Abstract
Tertiary lymphoid structures (TLS) existence is correlated with favorable prognosis in many types of cancer including non-small cell lung cancer (NSCLC). However, TLS formation and its relationship with treatment response remains unknown in NSCLC who received anti-PD-1 antibody plus chemotherapy as the neoadjuvant treatment (neoadjuvant chemoimmunotherapy). Here, we investigate TLS maturation and abundance in resectable NSCLC receiving neoadjuvant treatments. We retrospectively collected formalin-fixed paraffin embedded (FFPE) tissues from patients with resectable NSCLC (stage II–IIIA) from three cohorts based on treatment: naïve (N=40), neoadjuvant chemoimmunotherapy (N=40), and neoadjuvant chemotherapy (N=41). The TLS in tumor tissues was detected by immunohistochemical staining, and the differences in TLS maturation and abundance among different treatment groups were analyzed, as well as the relationship with pathological response and prognosis of patients. Multiplex immunofluorescence staining was used to explore the features of immune microenvironment. Higher major pathological response (MPR) rate and pathological complete response (pCR) rate were in the neoadjuvant chemoimmunotherapy group than in the neoadjuvant chemotherapy group (MPR: 45.0% vs 17.1%; pCR: 35.0% vs 4.9%). Among the three cohorts, neoadjuvant chemoimmunotherapy-treated NSCLCs displayed highest TLS maturation and abundance. Both the maturation and abundance of TLS were significantly correlated with MPR in both the neoadjuvant chemoimmunotherapy and the chemotherapy group. Patients with high maturation and abundance of TLS exhibited better disease-free survival (DFS) in all the three cohorts. TLS maturation was also an independent predictor for DFS in the neoadjuvant chemoimmunotherapy and treatment naïve group. Multiplex immunohistochemistry analysis using paired biopsy-surgery samples showed increased infiltration of CD8+T cell and decreased infiltration of M1 and M2 macrophages after neoadjuvant chemoimmunotherapy treatment in patients achieving MPR. There were no significant differences in features of immune cell infiltration for those with mature TLS achieving MPR when cross-compared across the three cohorts. These results demonstrate that TLS maturation is associated with MPR and an independent predictor for DFS in resectable neoadjuvant chemoimmunotherapy-treated NSCLC. The induction of TLS maturation may be a potential mechanism of action of neoadjuvant chemoimmunotherapy in resectable NSCLC.
Keywords: Tumor Biomarkers, Immunohistochemistry, Tumor Microenvironment
Introduction
Lung cancer is the leading cause of death by cancer worldwide.1 Most patients with resectable non-small cell lung cancer (NSCLC) relapsed and died from the disease despite curative surgery, highlighting the urgent need for therapeutic innovations in these patients. Immune checkpoint blockade targeting programmed death-1 (PD-1) in neoadjuvant therapy represents one of the next frontiers in cancer treatment. Clinical trials showed that neoadjuvant chemoimmunotherapy significantly prolonged event-free survival and improved major pathological response (MPR) rates compared with neoadjuvant chemotherapy in patients with NSCLC.2–4
The successful response to immunotherapy depends on the immunological composition of the tumor microenvironment.5 6 Tertiary lymphoid structures (TLS) also called ectopic lymphoid tissues are lymph node-like structures that develop in non-lymphoid tissues such as those sites affected by tumors, autoimmune diseases, and infectious diseases.7 Increasing studies have reported that TLS presence is closely correlated with favorable prognosis in many types of cancer including NSCLC.8 9 However, the effects of chemotherapy and immunotherapy on TLS and the maturation and abundance of TLS in NSCLC who received neoadjuvant chemoimmunotherapy remain largely unknown.
In this study, we detected TLS in tumor tissues from resected NSCLC patients with stage II-IIIA who received neoadjuvant chemoimmunotherapy, neoadjuvant chemotherapy and treatment naïve and analyzed the effects of chemotherapy and immunotherapy on TLS abundance and maturation, and the association of TLS abundance and maturation with pathological response and prognosis in the cohorts.
