Abstract
Serum C-reactive protein (CRP), a marker of systemic inflammation, is associated with increased risk for numerous inflammation-driven chronic diseases. A prior longitudinal study showed that the Low Inflammatory Foods Everyday (LIFE) diet, which is rich in dark green leafy vegetables (DGLV), lowered CRP over a mean follow-up period of 6 months. In this retrospective study, we investigate whether patients who consume the LIFE diet or their regular diet plus one component of the LIFE diet (LIFE smoothie), experience reductions in high-sensitivity CRP (hsCRP) in 7 days. Sixteen patients in a community practice met inclusion criteria. Patient compliance was assessed by patient interviews and measurements of beta-carotene, which is abundant in DGLV. Following the interventions, CRP decreased in both the LIFE diet (−0.47 mg/L, P = .02) and smoothie groups (−1.2 mg/L, P = .04). No statistically significant difference in reduction was observed between groups (P = .18). Plasma beta-carotene increased in both groups (+23.2, P = .02; +20.6, P = .006, respectively). These findings suggest that the LIFE diet or a regular American diet supplemented with the LIFE smoothie may quickly reduce systemic inflammation and the risk of many chronic diseases.
Keywords: C-reactive protein, CRP, beta-carotene, dark green leafy vegetables, DGLV, cardiovascular disease, CVD, whole food plant-based diet, WFPBD
Serum C-reactive protein (CRP) is a well-established indicator of systemic inflammation. 1 As a nonspecific acute-phase reactant, CRP is released in response to many inflammatory stimuli in the body, but it can also play a more direct proinflammatory role by activating endothelial cells and adhesion molecules itself.1,2 CRP levels, particularly high-sensitivity CRP (hsCRP) levels, also predict the risk of many chronic diseases involving inflammation, 3 such as myocardial infarction, 4 stroke, 4 sudden cardiac death, 4 peripheral artery disease, 5 type 2 diabetes, 6 autoimmune diseases such as rheumatoid arthritis, 7 and multiple forms of cancer.8-11 This relationship between CRP and future risk of cardiovascular events holds even after adjustment for age, gender, or preexisting cardiovascular disease (CVD). 3 Elevations in CRP are also associated with increased risk for death and severe infection from COVID-19 (as defined by Chinese National Health Commission criteria and computed tomographic [CT] severity scores),12-14 acute kidney injury, 15 chronic kidney disease, 15 age-related macular degeneration (AMD), 16 nonallergic asthma, 17 chronic obstructive pulmonary disease, 18 hypertension, 19 and all-cause mortality. 20
As a marker and mediator of inflammation that is also predictive of disease, CRP is a suitable target for primary disease prevention. This was proven in a secondary analysis of the CANTOS trial, which found that patients with CRP concentrations <2 mg/L after treatment with an interleukin-1β inhibitor had a 31% reduction in all-cause mortality and cardiovascular mortality versus no significant reduction in treated patients with CRP concentrations ≥2 mg/L. 21 Further analysis indicated that the predictive value of CRP for future cardiovascular events was strongly linear across all CRP values, meaning that the risk of CVD continually decreased the further CRP was reduced down to <0.5 mg/L. 22
CRP can also be diminished with lifestyle modifications such as diet and exercise.23-25 Specifically, the retinol precursor beta-carotene, an antioxidant found in many fruits and dark green leafy vegetables (DGLV),26,27 has a strong inverse relationship with CRP. 28 Most recently, this was demonstrated in the Low Inflammatory Foods Everyday (LIFE) diet study, which found that patients who adhered to a diet high in beta-carotene–rich vegetables for up to 1 year had decreased CRP levels and increased plasma beta-carotene levels (r = −0.68, P < .0001). 29 These results were consistent with an earlier randomized control trial, which reported a significant reduction in plasma CRP after a 4-week diet consisting of 8 servings of fruits and vegetables per day. 30
Herein, we build upon our earlier LIFE diet study by determining whether the LIFE diet (which includes 1 daily LIFE smoothie) or patients’ regular diets supplemented with 1 LIFE smoothie each day leads to increased beta-carotene and decreased CRP in only 7 days. We include the regular diet plus smoothie group in order to investigate the effects of a simple modification that may be easy for patients to implement in their daily lives. The smoothie component of the LIFE diet was isolated specifically because previous studies have shown that absorption of beta-carotene from DGLV is dependent on the physical state of the food consumed (ie, liquefied vs whole) due to differential disruption of the cellular food matrix.31,32 Castenmiller et al 31 showed that enzymatically liquefied spinach increased beta-carotene absorption by 86% compared to the whole food version. Moreover, in contrast to our first LIFE diet study, the present study evaluates the possible short-term benefit of the LIFE diet and the LIFE smoothie. To our knowledge, this is the first study to report diet-intervention-induced changes in CRP and beta-carotene in only 7 days.
We report a retrospective diet-intervention study examining CRP levels among 16 patients in a community practice who were instructed to eat the LIFE diet or their regular diet plus one LIFE smoothie per day for 7 days. The goal of this study was to determine whether 7 days on the full LIFE diet or the LIFE smoothie reduced CRP, which may indicate a rapid reduction in the risk of CVD and other inflammatory conditions.
