Abstract
The acquisition of cell identity is associated with developmentally regulated changes in the cellular histone methylation signatures. For instance, commitment to neural differentiation relies on the tightly controlled gain or loss of H3K27me3, a hallmark of polycomb-mediated transcriptional gene silencing, at specific gene sets. The KDM6B demethylase, which removes H3K27me3 marks at defined promoters and enhancers, is a key factor in neurogenesis. Therefore, to better understand the epigenetic regulation of neural fate acquisition, it is important to determine how Kdm6b expression is regulated. Here, we investigated the molecular mechanisms involved in the induction of Kdm6b expression upon neural commitment of mouse embryonic stem cells. We found that the increase in Kdm6b expression is linked to a rearrangement between two 3D configurations defined by the promoter contact with two different regions in the Kdm6b locus. This is associated with changes in 5-hydroxymethylcytosine (5hmC) levels at these two regions, and requires a functional ten-eleven-translocation (TET) 3 protein. Altogether, our data support a model whereby Kdm6b induction upon neural commitment relies on an intronic enhancer the activity of which is defined by its TET3-mediated 5-hmC level. This original observation reveals an unexpected interplay between the 5-hmC and H3K27me3 pathways during neural lineage commitment in mammals. It also questions to which extent KDM6B-mediated changes in H3K27me3 level account for the TET-mediated effects on gene expression.
Electronic supplementary material
The online version of this article (10.1007/s00018-020-03541-8) contains supplementary material, which is available to authorized users.
Keywords: H3K27me3, 5-Hydroxymethylcytosine, Kdm6b, Tet3, Enhancer, Neural stem cells, Neurogenesis
Introduction
Acquisition of cell identity upon differentiation is associated with developmentally regulated changes in the cellular histone methylation signature. This indicates the existence of an epigenetic mechanism to promote the establishment and maintenance of lineage-specific transcriptional patterns. Trimethylation of lysine 27 on histone H3 (H3K27me3), a hallmark of polycomb-mediated transcriptional gene silencing [1], is a central mark in this process. H3K27me3 co-existence with the active trimethylation of lysine 4 on histone H3 (H3K4me3) mark at promoters constitutes the so-called bivalent chromatin that is thought to repress transcription through H3K27me3, while keeping the promoter ‘poised’ for alternative fates induced by specific developmental cues [2–4]. This scheme, first described in pluripotent stem cells [2, 5, 6], might be important throughout development because bivalent domains have been identified also in multipotent progenitors and differentiated cells [7–13].
The contribution of H3K27me3 dynamics to the fine-tuned regulation of tissue-specific gene expression is best illustrated during neurogenesis. Commitment towards the neural lineage as well as the fate of neural progenitors and the specification of neuronal subtypes are regulated by polycomb group proteins [14–17]. These processes are associated with H3K27me3 gain or loss at specific sets of genes during neurogenesis. For instance, Albert et al. recently demonstrated at the Eomes locus that inhibition by genome editing of the transient decrease of H3K27me3 normally observed at this gene promoter in the developing cortex leads to lower expression of this transcription factor and altered neocortex development [13].
KDM6B is a key factor in the temporally regulated demethylation of H3K27me3. This JmjC-domain-containing protein is an H3K27me3-specific demethylase [18, 19] that is essential for neurogenesis [20]. Specifically, KDM6B critical role in embryonic stem (ES) cell commitment toward the neural lineage and in the activation of the lifelong neurogenic program relies on its capacity to catalyze H3K27me3 removal at gene promoters and enhancers [21–23].
Therefore, to better understand the epigenetic regulation of neural fate acquisition, it is important to determine how Kdm6b expression is regulated. Here, we investigated the molecular mechanisms involved in the control of Kdm6b expression upon mouse ES cell commitment towards neural lineages. Altogether, our data revealed a model; whereby, Kdm6b induction upon neural commitment relies on an intronic enhancer the activity of which is defined by its ten-eleven-translocation 3 (TET3)-mediated 5-hmC level.
Materials and methods
Cell culture and ES cell differentiation
Wildtype ES cells were derived from blastocysts obtained from crosses between C57BL/J (B) and JF1 (J) mice, and were maintained in gelatin-coated dishes with ESGRO complete plus medium (Millipore, SF001-500P) that contains LIF, BMP4, and a GSK3-β inhibitor. In vitro corticogenesis was performed as described previously [24], except that ES cells were plated on Matrigel-coated dishes (human ES cell-qualified matrix, Corning), and that Defined Default Medium was supplemented with B27 (without vitamin A, Gibco) to improve cell survival, and with 1 μM dorsomorphin homolog 1 (DMH1) (purified by C.C.H) to promote neurogenesis [25]. Using this protocol, neural precursors (NP) cells are the main cell population after 12 days (D12) of in vitro corticogenesis [24].
Expression analysis
Total RNA was extracted using TRIzol Reagent (Life Technologies, 15596018). After treatment with RNase-free DNase I (Life Technologies, 180868-015), first-strand cDNA was generated by reverse transcription with Superscript-IV (Life Technologies, 18090050) using random primers and 500 ng of RNA. Kdm6bos antisense RNA expression was analyzed by strand-specific reverse transcription. cDNA was then amplified by real-time PCR with a SYBR Green mixture (Roche) using a LightCycler R 480II (Roche) apparatus. The relative expression level was quantified with the 2-dCt method that gives the fold change variation in gene expression normalized to the geometrical mean of the expression of the housekeeping genes Gapdh, Tbp, and Gus (for Kdm6b, Tet1, Tet2, Tet3, Pou5f1, Nestin, and Pax6 expression analysis during corticogenesis), Rpl30 and Ppia (for Tet3 expression analysis in NP cells derived from ES cells incubated with retinoic acid), or Gapdh, Tbp, Rpl30, and Ppia (for Kdm6a and Kdm6b expression analysis in NP cells derived from ES cells incubated with retinoic acid). For Kdm6bos, gene expression was normalized to the geometrical mean of the strand-specific expression of the housekeeping genes Rpl30, Ppia, and Rps17. Each analysis was performed at least in triplicate. Details on the primers are in Supplementary Table 1.
Chromatin immunoprecipitation
Anti-H3K9ac (Millipore 06-942), -H3K4me3 (Diagenode 03-050), and -H3K27me3 (Millipore 07-449) antibodies were used for chromatin immunoprecipitation (ChIP) of native chromatin (N-ChIP) isolated from ES and NP cells (D12 of in vitro corticogenesis) [12].
