Abstract
A new approach is described for the synthesis of spiro[piperidine-3,2′-oxindoles] in 35–82 yields with excellent stereoselectivity via the [4 + 2] cycloaddition reaction of donor–acceptor cyclobutanes with iminooxindoles in the presence of 10–30 mol% Sc(OTf)3 at room temperature. This methodology provides great potential for building spiro-heterocycle compounds from simple building blocks.
A Lewis acid-catalyzed [4 + 2] cycloaddition reaction from D–A cyclobutanes and iminooxindoles, providing the corresponding spiro[piperidine-3,2′-oxindoles] under mild conditions.
Spirocyclic oxindole skeletons are present in numerous naturally occurring alkaloids and medicinal active molecules.1 Among them, spiro[piperidine-3,2′-oxindoles] molecular frameworks are attractive scaffolds because of their usefulness in potential biological activities. For instance, the drug KAE609 (formerly known as NITD609 or cipargamin), which has been identified as a potential treatment for malaria,2 and quinoline spirooxindoles, which is a potential antitumour molecular.3 Furthermore, some spiro[piperidine-3,2′-oxindoles] are being investigated as peptide isosteres that can mimetic type II β-turn in the search for new enzyme inhibitors (Fig. 1).4 Given the importance of this structural class, the great advances that have occurred,1d,g–j the continuous development of catalytic and versatile synthetic strategies that allow the direct formation of these frameworks is still needed. Herein, we report a general and efficient method for the synthesis of highly substituted spiro[piperidine-3,2′-oxindoles] from donor–acceptor (D–A) cyclobutanes and iminooxindoles catalyzed by Lewis acid under mild conditions.
Fig. 1. Some examples of bioactive spiro[piperidine-3,2′-oxindoles].
Cycloaddition reactions of strained cycloalkanes represent one of the most efficient ways for the synthesis of carbo- and heterocycles in organic chemistry.5 Cyclobutanes are found in a large number of biologically active natural products.6 Moreover, as an important type of four-membered all-carbon building block, donor–acceptor (D–A) cyclobutanes have been used in the ring-opening reaction7 and applied in the construction of various cyclic molecular frameworks by [4 + n] cycloaddition8 under the catalysis of Lewis acids.9 For example, in 2010, Pagenkopf group reported a Yb(OTf)3 catalyzed [4 + 2] cycloaddition reaction of D–A cyclobutanes and imines, giving highly substituted piperidines (Scheme 1a). However, to the best of our knowledge, examples of cycloaddition of D–A cyclobutanes with iminooxindoles for constructing spiro[piperidine-3,2′-oxindoles] under Lewis acid have not been reported. Thus, the development of new synthetic protocols to access such spiro-heterocycle skeleton is still desirable (Scheme 1b).
Scheme 1. Previous work and our strategy for this study.
Recently, we reported a Lu(OTf)3-catalyzed [4 + 4] cycloaddition reaction of D–A cyclobutanes with anthranils to deliver oxa-bridged eight-membered heterocycles.10 Owing to our continuous interest in Lewis acid catalyzed reactions,11 particularly in D–A cyclobutanes involved transformations, we envisioned that, upon suitable activation by acid, the reaction of D–A cyclobutanes and iminooxindoles would provide a new approach to deliver diverse spiro[piperidine-3,2′-oxindoles] (Scheme 1b).
We tested our hypothesis using iminooxindole 1a and dimethyl 2-(4 methoxyphenyl)cyclobutane-1,1-dicarboxylate 2a as model substrates. Initially, the use of 10 mol% Yb(OTf)3 as a catalyst did not yield good results (Table 1, entries 1 and 2). To our delight, spiro[piperidine-3,2′-oxindoles] 3aa was isolated in 82% yield as a single isomer and a trace amount of unidentified product when the reaction was carried out under the catalysis of 10 mol% Sc(OTf)3 at room temperature (Table 1, entry 3). Catalysts such as Y(OTf)3, Hf(OTf)4, In(OTf)3, Cu(OTf)2, Lu(OTf)3 and Nd(OTf)3 result in low efficiency (Table 1, entries 4–9). To improve the yield, Lewis acids, including iron salts, tin salts and other kinds of Lewis acids, were also tested, and the results are unsatisfactory (Table 1, entries 10–19). The yield decreased when the reaction was carried out in DCE, toluene and CHCl3 (Table 1, entries 20–22). Polar solvents were unsuitable for this cycloaddition reaction (Table 1, entries 23 and 24). Only a 32% isolated yield was obtained when the reaction was performed without 4 Å molecular sieves (MS), indicating that a trace amount of water might result in a side product (Table 1, entry 25).
