Abstract
Objectives
Eliminating mother-to-child transmission (MTCT) of hepatitis B virus (HBV) is central to WHO’s target of reducing hepatitis B infection in children to <0.1% by 2030. While Nigeria accounts for 8.3% of the global burden, interventional studies on prevention of MTCT of HBV are hardly available. This study aimed to assess the impact of prevention of MTCT interventions on vertical transmission of HBV among pregnant women in Nigeria.
Design
A prospective cohort study.
Setting
A University Teaching Hospitals Complex in Nigeria between 2015 and 2021.
Participants
10 866 pregnant women and their pre-existing children.
Interventions
Eligible pregnant women were screened for HBsAg using chromatographic immunoassay (Micropoint, USA). HbsAg-positive women had HBV serological assay done and their pre-existing children were screened. Women with HBV DNA ≥2 00 000 IU/mL and those positive for hepatitis B e-antigen (HBeAg) had 300 mg/day of Tenofovir Disoproxil Fumarate (TDF) in the third trimester. The newborns had hepatitis B vaccines and HB immunoglobulin (HBIG) administered, followed by testing for HBsAg at 9 months postnatally.
Primary outcome measures
Prevalence of chronic hepatitis B infection in pregnancy, and the incidence of MTCT of HBV.
Results
Overall, 395 women had chronic HBV infection, giving a prevalence of 3.64%. Their mean age was 31.51±5.71 years, with a median parity of 1.2. Thirteen women (5.2%) were positive for HBeAg, seven (3.1%) of the 225 pre-existing hepatitis B-exposed children were HbsAg positive and 17 women had prenatal TDF. Overall, 376 women completed the study, with mean birth weight of 3.21±1.86 kg and perinatal mortality rate of 29.2/1000 births. Hepatitis Bvaccine-HBIG combination was administered to 260 newborns, while the others had hepatitis B vaccine alone. All the children tested negative to the HbsAg at 9 months.
Conclusion
Eliminating MTCT of HBV infection through validated protocols in low and middle income countries with the highest burden of chronic HBV infections is feasible. National scale-up of such protocols is recommended.
Keywords: adult gastroenterology, fetal medicine, maternal medicine, hepatobiliary disease, perinatology
Strengths and limitations of this study.
This study involves the largest cohort of hepatitis B pregnant women to be prospectively recruited and actively managed in Nigeria.
The multidisciplinary nature of the managing team made implementation of the elimination-of-mother-to-child-transmission of hepatitis B interventions feasible.
This research is the only study to actively intervene, prospectively follow up and report the outcomes of hepatitis B exposed children till the age of 9 months in Nigeria.
Hepatitis B virus DNA assay could not be routinely done in the HBsAg-positive women due to financial constraints.
Introduction
The WHO estimates that 1.4 million deaths are recorded annually due to viral hepatitis, and hepatitis-associated mortality now ranks as the seventh leading cause of death globally, ahead of deaths due to HIV/AIDS, malaria and tuberculosis. This burden is mainly associated with hepatitis B virus (HBV) (48%) and hepatitis C virus (48%).1 Overall, about 2 billion people are estimated to have evidence of past or present hepatitis B infection, with Africa and the Western Pacific regions alone accounting for 67% of the global burden. Mother-to-child-transmission of HBV is a major route of transmitting the infection in low and middle-income countries (LMIC), due to its propensity to progress into chronicity.1–3
The Center for Disease Control estimated the prevalence of chronic hepatitis B (CHB) infection in Nigeria to be at least 8%.4 This was confirmed by the recent report from the Federal Ministry of Health, making Nigeria the country with the highest burden globally.5 Despite the high burden, most hospitals in Nigeria and other developing countries have no protocol in place to address the problem. In 2018, only 52.4% of Nigerian children reportedly received the birth dose of hepatitis B vaccine while only 50.3% completed the three additional doses of the vaccine.6 To achieve the Global Health Sector Strategy on viral hepatitis, the WHO has set the target of reducing HBsAg prevalence in children to 0.1% by 2030.7 Appropriately designed prospective studies to generate relevant data are imperative to achieve this goal.
