Abstract
Tobacco black shank caused by Phytophthora nicotianae is a serious disease in tobacco cultivation. We found that naringenin is a key factor that causes different sensitivity to P. nicotianae between resistant and susceptible tobacco. The level of basal flavonoids in resistant tobacco was distinct from that in susceptible tobacco. Of all flavonoids with different content, naringenin showed the best antimicrobial activity against mycelial growth and sporangia production of P. nicotianae in vitro. However, naringenin showed very low or no antimicrobial activity to other plant pathogens. We found that naringenin induced not only the accumulation of reactive oxygen species, but also the expression of salicylic acid biosynthesis‐related genes. Naringenin induced the expression of the basal pathogen resistance gene PR1 and the SAR8.2 gene that contributes to plant resistance to P. nicotianae. We then interfered with the expression of the chalcone synthase (NtCHS) gene, the key gene of the naringenin synthesis pathway, to inhibit naringenin biosynthesis. NtCHS‐RNAi rendered tobacco highly sensitive to P. nicotianae, but there was no change in susceptibility to another plant pathogen, Ralstonia solanacearum. Finally, exogenous application of naringenin on susceptible tobacco enhanced resistance to P. nicotianae and naringenin was very stable in this environment. Our findings revealed that naringenin plays a core role in the defence against P. nicotianae and expanded the possibilities for the application of plant secondary metabolites in the control of P. nicotianae.
Keywords: antimicrobial activity, naringenin, NtCHS, oomycete, Phytophthora nicotianae, plant defence
This study demonstrates that the plant secondary metabolite naringenin specifically inhibits Phytophthora nicotianae.
1. INTRODUCTION
Plant‐pathogenic oomycetes pose a serious threat to agriculture, horticulture, forestry, aquaculture and natural ecosystems (Wang, Tyler, et al., 2019). They encompass over 100 species that cause devastating diseases in both plants and animals. The Phytophthora species are the most serious pathogens. They infect a broad group of hosts and cause diseases such as root rot, late blight, downy blight, and downy mildew (Derevnina et al., 2016; Kamoun et al., 2015; Panabieres et al., 2016). Tobacco black shank (TBS) disease, caused by Phytophthora nicotianae, threatens production in tobacco‐producing areas across the world (Gallup et al., 2018). Infections mainly occur in the adult stage of tobacco, resulting in significant yield loss and quality reduction. Roots and stems are the main parts infected, leading to progressive decay of the diseased tissues, such as root and stem necrosis, wilting, and chlorosis, and finally death (Chen et al., 2020; Csinos & Minton, 1983; Gallup et al., 2006). Mycelia, oospores and chlamydospores from P. nicotianae are found in soil and plant tissues in compost through the winter and can survive for more than 3 years. Soil is therefore the main source of infection, while manure and irrigation promote the spread of pathogens (Antonopoulos et al., 2010; Gallup et al., 2018).
TBS is a very difficult disease to control due to the diversity and high adaptability of P. nicotianae (Gallup et al., 2018; Ji et al., 2014). Its management relies on the integration of different approaches, including planting resistant varieties, fungicide applications, crop rotation, management of water and fertilizer (Shew & Lucas, 1991). So far, four physiological races (0, 1, 2 and 3) of P. nicotianae have been reported. The undomesticated Nicotiana species are an important source of resistant varieties. Tobacco cultivars resistant to race 0 of P. nicotianae conferred by the genes Php or Phl have been widely used across the world; however, cultivars have become susceptible to P. nicotianae as there has been a gradual shift of pathogen populations from race 0 to race 1, making the strategy of TBS‐resistant cultivars ineffective in disease control (Johnson et al., 2002; Li et al., 2006; Sullivan et al., 2005). In addition, P. nicotianae has a large arsenal of secreted proteins, termed effectors, that act as weapons to overcome the host resistance and promote infection (Hou et al., 2019; Lee et al., 2018). These are the main reasons that crop rotation is highly recommended to avoid disease outbreaks and propagation of P. nicotianae in the field (Yong et al., 2010). Pesticide application is another important approach to managing TBS. Several fungicides such as mefenoxam and potassium phosphite have been applied for many years but resistance in Phytophthora has been reported for both compounds (Hao et al., 2019; Ji et al., 2014). Some new fungicides against oomycetes, such as fluopicolide, ethaboxam, oxathiapiprolin and mandipropamid, with different modes of action have been developed and they have shown higher efficiency in treatment of Phytophthora (Hao et al., 2019; Ji et al., 2014; Qu et al., 2016). These new fungicides provide another highly effective approach against pathogens in addition to rotation and proper use of all existing fungicides. However, more attention should be paid to the development of resistance to these new fungicides among the populations of P. nicotianae (Panabieres et al., 2016; Qu et al., 2016).
Biocontrol has been shown to be an alternative way to manage TBS efficiently, mainly using biocontrol strains and new compounds that have been isolated from natural organisms. Among biocontrol strains, Pseudomonas fluorescens and Bacillus spp. have been widely studied and used due to the antimicrobial components they release, such as peptides and enzymes (Choudhary & Johri, 2009; Fira et al., 2018; Rajaofera et al., 2018). These antimicrobial components can improve plant growth, as well as induce a plant immune response to abiotic stress (Choudhary & Johri, 2009; Sun et al., 2017). In addition, some biocontrol strains, such as the plant growth‐promoting rhizobacterium Bacillus amyloliquefaciens Ba168, secrete proteins and peptides that target pathogens directly, for example, by damaging the cell wall and membrane of P. nicotianae (Guo et al., 2020). In addition, various natural agents produced from plants are another source of antimicrobial components. Some secondary metabolites are not involved in growth, development or reproduction of plants directly. Rather, they perform special functions under a given set of conditions such as pathogen attack, water deficit or extreme temperature (Bartwal et al., 2012). The essential oil eugenol and diallyl disulphides have been confirmed to be effective against TBS by destroying mycelial cell membrane integrity, causing an increase in cell membrane permeability and leading to cell death (Jing et al., 2017; Wang et al., 2019). Phytoalexins with antiseptic, anti‐inflammatory, antioxidant or antimicrobial activities accumulate soon after pathogen infection (Chripkova et al., 2016; Kumar et al., 2006).
Over 9000 categories of flavonoids have been found in various plants, making them one of the largest families of secondary metabolites (Wang et al., 2011). They are essential factors that are involved in aspects of plant development and defence, and flower and fruit quality (Treutter, 2005; Wang et al., 2011). Flavonoids often accumulate in specialized cells and there are only a few studies on their function against pathogens (Kariu et al., 2017; Martínez‐Castillo et al., 2018; Paczkowski et al., 2017; Tattini et al., 2004). Naringenin is one of the major flavonoids and is mainly found in citrus fruits, including tangerine, lemon, orange and grapefruit (Manchope et al., 2017). Naringenin accumulates when plants are infected by Rhizobium leguminosarum bv. viceae, Pseudomonas syringae pv. pisi or Plasmodiophora brassicae (Makarova et al., 2016; Paesold et al., 2010). In addition, naringenin has shown anti‐inflammatory, antiviral and antifungal activities, for example against Fusarium spp. including F. poae, F. culmorum and F. graminearum, and the rice pathogen Magnaporthe grisea (Den Hartogh & Tsiani, 2019; Padmavati et al., 1997; Skadhauge et al., 1997). However, knowledge of the mechanism of how naringenin contributes to defence against P. nicotianae is still unknown. We found that naringenin conferred defence against P. nicotianae by inhibition of mycelial growth and sporangia production. Through metabolomics analysis, we found that basal flavanones and flavonols in resistant tobacco varieties were distinct from those in susceptible tobacco varieties. Of all flavanones and flavonols, we discovered that naringenin inhibited P. nicotianae most effectively. However, naringenin showed no or very low inhibition activity towards other plant pathogens. We also found that naringenin induced plant resistance to P. nicotianae by inducing the accumulation of O2 − and H2O2, the expression of genes regulating salicylic acid (SA) biosynthesis and SA signalling, and the expression of genes regulating plant basal defence and resistance to P. nicotianae. Knockdown of chalcone synthase (CHS), a gene encoding a rate‐limiting enzyme of the naringenin sythesis pathway, rendered tobacco highly susceptible to P. nicotianae, but it kept the same susceptibility to other plant pathogens. Interestingly, exogenous application of naringenin to tobacco enhanced resistance to TBS without affecting the agronomic traits. Our findings reveal that naringenin showed antimicrobial activity towards P. nicotianae and is therefore a potential botanical pesticide in tobacco disease control for the future.