An anti-TET3 antibody (polyclonal ABE290 Millipore), which targets the three TET3 isoforms (TET3FL, TET3s, TET3o; [26]), anti-H3K4me1 (Millipore, 07-436) and an anti-H3K27ac (Abcam Ab4729) antibody were used to assess their enrichment by ChIP of cross-linked chromatin (X-ChIP) isolated from ES and NP cells (D12). Briefly, cells were resuspended in PBS (1 million of cells per ChIP) and fixed in 1% formaldehyde (Sigma-Aldrich, F8775) by shaking at 700 rpm at room temperature for 6 min. The subsequent steps were performed using the Zymo-Spin ChIP Kit (Zymo Research, D5210) according to the manufacturer’s instructions. Chromatin was sonicated on a Bioruptor™ Next Gen for 4 × 11 cycles (30 s on/30 s off) at 4 °C.
Input and antibody-bound fractions were quantified by real-time PCR amplification with the SYBR Green mixture (Roche) using a LightCycler 480II (Roche) instrument. Background precipitation levels were determined by performing mock precipitations with a non-specific IgG antiserum (Sigma C-2288), and were only a fraction of the precipitation levels obtained with the specific antibodies. The bound/input ratios were calculated and normalized to the precipitation level at the Rpl30 promoter for anti-H3K9ac and -H3K4me3 ChIPs, at the Hoxa3 promoter for anti-H3K27me3 ChIPs, and at the IAP promoter for anti-H3K9me3 ChIPs. Normalization was not used for analysis of TET3, H3K4me1, and H3K27ac enrichment. The used primers are described in Supplementary Table 1.
Gene-specific bisulfite sequencing and combined bisulfite restriction analysis
Bisulfite conversion, PCR amplification, digestion for combined bisulfite restriction analysis (COBRA), cloning, and sequencing were performed as previously described [27]. Briefly, following bisulfite treatment, restriction sites are created (or maintained) only if their recognition site corresponds to a methylated cytosine in the native DNA (therefore, unconverted in the PCR product). For example, TCGA in the native sequence will become TTGA if the first cytosine is unmethylated, or will remain TCGA, if it is methylated. In this last case, the Taq1 site is maintained. Such sites can be found frequently in PCR products. Thus, digestion with Taq1 will indicate the ‘methylation pattern’ of the molecule population in the PCR products.
Details on the primers are in Supplementary Table 1.
5hmC detection
5hmC was analyzed using the EpiMark® 5-hmC and 5-mCAnalysis Kit (E33175, New England Biolabs) according to the manufacturer’s instructions. Briefly, genomic DNA was incubated with the T4-β-glucosyltransferase that protects against MspI digestion (CCGG) by adding a glucose moiety specifically to 5-hydroxymethylcytosine residues. The 5hmC levels were then determined by quantifying the MspI-resistant fraction in each sample by qPCR using primers designed around the analyzed MspI sites. The data were normalized to the amplification of the same region in the original DNA input. The obtained values were corrected relative to the basal rate of MspI digestion in untreated DNA. The primer sequences are in Supplementary Table 1.
Tet3 knockdown
Tet3 knockdown was performed as described in Ref. [28]. Briefly, shRNA-mir cassettes for the Tet3 gene were amplified from pSM2 retroviral vectors containing the shRNAmir sequences (Open Biosystems) and cloned into the p2Lox vector using HindII and NotI restriction sites. Then, to stably integrate these shRNAs (p2Lox constructs) were transfected into the A2lox.cre ES cells (derived from the E14 male cell line from the mouse strain 129P2/OlaHsd) [29] that express Cre after the addition of doxycycline (0.5 μg/ml) to the medium one day before transfection. ES cells were then transfected using Lipofectamine 2000 (Invitrogen) at a concentration of 5 × 105 cells/ml. One day after transfection, selection medium containing geneticin (G418, Melford—300 μg/ml active concentration) was added to the cells for 10 days. After selection, ES cell clones containing the shRNAmir were expanded in ES complete medium.
Neural differentiation of A2lox.cre ES cells was performed by incubation with retinoic acid from day 4 to day 8 of culture, resulting in a homogeneous NP population of PAX6-positive radial glial cells, as previously described in Ref. [30]. For shRNA expression after differentiation, doxycycline (2 μg/ml) was added to the medium for 5 days, and then cells were trypsinized and pellets stored at − 80 °C until analysis.
Western blotting
Proteins from ES and NP cell (D12) pellets were extracted with RIPA buffer containing Protease Inhibitor Cocktails (Roche, 04693159,001) (25 µl of buffer for 1 million cells). The lysate protein content was determined with the Bradford assay. Equal amounts of proteins (50 µg) were separated by SDS–PAGE, and then transferred to PVDF membranes (Bio-Rad). After membrane blocking, specific antibodies against KDM6B (Abcam Ab84200, rabbit, 1/500) and GAPDH (Sigma Ab2302, chicken, 1/10,000) were added overnight. Next, membranes were incubated with peroxidase-conjugated secondary antibodies against rabbit (Abliance Bl2407, 1/500) and chicken (Santa Cruz sc2428, 1/10,000) for 2 h. Finally, interactions were detected using the Clarity Western ECL substrate kit (Bio-Rad 1705060) according to the supplier, and the Chemidoc MP Imaging System apparatus (Bio-Rad).
Chromosome conformation capture (3C) analysis
The chromatin conformation capture (3C) protocol was performed as previously described [31] with minor modifications. Nuclei from ES and NP cells were extracted according the protocol described in Ref. [32], and purified on sucrose gradient. Between 1 and 5 M of formaldehyde-fixed nuclei were used for these experiments, and a total of 600 U of DpnII enzyme was used for chromatin digestion. Then, the 3C template was digested with 40U of NlaIII. Standards curves for qPCR were generated using BAC RP23-167O7. Primer sequences are in Supplementary Table 1.
Race-PCR
Race analyses were performed with Gene Racer™ (Invitrogen, L1500-01) according to the manufacturer’s instructions.
Raw data from ChIP-seqData mining
experiments in ES and NP cells were retrieved from GEO DataSets or from the Encode project (the accession numbers for the data are indicated in Supplemental Table 2). Reads were aligned to the mm9 genome using bowtie2 (version 2.3.4.3). Alignments with a MAPQ lower than 30 were filtered out using samtools (version 1.9). Coverage tracks of these alignments were produced with bamCoverage using RPKM normalization, a read extension of 200 pb, the ignoreDuplicates option, and a bin size of 20 pb. These coverage tracks were loaded on the UCSC genome browser for visualization.