Materials and methods
Patients
Resectable NSCLC patients from Tianjin Medical University Cancer Institute & Hospital (Tianjin, China) between June 2018 and January 2020 were classified into three cohorts based on preoperative treatment. One cohort included 40 operable NSCLC patients who underwent upfront surgery (treatment naïve cohort). One cohort contained 41 resectable NSCLC patients receiving surgery after neoadjuvant platinum-based chemotherapy (chemotherapy cohort), and another included 40 cases receiving surgery after neoadjuvant chemoimmunotherapy (chemoimmunotherapy cohort). The inclusion criteria and specific treatment regimens were shown in online supplemental table 1. Clinicopathological characteristics were collected. The rate of MPR (residual viable tumor in NSCLC ≤10%) and disease-free survival (DFS), which was defined by the symptom-free, metastasis-free, and recurrence-free survival time of patients after surgery were well calculated. The median follow-up time was 24 months.
jitc-2022-005531supp001.pdf (1.6MB, pdf)
Histopathological and immunohistopathological analyses
TLS were identified and quantified both by H&E and immunohistochemistry staining of multiple serial paraffin sections with a thickness of 4 μm. Sections were stained with anti-human antibodies against CD3 (Dako, 1:200), CD20 (Dako, 1:300), and CD21 (Dako, 1:100) to validate the presence of TLS by demonstrating the content of T cells, B cells, and follicular dendritic cells (FDCs). TLS can be divided into three maturation stages according to the previous study: early TLS (E-TLS, dense lymphocytic aggregates with no FDCs), primary follicle-like TLS (PFL-TLS, B cell clusters, FDC network without germinal centers) and secondary follicle-like TLS (SFL-TLS, B cell clusters, FDC network with germinal centers).10–12 The abundance of TLS within tumor tissues was scored as 0, 1, 2, and 3 as following: (A) a score of 0 indicates no TLS; (B) a score of 1 indicates the tumor with one or two TLS; (C) a score of 2 represents at least three TLS in the tumor but does not meet the score 3 standard; (D) a score of 3 represents a large number of TLS distributed throughout the tumor region and converges with each other.13 The TLS score was assessed independently by two experienced pathologists who were blinded to the clinical data.
Statistical analysis
SPSS V.24 (IBM) and GraphPad Prism (V.7.0; GraphPad Software) were used for statistical analysis. Categorical variables were compared using the χ2 test or Fisher’s exact test. Univariate logistic regression analyses were performed to assess the factors influencing pathological reactions. The Kaplan-Meier method was used to estimate the probability of DFS, and the log-rank test was used to investigate the significance of differences between different groups. Multivariate Cox regression analyses were done to assess the prognostic value of TLS. Mann-Whitney U test was used to analyze the differences of immune cells in different treatment groups. Wilcoxon test was used to analyze the changes of immune cells before and after neoadjuvant chemoimmunotherapy. A p<0.05 was considered statistically significant.
Results
Clinicopathological characteristics of patients
We retrospectively collected FFPE tissues from 121 resected NSCLC patients who received neoadjuvant chemoimmunotherapy (N=40), neoadjuvant chemotherapy (N=41), and treatment naïve (N=40). The clinicopathological characteristics of patients were presented in table 1.
Table 1.