Screening the reaction conditionsa.

| |||
|---|---|---|---|
| Entry | Catalyst | Solvent | Isolated yield (%) |
| 1 | Yb(OTf)3 | DCM | Trace |
| 2b | Yb(OTf)3 | DCM | n.r. |
| 3 | Sc(OTf) 3 | DCM | 82 |
| 4 | Y(OTf)3 | DCM | Trace |
| 5 | Hf(OTf)4 | DCM | Trace |
| 6 | In(OTf)3 | DCM | Trace |
| 7 | Cu(OTf)2 | DCM | Trace |
| 8 | Lu(OTf)3 | DCM | Trace |
| 9 | Nd(OTf)3 | DCM | Trace |
| 10 | Fe(OTf)3 | DCM | n.r. |
| 11 | FeCl3 | DCM | 30 |
| 12 | SnCl2 | DCM | n.r. |
| 13 | SnCl4 | DCM | Complex |
| 14 | Bi(OTf)3 | DCM | n.r. |
| 15 | Tm(OTf)3 | DCM | Trace |
| 16 | Gd(OTf)3 | DCM | Trace |
| 17 | Er(OTf)3 | DCM | Trace |
| 18 | Ho(OTf)3 | DCM | Trace |
| 19 | MgI2 | DCM | Trace |
| 20 | Sc(OTf)3 | DCE | 72 |
| 21 | Sc(OTf)3 | Toluene | 40 |
| 22 | Sc(OTf)3 | CHCl3 | 28 |
| 23 | Sc(OTf)3 | CH3CN | Trace |
| 24 | Sc(OTf)3 | Acetone | Trace |
| 25c | Sc(OTf)3 | DCM | 32 |
Reaction conditions: 1a (0.22 mmol), 2a (0.2 mmol), 10 mol% catalyst, and 60 mg of activated 4 Å MS in 2.0 mL of solvent at room temperature, DCM = CH2Cl2, DCE = ClCH2CH2Cl, n.r. = no reaction.
The reaction was performed according to the ref. 8d, at −50 °C for 1 h, then at 0 °C overnight.
Without 4 Å MS.
With the optimized reaction conditions in hand, the scope of this Lewis-acid catalyzed [4 + 2] cycloaddition reaction was explored with iminooxindole 1a and various D–A cyclobutanes 2, and the results are summarized in Table 2. 4-Phenyl D–A cyclobutane with an ethoxyl or benzyloxyl on the phenyl ring afforded the corresponding products in 67–72% yield (Table 2, 3ab–3ac). However, cyclobutane 2d without a substituent on the phenyl ring (R1 position) and 2e with a weak electron-withdrawing group (Br) on the phenyl ring (R1 position) could not undergo the cycloaddition reaction with 1a, and no desired cycloadducts were detected (Table 2, 3ad–3ae). The reaction of iminooxindole 1a and cyclobutanes 2f (R1 being 4-methylphenyl) also failed (Table 2, 3f). Di- and trisubstituted cyclobutanes 2g–2i with electron-donating groups on the phenyl ring (R1 position) also worked in this reaction, furnishing [4 + 2] cycloadditon products in 41–80% isolated yields albeit higher catalyst loading and longer reaction time were needed in some cases (Table 2, 3ag–3ai). For thienyl-substituted cyclobutane 3i, the desired [4 + 2] cycloaddition product 3aj was obtained in 46% yield (Table 2, 3aj). The aforementioned results indicate that the nature and position of the substituents on the aromatic rings significantly affect the reaction activity. Surprisingly, the dicarboxylates can be switched to other esters to give the desired spiro[piperidine-3,2′-oxindoles] 3ak–3al in 58–72% yields (Table 2, 3ak–3al). The structure and the relative stereochemistry of the products were established by X-ray crystallography analysis of 3aa (Fig. 2).
Substrate scopea.

|
Reaction conditions: 1a (0.22 mmol), 2 (0.2 mmol), 10 mol% Sc(OTf)3, and 60 mg of activated 4 Å MS in 2.0 mL of DCM at rt, isolated yield.