2. RESULTS
2.1. Metabolite profiling of basal flavanone and flavonol in resistant tobacco varieties is distinct from that in susceptible tobacco varieties
Typical resistant and susceptible cultivated tobacco varieties to TBS were used in this study. The typical cultivated tobacco varieties in China are Beinhart 1000‐1 (BH‐1) and Xiaohuangjin 1025 (XHJ), which show resistance and susceptibility to P. nicotianae, respectively (Figure 1a). The disease index of BH‐1 is significantly lower than that of XHJ after inoculation with P. nicotianae (Figure 1b), and the colonization of P. nicotianae in XHJ is higher than that in BH‐1 (Figure 1c). To test whether there is a difference in the basal level of metabolites between the resistant and susceptible tobacco varieties in uninfected roots, the flavonoids of BH‐1 and XHJ were analysed by liquid chromatography‐electrospray ionization‐tandem mass spectrometry (LC‐ESI‐MS/MS) to evaluate the metabolite profiles. As shown in Table S1, 166 metabolites were identified and characterized by its distinct retention time and mass‐to‐charge ratio (m/z). Compared to XHJ, the content of 15 metabolites in BH‐1 were significant lower when considering fold change and the variable importance in projection (VIP) value of an orthogonal partial least‐squares discriminant analysis (OPLS‐DA) model (fold change > 2, VIP > 1) (Figure 1d and Table S2). These metabolites could be divided into four categories: flavonoids, flavonols, flavanones and others; the proportion of metabolites with a higher content of flavonoids was different in each type of metabolite (Figure 1e,f). The proportions of flavanones and flavonols were 18.75% and 15.90%, respectively. Through metabolite analysis, 16 flavanones were characterized. The content of nine flavanones, including liquiritigenin and naringenin, was slightly higher and the content of three flavanones, including isosakuranetin and hesperetin, was significantly higher in BH‐1 compared to XHJ (Table S3). We also characterized 44 flavonols, of which the content of seven was significantly higher in BH‐1 than XHJ, such as kaempferol and quercetin (Table S4). These results indicate that there is a significant difference in the basal level of flavonoids between resistant and susceptible tobacco varieties, and this difference might be related to resistance to P. nicotianae in the plants.
FIGURE 1.

Metabolite profiling of resistant (BH‐1) and susceptible (XHJ) tobacco lines in response to Phytophthora nicotianae. Disease symptoms observed following root inoculation with a spore suspension of P. nicotianae at 5 days postinoculation (dpi). (a) Phenotypes of BH‐1 and XHJ in response to P. nicotianae. (b) Disease index of P. nicotianae on BH‐1 and XHJ (n = 3, error bars, SD). XHJ is significantly different from BH‐1 according to a Mann–Whitney test (**p < 0.01). (c) Biomass of P. nicotianae in BH‐1 and XHJ (n = 3, error bars, SD). XHJ is significantly different from BH‐1 by a Mann–Whitney test (**p < 0.01). (d) A volcano plot of metabolites of BH‐1 compared to XHJ. (e) The proportion of the number with a higher content of metabolites in each class of metabolites. The red numbers indicate the number of metabolites with significantly higher content, and the blue numbers indicate the total number of the metabolites detected in the metabolome. (f) A heatmap of 15 metabolites with higher content in BH‐1 than XHJ. The left three columns are from roots of XHJ and the right three columns are from roots of BH‐1; each column represents a biological replicate.
2.2. Naringenin showed antimicrobial activity on P. nicotianae in vitro
For the 16 flavanones and 44 flavonols described above, we tested their antimicrobial activity against P. nicotianae in vitro. P. nicotianae grew on potato dextrose agar (PDA) equally well in the presence of different concentrations of flavonoids. Only three flavanones inhibited the growth of P. nicotianae, and of these naringenin showed the strongest antimicrobial activity (Figure 2a,b). The half‐maximum effective concentration (EC50) of naringenin for inhibition of P. nicotianae mycelial growth was 22.01 mg/L, whereas the EC50 values of liquiritigenin and hesperetin were 51.43 and 30.20 mg/L (Table S5). In contrast, none of the flavonols, such as kaempferol, quercetin or reutinum, showed any inhibitory activity on the mycelial growth of even at concentrations up to 200 mg/L (Figure S1a,b and Table S5). Naringenin also inhibited growth of other oomycetes, such as Phytophthora capsici, with an EC50 value of 50.11 mg/L, but showed low inhibition activity on Pythium aphanidermatum (Figure S2 and Table S6). However, naringenin showed very low or no inhibitory activity on other plant pathogens such as Sclerotinia sclerotiorum, Botrytis cinerea, and Fusarium graminearum (Figure S3). We next tested whether naringenin affected the reproduction of P. nicotianae by microscopic observation. As shown in Figure 2c,d and Table S6, the EC50 and EC90 values of naringenin for inhibition of P. nicotianae sporangia production were 2.01 mg/L and 6.62 mg/L, respectively. These values are significantly lower than the EC50 for mycelial growth. Furthermore, we used reverse transcription‐quantitative PCR (RT‐qPCR) to confirm that cell growth and reproduction‐related genes were down‐regulated when P. nicotianae was treated with naringenin (Figure S4). These results indicate that naringenin inhibits not only the mycelial growth but also the reproduction of P. nicotianae.
FIGURE 2.

The antimicrobial activity of the different flavonoids on Phytophthora nicotianae. The control (CK) was added ethanol only. (a) Colony morphology of P. nicotianae on potato dextrose agarose amended with naringenin, liquiritigenin or hesperetin at 28°C for 7 days. (b) The inhibition rate of naringenin, liquiritigenin and hesperetin on mycelial growth (n = 3, error bars = SD). (c) The sporangia production of P. nicotianae is dramatically inhibited by naringenin under microscopic observation. (d) The inhibition rate of naringenin on the sporangia production (n = 3, error bars = SD).
2.3. Naringenin induces accumulation of reactive oxygen species
Normally, plants are resistant to the invasion of most pathogens through a burst of reactive oxygen species (ROS), including hydrogen peroxide (H2O2) and the superoxide anion (O2 −) (Bray, 2000). The increased H2O2 content promotes the up‐regulation of genes that are associated with the plant defence response (Bray, 2000). Flavonoids have been shown to be antioxidant agents by clearing away ROS (Turek & Stintzing, 2013). Quercetin, a flavonoid and powerful antioxidant, has been reported to activate Arabidopsis defence against Pseudomonas syringae pv. tomato DC3000 via an H2O2 burst (Jia et al., 2010). To determine if ROS accumulate in tobacco with naringenin treatment, we tested the content of H2O2 and O2 − in Honghuadajinyuan (HD) seedlings (Figure 3a,b). HD is an important flue‐cured tobacco variety that is susceptible to TBS. As expected, the accumulation of H2O2 and O2 − was significantly induced with naringenin treatment, indicating that naringenin induced an ROS burst in tobacco.
FIGURE 3.
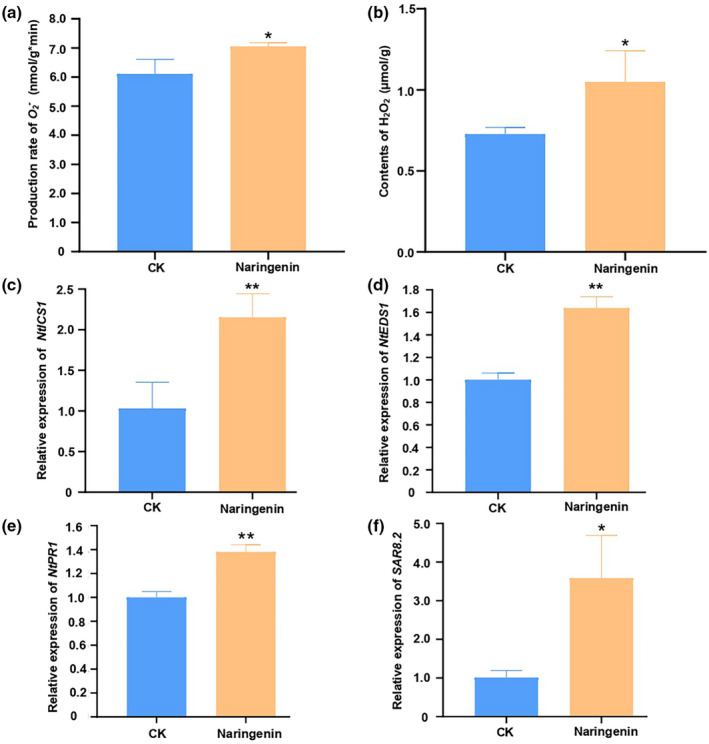
Naringenin induced plant resistance against Phytophthora nicotianae in Honghuadajinyuan (HD), the main flue‐cured tobacco variety, which is susceptible to tobacco black shank. Control (CK), the seedlings were treated without naringenin. (a) Statistical analysis for the rate of production of O2 − with or without treatment with naringenin 3 days postinoculation with P. nicotianae (n = 3, error bars = SD). (b) Statistical analysis for the content of H2O2 with or without treatment with naringenin 3 days postinoculation with P. nicotianae (n = 3, error bars = SD). (c) Relative expression levels of NtICS1 after treatment with naringenin (three biologically independent experiments, each with three technical replicates). (d) Relative expression levels of NtEDS1 after treatment with naringenin (three biologically independent experiments, each with three technical replicates). (e) Relative expression levels of NtPR1 in HD after treatment with naringenin (three biologically independent experiments, each with three technical replicates). (f) Relative expression levels of SAR8.2 after treatment with naringenin (three biologically independent experiments, each with three technical replicates). Significant differences compared to the control by Student's t test (*p < 0.05, **p < 0.01).
2.4. Naringenin induced expression of salicylic acid biosynthesis‐related genes and signalling in tobacco
The hormone salicylic acid (SA) is required for the activation of immune responses to biotrophic pathogens in plants (van Butselaar & Van den Ackerveken, 2020). Various flavonoids mediate the activation of plant‐pathogen resistance by the SA‐dependent pathway (Jia et al., 2010; Yang et al., 2016). To test whether SA biosynthesis‐related genes could be induced by naringenin, the expression of the representative SA biosynthesis‐related gene ICS1 was measured by RT‐qPCR. The expression of the ICS1 gene increased approximately 2‐fold with naringenin treatment (Figure 3c). This result suggests that naringenin induces an increase in SA biosynthesis by the isochorismate‐dependent pathway and prompted us to examine whether naringenin induces SA signalling. We measured the expression of the SA signalling gene EDS1 after treatment with naringenin by RT‐qPCR. The expression of EDS1 was increased 1.8‐fold by naringenin treatment (Figure 3d). This result indicates that naringenin induces SA biosynthesis and activates SA signalling in tobacco.