Materials and Methods
Subject Population
Charts were reviewed from new patients in the integrative medicine practice of DMD. The first criterion for eligibility was that subjects had adhered to the specific “dose” of ingredients and frequency of consumption required by the LIFE diet and/or LIFE smoothie. Specifying dose and frequency was essential to quantify the diets, allowing us to draw more accurate conclusions regarding efficacy. Second, subjects could not have an active infection, a flare-up of seasonal allergies, or physical trauma, as these events can alter CRP irrespective of dietary DGLV intake. Third, subjects had to use the same laboratory on day 0 and day 7 because CRP levels may differ between labs. Fourth, subjects in the LIFE diet group could not eat beta-carotene supplements, plant-based nutritional powders, or other supplements that could increase beta-carotene independent of dietary intake. Patients were also excluded if they ate more than 1 medium sweet potato or 4 cooked carrots during the study period, as these starchy vegetables are high in beta-carotene but are relatively low in phytonutrients, which are likely to be responsible for the anti-inflammatory effects of DGLV.27,33 These dietary restrictions did not apply to subjects in the smoothie group, whose only intervention was the addition of the LIFE smoothie to their regular American diet. In fact, these subjects were excluded if significant alterations were made to their routine diets, such as consuming more ultraprocessed foods than usual, as the study was not meant to investigate whether the LIFE smoothie could compensate for increased unhealthy eating. Fifth, patients could not start or stop a medication during the 1 week study period because some medications, such as rosuvastatin, alter CRP concentrations. 34 All new patients at a community practice were asked to follow one of these regimens as a component of their routine care in DMD’s integrative medicine practice. Laboratory data were collected on day 0 and day 7 of the study period.
Study Design
Thirty-two charts covering a 1-year period (March 2019 to March 2020) were reviewed for eligibility. Sixteen patients met inclusion criteria, and 16 were excluded. Failure to adhere to the LIFE diet or LIFE smoothie instructions was the most common reason for exclusion. Other excluded patients had flare-ups of seasonal allergies, stopped their medications, had acute infections, or physically injured themselves. Each patient who met inclusion criteria belonged to one of the following treatment arms: patients who followed the full LIFE diet (n = 7) or patients who added a once-daily LIFE smoothie to their regular American diet (n = 9).
Patients in the LIFE diet arm of the study followed all components of the LIFE diet as designed by one of the authors (DMD), an integrative physician. 29 This diet was adapted from Dr. Joel Fuhrman’s high nutrient density (HND) diet, which promotes the consumption of foods with a high nutrient per calorie ratio, particularly green vegetables. 33 Adherence to the LIFE diet involved (1) Consumption of one 32-ounce LIFE smoothie every day. The LIFE smoothie consists of 8 ounces (by weight) of DGLV (eg, spinach, baby bok choy, or baby kale), 2.25 cups of blueberries, 1 banana, 1 tablespoon of unsweetened cocoa powder, 1 tablespoon of ground flaxseed, ½ cup of soy milk (plain or vanilla) or unsweetened vanilla almond milk, and ½ cup of water. Patients were counseled by DMD on how to properly measure and combine the ingredients. (2) Consumption of at least 5 ounces by weight of DGLV in salad or cooked vegetables per day. Examples of DGLV are spinach, kale, collard greens, bok choy, broccoli, cauliflower, cabbage, Brussels sprouts, arugula, Swiss chard, endive, asparagus, mustard greens, beet greens, mache, broccolini, broccoli rabe, radish, watercress, escarole, romaine, and green leaf lettuce. Other non-starchy vegetables, such as onions, mushrooms, garlic, green and yellow zucchini, eggplants, peppers, or tomatoes were also recommended. (3) Daily consumption of fruit, specifically berries (the amount required per day is satisfied by the fruit in the LIFE smoothie, but additional fruit consumption was welcomed). (4) Daily consumption of at least ½ cup of beans or legumes or 3 ounces of bean pasta. (5) Limiting consumption of whole grains and starchy vegetables (eg, potatoes, sweet potatoes, winter squashes, peas, corn, and cooked carrots) to no more than 2 servings total per day. Patients could not eat more than 1 medium cooked or uncooked sweet potato or 4 cooked carrots during the study period because these foods contain beta-carotene and could confound beta-carotene levels derived from DGLV. (6) Limiting consumption of refined grains by consuming no more than 1 serving or 4 ounces once over 7 days. (7) Consuming no more than 24 g/day of sugar, which is in accordance with the American Heart Association and the World Health Organization’s guidelines. (8) Limiting consumption of raw seeds and nuts to no more than 2 ounces per day. (9) Limiting consumption of animal protein, including all types of meat, fish, eggs, or dairy products to no more than 4 to 6 ounces once per day. Consumption of cold cuts, bacon, butter, and cheese was prohibited during the study period. (10) Limiting consumption of oils to no more than 1 tablespoon per day (1 teaspoon or less was encouraged if possible). (11) Limiting consumption of dates to no more than 2 medium-sized dates per day. Finally, patients with low vitamin B12 (<550 pg/mL according to European standards) or methylmalonic acid (MMA) levels above the normal range as determined by their labs were advised to take 500 to 1000 µg vitamin B12 daily.
In the LIFE smoothie arm of the study, patients were required to consume a 32-oz LIFE smoothie as described above every day for 7 days without any other changes to their usual diet. None of the patients in this group were following special diets such as a vegetarian, vegan, paleo, or keto diet. Like the LIFE diet group, the smoothie group was intensively counseled on the proper way to measure and combine the smoothie ingredients by DMD.
Adherence to the requirements of each diet was subjectively assessed by DMD during patient interviews 1 week after the initiation of the diet. Compliance was also objectively evaluated using plasma beta-carotene levels on study day 7. As beta-carotene is abundant in DGLV, an increase in plasma levels would suggest at least some adherence to the diets. This retrospective analysis of de-identified patient data was approved by the University of Pennsylvania’s Institutional Review Board (IRB Protocol #: 831566).
Laboratory Measurements
Plasma CRP concentrations were measured using an hsCRP test at any one of the following labs: Quest, LabCorp, or BioReference. Plasma beta-carotene levels were also measured. Patients were required to undergo blood testing at the same lab on study days 0 and 7. Eight patients (50%) were tested at Quest, 7 patients (44%) were tested at LabCorp, and 1 patient (6%) was tested at BioReference.
