Abstract
Background and Objectives:
The pathologic substrates or neuroanatomic regions responsible for similarities in behavioral features seen in autism spectrum disorder and late-life dementia remain unknown. The present study examined the neuropathologic features of late-life dementia in research volunteers with and without antemortem behaviors characteristic of autism spectrum disorders.
Methods:
Antemortem cross-sectional assessment of autistic spectrum behaviors proximal to death in persons with diagnosis of mild cognitive impairment or dementia was completed using the Gilliam Autism Rating Scale, 2nd edition (GARS-2), followed by postmortem quantitative and semiquantitative neuropathologic assessment. All individuals who completed the GARS-2 prior to autopsy were included (n=56) and we note that no participants had known diagnosis of autism spectrum disorder. The GARS-2 was used as an antemortem screening tool to stratify participants into two groups: “Autism Possible/Very Likely” or “Autism Unlikely.” Data were analyzed using nonparametric statistics comparing location and scale to evaluate between-group differences in pathologic features.
Results:
Neurofibrillary tangles (NFT; p=0.028) density and tau burden (p=0.032) in the frontal region, the NFT density (p=0.048) and neuritic plaque burden (p=0.042), and the tau burden (p=0.032) of the temporal region, were significantly different in scale between groups. For measures with significant group differences, the medians of the Autism Possible/Very Likely group were roughly equal to the 75th percentile of the Autism Unlikely group (i.e., the distributions were shifted to the right).
Discussion:
This study links behaviors characteristic of autism to increased pathologic tau burden in the frontal and temporal lobes in persons with late-life dementia. Additional studies are needed to determine causal factors and treatment options for behaviors characteristic of autism behaviors in late-life dementias.
Keywords: Neuropathology, Autism, Alzheimer’s disease and related dementias
INTRODUCTION:
Behaviors characteristic of autism spectrum disorder (ASD), such as repetitive movements, impaired social awareness, and limited communication, have been observed in approximately 16% of late-onset dementia cases in individuals with no prior diagnosis of ASD.[1–3] Individuals with dementia exhibiting behaviors characteristic of ASD have clinical features different from those without such behaviors, including an earlier age of onset of cognitive impairment and lower cognitive functioning.[1] The neuropathologic substrates for ASD behaviors in late-life dementia remain poorly understood.
Increasing evidence suggests ASD and late-life dementia may involve neuroanatomic, pathologic, and/or neurochemical similarities.[4–10] Neuropathological analysis of persons with ASD suggests differences in frontal and temporal lobe brain development.[6–11] Frontotemporal dementia (FTD) has been described as a behavioral phenocopy of ASD with similarities of disturbances in social and emotional behaviors, as well as stereotypies (repetitive sounds and movements) associated with these conditions.[8,12] However, there is limited literature exploring these features across dementia types in persons with and without ASD-behaviors.
Understanding the clinicopathological relationships associated with antemortem behaviors characteristic of ASD in late-life dementia may help further define the shared neuroanatomic and or pathologic substrates for such behaviors. Such understanding may facilitate the development of shared intervention strategies designed to improve quality of life in both ASD and late-life dementia. The present study tested the hypothesis that individuals with late-onset mild cognitive impairment (MCI) or dementia and study partner (i.e., caregiver)-reported behaviors characteristic of ASD would demonstrate increased pathologic burden in frontotemporal association cortices compared to those without such behaviors, irrespective of the underlying pathological lesions.
METHODS:
Participants:
Study participants were drawn from the University of Kentucky Alzheimer’s Research Disease Center (UK-ADRC) longitudinal cohort.[13] Included individuals had antemortem diagnosis of MCI or dementia and Gilliam Autism Rating Scale, 2nd edition (GARS-2) score data. UK-ADRC standard clinical, genetic, and cognitive assessments were collected within 24 months of GARS-2 completion (n=56). No participants meeting these inclusion criteria were excluded from the present study. This study was approved by the UK Institutional Review Board.
Diagnostic criteria:
The diagnosis of MCI was determined according to the consensus guidelines adopted by the National Institute on Aging-Alzheimer’s Association Workgroup on Diagnostic Guidelines for Alzheimer’s Disease.[14] The diagnosis of dementia was based on the criteria set forth by the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV).[15] Additional details regarding etiology-specific dementia diagnostic criteria have been described elsewhere.[1] No participants had a known diagnosis of ASD.
