Abstract
Question addressed by the study
Do three coronavirus disease 2019 (COVID-19) vaccine doses induce a serological response in lung transplant recipients?
Methods
We retrospectively included 1071 adults (551 (52%) males) at nine transplant centres in France. Each had received three COVID-19 vaccine doses in 2021, after lung transplantation. An anti-spike protein IgG response, defined as a titre >264 BAU·mL−1 after the third dose (median (interquartile range (IQR)) 3.0 (1.7–4.1) months), was the primary outcome and adverse events were the secondary outcomes. Median (IQR) age at the first vaccine dose was 54 (40–63) years and median (IQR) time from transplantation to the first dose was 64 (30–110) months.
Results
Median (IQR) follow-up after the first dose was 8.3 (6.7–9.3) months. A vaccine response developed in 173 (16%) patients. Factors independently associated with a response were younger age at vaccination, longer time from transplantation to vaccination and absence of corticosteroid or mycophenolate therapy. After vaccination, 51 (5%) patients (47 non-responders (47/898 (5%)) and four (4/173 (2%)) responders) experienced COVID-19, at a median (IQR) of 6.6 (5.1–7.3) months after the third dose. No responders had severe COVID-19 compared with 15 non-responders, including six who died of the disease.
Conclusions
Few lung transplant recipients achieved a serological response to three COVID-19 vaccine doses, indicating a need for other protective measures. Older age and use of mycophenolate or corticosteroids were associated with absence of a response. The low incidence of COVID-19 might reflect vaccine protection via cellular immunity and/or good adherence to shielding measures.
Short abstract
Three mRNA COVID-19 vaccine doses rarely induced a serological response in lung transplant patients. COVID-19 was rare, suggesting cellular immunity and/or strong adherence to shielding measures. Other protective methods should be sought. https://bit.ly/3pr9Wox
Introduction
The repeated worldwide waves of coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) require urgent efforts to optimise vaccine responses in immunocompromised patients, including lung transplant recipients. In France, this population was among the first to be vaccinated, starting in January 2021, with the two mRNA COVID-19 vaccines approved by the European Medicines Agency: mRNA BNT162b2 (Comirnaty; Pfizer-BioNTech) and mRNA-1273 (Spikevax; Moderna). The two non-mRNA COVID-19 vaccines, ChAdOx1 nCoV-19 (Vaxzevria; AstraZeneca-Oxford University) and Ad26.COV2.S (Janssen; Janssen), were approved and used later.
Recent reports showed higher mortality rates in hospitalised transplant recipients than in non-transplant patients after adjustment for comorbidities. In a French matched case–control study comparing outcomes of kidney transplant recipients and non-transplanted patients, all of whom were admitted for COVID-19, the 30-day cumulative incidence of severe COVID did not differ between the two groups, whereas 30-day mortality was significantly higher in the transplant group (17.9% versus 11.4%, respectively; p=0.038); however, by multivariable analysis, kidney transplantation was not independently associated with mortality [1]. In an international cohort of 482 transplanted patients, COVID-19 mortality was 20.5% overall but was 33% in 30 lung transplant recipients [2]. In a French cohort of 35 lung transplant recipients who experienced COVID-19 during the first few months of the pandemic, hospital admission was usually required and mortality was 14.3% [3]. These data support the strong recommendation from the International Society for Heart and Lung Transplantation to vaccinate all transplant candidates and recipients, despite the exclusion of these from all phases of COVID-19 vaccine trials [4, 5].
Data on COVID-19 vaccine responses in solid-organ transplant recipients are emerging [6–10], with most studies using the production of anti-spike or anti-receptor binding domain antibodies as a marker for vaccine efficacy. These patients proved capable of generating a robust humoral response to natural SARS-CoV-2 infection, with 78% testing positive for antibodies >3 months after the diagnosis [6]. However, in several studies only 40–69% had positive serological tests after three doses of COVID-19 vaccine [7–13]. The response to COVID-19 vaccines may vary according to the type of organ transplant and data in the subgroup with lung transplantation remain sparse [11, 12]. In a previous study, the proportion of serological responders to two vaccine doses was 62% among heart transplant recipients but only 36% among lung transplant recipients, reflecting the greater intensity of immunosuppression required after lung transplantation [11]. Although the evaluation of vaccination efficacy usually relies on serology, further information on the humoral and cellular responses to, and clinical effectiveness of, COVID-19 vaccines in lung transplant recipients is needed to develop optimal vaccination guidelines. France was among the first countries to recommend a booster dose in immunocompromised patients, including transplant recipients, in early April 2021, based on preliminary evidence of a poor response to the first two doses [14–17].
The objective of this retrospective observational cohort study was to assess the spike protein IgG antibody response and clinical protection offered by three mRNA COVID-19 vaccine doses in lung transplant recipients.