Statistical Analysis
Patient characteristics were summarized using mean (standard deviation) for continuous measures and percentage for categorical measures. The comparisons of patient characteristics between 2 treatment groups were performed using the 2-sample t test for continuous measures and the Fisher exact test for categorical measures. The differences between day 0 and day 7 in CRP, beta-carotene, HbA1C, CO2, UpH (urinary pH), GFR (glomerular filtration rate), BUN (blood urea nitrogen), Cr (creatinine), BUN/Cr ratio, ferritin, and WBC (white blood cells) were tested within the same treatment group using the paired t test and compared between 2 treatment groups using the 2-sample t test. The Spearman correlation coefficient was used to evaluate the association between change of beta-carotene and change of CRP and other measures. All statistical analyses were performed in SAS v9.4 (SAS Institute Inc), and 2-sided P < .05 was considered to be statistically significant.
Results
Characteristics of Study Participants
Sixteen patient records met the inclusion criteria and were included in the analysis: 7 in the LIFE diet group and 9 in the smoothie group. Baseline characteristics of participants from each group are shown in Table 1. In the LIFE diet group, 3 (42.9%) patients were male and 4 (57.1%) were female. In the smoothie group, 4 (44.4%) patients were male and 5 (55.6%) patients were female. Patients in the LIFE diet group were significantly older than patients in the smoothie group, with mean ages of 65 and 49, respectively (P = .01). Both groups were racially diverse. In the LIFE diet group, 2 (28.6%) patients were Asian, 1 (14.3%) patient was Black, 2 (28.6%) patients were White, and 2 (28.6%) patients were Hispanic. In the smoothie group, 3 (33.3%) patients were Black and 6 (66.7%) patients were Hispanic. Common chronic diseases were represented in both patient populations. Four (57.1%) patients had diabetes, 3 (42.9%) patients had hypertension, and 6 (85.7%) patients had hyperlipidemia in the LIFE diet group. One (11.1%) patient had diabetes, 4 (44.4%) patients had hypertension, and 1 (11.1%) patient had hyperlipidemia in the smoothie group. There was no significant difference in baseline laboratory values between subjects in either study arm. In the LIFE diet group, baseline CRP ranged from <0.3 to 2.19 mg/L, with a mean of 1.32 mg/L. In the smoothie group, baseline CRP ranged from 1.2 to 7.6 mg/L, with a mean of 2.86 mg/L. Baseline beta-carotene ranged from 19.7 to 168 µg/dL, with a mean of 70.24 µg/dL in the LIFE diet group, and from 7 to 98 µg/dL, with a mean of 40.56 µg/dL in the smoothie group.
Table 1.
Characteristics of Study Participants (N = 16).
| Characteristic | LIFE diet group (n = 7) | Smoothie group (n = 9) | P value |
|---|---|---|---|
| Age (years), mean (SD) | 65 (9.5) | 49 (11.4) | .01 |
| Gender, male (%) | 3 (42.9%) | 4 (44.4%) | 1.00 |
| Race, n (%) | 0.10 | ||
| Asian | 2 (28.6%) | 0 (0.0%) | |
| Black | 1 (14.3%) | 3 (33.3%) | |
| White | 2 (28.6%) | 0 (0.0%) | |
| Latino | 2 (28.6%) | 6 (66.7%) | |
| Diabetes, n (%) | 4 (57.1%) | 1 (11.1%) | .11 |
| Hypertension, n (%) | 3 (42.9%) | 4 (44.4%) | 1.00 |
| Hyperlipidemia, n (%) | 6 (85.7%) | 1 (11.1%) | .009 |
| CRP (mg/L), mean (SD) | 1.32 (0.78) | 2.86 (2.03) | .06 |
| Beta-carotene (µg/dL), mean (SD) | 70.24 (47.65) | 40.56 (25.53) | .13 |
| HbA1C (%), mean (SD) | 6.45 (1.00) | 5.53 (0.53) | .08 |
| CO2 (mmol/L), mean (SD) | 26.0 (2.16) | 26.8 (3.63) | .63 |
| UpH, mean (SD) | 6.07 (0.53) | 6.25 (0.96) | .67 |
| GFR (mL/min/1.73 m2), mean (SD) | 87.1 (13.1) | 80.6 (12.9) | .33 |
| BUN (mg/dL), mean (SD) | 16.4 (8.58) | 17.2 (4.82) | .82 |
| Cr (mg/dL), mean (SD) | 0.80 (0.18) | 0.99 (0.19) | .06 |
| BUN-Cr ratio, mean (SD) | 20.4 (8.70) | 17.6 (4.33) | .40 |
| Ferritin (µg/L), mean (SD) | 89.8 (46.2) | 137 (210) | .56 |
| WBC (×109/L), mean (SD) | 5.55 (1.23) | 5.58 (0.74) | .96 |
| Weight (lbs), mean (SD) | 144 (33.9) | 203 (40.7) | .01 |
| % of fat, mean (SD) | 31.0 (5.63) | 32.9 (7.03) | .58 |
| BMI (kg/m2), mean (SD) | 25.22 (4.21) | 30.18 (4.54) | .05 |
Abbreviations: LIFE, Low Inflammatory Foods Everyday diet; CRP, C-reactive protein; UpH, urinary pH; GFR, glomerular filtration rate; BUN, blood urea nitrogen; CR, creatinine; WBC, white blood cells; BMI, body mass index.
