Abstract
Study Objectives
Sleep spindles are waxing and waning EEG waves exemplifying the main fast oscillatory activity occurring during NREM sleep. Several recent studies have established that sleep spindle abnormalities are present in schizophrenia spectrum disorders, including in early-course and first-episode patients, and those spindle deficits are associated with some of the cognitive impairments commonly observed in these patients. Cognitive deficits are often observed before the onset of psychosis and seem to predict poor functional outcomes in individuals at clinical high-risk for psychosis (CHR). Yet, the presence of spindle abnormalities and their relationship with cognitive dysfunction has not been investigated in CHR.
Methods
In this study, overnight high-density (hd)-EEG recordings were collected in 24 CHR and 24 healthy control (HC) subjects. Spindle density, duration, amplitude, and frequency were computed and compared between CHR and HC. Furthermore, WM was assessed for both HC and CHR, and its relationship with spindle parameters was examined.
Results
CHR had reduced spindle duration in centro-parietal and prefrontal regions, with the largest decrease in the right prefrontal area. Moderation analysis showed that the relation between spindle duration and spindle frequency was altered in CHR relative to HC. Furthermore, CHR had reduced WM performance compared to HC, which was predicted by spindle frequency, whereas in HC spindle frequency, duration, and density all predicted working memory performance.
Conclusion
Altogether, these findings indicate that sleep spindles are altered in CHR individuals, and spindle alterations are associated with their cognitive deficits, thus representing a sleep-specific putative neurophysiological biomarker of cognitive dysfunction in psychosis risk.
Keywords: working memory, clinical high-risk for psychosis, sleep spindle, sleep EEG, High-density EEG, prefrontal cortex
Graphical Abstract
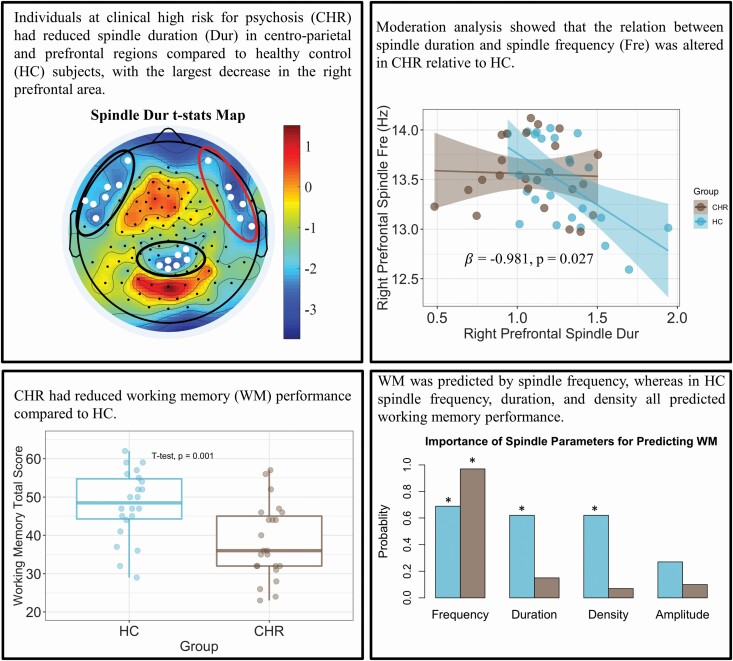
Graphical Abstract.
Statement of Significance.
Sleep spindles abnormalities have been reported in patients affected by schizophrenia spectrum disorders and have been associated with their cognitive impairments. Yet, the presence of such deficits before the onset of psychosis is unknown. By performing sleep high-density EEGs, here we found that clinical high-risk for psychosis (CHR) individuals had reduced spindle duration in centro-parietal and prefrontal regions, and an altered relationship between spindle duration and spindle frequency. CHR had also worse WM performance that was predicted only by spindle frequency, whereas in healthy controls, spindle frequency, duration, and density all predicted WM performance. Altogether, these findings indicate that sleep spindles are altered in CHR and associated with their cognitive deficits, thus representing a sleep-specific putative neurophysiological biomarker of cognitive dysfunction in psychosis risk.
Introduction
Sleep spindles are waxing and waning, 12–16 Hz EEG oscillations that represent the predominant fast oscillatory activity occurring during NREM sleep. Several studies have established sleep spindle abnormalities in patients with schizophrenia (SCZ) during different stages of the illness [1–4]. Specifically, reductions in spindle parameters have been reported in first-episode (e.g. density [1], duration [1]), early-course (e.g. amplitude [5], density [5, 6]), as well as chronic patients with SCZ (e.g. amplitude [2, 7], density [2, 7, 8], and duration [2, 7]), which were primarily localized in centro-parietal and prefrontal regions [1, 2, 4–8]. Furthermore, recent work has shown deficits in spindle parameters, including spindle frequency, in patients with childhood onset SCZ [9]. Individuals at clinical high-risk for psychosis (CHR) provide a unique window into the neurobiology of SCZ spectrum disorders, given their higher rate of transition to psychosis and SCZ compared to the general population [10]. Identifying sleep alterations in CHR individuals may therefore help to establish further the involvement of spindle abnormalities in the development and manifestation of SCZ. Yet, despite this growing body of evidence, only one study has investigated sleep spindles in CHR and reported no deficits in total spindle number and spindle density; the only spindle parameters assessed in these individuals relative to healthy control (HC) subjects [11]. Notably, in that study, sleep spindles were detected just in one channel (i.e. C4). In contrast, high-density EEG systems (hd-EEG) couple the high temporal resolution of standard EEG with enhanced spatial resolution, which allows to better characterize topographic differences in sleep spindles between CHR and HC.