Methods
Study design and population
We conducted a multicentre, retrospective, observational cohort study. Patients with three doses of COVID-19 vaccines approved in France were included between 1 January 2021 and 31 August 2021. Participants were recruited at nine lung transplant centres belonging to the Groupe de Travail de Transplantation de la Société Française de Pneumologie, a network composed of all lung transplant centres involved in biomedical research projects in France. Exclusion criteria were age <12 years, vaccination before transplantation, less or more than three vaccine doses, absence of data on post-vaccination SARS-CoV-2 antibody levels, COVID-19 infection before vaccination or before serological testing and treatment with monoclonal antibodies to SARS-CoV-2 before serological testing. Patients who received monoclonal antibodies to SARS-CoV-2 as prophylaxis due to vaccination failure were kept in the study if this treatment was delivered after serological testing.
The study was conducted in accordance with French legislation on biomedical research and the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Société Française de Chirurgie Thoracique et Cardio-Vasculaire (IRB00012919). Written informed consent was obtained from each patient before study inclusion. Details of the lung transplantation procedures and management of COVID-19 are available in the supplementary material.
Data collection
Data were entered into a secure and anonymised database. We recorded demographic features, characteristics of the transplantation procedure, medications, nature and timing of the vaccine doses, occurrence of COVID-19 infection after vaccination, and administration of monoclonal antibodies to treat or prevent COVID-19. The maintenance immunosuppressive regimen at the time of vaccination was collected, as well as whether intensified immunosuppression was given within 6 months before vaccination. Follow-up for the study was ended on 31 December 2021.
Vaccination protocol and vaccine response assessment
Starting in January 2021, all French lung transplant centres implemented a proactive strategy to provide COVID-19 vaccination to eligible lung transplant recipients. Due to the high level of immunosuppression required initially, the first vaccine dose was given no sooner than 3 months after lung transplantation. Three doses were given following French recommendations, with intervals of at least 4 weeks between doses [18]. A cut-off of 256 binding antibody units (BAU)·mL−1 was chosen by French health authorities to separate responders from non-responders [19], based on serological and clinical data from a UK trial of the AstraZeneca vaccine done before the emergence of variants [20]. In line with French recommendations [18], patients with titres between 51 and 264 BAU·mL−1 received a fourth vaccine dose consisting of the BNT162b2 mRNA Pfizer vaccine; the response to this dose was not investigated in the current study. Patients with titres ≤50 BAU·mL−1 were offered monoclonal antibody treatment against COVID-19.
SARS-CoV-2 spike protein IgG antibody assays
Blood samples were collected locally 3 weeks to 6 months after the third vaccine dose. Samples were analysed using locally available CE-marked enzyme immunoassays among the following: Architect SARS-CoV-2 (Abbott, Chicago, IL, USA), Liaison (DiaSorin, Saluggia, Italy), Elecsys Anti-SARS-CoV-2 (Roche Diagnostics, Basel, Switzerland), NovaLisa (NovaTec Immundiagnostica, Dietzenbach, Germany), Access SARS-CoV-2 IgG (Beckman Coulter, Brea, CA, USA) and Atellica IM SARS-CoV-2 (Siemens, Munich, Germany). All assays were used and results converted to BAU·mL−1 if needed, according to the instructions of the manufacturer.
Outcome measures
The primary outcome was the proportion of patients with a spike protein IgG antibody titre ≥264 BAU·mL−1.
The secondary outcome was the proportion of patients with COVID-19, defined as a positive nasopharyngeal SARS-CoV-2 PCR test after the third vaccine dose. Testing was done when there was a known contact with a patient who had COVID-19 or when symptoms consistent with COVID-19 developed. Moderate COVID-19 was defined as clinical signs of pneumonia (fever, cough, dyspnoea, high breathing rate) without signs of severe pneumonia, notably with peripheral oxygen saturation (SpO2) ≥90% on room air. Severe COVID-19 was defined as clinical signs of pneumonia plus at least one of the following: respiratory rate >30 breaths·min−1, severe respiratory distress and/or SpO2 <90% on room air. Critical COVID-19 was defined as the presence of acute respiratory distress syndrome [21].
Statistical analysis
Continuous variables were shown by the Shapiro–Wilk test to have a skewed distribution and were therefore described as median (interquartile range (IQR)) and compared between the groups with versus without a vaccine response using the Mann–Whitney U-test. Categorical variables were described as number (percentage) and compared by applying the Chi-squared test, with Monte Carlo simulations when counts were <5.
The robust Bianco–Yohai procedure was chosen to build a multivariable logistic regression model designed to identify factors associated with a vaccine response. Backward and forward stepwise selection was applied to variables associated with p-values <0.10 by univariate ANOVA. The odds ratios and 95% confidence intervals were computed. All tests were two-sided and p-values <0.05 were taken to indicate significant differences. The statistical analyses were conducted using R version 3.6.1 (www.r-project.org).
