Abstract
Background:
Many American Indian (AI) communities are in areas affected by environmental contamination, such as toxic metals. However, studies assessing exposures in AI communities are limited. We measured blood metals in AI communities to assess historical exposure and identify participant characteristics associated with these levels in the Strong Heart Study (SHS) cohort.
Method:
Archived blood specimens collected from 2,014 participants (all participants were 50 years of age and older) in Arizona, Oklahoma, and North and South Dakota during SHS Phase-III (1998–1999) were analyzed for cadmium, lead, manganese, mercury, and selenium using inductively coupled plasma triple quadrupole mass spectrometry. We conducted descriptive analyses for the entire cohort and stratified by selected subgroups, including selected demographics, health behaviors, income, waist circumference, and body mass index. Bivariate associations were conducted to examine associations between blood metal levels and selected socio-demographic and behavioral covariates. Finally, multivariate regression models were used to assess the best model fit that predicted blood metal levels.
Findings:
All elements were detected in 100% of study participants, with the exception of mercury (detected in 73% of participants). The SHS population had higher levels of blood cadmium and manganese than the general U.S. population 50 years and older. The blood mercury median of the SHS population was at about 30% of the U.S. reference population, potentially due to low fish consumption. Participants in North and South Dakota had the highest blood cadmium, lead, manganese, and selenium, and the lowest total mercury levels, even after adjusting for covariates. In addition, each of the blood metals was associated with selected demographic, behavioral, income, and/or weight-related factors in multivariate models. These findings will help guide the tribes to develop education, outreach, and strategies to reduce harmful exposures and increase beneficial nutrient intake in these AI communities.
Keywords: Metals, American Indians, Strong Heart Study, Lead, Manganese, Cadmium, Selenium
INTRODUCTION
Many American Indian (AI) communities are in areas affected by environmental contamination from both anthropogenic and natural sources. More than 160,000 abandoned hard rock mines are located on AI lands throughout the western United States, which has led to soil and water contamination and created a legacy of chronic exposures to metal mixtures in AI communities (Lewis et al., 2017). Numerous studies support that chronic exposure to metals (such as cadmium, lead, manganese, and mercury) is associated with a variety of adverse health outcomes, including cardiovascular disease, at reference levels in U.S. populations (Chowdhury et al., 2018; Cosselman et al., 2015; Khanam et al., 2021; Solenkova et al., 2014). The adverse health outcomes likely occur via numerous physiological mechanisms, including increased oxidative stress and inflammation, atherosclerosis, impaired calcium signaling (Potula et al., 2005), and impaired placental function (Alissa and Ferns, 2011; Khanam et al., 2021; Llanos and Ronco, 2009).
Metals persist in the environment, can accumulate in the body, and some of them have long half-lives and slow excretion rates (e.g., lead and cadmium) (Nordberg et al., 2007). Exposure to these toxic metals is widespread, as documented in the U.S. National Health and Nutrition Examination Survey (NHANES) (CDC, 2022). AI communities have raised concerns about potentially higher exposure to toxic metals due to historical mining activity (Lewis et al., 2017). Characterizing population-level metal exposures in AI communities can identify the magnitude of exposure, characteristics associated with exposure specific to AI communities, and adverse health outcomes related to metal exposure in AI communities. While some environmental health studies have documented disproportionate metal exposure in some AI populations, mainly using urinary samples, more research is needed to assess exposure to harmful chemicals in many other AI communities, including toxic metals and related potential adverse health effects (Hoover et al., 2020; Navas-Acien et al., 2009; Pang et al., 2016; Scheer et al., 2012).
The Strong Heart Study (SHS) is an ongoing, population-based, prospective epidemiologic study implemented in 1988 to study cardiovascular disease (CVD) and its risk factors among AI communities. The study population includes participants from 13 AI tribes and communities in four states (Arizona, Oklahoma, North Dakota, and South Dakota), and is the largest epidemiologic study of CVD and its risk factors in AI communities. Phase I of the study occurred in 1989–1991 and included 4,549 AI members. The study is now in Phase VII.
Within the SHS original cohort, several metals/metalloids (e.g., arsenic, cadmium, lead, selenium, tungsten, uranium, and zinc), were measured in urine samples collected at Phase I (all participants) and in subsets of samples collected at Phases II and III (around 300 samples) (Navas-Acien et al., 2009; Pang et al., 2016; Scheer et al., 2012). Urinary levels for some metals/metalloids (hereafter referred to as metals) in SHS participants were higher than those measured in the general U.S. population reported in NHANES and six urban communities, underscoring that AI communities remain disproportionately exposed to metals (Pang et al., 2016).
To complement the previous urine metal studies, we conducted a study to measure five elements in blood samples collected in the SHS original cohort during Phase III (1998 – 1999). The goals of this study included assessing blood lead, cadmium, and selenium exposure, measuring exposure to additional metals (manganese and mercury), and characterizing the association of blood metals with health outcomes in the cohort. This paper reports blood levels of the five elements with the following objectives: 1) report the distribution of blood metal levels in the SHS cohort, 2) compare levels in SHS participants to those in the general U.S. population as measured in NHANES, and 3) identify participant characteristics associated with blood metal levels.
METHODS
Study population
The detailed study design for SHS has been described elsewhere (Howard et al., 1992; Lee et al., 1990). In brief, SHS is a longitudinal cohort study with participants from 12 AI tribes and communities in four states. Recruitment of a cohort of 4,549 participants started in 1989. Approximately 1,036 participants were later removed because one community withdrew consent and another two individual participants withdrew their consents. The SHS completed three rounds of clinical examinations and morbidity and mortality surveillance of the original cohort (Phase I: 1989–1991; Phase II: 1993–1995; Phase III: 1998–1999). For the subsequent follow-ups from Phase IV to the current Phase VII, the study expanded into a family study (Strong Heart Family Study or SHFS); the original cohort members continued to participate in the morbidity and mortality surveillance, but most did not have clinical examinations.
Personal interviews, physical examinations, fasting blood draws, and urine specimen collections were conducted by centrally trained staff and nurses during Phase III. Morbidity and mortality surveillance was conducted by medical reviewers and adjudicated following a protocol for cardiovascular disease outcomes. The questionnaires included a variety of domains, such as demographics, socioeconomic status, health behaviors, physical activity, medication use, and medical history. Trained field staff conducted a large number of anthropometric and clinical measurements, including height, weight, waist circumference, systolic and diastolic blood pressure, cholesterol, fasting glucose, plasma creatinine, as detailed elsewhere (Howard et al., 1992; Lee et al., 1990). Education level was collected from study participants during Phase I.
For the blood metal study that we report in this paper, the study population consists of all SHS original cohort members during Phase III (1998–1999) who met the following inclusion criteria: 1) was an original cohort member, 2) consented to continue participating in SHS, 3) participated in both clinical exam and surveillance, 4) provided a whole blood sample that was archived, and 5) had archived blood samples that were not clotted and had sufficient volume for analysis. At the time of data collection in 1998–1999, all participants were aged 50 years and older. After the analytical lab at the Centers for Disease Control and Prevention (CDC) received samples and assessed the quality of the blood specimens, additional participants were excluded if clots were observed in blood samples.
This study was approved by the SHS Steering Committee, research review boards of participating tribes, University of Oklahoma Health Sciences Center, and Medstar Health Research Institute. CDC classified this study as not human subjects research and therefore did not require human subjects review. This manuscript has been reviewed and approved by the participating AI communities. Individual consents were obtained during each SHS exam.
Blood metal measurements
Aliquots of whole blood samples collected during the Phase III examination were stored at < −70ºC at Medstar Health Research Institute, then frozen whole blood samples were shipped on dry ice via overnight express shipments to CDC in 2019. Blood metal measurements were conducted by CDC’s Division of Laboratory Sciences in 2019 and 2020. Five elements (cadmium, lead, manganese, mercury, and selenium) were measured in the blood samples using a newly developed inductively coupled plasma triple quadrupole mass spectrometry (ICP-QQQ-MS) method (CDC, 2019). In brief, a 50 µL aliquot of whole blood was diluted to 1 mL in an aqueous solution of 1.0% v/v tetramethyl ammonia hydroxide, 1% ethanol, 0.01% ammonium pyrrolidine dithiocarbamate (APDC), 0.05% Triton™ X-100, and a 5 µg/L iridium, rhodium, and tellurium internal standards. The sample mixture was introduced to an Agilent 8900 ICP-QQQ-MS. Cadmium (111Cd), lead (206Pb + 207Pb + 208Pb), manganese (55Mn), mercury (200Hg + 202Hg), and selenium (as 80Se16O) along with the internal standards rhodium (103Rh, for manganese), iridium (193Ir, for cadmium and lead), and tellurium (130Te, for selenium and mercury) were all analyzed within a single tune mode in which the octopole reaction cell was pressurized with 50% oxygen and 1.0 mL/min hydrogen. Quantitation was accomplished using a matrix-matched, external calibration with calibration ranges of 0.48 – 400 µg/L for manganese, 10 – 2500 µg/L for selenium, 0.06 – 200 µg/L for cadmium, 0.24 – 200 µg/L for mercury, and 0.06 – 200 µg/dL for lead. The limits of detection for the method are 0.065 µg/L for cadmium, 0.049 µg/dL for lead, 0.52 µg/L for manganese, 0.17 µg/L for mercury, and 9.9 µg/L for selenium. Three custom-made, pre-characterized blood bench quality control materials were inserted at the beginning and end of each analytical run. The results were deemed valid after fulfilling a series of quality control checks in a multi-rule quality control system (Caudill et al., 2008).