Measures:
The GARS-2[16] was used to assess behaviors characteristic of ASD. Participants’ study partners completed the GARS-2, a 42-item assessment used to screen ASD in pediatric and adolescent populations,[16] ranking current behaviors on an ordinal scale from 0–3 (0= behavior is never observed, 1=seldom observed, 2=sometimes observed, and 3=frequently observed). GARS-2 scoring divides items into three subscales: behaviors, communication, and social interaction. Standard scores of the subscales were summed and used to determine the Autism Index Score (AIS) per standing scoring rules.[16] For this analysis, we followed the established ranges identified by the GARS-2 and classified participants as “Autism Possible/Very Likely” (AIS ≥ 70) or “Autism Unlikely” (AIS < 70). At the time of initial GARS-2 administration in 2014, there were no validated ASD assessments for older adults with cognitive impairment. The GARS-2 was selected due to the breadth of behaviors assessed.
Clinical Dementia Rating (CDR) Sum of Boxes[17,18] was used to characterize global cognition. The CDR uses semi-structured interviews by trained clinicians with primary informants (e.g., study partners/caregivers) to determine severity of impairment in six cognitive domains.[17,18] Following the interview, the clinician rates each domain on a 5-point scale indicating the level of impairment (0=none, 0.5=questionable, 1=mild, 2=moderate, 3=severe) based on standard rules.[18] Ratings from all domains are summed to create the CDR Sum of Boxes score, which has a range of 0 to 18, with larger values indicating increased impairment. CDR was administered to participants annually at UK-ADRC visits; for this study, we report on the CDR score obtained closest to GARS-2 completion.
The Neuropsychiatric Inventory (NPI) is an assessment of behavioral and psychiatric symptoms of dementia with high reliability and validity.[19–21] A trained interviewer asked study partners to indicate the presence of 12 behavioral and psychiatric domains. Each domain was rated as present or absent (yes/no). When study partners indicated the presence of a symptom, they were then asked to rate the severity of the symptom as mild, moderate, or severe. NPI was administered to participants annually at UK-ADRC visits; for this study, we report on the NPI score obtained closest to GARS-2 completion.
Pathological Analysis:
Comprehensive neuropathologic evaluation included assessment of common late-life dementia pathologies using both semi-quantitative rating scales and quantitative digital measures of regional pathological features. As previously described [22–24], at least 24 samples were taken from each brain. Areas for sample extraction included the middle frontal gyrus (area 9), superior and middle temporal gyri (areas 21 and 22), inferior parietal lobule (areas 39 and 40) and occipital lobe including primary visual area (areas 17 and 18). Amyloid plaques were separated into diffuse plaques (plaques without neurites) and neuritic Aβ plaques (NPs) in each region. An arithmetic mean was calculated from counts of diffuse plaques (number / 2.35 mm2), NPs (number / 2.35 mm2), and neurofibrillary tangle (NFTs; number / 0.586 mm2) for each region in the 5 fields that were subjectively determined to have the greatest involvement.[23] Braak NFT staging following established criteria [25] was used to characterize the extent of NFT distribution. Lewy body pathology was assessed per region in the brainstem, limbic system and amygdala, or neocortex. Chronic cerebrovascular disease (CVD) severity was graded by a neuropathologist (PTN), which incorporated assessment of cerebral amyloid angiopathy, arteriolosclerosis, atherosclerosis, and total infarcts to form a composite measure similar to that described previously.[26] Presence (= 1) or absence (= 0) of lacunar, gross, and micro infarcts, arteriolosclerosis, and cerebral amyloid angiopathy were summed for a global measure of CVD (range 0–5). CVD was further assessed by region as listed below (with a range of 0–5 for each region). Presence or absence of limbic-predominant age-related TDP-43 encephalopathy (LATE) [27] in the hippocampus (i.e., Stage 1 LATE) was also assessed. Clinical FTD was absent in our sample (as in other community-based cohorts)[28]. Digitally quantified AD-type pathologies, including amyloid burden, amyloid density, NFT density, neuritic plaques (NP) burden, and tau burden were assessed by region in the neocortex: frontal (Brodmann area [BA] 9), occipital (BAs 17/18/19), inferior parietal (BA 39), and superior and middle temporal (BAs 21/22) lobes.[22,29] The neuropathologist was blinded to ASD status during assessment and reporting of neuropathology results.
Immunohistochemical and quantitative digital pathological analysis:
Immunohistochemical stains were performed using the PHF-1 antibody and the combination of Aβ stains as described previously,[22,30] and sections were counterstained with hematoxylin. For digital pathologic assessments, slides were loaded into an Aperio/Leica ScanScope, scanned at 40X magnification via the semi-automated method, and the images stored on a dedicated server. Immunoreactive staining was quantified as previously described in detail.[22,30] Briefly, the readouts were Amyloid burden (percentage of area with Aβ immunoreactivity); Amyloid count (number of discrete Aβ plaques in highlighted area); Tau burden (% highlighted area with PHF-1 immunoreactivity); NFT density (number of discrete NFTs in highlighted area); and NP burden (number of counted neuritic plaques in highlighted area).