Several high-throughput alignments were obtained from the UCSC genome browser. The ChiP-seq alignments for H3K27ac, H3K4me1, and H3K4me3 in cortex samples were from the LICR histone track, DNase experiments in mouse ES cells and in mouse brain were from the UW DNaseI HS track, and DNA methylation data (WGBS and RRBS) were from the HUB DNA Methylation of UCSC and the Stadler 2011 and Booth 2012 tracks, respectively. BedGraph tracks of the GRO-seq data for ES cells were retrieved from the GEO dataset GSE48895. The Pearson’s correlation coefficients for Kdm6b and Tet expression data were obtained using the pseudo-counts associated with the GSE58523 dataset [33].
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.02, or R3.6.1. The number of independent experiments and the name of the statistical test used for each comparison are indicated in the legends.
Results
Kdm6b is upregulated upon neural commitment
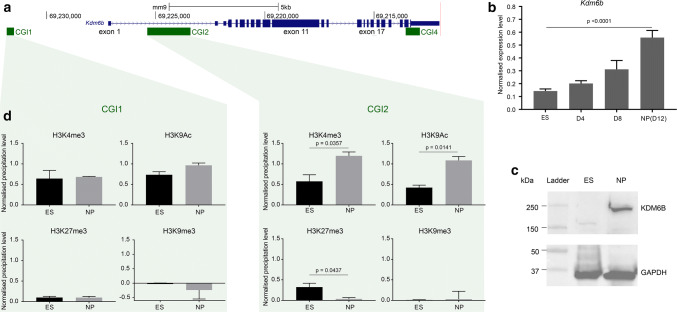
The canonical mouse Kdm6b gene is about 15 kb-long and is located on chromosome 11. It contains 23 exons, including three that are untranslated and located in the 5′ part (Fig. 1a). To determine its regulation upon neural commitment we chose an established in vitro protocol that uses ES cells to recapitulate the sequential steps of mouse in vivo corticogenesis [24, 33]. We focused on the first 12 days of in vitro corticogenesis. During this period, ES cells differentiated mainly into NP cells, as indicated by the marked downregulation of the pluripotency marker Pou5f1 and the upregulation of the neural progenitor markers Nestin and Pax6 (Supplementary Fig. 1a). Concomitantly, Kdm6b expression level increased by more than five times (Fig. 1b and Supplementary Fig. 1b), leading to a marked increase in its protein level in NP cells at day 12 (D12) (Fig. 1c). Hereafter, D12 of in vitro corticogenesis will be referred to as “NP cell stage”.
Fig. 1.
Kdm6b is upregulated upon neural commitment. a Map of the mouse Kdm6b locus showing the CpG islands (CGI) and exons as described in the UCSC Genome Browser. Positions are given according to the mm9 assembly. b Kdm6b expression level in ES cells (n = 6) and at day 4 (D4; n = 3), D8 (n = 3), and D12 (NP cell stage; n = 6) of in vitro corticogenesis. The expression was assessed with primers located on exons 5 and 6. Statistical significance was determined by one-way ANOVA (comparison of each differentiation point with ES cells). c KDM6B protein level evaluated by western blotting in ES and NP cells (D12). d ChIP analysis of H3K9ac, H3K4me3, H3K27me3, and H3K9me3 enrichment at CGI1 and CGI2 in ES (n = 3) and NP (n = 3) cells. The precipitation level was normalized to that obtained at the Rpl30 (for H3K4me3 and H3K9ac), HoxA3 (for H3K27me3), and IAP (for H3K9me3) promoters. Statistical significance was determined with the unpaired t test (p values in the figure). The data are presented as the mean ± SEM
To determine the molecular bases of this upregulation, we first wanted to delineate the Kdm6b promoter region(s) in ES cells and after their differentiation into NP cells using Race-PCR. This approach revealed that besides the documented transcription start site (TSS) in exon 1, a large subset of Kdm6b transcripts initiated from a CpG island (CGI) located in intron 1 and referred to as CGI2 (Fig. 1a, Supplementary Fig. 1c). CGI2 emerged as one of the main Kdm6b promoters. This observation was further supported by publicly available GRO-seq data from ES cells showing a cluster of initiation sites along CGI2 (Supplementary Fig. 1d). Accordingly, and unlike CGI1, this region displayed a dynamic histone modification profile between ES and NP cells. In ES cells, H3K4me3 and also H3K27me3, although at a lower level, were enriched at CGI2, constituting a “weak” bivalent domain. This signature was lost in NP cells due to the H3K27me3 decrease and the gain in H3K4me3 and acetylation of lysine 9 on histone H3 (H3K9ac), which in combination generally mark active promoters (Fig. 1d). DNA at both CGIs remained unmethylated throughout the experiment (Supplementary Fig. 1d).
These data indicate that Kdm6b upregulation upon neural commitment is associated with changes in the histone mark signature at CGI2, one of its main promoters.
Dynamic 3D conformation changes at the Kdm6b locus upon neural commitment
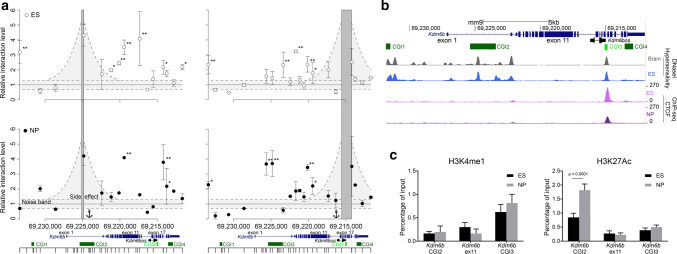
To identify putative regulatory regions, we looked for regions that physically interact with CGI2 in ES and NP cells by using the 3C approach. As such contacts are strongly favored between genomic regions that belong to the same topologically associating domain (TAD), we first checked using publicly available data [34, 35] that the Kdm6b locus was not close to a TAD boundary (Supplementary Fig. 2a). Then, we tested whether 15 regions distributed along a 22 kb window that includes the Kdm6b locus could interact with CGI2. We found that in ES cells, CGI2 mainly interacted with exon 11 and to a lesser extent with exons 17–21. Conversely, at the NP cell stage, the CGI2 interaction with exon 11 became restricted to the 5′ part of the exon, whilst the interaction between exons 17–21 and CGI2 was markedly increased (Fig. 2a). In human KDM6B, the region encompassed by exons 17–21 contains a CGI not described in the mouse genome (Supplementary Fig. 2b). However, we detected the presence of a “weak” CGI between exon 17 and 18 in the mouse gene that we called CGI3. CGI3 met all criteria for a CGI (GC content ≥ 50% and ratio of observed to expected number of CG dinucleotides > 0.6) but one (i.e., size > 200 bp), because it is 196 bp in size. Reciprocally, when CGI3 was used as anchor, this region mainly interacted with CGI2 in NP cells (Fig. 2a, right panels).