The clinicopathological characteristics of patients in the three different treatment cohort
| Characteristics | Neoadjuvant chemoimmunotherapy (%) |
Neoadjuvant chemotherapy (%) |
Treatment naïve (%) |
P value |
| Gender | ||||
| Male | 25(62.5) | 32 (78.0) | 22 (55.0) | 0.084 |
| Female | 15(37.5) | 9 (22.0) | 18 (45.0) | |
| Age | ||||
| <63 | 19 (47.5) | 23 (56.1) | 22 (55.0) | 0.702 |
| ≥63 | 21 (52.5) | 18 (43.9) | 18 (45.0) | |
| Smoking history | ||||
| Smoker or ex-smoker | 30 (75.0) | 33 (80.5) | 26 (65.0) | 0.278 |
| Never smoker | 10 (25.0) | 8 (19.5) | 14 (35.0) | |
| Histology | ||||
| Squamous cell carcinoma | 21 (52.5) | 25 (61.0) | 14 (35.0) | 0.111 |
| Adenocarcinoma | 15 (37.5) | 14 (34.1) | 24 (60.0) | |
| Large cell carcinoma | 4 (10.0) | 2 (4.9) | 2 (5.0) | |
| N stage | ||||
| N0 | 29 (72.5) | 24 (58.5) | 28 (70.0) | 0.361 |
| N1-2 | 11 (27.5) | 17 (41.5) | 12 (30.0) | |
| Neoadjuvant therapy no of cycles | ||||
| 2 | 16 (40.0) | 25 (61.0) | -- | 0.059 |
| >2 | 24 (60.0) | 16 (39.0) | -- | |
| EGFR mutation status | ||||
| Mutation-positive | 0 (0) | 2 (4.9) | 4 (10) | 0.106 |
| Mutation-negative | 9 (22.5) | 5 (12.2) | 11 (27.5) | |
| Unknown | 31 (77.5) | 34 (82.9) | 25 (62.5) | |
There were no statistical differences regarding age, sex, smoking history, neoadjuvant therapy cycle number, N stage, histology, and EGFR mutation status among three cohorts. In the neoadjuvant chemoimmunotherapy group, 45.0% (N=18) of patients achieved MPR, including 14 patients with pathological complete response (pCR). In the neoadjuvant chemotherapy group, only 17.1% (N=7) of patients achieved MPR, 2 with pCR.
The maturation and abundance of TLS in resectable NSCLC patients
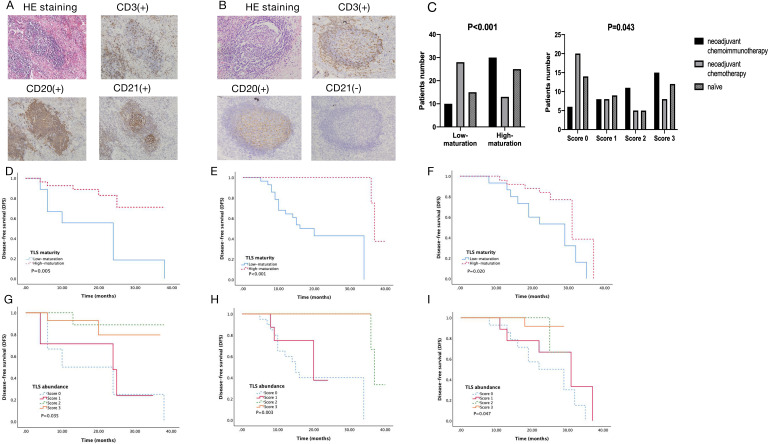
The presence of TLS within tumor tissues was initially evaluated based on H&E staining. TLS were found in 34 patients in the neoadjuvant chemoimmunotherapy group, 21 in the neoadjuvant chemotherapy group, and 26 in the treatment naïve group. In order to assess the lymphocytic organization and maturation stages of TLS, we performed immunohistochemical staining. As shown in figure 1A, CD20+B cells were the most abundant TLS component. CD3 staining showed that T lymphocytes were enriched in the peripheral areas of TLS. CD21 staining was used to detect FDCs.
Figure 1.