Although some Nigerian researchers have evaluated the outcomes of pregnancies among women with HBV infection, many of these studies were either retrospectively conducted or simply prevalence studies that did not control for or report the quality of prevention of mother to child transmission of hepatitis B care that the study subjects received8 9; none had, therefore, reported on the outcome of implementing a well-designed and implemented protocol. While Onakewhor et al reported the administration of hepatitis B vaccine and Hepatitis B Immunoglobulin (HBIG) immunoprophylaxis for prevention of mother-to-child transmission (PMTCT) among a Nigerian cohort, the study had a sample size of only 45 patients10. This study was, therefore, designed to meticulously implement the Elimination-of-Mother-to-Child-Transmission (EMTCT) of hepatitis B measures and to follow up the hepatitis B-exposed babies till the age of 9 months, for the purpose of identifying perinatal vertical and early childhood horizontal transmissions.
Methods
Study setting
The study was undertaken at the Perinatal Unit of the Department of Obstetrics, Gynaecology and Perinatology and the Gastroenterology Unit of the Department of Medicine, Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Osun State, Nigeria between November 2015, and March 2021.
Design
The study is a prospective cohort study.
Study participants
All consecutive pregnant women who booked for antenatal care at the institution were prospectively recruited for the study after appropriate counselling. As the study was descriptive without any hypothesis testing, a specific sample size was not calculated.
Inclusion criteria
All pregnant women who received antenatal care at the Obafemi Awolowo University Teaching Hospitals Complex, Ife, Osun State, Nigeria during the study period.
Exclusion criteria
Pregnant women who refused to consent to HBV screening.
Statistical analysis
The data were analysed with IBM SPSS V.20.0. Categorical variables were presented in frequencies and percentages while continuous variables were presented as means and SD.
Patient and public involvement
It was not possible to involve patients in the design and conduct of the study, but they will be involved in the dissemination of the study findings, for ease of widespread dissemination.
Intervention
After obtaining informed consent from each study participant, relevant data regarding the sociodemographic characteristics of the women were captured using a purpose-designed proforma. Provider-initiated counselling and testing of the participants’ serum for HBsAg was undertaken for all consecutive pregnant women by the Nurses at the antenatal clinic, using chromatographic immunoassay (Micropoint, USA) rapid diagnostic kit. In addition to HBsAg testing, all the women also had retroviral screening done after due counselling. HBsAg-positive women subsequently had the complete hepatitis B serological markers assay done, comprising the hepatitis B surface Antibody (HBsAb), Hepatitis B core antibody (HBcAb: total and IgG). Hepatitis B e-antigen (HBeAg) and the hepatitis B e-antibody (HBeAb), using Micropoint kit, for the purpose of categorisation and prognostication. To ensure compliance, 95% subsidy on the cost of the hepatitis B serological marker was provided by the research team. HBV DNA assay was also routinely requested for all the HBsAg-positive patients, but this was not subsidised. CHB infection was defined as the presence of HBsAg for more than 6 months in the participants, or the presence of the IgG fraction of the HBcAb, with absent HBsAb and IgM fraction. All the pregnant women diagnosed with CHB were encouraged to bring their pre-existing children for testing and those identified as HBsAg positive were referred for necessary treatment at the Paediatric and Child Health Department of the hospital. Three categories of women were considered for antenatal treatment with Tenofovir Disoproxil Fumarate (TDF), namely, those with serum HBV DNA≥2 00 000 IU/mL, women positive for HBeAg without serum HBeAb, and women with history of perinatal transmission in their previous pregnancies. Pregnant women in any of these categories were placed on TDF 300 mg orally per day starting from the 28th week of gestation till delivery, or as soon as possible, if the diagnosis was made after the 28th week of gestation. Adherence to medication was assessed during outpatient follow-up by the gastroenterologists, who were also members of the research team. The mode of delivery was determined strictly by obstetric indications. Serial phone calls were placed to all the study participants by dedicated research staff and community health workers as their pregnancies advanced, to ensure compliance with antenatal care and postpartum EMTCT of hepatitis B advisories. Those who delivered out-of-facility were, therefore, identified and necessary EMTCT of hepatitis B measures were implemented without delay. All the babies that were delivered within the study period, irrespective of the place of birth or the day of the week, had the birth dose of the hepatitis B vaccine administered within 24 hours of delivery by the National Programme on Immunization department. When affordable to the patient, 200 iu of the HBIG was also administered to the newborns of the hepatitis B-positive parturients within 24 hours of delivery, while the mothers were referred to the Gastroenterology Unit for continuity of care. All the babies were followed up on phone to ensure strict compliance with the national vaccination schedule, including the hepatitis B vaccination. The infants were tested at the age of 9 months, to assess their hepatitis B infection status. The primary outcome measure was the proportion of infants who tested positive to the HBsAg at the age of 9 months. Secondary outcomes were the proportion of hepatitis B-exposed babies that had the birth dose of hepatitis B vaccine within 24 hours, the proportion of babies that completed the three doses of the hepatitis B vaccine at the appropriate time, spontaneous miscarriage rate, preterm delivery rate, mean birth weight and the perinatal mortality rate of the hepatitis B-exposed infants.
