effect. There was no significant interaction between time and insomnia groups. Results of the associations between insomnia and the other cognitive tests are shown in Supplementary Table 9. Briefly, there was no significant interaction between time and insomnia groups in executive functions assessed with the MAT and the Stroop Test, in both model 1 and model 2. Furthermore, there was no significant interaction between time and insomnia groups in psychomotor speed assessed via the choice reaction time (CRT) task in all models.
Table 3.
Estimated fixed effects for declarative memory—Analysis 1
| Memory | Parameter | Estimates of fixed effects | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | df | t | p | 95% CI | ||
| RAVLT I | ||||||
| Model 2 | Time (baseline vs. follow-up) | 0.728 (0.02) | 11 460 | 39.621 | <.0001 | 0.69–0.76 |
| Group 2 | −0.015 (0.03) | 12 210 | −0.449 | 0.760 | −0.08–0.05 | |
| Group 3 | 0.044(0.10) | 12 100 | 0.452 | 0.760 | −0.15–0.23 | |
| Group 2: time | 0.025 (0.04) | 11 460 | 0.605 | 0.668 | −0.05–0.10 | |
| Group 3: time | −0.024 (0.12) | 11 310 | −0.209 | 0.899 | −0.26–0.21 | |
| RAVLT II | ||||||
| Model 2 | Time (baseline vs. follow-up) | 0.609 (0.02) | 11 330 | 31.421 | <.0001 | 0.57–0.65 |
| Group 2 | −0.027 (0.04) | 12 210 | −0.718 | 0.616 | −0.10–0.05 | |
| Group 3 | −0.141(0.11) | 12 100 | −1.273 | 0.373 | −0.36–0.08 | |
| Group 2: time | 0.013 (0.04) | 11 340 | 0.305 | 0.816 | −0.07–0.10 | |
| Group 3: time | −0.217(0.13) | 11 200 | −1.730 | 0.183 | −0.46–0.03 | |
SE, standard error; 95% CI, 95% confidence intervals; RAVLT I, Rey Auditory Verbal Learning Test—Encoding Phase; RAVLT II, Rey Auditory Verbal Learning Test–Recall Phase.
Group 1 = NIS to NIS; Group 2 = NIS to ISO; Group 3 = NIS to PID.
Model 2 = adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up, BMI, alcohol consumption (≥4 times per week), diagnosis of cancer, anxiety disorder, clinical depression, or hypertension, current level of smoking, presence of chronic pain, activity level, a report of daytime sleepiness, a witness report of breathing interruption during sleep, a self-report of RBD, a self-report of RLS, a report of using sleep-related medications including hypnotics, antidepressants, antipsychotics, antiepileptics, antihistamines, and analgesics, as well as insufficient sleep (<6 h per night).
All analyses are FDR adjusted to correct for multiple comparisons.
Analysis 2—improving insomnia status vs. worsening insomnia status
Sample characteristics.
To examine the interaction between the course of insomnia status (either worsening or improving) and changes in cognition, the second analysis was comprised of 24 170 participants separated into groups based on insomnia status trajectory. Overall, 5716 (23.6%) were classified in the “improving symptoms” group, which included participants who had improving insomnia status (ISO to NIS, PID to ISO, PID to NIS), 12 145 (50.2%) participants were classified in the “symptom-free” group, which included participants who remained without insomnia symptoms (NIS to NIS), and 6309 (26.1%) participants with worsening insomnia status were classified in the “worsening symptoms” group (NIS to ISO, NIS to PID, ISO to PID). Table 4 displays the baseline demographic, lifestyle, and medical characteristics for all groups. There were no significant differences between participants in the “improving symptoms” group compared to those in the “symptom-free” group for all measures except for the use of sedating antiepileptic drugs. Furthermore, consistent with the first analysis, participants in the “worsening symptoms” group exhibited a higher prevalence of daytime sleepiness, pRBD, RLS symptoms, chronic pain, increasing BMI, and were more likely to be smokers, with a greater prevalence of affective disorders such as anxiety and depression compared to participants who remained without symptoms or those with improving symptoms. Similarly, participants in the “worsening symptoms” group showed a larger prevalence of sleep-related medication use, specifically for hypnotics and analgesics compared to the other groups.
Table 4.