Cognitive dysfunction is a core feature of SCZ spectrum disorders that represents one of the strongest predictors of poor functional outcomes in these patients [12]. Furthermore, an increasing body of evidence has shown that cognitive deficits are present even before the onset of psychosis, including in CHR [13, 14]. Several recent studies have also established that sleep spindle features are associated with cognitive functioning in both HC subjects and patients with SCZ. In HC, a recent meta-analysis has shown that both spindle amplitude and spindle duration had a positive association with. general cognitive ability and that this association was stronger for spindle duration [15]. In patients with SCZ, a systematic review reported a positive correlation between sleep spindle activity and cognitive function [16]. Among cognitive deficits, impairments in working memory (WM) have been consistently reported in CHR individuals, and it has been shown that altered WM is an important predictor for worse clinical trajectories [17, 18]. Of note, sleep studies in healthy adolescents have reported a negative correlation between spindle frequency and WM performance [19, 20]. However, only a handful of sleep studies have looked at multiple spindle parameters, and none of them have examined how spindle parameters may interact with each other to account for WM performance in both healthy and clinical (i.e. CHR) populations. Furthermore, none of these sleep studies employed high-density EEG to assess the associations between topographic differences in spindle parameters and differences in performance in WM, a cognitive function that relies on prefrontal cortex activity [21], between these populations [16]. Topographical information about spindle parameters not only allows characterizing the involvement of specific brain regions (i.e. prefrontal cortex) in spindle alterations, but it can also help establish the implication of these regions in the WM deficits observed in CHR subjects.
In the present study, we employed high-density EEG to investigate several sleep spindle parameters in CHR relative to HC. Furthermore, we examined the relationship between spindle parameters and WM performance in HC and CHR individuals. We hypothesized that sleep spindle abnormalities suggestive of early involvement of the prefrontal cortex that would relate to cognitive dysfunction in CHR individuals.
Methods
Participants
Twenty-four CHR individuals (14 female) and 24 age-matched HC (13 female) subjects were recruited for this study. Table 1 presents the demographic variables and clinical measurements for each study group.
Table 1.
Demographic and clinical variables of study groups
| Clinical measures | Healthy controls | Clinical high-risk | P-value |
|---|---|---|---|
| Number of subjects | N = 24 | N = 24 | |
| Sex (# female) | N = 13 | N = 14 | .7711 |
| Age in years (average ± SD) | 20.7 ± 4.3 (14–33) | 20.6 ± 3.9 (14–30) | .9292 |
| SOPS positive symptoms (average ± SD) | 0.083 ± 0.400 | 12.3 ± 2.9 | <.0012 |
| SOPS negative symptoms (average ± SD) | 0.125 ± 0.331 | 12.7 ± 4.4 | <.0012 |
| SOPS disorganized symptoms (average ± SD) | 0.083 ± 0.400 | 5.4 ± 1.9 | <.0012 |
| SOPS general symptoms (average ± SD) | 0.083 ± 0.276 | 7.2 ± 2.9 | <.0012 |
SD represents the standard deviation.
1Pearson Chi-Square two-sided p-value.
2Unpaired two-tailed student’s t-tests p-value.
Recruitment, eligibility criteria, and assessments
The study was approved by the University of Pittsburgh Institutional Review Board and conducted at Western Psychiatric Hospital in Pittsburgh. All participants provided written informed consent prior to assessment and participating in the experiment and received financial compensation for their participation in the study. HC participants were enrolled from the local community using physical and online advertisements. CHR volunteers were recruited through UPMC clinical settings, referrals from other clinicians, and outside sources.