2.5. Naringenin enhanced plant resistance to P. nicotianae
Flavonoids have been shown to be critical to plant defence on pathogenic bacteria and fungi through the induction of PR genes (Mierziak et al., 2014). Quercetin and its derivatives are capable of inducing pathogen resistance to both bacteria and fungi (Jia et al., 2010; Parvez et al., 2004; Yang et al., 2016). To examine whether naringenin induces basal pathogen resistance, we analysed the expression of PR1 and SAR8.2 genes in tobacco after naringenin treatment using RT‐qPCR. The SAR8.2 gene is a gene that controls plant resistance to P. nicotianae (Shi et al., 2022). As expected, the expression levels of PR1 and SAR8.2 with naringenin treatment were approximately 1.4‐fold and 3.5‐fold higher than those of the control, respectively (Figure 3e,f). These results suggest that naringenin induces basal pathogen resistance.
2.6. Interference of naringenin biosynthesis led to more susceptibility to P. nicotianae in tobacco
The flavonoid biosynthesis pathway has been well studied and most intermediate enzyme steps have been characterized (see Figure 4a). The pathway starts with the conversion of phenylalanine to cinnamic acid by phenylalanine ammonia‐lyase (PAL). Under continuous catalysis by cinnamate 4‐hydroxylase (C4H) and 4‐coumaroyl coenzyme A (CoA) ligase (4CL), cinnamic acid is converted to p‐coumarinyl CoA, a substrate of flavonoids. Decarboxylative condensation of p‐coumarinyl CoA with three molecules of malonyl CoA into naringenin chalcones by chalcone synthase (CHS) provides substrates for the final synthesis of naringenin by chalcone isomerase (CHI) (Kreuzaler & Hahlbrock, 1972; Pandey et al., 2015). We first tested the expression of NtCHS, NtPAL, NtC4H and Nt4CL in BH‐1 and XHJ without and with inoculation of P. nicotianae. We found that, compared to XHJ, NtCHS was more highly expressed in BH‐1 even when infected with P. nicotianae, indicating that NtCHS might be a core regulatory gene in the biosynthesis of naringenin in tobacco (Figure 4b). The other three genes (NtPAL, NtC4H and Nt4CL) involved in the synthesis of naringenin did not show significant differences except in the later period (5 days) after tobacco was inoculated with P. nicotianae (Figure S5).
FIGURE 4.

Transgenic validation of NtCHS conferring resistance to Phytophthora nicotianae in tobacco. (a) The pathway of naringenin synthesis. (b) Reverse transcription‐quantitative PCR analysis of NtCHS expression. (c) Disease symptoms of the T2 progeny derived from three transformants (NtCHS‐RNAi‐1, NtCHS‐RNAi‐2 and NtCHS‐RNAi‐3) obtained from transformation of wild‐type (WT) tobacco with an ubi::Tachs RNAi construct at 5 days postinoculation (dpi). (d) Relative NtCHS expression levels detected in the T2 progenies derived from the three RNAi transformants (three biologically independent experiments, each with three technical replicates). (e) RNAi of NtCHS decreased naringenin levels in the entire plant. (f) Disease index of the T2 progenies derived from three transformants (NtCHS‐RNAi‐1, NtCHS‐RNAi‐2 and NtCHS‐RNAi‐3) 5 dpi. (b, d–f) (n = 3, error bars = SD). * Significant differences compared to WT by the Mann–Whitney test (*p < 0.05, **p < 0.01).
Next, to interfere with the naringenin synthesis pathway, we generated tobacco transgenic lines with NtCHS silenced via RNA interference (RNAi) in the resistant cultivar BH‐1 (Figure 4c). Three independent T2 transgenic lines comprising the NtCHS RNAi construct showed a significant decrease in the expression level of NtCHS (Figure 4d) and a dramatic decrease in naringenin content compared to the wild type (WT) (Figure 4e). These transgenic lines showed more susceptibility to P. nicotianae compared to the WT, but they kept the same sensitivity to Ralstonia solanacearum, a serious pathogen that causes tobacco bacterial wilt (Figures 4f and S6). Naringenin showed very low antimicrobial activity against R. solanacearum even at concentrations up to 400 mg/L (Table S7). Furthermore, the biomass of P. nicotianae in NtCHS‐RNAi mutants was up to six times more than that in WT by DNA quantification of the oomycete (Figure S7). These results show that interference in naringenin synthesis in tobacco induced more susceptibility to P. nicotianae and naringenin might be a specific antimicrobial agent to P. nicotianae because it showed very low or no activity towards other plant pathogens.
2.7. Exogenous application of naringenin enhanced resistance to P. nicotianae
To explore the possibility of applying naringenin as a natural antimicrobial agent in the management of TBS in the field, we treated susceptible tobacco HD seedlings with naringenin before they were inoculated with P. nicotianae. HD seedlings inoculated with P. nicotianae but without naringenin treatment were used as the control. After 10 days, we found that HD seedlings treated with naringenin were significantly more resistant to P. nicotianae compared to the control (Figure 5a,b). Accordingly, the disease index in HD treated with naringenin was dramatically lower than in the control (Figure 5c). More importantly, the application of naringenin did not negatively affect the agronomic traits of tobacco, such as plant height, leaf length, leaf width, knot spacing and stem girth (Figure S8). In addition, to test the impact of naringenin stability on infection by pathogens, we checked the susceptibility of HD tobacco to P. nicotianae 3, 7, 15 and 30 days after treatment with naringenin. The results showed that HD treated with naringenin for 30 days still had the same susceptibility to P. nicotianae as HD treated with naringenin for 3 days, and both were significantly more resistant to P. nicotianae compared to HD without naringenin treatment. These results show that exogenous application of naringenin improved resistance to P. nicotianae significantly and showed good stability of antimicrobial activity in the environment (Figure S9). Therefore, naringenin was shown to be a potential antimicrobial agent for the management of TBS in the field.
FIGURE 5.

Resistance to tobacco black shank was enhanced by exogenous application of naringenin. (a) Disease symptoms of Honghuadajinyuan (HD) supplied with 100 ml of a solution of 0.4 g/L naringenin 10 days postinoculation with Phytophthora nicotianae. HD was inoculated with P. nicotianae but without treatment of naringenin as a control. (b) Enlarged view of the neck with or without treatment of naringenin after HD was inoculated with P. nicotianae. (c) The disease index with or without the treatment of naringenin after HD was inoculated with P. nicotianae (n = 3, error bars = SD). HD is the main cultivated flue‐cured tobacco variety but is susceptible to tobacco black shank. Significant difference compared to control by the Mann–Whitney test (**p < 0.01).
3. DISCUSSION
Plants have evolved diverse biosynthetic routes to produce a range of small organic molecules referred to as secondary metabolites. Secondary metabolites are restricted to specific taxonomic groups and play important roles in diverse aspects of plant life activities, including as components of signalling cascades and in plant defence against herbivores (Pandey et al., 2015). Among all plant secondary metabolites, flavonoids are widely studied as they have a multitude of biological functions, including a function as defence molecules against biotic and abiotic stresses (Treutter, 2005; Zhang et al., 2020). Flavanones and flavonols are categories of flavonoids that differ by a hydroxyl moiety in the 3′ position in flavonols that is lacking in flavanones (Sun et al., 2019). Flavanones include phloretin, glyceollins and naringenin. Aminoethyl‐phloretin is a water‐soluble phloretin derivative that has been shown to possess antibacterial activity toward both gram‐positive and gram‐negative bacteria (Wei et al., 2020). Glyceollins are a group of phytoalexins that are mainly isolated from soybeans. Both aminoethyl‐phloretin and glyceollins have numerous functions in human health, especially as anticancer agents (Pham et al., 2019). Naringenin is a typical flavanone that has been found mostly in edible fruits such as citrus species, tomatoes and figs, and studies on naringenin have mainly focused on its biological effects on human health (Salehi et al., 2019). There are a few reports on naringenin and its antimicrobial activity on Escherichia coli O157:H7 in apple cider and its improvement of resistance to rice blast (Surendran Nair et al., 2020). However, there are no reports on the mechanism by which naringenin provides defence against plant pathogens.
In this study, we found that there was a significant difference in the basal flavonoid metabolite profile between resistant and susceptible tobacco varieties. We treated P. nicotianae in vitro with different concentrations of flavanones and flavonols, and found that only three flavanones showed antimicrobial activity, with naringenin showing the highest activity. However, none of the flavonols showed any antimicrobial activity, even at high concentrations up to 200 mg/L. Compared to its activity against P. nicotianae, naringenin had a lower antimicrobial activity against other oomycetes such as P. capsici. Naringenin showed no or very low inhibitory activity towards plant pathogens such as S. sclerotiorum and B. cinerea. Interestingly, there was dramatic inhibition of sporangia production when P. nicotianae was treated with a very low concentration of naringenin. The reason for the inhibition of sporangia production by naringenin might be that genes regulating the reproductive process in P. nicotianae are directly suppressed by naringenin. We used RT‐qPCR to confirm that genes regulating cell growth and reproduction were down‐regulated after P. nicotianae was treated with naringenin. Naringenin is thus the first flavonoid to be identified as a antimicrobial agent against P. nicotianae; it might affect the expression of genes regulating the growth and reproduction of P. nicotianae directly.