Data analyses
We first calculated detection rates in 2014 blood samples, plotted distributions, and tested normality for blood metal levels. Based on distribution plots (Supplemental Material, Figure S-1) and tests of normality (Shapiro-Wilk test), elemental levels were ln-transformed for linear regression analyses. Four of the metals had 100% detection rates, with only mercury impacted by significant left censoring due to non-detects (27%). Mercury levels below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2.
We conducted descriptive analyses of blood metal levels for the entire study population and stratified by selected participant subgroups, including geographic regions (defined by the three study centers: Arizona, Oklahoma, and North Dakota/South Dakota), age group (50–59 years, 60–69 years, 70+ years), sex (female, male), household income (less than $15K, $15K or above), income meeting family needs (needs met, needs not met), smoking status (never, former, current), education (less than 12 years, 12 years and more), secondhand smoke exposure (categorized as 0, 1–7, >8 hours per day), alcohol consumption (categorized as 0, 1–5, >5 drinks per week), body mass index (BMI, underweight <18.5, normal 18.5–24.9, overweight 25.0–29.9, and obese 30+), and waist circumference (normal, at risk defined as >40 in for male and >35 in for female (NHLBI, 2001)). Wald F statistics were calculated to determine if metal geometric mean levels across these subgroups were statistically different.
To determine whether the blood metal levels in the SHS at Phase III were elevated relative to the general U.S. population, we calculated blood metal medians for participants aged 50 years and older in NHANES as the national reference population. Since each analyte became available in NHANES during different years, we used the NHANES data from the closest years as the benchmark for the comparison, i.e., NHANES 1999–2000 for cadmium and lead, and NHANES 2011–2012 for manganese and selenium. Total mercury was only available for females younger than 50 years old in NHANES between years 1999–2002, therefore, we used NHANES 2003–2004 to calculate median total mercury for people aged 50 years and older as the general U.S. population reference level. Weight and design variables were used according to NHANES guidelines to account for complex survey design and to make results generalizable to the target group in the U.S. civilian, non-institutionalized population.
To identify participant characteristics associated with blood metal levels, we used linear regression models with ln-transformed blood metal level as the dependent variable to calculate adjusted geometric mean (GM) ratios while controlling for all available demographic, behavial, education, income and weight variables (Table 1). We included participant characteristics covered in the descriptive analyses and did not include any clinical measurements or health outcomes in the models, in accordance with the objectives of this analysis. Continuous variables were assessed to determine the need for transformation or addition of non-linear terms in the model.
Table 1.
Selected demographic information of 2,014 cohort participants with blood metal results, Strong Heart Study (Phase III, 1998–1999) for the entire cohort and by study center.
| Variable | Levels1 | All | Arizona | Oklahoma | North Dakota/ South Dakota |
|---|---|---|---|---|---|
| Total | 2014 | 221 | 869 | 924 | |
| Sex | Female | 1217 (60.4%) | 159 (71.9%) | 511 (58.8%) | 547 (59.2%) |
| Male | 797 (39.6%) | 62 (28.1%) | 358 (41.2%) | 377 (40.8%) | |
| Age Group | 50–59 years | 761 (37.8%) | 96 (43.4%) | 313 (36%) | 352 (38.1%) |
| 60–69 years | 781 (38.8%) | 78 (35.3%) | 326 (37.5%) | 377 (40.8%) | |
| 70+ years | 472 (23.4%) | 47 (21.3%) | 230 (26.5%) | 195 (21.1%) | |
| Smoker | Never | 663 (32.9%) | 115 (52%) | 260 (29.9%) | 288 (31.2%) |
| Former | 685 (34%) | 65 (29.4%) | 369 (42.5%) | 251 (27.2%) | |
| Current | 620 (30.8%) | 19 (8.6%) | 227 (26.1%) | 374 (40.5%) | |
| Missing | 46 (2.3%) | 22 (10%) | 13 (1.5%) | 11 (1.2%) | |
| Second-hand Smoke | 0 hours/day | 1190 (59.1%) | 163 (73.8%) | 572 (65.8%) | 455 (49.2%) |
| 1–7 hours/day | 581 (28.8%) | 36 (16.3%) | 245 (28.2%) | 300 (32.5%) | |
| 8+ hours/day | 204 (10.1%) | 0 | 49 (5.6%) | 155 (16.8%) | |
| Missing | 39 (1.9%) | 22 (10%) | 3 (0.3%) | 14 (1.5%) | |
| Alcohol Servings | No drinks/week | 1688 (83.8%) | 180 (81.4%) | 748 (86.1%) | 760 (82.3%) |
| 1–5 drinks/week | 149 (7.4%) | 16 (7.2%) | 68 (7.8%) | 65 (7%) | |
| >5 drinks/week | 174 (8.6%) | 25 (11.3%) | 50 (5.8%) | 99 (10.7%) | |
| Missing | 3 (0.1%) | 0 | 3 (0.3%) | 0 | |
| Waist Circumference | Normal | 607 (30.1%) | 38 (17.2%) | 271 (31.2%) | 298 (32.3%) |
| At risk (M>40in,F>35in) | 1332 (66.1%) | 182 (82.4%) | 561 (64.6%) | 589 (63.7%) | |
| Missing | 75 (3.7%) | 1 (0.5%) | 37 (4.3%) | 37 (4%) | |
| Body Mass Index | Underweight (<18.5) | 19 (0.9%) | 0 | 4 (0.5%) | 15 (1.6%) |
| Normal (18.5–24.9) | 293 (14.5%) | 21 (9.5%) | 118 (13.6%) | 154 (16.7%) | |
| Overweight (25–29.9) | 668 (33.2%) | 41 (18.6%) | 274 (31.5%) | 353 (38.2%) | |
| Obese (30+) | 964 (47.9%) | 152 (68.8%) | 438 (50.4%) | 374 (40.5%) | |
| Missing | 70 (3.5%) | 7 (3.2%) | 35 (4%) | 28 (3%) | |
| Education | 12 years and more | 1084 (53.8%) | 115 (52%) | 540 (62.1%) | 429 (46.4%) |
| <12 years | 665 (33%) | 82 (37.1%) | 218 (25.1%) | 365 (39.5%) | |
| Missing | 265 (13.2%) | 24 (10.9%) | 111 (12.8%) | 130 (14.1%) | |
| Household Income | $0-$15,000 | 943 (46.8%) | 91 (41.2%) | 277 (31.9%) | 575 (62.2%) |
| more than $15,000 | 490 (24.3%) | 57 (25.8%) | 180 (20.7%) | 253 (27.4%) | |
| Missing | 581 (28.8%) | 73 (33%) | 412 (47.4%) | 96 (10.4%) | |
| Needs Met | Needs met | 1568 (77.9%) | 177 (80.1%) | 745 (85.7%) | 646 (69.9%) |
| Needs not met | 288 (14.3%) | 18 (8.1%) | 82 (9.4%) | 188 (20.3%) | |
| Missing | 158 (7.8%) | 26 (11.8%) | 42 (4.8%) | 90 (9.7%) |
Missing numbers include don’t know, refuse to answer, and missing values.
Some continuous variables were recoded as categorical variables either for ease of interpretation (age, BMI), because the continuous distributions were highly skewed (weekly alcohol consumption), or the variable behaved like a discrete variable (second-hand smoke exposure). We categorized second-hand smoke exposure as 0 hour, 1–7 hours, or 8+ hours per day. The cut-off point (7 hours/day) was based on the median value of participants who had non-zero exposure. This value also approximately represents the amount of exposure one might get if they were only exposed in a workplace (Pirkle et al., 1996). The cut-off point for weekly alcohol consumption categories (5 drinks/week) was also based on the median value of those with non-zero alcohol consumption.
This analysis included all available anthropometric, behavioral, demographic, and socioeconomic variables to identify participant characteristics associated with blood metal levels. We employed a model-averaging technique (resampling the data with replacement, SAS PROC GLMSELECT). Using this method, the number of times that a regressor was selected in repeated samples served as an indication of its importance and was used to obtain a more parsimonious final model. Variable reduction and model selection at this step was based on a forward selection process, where effects entered the models based on Schwartz Bayesian Criteria, and the model choice was based on the adjusted R-square value. Final regressor candidates were obtained using 500 model averaging samples where final regressors were chosen based on a minimum model frequency of 35% in refit models.
