Abstract
We present a rare case of combined hepatocellular carcinoma-cholangiocarcinoma in a woman with a history of univentricular congenital heart disease requiring multiple corrective operations including Fontan procedure. During workup for elevated alpha fetal protein, a right hepatic lobe lesion was identified with biopsy showing poorly differentiated hepatocellular carcinoma. She underwent successful segment 5 liver resection. Final pathology demonstrated combined hepatocellular carcinoma-cholangiocarcinoma. She was treated with gemcitabine/oxaliplatin adjuvant chemotherapy and had no evidence of recurrent disease at her 12-month follow-up. To our knowledge, this is the first case reported in of successful treatment of this rare malignancy in the setting of Fontan-associated liver disease and highlights the importance of a robust screening protocol in this patient population. Semiannual screening for the development of primary liver malignancy should start by 10 years post-Fontan and continue until heart–liver transplantation may be performed. It is important to note that cirrhosis is not a pre-requisite for the development of hepatocellular carcinoma or cholangiocarcinoma in these patients.
Keywords: Gastrointestinal surgery, Cardiothoracic surgery, Transplantation, Surgical oncology, Hepatic cancer
Background
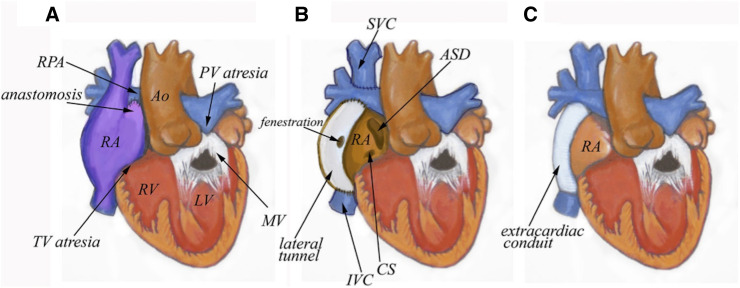
The Fontan procedure, first performed in 1968, is the surgical palliation of congenital univentricular heart disease.1 The operation directs systemic venous return from the inferior vena cava into the pulmonary circulation, achieving pulmonary circulatory filling in the absence of ventricular propulsion, as illustrated in figure 1. However, this rearrangement, known as Fontan physiology, creates iatrogenic end-organ disease due to chronic congestive circulation.2 The sequelae of Fontan physiology is becoming a growing area of interest as Fontan patients survive into adulthood. The mean age of Fontan patients is estimated to be 23 by 2025, with a population of 70 000, and is projected to double over the next decade.2
Figure 1.
Major Fontan subtypes for the treatment of congenital univentricular heart disease. (A) First iterations of the procedure utilised the right atrium to restore flow between the superior and inferior vena cava and pulmonary artery. (B) Lateral tunnel Fontan included a small fenestration in addition to connecting the superior vena cava directly to the right pulmonary artery (Glenn shunt). (C) Extracardiac Fontan connects only the inferior vena cava to the right pulmonary artery, bypassing the heart. author: Leigh Reardon. (RPA, Right Pulmonary Artery; PV, Pulmonic Valve; RA, Right Atrium; RV, Right Ventricle; LV, Left Ventricle; MV, Mitral Valve; IVC, Inferior Vena Cava; CS, Coronary Sinus; SVC, Superior Vena Cava; ASD, Atrial-Septal Defect; TV, Tricuspid Valve)
Fontan-associated liver disease (FALD) is becoming a well-recognised entity due to its rising prevalence and significance in overall mortality and morbidity in this patient population. Elevated central venous pressure and diminished cardiac output lead to sinusoidal dilation, edema and centrilobular hepatocyte hypoxia. These changes lead to hepatic fibrosis, a common feature found in Fontan patients.3–10 As in non-FALD patients, these changes ultimately may lead to cirrhosis and hepatocellular carcinoma (HCC). HCC is now recognised as an uncommon complication of FALD from a number of case reports and series with a reported incidence of 1.5%–5%.11 12 Diagnosis is predominantly based on imaging criteria,13 14 with biopsy reserved for indeterminant lesions to prevent the possibility of seeding.14
Cholangiocarcinoma is an even rarer complication of FALD. Ohuchi et al published a series of 14 liver cancers with 2 cholangiocarcinomas identified. However, both patients died quickly after their diagnosis, with one patient dying after surgical resection. To date, there has been no reported case of FALD associated combined HCC-cholangiocarcinoma (cHCC-CCA) or its successful treatment in North America.