Study participants underwent a comprehensive screening assessment, including the Structured Interview for Prodromal Symptoms (SIPS). The SIPS is an interview utilized to rate subthreshold-intensity symptoms along four major dimensions on the Scale of Prodromal Symptoms (SOPS): positive (5 items), negative (6 items), disorganized (4 items), and general (4 items) symptoms [22]. Each symptom is rated between 0 and 6, with scores between 3 and 5 considered sub-syndromal or at high-risk for psychosis, while scores of 6 are deemed to be of psychotic intensity. Eligibility criteria in both groups included: (1) ages between 12 and 35; (2) no lifetime history of head injury or neurological disorder resulting in loss of consciousness for more than 1 min, (3) being able to speak English fluently enough to participate in clinical assessments and study procedures; (4) able to travel to Western Psychiatric Hospital to participate in the study; (5) no pregnancy, (6) no history of drug or alcohol dependence in the past 12 months. Additional exclusion criteria for HC participants are as follows: (1) Axis-1 psychiatric disorder diagnosis, assessed with the structured clinical interview for DSM disorders (SCID); (2) high-risk syndrome diagnosis; (3) first-degree relatives with a diagnosed psychotic disorder. Additional eligibility criteria for the CHR group were a score of 3–5 on one of the five SOPS positive symptom items, along with a worsening trajectory over the previous year.
Cognitive assessment
WM performance was assessed using the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB) in CHR and HC individuals. MCCB scores in other cognitive domains (speed of processing, attention/vigilance, verbal learning, visual learning, reasoning/problem solving, and social cognition) for CHR and HC participants were also computed and are presented in the Supplementary Table S1. Two HC and one CHR participants did not complete the MCCB, and therefore they were excluded from the WM task analyses.
Sleep hd-EEG recordings
Two nights of sleep EEG were recorded for all participants at the University of Pittsburgh Sleep and Behavioral Neuroscience Center. The first night of sleep (i.e. adaptation night) was collected to familiarize participants with the overnight recording procedure as well as to help the participants adapt to the sleep laboratory environment [23]. Polysomnography (PSG) was also collected during the first night of sleep to screen for sleep disorders, such as obstructive sleep apnea, restless leg syndrome, or periodic limb movement disorder. No participants were identified to be at-risk of any sleep disorders. Since sleeping for the first time in a novel environment may alter the participants’ sleep, only the data from the second night was used for this study. A 128-channel EEG system (Electrical Geodesics INC., EGI, Eugene, Oregon) was utilized to collect overnight sleep EEG recordings. EEG data was originally recorded at a sampling rate of 250 Hz using the Cz electrode as a common reference. We applied a conductive gel to each electrode to obtain impedance values below 100 kΩ for all channels. American Academy of Sleep Medicine (AASM) criteria [24] were utilized by experienced sleep technicians to score overnight sleep EEG recordings.
Sleep hd-EEG data processing
We used MATLAB (The MathWorks Inc., Natick, MA) to analyze the sleep hd-EEG data. First, band-pass filters between 0.5 and 40 Hz were used to filter the EEG signals, which were then down-sampled from 250 to 128 Hz. Next, EEG data were re-referenced to the average of all electrodes and segmented into 6-s epochs for sleep EEG power spectra computation. Welch’s modified periodogram method in two second Hamming windows (with 50% overlap) was applied to transform the sleep EEG data time series into the frequency domain. We used a semi-automatic artifact rejection procedure to remove channels and epochs with high-frequency noise and/or other artifacts (e.g. muscle activity). Specifically, the thresholds for artifact rejection for low (1–4 Hz) and high (20–30 Hz) frequency ranges were automatically calculated at the 99.8th and 99.5th percentile, respectively, for each channel individually. The higher threshold was utilized to account for muscle artifacts because muscle activity is usually in that frequency band, and the lower threshold was used to detect low-frequency drift due to poor channel contact and sweating. Bad epochs were also removed based on a 36-s sliding window which was three times higher than low-frequency power and six times higher than the high-frequency power of that window. For each channel, we plotted the sleep EEG spectral power in the low- and high-frequency bands and visually inspected all 6-s NREM epochs. EEG channels in which artifacts affected most of the recording were rejected.
An automatic algorithm was employed to detect spindle parameters by using rectified filtered traces between 11 and 16 Hz as new time series for each EEG channel. Additional details regarding the spindle detection procedures are provided elsewhere [2, 7]. The number of sleep spindles per minute of NREM sleep (spindle density) as well as average spindle duration, amplitude, and frequency during all NREM sleep epochs were measured for each channel and compared between CHR and HC individuals.
As an exploratory analysis, we also conducted slow wave (SW)-spindle coordination analysis as performed in the previous studies in SCZ patients [25, 26]. Specifically, we identified the SW phase at the spindle peak to quantify the coupling of SW and spindle. The details of the SW detection are reported in previous work from our group [7, 27].