100 mol% Sc(OTf)3 was used at 90 °C, and no desired product was detected.
20 mol% Sc(OTf)3 was used.
30 mol% Sc(OTf)3 was used.
Fig. 2. X-ray crystal structure of compound 3aa (CCDC 2143778†).
We studied the scope of this reaction due to the variation of the iminooxindole component (Table 3). The data in Table 3 showed that various functional groups were introduced into the oxindole fragment of iminooxindole 1b–1g (R3 position), such as halides (F, Cl, Br), methyl, methoxyl and nitro group, were compatible, affording the desired [4 + 2] cycloaddition products 3ba–3ga in 67–76% isolated yields (Table 3, 3ba–3ga). Similarly, iminooxindole 1h–1m, bearing both electron-withdrawing and electron-donating groups in the aromatic substituent at the imine N atom (R′ group), also reacted with D–A cyclobutane 2a and yielded corresponding products 3ha–3ma (Table 3, 3ha–3ma). Unfortunately, when we used iminooxindole 1 with alkyl groups (e.g., Bu, 1n) at the imine N atom, no desired cycloadduct 3na was detected. The reaction of N-protected iminooxindole 1o (R = Me) with cyclobutane 2a, affording the desired product in 35% yield (Table 3, 3oa). It is noteworthy to mention that no other stereoisomer was detectable in all the reactions we carried out.
Substrate scopea.
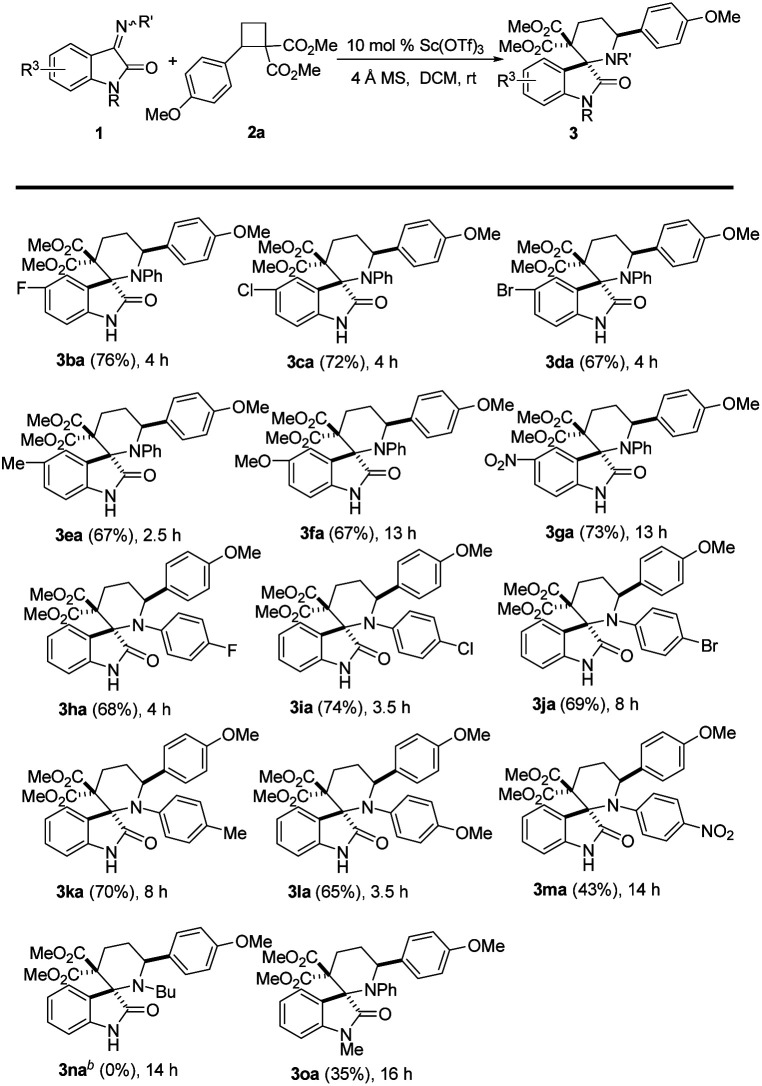
|
Reaction conditions: 1a (0.22 mmol), 2 (0.2 mmol), 10 mol% Sc(OTf)3, and 60 mg of activated 4 Å MS in 2.0 mL of DCM at rt, isolated yield.