Tertiary lymphoid structures (TLS) in NSCLC and its relationship with DFS. (A) High-maturation of TLS, dense lymphocytic aggregates (CD3+, CD20+) with follicular dendritic cells (CD21+) ×10; (B) Low-maturation of TLS, dense lymphocytic aggregates (CD3+, CD20+) without follicular dendritic cells (CD21-) ×10; (C) Comparison of TLS maturity (p<0.001) and TLS abundance (p=0.043) under different treatments; (D) Kaplan-Meier analysis of DFS curve of patients with the TLS maturity in the neoadjuvant chemoimmunotherapy group (p=0.005); (E) DFS curve of patients with the TLS maturity in the neoadjuvant chemotherapy group (p<0.001); (F) DFS curve of patients with the TLS maturity in the treatment naïve group (p=0.020); (G) DFS curve of patients with the TLS abundance in the neoadjuvant chemoimmunotherapy group (p=0.035); (H) DFS curve of patients with the TLS abundance in the neoadjuvant chemotherapy group (p=0.003); (I) DFS curve of patients with the TLS abundance in the treatment naïve group (p=0.047). NSCLC, non‒small cell lung cancer.
According to the maturation of TLS, we classified patients into the low-maturation group (no TLS and E-TLS, figure 1B) and the high-maturation group (PFL-TLS and SFL-TLS, figure 1A). In the neoadjuvant chemoimmunotherapy group, 30 out of the 40 patients had high-maturation TLS. In contrast, high-maturation TLS existed in only 13 patients in the neoadjuvant chemotherapy group. In the treatment naïve group, 25 cases displayed high-maturation TLS. It suggested that anti-PD-1 immunotherapy may induce TLS maturity, whereas chemotherapy may impair TLS maturity. As for the abundance of TLS, the TLS localized overall in the tissue, regardless of the compartment, were counted.13 Among 40 patients in the neoadjuvant chemoimmunotherapy group, 6, 8, 11, and 15 patients were scored as 0, 1, 2, and 3, respectively, while 20, 8, 5, and 8 patients in the neoadjuvant chemotherapy group, compared with 14, 9, 5, and 12 patients in the treatment naïve group. Both TLS abundance and TLS maturity were higher in the neoadjuvant chemoimmunotherapy group than in the other groups (figure 1C).
Correlations between TLS and the pathological response of neoadjuvant treatment
Next, we analyzed the correlation between pathological response and clinicopathological features in the neoadjuvant chemoimmunotherapy and neoadjuvant chemotherapy group. Patients with MPR had more mature TLS than those with non-MPR in both the neoadjuvant chemoimmunotherapy and the neoadjuvant chemotherapy group. Squamous cell carcinoma was significantly associated with MPR in the neoadjuvant chemoimmunotherapy group but not in the neoadjuvant chemotherapy group. There was a significant association between neoadjuvant therapy cycles and MPR in both groups. In neoadjuvant chemoimmunotherapy group, more cases with MPR received only 2 cycles of neoadjuvant therapy. More than two cycles did not show benefits for patients. On the contrary, rare patients receiving two cycles of neoadjuvant treatment achieved MPR and more than two cycles displayed relatively better response in the neoadjuvant chemotherapy group. The results indicated the close relationship between TLS maturation and the anti-cancer effect of neoadjuvant treatment in resectable NSCLC (online supplemental table 2).
Correlations between TLS and DFS
We further analyzed the correlations between the maturation and abundance of TLS and DFS in the three groups. A median follow-up was 24 months for the entire cohort. Twelve patients had disease relapse or progression in the neoadjuvant chemoimmunotherapy group, 17 in the neoadjuvant chemotherapy group, and 19 in the treatment naïve group. High-maturation and high abundance groups had better DFS than low-maturation and low-abundance groups in all the three cohorts (figure 1D–I). Multivariate analysis determined that TLS maturity, not TLS abundance (neoadjuvant chemoimmunotherapy group: p=0.014, HR, 4.527, 95% CI, 1.351 to 15.167; treatment naïve group: p=0.035, HR, 2.988, 95% CI, 1.079 to 8.276) was an independent predictor for DFS in the neoadjuvant chemoimmunotherapy group and treatment naïve group (table 2).
Table 2.