Results
Patients
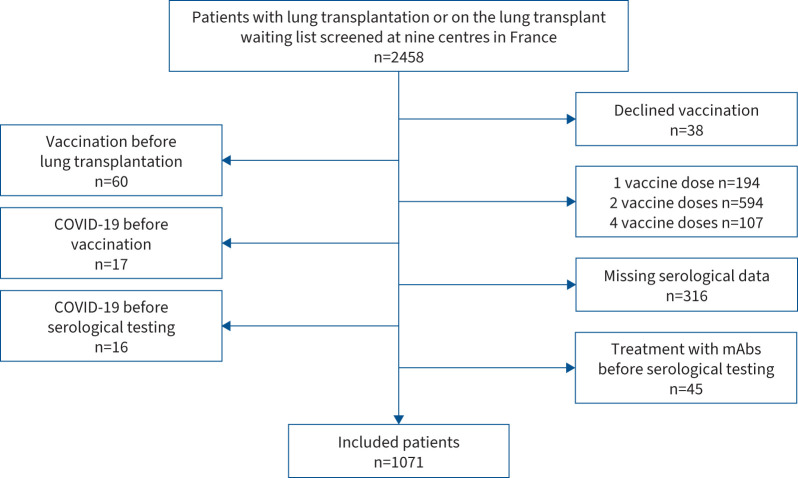
Figure 1 shows the patient flowchart. Between January and August 2021, 2458 patients were screened at the nine participating centres and 1071 patients were included in the study (table 1). The distribution of inclusions across centres was: Le Plessis-Robinson n=218 (20%), Suresnes n=208 (19%), Strasbourg n=170 (16%), Marseille n=112 (10%), Nantes n=109 (10%), Paris-Bichat n=97 (9%), Bordeaux n=65 (6%), Paris-Cochin n=63 (6%) and Grenoble n=29 (3%). Supplementary table S1 reports the main differences between included and excluded patients.
FIGURE 1.
Patient flowchart. mAb: monoclonal antibody.
TABLE 1.
Main features of the study patients
| Overall (n=1071) | Responders (n=173) | Non-responders (n=898) | p-value | |
| Male | 551 (52) | 82 (47) | 469 (52) | 0.27 |
| Age at transplantation (years) | 47 (31–58) | 32 (24–52) | 50 (34–58) | 0.001 |
| Age at vaccination (years) | 54 (40–63) | 45 (33–58) | 56 (43–64) | 0.002 |
| Transplantation indication | 0.0035 | |||
| CF | 327 (31) | 87 (50) | 240 (27) | |
| COPD | 323 (30) | 36 (21) | 287 (32) | |
| Fibrosis | 160 (15) | 13 (7) | 147 (16) | |
| PAH | 121 (11) | 18 (10) | 103 (11) | |
| Other | 140 (13) | 20 (11) | 120 (13) | |
| Transplantation procedure | 0.37 | |||
| Double lung | 918 (86) | 156 (90) | 762 (85) | |
| Single lung | 75 (7) | 9 (5) | 66 (7) | |
| Heart–lung | 62 (6) | 7 (4) | 55 (6) | |
| Multiorgan | 16 (1) | 2 (1) | 14 (2) | |
| Maintenance immunosuppression | ||||
| Cyclosporine | 197 (20) | 24 (15) | 173 (21) | 0.30 |
| Tacrolimus | 786 (79) | 132 (83) | 654 (78) | 0.49 |
| Corticosteroids | 903 (85) | 123 (71) | 780 (87) | 0.004 |
| Mycophenolate | 675 (63) | 89 (51) | 586 (66) | 0.03 |
| Azathioprine | 157 (15) | 36 (21) | 121 (14) | 0.15 |
| Everolimus | 195 (18) | 39 (23) | 156 (17) | 0.18 |
| Sirolimus | 11 (1) | 3 (2) | 8 (1) | 0.19 |
| Intensified immunosuppression within 6 months before vaccination (n=1064) | 90 (8) | 7 (4) | 83 (9) | 0.03 |
| Methylprednisolone | 52 (5) | 5 (3) | 47 (5) | 0.24 |
| Rituximab | 12 (1) | 0 (0) | 12 (1) | 0.24 |
| Anti-thymocyte globulins | 2 (0) | 0 | 2 (0) | 0.99 |
| Intravenous immunoglobulins | 25 (2) | 2 (1) | 23 (3) | 0.23 |
| Plasmapheresis | 1 (0) | 0 | 1 (0) | 0.99 |
| Follow-up (months) | 8.3 (6.7–9.3) | 8.6 (7.2–9.3) | 8.3 (6.7–9.3) | 0.08 |
| Death | 13 (1) | 0 | 13 (1) | 0.14 |
Data are presented as n (%) or median (interquartile range), unless otherwise stated. CF: cystic fibrosis; PAH: pulmonary arterial hypertension.