100 mol% Sc(OTf)3 was used at 90 °C, and no desired product was detected.
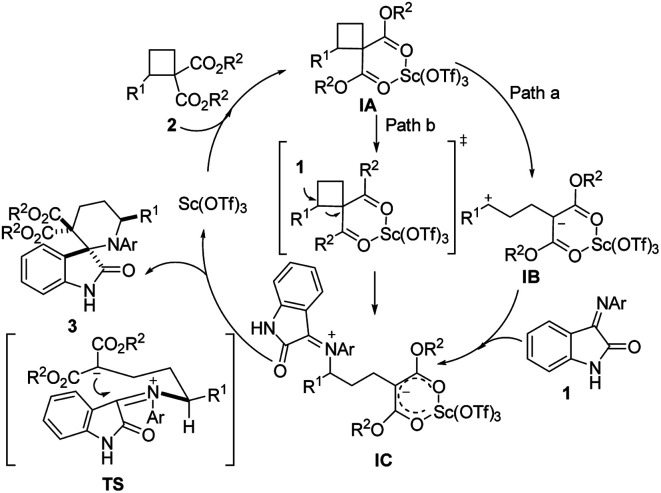
Based on these experimental results, a plausible mechanism that accounts for these results is proposed in Scheme 2. Initially, the Lewis acid Sc(OTf)3 binds to the ester moiety to produce intermediate IA. The direct C–C heterolysis of IA would yield intermediate IB (path a), which would readily react with iminooxindole to yield a zwitterionic intermediate IC. Subsequent cyclization via the favored conformation TS would afford cycloadducts 3 and regenerate the catalyst. The alternative reaction pathway (path b) to generate IC from IA is via an SN2-like nucleophilic attack of iminooxindole to the activated D–A cyclobutane offers the zwitterion IC, thereby causing the inversion of the stereochemistry at the activated R1 substituted carbon center of cyclobutane, which has been explored by Johnson group in donor–acceptor cyclopropanes.12 Thus, the reaction of enantioenriched cyclobutane would give an enantioenriched cycloadduct. To know which reaction pathway is the real one, enantioenriched 2a was prepared based on the method developed by Tang et al.13 After running for 4 h under standard reaction conditions, the reaction affords a racemic cycloadduct 3aa (Scheme 3), indicating that reaction path a may be a more possible reaction pathway.
Scheme 2. Plausible mechanism.
Scheme 3. Stereospecificity of the [4 + 2] cycloaddition reaction of enantioenriched cyclobutane 2a.
Finally, the derivatization of spiro[piperidine-3,2′-oxindoles] 3 was investigated by the selective transformations of the representative compound 3aa. The diester group of 3aa can be reduced by LiAlH4 in THF, yielding the desired product 4 in an 89% isolated yield (Scheme 4).
Scheme 4. Transformations of product 3aa.
In conclusion, we have developed an Sc(OTf)3-catalyzed [4 + 2] cycloaddition reaction from D–A cyclobutanes and N-unprotected iminooxindoles under mild reaction conditions, providing the corresponding spiro[piperidine-3,2′-oxindoles] with good yields and excellent diastereoselectivity. Further studies on Lewis acid-catalyzed cycloaddition reactions are ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was supported by National Natural Science Foundation of China (Grant No. 21861041) and Research Start-up Funding in University, China.