Fig. 2.
3D conformation of the Kdm6b locus in ES and NP cells. a 3C data quantification at the Kdmb6 locus. The left panels show the relative interaction frequencies between CGI2 (the anchor region) and the 15 tested sites along the locus, in ES cells (white circles) and NP cells (black circles). Four independent experiments were performed. Data points represent the mean ± SD of those that have provided a detectable quantification signal. The right panels show the relative interaction frequencies using the “weak” CGI3 area as anchor region. The noise band was defined using the basal interaction level (BIL [31]) ± 1 standard deviation (SD) of BIL (gray area); significant interactions were identified when the relative interaction level was higher than the BIL + 3 SD for (*) or 4 SD (**). b Data mining of DNase I hypersensitivity and CTCF recruitment at the Kdm6b locus in ES cells, whole brain, and NP cells. The Ensembl transcript ENSMUSG00000085178 (named Kdm6bos in the mm10 assembly) initiates from CGI3. c ChIP analysis of H3K4me1 (left panel) and H3K27ac (right panel) at CGI2, exon 11, and CGI3 in ES (n = 4) and NP cells (n = 4). Statistical significance was determined by two-way ANOVA (p values in the figure). The data are presented as the mean ± SEM
As supported also by GRO-seq data in ES cells (Supplementary Fig. 2d), CGI3 contained the documented transcriptional start site of Kdm6bos, and thus was the promoter for this Kdm6b small antisense transcript (Fig. 2b). This non-coding transcript was poorly expressed in ES and NP cells (Supplementary Fig. 2c). Data mining revealed that a DNase I hypersensitive site was located within CGI3 in ES cells and whole brain. These two features, generally found at enhancers, suggest that CGI3 could be a regulatory region. Data mining also showed a marked enrichment for the boundary protein CTCF at CGI3 in ES and NP cells (Fig. 2b). However, neither CGI3 nor exon 11 displayed the canonical chromatin signature generally observed at enhancers (Fig. 2c). ChIP analyses in ES and NP cells associated with data mining showed H3K4me1 enrichment at CGI3. However, this enrichment was not specific to that region, but rather present throughout the whole locus (Fig. 2c, Supplementary Fig. 2d). In addition, H3K27ac, which is indicative of an active enhancer and promoter, was enriched in the 5′ part of the locus that included CGI2, but was at basal level at exon 11 and CGI3, in both ES and NP cells (Fig. 2c, Supplementary Fig. 2d).
In conclusion, these approaches suggest that CGI3 is a putative regulatory region, despite the absence of a canonical enhancer chromatin signature.
5hmC enrichment switches from exon 11 to CGI3 during differentiation of ES cells into NP cells
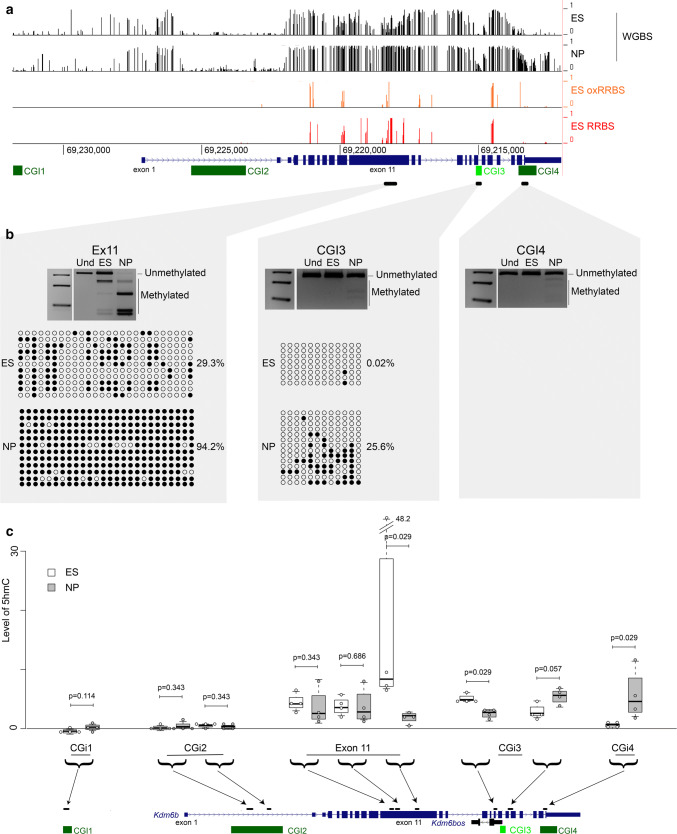
Due to the relative high level of 5-hydroxymethylation (5hmC) in ES cells and in the central nervous system [36, 37] and its enrichment at regulatory regions including enhancers [38, 39], we next investigated the 5mC/5hmC profile at exon 11 and CGI3 of Kdmb6. Bisulfite analysis, supported by publicly available whole-genome bisulfite sequencing (WGBS) data, showed that like CGI1 and CGI2, CGI3, and CGI4 (a CGI located in the last exon of Kdmb6) were not methylated in ES cells. In NP cells, CGI3 and CGI4 showed a limited gain of methylation, while CGI1 and CGI2 remained unmethylated. In contrast, we observed a marked methylation gain at exon 11 that was partially methylated in ES cells, and fully methylated in NP cells (Fig. 3a,b and Supplementary Fig. 1d).
Fig. 3.