Statistical analysis:
Participant demographics were compared between groups (“Autism Possible/Very Likely” [AIS ≥ 70] vs. “Autism Unlikely” [AIS < 70]) using two-sample t-tests for interval level variables and chi-squared tests for categorical measures. Neuropathologic features including Braak stage, global presence of Lewy body pathology, chronic cerebrovascular disease severity, presence of LATE, and regional cerebrovascular disease were compared between groups using chi-square or Fisher Exact tests. Global CVD burden was analyzed using a t-test. Location and scale for regional, quantitative neuropathologic features including amyloid burden, amyloid density, NFT density, NP burden, and tau burden were compared between groups using nonparametric statistics (Wilcoxon, Ansari-Bradley) because these variables were not normally distributed. Computations were done using PC SAS 9.4.
RESULTS:
Clinical descriptions of the cohort are provided in Table 1. The sample included 16 participants in the Autism Possible/Very Likely group and 40 participants in the Autism Unlikely group. Between-group analyses (Autism Possible/Very Likely versus Autism Unlikely) demonstrated no significant differences in age, education, sex, clinical diagnosis, or APOE4 carrier status in this sample (see Table 1). ASD symptoms were more frequent in severely impaired participants (p<0.001) determined by CDR Sum of Boxes scale collected closest to the time of the GARS-2 administration, as it has been suggested by previous works. [1,2] Participants in the Autism Possible/Very Likely group presented with greater overall severity of neuropsychiatric behaviors on the NPI (p=0.019) compared to those in Autism Unlikely group. The two groups did not significantly differ in length of time between assessments, GARS, and autopsy report.
Table 1:
Participant demographics by autism-behavior status
| Characteristic | Autism Unlikely (GARS AIS <70) Mean (SD) |
Autism Possible/ Very Likely (GARS AIS ≥ 70) Mean (SD) |
P value |
|---|---|---|---|
| N | 40 | 16 | |
| Sex: M/F | 19/21 | 8/8 | 0.87 |
| Age at death | 83.7 (7.9) | 79.2 (10.1) | 0.084 |
| Years of education | 17.2 (3.0) | 16.9 (3.9) | 0.76 |
| APOE4 carrier: No/Yes | 22/7 | 6/9 | 0.28 |
| GARS-2 AIS | 53.85 (9.04) | 80.50 (7.50) | N/A |
| CDR Sum of Boxes | 6.43 (4.92) | 11.53 (5.97) | 0.0017 |
| Neuropsychiatric Inventory Severity | 4.55 (4.91) | 9.63 (7.87) | 0.019 |
| Time between tests to GARS (n): | 0.3447 | ||
| 0–12 months | 22 | 11 | − |
| 13–24 months | 18 | 5 | − |
| Time between tests to autopsy (n): | 0.0538 | ||
| 0–12 months | 13 | 6 | − |
| 13–24 months | 7 | 6 | − |
| 24+ months | 20 | 4 | − |
| Clinical Diagnosis (n): | |||
| AD | 23 | 10 | − |
| VD | 2 | 1 | − |
| DLB | 2 | 2 | − |
| FTD | 2 | 2 | − |
| AD/VD | 3 | 1 | − |
| MCI/VCI | 8 | 0 | − |
Approximately 80% of cases had pathologic characteristics predominately of AD, with the remaining 20% of individuals demonstrating pathologic features consistent with LATE (16%), cerebrovascular (2%), or Lewy body disease (2%). No cases had frontotemporal lobar degeneration (FTLD) as a primary or comorbid pathology. There were no significant differences between groups in global measures of neuropathology (see Table 2).