Flavonoids are well known as ROS scavengers and powerful antioxidants (Pannala et al., 2001; Rice‐Evans, 2001). Flavonoids decrease the ROS level through inhibition of pro‐oxidant enzymes, including cyclo‐oxygenase and lipoxygenase (Eghbaliferiz & Iranshahi, 2016). Phenolics and carotenoids are chemical groups that prevent oxidative damage as a result of their ability to decrease the ROS level, and they also exhibit pro‐oxidant activities in vitro in the presence of metal ions (Eghbaliferiz & Iranshahi, 2016). Like phenolics and carotenoids, flavonoids act as pro‐oxidants at physiological pH. In this study, we showed that naringenin induced the accumulation of ROS, which suggests that naringenin might act as a pro‐oxidant. An et al. also showed that naringenin induced an ROS burst in plants as a defence against P. syringae (An et al., 2021). SA has been shown to induce pathogen resistance through ROS accumulation and increased expression of PR genes. We also found that genes regulating SA biosynthesis, SA signalling and basal pathogen resistance were enhanced at the transcriptional level by treatment with naringenin . Furthermore, SAR8.2, a gene in tobacco that regulates plant resistance to P. nicotianae (Shi et al., 2022), was substantially up‐regulated by treatment with naringenin. These results indicate that naringenin induces plant‐pathogen resistance. Naringenin accumulates soon after plants are infected with biotrophic pathogens such as Rhizobium leguminosarum bv. viceae, Pseudomonas syringae pv. pisi and Plasmodiophora brassicae (Makarova et al., 2016). However, we did not detect significant accumulation of naringenin in tobacco after infection with P. nicotianae by metabolite analysis. Interestingly, naringenin has been shown to induce pathogen resistance against P. syringae through the activation of NPR1 in Arabidopsis 2021, but did not show any antimicrobial activity against P. syringae even at high concentrations in vitro (An et al., 2021). Our results demonstrate that naringenin confers plant defence against P. nicotianae through induction of plant‐pathogen resistance in addition to its antimicrobial activity.
The key genes in the metabolic pathway of naringenin synthesis include PAL, 4CL, C4H, and CHS (Kreuzaler & Hahlbrock, 1972; Pandey et al., 2015). Our results showed that the expression level of NtPAL, NtC4H and Nt4CL did not show a significant change except in the later period (5 days) after BH‐1 had been infected by P. nicotianae (Figure S5). Remarkably, only NtCHS had a higher expression level in BH‐1 than that in XHJ, even after BH‐1 was treated with P. nicotianae. The accummulation of naringenin is positively correlated with the expression of CHS (Pandith et al., 2019). In this study, without infection of P. nicotianae, the basal expression level of NtCHS in resistant tobacco variety BH‐1 was higher than that in susceptible tobacco variety XHJ. Correspondingly, the content of naringenin in BH‐1 was higher than that in XHJ. CHS is a rate‐limiting enzyme that controls the supply of substrates in the flavonoid biosynthesis pathway, thus the amount of products downstream are affected by the efficiency of CHS. Naringenin plays a key role in both plant‐pathogen resistance and antimicrobial activity after the plant is infected by P. nicotianae; however, the plant is not able to synthesize unlimited amounts of naringenin as there is a lack of substrates in the biosynthesis pathway. The expression level of NtCHS was down‐regulated to keep the flavonoid biosynthesis pathway in order (Figure 4b). RNA interference of NtCHS led to a dramatic decrease in naringenin content and the biomass of P. nicotianae in NtCHS‐RNAi tobacco mutants was up to 6‐fold higher than that in WT plants. The level of RNA interference in different RNAi transgenic plant lines might vary. In this study, compared to the other two RNAi transgenic lines, the content of naringenin in the NtCHS‐RNAi‐3 transgenic line was much less and this may be the reason that the NtCHS‐RNAi‐3 transgenic line was more susceptible to P. nicotianae. Significantly, TBS severity is a quantitative trait and its phenotype might be affected by multiple factors in the environment. Therefore, genes that regulate the plant resistance to TBS and the plant phenotype do not correspond to each other, l, raising difficulties to study the mechanism of plant resistance to TBS.
CHS orchestrates the general flavonoid biosynthesis pathway in tobacco; thus, its down‐regulation influences the biosynthesis of all chalcones, flavones, anthocyanins and other derivatives and is not limited to naringenin. In fact, the accumulation of various flavonoids, including naringenin and rutin, decreases significantly in NtCHS1‐RNAi transgenic plants (Chen et al., 2019). However, only naringenin showed antimicrobial activity against P. nicotianae, and other flavonoids such as rutin showed very low or no antimicrobial activity. By contrast, compared to the WT, we found that the NtCHS‐RNAi tobacco mutants kept the same susceptibility to R. solanacearum, a serious pathogen which causes tobacco bacterial wilt. This indicates that RNAi of NtCHS induced a reduction in the content of naringenin, and specific antimicrobial activity against P. nicotianae by naringenin might be the main reason that NtCHS‐RNAi tobacco was more sensitive to P. nicotianae compared to other plant pathogens such as R. solanacearum. Naringenin showed no antimicrobial activity against R. solanacearum in vitro even with concentrations of naringenin up to 400 mg/L. Because naringenin induced resistance against both P. nicotianae and P. syringae, naringenin could also be expected to induce resistance to R. solanacearum. CHS regulates flower colour, fertility and gas substances, and we found that CHS was also involved in resistance to P. nicotianae. We also found that TBS‐susceptible tobacco variety HD became resistant to P. nicotianae after treatment with naringenin. Naringenin showed high activity with a good stability in the environment (Figure S9). This leads to the potential application of naringenin as a natural antimicrobial agent in the management of TBS in the field in the future.
In conclusion, for first time we found that naringenin might be an antimicrobial secondary metabolite against P. nicotianae. Naringenin inhibited P. nicotianae through not only its antimicrobial activity, but also its induction of defences against plant pathogens (Figure S10). Our discoveries will enrich the means of prevention and control for TBS. Future work needs to address the question of whether naringenin targets proteins or genes in P. nicotianae directly, and whether naringenin interferes in the interaction between P. nicotianae and tobacco. Notably, the development of naringenin as a plant‐derived fungicide against P. nicotianae is important work for the future.
4. EXPERIMENTAL PROCEDURES
4.1. Plant materials and P. nicotianae inoculation
BH‐1 shows a high level of resistance to TBS, whereas XHJ is extremely susceptible. Both of these were obtained from the Tobacco Research Institute, Chinese Academy of Agricultural Sciences. Plants were cultured using Holland nutrient solution and were grown in a growth chamber at 28°C with 16 h light and 8 h dark photoperiod cycles. Sixteen‐week‐old tobacco plants were infected with P. nicotianae by dipping tobacco in a spore suspension for 3 h, then cultured in water. After 5 days, the phenotype of TBS was evaluated using an empirical scale (YC/T39‐1996, China), where 0 represents a highly resistant response and 9 represents a highly susceptible response. Disease index scores based on disease severity were used for assessment and calculated using the following formula: disease index (%) = [∑(disease evaluation scale score × number of plants with each scale score)/(total number of plants observed × the highest disease evaluation scale score)] × 100. Three‐month‐old tobacco plants were infected with P. nicotianae according to a described method (Zhang et al., 2018). The biomass of P. nicotianae in infected tobacco plants was quantified using a slight modification of a previously described method (Park et al., 2012). Quantitative PCR was performed using a Light Cycler 96 Real‐Time PCR detection system (Roche, http://technical‐support.roche.com). The biomass of P. nicotianae was calculated using the threshold cycle value (C t) of P. nicotianae WS21 DNA against the C t of tobacco genomic actin DNA.
4.2. Inoculation of tobacco with R. solanacearum
After 12‐week‐old tobacco plants were infected with R. solanacearum, a small amount of bacteria was picked up with a toothpick and placed into 1 L of nutrient broth (NB) (Qingdao Hope Bio‐Technology Co., Ltd). The bacteria were grown in an incubator at 30°C and 200 rpm until the OD600 reached 1.0. Two hundred millilitres of suspension bacteria cells was added to each plate of tobacco seedlings. After 15 days, the phenotype of tobacco bacterial wilt was evaluated using an empirical five‐point scale (GB/T 23222–2008), where 0 represents a highly resistant response and 4 represents a highly susceptible response. Disease index scores based on the disease severity were used for assessment and were calculated using the following formula: disease index (%) = [∑(disease evaluation scale score × number of plants with each scale score)/(total number of plants observed × the highest disease evaluation scale score)] × 100.
4.3. Pathogen isolation and inoculum preparation
R. solanacearum and P. nicotianae race 0 were obtained from the Plant Protection Laboratory of the Chinese Academy of Agricultural Sciences. P. capsici, P. aphanidermatum, Pythium ultimum, S. sclerotiorum, Colletotrichum gloeosporioides, Fusarium moniliforme, B. cinerea, and F. graminearum were obtained from Nanjing Agricultural University. Each pathogen was grown separately in 25 ml of potato dextrose agar (PDA; potato 200 g, glucose 20 g, agar 16 g, in 1 L) at 28°C for 14 days. The inoculum method was used as described (Zhang et al., 2018). Then 0.1% KNO3 was added to the PDA plate containing P. nicotianae and kept at 4°C for 25 min, then kept in the light for 30 min at 25°C. The concentration of the spore suspension was determined by a cellometer Auto T4 (Nexcelom Cellometer) and adjusted to 104 spores/ml.