Results
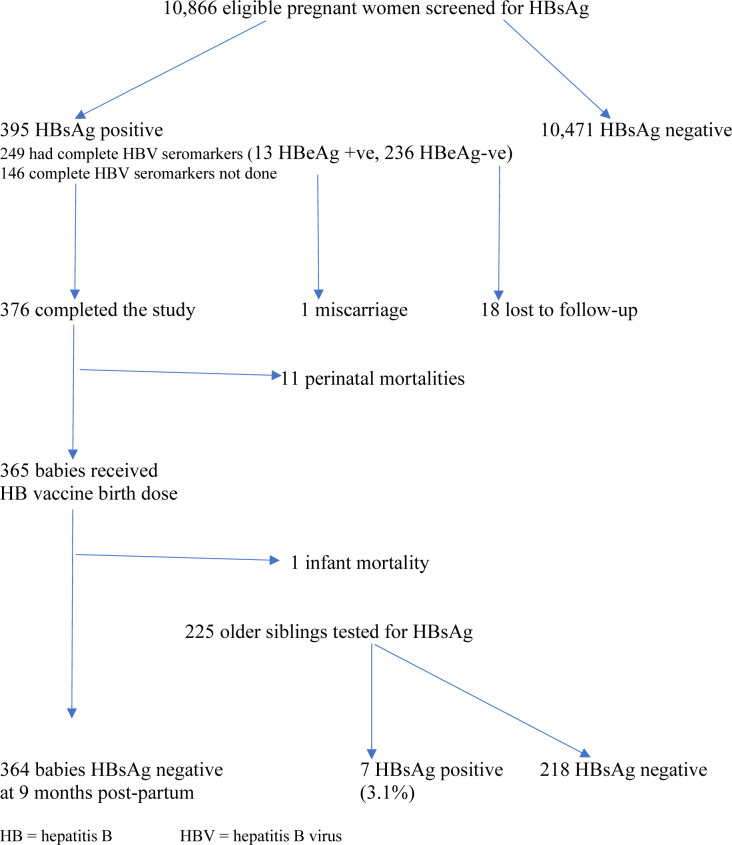
A total of 10 866 eligible pregnant women were screened during the study period, of which 395 tested positive to the HBsAg, giving a prevalence of 3.64%. The mean age of the women was 31.51±5.71 years, with a median parity of 1.2. More than half (53.9%) were screened before the 28th week of pregnancy, and 249 women (63%) had the complete hepatitis B viral assay done. The remaining expectant mothers (146:37%) could not do the complete hepatitis B serological markers due to financial constraints. All the HBsAg-positive women had CHB infection. In addition, 13 (5.2%) of the 249 HBsAg-positive women expressed HBeAg in their serum, while 162 women (65.1%) had developed the anti-HBe antibody. Three patients had both the HBeAg and the HBeAb contemporaneously. Only 21 patients had HBV DNA assay done, with values that ranged between 20 IU/mL and 7456 IU/mL. Consequently, none of them attained the viral load trigger for prenatal TDF. Of the 486 pre-existing hepatitis B-exposed children before the index pregnancy, 225 (46.3%), with ages ranging between 2 years and 22 years were tested and seven (3.1%) were HBsAg positive. The other children were not tested because their parents did not give consent. Seventeen women had prenatal TDF administered from 28 weeks gestation till delivery. Eight (2.0%) of the 395 women with chronic HBV infection had HBV-HIV coinfection.
Of the 395 women with positive HBsAg, there was one spontaneous miscarriage, while 18 patients were lost to follow-up (see figure 1). Further analysis was, therefore, based on 376 women who completed the study with known perinatal outcomes. With respect to mode of delivery, 219 women (58.2%) had spontaneous vaginal delivery, with a Caesarean section rate of 41.8%. The mean birth weight of the babies was 3.21±1.86 kg. Forty women (10.2%) had preterm births, defined as spontaneous or provider-initiated delivery for obstetric reasons, before 37 completed weeks, and there were 11 perinatal mortalities (comprising six stillbirths and five early neonatal deaths), with a stillbirth rate of 15.9/1000 births and a perinatal mortality rate of 29.2/1000 births. The details of the perinatal mortalities are depicted in table 1. One of the 17 women who had prenatal TDF had a stillbirth from complications of obstructed labour outside the facility; there was no other complication recorded among the remaining 16 women. All the babies were breastfed (figure 1).