Case presentation
A woman presented to the hepatobiliary surgery clinic after abdominal ultrasound revealed an asymptomatic right hepatic lobe lesion. Her medical history was significant for univentricular congenital heart disease, for which she underwent multiple corrective cardiac operations. Her first surgery was a pulmonary artery banding. This was followed by bidirectional Glenn shunt and a fenestrated extracardiac Fontan procedure the following year. She had normal oxygen saturations during her follow-up, suggesting closure of fenestrations at some point. She has been followed by the congenital cardiology team since childhood. A 24-hour Holter monitor study demonstrated normal conduction. Echocardiogram demonstrated normal single right ventricular function, moderate tricuspid regurgitation, and widely patent Glenn, right pulmonary artery, and Fontan conduits. Cardiac positron emission tomography demonstrated adequate exercise capacity with myocardial volume oxygen consumption of 29, which was 89% of predicted value, and cardiac MRI once again showed widely patent shunts with a normal sized liver without lesions. At her most recent cardiology follow-up prior to her presentation, she reported having good exercise capacity and functional status. There were no cardiopulmonary or abdominal symptoms.
Investigations
The patient had a history of thrombocytopaenia and underwent several abdominal ultrasound examinations. An ultrasound 4 years prior showed coarse hepatic parenchyma suggesting underlying liver disease without focal lesions. Elastography at that time estimated moderate fibrosis. Screening bloodwork revealed an elevated-fetoprotein (AFP 399 ng/mL) and repeat ultrasound revealed a new 27 mm right hepatic lobe lesion with otherwise normal aspartate aminotransferase (22 U/L), alanine aminotransferase (16 U/L), total bilirubin (1.0 mg/dL), and alkaline phosphatase (54 U/L). Platelet count was 85×109/L. Elastography reported shear wave liver stiffness of 2.04 m/s (12.6 kPa) suggesting compensated, advanced chronic liver disease. MRI with eovist contrast demonstrated a multiloculated segment five hepatic lesion with enhancing walls and septa with central areas of non-enhancement concerning for HCC. An ultrasound-guided biopsy showed poorly differentiated HCC. A CT scan of the chest was obtained and showed no evidence of intrathoracic metastatic disease. She was referred for surgical resection evaluation. Given her otherwise acceptable cardiac and hepatic function and the favourable anatomic location of the lesion, a partial right hepatectomy was recommended and the patient agreed to proceed.
Treatment
The patient was taken to the operating room, placed under endotracheal general anaesthesia with an epidural for postoperative pain control. Exploratory laparotomy was performed via midline incision. The mass was identified and demonstrated an exophytic component in the inferior aspect of the liver abutting the gallbladder. Intraoperative ultrasound showed no additional lesions. A near total segment 5 resection was completed with 15 mm margin circumferentially around the tumour. Particular care was given to haemostasis during the resection due to the congested nature of the liver. The patient was extubated immediately postoperatively and had an uncomplicated postoperative course.
Postoperatively, she had close monitoring of her central venous pressure via the central venous catheter, as well as air bubble filters on all intravenous lines due to the presence of right-to-left shunting and risk of air embolism. She was discharged 6 days after her operation.