Table 2:
Description of neuropathology by autism-behavior status
| Pathology | Autism Unlikely (GARS AIS <70) |
Autism Possible/ Very Likely (GARS AIS ≥ 70) |
P value |
|---|---|---|---|
| Braak Stage (rating 3–6) N (%) | 32 (82) | 12 (80) | 0.86 |
| Braak Stage 3 | 2 | 0 | − |
| Braak Stage 4 | 2 | 2 | − |
| Braak Stage 5 | 14 | 1 | − |
| Braak Stage 6 | 14 | 9 | − |
| Neocortical/Diffuse LBs | 6 | 2 | 0.59 |
| Limbic predominant & amygdala LBs | 15 | 6 | 0.61 |
| Brainstem predominant LBs | 2 | 0 | 0.50 |
| LATE N (%) | 19 (48) | 7 (44) | 0.80 |
| Vascular Severity Composite (> 0) N (%) |
32 (80) | 9 (56) | 0.070 |
| Global CVD (SD) | 2.37 (2.07) | 1.25 (1.44) | 0.052 |
| CVD: N present/absent | |||
| Frontal | 22/18 | 6/10 | 0.24 |
| Occipital | 14/26 | 3/13 | 0.79 |
| Parietal | 23/17 | 5/11 | 0.076 |
| Temporal | 23/17 | 3/13 | 0.0086 |
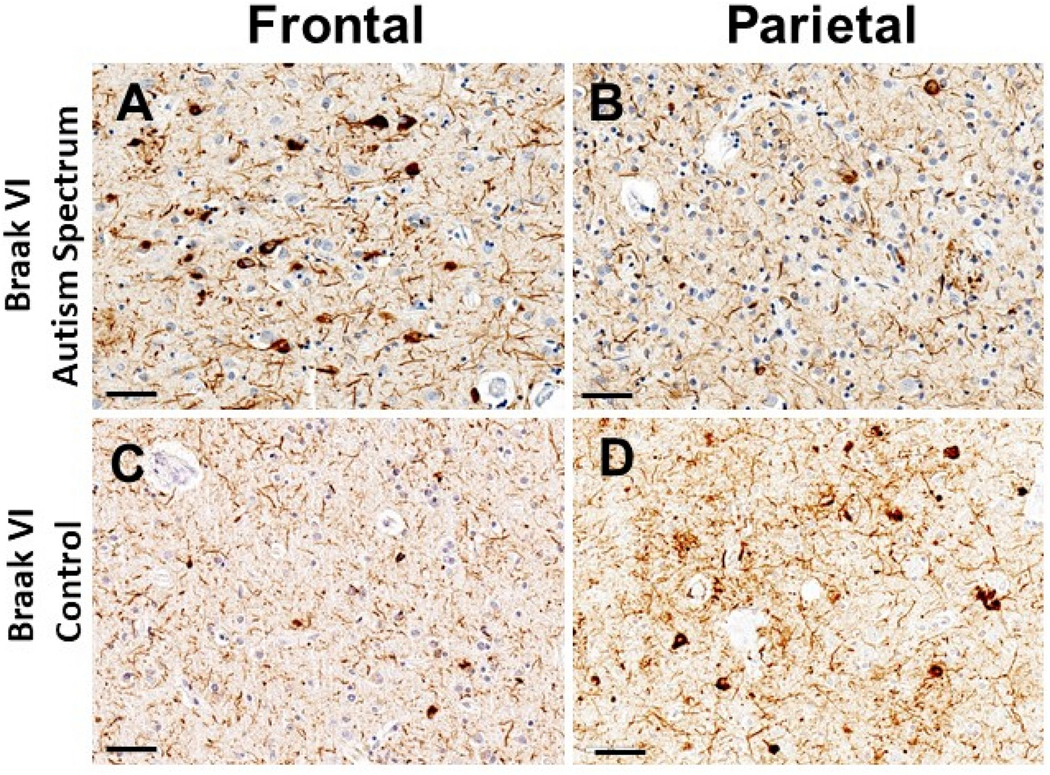
Additional nonparametric analyses between groups were conducted based on the regional distribution of pathologic features. Figure 1 illustrates pathology present in a subject with behaviors characteristic of ASD compared to control. As illustrated in Table 3, the two groups differed significantly on scale for 6 of the 20 endpoints, with significance clustered in the frontal region (NFT density p=0.028 and tau burden p= 0.032) and the temporal region (NFT density p=0.048, NP burden 0.042, tau burden p=0.032).). The medians of all significant variables of the Autism Possible/Very Likely group were roughly equal to the 75th percentile of the Autism Unlikely group. Overall, the Autism Possible/Very Likely group had a significantly wider interquartile range in significant variables, indicating increased variability, than the Autism Unlikely group.
Figure 1.
Figure Legend. Photomicrographs show p-Tau immunoreactive pathologic features in cortical regions of a person who died at age 72 years following new-onset autism spectrum symptoms (A,B), and a control subject, age 76 years at death (C,D). Both individuals had the APOE e4 risk allele, were clinically diagnosed with Probable Alzheimer’s disease, and subsequently had autopsy-confirmed Braak NFT Stage VI pathologic changes. For the individual with autism spectrum symptoms, the tau tangle pathology was more severe in the frontal (Panel A; Brodmann area 9) than parietal (Panel B; Brodmann area 39) cortical region. By contrast, in the individual lacking autism spectrum symptoms, the reverse was true: tau tangle pathology was more modest in the frontal cortex (Panel C) in comparison to that in the parietal cortex (Panel D). Notably, a large proportion of p-Tau-immunoreactive staining in all sections was in neuropil threads rather than intracytoplasmic tangles. Immunohistochemical stains were performed using the PHF-1 antibody (a gift from Dr. Peter Davies), and sections were counterstained with hematoxylin. Scale bars = 50microns.