Baseline demographic, lifestyle, and medical characteristics of participants in Analysis 2
| Improving symptoms group n = 5716 |
Symptom- free group n = 12 145 |
Worsening symptoms group n = 6309 |
sig. Free vs. improving |
sig. Worsening vs. improving |
sig. Worsening vs. free |
|
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years ± SD | 65.39 (10.04) | 65.47 (9.99) | 65.40 (10.15) | 0.969 | 0.998 | 0.969 |
| Sex, %F | 47.81 | 47.8 | 59.6 | 0.975 | <.0001 | <.0001 |
| English, % | 78.9 | 78.8 | 78.4 | 0.969 | 0.576 | 0.835 |
| Caucasian, % | 96.0 | 95.7 | 95.8 | 0.566 | 0.835 | 0.835 |
| >12 years education, % | 86.2 | 86.9 | 84.2 | 0.567 | <.0001 | <.0001 |
| Income >50,000$, % | 71.3 | 71.4 | 64.8 | 0.969 | <.0001 | <.0001 |
| Health & lifestyle factors | ||||||
| BMI, kg m2, SD | 27.9 (5.1) | 27.8 (5.1) | 28.1 (5.5) | 0.689 | 0.665 | .044 |
| Systolic BP, mmHg ± SD | 121.0 (16.5) | 120.6 (16.3) | 120.8 (16.7) | 0.567 | 0.894 | 0.969 |
| Diastolic BP, mmHg ± SD | 74.3 (9.9) | 73.9 (9.7) | 73.5 (10.1) | 0.148 | <.001 | 0.058 |
| Smoker, % | 5.5 | 5.6 | 7.9 | 0.969 | <.0001 | <.0001 |
| Alcohol≥4 times per week, % | 26.4 | 27.0 | 25.7 | 0.665 | 0.053 | <.0001 |
| Cancer, % | 15.1 | 14.5 | 15.5 | 0.518 | 0.802 | 0.145 |
| Diabetes mellitus, % | 8.7 | 7.8 | 9.8 | 0.847 | 0.871 | 0.265 |
| Current anxiety disorder, % | 6.8 | 7.3 | 10.9 | 0.512 | <.0001 | <.0001 |
| Current or history of depression, % | 14.1 | 14.0 | 20.6 | 0.969 | <.0001 | <.0001 |
| CESD score ± SD | 4.3 (4.9) | 4.3 (4.8) | 6.7 (6.1) | 0.975 | <.0001 | <.0001 |
| Chronic pain, % | 31.4 | 31.9 | 44.1 | 0.835 | <.0001 | <.0001 |
| Other sleep-related issues | ||||||
| Daytime sleepiness, % | 36.2 | 35.7 | 43.2 | 0.9975 | <.0001 | <.0001 |
| Breathing stops in sleep, % | 14.4 | 14.1 | 15.0 | 0.835 | 0.488 | 0.145 |
| Acting out on dreams while asleep, % | 10.0 | 10.3 | 12.6 | 0.835 | <.001 | <.0001 |
| Uncomfortable feelings or sensations in the legs, or urges to move their legs while sitting or lying down, % | 14.5 | 14.3 | 20.4 | 0.969 | <.0001 | <.0001 |
| Medications | ||||||
| Taking sleep-related medication, % | 7.1 | 6.8 | 10.1 | 0.736 | <.0001 | <.0001 |
| Taking hypnotic medication, % | 1.6 | 1.5 | 2.3 | 0.807 | .008 | <.0001 |
| Taking antidepressant medication, % | 1.0 | 1.0 | 1.3 | 0.998 | 0.171 | 0.084 |
| Taking antipsychotic medication, % | 0.4 | 0.4 | 0.4 | 0.856 | 0.975 | 0.835 |
| Taking antiepileptic medication, % | 2.2 | 1.7 | 2.4 | .043 | 0.577 | <.001 |
| Taking antihistamine medication, % | 0.4 | 0.5 | 0.6 | 0.835 | 0.176 | 0.219 |
| Taking analgesic medication, % | 3.1 | 3.1 | 4.8 | 0.917 | <.0001 | <.0001 |
CESD, Center for Epidemiologic Studies Depression Scale; BMI, body mass index; BP, blood pressure.
Tukey’s honest significance test or χ 2 test, as appropriate. All analyses are FDR adjusted to correct for multiple comparisons.
Bold and italicized text refers to values that pass the FDR adjusted threshold for statistical significance.
Associations between insomnia and subjective memory decline.