Statistical analyses
Unpaired two-sided Student’s t-tests were applied to assess for between-group age differences, while a chi-squared test was used to examine between-group sex differences. Unpaired t-tests were calculated to examine differences in individual sleep spindle features (i.e. amplitude, density, frequency, and duration) and SW phase at the spindle peak between CHR and HC groups at each channel. To account for multiple comparisons (i.e. number of channels), we conducted a clustering analysis on the channel t-map for each spindle feature using a Gaussian mixture model [28]. We set the number of clusters for each t-map to be the value that achieved the minimum adjusted Bayesian information criterion (BIC). These analyses were performed in R using the package mclust. A Bonferroni correction was applied to account for the topographic features (i.e. the scalp regions) that were part of the significant cluster identified with the Gaussian mixture model. The Cohen’s D effect size (ES) was also computed for the region showing the largest difference between CHR and HC participants. To assess whether the group label (CHR or HC) moderated the relationship between spindle parameters, we tested for two-way interactions between the group label and the spindle parameter treated as an independent variable. We used the lm function in R to test for moderation effects. Two-sample t-tests were utilized to assess differences in WM performance between CHR and HC. Moreover, we applied an L1-penalized regression (LASSO) model that regressed WM on all spindle parameters to identify which of those parameters were important for predicting WM performance in both groups. The penalty for the LASSO model was chosen by cross-validation by using the cv.glmnet function in R. Sleep spindle parameters with non-zero coefficients in the fitted LASSO model were deemed important variables for prediction based on the variable selection property of the LASSO method [29]. In detail, for each group, a LASSO model regressing WM performance on spindle frequency, duration, and density was fit on 100 training set splits of the data, where each training set contained a random sample of 90% of the observations in the sample. For each parameter, variable importance was measured as the proportion of splits (out of 100) for which that sleep spindle parameter had a non-zero coefficient in the model. To validate the findings of the LASSO strategy, we also performed model selection via stepwise regression (i.e. Akaike information criterion, AIC model) [30] using the adjusted R2 criterion for each model and compared the final fitted model with the variable importance determined by the LASSO model.
Results
Sleep architecture difference between CHR and HC
CHR individuals had a significantly higher wake after sleep onset (WASO) percentage compared to HC participants; p = .030, t-stat = 2.240. The sleep efficiency was lower in CHR compared to HC, p = .023, t-stat = ‐2.357. No differences in any other sleep architecture parameters, including total sleep time, the total number of NREM epochs, sleep onset latency, percentage of time spent in NREM (i.e. N1, N2, and N3), and rapid eye movement (REM) sleep stages were found between CHR and HC individuals (Supplementary Table S2).
CHR had reduced sleep spindle duration relative to HC
Topographic analyses established that spindle parameters were highest in both HC and CHR groups in centro-parietal and prefrontal regions (Supplementary Figure S1). Furthermore, there were no significant differences in sleep spindle amplitude, density, and frequency between HC and CHR groups.
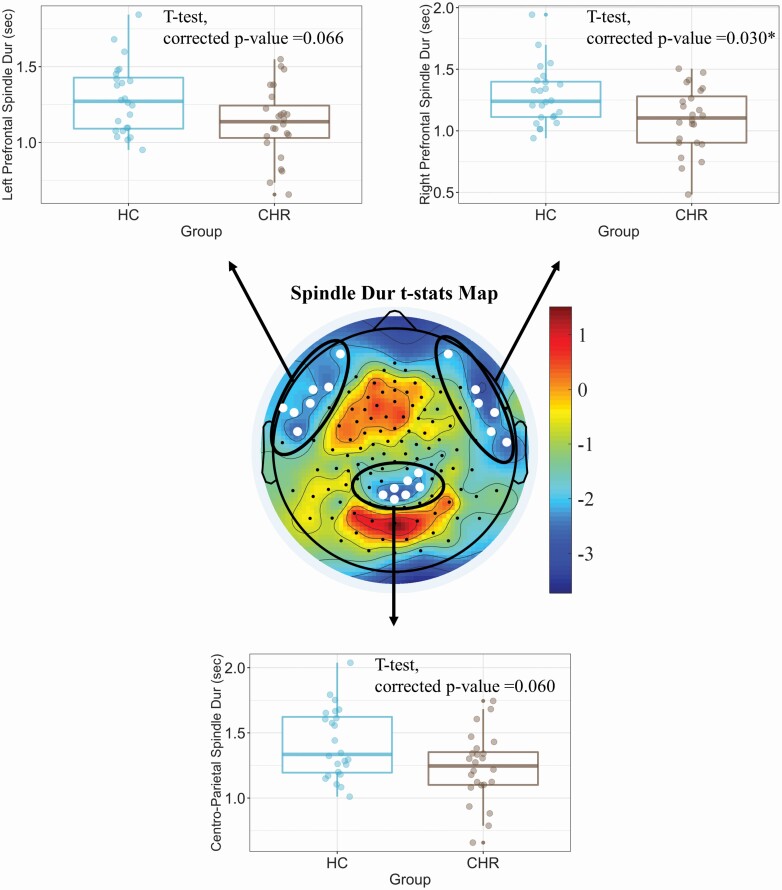
In contrast, CHR showed reduced sleep spindle duration in centro-parietal and lateral prefrontal regions (mean spindle duration across twenty electrodes in CHR vs HC groups, unpaired t-test: t-stat = ‐2.504; p = .016, Figure 1, topographic statistical plot). Although a reduction in spindle duration was observed in each of these regions (Figure 1, box plots), we found that CHR had the largest reduction in sleep spindle duration in the right prefrontal region relative to HC (t-stat = 2.682, p = .030 after Bonferroni’s correction for multiple comparisons). We also computed the Cohen’s D at this right prefrontal region, which yielded a large effect size (ES = 0.8).