Comparison of Outcomes Between Subjects in the LIFE Diet Group and Smoothie Group
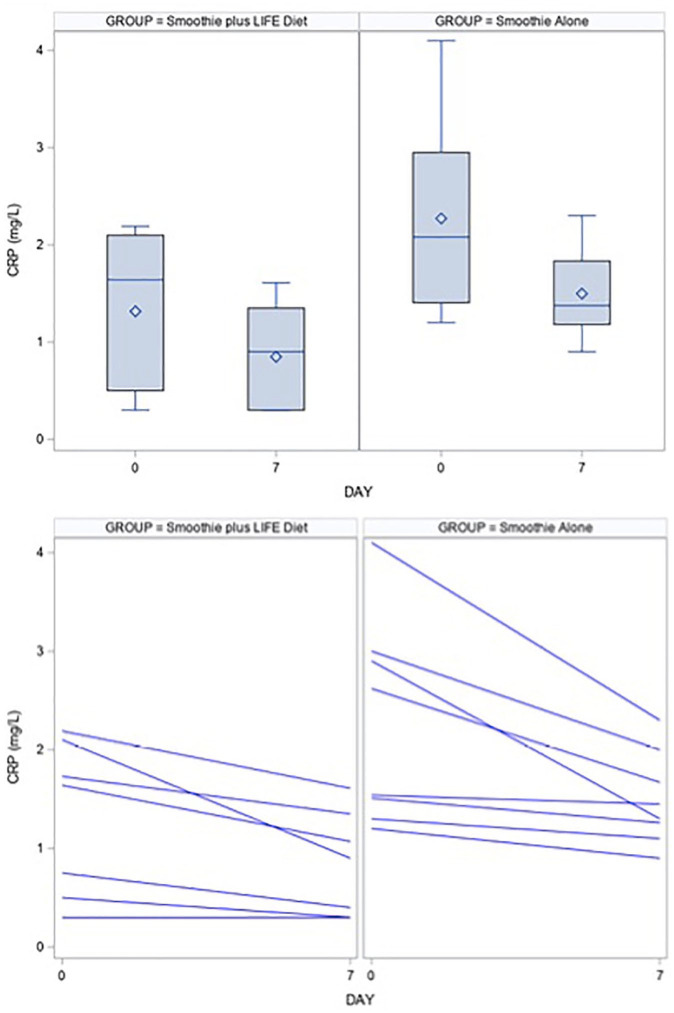
After the 7-day dietary interventions, the mean (SD) change in CRP from baseline was significantly reduced in both groups (Table 2). In the LIFE diet group, mean plasma CRP decreased by 0.47 (0.38) mg/L, from 1.32 to 0.85 mg/L (P = .02). In the smoothie group, mean plasma CRP decreased by 1.2 (1.50) mg/L, from 2.86 to 1.66 mg/L (Figure 1; P = .04). There was no significant difference in CRP reductions between groups (P = .18).
Table 2.
Comparison of Outcomes Between Treatment Groups.
| Change of outcome measures from baseline | LIFE diet group (n = 7) |
Smoothie group (n = 9) |
P value a | ||
|---|---|---|---|---|---|
| Mean (SD) | P value b | Mean (SD) | P value b | ||
| Change of beta-carotene | 23.2 (19.1) | .02 | 20.6 (16.5) | .006 | .78 |
| Change of CRP | −0.47 (0.38) | .02 | −1.2 (1.5) | .04 | .18 |
| Change of HbA1C | −0.02 (0.18) | .83 | NA | NA | NA |
| Change of CO2 | 0.71 (3.35) | .59 | 1.89 (2.62) | .06 | .44 |
| Change of UpH | 0.43 (1.02) | .31 | 0.14 (1.07) | .74 | .62 |
| Change of GFR | 2.57 (10.4) | .54 | 0.88 (5.03) | .64 | .69 |
| Change of BUN | −2.7 (5.3) | .22 | −2.0 (3.57) | .13 | .75 |
| Change of Cr | −0.01 (0.11) | .87 | −0.01 (0.06) | .69 | .99 |
| Change of BUN-Cr ratio | −3.1 (6.69) | .26 | −2.3 (3.77) | .10 | .76 |
| Change of ferritin | −14 (15.3) | .11 | −29 (52.6) | .17 | .57 |
| Change of WBC | −45 (0.52) | .06 | 0.29 (1.11) | .46 | .13 |
Abbreviations: LIFE, Low Inflammatory Foods Everyday diet; CRP, C-reactive protein; UpH, urinary pH; GFR, glomerular filtration rate; BUN, blood urea nitrogen; CR, creatinine; WBC, white blood cell.
To test whether the difference between 2 treatment groups is statistically significant or not using 2-sample t test.
To test whether there is statistically significant change from baseline using paired t test.
Figure 1.
Boxplots and line plots for the distribution of C-reactive protein (CRP) at baseline and day 7 in the LIFE diet and smoothie groups. For the purposes of maintaining the scale of the graph, we excluded one outlier in the smoothie group. Statistical analysis was performed using a 2-sample t test.