Results of the logistic regression analysis are shown in Table 5. On the first dichotomous dependent variable—subjective memory worsening (Yes/No), 57.2% of participants across all groups reported experiencing memory worsening at follow-up. For the minimally adjusted model (model 1), the odds of having memory worsening at follow-up were 27.3% greater (OR 1.27; 95% CI 1.17–1.39) for participants who exhibited deteriorating insomnia status (worsening symptoms group) compared to those who exhibited improving insomnia status (improving symptoms group) and 28.7% higher (OR 1.29; 95% CI 1.19–1.39) for participants in the “worsening symptoms” group compared to participants who remained without symptoms (symptom-free group). Similarly, after adjusting for model 2, the odds of having memory worsening at follow-up were 21.5% higher (OR 1.22; 95% CI 1.10–1.34) for participants in the “worsening symptoms” group compared to those in the “improving symptoms” group and 21.8% higher (OR 1.22; 95% CI 1.12–1.33) for the “worsening symptoms” group compared to the “symptom-free” group. Furthermore, there were no significant differences in the odds of having subjective memory worsening at follow-up for participants in the “symptom-free” group compared to the “improving symptoms” group in both model 1 and model 2.
Table 5.
Associations between insomnia and subjective memory decline at follow-up—Analysis 2
| Subjective memory | |||
|---|---|---|---|
| Symptom-free vs. improving symptoms† | Worsening symptoms vs. improving symptoms† | Worsening symptoms vs. symptom-free† | |
| Self-reported memory worsening | |||
| Model 1 OR [95% CI] | 0.99 [0.92–1.06] | 1.27*** [1.17–1.39] | 1.29*** [1.19–1.39] |
| Model 2 OR [95% CI] | 1.00 [0.92–1.08] | 1.21*** [1.10–1.34] | 1.22*** [1.12–1.33] |
| Self-reported diagnosed memory problem | |||
| Model 1 OR [95% CI] | 1.13 [0.74–1.74] | 1.35 [0.84–2.17] | 1.16 [0.78–1.72] |
| Model 2 OR [95% CI] | 1.09 [0.67–1.78] | 0.97 [0.55–1.72] | 0.85 [0.52–1.39] |
OR, odds ratio; 95% CI, 95% confidence intervals.
Model 1 = adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up; Model 2 = adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up, BMI, alcohol consumption (≥4 times per week), diagnosis of cancer, anxiety disorder, clinical depression, or hypertension, current level of smoking, presence of chronic pain, activity level, a report of daytime sleepiness, a witness report of breathing interruption during sleep, a self-report of RBD, a self-report of RLS, a report of using sleep-related medications including hypnotics, antidepressants, antipsychotics, antiepileptics, antihistamines, and analgesics, as well as insufficient sleep (<6 h per night).
Bold and italicized text refers to values that pass the threshold for statistical significance.
*p < .05; **p < .01; ***p < .001.
†Reference group.
Lastly, on the second dichotomous dependent variable—self-reported diagnosed memory problem (Yes/No), 0.8% of participants across all groups self-reported being diagnosed with a memory problem at follow-up. Results were not statistically significant after adjusting for model 1 or model 2.
Associations between insomnia and objective memory decline.
Results of the association between insomnia and declarative memory (RAVLT) assessed via linear mixed-effects modeling for model 2 are reported in Table 6, while results for model 1 are shown in Supplementary Table 10. On the immediate recall trial (RAVLT I), after adjusting for model 1, there was a significant performance improvement over time (β = 0.70 ± 0.01; 95% CI 0.68–0.73), as well as a significant group effect (i.e. insomnia groups). Participants who had worsening insomnia status (worsening symptoms group) performed significantly worse than participants who had improving insomnia status (improving symptoms group) across both assessments (β = −0.08 ± 0.03; 95% CI −0.14–[−0.01]). However, there were no significant differences between all other insomnia groups. There was no significant interaction between time and insomnia groups. After adjusting for model 2, the significant positive effect of time remained (β = 0.72 ± 0.02; 95% CI 0.69–0.75), but the insomnia group effect was no longer significant. Furthermore, there was no significant interaction between time and insomnia groups. On the delayed recall trial (RAVLT II), after adjusting for model 1, there was a significant positive effect of time (β = 0.59 ± 0.02; 95% CI 0.56–0.62), as well as a significant insomnia group effect. Similarly, participants in the “worsening symptoms” group performed significantly worse than participants in the “improving symptoms” group across both assessments (β = −0.11 ± 0.04; 95% CI −0.18–[−0.04]), however, no significant difference between other insomnia groups was found. There was no significant interaction between time and insomnia groups. After adjusting for model 2, the significant positive effect of time (β = 0.59 ± 0.02; 95% CI 0.57–0.62) remained, but the insomnia group effect was no longer significant. There was no significant interaction between time and insomnia groups. Results of the associations between insomnia and the other cognitive tests are shown in Supplementary Table 11. Briefly, there was no significant interaction between time and insomnia groups in executive functions in both models. Moreover, there was no significant interaction between time and insomnia groups in psychomotor speed in all models.