Figure 1.
Algorithm for HBsAg screening of pregnant women and hepatitis B birth dose vaccination of exposed babies. HB, hepatitis B; HBV, hepatitis B virus.
Table 1.
Causes of perinatal deaths among women with hepatitis B infection in pregnancy at OAUTHC
| Cause | Perinatal mortality N=11 | Total | |
| Stillbirths | Neonatal deaths | Frequency (%) | |
| Antepartum haemorrhage | 2 | 1 | 3 (27.3) |
| Eclampsia | 1 | 1 | 2 (18.2) |
| Obstructed labour* | 1 | 1 | 2 (18.2) |
| Out-of-facility delivery | 0 | 2 | 2 (18.2) |
| Fetal growth restriction | 1 | 0 | 1 (9.1) |
| Unexplained | 1 | 0 | 1 (9.1) |
| Total | 6 | 5 | 11 (100) |
*Patients presented late to the facility after initial management at unorthodox centres.
OAUTHC, Obafemi Awolowo University Teaching Hospitals Complex.
The birth dose of hepatitis B vaccine was administered to all the 365 newborns; 327 babies (89.6%) had the vaccine administered within 24 hours of delivery, while the remaining 38 babies (10.4%) had delayed vaccination within 2–4 days postpartum due to logistic reasons. There was a diarrhea-related infant mortality among the cohort, leaving 364 babies (99.7%) that completed the vaccination at the appropriate time. In addition, 260 (71.3%) babies had both hepatitis B vaccine and the HBIG combination; the remaining 105 babies (28.7%) did not have the HBIG administered due to financial constraints. Only eight (61.5%) of the 13 women with HBeAg had the hepatitis B vaccine-HBIG combination administered to their babies. The entire hepatitis-B exposed cohort of 364 children tested HbsAg negative at the exit-hepatitis B screening that was conducted at 9 months postnatally.
Discussion
This study met all the set targets of the WHO Interim guidance for the elimination of hepatitis B1, with absolute prevention of vertical and early childhood transmission of HBV up to the age of 9 months among the cohort. This conforms to the findings of some studies that had earlier reported 0% transmission rate following initiation of immunoprophylaxis within 24 hours of birth.9 10 Meanwhile, 7 (3.1%) of the 225 already existing children who were delivered to the same cohort of women before the commencement of this protocol were HBsAg positive.
Expression of HBeAg significantly heightens the risk of vertical transmission of HBV. In a systematic review of 15 articles from 11 African countries including Nigeria by Keane et al,11 the pooled risks of vertical transmission of HBV among 34 HBeAg-positive mothers was 38.3% (95% CI 7.0 to 74.4%) without prophylaxis, and 4.8% (95% CI 0.1 to 13.3%) among HBeAg-negative mothers, without prophylaxis. These rates are similar to the 40% and 5% transmission rates for HBeAg-positive and HBeAg-negative mothers, respectively, by WHO.3 This risk was, however, eliminated among the 13 HBeAg-positive women in the index study due to the combination of prenatal TDF from the 28th week of gestation and postpartum prophylaxis in the exposed children. This approach has been proven to be more effective among HBeAg-positive mothers than vaccination at birth alone, at preventing vertical transmission.3 12 Prenatal TDF is already supplied to HIV-positive patients at no cost. Such privilege is, however, not yet available to HBV-positive women.10 13 It is advisable that the country leverages on the supply chain that is already available for HIV PMTCT programmes, for the prevention of HBV as well.
Almost 30% of the children in this study did not have the HBIG administered due to financial constraints. This is not unexpected, as such medications are expensive and only available on an ‘out-of-pocket’ payment basis. There was, however, no difference in the outcome of the children in the hepatitis B vaccine–HBIG combination and the hepatitis B vaccine alone groups. This observation aligns with an earlier report that the efficacy of appropriately administered hepatitis B vaccine is comparable to that of hepatitis B vaccine–HBIG combination,14 and the WHO recommendation of HepB3 as the primary preventative measure for hepatitis B-exposed children, with expected immunity rate of 95%. This finding is of significant relevance, especially inLMICs such as Nigeria, where a dose (200 iu) of HBIG as at the time of the study costs about 70 000 Nigerian Naira, equivalent to US$166. Meanwhile, the hepatitis B vaccine is available at no cost to all newborns as a national policy through governmental efforts in Nigeria.