Spike protein IgG response (primary outcome)
Of the 1071 patients, 173 (16%) had an antibody response as defined by a titre >264 BAU·mL−1. Table 1 compares the main features in the responders and non-responders. In the subgroup of responders, age at vaccination was younger, more patients had cystic fibrosis as the reason for lung transplantation, fewer patients took corticosteroids and mycophenolate for maintenance immunosuppression, and fewer patients received intensified immunosuppression within 6 months before the first vaccine dose.
Details of the times of anti-spike IgG assays in the overall population and in subgroups defined by time from lung transplantation to vaccination are given in supplementary figure S1. Antibody titres did not decrease over time. In the subgroup vaccinated sooner after lung transplantation, antibody titres increased over time, perhaps due to a decrease in immunosuppressant therapy intensity.
Table 2 gives details of the vaccination protocols and serological testing methods. The time from lung transplantation was significantly longer in responders than in non-responders. Most patients received an mRNA vaccine.
TABLE 2.
Vaccination protocols and post-vaccination COVID-19
| Overall (n=1071) | Responders (n=173) | Non-responders (n=898) | p-value | |
| Vaccine manufacturer | 0.14 | |||
| BNT162b2 (Pfizer-BioNTech) | 777 (73) | 118 (68) | 659 (73) | |
| mRNA-1273 (Moderna) | 145 (13) | 28 (16) | 117 (13) | |
| ChAdOx1 nCoV-19 (AstraZeneca) | 16 (1) | 0 | 16 (2) | |
| Combined mRNA | 6 (1) | 2 (1) | 4 (0) | |
| Combined mRNA and AstraZeneca | 127 (12) | 25 (15) | 102 (12) | |
| Times to serology (months) | ||||
| Time from lung transplantation to first vaccine dose | 64 (30–110) | 96 (56–156) | 57 (26–104) | 0.002 |
| Time from third vaccination dose to serology | 3.0 (1.7–4.1) | 3.3 (2.1–4.1) | 2.8 (1.6–4.1) | 0.034 |
| Intervals between vaccine doses (days) | ||||
| First to second | 27 (27–30) | 27 (24–30) | 30 (27–31) | 0.18 |
| Second to third | 43 (30–64) | 46 (30–70) | 43 (30–61) | 0.10 |
| Type of immunoassay | 0.005 | |||
| Abbott | 635 (59) | 69 (40) | 566 (63) | |
| DiaSorin | 207 (19) | 52 (33) | 155 (17) | |
| Elecsys | 96 (9) | 6 (5) | 90 (10) | |
| Cerba | 75 (7) | 23 (12) | 52 (6) | |
| Roche Diagnostics | 43 (4) | 11 (7) | 32 (4) | |
| NovaTec | 15 (2) | 12 (3) | 3 (0) | |
| COVID-19 after vaccination | 51 (5) | 4 (2) | 47 (5) | 0.12 |
| Moderate | 36 (3) | 4 (2) | 32 (4) | 0.40 |
| Severe | 8 (1) | 0 | 8 (1) | 0.71 |
| Critical | 7 (1) | 0 | 7 (1) | 0.83 |
| Deaths due to COVID-19 | 6 (1) | 0 | 6 (1) | 0.99 |
| Curative monoclonal antibodies | 13 (1) | 1 (1) | 12 (1) | 0.99 |
| Time from third vaccine dose to COVID-19 (months) | 6.6 (5.1–7.3) | 7.1 (6.6–7.4) | 6.4 (4.9–7.3) | 0.33 |
Data are presented as n (%) or median (interquartile range), unless otherwise stated.
Factors associated with a vaccine response
Table 3 reports the results of the univariate and multivariate analyses performed to identify factors associated with an antibody response. By multivariate analysis, younger age at vaccination, longer time from lung transplantation to vaccination and not taking corticosteroid or antimetabolite therapy were significantly and independently associated with an antibody response.
TABLE 3. Univariate and multivariate analyses to identify factors associated with a vaccine response (titre ≥264 BAU·mL−1).