Figure 1.
CHR showed reduced sleep spindle duration (Dur) in centro-parietal and prefrontal regions (p = .015). The strongest reduction in spindle duration was observed in the right prefrontal region, which was significant after correction for multiple comparisons (p = .030 after Bonferroni’s correction). The corrected p-values for the other two regions are 0.06 and 0.066. *Represents a significant difference between groups after multiple comparison correction.
Furthermore, we found no significant differences in the SW phase at the spindle peak using clustering analysis (Supplementary Figure S2).
Group moderated the relationship between spindle duration and spindle frequency
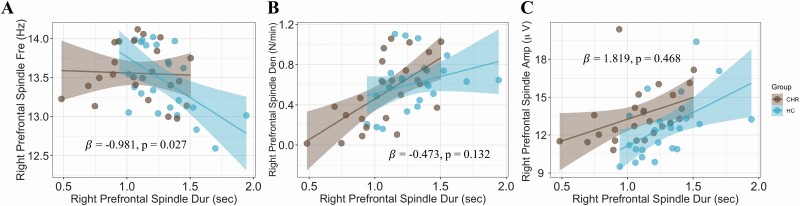
To investigate whether differences in sleep spindle duration affected the relationship between duration and the other spindle parameters in CHR and HC, we ran a moderation analysis. We found that group (CHR vs. HC) moderated the relationship between spindle duration and spindle frequency in the right prefrontal region (β = ‐0.981, p = .027, Figure 2A), whereas it did not moderate the relationship between spindle duration and spindle density (β = ‐0.473, p = .132; Figure 2B) or spindle amplitude (β = 1.819, p = .468; Figure 2C). Notably, this effect was observed only in the right prefrontal region, whereas it was not significant in the left prefrontal or centro-parietal regions (Supplementary Figure S3).
Figure 2.
Group moderated the relationship between spindle duration and spindle frequency (A), while it did not moderate the relation between spindle duration and spindle density (B) or spindle amplitude (C). Moderation coefficients (β) and p-value (p) are shown inside the plots.
Spindle parameters predicted working memory performance in HC and CHR individuals
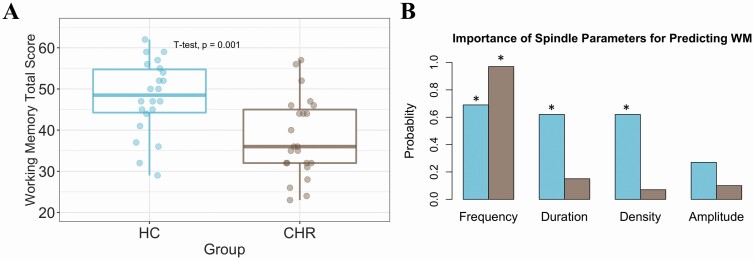
We found that CHR individuals had significantly reduced WM performance relative to HC (p = .001; Figure 3A). The LASSO analysis was then performed to examine the importance of spindle parameters for predicting WM performance in CHR and HC (Figure 3B). Spindle frequency was the most important predictive parameter for both groups (with a weight value of 0.69 for HC and 0.97 for CHR), followed by spindle duration (with variable importance of 0.62 for HC and 0.15 for CHR, respectively). Spindle density was moderately predictive for HC (0.62) but the least predictive parameter for CHR (0.07), while spindle amplitude was the least predictive parameter for HC (0.27) and similarly not predictive for CHR (0.10). Model selection via stepwise regression (AIC model) supported the findings of the LASSO model. For the HC group, the spindle frequency, duration, and density were identified as important and significantly related to WM performance (p-values from the AIC model = 0.003, 0.037, 0.012, respectively). In the CHR group, only spindle frequency (p-value = .005) was selected as an important predictor via stepwise regression.
Figure 3.
(A) Box plots display working memory performance in HC (blue) and CHR (brown), which was significantly reduced in the latter group (p = .001). (B) The importance of sleep spindle parameters in predicting WM performance. The asterisks indicate the sleep parameters that were important in predicting WM based on the Akaike information criterion (AIC) approach.
Discussion
In this study, we investigated differences in sleep spindle features (i.e. duration, density, amplitude, and frequency) by collecting hd-EEG recordings and cognitive performance using MCCB assessment between CHR and HC groups. We found reduced sleep spindle duration in centro-parietal and lateral prefrontal electrodes in CHR individuals, which was most pronounced in the right prefrontal region. We also established that the relation between spindle frequency and spindle duration was altered in CHR individuals relative to HC subjects, that WM performance was reduced in CHR compared to HC groups, and that different spindle parameters predicted WM in each group; specifically, spindle frequency, duration, and density predicted WM in HC, while only spindle frequency predicted WM in CHR subjects.