Changes in 5hmc level at the Kdm6b locus during ES cell differentiation into NP cells. a Data mining of 5mC by WGBS (ES and NP cells) and of 5hmC by comparing the RRBS and oxRRBS datasets (ES cells) at the Kdm6b locus. b Bisulfite-based DNA methylation analyses at exon 11, CGI3, and CGI4 by COBRA and/or sequencing in ES and NP cells. For each sequenced region, the methylation patterns are symbolized by lollipops (black: methylated CpG; white: unmethylated CpG). For each region, PCR analysis was repeated using 3 independent samples for ES and for NP cells that gave similar results. The results of one representative experiment are shown. c 5hmC distribution along the Kdm6b locus in ES (white boxes; n = 4) and NP cells (gray boxes; n = 4), respectively. Levels of 5hmC are given as the level of the MspI-resistant fraction at each analyzed region. Statistical significance was determined with the Mann–Whitney test (p values in the figure). The data are presented as the mean ± SEM
As bisulfite sequencing cannot distinguish between 5mC and 5hmC, we wanted to know whether the low level of DNA methylation at exon 11, CGI3 and CGI4 could be explained by 5hmC deposition. To this aim, first we analyzed publicly available oxidative reduced representation bisulfite sequencing (oxRRBS) data for ES cells. In this approach, the level of 5mC and 5hmC are obtained by comparing the data generated using the same material by reduced representation bisulfite sequencing (RRBS), which detects both modifications, and by oxRRBS, which only detects 5mC [40]. This analysis highlighted 5hmC enrichment at exon 11 in ES cells (Fig. 3a). Then, we took advantage of the fact that following glucosylation, the C(5hm)CGG site becomes insensitive to MspI. Thus, analyses of glucosylated and untreated DNA from ES and NP cells allowed us to evaluate 5hmC level at nine MspI-containing regions throughout the Kdm6b locus. This approach confirmed 5hmC enrichment at exon 11 (covered by three sites) in ES cells, and indicated that 5hmC level was decreased in NP cells, particularly in its 3′ part. We observed the same dynamics at the region located immediately upstream of CGI3. Conversely, at the region located immediately downstream of CGI3 and within CGI4, 5hmC increased from the ES to the NP cell stage (Fig. 3c), suggesting again that the limited methylation gain observed at CGI3 and CGI4 by classic bisulfite sequencing (Fig. 3a, b) was, at least in part, a 5hmC gain.
These data revealed that at exon 11 and in the CGI3–CGI4 area of the Kdm6b locus, 5hmC level changes during ES cell differentiation into NP cells and parallels the changes in CGI2 interactions.
TET3 is required for Kdm6b upregulation
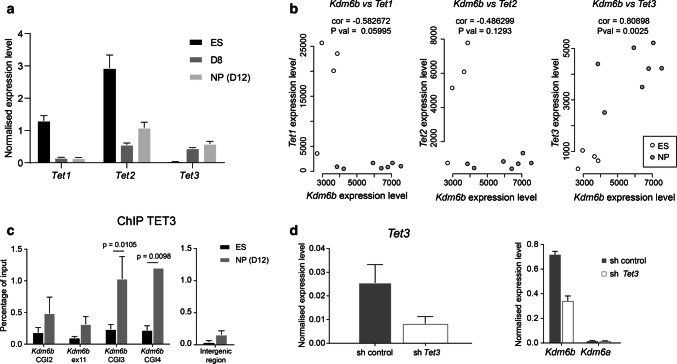
Deposition of 5hmC is catalyzed by the TET methylcytosine oxidase family that includes three members in mammals (TET1, TET2, and TET3). In agreement with the literature [41], we observed strong expression of Tet1 and Tet2, but not of Tet3 in ES cells. In addition, as documented in the developing brain [39], all three TETs were expressed during in vitro corticogenesis, although at very low level for Tet1 (Fig. 4a). b is upregulated u We found that only Tet3 expression was significantly correlated with Kdm6b expression (R = 0.8) (Fig. 4b).
Fig. 4.
A role for TET3 in Kdm6b upregulation. a Tet1, Tet2, and Tet3 expression level at D0 (ES cells) (n = 7), D8 (n = 5), and D12 (NP cells) (n = 7) during in vitro corticogenesis. b Correlation analysis of Kdm6b and Tet1 (left panel), Tet2 (middle panel), and Tet3 (right panel) expression levels in ES (white dots) and NP cells (gray dots). c ChIP analysis of TET3 enrichment at CGI2, exon 11, CGI3, and CGI4 of Kdm6b in ES (n = 3) and NP cells (D12 of in vitro corticogenesis, n = 3). An intergenic region (chr5:42,282,264–42,282,413 in mm9) was used as negative control. Statistical significance was determined by two-way ANOVA (p values in the figure). The data are presented as the mean ± SEM. d The expression level of Kdm6b and Kdm6a after shRNA-mediated Tet3 knockdown in NP cells. NP cells that stably integrated a scramble shRNA were used as control (sh control)
Moreover, ChIP analyses showed that at the NP cell stage, TET3 was recruited at the Kdm6b locus, particularly at CGI3 and CGI4, precisely where we observed a 5hmC gain (Fig. 4c). Finally, shRNA-mediated knockdown of Tet3 in NP cells derived from ES cells using a retinoic acid-based protocol showed that Tet3 downregulation was associated with reduced Kdm6b expression, without any effect on Kdm6a, the other H3K27me3 demethylase (Fig. 4d). When combined, these observations suggest that Kdm6b upregulation during neural commitment is dependent on TET3 recruitment to CGI3, its putative regulatory region.
Discussion
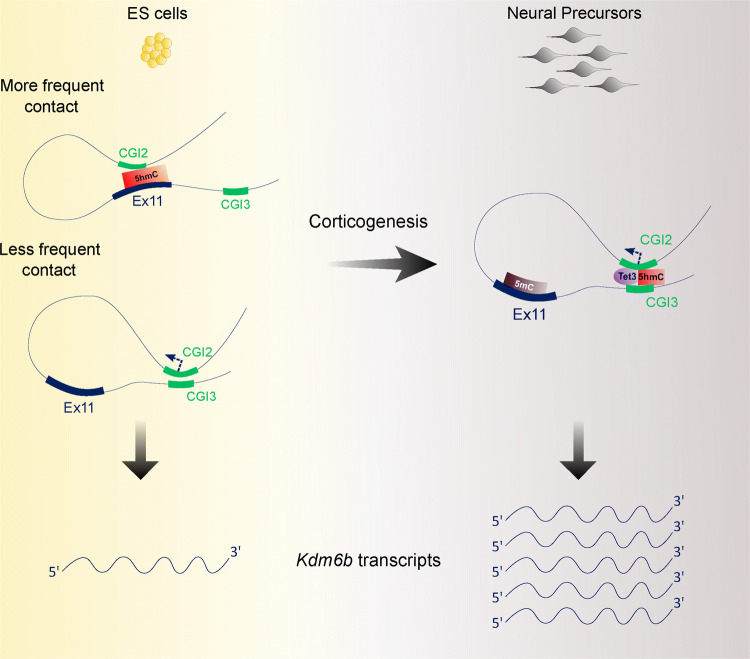
Taken together, our data suggest that TET3 and 5hmC dynamics contribute to the control of Kdm6b expression during neural commitment.