| Univariate | Multivariate | |||||||
| Reference | Modality | HR (95% CI) | p-value | Reference | Modality | HR (95% CI) | p-value | |
| Sex | Female | Male | 0.82 (0.59–1.14) | 0.24 | ||||
| Age at vaccination | Continuous | 0.97 (0.96–0.98) | 0.0045 | Continuous | 0.97 (0.96–0.98) | 0.0056 | ||
| Time from lung transplantation to first vaccine dose | Continuous | 1.01 (1.00–1.01) | 0.009 | Continuous | 1.01 (1.00–1.01) | 0.0094 | ||
| Lung transplantation indication | Fibrosis | COPD | 1.42 (0.49−1.22) | 0.30 | ||||
| CF | 4.10 (2.21–2.60) | 0.005 | ||||||
| PAH | 1.98 (0.93–4.21) | 0.08 | ||||||
| Other | 1.79 (0.85−3.77) | 0.13 | ||||||
| Lung transplant procedure | Single | Double | 1.50 (0.73–3.07) | 0.27 | ||||
| Heart–lung | 0.93 (0.33–2.67) | 0.90 | ||||||
| Multiorgan | 1.05 (0.20–5.39) | 0.95 | ||||||
| Increased immunosuppression within 6 months before vaccination | No | Yes | 0.41 (0.19–0.90) | 0.03 | ||||
| Methylprednisolone | No | Yes | 0.53 (0.21–1.36) | 0.19 | ||||
| Rituximab | No | Yes | 0.01 (0.00– >100) | 0.97 | ||||
| Anti-thymocyte globulins | No | Yes | 0.01 (0.00– >100) | 0.97 | ||||
| Intravenous immunoglobulins | No | Yes | 0.44 (0.10–1.89) | 0.27 | ||||
| Plasmapheresis | No | Yes | 0.01 (0.00– >100) | 0.98 | ||||
| Calcineurin inhibitors | No | Cyclosporine | 0.76 (0.16–3.65) | 0.73 | ||||
| No | Tacrolimus | 1.11 (0.24–5.07) | 0.89 | |||||
| Corticosteroids | No | Yes | 0.36 (0.25–0.53) | 0.005 | No | Yes | 0.43 (0.28–0.66) | 0.005 |
| Mycophenolate | No | Yes | 0.57 (0.39–0.84) | 0.0037 | No | Yes | 0.66 (0.42–1.02) | 0.06 |
| Azathioprine | No | Yes | 1.12 (0.69–1.83) | 0.96 | No | Yes | 0.73 (0.41–1.32) | 0.30 |
| mTOR inhibitors | No | Everolimus | 1.40 (0.94–2.08) | 0.10 | ||||
| No | Sirolimus | 2.09 (0.55–7.99) | 0.28 | |||||
| Vaccine manufacturer | BNT162b2 (Pfizer-BioNTech) | mRNA-1273 (Moderna) | 1.34 (0.85–2.11) | 0.21 | ||||
| ChAdOx1 nCoV-19 (AstraZeneca) | 0.00 (0.15– >100) | 0.98 | ||||||
| Combined mRNA | 2.79 (0.51–15.42) | 0.24 | ||||||
| Combined mRNA and Astra | 1.99 (0.71–5.64) | 0.11 | ||||||
| Serology assay | Abbott | DiaSorin | 3.13 (2.08–4.72) | <0.01 | ||||
| Elecsys | 0.81 (0.39–1.68) | 0.57 | ||||||
| Cerba | 3.60 (1.81–7.15) | <0.01 | ||||||
| Roche Diagnostics | 2.22 (0.97–5.07) | 0.06 | ||||||
| NovaTec | 5.40 (1.19–24.60) | 0.03 | ||||||
| Time from third vaccine dose to serological testing | Continuous | 1.15 (1.04–1.29) | 0.0025 | |||||
CF: cystic fibrosis; PAH: pulmonary arterial hypertension; mTOR: mechanistic target of rapamycin.
COVID-19 infection and other events
Median (IQR) follow-up was 8.3 (6.7–9.3) months after the first vaccine dose. No patient was lost to follow-up. Among 777 patients with titres ≤50 BAU·mL−1 after the third vaccine dose, 221 (28%) were given monoclonal antibody treatment against COVID-19. COVID-19 occurred after the third vaccine dose in 51 (5%) patients: 47 non-responders and four responders, a nonsignificant difference (p=0.12). Among the 47 non-responders who experienced COVID-19 infection, 26 had no detectable anti-spike IgG antibodies and the median (IQR) titre in the remaining 21 patients was 31 (10–65) BAU·mL−1. The disease was severe and critical in eight and seven non-responders, respectively; no responders had severe or critical disease. Six non-responders, all of whom had critical disease, died of COVID-19, yielding a mortality rate of 13% (6/47) compared with none of the responders, although the difference was not statistically significant (table 2). This 13% mortality rate was lower than the 29% rate in the 17 patients who experienced COVID-19 before vaccination, although the difference was not statistically significant (p=0.14). No patients experienced COVID-19 during the vaccination protocol.
The proportion of patients with COVID-19 was significantly higher in patients who were excluded because they received none, one or two vaccine doses than in the study patients (14% versus 5%; p<0.001), whereas mortality was similar in these two groups (14%; p=0.25).
Of the 1071 patients, 12 died. In addition to the six patients who died of COVID-19, two patients died of chronic lung allograft dysfunction and one each of pulmonary embolism, lung cancer, kidney failure and septic shock.