Electronic supplementary information (ESI) available. CCDC 2143778. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ra04730f
Notes and references
- (a) Akaev A. A. Bezzubov S. I. Desyatkin V. G. Vorobyeva N. S. Majouga A. G. Melnikov M. Y. Budynina E. M. J. Org. Chem. 2019;84:3340–3356. doi: 10.1021/acs.joc.8b03208. [DOI] [PubMed] [Google Scholar]; (b) Boddy A. J. Bull J. A. Org. Chem. Front. 2021;8:1026–1084. [Google Scholar]; (c) Yu B. Yu D.-Q. Liu H.-M. Eur. J. Med. Chem. 2015;97:673–698. doi: 10.1016/j.ejmech.2014.06.056. [DOI] [PubMed] [Google Scholar]; (d) Singh G. S. Desta Z. Y. Chem. Rev. 2012;112:6104–6155. doi: 10.1021/cr300135y. [DOI] [PubMed] [Google Scholar]; (e) Santos M. M. M. Tetrahedron. 2014;70:9735–9757. [Google Scholar]; (f) Pavlovska T. L. Redkin R. G. Lipson V. V. Atamanuk D. V. Mol. Diversity. 2016;20:299–344. doi: 10.1007/s11030-015-9629-8. [DOI] [PubMed] [Google Scholar]; (g) Mei G.-J. Shi F. Chem. Commun. 2018;54:6607–6621. doi: 10.1039/c8cc02364f. [DOI] [PubMed] [Google Scholar]; (h) Hong L. Wang R. Adv. Synth. Catal. 2013;355:1023–1052. [Google Scholar]; (i) Cao Z.-Y. Zhou F. Zhou J. Acc. Chem. Res. 2018;51:1443–1454. doi: 10.1021/acs.accounts.8b00097. [DOI] [PubMed] [Google Scholar]; (j) Ball-Jones N. R. Badillo J. J. Franz A. K. Org. Biomol. Chem. 2012;10:5165–5181. doi: 10.1039/c2ob25184a. [DOI] [PubMed] [Google Scholar]
- (a) Takada H. Kumagai N. Shibasaki M. Org. Lett. 2015;17:4762–4765. doi: 10.1021/acs.orglett.5b02300. [DOI] [PubMed] [Google Scholar]; (b) Rottmann M. McNamara C. Yeung B. K. S. Lee M. C. S. Zou B. Russell B. Seitz P. Plouffe D. M. Dharia N. V. Tan J. Cohen S. B. Spencer K. R. González-Páez G. E. Lakshminarayana S. B. Goh A. Suwanarusk R. Jegla T. Schmitt E. K. Beck H.-P. Brun R. Nosten F. Renia L. Dartois V. Keller T. H. Fidock D. A. Winzeler E. A. Diagana T. T. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yeung B. K. S. Zou B. Rottmann M. Lakshminarayana S. B. Ang S. H. Leong S. Y. Tan J. Wong J. Keller-Maerki S. Fischli C. Goh A. Schmitt E. K. Krastel P. Francotte E. Kuhen K. Plouffe D. Henson K. Wagner T. Winzeler E. A. Petersen F. Brun R. Dartois V. Diagana T. T. Keller T. H. J. Med. Chem. 2010;53:5155–5164. doi: 10.1021/jm100410f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsov V. V. Bello Forero J. S. Amado Torres D. F. Tetrahedron Lett. 2008;49:5855–5857. [Google Scholar]
- Lesma G. Landoni N. Sacchetti A. Silvani A. Tetrahedron. 2010;66:4474–4478. [Google Scholar]
- (a) Grover H. K. Emmett M. R. Kerr M. A. Org. Biomol. Chem. 2015;13:655–671. doi: 10.1039/c4ob02117g. [DOI] [PubMed] [Google Scholar]; (b) Vemula N. Pagenkopf B. L. Org. Chem. Front. 2016;3:1205–1212. [Google Scholar]; (c) Schneider T. F. Kaschel J. Werz D. B. Angew. Chem., Int. Ed. 2014;53:5504–5523. doi: 10.1002/anie.201309886. [DOI] [PubMed] [Google Scholar]; (d) Rassadin V. A. Six Y. Tetrahedron. 2016;72:4701–4757. [Google Scholar]; (e) Pagenkopf B. L. Vemula N. Eur. J. Org. Chem. 2017;2017:2561–2567. [Google Scholar]; (f) de Nanteuil F. De Simone F. Frei R. Benfatti F. Serrano E. Waser J. Chem. Commun. 