With respect to obstetric outcomes, the prematurity rate is comparable with the report for the general obstetric population. The Caesarean section rate was comparable to the 47% reported from an earlier study, and the mean birth weight was also comparable to the 3210±490 g reported by Awowole et al from the same centre 3 years earlier.15 Hepatitis B infection in pregnancy was not associated with increased stillbirth or perinatal mortality rate among the cohort in this study, as the findings were comparable to the stillbirth rate of 15/1000 births recorded by Kuti et al among unselected booked patients at the same facility.16 The perinatal mortality rate of 29.2/1000 births from this study, though high, is comparable to the perinatal mortality rate of 38/1000 births reported by the Nigerian Demographic and Health Survey for the southwest geopolitical zone, where the OAUTHC is situated.6 Chronic asymptomatic hepatitis B infection in pregnancy was, therefore, not associated with increased caesarean section, low birth weight, stillbirth and perinatal mortality rates in this study.
The mother-to-child-transmission of hepatitis B infection rate of 0% at 9 months in this study is comparable to the findings from some earlier studies that reported similar transmission rates, following initiation of the birth dose of the hepatitis B vaccine within 24 hours of delivery.10 11 13 Meanwhile, seven (3.1%) of the 225 children delivered to the same cohort of women before the commencement of this protocol was HBsAg positive. This further underpins the immense role of the hepatitis B preventative measures at eliminating vertical transmission.
One of the strengths of this study is the sample size, which involves the largest cohort of HBV-positive pregnant women to be prospectively recruited and actively managed in Nigeria, translating to generation of reliable and credible data. The multidisciplinary nature of the study, with involvement of Specialists from Obstetrics, Gastroenterology, Paediatrics and Child Health, Public Health, the Childhood Immunization Sub-unit and the Community Health Extension Workers made in-facility and community monitoring, surveillance and painstaking implementation of the hepatitis B EMTCT interventions feasible in the study cohort. This research is also the only study in Nigeria, to prospectively follow-up and report the outcomes of hepatitis B-exposed children till the age of 9 months, following implementation of HBV EMTCT measures. In addition, this is the first study from Nigeria that endeavoured to screen all the pre-existing children of consenting hepatitis B pregnant women, with appropriate follow-up at the Paediatric and Child Health Unit of the Teaching Hospital, thereby using the antenatal period as a window of opportunity to provide a family-centred, holistic care to the affected women.
This study also has some identifiable limitations. Since 2020, WHO has updated its recommendation to include the administration of TDF to pregnant women with HBV DNA ≥2 00 000 IU/mL to PMTCT of HBV.1 17 One of the limitations of this study is, however, the inability to undertake the quantitative HBV DNA in all the women due to financial constraints. This, however, did not significantly affect the outcome of the study. Second, the additional benefit of HBIG among HBV-positive women in low-resource settings could have been better assessed using a randomised controlled trial, as this study was not designed to specifically detect the comparative effectiveness of hepatitis B vaccine alone versus hepatitis B vaccine-HBIG combination therapy. Withholding HBIG from the control group, especially for patients who could afford, it would, however, have been unethical. Nevertheless, further trials to appraise this observation will be needed in the future. It is also not clear whether the pre-existing children that tested positive to the HBV acquired the infections vertically at birth or horizontally in early childhood. Discerning this may, however, not be significant, as the endpoint of chronic childhood hepatitis B infection is similar. Furthermore, the vaccination status of the pre-existing children could not be evaluated with certainty. Finally, screening for anti-HBs titre has been recommended for HBsAg-negative infants as anti-HBs levels ≥100 mIU/ml are considered protective with no need for further medical management.18 Screening for anti-HBs was not done in this study for the 364 babies who were HBsAg negative at 9 months of age due to financial constraints.
In conclusion, this study demonstrates that achieving the set goals for eliminating mother-to-child transmission of hepatitis B infection is feasible, even in the absence of high-end investigations such as HBV DNA assay and expensive interventions such as the HBIG, especially in LMICs with the highest burden of chronic HBV infections and limited resources. Chronic HB infection did not demonstrate adverse effect on the outcome of the pregnancies. A national scale-up of these interventions in Nigeria through committed vaccination policies, sustained governmental funding and commitment as well as universal health coverage are required to bridge the gap for the patients who live below poverty line in Nigeria and would, therefore, not be able to afford even the most basic of these interventions.