Candidate variables, identified above, were then further analyzed for potential collinearity, interaction, and confounding effects using a manual backward elimination approach (SAS PROC GLM), where variables were removed if deletion improved the model. Variables that were eliminated in the model averaging step, but appeared important in preliminary analyses or plots, were reassessed. Once final models were constructed, regression beta parameters were exponentiated to represent adjusted GM ratios.
To account for the high left-censoring of mercury due to non-detects, we also modeled the median mercury level using quantile regression (SAS PROC QUANTREG), a robust regression procedure in the presence of censoring due to non-detects.Since we arrived at the same set of regressors using both quantile regression and linear regression, only the latter is reported for mercury.
Regression modeling was followed by a diagnostic assessment. Potential influential observations (those that have a large influence on the regression parameter estimates) were assessed using scaled measures of change in parameter estimates and predicted values. We used leverage values exceeding 2p/n (where p is the number of regressors, and n is the number of observations used in the analysis) and Cook’s D values exceeding 4/n as indicators of influence. Studentized residuals exceeding a value of 2 were investigated as potential outliers. SAS Version 9.4 TS Level 1M3 was used for all statistical analyses (SAS Institute Inc., Cary, NC, USA). An α of 0.05 was used in hypothesis testing and statistical estimation.
RESULTS
A total of 2,204 archived whole blood samples from Phase III met the initial inclusion criteria and were shipped to the CDC lab for analysis. Of these, 188 (8.5%) were clotted and 2 (0.1%) had insufficient quantity for analysis, resulting in 2,014 samples analyzed for blood metals. Table 1 gives the demographic information for the 2,014 participants who were included in this study. All participants were over 50 years old at the time of blood collection. The participants were primarily female (60.4%), had BMI 25–29 kg/m2 (33.2%) or BMI ≥ 30 kg/m2 (47.9%), and had low income (943/1,433 or 65.8% who reported income had annual household income less than $15,000), although most (77.9%) reported that income met their family needs. Participants were evenly distributed among never, former, and current smokers; 59.1% reported no exposure to second-hand smoke; 83.8% reported no alcohol drinking (Table 1).
Figure 1 presents the box plots for blood metal levels in this study (entire cohort and by study center), as well as the median levels in the U.S. reference population (adults 50 years or older) as reported in NHANES. Descriptive statistics (GM and selected percentiles, with 95% CI) are givens in Supplemental Material, Table S-1. We observed a clear regional pattern on all blood metal levels. Cadmium, lead, manganese, and selenium levels were highest in AI communities from North Dakota and South Dakota, followed by Oklahoma, and then Arizona (p-values<0.0001, Table 2). Total blood mercury levels, on the contrary, were lowest in the participating North Dakota and South Dakota tribes (p=0.0007). Compared to the NHANES older adult reference levels, the median level in this study was 35% higher for blood cadmium (driven by North Dakota and South Dakota; SHS 0.617 [95% CI: 0.589–0.645] μg/L, vs. NHANES 0.458 (95% CI: 0.418 −0.498) μg/L) and 44% higher for blood manganese (elevated in all three study centers; SHS 12.6 [95% CI 12.3–12.8] μg/L, vs. NHANES 8.77 [95% CI: 8.50 −9.04] μg/L). Blood lead level (BLL) in the entire cohort was similar to NHANES, although the median level in North Dakota and South Dakota (2.58 [95% CI: 2.48–2.71 μg/dL] was higher than NHANES (2.17 [95% CI: 2.08–2.26] μg/dL). For total mercury, the median in the SHS cohort (0.325 [95% CI: 0.299–0.340] μg/L) was substantively lower at 31% of NHANES (1.06 [95% CI: 0.859 −1.27] μg/L), both for the entire cohort and the three individual study centers. For selenium, the SHS median level was statistically lower in Arizona (174 [95% CI: 170–177] μg/L) and Oklahoma (181 [95% CI: 180–183] μg/L) than NHANES (193 [95% CI: 188–198] μg/L).
Figure 1.
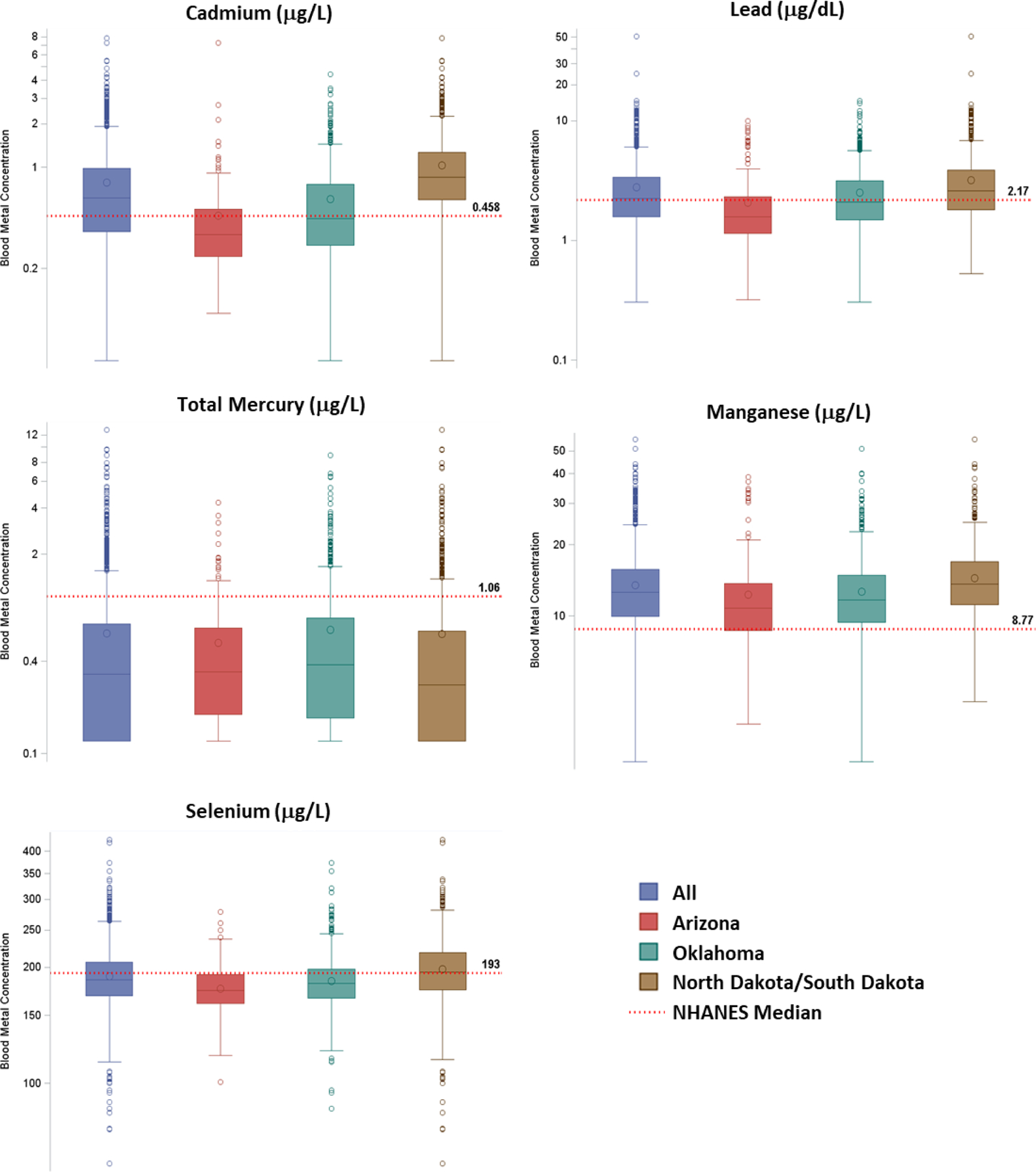
Box plots for blood metal levels in the Strong Heart Study cohort (all and by study center, 1998–1999), in comparison to median levels for U.S. adults aged 50 years and older, from NHANES 1999–2000 (cadmium and lead), NHANES 2003–2004 (total mercury), or NHANES 2011–2012 (manganese and selenium).
Table 2.