2014;50:10912–10928. doi: 10.1039/c4cc03194f. [DOI] [PubMed] [Google Scholar]; (g) De N. Yoo E. J. ACS Catal. 2018;8:48–58. [Google Scholar]; (h) Werz D. B. Biju A. T. Angew. Chem., Int. Ed. 2020;59:3385–3398. doi: 10.1002/anie.201909213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Pirenne V. Muriel B. Waser J. Chem. Rev. 2021;121:227–263. doi: 10.1021/acs.chemrev.0c00109. [DOI] [PubMed] [Google Scholar]; (j) Namyslo J. C. Kaufmann D. E. Chem. Rev. 2003;103:1485–1538. doi: 10.1021/cr010010y. [DOI] [PubMed] [Google Scholar]; (k) Reissig H.-U. Zimmer R. Angew. Chem., Int. Ed. 2015;54:5009–5011. doi: 10.1002/anie.201501135. [DOI] [PubMed] [Google Scholar]
- (a) Wang M. Lu P. Org. Chem. Front. 2018;5:254–259. [Google Scholar]; (b) Fan Y.-Y. Gao X.-H. Yue J.-M. Sci. China: Chem. 2016;59:1126–1141. [Google Scholar]; (c) Li J. Gao K. Bian M. Ding H. Org. Chem. Front. 2020;7:136–154. [Google Scholar]
- (a) Kolb S. Petzold M. Brandt F. Jones P. G. Jacob C. R. Werz D. B. Angew. Chem., Int. Ed. 2021;60:15928–15934. doi: 10.1002/anie.202101477. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kreft A. Ehlers S. Jones P. G. Werz D. B. Org. Lett. 2019;21:6315–6319. doi: 10.1021/acs.orglett.9b02197. [DOI] [PubMed] [Google Scholar]; (c) Ahlburg N. L. Freese T. Kolb S. Mummel S. Schmidt A. Werz D. B. Eur. J. Org. Chem. 2021;2021:1603–1606. [Google Scholar]; (d) Kolb S. Ahlburg N. L. Werz D. B. Org. Lett. 2021;23:5549–5553. doi: 10.1021/acs.orglett.1c01890. [DOI] [PubMed] [Google Scholar]; (e) Mondal B. Das D. Saha J. Org. Lett. 2020;22:5115–5120. doi: 10.1021/acs.orglett.0c01702. [DOI] [PubMed] [Google Scholar]
- (a) Shigeru S. Kazuhiko S. Hideyuki N. Masaki H. Chem. Lett. 1991;20:1149–1152. [Google Scholar]; (b) Allart E. A. Christie S. D. R. Pritchard G. J. Elsegood M. R. J. Chem. Commun. 2009:7339–7341. doi: 10.1039/b917332c. [DOI] [PubMed] [Google Scholar]; (c) Parsons A. T. Johnson J. S. J. Am. Chem. Soc. 2009;131:14202–14203. doi: 10.1021/ja906755e. [DOI] [PubMed] [Google Scholar]; (d) Moustafa M. M. A. R. Pagenkopf B. L. Org. Lett. 2010;12:4732–4735. doi: 10.1021/ol102062t. [DOI] [PubMed] [Google Scholar]; (e) Moustafa M. M. A. R. Stevens A. C. Machin B. P. Pagenkopf B. L. Org. Lett. 2010;12:4736–4738. doi: 10.1021/ol102063f. [DOI] [PubMed] [Google Scholar]; (f) Stevens A. C. Palmer C. Pagenkopf B. L. Org. Lett. 2011;13:1528–1531. doi: 10.1021/ol200220d. [DOI] [PubMed] [Google Scholar]; (g) de Nanteuil F. Waser J. Angew. Chem., Int. Ed. 2013;52:9009–9013. doi: 10.1002/anie.201303803. [DOI] [PubMed] [Google Scholar]; (h) Kawano M. Kiuchi T. Negishi S. Tanaka H. Hoshikawa T. Matsuo J.-i. Ishibashi H. Angew. Chem., Int. Ed. 2013;52:906–910. doi: 10.1002/anie.201206734. [DOI] [PubMed] [Google Scholar]; (i) Vemula N. Stevens A. C. Schon T. B. Pagenkopf B. L. Chem. Commun. 2014;50:1668–1670. doi: 10.1039/c3cc47775d. [DOI] [PubMed] [Google Scholar]; (j) Hu J.