Outcome and follow-up
Final pathology from her resection demonstrated periportal and bridging fibrosis with patchy sinusoidal dilation within parenchymal samples. The mass was determined to be a cHCC-CCA measuring 27×25×21 mm, stage T2Nx. There was no steatosis, cholestasis, alpha-1 antitrypsin globules or iron deposition. Gallbladder was unremarkable pathologically. During this time, genetic analysis returned with loss of BRCA2 copies, significant for mutation. In light of this combined carcinoma and genetic diagnosis, she was referred to oncology, who recommended gemcitabine and cisplatin adjuvant chemotherapy for 6 months. This was revised to gemcitabine and oxaliplatin as the patient wished to avoid any potential long-term hearing loss associated with cisplatin. She underwent eight cycles of adjuvant chemotherapy complicated by repeated episodes of thrombocytopaenia necessitating delays in treatment, tapering of gemcitabine and complete cessation of oxaliplatin.
CT chest and MRI abdomen performed in this past year showed no evidence of recurrent or metastatic disease at 12-month follow-up. She is currently doing well and consulting a breast surgeon for possible bilateral prophylactic mastectomies after screening mammography and MRI later this year.
Discussion
FALD is associated with congestive hepatopathy, leading to fibrosis, cirrhosis and increased risk of primary liver malignancy.1 The proposed pathophysiology is related to the dual blood supply of the liver, with the hepatic vein in direct continuity with the pulmonary artery, leading to increased susceptibility of hepatic parenchymal injury from decreased portal vein oxygenation and increased intrahepatic congestion, ultimately leading to ischaemic hepatitis, characterised by centrilobular necrosis around the hepatic veins. With each injury cycle, increased fibrosis and cirrhosis occurs, leading to FALD presentation.2–5 15
While the risk of HCC is now acknowledged as a complication of chronic hepatic congestion in the context of failing Fontan physiology,16 17 this damage profile may also lead to other rare primary liver cancers such as CCA and cHCC-CCA.18 CCA is a rare cancer with increased prevalence in certain areas around the world, usually secondary to chronic cholangitis, liver flukes, viral hepatitis or aflatoxin exposures. The cellular origin of intrahepatic CCA remains controversial given mixed tumour types that suggest a possible common hepatic progenitor.19 Of note, germline DNA mismatch repair deficiency (Lynch syndrome) is associated with CCA as well. DNA damage repair mutations were significantly correlated with higher tumour mutation burden regardless of primary liver cancer subtypes, including CCA, with increased tumour susceptibility when DNA damage repair genes exhibit oncogenic mutations.20 21 However, no direct link has been established between BRCA2 mutations and the risk of developing HCC or CCA.
To our knowledge, this is the first North American report of FALD associated cHCC-CCA and the first report of successful surgical resection of this tumour. To date, only one study examining 339 Fontan patients in Japan between 2005 and 2019 published by Ohuchi et al revealed 1 case of cHCC-CCA out of 10 patients who developed primary liver cancer. Unfortunately, this patient died from a cerebellar haemorrhage after tumour treatment with transarterial chemoembolisation. Another report detailed a patient of similar age with visceral heterotaxy requiring a Fontan procedure at age 4 who presented with abdominal and shoulder pain with workup delineating a right liver mass. Biopsy and resection demonstrated pleomorphism with predominant intrahepatic CCA features. Of note, routine screening was not reported prior to her diagnosis in this publication.22
The optimal treatment strategy for primary liver cancers in the setting of FALD remains controversial.23–26 Surgical resection may be feasible if liver disease and cardiac function is otherwise acceptable.27 Liver transplantation may be considered for HCC, and recommendations favouring combined heart–liver transplantation have also been published as definitive treatment.