4.4. Antimicrobial activity test for flavonoids
All flavonoid standards used in this study were purchased from the Solarbio Company. First, 20 mg of standard flavonoid was dissolved in 1 ml of ethanol to make 20 mg/ml stock solution, which was filtered and sterilized before use. PDA containing different concentrations of different flavonoids was prepared for the antimicrobial tests. A single agar plug (5 mm diameter) was removed from the actively growing edge of the fungal culture and placed in a new PDA culture containing flavonoids. Four different pathogens inoculated with PDA or with only ethanol added served as positive controls. The medium was observed after incubating at 28°C for 7 days. The efficacy of each treatment was evaluated by measuring the diameter of each colony. Each treatment contained three replicates. The inhibition rate was calculated as follows: inhibition rate (%) = (control hyphae diameter − treatment hyphae diameter)/control hyphae diameter × 100.
4.5. Antimicrobial activity of naringenin on R. solancearum
First, 0.1 ml of 108 cfu/ml R. solancearum suspension was pipetted onto a Petri dish (diameter of 9 cm). Then the cooled and melted PDA medium was mixed thoroughly with the R. solancearum suspension. Filter paper was punched into 6‐mm diameter discs with a hole punch, immersed in different concentrations of naringenin, then moved to a flat plate. The plates were placed in an incubator at 28°C for 3 days. The diameter of the bacteriostatic circle was measured by the cross‐crossing method, and the average weight of each treatment was calculated. Each treatment contained three replicates. The inhibition rate was calculated as follows: inhibition rate (%) = (treatment bacteriostatic circle − control bacteriostatic circle)/control bacteriostatic circle × 100.
4.6. Metabolite estimation and analysis
Eight‐week‐old seedlings of BH‐1 and XHJ grown in Hoagland hydroponic nutrient solution were used for flavonoid metabolomic analysis. The freeze‐dried leaves were crushed using a mixer mill (MM 400; Retsch) with a zirconia bead for 1.5 min at 30 Hz. One hundred milligrams of powder extracted overnight at 4°C with 1 ml of 70% methanol. Following centrifugation at 10,000 × g for 10 min, the extracts were absorbed and filtrated (SCAA‐104, 0.22 μm pore size) before LC‐ESI‐MS/MS (HPLC, Shim‐pack UFLC SHIMADZU CBM30A system; MS, Applied Biosystems 4500 Q TRAP) analysis. The sample extracts were analysed by LC‐ESI‐MS/MS according to methods described previously (Gong et al., 2013). The effluent was connected to an ESI‐triple quadrupole‐linear ion trap (QTRAP)‐MS. Linear ion trap (LIT) and triple quadrupole (QQQ) scans were acquired on a triple quadrupole‐linear ion trap mass spectrometer (Q TRAP; API 4500 Q TRAP LC/MS/MS System) equipped with an ESI Turbo ion‐spray interface operating in a positive ion mode and controlled by Analyst v. 1.6.3 software (AB Sciex). The ESI source operation parameters were as follows: ion source, turbo spray; source temperature 550°C; ion spray voltage (IS) 5500 V; ion source gas I (GSI), gas II (GSII), curtain gas (CUR) set at 55, 60 and 25.0 psi, respectively; collision gas (CAD) high. Instrument tuning and mass calibration were performed with 10 and 100 μM polypropylene glycol solutions in QQQ and LIT modes, respectively. QQQ scans were acquired as multiple reaction monitoring (MRM) experiments with collision gas (nitrogen) set to 5 psi. DP (declustering potential) and CE (collision energy) for individual MRM transitions was done with further optimization of DP and CE. A specific set of MRM transitions was monitored for each period according to the metabolites eluted within this period.
Based on the self‐built database MWDB (Metware database) and the public database of metabolite information, qualitative analysis was performed on the primary and secondary mass spectrometry data. Metabolite quantification was carried out via the mode of MRM. After obtaining metabolite spectrum analysis data for different samples, peak area integration was performed on the mass spectrum peaks of all substances and integration correction was performed on the mass spectrum peaks of the same metabolite in different samples. To screen out the differential metabolites between the BH‐1 and XHJ groups, the mass spectrum peak of each metabolite in each sample was corrected according to the retention time and peak shape of each metabolite, thereby ensuring the accuracy of the qualitative and quantitative analyses. Supervised multiple regression orthogonal partial least‐squares discriminant analysis (OPLS‐DA) was conducted to estimate the stability and reliability of the model. The threshold variable importance in projection (VIP) value ≥1 and fold change ≥2 (up‐regulated) or ≤0.5 (down‐regulated) were used for screening the differential metabolites and drawing the heatmap.
4.7. RNA extraction of plants, cDNA synthesis and RT‐qPCR
Total RNA was extracted with a Plant RNA pure kit (ZOMANBIO, ZP405‐2), then the reverse transcription of the cDNA was synthetized with HiScript III RT SuperMix for qPCR (Vazyme). The primers for amplification of all genes were designed by Primer Premier v. 5 software. The qPCR was performed by a LightCycler 96 Real‐Time PCR detection system (Roche, http://technical‐support.roche.com) using ChamQ SYBR Colour qPCR Master Mix (Vazyme, Q411‐02/03). The relative expression levels of genes were calculated by the 2−ΔΔCt method and all reactions were repeated three times. Three independent biological replicates were used. The sequences of all primers are shown in Table S8.
4.8. The production of NtCHS‐RNAi lines
The construction of NtCHS‐RNAi and generation of transgenic tobacco lines have been described previously (Chen et al., 2019). PCR identification was used to amplify the transformed seedling DNA with RNAi‐F and RNAi‐R primers to determine whether the RNAi vector was successfully introduced. For the transformed lines, the silencing efficiency of NtCHS was identified by RT‐qPCR. The sequences for all primers used in this experiment are shown in Table S8.
4.9. Naringenin measurement by LC‐ESI‐MS/MS
Eight‐week‐old tobacco plants of WT and NtCHS‐RNAi lines were sampled and ground into homogenate using liquid nitrogen. After freeze‐drying, the 20‐mg samples were suspended in 1 ml of extraction solution (methanol:chloroform:water = 5:2:2) and treated by ultrasonic vibration for 45 min to extract naringenin. The extracted naringenin was analysed using LC‐ESI‐MS/MS (HPLC, Shim‐pack UFLC Shimadzu CBM‐20A system; MS, Applied Biosystems 4000 Q TRAP) according to the methods described previously (Zhao et al., 2020). Three biological replicates of each treatment were performed. The chromatographic conditions were as follows: Waters BEH C18 chromatographic column (150 mm × 2.1 mm, 1.7 μm); mobile phase: A phase water, B phase acetonitrile, 0.1% (vol/vol) formic acid and 0.2 mM ammonium acetate were added t both phases. Step‐stripping sequence: 0–1.0 min, 10% B; 0–9.0 min, 10% B–90% B; 9.0–11.0 min, 90% B–100% B; 11.0–11.1 min, 100% B–10% B; 11.1–13.0 min, 10% B. Column temperature: 30°C; injection volume: 2 μl; flow rate: 0.25 ml/min. Mass spectrum conditions: retention time 4.94 min−1; ion pair l: quantitative ion 433.1/271.1, collision energy −22 V; ion pair 2: quantitative ion 433.1/150.9, collision energy −42 V.
4.10. Inhibition of sporangia production
The effect of different concentrations of naringenin on sporangia production of P. nicotianae was assayed. A fresh mycelial block was immersed in V8 liquid medium and cultivated at 28°C for 48 h. Mycelium was washed with sterile deionized water, then soaked in sterile deionized water containing 0, 0.78, 1.56, 3.13, 6.25, and 12.50 mg/L of naringenin. Following a 24‐h incubation period at 28°C in the light, the sporangia were counted by observation with a microscope using three fields. Each treatment contained three replicates. The inhibition rate was calculated as follows: inhibition rate (%) = (control number of sporangia − treatment number of sporangia)/control number of sporangia × 100.
4.11. Measurement of H2O2 content and production rate of O2 − content
Three days after inoculation with P. nicotianae, HD roots treated with naringenin were used to measure ROS levels. The H2O2 content was determined by measuring the yellow titanium peroxide compound, which had specific absorption peak at 415 nm, according to the instruction of H2O2 Content Detection Kit (Suzhou Keming Biotechnology Ltd Co.). The production rate of O2 − was determined by measuring the formation of red azo compound, which had a specific absorption peak at 530 nm, according to the O2 − Detection Kit (Suzhou Keming Biotechnology Ltd Co.).
4.12. Exogenous application of naringenin
Under simulated field conditions, Nicotiana tabacum ‘Honghuadajinyuan’ (HD) was cultured for 3 months in pots. Then, the stem base of the HD seedlings was treated with 100 ml of solution including 0.04 g of naringenin 1 week prior their inoculation with P. nicotianae. Naringenin was added only once. HD seedlings with inoculation by P. nicotianae but without prior treatment of naringenin were used as a negative control. After 10 days, the phenotype of TBS was evaluated using an empirical six‐point scale (YC/T39‐1996, China), where 0 represents a highly resistant response and 9 represents a highly susceptible response. Disease index scores based on disease severity were used for assessment and calculated using the following formula: disease index (%) = [∑(disease evaluation scale score × number of plants with each scale score)/(total number of plants observed × the highest disease evaluation scale score)] × 100.
CONFLICT OF INTEREST
No conflict of interest declared.