Univariate and multivariate analysis of DFS for the three different groups
| Characteristics | Neoadjuvant chemoimmunotherapy | Neoadjuvant chemotherapy | Treatment naïve | ||||
| Univariate analysis P value |
Multivariate analysis P value OR (95% CI) |
Univariate analysis P value |
Multivariate analysis P value OR (95% CI) |
Univariate analysis P value |
Multivariate analysis P value OR (95% CI) |
||
| Gender | Male or female | 0.664 | – | 0.655 | – | 0.124 | – |
| Age | <61 or ≥61 | 0.708 | – | 0.183 | – | 0.355 | – |
| Smoking history | Smoker, ex-smoker or never smoker | 0.380 | – | 0.453 | – | 0.739 | – |
| Histology | Squamous cell carcinomas, Adenocarcinoma, or large cell carcinoma | 0.775 | – | 0.765 | – | 0.186 | – |
| N stage | N0 or N1-2 | 0.016 | 0.037 0.259 (0.073 to 0.923) |
0.001 | 0.003 0.177 (0.056 to 0.558) |
0.018 | 0.034 0.341 (0.126 to 0.922) |
| Neoadjuvant therapy no of cycles | 2 or >2 | 0.379 | – | 0.345 | – | – | – |
| TLS maturation | Low maturationor high maturation | 0.005 | 0.014 4.527 (1.351 to 15.167) |
0.884 38.84 (0.001 to 4.870) |
0.020 | 0.035 2.988 (1.079 to 8.276) |
|
| TLS abundance | 0.035 | 0.054 | 0.003 | 0.965 | 0.047 | 0.104 | |
| Score 0 | 1 | 1 | 1 | ||||
| Score 1 | 0.059 5.764 (0.937 to 35.452) |
0.882 8.113 (0.001 to 6.853) |
0.026 10.509 (1.326 to 83.259) |
||||
| Score 2 | 0.027 10.410 (1.300 to 83.363) |
0.885 5.813 (0.001 to 4.952) |
0.068 8.428 (0.854 to 82.213) |
||||
| Score 3 | 0.995 1.007 (0.082 to 12.413) |
0.928 6.785 (0.001 to 1.330) |
0.496 2.622 (0.164 to 42.011) |
||||
DFS, disease-free survival; TLS, tertiary lymphoid structures.
And N stage (neoadjuvant chemoimmunotherapy group: p=0.037, HR 0.259, 95% CI 0.073 to 0.923; neoadjuvant chemotherapy group: p=0.003, HR 0.177, 95% CI 0.056 to 0.558; treatment naïve group: p=0.034, HR 0.341, 95% CI 0.126 to 0.922) independently predicted DFS in all the three cohorts (table 2).
Analysis of tumor immune microenvironment
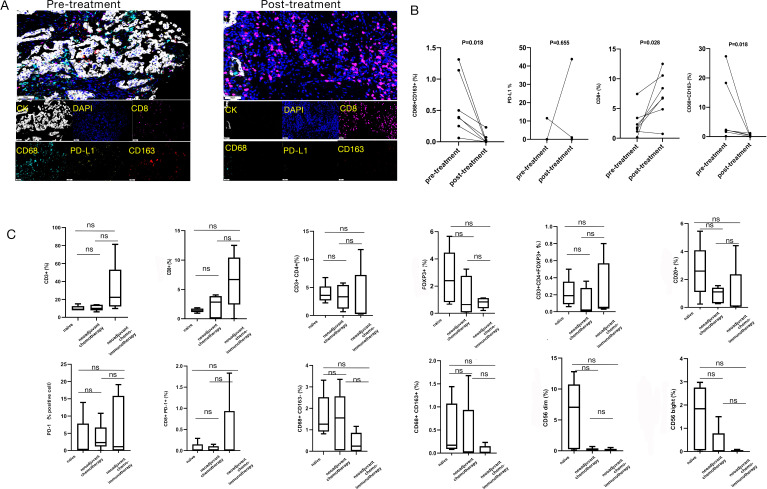
To explore the potential mechanisms of chemoimmunotherapy, we compared the differences in tumor immune microenvironment before and after neoadjuvant chemoimmunotherapy (figure 2A). Multiplex immunohistochemistry was performed on seven paired biopsy and resection specimens from NSCLC patients who achieved MPR after neoadjuvant chemoimmunotherapy. CD8+T cells in tumor stroma was elevated after treatment (p=0.028, figure 2B). While both CD68+CD163- M1 and CD68+CD163+ M2 macrophages were declined in tumor stroma after treatment (p=0.018, figure 2B). PD-L1+cells in tumor stroma showed no obvious change after treatment (p=0.655, figure 2B).