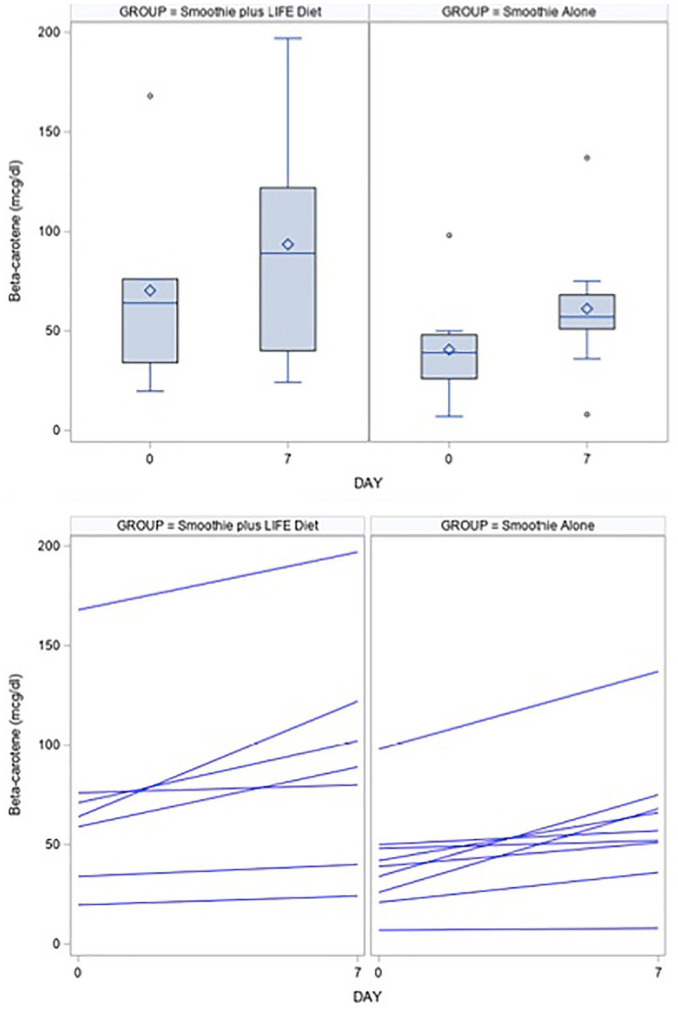
Change in mean beta-carotene from baseline was also statistically significant in both the LIFE diet group and the smoothie group after 7 days (Table 2). In the LIFE diet group, mean (SD) beta-carotene increased by 23.2 (19.1) µg/dL, from 70.2 to 93.4 µg/dL (P = .02). In the smoothie group, mean beta-carotene increased by 20.6 µg/dL (16.5), from 40.5 to 61.1 µg/dL (Figure 2; P = .006). There was no significant difference in beta-carotene increases between groups (P = .78).
Figure 2.
Boxplots and line plots for the distribution of beta-carotene at baseline and day 7 in the LIFE diet and smoothie groups. Statistical analysis was performed using a 2-sample t test.
Changes in HbA1C, CO2, UpH, GFR, BUN, Cr, BUN/Cr ratio, and ferritin were not statistically significant in either group or between groups. There was a reduction in WBC from baseline in the LIFE diet group, though this decline did not quite reach statistical significance (P = .06). There was no significant change in WBC in the smoothie group or between the 2 groups (Table 2).
Association Between Changes in Plasma Beta-Carotene Levels and CRP Levels
Table 3 shows the correlation between change in beta-carotene and change in CRP and other measures for all subjects not separated by treatment group. As expected, the change in mean beta-carotene was inversely correlated with the change in CRP levels for all patients, though this relationship was not significantly significant (r = −0.3, P = .26). Beta-carotene was again inversely related to CRP when patients were separated into the LIFE diet (r = −0.21) or smoothie group (r = −0.35; Table 4). In the LIFE diet group, beta-carotene increased 33.0% from baseline, which corresponded to a 35.8% decrease in CRP. Likewise, in the smoothie group, beta-carotene increased 50.7% from baseline, which corresponded to a 43.4% decrease in CRP (Table 5). Overall, however, the inverse relationship between CRP and beta-carotene was not statistically significant in either group (P = .65; P = .36; Table 4).
Table 3.
Correlation Between Change of Beta-Carotene and Change of CRP and Other Measures (N = 16).
| Change of measures from baseline | # of subjects included for correlation analysis | Spearman correlation coefficient (P value) |
|---|---|---|
| Change of CRP | 16 | −0.30 (.26) |
| Change of HbA1C | 6 | 0.50 (.31) |
| Change of CO2 | 16 | 0.13 (.62) |
| Change of UpH | 14 | −0.15 (.61) |
| Change of GFR | 15 | 0.16 (.58) |
| Change of BUN | 16 | 0.31 (.24) |
| Change of Cr | 16 | −0.20 (.46) |
| Change of BUN-Cr ratio | 16 | 0.37 (.16) |
| Change of ferritin | 13 | 0.09 (.76) |
| Change of WBC | 16 | 0.33 (.21) |
Abbreviations: LIFE, Low Inflammatory Foods Everyday diet; CRP, C-reactive protein; UpH, urinary pH; GFR, glomerular filtration rate; BUN, blood urea nitrogen; CR, creatinine; WBC, white blood cells.
Table 4.
Correlation Between Change of Beta-Carotene and Change of CRP and Other Measures by Treatment Group.
| Change of measures from baseline | LIFE diet group (n = 7) |
Smoothie group (n = 9) |
||
|---|---|---|---|---|
| # of subjects included for correlation analysis | Spearman correlation coefficient (P value) | # of subjects included for correlation analysis | Spearman correlation coefficient (P value) | |
| Change of CRP | 7 | −0.21 (.65) | 9 | −0.35 (.36) |
| Change of HbA1C | 6 | 0.50 (.31) | 0 | NA |
| Change of CO2 | 7 | −0.11 (.82) | 9 | 0.21 (.58) |
| Change of UpH | 7 | −0.13 (.79) | 7 | −0.07 (.88) |
| Change of GFR | 7 | 0.11 (.82) | 8 | 0.18 (.67) |
| Change of BUN | 7 | −0.05 (.91) | 9 | 0.60 (.09) |
| Change of Cr | 7 | −0.11 (.82) | 9 | −0.19 (.62) |
| Change of BUN-Cr ratio | 7 | 0.00 (1.00) | 9 | 0.69 (.04) |
| Change of ferritin | 5 | 0.50 (.39) | 8 | 0.05 (.91) |
| Change WBC | 7 | 0.20 (.67) | 9 | 0.45 (.22) |
Abbreviations: LIFE, Low Inflammatory Foods Everyday diet; CRP, C-reactive protein; UpH, urinary pH; GFR, glomerular filtration rate; BUN, blood urea nitrogen; CR, creatinine; WBC, white blood cell.
Table 5.