Supporting information
Figure S1 The antimicrobial activity of the representative flavonols on Phytophthora nicotianae. The control (CK) was added ethanol only. (a) Colony morphology of P. nicotianae on potato dextrose agarose at 28°C for 7 days amended with kaempferol, quercetin or rutin. The concentrations were 12.5, 25, 50, 100 and 200 mg/L. (b) The inhibition rate of kaempferol, quercetin and rutin on P. nicotianae. (n = 3, error bars, SD).
Figure S2 The antimicrobial activity of three other pathogens treated with naringenin. (a) Colony morphology of Phytophthora capsici on potato dextrose agarose (PDA) amended with a different concentration of naringenin at 28°C. The concentrations of naringenin were 12.5, 25, 50, 100, 200, and 400 mg/L. The control (CK) was added ethanol only. (b) Colony morphology of Pythium aphanidermatum and Pythium ultimum on PDA amended with different concentrations of naringenin at 28°C. The concentrations of naringenin were 25, 50, 75, 100, 125, 150 and 175 mg/L. The control (CK) was added ethanol only. (c,d) The inhibition rate of naringenin on Phytophthora capsici, Pythium aphanidermatum and Pythium ultimum (n = 3, error bars, SD).
Figure S3 The inhibitory test of other phytopathogens treated with naringenin. The concentration of naringenin was 200 mg/L. The control (CK) was added ethanol only.
Figure S4 The result of reverse transcription‐quantitative PCR (RT‐qPCR) validation for differentially expressed genes on cell growth and reproduction of Phytophthora nicotianae. The control (CK) means P. nicotianae was treated with solvent only. The NG means P. nicotianae was treated with 20 mg/L naringenin. Gene expression was determined by RT‐qPCR after sampling (n = 3, error bars, SD).
Figure S5Reverse transcription‐quantitative PCR (RT‐qPCR) analysis of NtPAL, NtC4H and Nt4CL expression levels. (a) RT‐qPCR analysis of NtPAL. (b) RT‐qPCR analysis of NtC4H expression levels. (c) RT‐qPCR analysis of Nt4CL expression levels (n = 3, error bars, SD). * indicates that gene expression level in BH‐1 has differences comparing to it in XHJ at 120 postinoculation (hpi) by Student’s t test (p < 0.05). ** indicates that gene expression level in BH‐1 has significant differences comparing to it in XHJ at 120 hpi by Student’s t test (p < 0.01).
Figure S6 Disease index of the T2 progenies derived from three transformants (NtCHS‐RNAi‐1, NtCHS‐RNAi‐2 and NtCHS‐RNAi‐3) 5 days after inoculation of Ralstonia solanacearum. Disease index of the transgenic lines comparing to that of the wild type was showed as p values by Student’s t test.
Figure S7 Biomass of Phytophthora nicotianae in the T2 progenies derived from three NtCHS‐RNAi transformants for 5 days after inoculation of P. nicotianae. n = 3, error bars, SD. * indicate that NtCHS‐RNAi‐1 and NtCHS‐RNAi‐2 has differences comparing to the wild type (WT) by Student’s t test (p < 0.05). ** indicates that NtCHS‐RNAi‐3 has significant differences comparing to WT by Student’s t test (p < 0.01).
Figure S8 Agronomic traits of tobacco var. Honghuadajinyuan (HD) treated with naringenin. All agronomic trails were determined at 60 days after transplanting HD. (a) Plant height of HD treated with naringenin. (b) Leaf length of HD treated with naringenin. (c) Leaf width of HD treated with naringenin. (d) Knot spacing of HD treated with naringenin. (e) Stem girth of HD treated with naringenin. n = 6, error bars, SD. p values were calculated the transgenic lines comparing to the wild type by Student’s t test.
Figure S9 The stability of naringenin. Honghuadajinyuan (HD) was inoculated with Phytophthora nicotianae after treated with a solution of 0.4 g/L naringenin for 3, 7, 15 and 30 days. HD not treated with naringenin was concurrently inoculated with P. nicotianae as a control. Statistical analysis for the disease index of HD with or without naringenin treatment postinoculation with P. nicotianae (n = 3, error bars, SD). * indicates that the desease index of HD treated with naringenin has differences compared to control by Mann–Whitney test (p < 0.05). ** indicates that the desease index of HD treated with naringenin has ignificant differences compared to control by Mann–Whitney test (p < 0.01).
Figure S10 A model of how naringenin affects pathogen resistance to Phytophthora nicotianae. Once plants are under the attack from P. nicotianae, naringenin produced from plants will not only inhibit mycelial growth, but also sporangia production of P. nicotianae. Meanwhile, naringenin induced not only the accumulation of reactive oxygen species (ROS), but also salicylic acid (SA) induced basal plant pathogen resistance. Both antimicrobial activity and induction of plant defence by naringenin lead to plant resistance to P. nicotianae.
Table S1 A total of 166 metabolites was characterized by their distinct retention times and mass‐to‐charge ratios (m/z)
Table S2 Significant up‐regulated metabolites in Beinhart 1000‐1 (BH‐1) relative to Xiaohuangjin (XHJ)
Table S3 A total of 16 flavanones were characterized in metabolite analysis
Table S4 A total of 44 flavonols were characterized in metabolite analysis
Table S5 The inhibitory activity of the different flavonoids on Phytophthora nicotianae
Table S6 The inhibitory test of the three pathogens and sporangia production of Phytophthora nicotianae treated with naringenin
Table S7 The inhibitory activity of naringenin on Ralstonia solancearum
Table S8 All primer sequences used for this research
ACKNOWLEDGEMENTS
The research was funded by the Science Foundation for Young Scholars of the Tobacco Research Institute of the Chinese Academy of Agricultural Sciences (grant no. 2017A01), the Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) (CX[20]3129), the Fundamental Research Funds for the Central Universities (KYXK202010, KYGD202110), the National Science Foundation of China (31871996), the Fundamental Research Funds for Central Non‐profit Scientific Institution (1610232020001 and 1610232019002), the Key Technology Program of Chongqing Tobacco Corporation and the Sichuan Tobacco Corporation (grant no. A20201NY02‐1305 and SCY202003). We are grateful to Dr Shu Xu for providing the strains of P. capsici, P. aphanidermatum and P. ultimum. We thank Dr Karsten Melcher and Dr Yiming Wang for their careful reading of this manuscript and their critical suggestions. We also thank Dr Chen Shuai for providing seeds of NtCHS‐RNAi lines of tobacco plants.
Sun, M. , Li, L. , Wang, C. , Wang, L. , Lu, D. & Shen, D. et al. (2022) Naringenin confers defence against Phytophthora nicotianae through antimicrobial activity and induction of pathogen resistance in tobacco. Molecular Plant Pathology, 23, 1737–1750. Available from: 10.1111/mpp.13255
Mingming Sun and Lei Li contributed equally to this article.
Contributor Information
Yiting Li, Email: liyiting@caas.cn.