The successful treatment of this patient was due to regular screening, with workup for elevated AFP leading to early detection and resection of the tumour while her physiology allowed. Despite seemingly acceptable cardiac parameters, there remains an element of hepatic congestion leading to increased malignancy risk in the absence of frank cirrhosis in the FALD population. This highlights the importance of early and ongoing screening in this patient population even in the absence of cirrhosis. There are currently no standardised guidelines on cancer screening in this population. Gordon-Walker et al recommended that screening may be started any time after the Fontan procedure, but the intensity of screening should increase after 10 years as cirrhosis and HCC have been consistently described in this timeframe. Patients should undergo semiannual clinical examination and blood tests including cell count, liver function and tumour marker. It should be noted that the sensitivity of AFP in diagnosing HCC is only about 60%28 and AFP is not a practical diagnostic marker of CCA.29 Liver ultrasound should be performed every 6 months. Longitudinal data with regular surveillance are essential to allow for correlation and validation of scoring systems for Fontan patients. Elastography, whether MR or ultrasound, may be repeated every 2 years. If abnormalities are detected on screening ultrasound, this should be investigated further with dedicated liver protocol contrast MRI. Biopsies should be reserved for patients for whom the presence of liver cirrhosis may be in doubt or when dual pathology is suspected. Overarching recommendations are to assess whether the progression of liver disease, including fibrosis and cirrhosis, are proportional to the estimated failing Fontan physiology, as an unexpectantly non-elastic liver, portal hypertension or worrisome features, particularly earlier in post-Fontan course, should prompt haemodynamic studies as this may portent to poor cardiac function. Emamaullee et al proposed a slightly different surveillance schedule, starting with a liver biopsy at 10 years post-Fontan, tumour markers, elastography and cross-sectional imaging to establish baseline levels of stiffness, nodularity, portal hypertension and splenomegaly. Longitudinal lab values may be obtained to establish trends and upper endoscopy may be helpful for patients with cirrhosis demonstrated on biopsy to assess for oesophageal varices. Patients should be informed of modifiable risk factors to optimise liver function and health including management of obesity, avoidance of hepatotoxic medications and alcohol use. In terms of direct monitoring of HCC, serial ultrasound and ⍺-fetoprotein according to the American Association for the Study of Liver Disease guidelines may be adapted until FALD specific values are validated. Ultimately, screening should be continued until a combined heart–liver transplantation may be performed, as this is the only curative procedure for failing Fontan physiology combined with FALD.
Patient’s perspective.
This patient perspective was written seven months after hepatic resection.
The first indication that something was wrong was an abnormal blood test. Then, after two months of additional tests, scans, and a biopsy, I was diagnosed with hepatocellular carcinoma. Even though my doctors had discussed the possibility of cancer over the previous two months, I was still shocked with the diagnosis. Growing up with my heart condition, I was a pretty active kid, and aside from the occasional need to sit out during physical education, I had not felt too different from my friends. I had been (and still am!) receiving excellent care from my cardiologists, and my annual appointments had always been positive. I had known that the Fontan circulation was affecting my liver, but I didn’t expect to develop cancer at such a young age.
After the diagnosis, everything moved very quickly. I met with the surgeon the following week; he showed me my scans and talked about the plan for the operation. He explained that the location of the mass made it not too difficult to resect and that my gallbladder may need to be removed as well due to its proximity to the tumor. I agreed to proceed with the surgery as recommended, and it was scheduled for the following week. For some reason, I wasn’t very nervous in the days following up to my surgery. Perhaps because everything had moved so quickly, it felt as if the reality of undergoing surgery had not quite sunk in.
The operation was scheduled for early morning, so I arrived at the hospital at dawn. Compared toCompared with the quiet and dark outside world, the pre-operative room was brightly lit and bustling with doctors, nurses, and other healthcare staff. Seeing all of this suddenly made everything feel very real, and I started to feel anxious. Before going into surgery, I met a lot of people − surgeon, anesthesiologists, operating room nurses − who were all very nice and explained everything very clearly.
I received excellent care from all of my doctors and nurses during my hospital stay. Thanks to the epidural, I felt almost no pain, and I was able to start walking around the day after my operation. The surgeon came by and told me that the tumor had been taken out successfully, with clear margins (and even showed me photos!). I was discharged after a week, and at first, the recovery at home was tough due to limited mobility and pain. Mentally, I was frustrated because the restrictions set by my limited mobility made it difficult to perform simple tasks. Still, I was grateful for having received such wonderful care and thankful that my operation had gone well without complications.