Safety
Information on vaccine safety was collected retrospectively at each transplant centre. Symptoms occurring mainly within 3 days after the second injection were considered vaccine-related. Most vaccine-related symptoms occurred after the second injection and were mild, with the most common being tiredness (25%), headache (16%), fever (11%) and chills (7%). No serious vaccine-related adverse events were reported.
Discussion
We report data on the spike protein IgG antibody response to three doses of COVID-19 vaccine in the largest cohort to date of lung transplant recipients with no history of COVID-19. The humoral response to vaccination was poor, with only 16% of patients producing protective levels of anti-spike IgG antibody. Clinically, severe COVID-19 did not occur in any of the responders. Of the non-responders, 1.7% had severe COVID-19 and 0.7% died of COVID-19 during study follow-up.
The recruitment at multiple transplant centres involved in research and the sample size of over 1000 patients are major strengths of our study. No patients with a history of COVID-19 or anti-SARS-CoV-2 antibody treatment before vaccination, or of antibody treatment before serological testing, were included. All study centres used the same vaccination protocol. Our study had no control group of healthy vaccinated adults. There was also no control group of unvaccinated lung transplant recipients for comparison of clinical events, as vaccination is recommended to all immunocompromised individuals in France. Anti-spike IgG antibodies, but not neutralising antibodies or memory T-cell response, were investigated. However, anti-spike IgG titres correlate closely with geometric mean titres of neutralising antibodies [22]. Since patients were tested for COVID-19 only in the event of a documented contact or compatible symptoms, we may have missed cases of asymptomatic COVID-19. It is the development of symptoms, however, that is of greatest concern and, given the very close monitoring offered to all lung transplant recipients in France, symptomatic cases are very unlikely to have been missed. Although titres of anti-spike IgG antibodies correlate with those of neutralising antibodies, no threshold or correlate of protection has been established for variants of concern. Lower levels of neutralising antibodies have been reported with variants compared with the ancestral strain [23]. Data on correlations between antibody titres and disease severity are lacking [24]. No change in the 264 BAU·mL−1 anti-spike IgG antibody threshold has been recommended since the emergence of variants of concern. The use of monoclonal antibodies in a quarter of the non-responders may have decreased the number of COVID-19 cases. Several different immunoassay brands were used to determine the antibody titres. Nonetheless, manufacturers have established standardised results [25]. Due to the start of the omicron wave, follow-up was ended on 31 December 2021, limiting our ability to diagnose subsequent COVID-19 episodes. Finally, safety of the vaccination protocol was not specifically assessed, although no serious adverse effects of the vaccine were recorded and neither was the frequency of transplant rejection unusually high.
Our findings are consistent with published data on the humoral response to three COVID-19 vaccine doses in solid-organ transplant recipients [7–13] and add to the scant information available on lung transplant recipients [6, 14–16], for whom the effect of three vaccine doses had not yet been reported in substantial cohorts. The marker for vaccine efficacy was usually anti-spike antibody production [6–8, 11–13, 15–17], with only a few studies assaying anti-receptor binding domain [10, 17] and neutralising antibodies [10]. With two doses of mRNA vaccine, the proportion of lung transplant recipients who achieved a serological response was only 10–39% in earlier work [11, 15, 17]. In populations of solid-organ transplant recipients, most of whom had received kidney transplants, the proportion of responders to three doses ranged from 40% to 69% [7–13]. In our cohort, this proportion was only 16%, in marked contrast to the high proportions of responders in the original clinical trials in immunocompetent individuals [26, 27]. Two factors associated with less immunosuppression, i.e. a longer time since lung transplantation and absence of current exposure to corticosteroid or mycophenolate therapy, were significantly associated with a better response in our study, although our study design did not allow an assessment of potential causality. Treatment with antimetabolites such as mycophenolate was also associated with a poorer vaccine response in lung transplant recipients in two earlier studies [15, 16], suggesting that decreasing the dosages of these drugs during vaccination might improve the immune response. Consistent with this possibility, the subgroup of patients vaccinated sooner after lung transplantation experienced increasing anti-spike IgG titres over time (supplementary figure S1b). Younger patients more often achieved a vaccine response, in keeping with other studies of the effects of mRNA COVID-19 vaccines [6, 15, 16].