Table 6.
Estimated fixed effects for declarative memory—Analysis 2
| Memory | Parameter | Estimates of fixed effects | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | df | t | p | 95% CI | ||
| RAVLT I | ||||||
| Model 2 | Time (baseline vs. follow-up) | 0.722 (0.02) | 15 250 | 45.440 | <.0001 | 0.69–0.75 |
| Symptom-free group | −0.039 (0.03) | 16 210 | −1.292 | 0.325 | −0.10–0.02 | |
| Worsening symptoms group | −0.046 (0.04) | 16 230 | −1.261 | 0.334 | −0.12–0.03 | |
| Symptom-free: time | 0.021 (0.04) | 15 220 | 0.529 | 0.700 | −0.06–0.10 | |
| Worsening symptoms: time | 0.042 (0.05) | 15 220 | 0.910 | 0.527 | −0.05–0.13 | |
| RAVLT II | ||||||
| Model 2 | Time (baseline vs. follow-up) | 0.592 (0.02) | 15 070 | 35.186 | <.0001 | 0.56–0.62 |
| Symptom-free group | −0.054 (0.03) | 16 210 | −1.586 | 0.198 | −0.12–0.01 | |
| Worsening symptoms group | −0.092 (0.04) | 16 230 | −2.210 | 0.075 | −0.17–(−0.01) | |
| Symptom-free: time | 0.066 (0.04) | 15 060 | 1.598 | 0.194 | −0.02–0.15 | |
| Worsening symptoms: time | 0.054 (0.05) | 15 060 | 1.125 | 0.382 | −0.04–0.15 | |
SE, standard error; 95% CI, 95% confidence intervals; RAVLT I, Rey Auditory Verbal Learning Test—Encoding Phase; RAVLT II, Rey Auditory Verbal Learning Test—Recall Phase.
Model 2 = adjusted for age, sex, a binary variable of total household income (>50 000 $ or ≤50 000 $), years of education, ethnicity, language, time to follow-up, and percentage loss to follow-up, BMI, alcohol consumption (≥4 times per week), diagnosis of cancer, anxiety disorder, clinical depression, or hypertension, current level of smoking, presence of chronic pain, activity level, a report of daytime sleepiness, a witness report of breathing interruption during sleep, a self-report of RBD, a self-report of RLS, a report of using sleep-related medications including hypnotics, antidepressants, antipsychotics, antiepileptics, antihistamines, and analgesics, as well as insufficient sleep (<6 h per night).
All analyses are FDR adjusted to correct for multiple comparisons.
Sex differences in the associations between insomnia and both subjective and objective memory decline.
Results on the association between insomnia and subjective memory decline assessed via logistic regression models were compared between both sexes. On the first analysis (Analysis 1), similar results were observed for the association between PID and subjective memory worsening at follow-up (Supplementary Table 12). However, compared to men, the odds of being diagnosed with a memory problem at follow-up were increasingly higher for women NIS participants who developed PID than those who converted to ISO or remained NIS, after adjusting for age-related demographic, lifestyle, and medical factors. On the second analysis (Analysis 2) —investigating the effects of insomnia symptoms trajectories (improving or worsening) and changes in cognition—the results did not differ between men and women (Supplementary Table 13).
Furthermore, sex differences on the association between insomnia and declarative memory (RAVLT) assessed via linear mixed-effects modeling were also reported. On the first analysis (Analysis 1), there was a significant interaction between time and insomnia groups in memory performance for men only. Men who had NIS at baseline and developed PID at follow-up showed a significant decrease in performance over time on the delayed memory recall phase of the RAVLT (RAVLT II) compared to those who developed ISO or remained NIS, before controlling for lifestyle and medical factors (Supplementary Figure 1). However, this interaction between time and the probable insomnia disorder (PID) group was no longer significant after adjusting for age-related demographic, lifestyle, and medical factors. For women, there was no significant interaction between time and insomnia groups in memory performance. Lastly, on the second analysis (Analysis 2), there was no significant interaction between time and insomnia groups in memory performance for either men or women in all models.