Two weeks after I was discharged, I had a follow-up visit with my surgeon. He went over the pathology of the resected tumor, which turned out to be combined hepatocellular-cholangiocarcinoma. To me, this seemed just as shocking as the initial diagnosis because I had not known that the mixed cancer was a possibility. I had an extensive discussion with my surgeon and oncologist regarding the best course of action, and they explained that due to the aggressiveness of cholangiocarcinoma and my age, adjuvant chemotherapy could be a good choice. After some deliberation and discussion with my family, I decided to proceed with chemotherapy.
At first, managing the side effects was difficulty. But my oncologist and the nurses at the infusion center were attentive to my concerns and provided tips to help manage the side effects. My family was also a huge support, and they helped me in any way they could. After some adjustment of medications and some trial and error on my part, the effects became much easier to tolerate. By this time, it was about two months after the operation, and I was able to resume some of my normal activities, which helped both physically and mentally.
The care and support that I received from my medical team and my family throughout this entire process has been truly wonderful. I am grateful that this cancer was caught early, and I appreciate that my doctors were so attentive to my questions and concerns and involved me in all the decisions every step of the way. I still have some time let until the end of my treatment, and I will have to follow upfollow-up frequently, but I know that I will continue to receive excellent care in their hands.
Learning points.
Fontan-associated liver disease is gaining prevalence as Fontan patients survive into adulthood with increased risk of primary hepatic malignancy.
Despite seemingly acceptable cardiac parameters, there remains an element of hepatic congestion leading to increased malignancy risk even in the absence of cirrhosis in this patient population, including rare tumours such as combined hepatocellular-cholangiocarcinoma.
Timely and frequent screening in this population may lead to early detection of tumours and allow intervention while liver disease and cardiac function permit. At the minimum, screening should be started at 10 years after Fontan procedure with semiannual bloodwork including hepatic function tests and tumour marker, and screening ultrasound every 6 months.
Footnotes
Contributors: JJ, JL and FMK contributed equally to the drafting of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Emamaullee J, Zaidi AN, Schiano T, et al. Fontan-Associated liver disease: screening, management, and transplant considerations. Circulation 2020;142:591–604. 10.1161/CIRCULATIONAHA.120.045597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon-Walker TT, Bove K, Veldtman G. Fontan-associated liver disease: a review. J Cardiol 2019;74:223–32. 10.1016/j.jjcc.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 3.Ohuchi H, Hayama Y, Nakajima K, et al. Incidence, predictors, and mortality in patients with liver cancer after Fontan operation. J Am Heart Assoc 2021;10:e016617. 10.1161/JAHA.120.016617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg DJ, Surrey LF, Glatz AC, et al. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc 2017;6. 10.1161/JAHA.116.004809. [Epub ahead of print: 26 Apr 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae JM, Jeon TY, Kim JS, et al. Fontan-associated liver disease: spectrum of US findings. Eur J Radiol 2016;85:850–6. 10.1016/j.ejrad.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 6.Kim T-H, Yang HK, Jang H-J, et al. Abdominal imaging findings in adult patients with Fontan circulation. Insights Imaging 2018;9:357–67. 10.1007/s13244-018-0609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulut OP, Romero R, Mahle WT, et al. Magnetic resonance imaging identifies unsuspected liver abnormalities in patients after the Fontan procedure. J Pediatr 2013;163:201–6. 10.1016/j.jpeds.2012.12.071 [DOI] [PubMed] [Google Scholar]
- 8.Wolff D, van Melle JP, Dijkstra H, et al. The Fontan circulation and the liver: a magnetic resonance diffusion-weighted imaging study. Int J Cardiol 2016;202:595–600. 10.1016/j.ijcard.2015.09.088 [DOI] [PubMed] [Google Scholar]