Geometric mean concentrations of blood cadmium, lead, mercury, manganese, and selenium in Strong Heart Study cohort (Phase-III, 1998–1999), stratified by covariates.
| Covariate | Levels | N | Cadmium (μg/L) | Lead (μg/dL) | Total Mercury (μg/L) | Manganese (μg/L) | Selenium (μg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geomean | p-value1 | Geomean | p-value1 | Geomean | p-value1 | Geomean | p-value1 | Geomean | p-value1 | |||
| Total | All | 2014 | 0.605 | . | 2.3 | . | 0.359 | . | 12.6 | . | 187 | . |
| Center | Arizona | 221 | 0.363 | <.0001 | 1.66 | <.0001 | 0.359 | 0.0007 | 11.3 | <.0001 | 174 | <.0001 |
| Oklahoma | 869 | 0.472 | 2.13 | 0.393 | 11.9 | 182 | ||||||
| Dakotas | 924 | 0.864 | 2.67 | 0.33 | 13.7 | 194 | ||||||
| Sex | Female | 1217 | 0.631 | 0.0019 | 1.97 | <.0001 | 0.337 | 0.0003 | 12.8 | 0.0058 | 186 | 0.053 |
| Male | 797 | 0.569 | 2.91 | 0.396 | 12.3 | 188 | ||||||
| Age Group | 50–59 years | 761 | 0.627 | 0.114 | 2.23 | 0.139 | 0.435 | <.0001 | 12.7 | 0.332 | 187 | 0.0008 |
| 60–69 years | 781 | 0.604 | 2.32 | 0.333 | 12.6 | 189 | ||||||
| 70+ years | 472 | 0.574 | 2.38 | 0.300 | 12.4 | 182 | ||||||
| Education | <12 years | 665 | 0.673 | <.0001 | 2.52 | <.0001 | 0.278 | <.0001 | 13.1 | 0.0005 | 186 | 0.578 |
| 12+ years | 1084 | 0.566 | 2.16 | 0.421 | 12.3 | 187 | ||||||
| Income Meets Needs | Needs met | 1568 | 0.586 | <.0001 | 2.28 | 0.337 | 0.380 | <.0001 | 12.4 | 0.0010 | 187 | 0.862 |
| Needs not met | 288 | 0.720 | 2.39 | 0.273 | 13.1 | 187 | ||||||
| Missing2 | 158 | 0.613 | 2.37 | 0.340 | 13.6 | 185 | ||||||
| Annual Household Income | <$15,000 | 943 | 0.669 | <.0001 | 2.49 | <.0001 | 0.296 | <.0001 | 13.1 | <.0001 | 187 | <.0001 |
| $15,000+ | 491 | 0.594 | 2.13 | 0.518 | 12.3 | 191 | ||||||
| Missing2 | 581 | 0.523 | 2.15 | 0.362 | 12.1 | 182 | ||||||
| Smoker | Never | 663 | 0.487 | <.0001 | 1.99 | <.0001 | 0.340 | .0005 | 12.8 | .154 | 186 | .197 |
| Former | 685 | 0.461 | 2.19 | 0.401 | 12.3 | 189 | ||||||
| Current | 620 | 1.05 | 2.83 | 0.332 | 12.7 | 187 | ||||||
| Second-hand Smoke | 0 hours | 1190 | 0.529 | <.0001 | 2.16 | <.0001 | 0.355 | 0.828 | 12.5 | 0.087 | 187 | 0.513 |
| 1–7 hours | 581 | 0.685 | 2.44 | 0.366 | 12.7 | 187 | ||||||
| 8+ hours | 204 | 0.956 | 2.87 | 0.358 | 13.2 | 190 | ||||||
| Weekly Alcohol | No drinks | 1688 | 0.586 | <.0001 | 2.19 | <.0001 | 0.351 | 0.010 | 12.6 | 0.156 | 186 | 0.615 |
| 1–5 drinks | 149 | 0.642 | 2.57 | 0.458 | 12.5 | 190 | ||||||
| >5 drinks | 174 | 0.781 | 3.28 | 0.369 | 13.2 | 187 | ||||||
| BMI Category | Underweight (<18.5) | 19 | 0.844 | <.0001 | 3.99 | <.0001 | 0.221 | 0.012 | 12.1 | 0.892 | 156 | <.0001 |
| Normal (18.5–24.9) | 293 | 0.789 | 2.84 | 0.328 | 12.5 | 183 | ||||||
| Overweight (25–29.9) | 668 | 0.663 | 2.49 | 0.362 | 12.7 | 192 | ||||||
| Obese (30+) | 964 | 0.523 | 2.00 | 0.369 | 12.6 | 185 | ||||||
| Waist Circumference | At risk (M>40in, F>35in) | 1333 | 0.576 | <.0001 | 2.07 | <.0001 | 0.341 | 0.0008 | 12.8 | 0.0005 | 187 | 0.702 |
| Normal | 608 | 0.676 | 2.85 | 0.401 | 12.1 | 188 | ||||||
Wald F Statistics to test differences in Geometric mean concentration between covariate levels.
Missing numbers include don’t know, refuse to answer, and missing values. For the two income variables, “Missing” was kept as a level due to its large number and potential association between missing income information and outcomes.
Of note, some levels observed in this cohort were unusual. For example, 275 (14%) participants’ blood cadmium levels were higher than the 95th percentile of adults in the U.S. population (1.35 µg/L); moreover, levels in five participants were even higher than a concentration trigger level (5 µg/L) used by the Occupational Safety and Health Administration (OSHA) in the process of determining if removal from the workplace is necessary (OSHA, 2020b). For lead exposure, 177 (8.8%) had BLL over the level that the Adult Blood Lead Epidemiology and Surveillance (ABLES) used to indicate an elevated BLL for surveillance purposes (5 µg/dL). The maximum BLL was 50.7 µg/dL, which was ten times higher than the ABLES elevated BLL case classification, and even higher than the threshold (50 µg/dL) at which OSHA regulations require removal from the workplace for workers in construction (CDC, 2021; OSHA, 2020a). For the essential element selenium, one participant’s whole blood selenium level (61.9 µg/L) was less than the 70 µg/L serum level that is used as a threshold for diagnosing selenium deficiency (CDC, 2012), however, it is not clear how well that serum-matrix threshold may apply to interpreting whole blood selenium concentrations. The results that were unusually high (for cadmium and lead) or low (selenium) were communicated to the SHS Steering Committee for potential follow-up.
Table 2 shows the GM with 95% CI of blood metal results in the entire SHS cohort and stratified by participant characteristics, including study center, sex, age, smoking, second-hand smoke, alcohol consumption, education, BMI, waist circumference, and the two income variables. Table 3 gives the multivariate linear regression model results on the association of each of the five blood metals with all covariates (same list as in the univariate analyses), adjusting for other variables. Both univariate analyses and multivariate models confirmed that study center was the biggest factor associated with the blood levels for cadmium, lead, manganese, and selenium, although it was not associated with total mercury after adjusting for covariates (Table 3). A summary of associations with non-location factors for each analyte is described below.
Table 3.
Multivariate linear regression model results on blood cadmium, lead, manganese, total mercury, and selenium levels (N=1,637 study participants who had non-missing values for all covariates). Data shown are regression-adjusted geometric mean ratios (i.e., ratio of the current level geometric mean to that of the reference level, adjusted for all other variables in the model) and 95% CI.
| Variable | Level | Cadmium1 | Lead1 | Total Mercury1,2 | Manganese1 | Selenium1 |
|---|---|---|---|---|---|---|
| Sex (Ref: Male) | Female | 1.25 (1.18–1.33) | 0.71 (0.67–0.75) | - | 1.03 (0.99–1.07) | - |
| Age group (Ref: 70+) | 50–59 years | 0.93 (0.86–1.00) | - | - | - | - |
| 60–69 years | 0.91 (0.85–0.98) | - | - | - | - | |
| Center (Ref: Arizona) | North Dakota/ South Dakota | 2.04 (1.86–2.24) | 1.45 (1.33–1.59) | - | 1.25 (1.18–1.32) | 1.12 (1.09–1.15) |
| Oklahoma | 1.23 (1.12–1.35) | 1.19 (1.09–1.30) | - | 1.10 (1.04–1.17) | 1.06 (1.03–1.09) | |
| Body Mass Index (Ref: Normal, 18.5–24.9 kg/m2) | <18.5 kg/m2 | - | 1.32 (1.01–1.73) | - | - | 0.80 (0.73–0.87) |
| 25.0–29.9 kg/m2 | - | 0.90 (0.83–0.97) | - | - | 1.04 (1.02–1.07) | |
| ≥30 kg/m2 | - | 0.83 (0.77–0.89) | - | - | 1.02 (0.99–1.04) | |
| Waist circumference (Ref: Normal) | At risk | 0.93 (0.88–0.99) | - | 0.89 (0.81–0.98) | 1.07 (1.03–1.11) | - |
| Household income (Ref: <15,000/year) | $15,000+/year | - | 0.93 (0.87–1.00) | 1.37 (1.21–1.54) | - | - |
| Income meets needs (Ref: Needs met) | Needs not met | - | - | 0.75 (0.66–0.85) | - | - |
| Smoker (Ref: Never) | Former | 1.04 (0.98–1.11) | 1.03 (0.97–1.09) | - | - | - |
| Current | 1.99 (1.85–2.14) | 1.20 (1.13–1.29) | - | - | - | |
| Second-hand smoke (Ref: 0h/d) | 1–7 hours/day | 0.99 (0.93–1.06) | - | - | - | - |
| 8+ hours/day | 1.14 (1.03–1.25) | - | - | - | - | |
| Alcohol consumption (Ref: No drinks) | 1–5 drinks/week | 1.06 (0.96–1.17) | 1.13 (1.03–1.24) | 1.21 (1.03–1.43) | 1.00 (0.95–1.07) | - |
| >5 drinks/week | 1.12 (1.02–1.23) | 1.24 (1.13–1.35) | 0.98 (0.84–1.15) | 1.06 (1.00–1.13) | - | |
| Education (Ref: 12+ years) | <12 years | 1.07 (1.01–1.13) | 1.07 (1.01–1.13) | 0.77 (0.70–0.85) | 1.05 (1.01–1.09) | - |
| Model Fit (R2) | 0.44 | 0.26 | 0.10 | 0.07 | 0.07 | |
Variables with blank cells were not included in the final model.