Despite the low proportion of vaccine responders, COVID-19 was uncommon, occurring in 5% of patients overall, 5% of non-responders and 2% of responders. This low incidence may reflect good patient education about, and adherence to, protective measures such as mask wearing and hand hygiene, in addition to the limited overall incidence of COVID-19 during the study period in France [28]. Moreover, monoclonal antibodies to SARS-CoV-2 were given to 25% of non-responders and may have contributed to decrease the risk of COVID-19. The routine use of monoclonal antibodies early in the course of COVID-19, starting in June 2021, may have diminished the proportion of severe and fatal cases. Finally, vaccination confers immunity via mechanisms other than the production of anti-spike IgG antibodies. A robust CD8+ T-cell response was documented 1 week after the first dose of BNT162b2 mRNA vaccine in immunocompetent healthcare workers [29]. Importantly, T-cell responses in immunocompetent individuals after two vaccine doses were of similar magnitude to those seen after natural infection, although they seemed somewhat more differentiated [30]. Although not observed in our population (supplementary figure S1), antibody waning after vaccination remains a concern [31]. T memory stem cells develop after vaccination and may be more long lasting. However, severe or fatal COVID-19 was seen only among non-responders in our study, suggesting a possible protective role for cellular immunity. Last, during the study period, the alpha and delta SARS-CoV-2 variants predominated. Although recent data suggest that boosters may increase effectiveness against severe omicron variant disease in immunocompetent patients [32], whether our results apply to current and emerging variants in immunocompromised populations remains to be investigated.
In conclusion, lung transplant recipients had an impaired anti-spike IgG response to three COVID-19 vaccine doses. Older age and stronger immunosuppressive regimens including mycophenolate or corticosteroids were significantly associated with failure to achieve a vaccine response. During the study period, when the alpha and delta SARS-CoV-2 variants predominated, COVID-19 was very uncommon despite the low frequency of vaccine responses, and the few fatal or non-fatal severe cases occurred only in non-responders. However, a protective role for monoclonal antibody prophylaxis is possible, and whether our results apply to current and emerging variants is unclear. Additional research is needed to clarify these findings, characterise the T-cell response to COVID-19 vaccines and determine the extent to which cellular immunity contributes to protect immunocompromised patients against COVID-19.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00502-2022.Supplement (374KB, pdf)
Shareable PDF
Acknowledgements
We thank Stéphane Morisset (Stéphane Morisset Etudes and Consulting, Pérouges, France) for the statistical analysis, A. Wolfe (Chaumont, France) for revising the draft and Olaf Mercier (Hôpital Marie Lannelongue, Le Plessis-Robinson, France) for supporting this project.
Footnotes
Data sharing: Anonymised participant-level data, the study protocol and informed consent form, and the statistical analysis plan will be available from the corresponding author upon reasonable request, after study publication and after signature of a data-sharing agreement.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01723-2022
Author contributions: G. Dauriat and J. Le Pavec: study conception and design; acquisition, analysis and interpretation of data; article drafting and revising. L. Beaumont, B. Renaud Picard, M. Penhouet, B. Coiffard, M. Salpin, X. Demant, N. Carlier, J. Messika, M. Reynaud Gaubert and A. Roux: acquisition, analysis and interpretation of data; article drafting and revising. L.B. Luong Nguyen: study conception and design; analysis and interpretation of data; article drafting and revising. C. Saint Raymond, I. Danner and F. Gallais: acquisition, analysis and interpretation of data. The corresponding author, J. Le Pavec, as the guarantor of the study, accepts full responsibility for the work and the conduct of the study. He had access to the data and controlled the decision to publish. The corresponding author also attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J. Le Pavec takes responsibility for the content of the manuscript, including the data and analysis, and affirms that the manuscript is an honest, accurate and transparent account of the reported study and omits no important aspects of the study. No discrepancies from the original study protocol occurred.
Conflict of interest: None for any authors.
References
- 1.Caillard S, Chavarot N, Francois H, et al. . Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant 2021; 21: 1295–1303. doi: 10.1111/ajt.16424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kates OS, Haydel BM, Florman SS, et al. . Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis 2021; 73: e4090–e4099. doi: 10.1093/cid/ciaa1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messika J, Eloy P, Roux A, et al. . COVID-19 in lung transplant recipients. Transplantation 2021; 105: 177–186. doi: 10.1097/TP.0000000000003508 [DOI] [PubMed] [Google Scholar]
- 4.BioNTech SE . A phase 1/2/3, placebo-controlled, randomized, observer-blind, dose-finding study to evaluate the safety, tolerability, immunogenicity, and efficacy of SARS-COV-2 RNA vaccine candidates against COVID-19 in healthy individuals. 2021. https://clinicaltrials.gov/ct2/show/NCT04368728 Date last accessed: 17 January 2022.
- 5.ModernaTX, Inc. A phase 3, randomized, stratified, observer-blind, placebo-controlled study to evaluate the efficacy, safety, and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine in adults aged 18 years and older. 2021. https://clinicaltrials.gov/ct2/show/NCT04470427 Date last accessed: 17 January 2022.