Table 3:
Regional analysis of neuropathology by autism-behavior status.
| Autism Unlikely | Autism Possible/ Very Likely |
P Value | |||
|---|---|---|---|---|---|
| Region | Burden | Median (Q1–Q3) | Median (Q1 – Q3) | Loc. Scale | |
|
| |||||
| Frontal | Amyloid burden | 95.58 (50.36 − 129.16) | 94.88 (43.88 − 130.09) | 0.93 | 0.69 |
| Amyloid density | 72.84 (45.68 − 105.22) | 85.48 (34.09 − 109.24) | 0.60 | 0.77 | |
| NFT density | 3.24 (0.43 − 22.93) | 21.79 (0.19 − 36.89) | 0.58 | 0.028 | |
| NP burden | 13.39 (1.26 − 89.79) | 84.06 (1.33 − 128.78) | 0.55 | 0.17 | |
| Tau burden | 14.47 (2.50 − 89.79) | 76.91 (2.73 − 137.54) | 0.29 | 0.032 | |
|
| |||||
| Occipital | Amyloid burden | 30.95 (13.20 − 46.73) | 44.06 (29.18 − 71.96) | 0.04 | 0.74 |
| Amyloid density | 37.09 (15.62 − 57.55) | 50.45 (30.31 − 74.58) | 0.09 | 0.66 | |
| NFT density | 1.57 (0.03 − 12.91) | 2.65 (0.18 − 26.78) | 0.35 | 0.65 | |
| NP burden | 8.20 (0.75 − 80.60) | 18.95 (1.10 − 110.72) | 0.62 | 0.35 | |
| Tau burden | 8.66 (1.30 − 67.22) | 30.66 (1.91 − 115.83) | 0.36 | 0.47 | |
|
| |||||
| Parietal | Amyloid burden | 82.05 (26.46 − 111.00) | 75.55 (36.05 − 99.41) | 0.74 | 0.13 |
| Amyloid density | 67.66 (24.60 − 98.04) | 56.83 (32.28 − 84.16) | 0.93 | 0.042 | |
| NFT density | 5.40 (0.73 − 19.21) | 14.42 (0.48 − 39.60) | 0.22 | 0.46 | |
| NP burden | 23.81 (5.22 − 88.59) | 61.06 (1.94 − 123.37) | 0.35 | 0.20 | |
| Tau burden | 24.41 (5.59 − 92.73) | 43.82 (3.38 − 144.73) | 0.37 | 0.28 | |
|
| |||||
| Temporal | Amyloid burden | 70.31 (39.08 − 99.34) | 78.19 (39.12 − 111.25) | 0.68 | 0.51 |
| Amyloid density | 51.65 (28.49 − 88.39) | 61.59 (34.08 − 91.09) | 0.65 | 0.51 | |
| NFT density | 15.14 (1.70 − 36.15) | 32.09 (1.05 − 57.44) | 0.30 | 0.048 | |
| NP burden | 78.69 (11.25 − 95.76) | 91.06 (3.43 − 136.09) | 0.60 | 0.042 | |
| Tau burden | 55.36 (10.23 − 96.61) | 93.90 (5.56 − 137.75) | 0.39 | 0.032 | |
Values represent the pixel and lesion counts by pathologic type per region. P-values calculated by comparing the location (Loc.) using Wilcoxon statistic (as illustrated by the median) and scale by using the Ansari-Bradley statistic (as illustrated by the range from Q1 to Q3, the middle 50% of the measurements, where Q1 is the lower quartile while Q3 is the upper quartile). Red indicates statistical significance.
DISCUSSION:
This study reports a positive relationship between tau pathology in the frontal and temporal regions and behaviors characteristic of ASD in later life dementia. CVD burden was also found to be higher in temporal regions for those with behaviors characteristic of ASD. Other pathologic features or regions of neuroanatomic involvement were not associated with ASD behaviors in this sample of community-dwelling participants with dementia. While use of the GARS-2 in this study was exploratory, these findings support several hypotheses regarding both neuroanatomic and potential neuropathologic involvement that link ASD and late-life dementia.