Figure 2.
Multiplex immunohistochemistry assay of immune microenvironment in three different treatment groups. (A) Establishment of a multiplex immunofluorescence staining assay to detect 4 different indices (CD8, CD68, CD163, and PD-L1) of immunomicroenvironment before and after neoadjuvant chemoimmunotherapy. (B) The difference of each index in immune microenvironment before and after neoadjuvant chemoimmunotherapy by Wilcoxon test. CD8+ cytotoxic T cells in the tumor stroma area showed a consistent upward trend after treatment; CD68+CD163- M1 macrophages in the tumor stroma showed a downward trend in each sample after treatment; CD68+CD163+ M2 macrophages in the tumor stroma showed a downward trend in each sample after treatment; PD-L1+ cells in the tumor stroma showed no significant difference before and after treatment (four out of seven patients with PD-L1 TPS < 1% or negative before neoadjuvant chemoimmunotherapy (0, 0.05%, 0.04%, 0), and 5 patients had PD-L1 TPS < 1% or negative after treatment (0, 0, 0, 1.04%, 0)). (C) The differences of immune microenvironment in 3 different treatment groups. There were no obvious differences by multiple comparisons (Mann-Whitney test) among the three groups. ns, P>0.05). (TPS, tumor proportion score)
Next, we interrogated features of immune cell infiltration in the mature TLS microenvironment. Total of 15 samples (5 samples per cohort) with high-maturation TLS were analyzed by multiplex immunohistochemistry in CD3+T cells, CD8+cytotoxic T cells, PD-1+CD8+ T cells, CD3+CD4+ T cells, CD3+CD4+FOXP3+ Treg cells, CD20+B cells, CD68+CD163- M1 macrophages, CD68+CD163+ M2 macrophages, and NK cells among different treatment groups (figure 2C). Our results suggested that mature TLS could exhibit similar features of immune cell infiltration regardless of neoadjuvant treatment.
Discussion
The roles of TLS in neoadjuvant chemoimmunotherapy of NSCLC are unknown. In this study, we analyzed TLS abundance and maturation in resectable NSCLC patients with stage II–IIIA who received neoadjuvant chemoimmunotherapy and neoadjuvant chemotherapy, followed by surgery, and underwent upfront surgery and the changes in tumor immune microenvironment before and after neoadjuvant chemoimmunotherapy. To our best knowledge, this is the first study that reveals the correlation of TLS maturation with MPR and DFS in resectable NSCLC patients who received neoadjuvant chemoimmunotherapy.
It reported that the density of mature DCs in TLS was highly associated with favorable overall survival and DFS.14 It is still largely unknown whether immunotherapy and chemotherapy affect TLS formation in resectable NSCLC. We found that the number of high-maturation TLS was higher in the neoadjuvant chemoimmunotherapy group, and lower in the neoadjuvant chemotherapy group than treatment naïve group. TLS abundance was higher in the neoadjuvant chemoimmunotherapy group than in the other groups, but there was no significant difference in TLS abundance between the neoadjuvant chemotherapy group and treatment naïve group. Supporting our findings that Silina et al reported the proportion of mature TLS was lower in lung squamous cell carcinoma patients who received neoadjuvant chemotherapy than the treatment naïve patients, while the density of TLS was similar.15 There have been no reports about the roles of neoadjuvant immunotherapy alone on the maturation of TLS in resectable NSCLC. However, Helmink et al found increased numbers of TLS-mainly mature TLS in resectable melanoma with responders treated with neoadjuvant immunotherapy.12 In addition, several studies have demonstrated that immunotherapy induces TLS formation and maturation using cancer mouse models.16 17 Sánchez-Alonso et al reported that the number of TLS and the degree of organization were significantly increased in the lung cancer-bearing mice treated with anti-PD-1 compared with the controls.16 Thus, we inferred that the effect of chemoimmunotherapy on TLS maturation is neither additive nor synergistic, because anti-PD-1 immunotherapy may induce TLS maturity, whereas chemotherapy may impair TLS maturity in resectable NSCLC. The correlation between TLS maturation and clinicopathological features was analyzed. TLS maturation had no relationship with gender, smoking status and histology (online supplemental table 3). Our work suggests induction of TLS is a potential key anti-cancer mechanism of neoadjuvant chemoimmunotherapy in resectable NSCLC and further research on the biological mechanism is necessary.