- 9.Wells ML, Fenstad ER, Poterucha JT, et al. Imaging findings of congestive hepatopathy. Radiographics 2016;36:1024–37. 10.1148/rg.2016150207 [DOI] [PubMed] [Google Scholar]
- 10.Wallihan DB, Podberesky DJ, Marino BS, et al. Relationship of MR elastography determined liver stiffness with cardiac function after Fontan palliation. J Magn Reson Imaging 2014;40:1328–35. 10.1002/jmri.24496 [DOI] [PubMed] [Google Scholar]
- 11.Fidai A, Dallaire F, Alvarez N, et al. Non-invasive investigations for the diagnosis of Fontan-Associated liver disease in pediatric and adult Fontan patients. Front Cardiovasc Med 2017;4:15. 10.3389/fcvm.2017.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohuchi H, Inai K, Nakamura M, et al. Mode of death and predictors of mortality in adult Fontan survivors: a Japanese multicenter observational study. Int J Cardiol 2019;276:74–80. 10.1016/j.ijcard.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M, Oka H, Kajihama A, et al. Non-invasive assessment of liver fibrosis by magnetic resonance elastography in patients with congenital heart disease undergoing the Fontan procedure and intracardiac repair. J Cardiol 2016;68:202–8. 10.1016/j.jjcc.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 14.Poterucha JT, Johnson JN, Qureshi MY, et al. Magnetic resonance elastography: a novel technique for the detection of hepatic fibrosis and hepatocellular carcinoma after the Fontan operation. Mayo Clin Proc 2015;90:882–94. 10.1016/j.mayocp.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surrey LF, Russo P, Rychik J, et al. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol 2016;57:106–15. 10.1016/j.humpath.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 16.Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg 2005;129:1348–52. 10.1016/j.jtcvs.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med 2013;368:1756–7. 10.1056/NEJMc1214222 [DOI] [PubMed] [Google Scholar]
- 18.Song J, Kim K, Huh J, et al. Imaging assessment of hepatic changes after Fontan surgery. Int Heart J 2018;59:1008–14. 10.1536/ihj.17-349 [DOI] [PubMed] [Google Scholar]
- 19.Wardell CP, Fujita M, Yamada T, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol 2018;68:959–69. 10.1016/j.jhep.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 20.Gamboa AC, Maithel SK. The landmark series: gallbladder cancer. Ann Surg Oncol 2020;27:2846–58. 10.1245/s10434-020-08654-9 [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Shi J, Guo H, et al. Alterations in DNA damage repair genes in primary liver cancer. Clin Cancer Res 2019;25:4701–11. 10.1158/1078-0432.CCR-19-0127 [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Marshall D, Veldtman G, et al. Intrahepatic cholangiocarcinoma after Fontan procedure in an adult with visceral heterotaxy. Pathol Res Pract 2018;214:914–8. 10.1016/j.prp.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 23.Valle J, Wasan H, Palmer DH, et al. ABC-02 trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. [DOI] [PubMed] [Google Scholar]
- 24.Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (prodige 12-accord 18-unicancer GI): a randomized phase III study. J Clin Oncol 2019;37:658–67. 10.1200/JCO.18.00050 [DOI] [PubMed] [Google Scholar]
- 25.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663–73. 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 26.Ebata T, Hirano S, Konishi M, et al. Bile duct cancer adjuvant trial (BCAT) Study Group. randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192–202. [DOI] [PubMed] [Google Scholar]
- 27.Takuma Y, Fukada Y, Iwadou S, et al. Surgical resection for hepatocellular carcinoma with cardiac cirrhosis after the Fontan procedure. Intern Med 2016;55:3265–72. 10.2169/internalmedicine.55.6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruix J, Sherman M. American association for the study of liver D. management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Li D-J, Chen J, et al. Application of joint detection of AFP, CA19-9, CA125 and CEA in identification and diagnosis of cholangiocarcinoma. Asian Pac J Cancer Prev 2015;16:3451–5. 10.7314/APJCP.2015.16.8.3451 [DOI] [PubMed] [Google Scholar]