Previous works from our and other research groups have demonstrated reductions in sleep spindle duration in patients with SCZ, from the early-course [1, 4] to chronic stages [2, 5, 7] in central and frontal/prefrontal regions. By performing hd-EEG recordings, here we found significantly reduced spindle duration in the right and left prefrontal channels as well as centro-parietal regions in CHR individuals compared to HC. Thus, the present finding indicates that altered spindle duration is present in at-risk individuals even before the onset of full-blown psychosis. The first potential implication of this result is that spindle duration may in part contribute to the identification of CHR individuals. Consistent with this assumption, we found that reduced spindle duration in the right prefrontal region yielded a large Effect Size (ES = 0.8), which corresponded to 72% separation between CHR and HC groups. Another interesting aspect of this finding is the localization of reduced spindle duration in the prefrontal and parietal regions. Alterations in the fronto-parietal network have been consistently reported in SCZ, including first-episode and early-course patients [31, 32] and abnormalities in this network are thought to critically contribute to the development and manifestation of SCZ spectrum disorders [33]. Our findings are in line with this body of evidence and suggest that altered spindle duration could be a functional readout of these fronto-parietal network abnormalities that can be detected early in the course of psychosis.
In several recent sleep studies, we and others have shown alterations in spindle amplitude and density in chronic [2, 7, 8, 34] early-course [1, 5, 6, 35, 36], as well as early onset [9] patients with SCZ. In the present study, we found no significant differences in these spindle parameters in CHR individuals compared to HC. One intriguing explanation for these findings is that alterations in sleep spindle parameters other than duration may occur only after the manifestation of the first psychotic episode and/or later through the course of a chronic SCZ spectrum disorder.
We were also interested in examining whether alterations in one of the spindle measures (i.e. spindle duration) in CHR would affect their relationships with the other spindle parameters relative to HC participants. We found that the group moderated the relationship between spindle frequency and duration. Specifically, in HC, there was a strong negative association between spindle frequency and duration, whereas this association was lost in the CHR group. Given that spindle duration, but not frequency, was reduced in CHR, this analysis can be used to identify spindle abnormalities above and beyond simple comparisons of individual spindle features between healthy and psychiatric groups.
Consistent with previous work in at-risk individuals [17, 18], we found a significant reduction in WM performance in CHR individuals compared to HC subjects. WM is one of the cognitive domains most consistently found to be altered in CHR, which has been linked to worse clinical trajectories in these individuals. For example, a meta-analysis found that WM and visual learning are the only two different cognitive domains between CHR converters and CHR non-converters [17]. Of note, in the present study we did not establish a difference in visual learning performance between CHR and HC, which could be related to the heterogeneity of the CHR population. Moreover, several studies have found that the prefrontal cortex, especially in the right hemisphere, plays a crucial role in regulating WM [21, 37, 38]. In the present study, we found that the most significant alteration of spindle features (including the duration and association between frequency and duration) was established in the right prefrontal electrodes. To further examine the relationship between spindle parameters and WM, we employed both LASSO prediction and the AIC approach and found that frequency was the most important spindle parameter in predicting WM in both groups. This finding is in line with two previous studies in adolescents that found strong associations between WM and spindle frequency [19, 20]. These findings also indicate the need for further exploring the relationship between spindle parameters, and especially spindle frequency and cognitive functioning in relation to age-related developmental trajectory. Spindle density and duration were also identified as important predictors of WM in HC but not in CHR individuals. These results corroborate the implication of spindle density in executive function and WM performance in healthy individuals, as shown by other recent sleep studies in nonclinical populations [39, 40] Our findings also suggest that spindle duration plays an important role in predicting WM performance in HC, but not in CHR, likely due to the spindle duration deficits observed in CHR individuals in the present study.
Sleep spindle alterations point to abnormalities of the underlying neuronal circuits. Converging electrophysiological and neuroimaging evidence indicates that dysfunctions in a thalamic reticular nucleus-mediodorsal (TRN-MD)-prefrontal cortex (PFC) network are especially implicated in spindle deficits [41]. The TRN, which is strategically located between the dorsal thalamus and the cortex, plays a crucial role in triggering spindle oscillations. Specifically, the TRN receives excitatory afferents from both cortical and thalamic neurons while sending inhibitory projections to all thalamic nuclei, which enables to ignite and maintain the spindle oscillatory activity [42, 43]. Computer simulations and in vivo multisite recordings have also demonstrated that sleep spindle duration is critically dependent on the cortico-thalamic connections that sustain the sleep oscillation over time [44, 45]. Furthermore, several recent neuroimaging studies have shown thalamo-cortical alterations (e.g. based on functional connectivity findings) in both CHR, early-course, and chronic SCZ patients, which included the MD-PFC network [46–49]. In previous work, we found that chronic patients with SCZ had reduced MD thalamic volumes, that this reduction correlated with decreased sleep spindles in the PFC, and that decreased PFC spindle activity was associated with worse cognitive performance in these patients relative to HC [8]. In the present study, we showed that dysfunctions in this thalamo-cortical circuitry, as reflected by altered PFC sleep spindles, occur even before the manifestation of full-blown psychosis and are implicated in the cognitive impairments observed in CHR individuals, thus representing a unique window for early intervention in this at-risk population.