Studies have shown the close relationship between TLS presence and favorable survival in many cancer types.12 18 19 However, the relevance of TLS abundance and maturation for predicting DFS has not been studied in resectable NSCLC receiving neoadjuvant chemoimmunotherapy. Our analysis demonstrated that patients with a high abundance of TLS had significantly better DFS than those with none or low abundance of TLS in neoadjuvant chemoimmunotherapy group, the same as in the neoadjuvant chemotherapy group and treatment naïve group. TLS exists with different maturation stages in tumors, which may reflect its different anti-cancer effects. Some studies reported comparing with TLS numbers, TLS maturity could have higher prognostic value in several cancer types.20 21 We classified patients into low-maturation and high-maturation groups. High-maturation group showed better DFS in all the three cohorts. And TLS maturity was an independent predictor for favorable DFS in the neoadjuvant chemoimmunotherapy and treatment naïve group. The results suggest that TLS maturity could be better than TLS abundance, which contributes to postoperative risk stratification for resectable NSCLC receiving neoadjuvant chemoimmunotherapy.
We analyzed features of tumor immune microenvironment in patients achieving MPR after neoadjuvant chemoimmunotherapy using paired pretreatment and post-treatment tumor tissues with high-maturation TLS. Not surprisingly, the density of CD8+T cells was higher in the tumor stroma of post-treatment samples. Interestingly, both the density of CD68+CD163- M1 and CD68+CD163+ M2 macrophages decreased in the tumor stroma of post-treatment samples. In agreement with most studies that CD8+T cells increased,22 while M2 macrophages, one of the immune suppressor cells,23 reduced after neoadjuvant anti-PD-1 therapy in NSCLC. Studies reported that anti-PD-1 treatment increased population of M1 macrophages and high level of M1 macrophages was associated with better response in NSCLC.24 25 Notably, the findings were mostly based on anti-PD-1 monotherapy in advanced NSCLC, and what they analyzed was the infiltration of M1 macrophages in the tumor islets. In our study, we analyzed M1 macrophages in the tumor stroma as the post-treatment specimens were from patients achieving pCR and MPR with no or less than 10% tumor cells. It is deserved to further study the association of M1 macrophages with TLS maturity.
The limitations of the study include the small sample size in a single center. In addition, only CD8+T cells and macrophages were examined because of the limited number of tissue slides. Analysis of more immune cell subsets would contribute to understand the constituent cells of TLS maturity under neoadjuvant chemoimmunotherapy in resectable NSCLC.
In conclusion, our study indicates immunotherapy could induce TLS formation and TLS maturity may be used as a biomarker to improve the predictability of DFS in NSCLC patients undergoing neoadjuvant chemoimmunotherapy.
Acknowledgments
We thank all the participants who contributed to the article.
Footnotes
XS, WL, LS and HM contributed equally.
Contributors: XS, WL, LS and HM were the primary experimenter and data analyser and wrote the manuscript. YF and XW collected the clinicopathological information of the patients. CL, CC, JL, YX and ZZ made guidance on data analysis methods and experiment technology. CW, BZ and DY corrected the manuscript critically for important intellectual content and final approval of the version to be published. All authors read and approved the final manuscript.