Growing evidence identifies various abnormalities in the neuroanatomical development of ASD, especially in the frontotemporal regions.[6,31–33] Previous studies have demonstrated overlap in behaviors and anatomical involvement in ASD and FTD.[2,8,12] Frontal and temporal lobes are associated with behavior, language, emotional regulation, and social awareness, all of which are commonly afflicted in both conditions.[8,34–37] It is noteworthy that no participants in our sample had neuropathological evidence of FTD. It is not surprising that FTLD pathology, the neuropathological substrate of FTD, which has a ∼1:750 lifetime risk, [28] is not a major contributor to such behaviors in a community-based cohort. Instead, AD-related tauopathy in the frontal and temporal cortices and CVD pathology in the temporal cortex were significantly higher in the Autism Possible/Very Likely group, underscoring that behaviors characteristic of ASD in dementia are not confined to FTD. Findings presented here demonstrate increased pathology in frontotemporal regions, supporting the hypothesis that neuropathologic changes in these regions may underlie ASD behaviors irrespective of distinct pathologic lesion type. It is possible that those who exhibit antemortem ASD behaviors have a more severe form of dementia than those who do not exhibit such behavior, as suggested previously.[38] Future analyses of anatomical substrates of these behavior may strengthen understanding of these relationships.
Interestingly, our analysis further defined localization by pathologic type and tau-related pathologies were found at increased levels in the ASD group in both frontal and temporal regions, as opposed to CVD that was found to only be increased in temporal but not in frontal cortices. CVD pathology was identified as trending toward significance in parietal and global measures in this sample. Gross pathological distribution suggests that, in addition to neuroanatomic localization of pathology, the specific type of pathology may play an important role in the development of ASD behaviors in late-life dementia. Recent evidence suggests that tauopathic changes may be associated with ASD.[4,39] A variety of neuronal insults have been shown to increase tauopathy in young individuals, including infectious, neoplastic, and trauma-related conditions, as well as a number of congenital conditions, such as myotonic dystrophy, lipofuscinosis, and Perry syndrome, many of which demonstrate ASD characteristics.[40] These data suggest that there may be shared mechanisms of neurodegeneration and behavioral phenotype between ASD in children and late-life tauopathic lesions associated with ASD behaviors. This may possibly be mediated through convergent pathways of cellular dysfunction, such as cytoskeletal integrity,[41] neurotransmitter dysregulation,[42] interference with synaptic function, [43,44] and/or other neuronal abnormalities. [45–47]
Generalizability of the present findings is limited due to the small sample size, the highly educated and predominantly white cohort, and biases inherent in only studying those agreeing to autopsy.[48] Use of the GARS-2 was exploratory as this assessment has yet to be validated in an older adult population. Additional analyses of validity are needed in future studies using the GARS-2 in older adults. FTD and dementia with Lewy bodies (DLB) present with challenging behavioral phenotypes that have been linked to behaviors characteristic of ASD; however, our analysis was based on a community-based cohort, which naturally limits the numbers of FTD and pure DLB cases. As such, the present study may have simply been underpowered to detect associations. Additionally, we did not attempt to adjust analyses for confounding, for two reasons: 1) the small sample size; and 2) given a confounding variable is a shared cause of the outcome (here AIS score) and the exposure [49] (here neuropathology; i.e., we hypothesized that AIS score is driven by neuropathology), other potential causes of AIS score (e.g., dementia severity, home environment, relationship with caregiver, other health conditions) are either causal intermediaries between neuropathology and AIS score (i.e., dementia severity), not causes of neuropathology (i.e., home environment, relationship with caregiver), or not well understood (i.e., other health conditions in late-life that cause behaviors characteristic of ASD are not well documented).
The strengths of the current study include the use of a community rather than clinic-based sample, reducing the bias that can be associated with studies from selected clinics and subspecialists, suggesting a broader generalizability of our findings in relation to the population burden of dementia and the relationship with behaviors characteristic of ASD. Another major strength of the present study is the use of an extremely well-characterized cohort who have undergone longitudinal assessment and have agreed to autopsy a priori, thereby possibly limiting the extent of autopsy bias in our study.
In conclusion, these data report a novel link behaviors characteristic of ASD to increased levels of neuropathologic tau and CVD in the frontotemporal regions in subjects with late-life dementia. Identification of similar behavioral sequalae between neurodevelopmental and neurodegenerative conditions, further characterized by postmortem neuropathological patterns, may provide insights that will allow the development of innovative treatment approaches for conditions at both ends of the life spectrum. Further work toward understanding potential links between non-FTD tau-mediated neurodegeneration and ASD behavior is warranted.