Discussion
This study examined the association of longitudinal change in probable insomnia disorder status with longitudinal change in subjective and objective memory decline in cognitively healthy middle-aged and older Canadian adults. We tested this association using data from baseline and first follow-up 3 years after baseline from a large-scale cohort study [14]. Based on symptoms mirroring standardized DSM-5 criteria for insomnia disorder [1], participants were defined at each timepoint as having probable insomnia disorder (PID), while those who demonstrated lesser insomnia symptoms and/or without sustaining impairments of daytime functioning were identified as insomnia symptoms only (ISO). Such classification allowed for a distinction between the effects of insomnia symptoms and insomnia as a disorder modeled on clinical diagnostic criteria.
Participants with no baseline symptoms who developed PID by the end of the first follow-up were more likely to exhibit subjective memory decline at follow-up compared with those who either developed ISO or remained symptom-free. This is likely represented by the diagnostic criteria for insomnia, which requires a self-report of sleep-related daytime impairment. Nevertheless, in older adults, subjective memory complaints (SMC) have been shown to be an early predictor of objective cognitive decline, leading to an increased risk of converting to MCI and dementia in later life [23]. Thus, the direct association between PID and SMC in older adults may place these adults at a greater risk for dementia than adults without insomnia.
Additionally, adults who developed PID also showed higher prevalence of anxiety, depression, daytime sleepiness, witnessed breathing interruptions during sleep (as a proxy for probable obstructive sleep apnea), other sleep-related issues (i.e. pRBD or symptoms of RLS), smoking and greater BMI. These are all independently considered as risk factors for cognitive decline and dementia [25, 40–42]. Increasing evidence supports the important contribution of these modifiable risk factors to cognitive decline and dementia [42]. The association of PID in later life with these comorbidities therefore further places it as an important marker of increased risk for dementia. Insomnia has also been shown to have a complex bidirectional relationship with neurodegenerative diseases and several risk factors of dementia (e.g. depression, diabetes, obesity), thus contributing indirectly to cognitive decline and dementia [42, 43]. Nevertheless, following adjustment of these risk factors, our results demonstrated that symptom-free adults who developed PID remained at increased odds of subjective memory decline compared with adults who converted to ISO or remained NIS. This suggests that there also still exists an independent association between probable insomnia disorder and subjective memory decline.
In contrast, objective neuropsychological testing did not reveal any significant associations between insomnia and memory performance, or other cognitive domains. Given the short time interval between both assessments in this study (~3 years) and considering the age distribution of the CLSA cohort, it is plausible that the current follow-up period is not a long enough duration for the association between insomnia and objective cognitive decline to be determined yet within this study cohort. Participants who developed PID and exhibited subjective memory decline at this stage could potentially show further cognitive decline in the upcoming years. The examination of this cohort in future will be important to understand if probable insomnia disorder may increase the risk of development of neurodegenerative diseases and dementia in older adults over time.
However, our results revealed that compared to female participants, male adults with NIS at baseline who developed PID exhibited significantly poorer memory performance (RAVLT II) over time compared with those who converted to ISO or remained symptom-free, despite females with PID showing a greater prevalence of SMC. One possible explanation for this disparity could be that men are often more reluctant to seek help and express their symptoms related to health issues [44, 45], however, the prevalence of SMC among male and female older adults is mixed in the literature [46–49]. Another explanation could be that women have previously been shown to outperform men on verbal memory tasks, specifically in adults with SMC [50] which could potentially indicate a sex-specific form of cognitive reserve delaying memory impairment prior to further cognitive decline [51, 52]. However, the interaction between sex and insomnia status on objective memory change in this study was no longer significant after adjusting for age-related demographic, lifestyle, and medical factors. Therefore, there appears to be a complex relationship between subjective and objective memory impairments in older adults.
Taken together, these findings suggest that insomnia as a disorder, rather than symptoms of insomnia alone, is important in the context of early changes in cognitive function in later life. Subjective reports of daytime difficulties should be taken into consideration when monitoring cognitive decline in older adults with sleep complaints. Insomnia disorder thus may be considered part of a combination of adverse factors that occur in middle age and older adults, which are linked to higher risk of cognitive decline and may lead to MCI and dementia in later life. Importantly, adults who had improving sleep symptoms did not develop subjective memory decline at follow-up, suggesting that the adverse cognitive effects of insomnia disorder may be halted. This highlights the importance of properly diagnosing and managing insomnia as early as possible in older adults. Adequately treating insomnia disorder might become an important preventive measure for cognitive decline and mitigate the incidence of dementias in later life.