-L. Wang L. Xu H. Xie Z. Tang Y. Org. Lett. 2015;17:2680–2683. doi: 10.1021/acs.orglett.5b01077. [DOI] [PubMed] [Google Scholar]; (k) Perrotta D. Racine S. Vuilleumier J. de Nanteuil F. Waser J. Org. Lett. 2015;17:1030–1033. doi: 10.1021/acs.orglett.5b00149. [DOI] [PubMed] [Google Scholar]; (l) Vemula N. Pagenkopf B. L. Eur. J. Org. Chem. 2015;2015:4900–4906. [Google Scholar]; (m) Feng L.-W. Ren H. Xiong H. Wang P. Wang L. Tang Y. Angew. Chem., Int. Ed. 2017;56:3055–3058. doi: 10.1002/anie.201611734. [DOI] [PubMed] [Google Scholar]; (n) Garve L. K. B. Kreft A. Jones P. G. Werz D. B. J. Org. Chem. 2017;82:9235–9242. doi: 10.1021/acs.joc.7b01631. [DOI] [PubMed] [Google Scholar]; (o) Kuang X.-K. Zhu J. Zhou L. Wang L. Wang S. R. Tang Y. ACS Catal. 2018;8:4991–4995. [Google Scholar]; (p) Igarashi E. Sakamoto K. Yoshimura T. Matsuo J.-i. Tetrahedron Lett. 2019;60:13–15. [Google Scholar]; (q) Tong D. Wu J. Bazinski N. Koo D. Vemula N. Pagenkopf B. L. Chem.–Eur. J. 2019;25:15244–15247. doi: 10.1002/chem.201903833. [DOI] [PubMed] [Google Scholar]; (r) Wei S. Yin L. Wang S. R. Tang Y. Org. Lett. 2019;21:1458–1462. doi: 10.1021/acs.orglett.9b00209. [DOI] [PubMed] [Google Scholar]; (s) Wu J. Winiarz P. Patel D. de Jong J. Tong D. Chidley T. Vemula N. Pagenkopf B. L. Org. Lett. 2020;22:3140–3144. doi: 10.1021/acs.orglett.0c00896. [DOI] [PubMed] [Google Scholar]; (t) Manel A. Berreur J. Leroux F. R. Panossian A. Org. Chem. Front. 2021;8:5289–5295. [Google Scholar]
- (a) Dalpozzo R. Bartoli G. Sambri L. Melchiorre P. Chem. Rev. 2010;110:3501–3551. doi: 10.1021/cr9003488. [DOI] [PubMed] [Google Scholar]; (b) Kobayashi S. Sugiura M. Kitagawa H. Lam W. W. L. Chem. Rev. 2002;102:2227–2302. doi: 10.1021/cr010289i. [DOI] [PubMed] [Google Scholar]; (c) Wang X.-M. Zhang P. Xu Q. Guo C.-Q. Zhang D.-B. Lu C.-J. Liu R.-R. J. Am. Chem. Soc. 2021;143:15005–15010. doi: 10.1021/jacs.1c07741. [DOI] [PubMed] [Google Scholar]; (d) Chen Z. Tian Z. Zhang J. Ma J. Zhang J. Chem.–Eur. J. 2012;18:8591–8595. doi: 10.1002/chem.201201453. [DOI] [PubMed] [Google Scholar]; (e) Baudry D. B. Dormond A. Duris F. Bernard J. M. Desmurs J. R. J. Fluorine Chem. 2003;121:233–238. [Google Scholar]; (f) Zhang P. Wang X.-M. Xu Q. Guo C.-Q. Wang P. Lu C.-J. Liu R.-R. Angew. Chem., Int. Ed. 2021;60:21718–21722. doi: 10.1002/anie.202108747. [DOI] [PubMed] [Google Scholar]; (g) Antoniotti S. Dalla V. Duñach E. Angew. Chem., Int. Ed. 2010;49:7860–7888. doi: 10.1002/anie.200906407. [DOI] [PubMed] [Google Scholar]
- Hou M. Li J. Rao F. Chen Z. Wei Y. Chem. Commun. 2022;58:5865–5868. doi: 10.1039/d2cc00829g. [DOI] [PubMed] [Google Scholar]
- (a) Yan J. Luo H. Chen Z. Wei Y. Zhan H. Mei Y. Tetrahedron Lett. 2020;61:151453. [Google Scholar]; (b) Luo H. Yan J. Chen Z. Wei Y. Chen B. Liu Y. ChemistrySelect. 2020;5:4074–4077. [Google Scholar]; (c) Zhan H. Hou M. Li Y. Chen Z. Wei Y. Liu S. ChemistrySelect. 2021;6:11537–11540. [Google Scholar]
- Pohlhaus P. D. Sanders S. D. Parsons A. T. Li W. Johnson J. S. J. Am. Chem. Soc. 2008;130:8642–8650. doi: 10.1021/ja8015928. [DOI] [PubMed] [Google Scholar]
- Hu J.-L. Feng L.-W. Wang L. Xie Z. Tang Y. Li X. J. Am. Chem. Soc. 2016;138:13151–13154. doi: 10.1021/jacs.6b08279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.