For blood mercury, quantile regression was also used to model the median mercury level for the left-censoring due to non-detects. Both quantile regression and multi-variate linear regression models indicated the same set of regressors, so only the latter is reported in this table to produce the regression-adjusted geometric mean ratios
In bivariate analyses (Table 2), we observed higher blood cadmium GM in women, participants with lower income, lower education level, those who indicated that income does not meet family needs, and current smokers. A trend of higher cadmium levels was also observed by hours of exposure to second-hand smoke, as well as by the servings of alcohol per week. Cadmium levels were inversely related to BMI and waist circumference. These associations were confirmed in the multivariate regression model (Table 3). Higher blood cadmium was associated with smoking (GM ratio of current smoker vs. never-smoking: 1.99 [95% CI: 1.86–2.14], being female (female vs. male: 1.25 [95% CI: 1.18–1.33]), second-hand smoke exposure (8+ hours/day vs. none: 1.14 [95% CI: 1.03–1.25]), alcohol consumption (5 drinks/week vs. none: 1.12 [95% CI: 1.02–1.23], and lower education level (<12 years vs. 12+ years: 1.07 [95% CI: 1.01–1.13]).
Geometric means of BLL (Table 2) were higher in men, those reporting lower income, those with lower education levels, and current smokers. Lead levels also increased with increasing hours of exposure to second-hand smoke as well as with increasing servings of alcohol per week. Like cadmium, lead levels were inversely related to BMI and waist circumference. After adjusting for covariates in multivariate analyses, (Table 3), high BLL was associated with being male (female vs. male: 0.71 [95% CI: 0.67–0.75]), smoker (current vs. never: 1.20 [95% CI: 1.13–1.29]), lower-income ($15K+/year vs. <15K/years: 0.93 [95% CI: 0.87–1.00]), and lower education level (<12 years vs. 12+ years: 1.07 [95% CI: 1.01–1.13]). BLLs also displayed a clear trend, i.e., increasing BLL with increasing alcohol consumption and decreasing BMI (Table 3).
Bivariate analysis indicated that blood total mercury level was associated with all factors assessed, except for second-hand smoke exposure (Table 2). In multivariate regression (Table 3), both income and education variables had large impacts. Higher blood mercury was associated with higher annual household income ($15K+ vs. <$15K: 1.37 [95% CI: 1.21–1.54]), income meeting needs (not met vs. met: 0.75 [95% CI: 0.66–0.85]), and higher education (<12 years vs. 12+ years: 0.77 [95% CI: 0.70–0.85]). In addition, higher blood mercury was associated with lower waist circumference (at-risk vs. normal: 0.89 [95% CI: 0.81–0.98]).
Blood manganese GM level were slightly higher in women, those reporting lower household income, lower education, and those who had larger waist circumference (Table 2). Multivariate analyses showed higher blood manganese levels were statistically associated with lower education level, larger waist circumference, and more alcohol drinking, although the effects for these associations were small (GM ratios were all below 10% compared to reference levels, Table 3).
Bivariate analyses showed blood selenium GMs differed by age and household income, though the differences by variable level were very small (Table 2). Differences were also observed among BMI categories, though no trend was detected (Table 2). The multivariate linear regression model indicated that blood selenium trended higher with higher BMI (Table 3). Notably, the associations were small (less than 5% difference compared to reference levels), except for underweight BMI (vs. normal: 0.80 [95% CI: 0.73–0.87]).
DISCUSSION
In this study on the SHS cohort, we measured five elements in archived blood specimens collected from over 2,000 AI participants, 12 AI communities, 4 states in the Southwest and the Great Plains U.S., during 1998–1999 (Phase III). To our knowledge, this study was among the largest epidemiological studies assessing historical metal exposure using blood metal measurements on AI communities. Biomonitoring allows for the characterization of contamination levels and potential risk factors, and provides a foundation for subsequent analyses and activities, including examination of temporal trends, investigation of associated health effects, and informing the tribes on the development of potential public health activities for the AI communities.
Environmental exposure to metals is known to be unevenly distributed across the U.S (Dignam et al., 2019; Eagles-Smith et al., 2016; Sobel et al., 2021). Although regulations are in place to improve the quality of water delivered via public water systems, many AI members rely on non-public water sources that are mostly not monitored (Bonogofsky et al., 2013). Even for those using public water systems, AI communities, especially those in the Southwest and Midwest, had higher pollutants, such as arsenic, in public water systems (Nigra et al., 2020). This is consistent with higher urinary arsenic in the SHS cohort compared to the U.S. population (Navas-Acien et al., 2009). In addition, AI communities engage in various aspects of traditional lifestyles which are inextricably linked to the environment, e.g., local gathering of plants and game for food, medicines, and spiritual and traditional healing practices (Harris and Harper, 2001). Given these deep cultural ties to the earth and reliance on it, any environmental contamination may contribute to AI populations being disproportionally exposed (Hoover et al., 2012). The geographical differences across study centers may be related to multiple factors. For some metals, this can be related to differences in metal levels in the air, water, and soil in the local environment. This is likely to be the case for selenium, an element that is common in soils and water of the Northern Great Plains (North and South Dakota) (Boon, 1989; Franzen et al., 2006; Ramirez and Rogers, 2002). For other metals, differences in lifestyle factors and cultural practices could also play a role. For instance, smoking is more prevalent in North and South Dakota based on self-reporting (Table 1), which could contribute to higher exposure to cadmium and lead. Additional research is needed to understand differences in metal exposure across the SHS centers.
Cadmium
Cadmium has been linked to bone disease, cardiovascular disease, cancers, kidney damage, hypertension, and lung illnesses. Cadmium is found in the earth’s crust and can come from manufacturing byproducts and consumer products such as batteries, coatings, pigments, and plastics (ATSDR, 2015). Cigarette smoking is a known major source of cadmium exposure in people and leads to increased blood cadmium levels (Järup and Åkesson, 2009; Waalkes, 2003; Yassin and Martonik, 2004). Smokers typically have cadmium body burden levels twice as high as those of nonsmokers (Waalkes, 2003).
The SHS’s blood cadmium levels were statistically higher than that of the NHANES 1999–2000 both overall (Figure 1) and when stratified by smoking status, i.e., current smokers (GM: 1.05 vs. 0.896 μg/L) and non-smokers (GM: 0.487 vs. 0.394 μg/L). These indicate higher cadmium exposure in the AI communities than the general U.S. population among self-reported smokers and non-smokers. The elevated cadmium exposure was primarily driven by the participants from North Dakota and South Dakota, indicating possible differences in smoking rates (e.g., number of cigarettes per day) and/or local sources for cadmium exposure. In addition to smoking status and location being major factors, high second-hand smoke exposure was also associated with blood cadmium levels even after adjusting for all factors, further demonstrating cigarette smoke as a major source of cadmium. Other factors have been reported to influence cadmium exposure, such as diet, especially among non-smokers (ATSDR, 2015). An earlier study found that the consumption of processed meat was associated with higher urinary levels of cadmium in the cohort at a later phase for a different group of participants (Olmedo et al., 2017). Although the dietary recall was not conducted during the data collection of Phase III, the overall high cadmium exposure and large regional variation on blood cadmium levels were likely due to different environmental exposures and dietary intakes in these communities.
Women had statistically higher blood cadmium levels than men, which is consistent with the U.S. population based on NHANES 2011 – 2016 (CDC, 2022). Although estimated daily intakes of cadmium in nonsmoking adult males living in the U.S. were slightly higher than females (0.35 and 0.30 μg cadmium/kg body weight/day, respectively), females generally absorb greater amounts of cadmium in the gastrointestinal tract (ATSDR, 2015), which may contribute to the higher blood cadmium levels in women.
We found an inverse relationship between educational level and cadmium concentrations, which is consistent with an analysis on NHANES 1999–2018 that examined educational status across three levels and observed a similar relationship (Wen et al., 2021). We also observed blood cadmium increased with alcohol consumption, consistent with studies conducted in Korea and Brazil (Lee and Ha, 2011; Martins et al., 2020). A study using NHANES data reported that beer contributes an estimated 2.7% of daily cadmium intake for adults 20+ years old (Kim et al., 2018).