There are some limitations that must be considered in this study. First, causation cannot be inferred from an observational study. Further, the question surrounding subjective memory worsening was only asked during the follow-up assessment and was not available at baseline. As such, it is not possible to distinguish whether participants already had subjective memory complaints at baseline. However, based on the phrasing of the question (“Do you feel like your memory is becoming worse?”), it is implied that their memory deteriorated since the baseline assessment. Furthermore, the classification of PID was seemingly unstable from baseline to follow-up as only a few participants (2.88%) who had PID at baseline remained PID at follow-up. Therefore, the long-term consequences of persistent insomnia disorder could not yet be investigated within this cohort. This is surprising as studies have shown that insomnia as a disorder is often a persistent condition, with a remission rate of only 14%–41% over 1–5 years [53]. The low rate of persistent insomnia disorder within this cohort could partly be due to the evaluation methods. A classification of PID was measured using questions reflecting the DSM-5 criteria, but these were not part of a standardized clinical interview conducted by a qualified clinician. Thus, this cannot be regarded as a definite in-person diagnosis of insomnia disorder, although this would be difficult to achieve in such a large sample size. Additionally, despite including percentage lost to follow up as a confound, participants who withdrew from the study before the follow-up period were likely to be older and a higher proportion reported worse insomnia symptoms at baseline, which may have influenced these findings. Lastly, another limitation could involve the CLSA study design and stringent selection criteria of incorporating only cognitively healthy adults with an absence of cognitive impairment, dementia, or history of brain trauma at baseline. Participants also seemed to be healthier, more educated, and have higher household income than the general Canadian population [39]. Given the profile of the CLSA cohort, these associations may differ in a sample more representative of the general population. Nonetheless, the fact that significant differences in subjective memory performance were still able to be detected demonstrates the sensitive relationship between insomnia and memory in aging. Stronger associations may become more prevalent as cognitive function declines over time, and more diagnoses of dementia occur within the cohort.
In conclusion, these findings suggest that insomnia disorder in middle-aged and older adults is associated with higher proportions of subjective memory decline, even after controlling for lifestyle factors and comorbidities. Objective memory deficits were found only in men who developed probable insomnia disorder and could indicate a potential sex effect specific to the onset of insomnia in older adults rather than the worsening of sleep, however this relationship appears complex and any sex differences require further targeted investigation. Insomnia disorder may be a predictor of further cognitive decline and early signs of dementia, however more longitudinal evidence is required to confirm this.
Supplementary Material
Acknowledgments
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation, as well as the following provinces, Newfoundland, Nova Scotia, Quebec, Ontario, Manitoba, Alberta, and British Columbia. This research has been conducted using the CLSA Baseline Comprehensive Dataset version 4.0 and Follow-up 1 Comprehensive Dataset version 1.0, under Application ID 1906008. The CLSA is led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland. The opinions expressed in this manuscript are the author’s own and do not reflect the views of the Canadian Longitudinal Study on Aging.
Contributor Information
Jean-Louis Zhao, Institut Universitaire de Gériatrie de Montréal and CRIUGM, CIUSSS du Centre-Sud-de-l’Ile-de-Montréal, Montreal, Canada; Department of Neuroscience, Université de Montreal, Montreal, Canada.
Nathan Cross, Institut Universitaire de Gériatrie de Montréal and CRIUGM, CIUSSS du Centre-Sud-de-l’Ile-de-Montréal, Montreal, Canada; PERFORM Centre, Concordia University, Montreal, Canada; Department of Health, Kinesiology and Applied Physiology, Center for Studies in Behavioral Neurobiology, Concordia University, Montreal, Canada; Canadian Sleep and Circadian Network, Montreal, Canada.
Chun W Yao, Canadian Sleep and Circadian Network, Montreal, Canada; Integrated Program in Neuroscience, McGill University, Montreal, Canada; Research Institute of McGill University Health Center, Montreal, Canada.
Julie Carrier, Institut Universitaire de Gériatrie de Montréal and CRIUGM, CIUSSS du Centre-Sud-de-l’Ile-de-Montréal, Montreal, Canada; Canadian Sleep and Circadian Network, Montreal, Canada; Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Coeur de Montreal, CIUSSS du Nord-de-l’Ile-de-Montréal, Montreal, Canada; Department of Psychology, Université de Montréal, Montreal, Canada.
Ronald B Postuma, Canadian Sleep and Circadian Network, Montreal, Canada; Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Coeur de Montreal, CIUSSS du Nord-de-l’Ile-de-Montréal, Montreal, Canada; Department of Neurology and Neurosurgery, McGill University—Montreal General Hospital, Montreal, Canada.
Nadia Gosselin, Canadian Sleep and Circadian Network, Montreal, Canada; Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Coeur de Montreal, CIUSSS du Nord-de-l’Ile-de-Montréal, Montreal, Canada; Department of Psychology, Université de Montréal, Montreal, Canada.