This study left several unanswered questions while also paving the way for future studies. First, the present findings will need to be replicated in larger cohorts of CHR individuals. A larger sample size would help investigating further the effects of age on sleep spindles in CHR individuals, as it has been shown in previous work that spindle activity tends to be higher in females compared to males [50]. Second, it would be important to establish whether the reduced spindle duration found here can be utilized to predict and track the trajectory of illness in CHR individuals. To do so, future longitudinal studies are needed to investigate whether reduced spindle duration during NREM sleep can be utilized as an index to predict transition to psychosis and/or to help differentiating CHR individuals showing a persistent/worsening clinical course relative to the individuals experiencing partial/full remission of at-risk symptoms. Third, future studies should investigate whether pharmacological and non-pharmacological interventions in CHR individuals may lead to ameliorating spindle abnormalities and related cognitive dysfunctions in CHR individuals. Finally, unlike previous studies on first-episode psychosis and chronic SCZ, we could not find any deficits in spindle density in CHR. Several factors might have contributed to these negative findings. For example, it is possible that different subgroups of CHR individuals may have distinct spindle deficits and/or that the duration of clinical symptoms affects the type and extent of spindle deficits. Thus, to further evaluate if spindle duration deficit is the only spindle feature alteration in CHR, the heterogeneity and duration of clinical symptomatology should be evaluated in larger samples of at-risk individuals over extended periods of time. Another interesting possibility is that reduced spindle duration is an early sign of illness that precedes the manifestation of full-blown psychosis, which is then associated with decreased spindle density.
Altogether, in the present study, we established that spindle duration deficits are present in CHR individuals, which were most prominent in the right prefrontal region. We further demonstrated that altered prefrontal spindle activity was associated with worse WM performance in CHR individuals relative to HC subjects. Building on these findings, future work will contribute to further establishing a critical role of sleep anomalies in the neurobiology and cognitive deficits of the prodromal phase of psychosis. It may also lead to the discovery of more effective, early treatment interventions in at-risk individuals as well as in patients with psychosis, especially schizophrenia spectrum disorders, based on sleep neurophysiology.
Supplementary Material
Acknowledgments
This research was funded by National Institute of Mental Health (NIMH), grant number R01 MH113827 awarded to Fabio Ferrarelli.
Contributor Information
Ahmad Mayeli, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
James D Wilson, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Francesco L Donati, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Alice D LaGoy, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Fabio Ferrarelli, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Conflict of Interest statement
The authors declare no conflict of interest.
Disclosure Statement
The authors declare no financial arrangements or connections that are pertinent to the submitted manuscript. Further, the authors declare no non-financial interests that could be relevant to the submitted manuscript.
References
- 1. Kaskie RE, et al. . Topographic deficits in sleep spindle density and duration point to frontal thalamo-cortical dysfunctions in first-episode psychosis. J Psychiatr Res. 2019;113:39–44. doi: 10.1016/j.jpsychires.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 2. Ferrarelli F, et al. . Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 3. Ferrarelli F. Sleep abnormalities in schizophrenia: state of the art and next steps. Am J Psychiatry. 2021;178(9):903–913. doi: 10.1176/appi.ajp.2020.20070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schilling C, et al. . Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. Eur Arch Psychiatry Clin Neurosci. 2017;267(3):213–224. doi: 10.1007/s00406-016-0725-2. [DOI] [PubMed] [Google Scholar]
- 5. Manoach DS, et al. . Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. doi: 10.3389/fnhum.2014.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerstenberg M, et al. . Reduced sleep spindle density in adolescent patients with early-onset schizophrenia compared to major depressive disorder and healthy controls. Schizophr Res. 2020;221:20–28. doi: 10.1016/j.schres.2019.11.060. [DOI] [PubMed] [Google Scholar]
- 7. Ferrarelli F, et al. . Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchmann A, et al. . Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage. 2014;102:540–547. doi: 10.1016/j.neuroimage.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Markovic A, et al. . Sleep spindle activity in childhood onset schizophrenia: diminished and associated with clinical symptoms. Schizophr Res. 2020;223:327–336. doi: 10.1016/j.schres.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Pablo GS, et al. . Probability of transition to psychosis in individuals at clinical high risk: an updated meta-analysis. JAMA Psychiatry. 2021;78(9):970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purple R, et al. . Sleep-related memory consolidation in the psychosis spectrum phenotype. Neurobiol Learn Mem. 2020;174:107273. doi: 10.1016/j.nlm.2020.107273. [DOI] [PubMed] [Google Scholar]