Supplementary Material
Acknowledgments
The research team wishes to acknowledge the immense contributions of Matron Lawal G.O, Mrs Morakinyo, Mrs Omiwole O. and Miss Olaiwon S. to the successful completion of this research.
Footnotes
Contributors: DN and OK conceived the study. DN, OK, IA, OA, OI, OM, AA, CA and MI all participated in literature search, proposal writing, study design, supervision of sample collection, patient management, critical review of the manuscript for intellectual content, and approval of the final draft and agree to take responsibility for the integrity of the research. IA is responsible for the overall content of the manuscript, and serves as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Research approval for the study was obtained from the Obafemi Awolowo University Teaching Hospitals Complex Ethics and Research Board (IRB/IEC/0004553: ERC/20/02/010). Participants gave informed consent to participate in the study before taking part.
References
- 1.World Health Organization . Interim guidance for country validation of viral hepatitis elimination. Geneva: World Health Organization, 2021. [Google Scholar]
- 2.World Health Organization . Regional action plan for viral hepatitis in the Western Pacific 2016–2020. Geneva: World Health Organization, 2016. [Google Scholar]
- 3.World Health Organization . Global hepatitis report, 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 4.Center for Disease Control . Global viral hepatitis: millions of people are affected, 2021. Available: https://www.cdc.gov/hepatitis/global/index.htm
- 5.Federal Ministry of Health Nigeria . National guidelines for the prevention, treatment and care of viral hepatitis in Nigeria. FMOH; Abuja: Nigeria, 2016. [Google Scholar]
- 6.National Population Commission (NPC) [Nigeria] and ICF . Nigeria DHS key findings. Abuja, Nigeria and Rockville, Maryland, USA: National Population Commission, 2019. [Google Scholar]
- 7.World Health Organization . Global health sector strategy on viral hepatitis 2016-2021: towards ending viral hepatitis. Geneva: World Health Organization, 2016. [Google Scholar]
- 8.Mbaawuaga EM, Enenebeaku MNO, Okopi JA, et al. Hepatitis B virus (HBV) infection among pregnant women in Makurdi, Nigeria. Afri J Biomed Res 2008;11:155–9. 10.4314/ajbr.v11i2.50700 [DOI] [Google Scholar]
- 9.Utoo BT. Hepatitis B surface antigenemia (HBsAg) among pregnant women in southern Nigeria. Afr Health Sci 2013;13:1139–43. 10.4314/ahs.v13i4.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onakewhor JUE, Charurat M, Matthew O, et al. Serologic pattern of hepatitis B infection among exposed and non-exposed babies in a PMTCT program in low resource setting: does every exposed newborn require 200IU of hepatitis B immunoglobulin? J Vaccines Vaccin 2013;4:7. [Google Scholar]
- 11.Keane E, Funk AL, Shimakawa Y. Systematic review with meta-analysis: the risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Aliment Pharmacol Ther 2016;44:1005–17. 10.1111/apt.13795 [DOI] [PubMed] [Google Scholar]
- 12.Brown RS, McMahon BJ, Lok ASF, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology 2016;63:319–33. 10.1002/hep.28302 [DOI] [PubMed] [Google Scholar]
- 13.Ekra D, Herbinger K-H, Konate S, et al. A non-randomized vaccine effectiveness trial of accelerated infant hepatitis B immunization schedules with a first dose at birth or age 6 weeks in Côte d'Ivoire. Vaccine 2008;26:2753–61. 10.1016/j.vaccine.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 14.Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis 2016;16:1399–408. 10.1016/S1473-3099(16)30204-3 [DOI] [PubMed] [Google Scholar]
- 15.Awowole IO, Kuti O, Asaleye CM, et al. Normative references and clinical correlates of fetal umbilical artery Doppler indices in southwestern Nigeria. Int J Gynaecol Obstet 2020;151:134–40. 10.1002/ijgo.13294 [DOI] [PubMed] [Google Scholar]
- 16.Kuti O, Awowole I, Okunola T. Audit of stillbirths in a Nigerian teaching hospital. Trop J Obstet Gynaecol 2017;34:188–94. 10.4103/TJOG.TJOG_65_17 [DOI] [Google Scholar]
- 17.World Health Organization . Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
- 18.Hou J, Cui F, Ding Y, et al. Management algorithm for interrupting mother-to-child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2019;17:1929–36. 10.1016/j.cgh.2018.10.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.