- 6.Boyarsky BJ, Ou MT, Werbel WA, et al. . Early development and durability of SARS-CoV-2 antibodies among solid organ transplant recipients: a pilot study. Transplantation 2021; 105: e52–e53. doi: 10.1097/TP.0000000000003637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masset C, Kerleau C, Garandeau C, et al. . A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int 2021; 100: 1132–1135. doi: 10.1016/j.kint.2021.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benotmane I, Gautier G, Perrin P, et al. . Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021; 326: 1063–1065. doi: 10.1001/jama.2021.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Bello A, Abravanel F, Marion O, et al. . Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant 2022; 22: 322–323. doi: 10.1111/ajt.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall VG, Ferreira VH, Ku T, et al. . Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385: 1244–1246. doi: 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamar N, Abravanel F, Marion O, et al. . Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385: 661–662. doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stumpf J, Tonnus W, Paliege A, et al. . Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation 2021; 105: e267–e269. doi: 10.1097/TP.0000000000003903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werbel WA, Boyarsky BJ, Ou MT, et al. . Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021; 174: 1330–1332. doi: 10.7326/L21-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyarsky BJ, Werbel WA, Avery RK, et al. . Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325: 2204–2206. doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallett AM, Greenberg RS, Boyarsky BJ, et al. . SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant 2021; 40: 1579–1588. doi: 10.1016/j.healun.2021.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shostak Y, Shafran N, Heching M, et al. . Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med 2021; 9: e52–e53. doi: 10.1016/S2213-2600(21)00184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyarsky BJ, Werbel WA, Avery RK, et al. . Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021; 325: 1784–1786. doi: 10.1001/jama.2021.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conseil d'Orientation de la Stratégie Vaccinale . Recommandations pour la protection des personnes sévèrement immunodéprimées contre le Covid-19 (Vaccination et prophylaxie primaire) – 19 Novembre 2021. [Recommendations for the protection of severely immunocompromised people against Covid-19 (Vaccination and primary prophylaxis) – 19 November 2021.] 2021. https://solidarites-sante.gouv.fr/IMG/pdf/cosv_-_recommandations_pour_la_protection_des_personnes_severement_immunodeprimees_-_19_novembre_2021.pdf Date last accessed: 2 February 2022.
- 19.Conseil d'Orientation de la Stratégie Vaccinale . Avis du 13 avril 2021 – La sérologie Covid-19 post-vaccination en population générale est-elle justifiée? – Mise à jour du 26 novembre 2021. [Opinion of 13 April 2021 – Is post-vaccination Covid-19 serology in the general population justified? – Update of 26 November 2021.] 2021. https://solidarites-sante.gouv.fr/IMG/pdf/cosv_-_mise_a_jour_du_26_novembre_2021_de_l_avis_du_cosv_13_avril_2021_-_serologies_en_population_generale.pdf Date last accessed: 10 June 2022.
- 20.Feng S, Phillips DJ, White T, et al. . Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27: 2032–2040. doi: 10.1038/s41591-021-01540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Living guidance for clinical management of COVID-19. Living guidance: 23 November 2021. 2021. https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf Date last accessed: 26 May 2022.
- 22.Sahin U, Muik A, Vogler I, et al. . BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. Nature 2021; 595: 572–577. doi: 10.1038/s41586-021-03653-6 [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Sendra B, Albert E, Zulaica J, et al. . Neutralizing antibodies against SARS-CoV-2 variants of concern elicited by the comirnaty COVID-19 vaccine in nursing home residents. Sci Rep 2022; 12: 3788. doi: 10.1038/s41598-022-07849-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert PB, Montefiori DC, McDermott AB, et al. . Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375: 43–50. doi: 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Infantino M, Pieri M, Nuccetelli M, et al. . The WHO international standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int Immunopharmacol 2021; 100: 108095. doi: 10.1016/j.intimp.2021.108095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baden LR, El Sahly HM, Essink B, et al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santé Publique France . Coronavirus: chiffres clés et évolution de la COVID-19 en France et dans le Monde. [Coronavirus: key figures and evolution of COVID-19 in France and around the world.] 2022. www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde Date last accessed: 14 February 2022.
- 29.Oberhardt V, Luxenburger H, Kemming J, et al. . Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021; 597: 268–273doi: 10.1038/s41586-021-03841-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022; 23: 186–193. doi: 10.1038/s41590-021-01122-w [DOI] [PubMed] [Google Scholar]
- 31.Lassaunière R, Polacek C, Frische A, et al. . Neutralizing antibodies against the SARS-CoV-2 omicron variant (BA.1) 1 to 18 weeks after the second and third doses of the BNT162b2 mRNA vaccine. JAMA Netw Open 2022; 5: e2212073. doi: 10.1001/jamanetworkopen.2022.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higdon MM, Baidya A, Walter KK, et al. . Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect Dis 2022; 22: 1114–1116. doi: 10.1016/S1473-3099(22)00409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00502-2022.Supplement (374KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00502-2022.Shareable (489.3KB, pdf)