Comparison of Percent Change in Mean CRP and Mean Beta-Carotene by Treatment Group.
| LIFE diet group day 0 | LIFE diet group day 7 | % Change | |
|---|---|---|---|
| Beta-carotene mean | 70.24 | 93.45 | +33.04 |
| CRP mean | 1.32 | 0.847 | −35.83 |
| Smoothie group day 0 | Smoothie group day 7 | % Change | |
| Beta-carotene mean | 40.56 | 61.11 | +50.67 |
| CRP mean | 2.86 | 1.62 | −43.36 |
Abbreviations: LIFE, Low Inflammatory Foods Everyday diet; CRP, C-reactive protein.
When all patients were analyzed together, there was no statistically significant correlation between change in plasma beta-carotene and change in HbA1C, CO2, UpH, GFR, BUN, Cr, BUN/Cr ratio, ferritin, or WBC (Table 4). When subjects were separated by their respective groups, a statistically significant positive correlation was observed between change in beta-carotene and change in BUN/Cr ratio for the smoothie group (r = 0.69, P = .04). Changes in beta-carotene were not associated with changes in any of the other laboratory measures for either group (Table 4).
Discussion
In this retrospective analysis, we showed that CRP was significantly reduced in a small group of patients over a week-long dietary intervention. The decrease in CRP, and therefore decrease in systemic inflammation, was apparent in patients who adhered to the LIFE diet as well as in patients who maintained their regular diet with the addition of a 32-oz LIFE smoothie per day. Serum beta-carotene levels significantly increased from baseline in both groups, indicating that all patients were objectively adherent to the diet. As expected, decreases in CRP corresponded with increases in plasma beta-carotene, although the inverse correlation between beta-carotene and CRP did not reach statistical significance in this small patient population.
Importantly, patients in this study were excluded if they made any alterations to their medications (ie, starting, stopping, changing dose) or plant-based nutritional powder intake over the course of the study, as these behaviors could have confounded our findings. While plant-based supplements increase plasma beta-carotene, they fail to lower CRP because they lack the other phytonutrients found in DGLV. Moreover, supplement-induced increases in beta-carotene have been associated with either no effect or adverse effects on the risk of death from lung cancer and CVD. 35 One patient in the smoothie group had been regularly taking beta-carotene supplements prior to and during the study period. However, this did not confound results as her plasma beta-carotene levels increased from her baseline, while her CRP levels decreased, with no additional changes made to her supplement or dietary routine aside from the LIFE smoothie. Similarly, CRP levels were not confounded by medications or smoking because no patients added and/or increased their doses of medications, and no patients were smokers.
Overall, our findings were consistent with previous studies that have reported decreased plasma CRP with increased fruit and vegetable intake,30,36 including our first LIFE diet study published in 2019. 29 The study presented herein adds new information in several ways. First, it shows that the LIFE diet and LIFE smoothie can produce a 30% to 40% reduction in CRP in just 1 week. This rate of statistically significant change is the fastest diet-induced reduction in CRP we found in the literature.
Rapid CRP reduction could have important implications for patients in the current health care climate. Studies published in April and May of 2020 found that CRP is an independent risk factor that predicts mortality and severity of infection from COVID-19 (as defined by Chinese National Health Commission criteria and CT severity scores).12-14 Therefore, dietary modification with the LIFE diet or the LIFE smoothie may offer accessible and rapidly effective options for patients to reduce their plasma CRP levels, which may help decrease their risk of death and severe infection. At the very least, these findings warrant future studies to examine the association between a diet, such as the LIFE diet, and COVID-19 disease mortality and severity more specifically.
Second, patients were able to significantly decrease their inflammatory load by adding one 32-oz LIFE smoothie per day to their normal routine diet. Food often has intimate social, emotional, and cultural undertones, making dietary change a long and challenging process for many patients. Dr. Joel Fuhrman maintains that most overweight Americans, and therefore most Americans in general, are addicted to the food they eat, 33 and the difficulty in overcoming this addiction has been widely established in current literature.37-41 The present study suggests that a single dietary modification can provide quick and substantial health benefits by lowering CRP. While we do not suggest that a daily LIFE smoothie can compensate for a diet high in ultra-processed and fatty foods, it may offer a more practical first step for patients interested in improving their health through diet, but who are not ready to implement the more sweeping changes required by the entire LIFE diet.
Third, the baseline characteristics of patients in the present study differed from those in the original LIFE diet study. In the previous study, most patients were White, with only one Hispanic patient and no Asian patients included in the analysis. The current study includes a racially diverse patient population. Eight patients were Hispanic, 4 were Black, 2 were Asian, and 2 were White. The increased diversity suggests that the LIFE diet and LIFE smoothie may benefit patients of more diverse races than previously demonstrated. Furthermore, our population had a substantially lower baseline CRP than the patient population in the initial LIFE diet study. Mean baseline CRPs were 1.32 mg/L and 2.86 mg/L in the current LIFE diet and smoothie groups, respectively, compared to 6.67 mg/L among patients in the initial LIFE diet study (“normal” CRP ≤3 mg/L). Regardless of these low baseline values, we know from Ridker et al 22 that decreasing CRP from baseline levels even as low as 0.5 mg/L produces a linear reduction in the risk of future cardiovascular events. Because there is no threshold to the benefits reached once patients attain or even start with lower than normal CRP levels, our goal should still be continued reduction. Here we showed that a significant decrease in CRP achievable in only 1 week on the LIFE diet or on a regular diet supplemented with the LIFE smoothie, offers a spectrum of effective interventions for patients seeking to rapidly alleviate systemic inflammation and associated risks.