Funding: This work was supported by National Natural Science Foundation of China (grant number 82173038 to DY and 82273428 to BZ and 82273119 to ZZ).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute & Hospital (No. bc2021247). Participants gave informed consent to participate in the study before taking part.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413–22. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- 3. Zhao Z, Chen S, Qi H, et al. Phase II trial of toripalimab plus chemotherapy as neoadjuvant treatment in resectable stage III non-small cell lung cancer (NeoTPD01 study). JCO 2021;39:8541. 10.1200/JCO.2021.39.15_suppl.8541 [DOI] [Google Scholar]
- 4. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trujillo JA, Sweis RF, Bao R, et al. T cell–inflflamed versus non-T cell–inflflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res 2018;6:990–1000. 10.1158/2326-6066.CIR-18-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park Y-J, Kuen D-S, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med 2018;50:1–13. 10.1038/s12276-018-0130-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sautès-Fridman C, Lawand M, Giraldo NA, et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol 2016;7:407. 10.3389/fimmu.2016.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Caro G, Bergomas F, Grizzi F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res 2014;20:2147–58. 10.1158/1078-0432.CCR-13-2590 [DOI] [PubMed] [Google Scholar]
- 9. Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014;189:832–44. 10.1164/rccm.201309-1611OC [DOI] [PubMed] [Google Scholar]
- 10. Posch F, Silina K, Leibl S, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 2018;7:e1378844. 10.1080/2162402X.2017.1378844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren F, Xie M, Gao J, et al. Tertiary lymphoid structures in lung adenocarcinoma: characteristics and related factors. Cancer Med 2022;11:2969–77. 10.1002/cam4.4796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rakaee M, Kilvaer TK, Jamaly S, et al. Tertiary lymphoid structure score: a promising approach to refine the TNM staging in resected non-small cell lung cancer. Br J Cancer 2021;124:1680–9. 10.1038/s41416-021-01307-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dieu-Nosjean M-C, Antoine M, Danel C, et al. Long term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008;26:4410–7. 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 15. Siliņa K, Soltermann A, Attar FM, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res 2018;78:1308–20. 10.1158/0008-5472.CAN-17-1987 [DOI] [PubMed] [Google Scholar]
- 16. Sánchez-Alonso S, Setti-Jerez G, Arroyo M, et al. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J Immunother Cancer 2020;8:e001187. 10.1136/jitc-2020-001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez AB, Peske JD, Woods AN, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep 2021;36:109422. 10.1016/j.celrep.2021.109422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Domblides C, Rochefort J, Riffard C, et al. Tumor-associated tertiary lymphoid structures: from basic and clinical knowledge to therapeutic manipulation. Front Immunol 2021;12:698604. 10.3389/fimmu.2021.698604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maibach F, Sadozai H, Seyed Jafari SM, Jafari MS, et al. Tumor-infiltrating lymphocytes and their prognostic value in cutaneous melanoma. Front Immunol 2020;11:2105. 10.3389/fimmu.2020.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goc J, Germain C, Vo-Bourgais TKD, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705–15. 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- 21. Masuda T, Tanaka N, Takamatsu K, et al. Unique characteristics of tertiary lymphoid structures in kidney clear cell carcinoma: prognostic outcome and comparison with bladder cancer. J Immunother Cancer 2022;10:e003883. 10.1136/jitc-2021-003883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang I, Kim JW, Ylaya K, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med 2020;18:443. 10.1186/s12967-020-02618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larroquette M, Guegan J-P, Besse B, et al. Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies. J Immunother Cancer 2022;10:e003890. 10.1136/jitc-2021-003890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mediavilla-Varela M, Page MM, Kreahling J, et al. Anti-Pd1 treatment to induce M1 polarization of tumor infiltrating macrophages in a 3D ex vivo system of lung cancer patients. JCO 2017;35:e23090. 10.1200/JCO.2017.35.15_suppl.e23090 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005531supp001.pdf (1.6MB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.