We evidenced that Kdm6b shows two main 3D configurations during in vitro corticogenesis. In ES cells, where Kdm6b expression is low, the predominant 3D configuration was defined by the contact between one of its main promoters (CGI2) and exon 11. The second 3D configuration is mainly observed in NP cells, and is characterized by the contact between the promoter and CGI3, a previously uncharacterized intragenic CGI that displays several enhancer features. This promoter–enhancer contact might explain Kdm6b upregulation in NP cells. Moreover, we found that the change between 3D configurations correlates with a switch in 5hmC enrichment from exon 11 in ES cells to the CGI3 area (up to CGI4) in NP cells.
The 5hmC gain at the CGI3 area in NP cells is reminiscent of what observed at tissue-specific enhancers during cardiomyocyte and hematopoietic cell differentiation, for instance [42, 43]. This event might constitute an early step in the enhancer activation that could facilitate chromatin opening and the recruitment of pioneer transcription factors [38, 44]. The 5hmC enrichment at exon 11 upon differentiation suggests that 5hmC deposition might also have a more structural function, by stabilizing the contact between two distal genomic regions. In this scheme, 5hmC enrichment at exon 11, likely controlled by TET1 or TET2, would favor its unproductive interaction with the Kdm6b promoter in ES cells. The low Kdm6b expression observed at this stage could be explained by contacts between CGI2 and CGI3 in a subset of cells. Upon differentiation, 5mC gain at exon 11, possibly due to the decrease in TET1/2 levels, associated with TET3-mediated 5hmC gain at the CGI3 area would favor CGI2–CGI3 interactions. This could be further promoted by CTCF binding at CGI3. Indeed, it has been recently shown that CTCF reduces cell-to-cell variations by stimulating enhancer–promoter activity [45]. Therefore, CTCF could act upstream or conjointly with 5hmC to stabilize the interaction between CGI2 and CGI3 and ensure robust Kdm6b expression in NP cells (Fig. 5).
Fig. 5.
Model. 5hmC enrichment at exon 11 would favor its unproductive interaction with the Kdm6b promoter in ES cells. Productive contacts between CGI2 and CGI3 in a subset of cells would account for the basal Kdm6b expression detected at this stage. Upon differentiation, 5mC gain at exon 11 and TET3-mediated 5hmC gain at the CGI3 area would stabilize the CGI2–CGI3 productive interactions and ensure robust Kdm6b expression in NP cells
Besides providing information on how Kdm6b expression is regulated, our study unravels the tight interplay between the 5hmC and H3K27me3 pathways during neural commitment. These two key epigenetic pathways are crucial for neural development. For instance, 5hmC is very abundant in brain compared with other organs. Moreover, TET3 plays a critical role in neurogenesis [39, 46]. Our original observation raises the question of whether and to which extent KDM6B-mediated changes in H3K27me3 level can account for TET-mediated effects on gene expression during neural commitment. Functional studies on TETs in ES cells indirectly support such interplay with other epigenetic programs for regulating gene expression. In these studies, there is little correlation between changes in DNA methylation at the promoter and changes in gene expression following TET depletion [47–51]. For instance, in embryoid bodies differentiated from TET triple-knockout ES cells, about 70% of genes with reduced expression do not show any DNA methylation defect [52], suggesting that TET could indirectly control a subset of these genes. TET3-mediated control of Kdm6b might also be part of a more general developmental program. Indeed, TET1 is involved in the repression of polycomb–target genes in ES cells [53, 54]. Therefore, together with TET1 loss, KDM6B, through its H3K27me3 demethylase activity, could contribute to induce the expression of a subset of these genes upon neural differentiation. Similarly, TET3 could potentiate its genome-wide action by activating Kdm6b, as illustrated by the finding that 5hmC gain in the body of active genes is associated with H3K27me3 loss in neurogenesis [39]. The observation that both TET3 and KDM6B belong to the 2-oxoglutarate-dependant dioxygenase enzyme family raises the question of whether their interplay could be a component of a more general program contributing to cellular fitness in response to changes in oxygen levels and/or availability of metabolic co-factors, including iron [55].
Altogether, our observations support the hypothesis that TET3-mediated control of Kdm6b expression upon neural commitment could be the central component of a wider epigenetic program whereby 5hmC and H3K27me3 cooperate to control neurogenesis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supp Figure 1: Characterization of the Kdm6b locus. a Pou5f1, Nestin and Pax6 expression levels in ES cells (n=6) and in NP cells at day 12 (n=6) of in vitro corticogenesis. b Kdm6b expression level in ES cells (n=4) and in NP cells (n=4) at day 12 of in vitro corticogenesis. Expression was assessed with primers located in exon 11 (left panel) and with primers between exon 21 and 23 (right panel). c Race-PCR mapping along the Kdm6b locus in ES cells, neonate brain, and at D8 of in vitro corticogenesis. d CGI1 and CGI2 bisulphite-based DNA methylation analyses by COBRA and/or sequencing in ES and NP cells. For each sequenced region, the methylation patterns are symbolized by lollipops (black: methylated CpG; white: unmethylated CpG). In a and b, statistical significance was determined with the unpaired t-test (p values in the figure). Data are presented as the mean ± SEM (TIF 2047 kb)
Supp Figure 2: Genomic features of the Kdm6b locus. a Position of the Kdm6b locus relative to the topological associated domains (TAD), as defined by Dixon et al [34]. b The main genomic features are conserved at the mouse Kdm6b and human KDM6B loci, including the CpG island (CGI3) between exons 17 and 18. c Kdm6bos expression level in ES cells (n=3) and at D8 (n=3) and D12 (NP stage; n=3) of in vitro corticogenesis. Statistical significance was determined by one-way ANOVA (comparison of each differentiation point with ES cells). Data are presented as the mean ± SEM. There was no significant difference between ES and the differentiation points. d Data-mining analysis of H3K4me1, H3K27ac, and H3K4me3 at the Kdm6b locus in ES cells, whole brain, and NP cells. The GRO-seq signals obtained from ES cell are also shown. Signal are shown for Kdm6b sense (upper panel) and antisense (lower panel) transcription (TIF 3262 kb)
Acknowledgements
We thank Claire Chazaud for advice in ES cell derivation and all members of P.A.’s team for critical reading of the manuscript.
Author contributions
PA initiated and supervised the study. BM, FC and PA designed the study. BM, JC, TB, AC, SMM, DN, CGG, SC, NA, CCH, CC, IV, CJM, FC and PA performed the experiments. FC performed the bioinformatics analyses. BM, JC, AC, DN, CGG, IV, JM, FC and PA analyzed the data. BM and FC produced the figures with JC’s and IV’s input. PA wrote the paper. All authors read and approved the final manuscript.