Fourth, we observed that WBC count trended downward for patients who consumed the LIFE diet for 7 days. Though this decrease was not statistically significant, it is important to mention because WBC count, like plasma CRP, is a widely accepted biomarker of systemic inflammation 42 that is also predictive of ischemic heart disease independent of CVD risk factors.43-45 Patients in this study with an already low-normal mean baseline WBC count (5.5 × 109/L), experienced an even further reduction in 7 days on the LIFE diet. These results are consistent with a previous diet-based population cohort study, which reported that of 14 000 patients who consumed the Mediterranean diet, those with the strictest adherence based on the Italian Mediterranean Index had increased odds of being in the lowest WBC count group (WBC < 4 × 109/L; odds ratio = 1.41; 95% confidence interval = 1.07-1.86). 46 Our findings suggest that the LIFE diet may provide an additional diet-based means of reducing WBC count and chronic low-grade inflammation.
In this study, we decided to isolate the smoothie component of the LIFE diet because the LIFE smoothie itself, as a homogenized liquid, may have inherent properties that support phytonutrient absorption, and therefore, CRP reduction. In a prospective randomized controlled trial, Castenmiller et al 31 investigated the effects of variously processed spinach products on serum carotenoid concentrations. In the study, subjects consumed whole leaf spinach, minced spinach, or enzymatically liquefied spinach from a single batch for 3 weeks after completion of a 3-week washout period in which subjects avoided carotenoid-rich foods. Results of the study showed a 48% increase in beta-carotene bioavailability in the liquefied spinach group versus the minced spinach group (P = .05), and an 86% increase in beta-carotene bioavailability in the liquefied spinach group compared to the whole leaf spinach group (P = .03). The authors attributed the impressive bioavailability of liquefied spinach to the complete disruption of cellular integrity achieved through the liquefaction processes. The increased beta-carotene absorption observed after enzymatic liquefaction of spinach suggests that high-intensity mechanical liquefaction may also promote absorption; however, a study directly comparing the 2 methods of liquefaction is still needed. Nevertheless, the rapid increase in beta-carotene following smoothie consumption indicates that beta-carotene was well absorbed. Thus, by liquefying spinach with a high-intensity blender, the LIFE smoothie may provide ample benefit (high absorption of beta-carotene and hundreds of other phytonutrients) with modest behavioral change (consumption of 1 smoothie daily).
Importantly, the LIFE diet and LIFE smoothie are highly specific and readily quantifiable dietary modifications. Patients were counseled on how often to consume the respective components of their diets and the precise way to measure ingredients. This was done purposefully so that evaluations of efficacy were based on measurable doses and frequencies, similar to the dose responses exhibited by medications. For example, in a multicenter randomized double-blind placebo-control trial, when patients received 2.5, 5, and 10 mg of amlodipine, target blood pressure goals were reached in 41%, 56%, and 73% of patients, respectively. 47 Similar linear dose responses have been observed for lisinopril, 48 simvastatin, 49 valsartan, 50 and dabigatran. 51 Diet studies exhibit dose-response associations as well. 52 Fruit consumption, for example, has a powerful dose-response relationship with reduced mortality from cerebrovascular disease, heart disease, and all-cause mortality. 53 Furthermore, the initial LIFE diet study showed that patients classified as adherent (ie, consumed the proper dose at the correct frequency) saw decreases in CRP versus patients classified as nonadherent who did not. 29 Overall, the concept presented here can be represented in the following equation: health benefit = frequency × dose. This equation is meant to demonstrate that the health benefit derived from a specific and quantifiable dietary modification, just like medication, can be directly related to the frequency of consumption and dose of ingredients, up to the recommended dose. Importantly, doses in dietary interventions can be significantly affected by the physical state of food. The liquefied DGLV in the LIFE smoothie, for example, may have increased absorption capability compared to whole vegetables. Consumption of the liquefied ingredients in the smoothie, therefore, could represent higher dosages, which should result in increased efficacy.
It is important to acknowledge that our study is small in sample size and retrospective in nature, but our achievement of CRP reduction to <2 mg/L in 7 days justifies larger, prospective follow-up studies.
Overall, this study shows that the LIFE diet and a regular diet supplemented with the LIFE smoothie are associated with increased beta-carotene and decreased systemic CRP in 7 days. Lower CRP concentrations have been associated with reduced risk of inflammation-driven conditions, such as AMD, CVD, autoimmune diseases, and COVID-19. The easily quantifiable, beta-carotene-rich LIFE diet and/or LIFE smoothie may offer fast, effective, and practical ways to reduce this risk and improve overall health.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Adele Niessen Endowed Chair (JLD).
Ethical Approval: This retrospective analysis of de-identified patient data was approved by the University of Pennsylvania’s Institutional Review Board (IRB Protocol #: 831566).