Feng Zhang, Email: fengz@njau.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- An, J. , Kim, S.H. , Bahk, S. , Vuong, U.T. , Nguyen, N.T. , Do, H.L. et al. (2021) Naringenin induces pathogen resistance against Pseudomonas syringae through the activation of NPR1 in Arabidopsis . Frontiers in Plant Science, 12, 672552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos, D.F. , Melton, T. & Mila, A.L. (2010) Effects of chemical control, cultivar resistance, and structure of cultivar root system on black shank incidence of tobacco. Plant Disease, 94, 613–620. [DOI] [PubMed] [Google Scholar]
- Bartwal, A. , Mall, R. , Lohani, P. , Guru, S.K. & Arora, S. (2012) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. Journal of Plant Growth Regulation, 32, 216–232. [Google Scholar]
- Bray, E.A. (2000) Response to abiotic stress. In: Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists, pp. 1158–1203. [Google Scholar]
- van Butselaar, T. & Van den Ackerveken, G. (2020) Salicylic acid steers the growth–immunity tradeoff. Trends in Plant Science, 25, 566–576. [DOI] [PubMed] [Google Scholar]
- Chen, X. , He, B. , Yang, H. & Cernava, T. (2020) Bacteriome and mycobiome in Nicotiana tabacum fields affected by black shank disease. Plant Disease, 104, 315–319. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Wu, F. , Li, Y. , Qian, Y. , Pan, X. , Li, F. et al. (2019) NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Frontiers in Plant Science, 10, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary, D.K. & Johri, B.N. (2009) Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiological Research, 164, 493–513. [DOI] [PubMed] [Google Scholar]
- Chripkova, M. , Zigo, F. & Mojzis, J. (2016) Antiproliferative effect of indole phytoalexins. Molecules, 21, 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csinos, A. & Minton, N. (1983) Control of tobacco black shank with combinations of systemic fungicides and nematicides or fumigants. Plant Disease, 67, 204–207. [Google Scholar]
- Den Hartogh, D.J. & Tsiani, E. (2019) Antidiabetic properties of naringenin: a citrus fruit polyphenol. Biomolecules, 9, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derevnina, L. , Petre, B. , Kellner, R. , Dagdas, Y.F. , Sarowar, M.N. , Giannakopoulou, A. et al. (2016) Emerging oomycete threats to plants and animals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371, 20150459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbaliferiz, S. & Iranshahi, M. (2016) Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: updated review of mechanisms and catalyzing metals. Phytotherapy Research, 30, 1379–1391. [DOI] [PubMed] [Google Scholar]
- Fira, D. , Dimkić, I. , Berić, T. , Lozo, J. & Stanković, S. (2018) Biological control of plant pathogens by Bacillus species. Journal of Biotechnology, 285, 44–55. [DOI] [PubMed] [Google Scholar]
- Gallup, C.A. , McCorkle, K.L. , Ivors, K.L. & Shew, D. (2018) Characterization of the black shank pathogen, Phytophthora nicotianae, across North Carolina tobacco production areas. Plant Disease, 102, 1108–1114. [DOI] [PubMed] [Google Scholar]
- Gallup, A. , Sullivan, M. & Shew, H. (2006) Black shank of tobacco. St Paul, MN: APS Press. [Google Scholar]
- Gong, L. , Chen, W. , Gao, Y. , Liu, X. , Zhang, H. , Xu, C. et al. (2013) Genetic analysis of the metabolome exemplified using a rice population. Proceedings of the National Academy of Sciences of the United States of America, 110, 20320–20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D. , Yuan, C. , Luo, Y. , Chen, Y. , Lu, M. , Chen, G. et al. (2020) Biocontrol of tobacco black shank disease (Phytophthora nicotianae) by Bacillus velezensis Ba168. Pesticide Biochemistry and Physiology, 165, 104523. [DOI] [PubMed] [Google Scholar]
- Hao, W. , Gray, M.A. , Förster, H. & Adaskaveg, J.E. (2019) Evaluation of new oomycota fungicides for management of phytophthora root rot of citrus in California. Plant Disease, 103, 619–628. [DOI] [PubMed] [Google Scholar]
- Hou, Y. , Zhai, Y. , Feng, L. , Karimi, H.Z. , Rutter, B.D. , Zeng, L. et al. (2019) A Phytophthora effector suppresses trans‐kingdom RNAi to promote disease susceptibility. Cell Host & Microbe, 25, 153–165.e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, P. , Csinos, A.S. , Hickman, L.L. & Hargett, U. (2014) Efficacy and application methods of oxathiapiprolin for management of black shank on tobacco. Plant Disease, 98, 1551–1554. [DOI] [PubMed] [Google Scholar]
- Jia, Z. , Zou, B. , Wang, X. , Qiu, J. , Ma, H. , Gou, Z. et al. (2010) Quercetin‐induced H2O2 mediates the pathogen resistance against Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana . Biochemical and Biophysical Research Communications, 396, 522–527. [DOI] [PubMed] [Google Scholar]
- Jing, C. , Gou, J. , Han, X. , Wu, Q. & Zhang, C. (2017) In vitro and in vivo activities of eugenol against tobacco black shank caused by Phytophthora nicotianae . Pesticide Biochemistry and Physiology, 142, 148–154. [DOI] [PubMed] [Google Scholar]
- Johnson, E.S. , Wolff, M.F. , Wernsman, E.A. , Atchley, W.R. & Shew, H.D. (2002) Origin of the black shank resistance gene, Ph, in tobacco cultivar Coker 371‐Gold. Plant Disease, 86, 1080–1084. [DOI] [PubMed] [Google Scholar]
- Kamoun, S. , Furzer, O. , Jones, J.D. , Judelson, H.S. , Ali, G.S. , Dalio, R.J. et al. (2015) The Top 10 oomycete pathogens in molecular plant pathology. Molecular Plant Pathology, 16, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariu, T. , Nakao, R. , Ikeda, T. , Nakashima, K. , Potempa, J. & Imamura, T. (2017) Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. Journal of Periodontal Research, 52, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler, F. & Hahlbrock, K. (1972) Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5,7,4′‐trihydroxyflavanone) from p‐coumaroyl coenzyme A and malonyl coenzyme A. FEBS Letters, 28, 69–72. [DOI] [PubMed] [Google Scholar]
- Kumar, R. , Shanthala, L. , Kumar, T. & Sudharshana, L. (2006) Phytoalexins from finger millet leaves infected with Pyricularia grisea . Journal of Plant Biology‐New Delhi, 32, 177. [Google Scholar]
- Lee, J.H. , Lee, S.E. , Oh, S. , Seo, E. & Choi, D. (2018) HSP70s enhance a Phytophthora infestans effector‐induced cell death via an MAPK cascade in Nicotiana benthamiana . Molecular Plant‐Microbe Interactions, 31, 356–362. [DOI] [PubMed] [Google Scholar]
- Li, B.C. , Bass, W.T. & Cornelius, P.L. (2006) Resistance to tobacco black shank in Nicotiana species. Crop Science, 46, 554–560. [Google Scholar]
- Makarova, L. , Dudareva, L. , Petrova, I. & Vasil'eva, G. (2016) Secretion of phenolic compounds into root exudates of pea seedlings upon inoculation with Rhizobium leguminosarum bv. viceae or Pseudomonas siringae pv. pisi . Applied Biochemistry and Microbiology, 52, 205–209. [PubMed] [Google Scholar]
- Manchope, M.F. , Casagrande, R. & Verri, W.A., Jr. (2017) Naringenin: an analgesic and anti‐inflammatory citrus flavanone. Oncotarget, 8, 3766–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Castillo, M. , Pacheco‐Yepez, J. , Flores‐Huerta, N. , Guzmán‐Téllez, P. , Jarillo‐Luna, R.A. , Cárdenas‐Jaramillo, L.M. et al. (2018) Flavonoids as a natural treatment against Entamoeba histolytica . Frontiers in Cellular and Infection Microbiology, 8, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierziak, J. , Kostyn, K. & Kulma, A. (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules, 19, 16240–16265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowski, J.E. , Mukherjee, S. , McCready, A.R. , Cong, J.P. , Aquino, C.J. , Kim, H. et al. (2017) Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum‐sensing receptors. Journal of Biological Chemistry, 292, 4064–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmavati, M. , Sakthivel, N. , Thara, K. & Reddy, A.R. (1997) Differential sensitivity of rice pathogens to growth inhibition by flavonoids. Phytochemistry, 46, 499–502. [Google Scholar]
- Paesold, S. , Siegel, I. , Seidel, C. & Ludwig‐Müller, J. (2010) Flavonoid accumulation in Arabidopsis thaliana root galls caused by the obligate biotrophic pathogen Plasmodiophora brassicae . Molecular Plant Pathology, 11, 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panabieres, F. , Ali, G.S. , Allagui, M.B. , Dalio, R.J.D. , Gudmestad, N.C. , Kuhn, M.L. et al. (2016) Phytophthora nicotianae diseases worldwide: new knowledge of a long‐recognised pathogen. Phytopathologia Mediterranea, 55, 20–40. [Google Scholar]
- Pandey, A. , Misra, P. & Trivedi, P.K. (2015) Constitutive expression of Arabidopsis MYB transcription factor, AtMYB11, in tobacco modulates flavonoid biosynthesis in favor of flavonol accumulation. Plant Cell Reports, 34, 1515–1528. [DOI] [PubMed] [Google Scholar]
- Pandith, S.A. , Ramazan, S. , Khan, M.I. , Reshi, Z.A. & Shah, M.A. (2019) Chalcone synthases (CHSs): the symbolic type III polyketide synthases. Planta, 251, 15. [DOI] [PubMed] [Google Scholar]
- Pannala, A.S. , Chan, T.S. , O'Brien, P.J. & Rice‐Evans, C.A. (2001) Flavonoid B‐ring chemistry and antioxidant activity: fast reaction kinetics. Biochemical and Biophysical Research Communications, 282, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Park, C.‐H. , Chen, S. , Shirsekar, G. , Zhou, B. , Khang, C.H. , Songkumarn, P. et al. (2012) The Magnaporthe oryzae effector AvrPiz‐t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen‐associated molecular pattern–triggered immunity in rice. The Plant Cell, 24, 4748–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez, M.M. , Tomita‐Yokotani, K. , Fujii, Y. , Konishi, T. & Iwashina, T. (2004) Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa . Biochemical Systematics and Ecology, 32, 631–635. [Google Scholar]
- Pham, T.H. , Lecomte, S. , Efstathiou, T. , Ferriere, F. & Pakdel, F. (2019) An update on the effects of glyceollins on human health: possible anticancer effects and underlying mechanisms. Nutrients, 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, T. , Shao, Y. , Csinos, A.S. & Ji, P. (2016) Sensitivity of Phytophthora nicotianae from tobacco to fluopicolide, mandipropamid, and oxathiapiprolin. Plant Disease, 100, 2119–2125. [DOI] [PubMed] [Google Scholar]