Funding
This research has been financed by the French government IDEX-ISITE initiative 16- IDEX-0001 (CAP 20–25) (Emergence, Challenge 3-recherche) (to F. C. and P. A.), the Ligue Contre le Cancer comité de l’Ardéche, du Puy de Dôme and du Cantal (to F. C. and P. A.) and “Conseil Régional d’Auvergne” (to PA). B. M. had a fellowship from Fondation pour la recherche médicale (FRM). C.J.M. is funded by the Portuguese Foundation for Science and Technology (FCT-CEECIND/00371/2017).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Franck Court, Email: franck.court@uca.fr.
Philippe Arnaud, Email: philippe.arnaud@uca.fr.
References
- 1.Conway E, Healy E, Bracken AP. PRC2 mediated H3K27 methylations in cellular identity and cancer. Curr Opin Cell Biol. 2015;37:42–48. doi: 10.1016/j.ceb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes inembryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Lien WH, Guo X, Polak L, Lawton LN, Young RA, Zheng D, Fuchs E. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell. 2011;9:219–232. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes Dev. 2013;27:1318–1338. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azuara V, Perry P, Sauer S, Spivakov M, Jørgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 6.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O'Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Mohn F, Weber M, Rebhan M, Roloff TC, Richter J, Stadler MB, Bibel M, Schübeler D. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30:755–766. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Sanz LA, Chamberlain S, Sabourin JC, Henckel A, Magnuson T, Hugnot JP, Feil R, Arnaud P. A mono-allelic bivalent chromatin domain controls tissue-specific imprinting at Grb10. EMBO J. 2008;27:2523–2532. doi: 10.1038/emboj.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, Zhao K. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maupetit-Méhouas S, Montibus B, Nury D, Tayama C, Wassef M, Kota SK, Fogli A, Cerqueira Campos F, Hata K, Feil R, Margueron R, Nakabayashi K, Court F, Arnaud P. Imprinting control regions (ICRs) are marked by mono-allelic bivalent chromatin when transcriptionally inactive. Nucleic Acids Res. 2016;44:621–635. doi: 10.1093/nar/gkv960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert M, Kalebic N, Florio M, Lakshmanaperumal N, Haffner C, Brandl H, Henry I, Huttner WB. Epigenome profiling and editing of neocortical progenitor cells during development. EMBO J. 2017;36:2642–2658. doi: 10.15252/embj.201796764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, Koseki H, Vidal M, Gotoh Y. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63:600–613. doi: 10.1016/j.neuron.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Pereira JD, Sansom SN, Smith J, Dobenecker MW, Tarakhovsky A, Livesey FJ. Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc Natl Acad Sci USA. 2010;107:15957–15962. doi: 10.1073/pnas.1002530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morimoto-Suzki N, Hirabayashi Y, Tyssowski K, Shinga J, Vidal M, Koseki H, Gotoh Y. The polycomb component Ring1B regulates the timed termination of subcerebral projection neuron production during mouse neocortical development. Development. 2014;141(4343):53. doi: 10.1242/dev.112276. [DOI] [PubMed] [Google Scholar]
- 17.Yao M, Zhou X, Zhou J, Gong S, Hu G, Li J, Huang K, Lai P, Shi G, Hutchins AP, Sun H, Wang H, Yao H. PCGF5 is required for neural differentiation of embryonic stem cells. Nat Commun. 2018;9:1463. doi: 10.1038/s41467-018-03781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 19.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, Hermanson O, Rosenfeld MG. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 21.Burgold T, Spreafico F, De Santa F, Totaro MG, Prosperini E, Natoli G, Testa G. The histone H3 lysine 27-specific demethylase Jmjd3 is required for neural commitment. PLoS ONE. 2008;3:e3034. doi: 10.1371/journal.pone.0003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgold T, Voituron N, Caganova M, Tripathi PP, Menuet C, Tusi BK, Spreafico F, Bévengut M, Gestreau C, Buontempo S, Simeone A, Kruidenier L, Natoli G, Casola S, Hilaire G, Testa G. The H3K27 demethylase JMJD3 is required for maintenance of the embryonic respiratory neuronal network, neonatal breathing, and survival. Cell Rep. 2012;2:1244–1258. doi: 10.1016/j.celrep.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Park DH, Hong SJ, Salinas RD, Liu SJ, Sun SW, Sgualdino J, Testa G, Matzuk MM, Iwamori N, Lim DA. Activation of neuronal gene expression by the JMJD3 demethylase is required for postnatal and adult brain neurogenesis. Cell Rep. 2014;8:1290–1299. doi: 10.1016/j.stem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaspard N, Bouschet T, Herpoel A, Naeije G, van den Ameele J, Vanderhaeghen P. Generation of cortical neurons from mouse embryonic stem cells. Nat Protoc. 2009;4:1454–1463. doi: 10.1038/nprot.2009.157. [DOI] [PubMed] [Google Scholar]
- 25.Neely MD, Litt MJ, Tidball AM, Li GG, Aboud AA, Hopkins CR, Chamberlin R, Hong CC, Ess KC, Bowman AB. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and SOX1 expression during neural induction. ACS Chem Neurosci. 2012;3:482–491. doi: 10.1021/cn300029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin SG, Zhang ZM, Dunwell TL, Harter MR, Wu X, Johnson J, Li Z, Liu J, Szabó PE, Lu Q, Xu GL, Song J, Pfeifer GP. Tet3 reads 5-carboxylcytosine through its CXXC domain and is a potential guardian against neurodegeneration. Cell Rep. 2016;14:493–505. doi: 10.1016/j.celrep.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnaud P, Hata K, Kaneda M, Li E, Sasaki H, Feil R, Kelsey G. Stochastic imprinting in the progeny of Dnmt3L−/− females. Hum Mol Genet. 2006;15:589–598. doi: 10.1093/hmg/ddi475. [DOI] [PubMed] [Google Scholar]
- 28.Santiago M, Antunes C, Guedes M, Iacovino M, Kyba M, Reik W, Sousa N, Pinto L, Branco MR, Marques CJ. Tet3 regulates cellular identity and DNA methylation in neural progenitor cells. Cell Mol Life Sci. 2019 doi: 10.1007/s00018-019-03335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iacovino M, Bosnakovski D, Fey H, Rux D, Bajwa G, Mahen E, Mitanoska A, Xu Z, Kyba M. Inducible cassette exchange: a rapid and efficient system enabling conditional gene expression in embryonic stem and primary cells. Stem Cells. 2011;29:1580–1588. doi: 10.1002/stem.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bibel M, Richter J, Lacroix E, Barde YA. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc. 2007;2:1034–1043. doi: 10.1038/nprot.2007.147. [DOI] [PubMed] [Google Scholar]