Lead
Exposure to lead during adulthood can contribute to several adverse health outcomes, including damage to the neurological system, reduction in renal function, inhibition of hemoglobin production, increase in the risk of heart disease, and decreased sperm counts (ATSDR, 2020). The sources of exposure to lead in the U.S. have varied over time. Before 1996, the most common source of exposure was leaded gasoline which was phased out for on road vehicles in 1996 (DOE, 2020; EPA, 2022). Other sources of exposure include lead-based paints, lead pipes, lead-soldered joints in plumbing, tobacco, mining, and smelting (ATSDR, 2020).
Overall, BLL in our cohort was similar to the NHANES 1999–2000 participants ages 50 years and above. However, BLLs in the North Dakota and South Dakota participants were higher than the U.S. reference population. BLLs had been reported for several other AI populations. In Canada, the First Nations Biomonitoring Initiative (FNBI) collected biological samples from First Nations peoples living in reservations in 2011 (La Corte and Wuttke, 2012; Wuttke et al., 2013). Our cohort had a higher median BLL (2.24 μg/dL) than the overall FNBI study (1.08 μg/dL) and the Prairie Region sub-sample of the FNBI cohort, which is on the northern border of North Dakota (1.01 μg/dL). BLL was also measured in the Fond Du Lac (FDL) tribe that was located within the Great Lakes Region in 2013–2014. SHS cohort had higher BLLs than those of the FDL tribe (1.18 ug/dL) (FDL, 2014). It should be noted that our study was conducted earlier than the other AI studies, and BLL has been decreasing over time.
In this cohort, current smokers had higher BLL relative to those who were not smoking at the time. Additionally, GM BLL increased with increasing hours of second-hand smoke exposure, although the association did not hold in the multivariate analysis. A similar pattern in increasing BLL has also been observed in NHANES data (1994–2004) comparing nonsmokers with varied levels of second-hand smoke exposure (Apostolou et al., 2012; Richter et al., 2009).
We found that BLL increased with alcohol consumption in this cohort, a finding that is consistent with previous studies using NHANES 2011–2016 (Reja et al., 2020) and on other populations in different countries (Grandjean et al., 1981; Weyermann and Brenner, 1997). A study in Denmark concluded that one alcoholic drink (1.35 centiliter pure ethanol per day) can contribute 0.5–1.0 µg/dL to BLLs (Grandjean et al., 1981).
In this cohort, less educated individuals (<12 years education) had higher BLLs, relative to those who were more educated (>12 years education). A population-based cross-sectional survey conducted in New York City similarly found that participants who reported lower educational achievement (high school diploma or less) had higher BLLs relative to those who reported higher educational achievement (some college or more) (McKelvey et al., 2007).
We observed an inverse association between BLLs and BMI categories. Both in unadjusted and adjusted models, lower BMI categories were associated with higher GM BLLs. Lower BMI scores were associated with higher BLLs in many other studies, such as NHANES 1999 to 2006 (Scinicariello et al., 2013). Another study similarly found an inverse association between BMI and BLLs, however, this association only held for women (Dhooge et al., 2010). Interestingly, a study in China also found a sex-specific association between BLLs and BMI (Wang et al., 2015). The sex-specific association between BLL and BMI may be explained in part due to sex hormones, which are known to influence adiposity (Tchernof and Després, 2000).
Manganese
Manganese is a naturally occurring substance and is an essential metal for human health. Both inadequate and excess exposure to manganese can cause several serious health outcomes (ATSDR, 2012; Crossgrove and Zheng, 2004; Racette et al., 2012). Manganese deficiency is associated with both skin lesions and bone modeling and remodeling diseases (Keen et al., 2000; Watts, 1990), and overexposure to manganese can cause neurodegenerative damage (Barbeau, 1984; Cowan et al., 2009; Crossgrove and Zheng, 2004). Manganese is contained in groundwater and soil at low levels and people are frequently exposed via drinking water, air, soil, and food (ATSDR, 2012).
Our study reports median blood manganese of 12.6 μg/L, which was about 40% higher than the U.S. reference population (8.77 μg/L) and those reported in other countries, e.g., China, Italy, Korea, and Canada (ranging 8.9–10.8 μg/L) (Bocca et al., 2011; Clark et al., 2007; Lee et al., 2016; Oulhote et al., 2014; Pan et al., 2014; Zhang et al., 2015). Communities living near mining activities and industries using manganese may be exposed via drinking water, air, soil, and food (ATSDR, 2012).
In our study, women had slightly higher blood manganese levels than men which is consistent with the literature spanning across the U.S., Italy, Canada, Korea, and China (Bocca et al., 2011; Clark et al., 2007; Lee et al., 2012; Oulhote et al., 2014; Pan et al., 2014). One study found that women had higher absorption of manganese but a shorter biological half-life compared to men, which may be related to homeostatic controls (gut absorption, hepatic metabolism, and excretion) (Finley et al., 1994). We found increased waist circumference was associated with higher blood manganese. A study conducted in China found that higher manganese intake was associated with a lower risk of abdominal obesity (defined as weight circumference ≥90 cm for men and weight circumference ≥80 cm for women) among men, but not women (Zhou et al., 2016).
We also found that those with lower education levels had higher blood manganese, which may be related to the known neurologic effects from manganese exposure that could impact cognitive and intellectual functions (ATSDR, 2012; Zhang et al., 2021). Moreover, studies had reported potential joint or synergistic toxic actions of lead and manganese exposure on mental, intellectual, and psychomotor skills in children. Although we could not evaluate the potential mixture effect in this analysis, the findings that education level was inversely associated with BLL, manganese, and cadmium are consistent with other reports described above and warrant further research.
Selenium
Selenium is an essential element for adequate human nutrition but is toxic at higher levels, with a narrow safety margin (Vinceti et al., 2018). The health effects of selenium deficiency are wide-reaching and affect both male and female fertility, and long-term deficiency can result in necrotic cardiomyopathy, peripheral myopathy, alterations in the skin, hair, and nails, and anemia (Brown and Arthur, 2001; Mehdi et al., 2013). Humans are exposed to selenium via inhalation and ingestion, with the most common source being food, followed by water. In addition to natural sources of selenium, it can also be released into the environment through anthropogenic actions including mining, coal and oil combustion, and agricultural phosphate fertilizers (Favorito et al., 2021).
Overall, participants in our cohort had a similar median blood selenium level (186 µg/L) as that of the Canadian FNBI cohort (182 µg/L) (Wuttke et al., 2013), but was statistically lower than that of U.S. NHANES participants over the age of 50 years (193 µg/L). These findings suggest that the essential element in the AI population was lower than the national reference range.
Blood selenium in this cohort increased with BMI categories, from underweight, normal weight, to overweight, then decreased among obese participants. Notably, the GM level among underweight participants was 156 μg/L, lower than those with normal weight in the cohort (183 μg/L) and the U.S. reference levels for older adults (193 μg/L). This association remained in the multivariate models after adjusting for other factors, with the adjusted GM for underweight at 20% lower than those with normal weight, indicating potentially low nutrient level among underweight participants in the AI community. Interestingly, a study using NHANES data found that serum selenium levels were inversely associated with BMI (Zhong et al., 2018). A study in Kuwait found that serum selenium levels were reduced among their morbidly obese participants, relative to their age-matched overweight controls (Alasfar et al., 2011). Conversely, a French study found that non-obese women had higher blood serum selenium levels than those who were obese (Arnaud et al., 2006).
We found that blood selenium levels were slightly lower in the oldest age group (70 years and older) in univariate analysis, but not in the multivariate regression model. NHANES data from 1988–1994 found no differences in serum selenium levels by age group (Niskar et al., 2003), and a French cohort found that serum selenium levels among women increased with age, but the association did not hold for men (Arnaud et al., 2006).
Total Mercury
Mercury is a neurotoxicant and the related health effects depend on both the form of mercury and the exposure route. Total blood mercury is mainly a measure of methyl mercury exposure, which occurs almost exclusively via the consumption of seafood, especially predatory fish and large marine mammals (ATSDR, 2022). Our cohort had markedly lower total blood mercury than the NHANES population (Figure 1). Contrary to the other four elements that exhibited clear, large geographic differences, blood mercury levels were similar across the three study centers and were not associated with the location in multivariate analysis. Although we did not conduct speciated laboratory tests to measure inorganic and organic mercury in this study, the three times lower total mercury level, lack of differences among study centers, and the landlocked locations for all AI communities in this cohort are expected to be due to limited seafood consumption in these communities. This is consistent with low levels of urinary arsenobetaine, a specific arsenic compound found in seafood, measured in the SHS in phases I through III (Navas-Acien et al., 2009; Scheer et al., 2012). Furthermore, shellfish and fish were among the lowest consumed food categories in 1,727 SHFS participants during phase IV (Nigra et al., 2019). While it is reassuring that the SHS cohort has low mercury exposure, the presumably limited fish intake might also be a concern as sufficient fish consumption has been related to associated dietary nutrient benefits (e.g., protein and omega-3 fatty acids).