- 12. Kahn RS, et al. . Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 13. Guo J, et al. . Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24(5):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng W, et al. . Neurocognitive dysfunction in subjects at clinical high risk for psychosis: a meta-analysis. J Psychiatr Res. 2018;103:38–45. [DOI] [PubMed] [Google Scholar]
- 15. Ujma PP. Sleep spindles and general cognitive ability–a meta-analysis. Sleep Spindles Cortical Up States. 2021;2(1):1–17. [Google Scholar]
- 16. Au CH, et al. . Systematic review: the relationship between sleep spindle activity with cognitive functions, positive and negative symptoms in psychosis. Sleep Med X. 2020;2:100025. doi: 10.1016/j.sleepx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Herdt A, et al. . Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: a meta-analysis. Schizophr Res. 2013;149(1–3):48–55. [DOI] [PubMed] [Google Scholar]
- 18. Fusar-Poli P, et al. . Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562–571. [DOI] [PubMed] [Google Scholar]
- 19. Chatburn A, et al. . Sleep spindle activity and cognitive performance in healthy children. Sleep. 2013;36(2):237–243. doi: 10.5665/sleep.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gruber R, et al. . The association between sleep spindles and IQ in healthy school-age children. Int J Psychophysiol. 2013;89(2):229–240. [DOI] [PubMed] [Google Scholar]
- 21. Smucny J, et al. . Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology. 2022;47(1):292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller TJ, et al. . Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 23. Mayeli A, et al. . Examining first night effect on sleep parameters with hd-EEG in healthy individuals. Brain Sci. 2022;12(2):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silber MH, et al. . The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(02):121–131. [PubMed] [Google Scholar]
- 25. Demanuele C, et al. . Coordination of slow waves with sleep spindles predicts sleep-dependent memory consolidation in schizophrenia. Sleep. 2017;40(1) :369–465. doi: 10.1093/sleep/zsw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kozhemiako N, et al. . Non-rapid eye movement sleep and wake neurophysiology in schizophrenia. Elife. 2022;11:e76211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaskie RE, et al. . Reduced frontal slow wave density during sleep in first-episode psychosis. Schizophr Res. 2019;206:318–324. [DOI] [PubMed] [Google Scholar]
- 28. Fraley C, et al. . Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97(458):611–631. [Google Scholar]
- 29. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodological). 1996;58(1):267–288. [Google Scholar]
- 30. Henderson DA, et al. . Stepwise regression in social and psychological research. Psychol Rep. 1989;64(1):251–257. [Google Scholar]
- 31. Kirkpatrick B, et al. . Deficit schizophrenia: an update. World Psychiatry. 2008;7(3):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong Q, et al. . A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am J Psychiatry. 2016;173(3):232–243. [DOI] [PubMed] [Google Scholar]
- 33. Sheffield JM, et al. . Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manoach DS, et al. . Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010;44(2):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poulin J, et al. . Sleep architecture and its clinical correlates in first episode and neuroleptic-naive patients with schizophrenia. Schizophr Res. 2003;62(1–2):147–153. [DOI] [PubMed] [Google Scholar]
- 36. Yazıhan N, et al. . Sleep, sleep spindles, and cognitive functions of first-episode drug naïve patients with psychosis. J Clin Sleep Med. 16(12):2079–2087. doi: 10.5664/jcsm.8776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D’Ardenne K, et al. . Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci USA. 2012;109(49):19900–19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Curtis CE, et al. . Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7(9):415–423. [DOI] [PubMed] [Google Scholar]
- 39. Clemens Z, et al. . Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. [DOI] [PubMed] [Google Scholar]
- 40. Reynolds C, et al. . Sleep spindles and cognitive performance across adolescence: a meta-analytic review. J Adolesc. 2018;66:55–70. [DOI] [PubMed] [Google Scholar]
- 41. Ferrarelli F, et al. . Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr Res. 2017;180:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bazhenov M, et al. . Spiking-bursting activity in the thalamic reticular nucleus initiates sequences of spindle oscillations in thalamic networks. J Neurophysiol. 2000;84(2):1076–1087. [DOI] [PubMed] [Google Scholar]
- 43. Steriade M, et al. . The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57(1):260–273. [DOI] [PubMed] [Google Scholar]
- 44. Bonjean M, et al. . Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31(25):9124–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Timofeev I, et al. . Contribution of intrinsic and synaptic factors in the desynchronization of thalamic oscillatory activity. Thalamus Relat Syst. 2001;1(1):53–69. [Google Scholar]
- 46. Cao H, et al. . Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anticevic A, et al. . Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72(9):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cho KIK, et al. . Altered thalamo-cortical white matter connectivity: probabilistic tractography study in clinical-high risk for psychosis and first-episode psychosis. Schizophr Bull. 2016;42(3):723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harrisberger F, et al. . Alterations in the hippocampus and thalamus in individuals at high risk for psychosis. Npj Schizophr. 2016;2(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Purcell S, et al. . Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun. 2017;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.