Lisa Kakinami, PERFORM Centre, Concordia University, Montreal, Canada; Department of Mathematics and Statistics, Concordia University, Montreal, Canada.
Thien Thanh Dang-Vu, Institut Universitaire de Gériatrie de Montréal and CRIUGM, CIUSSS du Centre-Sud-de-l’Ile-de-Montréal, Montreal, Canada; Department of Neuroscience, Université de Montreal, Montreal, Canada; PERFORM Centre, Concordia University, Montreal, Canada; Department of Health, Kinesiology and Applied Physiology, Center for Studies in Behavioral Neurobiology, Concordia University, Montreal, Canada; Canadian Sleep and Circadian Network, Montreal, Canada.
Funding
Financial disclosure: RP reports grants and personal fees from Fonds de Recherche du Quebec-Sante, the Canadian Institute of Health Research, Parkinson Canada, the Weston-Garfield Foundation, the Michael J. Fox Foundation, the Webster Foundation, and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis Canada, Biogen, Boehringer Ingelheim, Theranexus, GE HealthCare, Jazz Pharmaceuticals, Abbvie, Jannsen, Phytopharmics, Inception Sciences, and Otsuko, outside the submitted work. NG is funded by a CIHR Foundation grant, #FDN154291 and FRQS Chercheur boursier, #34776. TDV reports personal fees from Eisai, Paladin Labs and Jazz Pharmaceuticals outside the submitted work, and grants from the Natural Sciences and Engineering Research Council of Canada (RGPIN 6990-2019), the Canadian Institutes of Health Research (MOP 142191, PJT 153115, PJT 156125 and PJT 166167) and the Fonds de Recherche du Quebec-Sante. JC reports consultant fees from Eisai and Haleo and grants from NSERC (RGPIN-2016-05149) and CIHR PJT-153259.
Conflict of Interest
Nonfinancial disclosure: The authors have no conflicts of interest.
Data Availability
Data are available from the Canadian Longitudinal Study on Aging (www.clsa-elcv.ca) for researchers who meet the criteria for access to de-identified CLSA data.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2. Morin CM, et al. . Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 3. Ohayon MM, et al. . Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the international classification of sleep disorders (ICSD). Sleep Med. 2009;10(9):952–960. doi: 10.1016/j.sleep.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel D, et al. . Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017–1024. doi: 10.5664/jcsm.7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altena E, et al. . Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17(3):335–343. doi: 10.1111/j.1365-2869.2008.00671.x [DOI] [PubMed] [Google Scholar]
- 6. Crenshaw MC, et al. . Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66(3):485–492. doi: 10.1016/s0031-9384(98)00316-3 [DOI] [PubMed] [Google Scholar]
- 7. Haimov I, et al. . Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. doi: 10.1080/15402000701796080 [DOI] [PubMed] [Google Scholar]
- 8. Orff HJ, et al. . Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30(9):1205–1211. doi: 10.1093/sleep/30.9.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varkevisser M, et al. . Chronic insomnia and daytime functioning: an ambulatory assessment. Behav Sleep Med. 2007;5(4):279–296. doi: 10.1080/15402000701557425 [DOI] [PubMed] [Google Scholar]
- 10. Fortier-Brochu E, et al. . Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 11. Murman DL. The impact of age on cognition. Semin Hear. 2015;36(3):111–121. doi: 10.1055/s-0035-1555115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: CRC Press/Taylor & Francis; 2007. Chapter 1. [PubMed] [Google Scholar]
- 13. Cross N, et al. . A human neuroimaging perspective on sleep in normative and pathological ageing. Curr Sleep Med Rep. 2019;5:1–12. [Google Scholar]
- 14. Raina PS, et al. . The Canadian Longitudinal Study on Aging (CLSA). Can J Aging. 2009;28(3):221–229. doi: 10.1017/S0714980809990055 [DOI] [PubMed] [Google Scholar]
- 15. Cross NE, et al. . Association between insomnia disorder and cognitive function in middle-aged and older adults: a cross-sectional analysis of the Canadian Longitudinal Study on Aging. Sleep. 2019;42(8). doi: 10.1093/sleep/zsz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrie JE, et al. . Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34(5):565–573. doi: 10.1093/sleep/34.5.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Potvin O, et al. . Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35(4):491–499. doi: 10.5665/sleep.1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tworoger SS, et al. . The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20(1):41–48. doi: 10.1097/01.wad.0000201850.52707.80 [DOI] [PubMed] [Google Scholar]
- 19. Ma Y, et al. . Association between sleep duration and cognitive decline. JAMA Netw Open. 2020;3(9):e2013573. doi: 10.1001/jamanetworkopen.2020.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osorio RS, et al. . Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59(3):559–562. doi: 10.1111/j.1532-5415.2010.03288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foley D, et al. . Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49(12):1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x [DOI] [PubMed] [Google Scholar]