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: Brittany Perzia  https://orcid.org/0000-0003-0528-5247
https://orcid.org/0000-0003-0528-5247
References
- 1.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363-369. [DOI] [PubMed] [Google Scholar]
- 2.Yeh ET. CRP as a mediator of disease. Circulation. 2004;109(21 Suppl 1):II11-II14. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:e81-e85. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836-843. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481-2485. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334. [DOI] [PubMed] [Google Scholar]
- 7.Dessein PH, Joffe BI, Stanwix AE. High sensitivity C-reactive protein as a disease activity marker in rheumatoid arthritis. J Rheumatol. 2004;31:1095-1097. [PubMed] [Google Scholar]
- 8.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217-2224. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Caporaso NE, Katki HA, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585-590. [DOI] [PubMed] [Google Scholar]
- 11.Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216-5222. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan C, Huang Y, Shi F, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92:856-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan L, Zhang HT, Goncalves J, et al. An interpretable mortality prediction model for COVID-19 patients. Nature Mach Intell. 2020;2:283-288. [Google Scholar]
- 15.Fu EL, Franko MA, Obergfell A, et al. High-sensitivity C-reactive protein and the risk of chronic kidney disease progression or acute kidney injury in post-myocardial infarction patients. Am Heart J. 2019;216:20-29. [DOI] [PubMed] [Google Scholar]
- 16.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291:704-710. [DOI] [PubMed] [Google Scholar]
- 17.Olafsdottir IS, Gislason T, Thjodleifsson B, et al. C reactive protein levels are increased in non-allergic but not allergic asthma: a multicentre epidemiological study. Thorax. 2005;60:451-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl M, Vestbo J, Zacho J, Lange P, Tybjaerg-Hansen A, Nordestgaard BG. C reactive protein and chronic obstructive pulmonary disease: a Mendelian randomisation approach. Thorax. 2011;66:197-204. [DOI] [PubMed] [Google Scholar]
- 19.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945-2951. [DOI] [PubMed] [Google Scholar]
- 20.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and all-cause mortality—the Copenhagen City Heart Study. Eur Heart J. 2010;31:1624-1632. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319-328. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham risk scores. Circulation. 2004;109:1955-1959. [DOI] [PubMed] [Google Scholar]
- 23.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haghighatdoost F, Bellissimo N, Totosy de, Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira A, Rodriguez-Artalejo F, Lopes C. The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur J Clin Nutr. 2009;63:1345-1352. [DOI] [PubMed] [Google Scholar]
- 26.Persson V, Ahmed F, Gebre-Medhin M, Greiner T. Increase in serum beta-carotene following dark green leafy vegetable supplementation in mebendazole-treated school children in Bangladesh. Eur J Clin Nutr. 2001;55:1-9. [DOI] [PubMed] [Google Scholar]
- 27.Stuetz W, Gowele V, Kinabo J, et al. Consumption of dark green leafy vegetables predicts vitamin A and iron intake and status among female small-scale farmers in Tanzania. Nutrients. 2019;11:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlinger TP, Guallar E, Miller ER, 3rd, Stolzenberg-Solomon R, Appel LJ. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med. 2001;161:1903-1908. [DOI] [PubMed] [Google Scholar]
- 29.Schultz H, Ying GS, Dunaief JL, Dunaief DM. Rising plasma beta-carotene is associated with diminishing c-reactive protein in patients consuming a dark green leafy vegetable–rich, low inflammatory foods everyday (LIFE) diet. Am J Lifestyle Med. Published online December 21, 2019. doi: 10.1177/1559827619894954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watzl B, Kulling SE, Moseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82:1052-1058. [DOI] [PubMed] [Google Scholar]
- 31.Castenmiller JJ, West CE, Linssen JP, van het Hof KH, Voragen AG. The food matrix of spinach is a limiting factor in determining the bioavailability of beta-carotene and to a lesser extent of lutein in humans. J Nutr. 1999;129:349-355. [DOI] [PubMed] [Google Scholar]
- 32.Kolodziejczyk JK, Flatt SW, Natarajan L, Patterson R, Pierce JP, Norman GJ. Associations of soluble fiber, whole fruits/vegetables, and juice with plasma beta-carotene concentrations in a free-living population of breast cancer survivors. Women Health. 2012;52:731-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuhrman J. Eat to Live: The Amazing Nutrient-Rich Program for Fast and Sustained Weight Loss: The Amazing Nutrient-Rich Program for Fast and Sustained Weight Loss. Little, Brown; 2011. [Google Scholar]
- 34.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-2207. [DOI] [PubMed] [Google Scholar]
- 35.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. [DOI] [PubMed] [Google Scholar]
- 36.Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J Nutr. 2004;134:913-918. [DOI] [PubMed] [Google Scholar]
- 37.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shriner RL. Food addiction: detox and abstinence reinterpreted? Exp Gerontol. 2013;48:1068-1074. [DOI] [PubMed] [Google Scholar]
- 39.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellisle F, Drewnowski A, Anderson GH, Westerterp-Plantenga M, Martin CK. Sweetness, satiation, and satiety. J Nutr. 2012;142:1149S-1154S. [DOI] [PubMed] [Google Scholar]
- 41.Adams RC, Sedgmond J, Maizey L, Chambers CD, Lawrence NS. Food addiction: implications for the diagnosis and treatment of overeating. Nutrients. 2019;11:2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rienstra M, Sun JX, Magnani JW, et al. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2012;109:533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120:1409-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658-670. [DOI] [PubMed] [Google Scholar]
- 45.Grau AJ, Boddy AW, Dukovic DA, et al. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke. 2004;35:1147-1152. [DOI] [PubMed] [Google Scholar]
- 46.Bonaccio M, Di Castelnuovo A, De Curtis A, et al. Adherence to the Mediterranean diet is associated with lower platelet and leukocyte counts: results from the Moli-sani study. Blood. 2014;123:3037-3044. [DOI] [PubMed] [Google Scholar]
- 47.Frick MH, McGibney D, Tyler HM. A dose-response study of amlodipine in mild to moderate hypertension. J Intern Med. 1989;225:101-105. [DOI] [PubMed] [Google Scholar]
- 48.Gomez HJ, Cirillo VJ, Sromovsky JA, et al. Lisinopril dose-response relationship in essential hypertension. Br J Clin Pharmacol. 1989;28:415-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuomilehto J, Guimaraes AC, Kettner H, et al. Dose-response of simvastatin in primary hypercholesterolemia. J Cardiovasc Pharmacol. 1994;24:941-949. [DOI] [PubMed] [Google Scholar]
- 50.Pool JL, Glazer R, Chiang YT, Gatlin M. Dose-response efficacy of valsartan, a new angiotensin II receptor blocker. J Hum Hypertens. 1999;13:275-281. [DOI] [PubMed] [Google Scholar]
- 51.Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103-111. [DOI] [PubMed] [Google Scholar]
- 52.Aune D, Giovannucci E, Boffetta P, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Key TJ, Thorogood M, Appleby PN, Burr ML. Dietary habits and mortality in 11 000 vegetarians and health conscious people: results of a 17 year follow up. BMJ. 1996;313:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]