In the SHS cohort, both income variables were associated with blood mercury, i.e., higher mercury levels among the higher income group and those reporting income meeting their needs. This is consistent with multiple studies reporting the positive association of mercury levels and income groups. For example, a Korean study that examined income quartiles found that blood mercury levels increased as gross household income rose (Kim et al., 2019). Participants with higher education levels had higher levels of total mercury blood levels, which is consistent with a population-based cross-sectional survey conducted in NYC (McKelvey et al., 2007).
In recent years, several social services, public health programs and policies have been implemented to improve the health of AI communities. For example, tobacco control programs have been established to provide educational presentations and workshops to community members (AZDHS, 2008). Tribes have enacted policies and regulations to require smoke-free air in all indoor public places (SDDOH, 2015), and a study examining the impact of such policy found a decrease in secondhand smoke exposures shortly after enacting the tribal tobacco control policy (Donald et al., 2020). Furthermore, tribes have also addressed emerging use of e-cigarettes by banning the possession and sale of e-cigarettes on reservations (Kaczke, 2019).
This study has several limitations. First, about 10% of the archived blood samples were clotted and could not be analyzed, which impacted the sample size and power to detect effects. Second, as do other surveys, recall bias may affect the accuracy of several variables, such as second-hand smoking. Third, for the comparison to the U.S. general population, NHANES data for mercury, manganese and selenium were only available several years after the time of samples collection for this study, which was not optimal. Fourth, some potentially important sources or risk factors were not collected, e.g., food intake and drinking water source. Lastly, the blood metal levels reported in the study reflect historical exposure from two decades ago and do not necessarily reflect current exposure levels. In recent years, AI tribes have implemented several social services and public health programs to promote healthy living, such as programs and policies on smoking cessation, tobacco and e-cigarette control, and physical activity promotion. A more recent assessment in the cohort would be beneficial to provide current exposure levels and inform future activities to reduce exposure and improve health.
In this cross-sectional study of the SHS cohort, we found that the SHS study population had statistically higher levels of cadmium and manganese and comparable BLL as compared to a general U.S. reference population. The highest exposed participants in this cohort had levels over established action levels for occupational populations with known high exposures. Meanwhile, selenium, an essential element, was lower in SHS, particularly among underweight participants. The lowest selenium level in the cohort indicated selenium deficiency. Elevated exposure to toxic heavy metals (e.g., cadmium and manganese) and lower levels of selenium (an essential element) may lead to long-term adverse health outcomes in the AI population. Blood mercury was only about 30% of the general U.S. reference population; the low mercury level, though positive from the mercury toxicity aspect, may indicate low fish consumption and lack of seafood-related nutrients (e.g., omega-3 fatty acid) in the population. Geographic difference was a major factor with participants from North Dakota and South Dakota having the highest median blood levels of cadmium, lead, manganese, and selenium. In addition, we found that each of the blood metals was associated with numerous factors, including selected demographics (age, sex), health behavior (smoking, second-hand smoke, alcohol consumption), income, education, waist circumference, and BMI. This study identified potential risk factors for metal exposure and will guide the subsequent analyses on association between exposure and health outcomes in the AI communities. In addition, the findings will help guide the tribes in the development of policies, educational, and community outreach programs to reduce harmful exposure and increase beneficial nutrient intake in these AI communities.
Supplementary Material
Acknowledgements, Disclaimer, and Funding
We thank all the study participants and Tribal Nations that made this study possible. We thank all SHS staff, including steering committee members, staff in the Coordinating Center at the University of Oklahoma Health Sciences Center, the SHS Central Lab at the MedStar Research Institute, and the three field centers in Arizona, Oklahoma, North Dakota, and South Dakota. We appreciate the high-quality laboratory analyses conducted by the CDC’s Division of Laboratory Sciences’ staff, through the challenging time during the pandemic. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
The Strong Heart Study was supported by grants from the National Heart, Lung, and Blood Institute contracts 75N92019D00027, 75N92019D00028, 75N92019D00029, and 75N92019D00030; previous grants R01HL090863, R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319; and cooperative agreements U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521; and by National Institute of Environmental Health Sciences grants R01ES021367, R01ES025216, R01ES032638, P42ES010349, and P30ES009089.
References
- Alasfar F, et al. , 2011. Selenium Is Significantly Depleted Among Morbidly Obese Female Patients Seeking Bariatric Surgery. Obesity Surgery 21, 1710–1713. [DOI] [PubMed] [Google Scholar]
- Alissa EM, Ferns GA, 2011. Heavy metal poisoning and cardiovascular disease. J Toxicol 2011, 870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou A, et al. , 2012. Secondhand tobacco smoke: a source of lead exposure in US children and adolescents. Am J Public Health 102, 714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud J, et al. , 2006. Serum selenium determinants in French adults: the SU.VI.M.AX study. Br J Nutr 95, 313–20. [DOI] [PubMed] [Google Scholar]
- ATSDR, Toxicological profile for Manganese U.S. Department of Health and Human Services, Public Health Services, Atlanta, GA, 2012. [Google Scholar]
- ATSDR, Toxicological profile for Cadmium U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Atlanta, GA, 2015. [Google Scholar]
- ATSDR, Toxicological profile for Lead U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Atlanta, GA, 2020. [Google Scholar]
- ATSDR, Toxicological profile for Mercury U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Atlanta, GA, 2022. [Google Scholar]
- AZDHS, 2008 Biennial Evaluation Report FY 2007–2008, A Report on Tobacco Control Programs and Services Arizona Department of Health Services, 2008. [Google Scholar]
- Barbeau A, 1984. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias). Neurotoxicology 5, 13–35. [PubMed] [Google Scholar]
- Bocca B, et al. , 2011. Assessment of reference ranges for blood Cu, Mn, Se and Zn in a selected Italian population. J Trace Elem Med Biol 25, 19–26. [DOI] [PubMed] [Google Scholar]
- Bonogofsky A, et al. , Honoring the river: how hardrock mining impacts tribal communities Tribal Partnerships Program, Boulder, CO, 2013. [Google Scholar]
- Boon DY, Potential selenium problems in Great Plains Soils. In: Jacobs LW, (Ed.), Selenium in Agriculture and the Environment American Society of Agronomy, Inc., Soil Science Society of America, Inc., 1989. [Google Scholar]
- Brown KM, Arthur JR, 2001. Selenium, selenoproteins and human health: a review. Public Health Nutr 4, 593–9. [DOI] [PubMed] [Google Scholar]
- Caudill SP, et al. , 2008. Multi-rule quality control for the age-related eye disease study. Stat Med 27, 4094–106. [DOI] [PubMed] [Google Scholar]
- CDC, Second National report on biochemical indicators of diet and nutrition in the U.S. population, 2012 Dept. of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA, 2012. [Google Scholar]
- CDC, Laboratory Procedure Manual; Analytes: Cadmium, Lead, Manganese, Mercury, and Selenium; Matrix: Whole Blood; Method No: DLS 3040.1–02 In: D. o. L. Sciences, (Ed.). U.S. Centers for Disease Control and Prevention, 2019. [Google Scholar]
- CDC, Adult Blood Lead Epidemiology and Surveillance (ABLES) U.S. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 2021. [Google Scholar]
- CDC, National Report on Human Exposure to Environmental Chemicals Atlanta, GA, 2022. [Google Scholar]
- Chowdhury R, et al. , 2018. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. Bmj 362, k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NA, et al. , 2007. Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere 70, 155–64. [DOI] [PubMed] [Google Scholar]
- Cosselman KE, et al. , 2015. Environmental factors in cardiovascular disease. Nat Rev Cardiol 12, 627–42. [DOI] [PubMed] [Google Scholar]
- Cowan DM, et al. , 2009. Manganese exposure among smelting workers: relationship between blood manganese-iron ratio and early onset neurobehavioral alterations. Neurotoxicology 30, 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W, 2004. Manganese toxicity upon overexposure. NMR Biomed 17, 544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhooge W, et al. , 2010. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int 36, 330–337. [DOI] [PubMed] [Google Scholar]
- Dignam T, et al. , 2019. Control of Lead Sources in the United States, 1970–2017: Public Health Progress and Current Challenges to Eliminating Lead Exposure. Journal of public health management and practice : JPHMP 25 Suppl 1, Lead Poisoning Prevention, S13–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOE, U. S., Gasoline Explained: Gasoline and the environment. Gasoline Explained U.S. Department of Energy, Energy Information Administration, 2020. [Google Scholar]
- Donald ER, et al. , 2020. Active smoking, secondhand smoke exposure and serum cotinine levels among Cheyenne River Sioux communities in context of a Tribal Public Health Policy. Tobacco Control 29, 570. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, et al. , 2016. Mercury in western North America: A synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci Total Environ 568, 1213–1226. [DOI] [PubMed] [Google Scholar]
- EPA, U. S., Timeline of Major Accomplishments in Transportation, Air Pollution, and Climate Change 2022.