- 22. Sabia S, et al. . Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12:2289. doi: 10.1038/s41467-021-22354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jessen F, et al. . Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30 [DOI] [PubMed] [Google Scholar]
- 24. Mitchell AJ, et al. . Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130(6):439–451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 25. Norton S, et al. . Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 26. Rabin LA, et al. . Predicting Alzheimer’s disease: neuropsychological tests, self reports, and informant reports of cognitive difficulties. J Am Geriatr Soc. 2021;60(6):1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bondi MW, et al. . Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev. 2008;18(1):73–90. doi: 10.1007/s11065-008-9054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bastien CH, et al. . Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 29. Buysse DJ, et al. . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 30. Ikehara S, et al. . Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32(3):295–301. doi: 10.1093/sleep/32.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neto AS, et al. . Subjective cognitive decline: the first clinical manifestation of Alzheimer’s disease? Dement Neuropsychol. 2016;10(3):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reid LM, et al. . Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- 33. Jahn H. Memory loss in Alzheimer’s disease. Dialogues Clin Neurosci. 2013;15(4):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1982. [Google Scholar]
- 35. Tuokko H, et al. . Cognitive measures in the Canadian Longitudinal Study on Aging. Clin Neuropsychol. 2016;31(1):233–250. doi: 10.1080/13854046.2016.1254279 [DOI] [PubMed] [Google Scholar]
- 36. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2013. Accessed June 26, 2020. http://www.r-project.org/ [Google Scholar]
- 37. Benjamini Y, et al. . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 38. Fernandez-Mendoza J, et al. . Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. doi: 10.1093/sleep/33.4.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raina P, et al. . Cohort profile: the Canadian Longitudinal Study on Aging (CLSA). Int J Epidemiol. 2019;48(6):1752–1753j. doi: 10.1093/ije/dyz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baumgart M, et al. . Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimer’s Dementia. 2015;11(6):718–726. [DOI] [PubMed] [Google Scholar]
- 41. Jee HJ, et al. . Impact of sleep disorder as a risk factor for dementia in men and women. Biomol Ther. 2020;28(1):58–73. doi: 10.4062/biomolther.2019.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Livingston G, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shamim SA, et al. . Insomnia: risk factor for neurodegenerative diseases. Cureus. 2019;11(10):e6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banks I. No man’s land: men, illness, and the NHS. BMJ. 2001;323(7320):1058–1060. doi: 10.1136/bmj.323.7320.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seidler ZE, et al. . The role of masculinity in men’s help-seeking for depression: a systematic review. Clin Psychol Rev. 2016;49:106–118. doi: 10.1016/j.cpr.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 46. Holmen J, et al. . Gender differences in subjective memory impairment in a general population: the HUNT study. Norway. BMC Psychol. 2013;1(1):1-9. [Google Scholar]
- 47. Iliffe S, et al. . Subjective memory problems. BMJ. 2010;340:703-706. [DOI] [PubMed] [Google Scholar]
- 48. Genziani M, et al. . Subjective memory impairment, objective cognitive functionning and social activity in French older people: findings from the Three Cities study. Geriatr Gerontol Int. 2013;13(1):139–145. doi: 10.1111/j.1447-0594.2012.00873.x [DOI] [PubMed] [Google Scholar]
- 49. Paradise MB, et al. . Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry. 2011;11:108–110. doi: 10.1186/1471-244X-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang L, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Gender differences in elderly with subjective cognitive decline. Front Aging Neurosci. 2018;10:166. 10.3389/fnagi.2018.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levine DA, et al. . Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4(2):e210169. doi: 10.1001/jamanetworkopen.2021.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sundermann EE, et al. . Female advantage in verbal memory. Neurology. 2016;87(18):1916–1924. doi: 10.1212/WNL.0000000000003288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morin CM, et al. . Incidence, persistence, and remission rate of insomnia over 5 years. JAMA Netw Open. 2020;3(11):e2018782e2018782. doi: 10.1001/jamanetworkopen.2020.18782 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Canadian Longitudinal Study on Aging (www.clsa-elcv.ca) for researchers who meet the criteria for access to de-identified CLSA data.