- Rajaofera, M. , Jin, P. , Fan, Y. , Sun, Q. , Huang, W. , Wang, W. et al. (2018) Antifungal activity of the bioactive substance from Bacillus atrophaeus strain HAB‐5 and its toxicity assessment on Danio rerio . Pesticide Biochemistry and Physiology, 147, 153–161. [DOI] [PubMed] [Google Scholar]
- Rice‐Evans, C. (2001) Flavonoid antioxidants. Current Medicinal Chemistry, 8, 797–807. [DOI] [PubMed] [Google Scholar]
- Salehi, B. , Fokou, P.V.T. , Sharifi‐Rad, M. , Zucca, P. , Pezzani, R. , Martins, N. et al. (2019) The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals (Basel), 12, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew, H.D. & Lucas, G.B. (1991) Compendium of tobacco diseases. St Paul, MN,: American Phytopathological Society. [Google Scholar]
- Shi, R. , Jin, J. , Nifong, J.M. , Shew, D. & Lewis, R.S. (2022) Homoeologous chromosome exchange explains the creation of a QTL affecting soil‐borne pathogen resistance in tobacco. Plant Biotechnology Journal, 20, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skadhauge, B. , Thomsen, K.K. & Von Wettstein, D. (1997) The role of the barley testa layer and its flavonoid content in resistance to Fusarium infections. Hereditas, 126, 147–160. [Google Scholar]
- Sullivan, M. , Melton, T. & Shew, H. (2005) Managing the race structure of Phytophthora parasitica var. nicotianae with cultivar rotation. Plant Disease, 89, 1285–1294. [DOI] [PubMed] [Google Scholar]
- Sun, Y.J. , He, J.M. & Kong, J.Q. (2019) Characterization of two flavonol synthases with iron‐independent flavanone 3‐hydroxylase activity from Ornithogalum caudatum Jacq. BMC Plant Biology, 19, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, G. , Yao, T. , Feng, C. , Chen, L. , Li, J. & Wang, L. (2017) Identification and biocontrol potential of antagonistic bacteria strains against Sclerotinia sclerotiorum and their growth‐promoting effects on Brassica napus . Biological Control, 104, 35–43. [Google Scholar]
- Surendran Nair, M. , Ma, F. , Lau, P. , Upadhyaya, I. & Venkitanarayanan, K. (2020) Inactivation of Escherichia coli O157:H7 in apple cider by resveratrol and naringenin. Food Microbiology, 86, 103327. [DOI] [PubMed] [Google Scholar]
- Tattini, M. , Galardi, C. , Pinelli, P. , Massai, R. , Remorini, D. & Agati, G. (2004) Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytologist, 163, 547–561. [DOI] [PubMed] [Google Scholar]
- Treutter, D. (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biology (Stuttgart, Germany), 7, 581–591. [DOI] [PubMed] [Google Scholar]
- Turek, C. & Stintzing, F.C. (2013) Stability of essential oils: a review. Comprehensive Reviews in Food Science and Food Safety, 12, 40–53. [Google Scholar]
- Wang, Y. , Chen, S. & Yu, O. (2011) Metabolic engineering of flavonoids in plants and microorganisms. Applied Microbiology and Biotechnology, 91, 949–956. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Tyler, B.M. & Wang, Y. (2019) Defense and counterdefense during plant‐pathogenic oomycete infection. Annual Review of Microbiology, 73, 667–696. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Wei, K. , Han, X. , Zhao, D. , Zheng, Y. , Chao, J. et al. (2019) The antifungal effect of garlic essential oil on Phytophthora nicotianae and the inhibitory component involved. Biomolecules, 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, L. , Zhao, J. , Meng, Y. , Guo, Y. & Luo, C. (2020) Antibacterial activity, safety and preservative effect of aminoethyl‐phloretin on the quality parameters of salmon fillets. LWT, 118, 108874. [Google Scholar]
- Yang, W. , Xu, X. , Li, Y. , Wang, Y. , Li, M. , Wang, Y. et al. (2016) Rutin‐mediated priming of plant resistance to three bacterial pathogens initiating the early SA signal pathway. PLoS One, 11, e0146910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong, C. , Qiming, X. & Guiping, D. (2010) [Advances in biological control of tobacco black shank]. Journal of Anhui Agricultural Sciences, 11, 5708–5710. [Google Scholar]
- Zhang, C. , Feng, C. , Zheng, Y. , Wang, J. & Wang, F. (2020) Root exudates metabolic profiling suggests distinct defense mechanisms between resistant and susceptible tobacco cultivars against black shank disease. Frontiers in Plant Science, 11, 559775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Guo, X. , Yan, X. , Ren, M. , Jiang, C. , Cheng, Y. et al. (2018) Identification of stably expressed QTL for resistance to black shank disease in tobacco (Nicotiana tabacum L.) line Beinhart 1000‐1. The Crop Journal, 6, 282–290. [Google Scholar]
- Zhao, X. , Zhang, S. , Liu, D. , Yang, M. & Wei, J. (2020) Analysis of flavonoids in Dalbergia odorifera by ultra‐performance liquid chromatography with tandem mass spectrometry. Molecules, 25, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The antimicrobial activity of the representative flavonols on Phytophthora nicotianae. The control (CK) was added ethanol only. (a) Colony morphology of P. nicotianae on potato dextrose agarose at 28°C for 7 days amended with kaempferol, quercetin or rutin. The concentrations were 12.5, 25, 50, 100 and 200 mg/L. (b) The inhibition rate of kaempferol, quercetin and rutin on P. nicotianae. (n = 3, error bars, SD).
Figure S2 The antimicrobial activity of three other pathogens treated with naringenin. (a) Colony morphology of Phytophthora capsici on potato dextrose agarose (PDA) amended with a different concentration of naringenin at 28°C. The concentrations of naringenin were 12.5, 25, 50, 100, 200, and 400 mg/L. The control (CK) was added ethanol only. (b) Colony morphology of Pythium aphanidermatum and Pythium ultimum on PDA amended with different concentrations of naringenin at 28°C. The concentrations of naringenin were 25, 50, 75, 100, 125, 150 and 175 mg/L. The control (CK) was added ethanol only. (c,d) The inhibition rate of naringenin on Phytophthora capsici, Pythium aphanidermatum and Pythium ultimum (n = 3, error bars, SD).
Figure S3 The inhibitory test of other phytopathogens treated with naringenin. The concentration of naringenin was 200 mg/L. The control (CK) was added ethanol only.
Figure S4 The result of reverse transcription‐quantitative PCR (RT‐qPCR) validation for differentially expressed genes on cell growth and reproduction of Phytophthora nicotianae. The control (CK) means P. nicotianae was treated with solvent only. The NG means P. nicotianae was treated with 20 mg/L naringenin. Gene expression was determined by RT‐qPCR after sampling (n = 3, error bars, SD).
Figure S5Reverse transcription‐quantitative PCR (RT‐qPCR) analysis of NtPAL, NtC4H and Nt4CL expression levels. (a) RT‐qPCR analysis of NtPAL. (b) RT‐qPCR analysis of NtC4H expression levels. (c) RT‐qPCR analysis of Nt4CL expression levels (n = 3, error bars, SD). * indicates that gene expression level in BH‐1 has differences comparing to it in XHJ at 120 postinoculation (hpi) by Student’s t test (p < 0.05). ** indicates that gene expression level in BH‐1 has significant differences comparing to it in XHJ at 120 hpi by Student’s t test (p < 0.01).
Figure S6 Disease index of the T2 progenies derived from three transformants (NtCHS‐RNAi‐1, NtCHS‐RNAi‐2 and NtCHS‐RNAi‐3) 5 days after inoculation of Ralstonia solanacearum. Disease index of the transgenic lines comparing to that of the wild type was showed as p values by Student’s t test.
Figure S7 Biomass of Phytophthora nicotianae in the T2 progenies derived from three NtCHS‐RNAi transformants for 5 days after inoculation of P. nicotianae. n = 3, error bars, SD. * indicate that NtCHS‐RNAi‐1 and NtCHS‐RNAi‐2 has differences comparing to the wild type (WT) by Student’s t test (p < 0.05). ** indicates that NtCHS‐RNAi‐3 has significant differences comparing to WT by Student’s t test (p < 0.01).
Figure S8 Agronomic traits of tobacco var. Honghuadajinyuan (HD) treated with naringenin. All agronomic trails were determined at 60 days after transplanting HD. (a) Plant height of HD treated with naringenin. (b) Leaf length of HD treated with naringenin. (c) Leaf width of HD treated with naringenin. (d) Knot spacing of HD treated with naringenin. (e) Stem girth of HD treated with naringenin. n = 6, error bars, SD. p values were calculated the transgenic lines comparing to the wild type by Student’s t test.
Figure S9 The stability of naringenin. Honghuadajinyuan (HD) was inoculated with Phytophthora nicotianae after treated with a solution of 0.4 g/L naringenin for 3, 7, 15 and 30 days. HD not treated with naringenin was concurrently inoculated with P. nicotianae as a control. Statistical analysis for the disease index of HD with or without naringenin treatment postinoculation with P. nicotianae (n = 3, error bars, SD). * indicates that the desease index of HD treated with naringenin has differences compared to control by Mann–Whitney test (p < 0.05). ** indicates that the desease index of HD treated with naringenin has ignificant differences compared to control by Mann–Whitney test (p < 0.01).
Figure S10 A model of how naringenin affects pathogen resistance to Phytophthora nicotianae. Once plants are under the attack from P. nicotianae, naringenin produced from plants will not only inhibit mycelial growth, but also sporangia production of P. nicotianae. Meanwhile, naringenin induced not only the accumulation of reactive oxygen species (ROS), but also salicylic acid (SA) induced basal plant pathogen resistance. Both antimicrobial activity and induction of plant defence by naringenin lead to plant resistance to P. nicotianae.
Table S1 A total of 166 metabolites was characterized by their distinct retention times and mass‐to‐charge ratios (m/z)
Table S2 Significant up‐regulated metabolites in Beinhart 1000‐1 (BH‐1) relative to Xiaohuangjin (XHJ)
Table S3 A total of 16 flavanones were characterized in metabolite analysis
Table S4 A total of 44 flavonols were characterized in metabolite analysis
Table S5 The inhibitory activity of the different flavonoids on Phytophthora nicotianae
Table S6 The inhibitory test of the three pathogens and sporangia production of Phytophthora nicotianae treated with naringenin
Table S7 The inhibitory activity of naringenin on Ralstonia solancearum
Table S8 All primer sequences used for this research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