- Favorito JE, et al. , 2021. Soil-plant-animal relationships and geochemistry of selenium in the Western Phosphate Resource Area (United States): A review. Chemosphere 266, 128959. [DOI] [PubMed] [Google Scholar]
- FDL, Community Report for Cadmium, Lead, and Mercury, Fond du Lac Community Biomonitoring Study Fond du Lac Human Services Division, Minnesota Department of Health; 2014. [Google Scholar]
- Finley JW, et al. , 1994. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am J Clin Nutr 60, 949–55. [DOI] [PubMed] [Google Scholar]
- Franzen DW, et al. , 2006. A Survey of Soil Attributes in North Dakota by Landscape Position. Agronomy Journal 98, 1015–1022. [Google Scholar]
- Grandjean P, et al. , 1981. Influence of smoking and alcohol consumption on blood lead levels. Int Arch Occup Environ Health 48, 391–7. [DOI] [PubMed] [Google Scholar]
- Harris S, Harper BL, 2001. Lifestyles, Diets, and Native American Exposure Factors Related to Possible Lead Exposures and Toxicity. Environmental Research 86, 140–148. [DOI] [PubMed] [Google Scholar]
- Hoover E, et al. , 2012. Indigenous peoples of North America: environmental exposures and reproductive justice. Environ Health Perspect 120, 1645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JH, et al. , 2020. Exposure to uranium and co-occurring metals among pregnant Navajo women. Environ Res 190, 109943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, et al. , 1992. Risk factors for coronary heart disease in diabetic and nondiabetic Native Americans. The Strong Heart Study. Diabetes 41 Suppl 2, 4–11. [DOI] [PubMed] [Google Scholar]
- Järup L, Åkesson A, 2009. Current status of cadmium as an environmental health problem. Toxicology and Applied Pharmacology 238, 201–208. [DOI] [PubMed] [Google Scholar]
- Kaczke L, Oglala Tribal Council bans vaping on Pine Ridge Reservation. Argus Leader, Argus Leader, 2019.
- Keen CL, et al. , 2000. Manganese metabolism in animals and humans including the toxicity of manganese. Met Ions Biol Syst 37, 89–121. [PubMed] [Google Scholar]
- Khanam R, et al. , 2021. Prenatal Environmental Metal Exposure and Preterm Birth: A Scoping Review. Int J Environ Res Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, et al. , 2019. A study of relationship between blood mercury concentration and hypertension in residents living in old mine fields and related factors. Ann Occup Environ Med 31, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, et al. , 2018. Dietary Cadmium Intake and Sources in the US. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Corte E, Wuttke S, 2012. The First Nations Biomonitoring Initiative – FNBI. International Journal of Hygiene and Environmental Health 215, 168–171. [DOI] [PubMed] [Google Scholar]
- Lee B, Ha J, 2011. The effects of smoking and drinking on blood lead and cadmium levels: data from the fourth Korea national health and nutrition examination survey. Occupational and Environmental Medicine 68, A93. [Google Scholar]
- Lee ET, et al. , 1990. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 132, 1141–55. [DOI] [PubMed] [Google Scholar]
- Lee JW, et al. , 2012. Korea National Survey for Environmental Pollutants in the Human Body 2008: heavy metals in the blood or urine of the Korean population. Int J Hyg Environ Health 215, 449–57. [DOI] [PubMed] [Google Scholar]
- Lee S, et al. , 2016. The Association Between Blood Mercury Levels and Risk for Overweight in a General Adult Population: Results from the Korean National Health and Nutrition Examination Survey. Biol Trace Elem Res 171, 251–261. [DOI] [PubMed] [Google Scholar]
- Lewis J, et al. , 2017. Mining and Environmental Health Disparities in Native American Communities. Curr Environ Health Rep 4, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos MN, Ronco AM, 2009. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod Toxicol 27, 88–92. [DOI] [PubMed] [Google Scholar]
- Martins AC, et al. , 2020. Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ Res 187, 109618. [DOI] [PubMed] [Google Scholar]
- McKelvey W, et al. , 2007. A Biomonitoring Study of Lead, Cadmium, and Mercury in the Blood of New York City Adults. Environmental Health Perspectives 115, 1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi Y, et al. , 2013. Selenium in the environment, metabolism and involvement in body functions. Molecules 18, 3292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, et al. , 2009. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect 117, 1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI, ATP III Guidelines At-A-Glance Quick Desk Reference U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, 2001. [Google Scholar]
- Nigra AE, et al. , 2020. Inequalities in Public Water Arsenic Concentrations in Counties and Community Water Systems across the United States, 2006–2011. Environ Health Perspect 128, 127001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigra AE, et al. , 2019. Dietary determinants of inorganic arsenic exposure in the Strong Heart Family Study. Environ Res 177, 108616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskar AS, et al. , 2003. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol Trace Elem Res 91, 1–10. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, et al. , 2007. Handbook on the Toxicology of Metals Academic Press, Burlington. [Google Scholar]
- Olmedo P, et al. , 2017. Dietary determinants of cadmium exposure in the Strong Heart Family Study. Food Chem Toxicol 100, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA, Occupational Safety and Health Standards, 1910.1025 - Lead U.S. Department of Labor, Occupational Safety and Health Administration; 2020a. [Google Scholar]
- OSHA, Occupational Safety and Health Standards, 1910.1027 - Cadmium U.S. Department of Labor, Occupational Safety and Health Standards, 2020b. [Google Scholar]
- Oulhote Y, et al. , 2014. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environ Health 13, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, et al. , 2014. Distribution of manganese, cobalt and molybdenum in blood and urine among general population in 8 provinces of China. Zhonghua Yu Fang Yi Xue Za Zhi 48, 784–90. [PubMed] [Google Scholar]
- Pang Y, et al. , 2016. Metal mixtures in urban and rural populations in the US: The Multi-Ethnic Study of Atherosclerosis and the Strong Heart Study. Environ Res 147, 356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle JL, et al. , 1996. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. Jama 275, 1233–40. [PubMed] [Google Scholar]
- Potula V, et al. , 2005. Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Arch Environ Occup Health 60, 195–204. [DOI] [PubMed] [Google Scholar]
- Racette BA, et al. , 2012. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 33, 881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JP, Rogers BP, 2002. Selenium in a Wyoming Grassland Community Receiving Wastewater from an In Situ Uranium Mine. Archives of Environmental Contamination and Toxicology 42, 431–436. [DOI] [PubMed] [Google Scholar]
- Reja D, et al. , 2020. Blood lead level is associated with advanced liver fibrosis in patients with non-alcoholic fatty liver disease: A nationwide survey (NHANES 2011–2016). Ann Hepatol 19, 404–410. [DOI] [PubMed] [Google Scholar]
- Richter PA, et al. , 2009. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999–2004. International journal of environmental research and public health 6, 1930–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer J, et al. , 2012. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods 4, 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, et al. , 2013. Blood lead level association with lower body weight in NHANES 1999–2006. Toxicology and Applied Pharmacology 273, 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SDDOH, Cheyenne River Sioux Tribe: the first reservation in South Dakota to become smoke-free in all indoor public places Good & Healthy South Dakota Tribes, South Dakota Department of Health, 2015. [Google Scholar]
- Sobel M, et al. , 2021. Spatial relationship between well water arsenic and uranium in Northern Plains native lands. Environ Pollut 287, 117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solenkova NV, et al. , 2014. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J 168, 812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A, Després JP, 2000. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res 32, 526–36. [DOI] [PubMed] [Google Scholar]
- Vinceti M, et al. , 2018. Environmental Selenium and Human Health: an Update. Current Environmental Health Reports 5, 464–485. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, 2003. Cadmium carcinogenesis. Mutat Res 533, 107–20. [DOI] [PubMed] [Google Scholar]
- Wang N, et al. , 2015. Blood lead level and its association with body mass index and obesity in China - Results from SPECT-China study. Sci Rep 5, 18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DL, 1990. The nutritional relationships of manganese. J. Orthomolec. Med 5, 219–222. [Google Scholar]
- Wen X, et al. , 2021. Cadmium exposure in US adults, research based on the National Health and Nutrition Examination Survey from 1988 to 2018. Environ Sci Pollut Res Int [DOI] [PubMed]
- Weyermann M, Brenner H, 1997. Alcohol consumption and smoking habits as determinants of blood lead levels in a national population sample from Germany. Arch Environ Health 52, 233–9. [DOI] [PubMed] [Google Scholar]
- Wuttke S, et al. , First Nations Biomonitoring Initiative: National Results (2011) Assembly of First Nations, 2013. [Google Scholar]
- Yassin AS, Martonik JF, 2004. Urinary cadmium levels in the U S working population, 1988–1994. J Occup Environ Hyg 1, 324–33. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. , 2021. Influence of manganese exposure on cognitive function, plasma APP and Aβ levels in older men. J Trace Elem Med Biol 67, 126788. [DOI] [PubMed] [Google Scholar]
- Zhang LL, et al. , 2015. Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environ Res 140, 10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, et al. , 2018. Adiposity and Serum Selenium in U.S. Adults. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, et al. , 2016. Dietary intake of manganese and the risk of the metabolic syndrome in a Chinese population. Br J Nutr 116, 853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