Acknowledgements
This study utilized data provided by University of Kentucky Alzheimer’s Disease Research Center longitudinal cohort participants. Researchers acknowledge and thank all participants. Funding for the longitudinal cohort is provided by NIH/NIA under Grant P30 AG072946; and the first author is funded by the NIH/NIA under Grant T32 AG057461: “Training in Translational Research in Alzheimer’s and Related Dementias (TRIAD).”
Footnotes
Competing Interests
The authors have no conflicts of interest to report.
References
- 1.Rhodus EK, Barber J, Abner EL, et al. Behaviors Characteristic of Autism Spectrum Disorder in a Geriatric Cohort With Mild Cognitive Impairment or Early Dementia. Alzheimer Dis Assoc Disord. 2020;34(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodus EK, Barber J, Abner EL, Bardach SH, Gibson A, Jicha GA. Comparison of behaviors characteristic of autism spectrum disorder behaviors and behavioral and psychiatric symptoms of dementia. Aging & mental health. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caselli RJ, Langlais BT, Dueck AC, Locke DEC, Woodruff BK. Subjective Cognitive Impairment and the Broad Autism Phenotype. Alzheimer Disease & Associated Disorders. 2018;32(4):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai C, Chang C-W, Yu G-Q, et al. Tau Reduction Prevents Key Features of Autism in Mouse Models. Neuron. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol DK, Maloney B, Westmark CJ, Lahiri DK. Novel Contribution of Secreted Amyloid-β Precursor Protein to White Matter Brain Enlargement in Autism Spectrum Disorder. Front Psychiatry. 2019;10:165–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ecker C, Schmeisser MJ, Loth E, Murphy DG. Neuroanatomy and Neuropathology of Autism Spectrum Disorder in Humans. Adv Anat Embryol Cell Biol. 2017;224:27–48. [DOI] [PubMed] [Google Scholar]
- 7.Rossignol DA, Frye RE. The Use of Medications Approved for Alzheimer’s Disease in Autism Spectrum Disorder: A Systematic Review. Frontiers in Pediatrics. 2014;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midorikawa A, Kawamura M. The Relationship between Subclinical Asperger’s Syndrome and Frontotemporal Lobar Degeneration. Dement Geriatr Cogn Dis Extra. 2012;2:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eissa N, Sadeq A, Sasse A, Sadek B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Frontiers in Pharmacology. 2020;11(886). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chez MG, Aimonovitch M, Buchanan T, Mrazek S, Tremb RJ. Treating autistic spectrum disorders in children: utility of the cholinesterase inhibitor rivastigmine tartrate. Journal of child neurology. 2004;19(3):165–169. [PubMed] [Google Scholar]
- 11.Raznahan A, Toro R, Daly E, et al. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cerebral cortex (New York, NY : 1991). 2010;20(6):1332–1340. [DOI] [PubMed] [Google Scholar]
- 12.Johnen A, Bertoux M. Psychological and Cognitive Markers of Behavioral Variant Frontotemporal Dementia–A Clinical Neuropsychologist’s View on Diagnostic Criteria and Beyond. Frontiers in Neurology. 2019;10(594). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt FA, Nelson PT, Abner E, et al. University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Current Alzheimer research. 2012;9(6):724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment - Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Vol 2562004. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-4. 1994. [Google Scholar]
- 16.Gilliam JW. GARS-2: Gilliam Autism Rating Scale–Second Edition. . Austin, TX: PRO-ED; 2006. [Google Scholar]
- 17.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. The British journal of psychiatry : the journal of mental science. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DK, Watts AS, Chapin BA, Anderson R, Burns JM. Neuropsychiatric profiles in dementia. Alzheimer Dis Assoc Disord. 2011;25(4):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord. 2012;5(6):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–16. [DOI] [PubMed] [Google Scholar]
- 22.Neltner JH, Abner EL, Schmitt FA, et al. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. J Neuropathol Exp Neurol. 2012;71(12):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson PT, Abner EL, Schmitt FA, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(7):774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith VD, Bachstetter AD, Ighodaro E, et al. Overlapping but distinct TDP-43 and tau pathologic patterns in aged hippocampi. Brain pathology (Zurich, Switzerland). 2018;28(2):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 26.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62(1):59–66. [DOI] [PubMed] [Google Scholar]
- 27.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain : a journal of neurology. 2019;142(6):1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coyle-Gilchrist IT, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abner EL, Neltner JH, Jicha GA, et al. Diffuse Amyloid-β Plaques, Neurofibrillary Tangles, and the Impact of APOE in Elderly Persons’ Brains Lacking Neuritic Amyloid Plaques. J Alzheimers Dis. 2018;64(4):1307–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsumata Y, Fardo DW, Bachstetter AD, et al. Alzheimer Disease Pathology-Associated Polymorphism in a Complex Variable Number of Tandem Repeat Region Within the MUC6 Gene, Near the AP2A2 Gene. J Neuropathol Exp Neurol. 2020;79(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margari L, De Giacomo A, Craig F, et al. Frontal lobe metabolic alterations in autism spectrum disorder: a (1)H-magnetic resonance spectroscopy study. Neuropsychiatr Dis Treat. 2018;14:1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solso S, Xu R, Proudfoot J, et al. Diffusion Tensor Imaging Provides Evidence of Possible Axonal Overconnectivity in Frontal Lobes in Autism Spectrum Disorder Toddlers. Biol Psychiatry. 2016;79(8):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira AM, Campos BM, Coan AC, et al. Differences in Cortical Structure and Functional MRI Connectivity in High Functioning Autism. Front Neurol. 2018;9:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakuta S, Hashimoto M, Ikeda M, et al. Clinical features of behavioral symptoms in patients with semantic dementia: Does semantic dementia cause autistic traits? PLoS One. 2021;16(2):e0247184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigenobu K, Ikeda M, Fukuhara R, et al. The Stereotypy Rating Inventory for frontotemporal lobar degeneration. Psychiatry research. 2002;110(2):175–187. [DOI] [PubMed] [Google Scholar]
- 36.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yildirim E, Soncu Buyukiscan E, Demirtas-Tatlidede A, Bilgiç B, Gurvit H. An investigation of affective theory of mind ability and its relation to neuropsychological functions in Alzheimer’s disease. Journal of Neuropsychology. 2020;14(3):399–415. [DOI] [PubMed] [Google Scholar]
- 38.Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci. 2005;236(1–2):43–48. [DOI] [PubMed] [Google Scholar]
- 39.Grigg I, Ivashko-Pachima Y, Hait TA, et al. Tauopathy in the young autistic brain: novel biomarker and therapeutic target. Translational psychiatry. 2020;10(1):228–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madaan P, Jauhari P, Luhar ZM, Chakrabarty B, Gulati S. Autism, Epilepsy, and Neuroregression: Photosensitivity on Electroencephalography Solved the Riddle. Clin EEG Neurosci. 2020;51(6):399–402. [DOI] [PubMed] [Google Scholar]
- 41.Chang Q, Yang H, Wang M, Wei H, Hu F. Role of Microtubule-Associated Protein in Autism Spectrum Disorder. Neurosci Bull. 2018;34(6):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho Pereira A, Violante IR, Mouga S, Oliveira G, Castelo-Branco M. Medial Frontal Lobe Neurochemistry in Autism Spectrum Disorder is Marked by Reduced N-Acetylaspartate and Unchanged Gamma-Aminobutyric Acid and Glutamate + Glutamine Levels. J Autism Dev Disord. 2018;48(5):1467–1482. [DOI] [PubMed] [Google Scholar]
- 43.Guang S, Pang N, Deng X, et al. Synaptopathology Involved in Autism Spectrum Disorder. Frontiers in cellular neuroscience. 2018;12:470–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadak MT, Cetin I, Tarakçıoğlu MC, Özer ÖF, Kaçar S, Çimen B. Low Serum Level α-Synuclein and Tau Protein in Autism Spectrum Disorder Compared to Controls. Neuropediatrics. 2015;46(6):410–415. [DOI] [PubMed] [Google Scholar]
- 45.Hashemi E, Ariza J, Rogers H, Noctor SC, Martínez-Cerdeño V. The Number of Parvalbumin-Expressing Interneurons Is Decreased in the Prefrontal Cortex in Autism. Cerebral cortex (New York, NY : 1991). 2017;27(3):1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courchesne E, Mouton PR, Calhoun ME, et al. Neuron number and size in prefrontal cortex of children with autism. Jama. 2011;306(18):2001–2010. [DOI] [PubMed] [Google Scholar]
- 47.Zahra A, Jiang J, Chen Y, Long C, Yang L. Memantine rescues prenatal citalopram exposure-induced striatal and social abnormalities in mice. Experimental Neurology. 2018;307:145–154. [DOI] [PubMed] [Google Scholar]
- 48.Tsuang D, Simpson KL, Li G, et al. Evaluation of selection bias in an incident-based dementia autopsy case series. Alzheimer disease and associated disorders. 2005;19(2):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernán MA. Confounding – Structure. In: Wiley StatsRef: Statistics Reference Online.1–7. [Google Scholar]