- 31.Court F, Baniol M, Hagege H, Petit JS, Lelay-Taha MN, Carbonell F, Weber M, Cathala G, Forne T. Long-range chromatin interactions at the mouse Igf2/H19 locus reveal a novel paternally expressed long non-coding RNA. Nucleic Acids Res. 2011;39:5893–5906. doi: 10.1093/nar/gkr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagschal A, Delaval K, Pannetier M, Arnaud P, Feil R. Chromatin immunoprecipitation (ChIP) on unfixed chromatin from cells and tissues to analyze histone modifications. Cold Spring Harbor Protocols. 2007 doi: 10.1101/pdb.prot4767. [DOI] [PubMed] [Google Scholar]
- 33.Bouschet T, Dubois E, Reynès C, Kota SK, Rialle S, Maupetit-Méhouas S, Pezet M, Le Digarcher A, Nidelet S, Demolombe V, Cavelier P, Meusnier C, Maurizy C, Sabatier R, Feil R, Arnaud P, Journot L, Varrault A. In vitro corticogenesis from embryonic stem cells recapitulates the in vivo epigenetic control of imprinted gene expression. Cereb Cortex. 2017;27:2418–2433. doi: 10.1093/cercor/bhw102. [DOI] [PubMed] [Google Scholar]
- 34.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonev B, Mendelson Cohen N, Szabo Q, Fritsch L, Papadopoulos GL, Lubling Y, Xu X, Lv X, Hugnot JP, Tanay A, Cavalli G. Multiscale 3D genome rewiring during mouse neural development. Cell. 2017;171:557–572. doi: 10.1016/j.cell.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sérandour AA, Avner S, Oger F, Bizot M, Percevault F, Lucchetti-Miganeh C, Palierne G, Gheeraert C, Barloy-Hubler F, Péron CL, Madigou T, Durand E, Froguel P, Staels B, Lefebvre P, Métivier R, Eeckhoute J, Salbert G. Dynamic hydroxymethylation of deoxyribonucleic acid marks differentiation-associated enhancers. Nucleic Acids Res. 2012;40:8255–8265. doi: 10.1093/nar/gks595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, Jui J, Jin SG, Jiang Y, Pfeifer GP, Lu Q. Dynamics of 5-hydroxymethylcytosine and chromatin marks in mammalian neurogenesis. Cell Rep. 2013;3:291–300. doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth MJ, Branco MR, Ficz G, Oxley D, Krueger F, Reik W, Balasubramanian S. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science. 2012;336:934–937. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Zhang Y. Tet1 and 5-hydroxymethylation: a genome-wide view in mouse embryonic stem cells. Cell Cycle. 2011;10:2428–2436. doi: 10.4161/cc.10.15.16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greco CM, Kunderfranco P, Rubino M, Larcher V, Carullo P, Anselmo A, Kurz K, Carell T, Angius A, Latronico MV, Papait R, Condorelli G. DNA hydroxymethylation controls cardiomyocyte gene expression in development and hypertrophy. Nat Commun. 2016;7:12418. doi: 10.1038/ncomms12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsagaratou A, Äijö T, Lio CW, Yue X, Huang Y, Jacobsen SE, Lähdesmäki H, Rao A. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc Natl Acad Sci USA. 2014;111:E3306–E3315. doi: 10.1073/pnas.1412327111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahé EA, Madigou T, Sérandour AA, Bizot M, Avner S, Chalmel F, Palierne G, Métivier R, Salbert G. Cytosine modifications modulate the chromatin architecture of transcriptional enhancers. Genome Res. 2017;27:947–958. doi: 10.1101/gr.211466.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren G, Jin W, Cui K, Rodrigez J, Hu G, Zhang Z, Larson DR, Zhao K. CTCF Mediated enhancer–promoter interaction is a critical regulator of cell-to-cell variation of gene expression. Mol Cell. 2017;67:1049–1058. doi: 10.1016/j.molcel.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li T, Yang D, Li J, Tang Y, Yang J, Le W. Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Mol Neurobiol. 2015;51:142–154. doi: 10.1007/s12035014-8734-5. [DOI] [PubMed] [Google Scholar]
- 47.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, Lahesmaa R, Orkin SH, Rodig SJ, Daley GQ, Rao A. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, Gao Q, Kim J, Choi SW, Page DC, Jaenisch R. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9:166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, Faull KF, Lyko F, Jaenisch R. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev Cell. 2013;24:310–323. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reimer M, Jr, Pulakanti K, Shi L, Abel A, Liang M, Malarkannan S, Rao S. Deletion of Tet proteins results in quantitative disparities during ESC differentiation partially attributable to alterations in gene expression. BMC Dev Biol. 2019;19:16. doi: 10.1186/s12861-019-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawlaty MM, Breiling A, Le T, Barrasa MI, Raddatz G, Gao Q, Powell BE, Cheng AW, Faull KF, Lyko F, Jaenisch R. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev Cell. 2014;29:102–111. doi: 10.1016/j.devcel.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H, D'Alessio AC, Ito S, Xia K, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011;473:389–393. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, D'Alessio AC, Ito S, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Genome wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 2011;25:679–684. doi: 10.1101/gad.2036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karuppagounder SS, Kumar A, Shao DS, Zille M, Bourassa MW, Caulfield JT, Alim I, Ratan RR. Metabolism and epigenetics in the nervous system: creating cellular fitness and resistance to neuronal death in neurological conditions via modulation of oxygen-, iron-, and 2-oxoglutarate-dependent dioxygenases. Brain Res. 2015;1628(Pt B):273–287. doi: 10.1016/j.brainres.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Figure 1: Characterization of the Kdm6b locus. a Pou5f1, Nestin and Pax6 expression levels in ES cells (n=6) and in NP cells at day 12 (n=6) of in vitro corticogenesis. b Kdm6b expression level in ES cells (n=4) and in NP cells (n=4) at day 12 of in vitro corticogenesis. Expression was assessed with primers located in exon 11 (left panel) and with primers between exon 21 and 23 (right panel). c Race-PCR mapping along the Kdm6b locus in ES cells, neonate brain, and at D8 of in vitro corticogenesis. d CGI1 and CGI2 bisulphite-based DNA methylation analyses by COBRA and/or sequencing in ES and NP cells. For each sequenced region, the methylation patterns are symbolized by lollipops (black: methylated CpG; white: unmethylated CpG). In a and b, statistical significance was determined with the unpaired t-test (p values in the figure). Data are presented as the mean ± SEM (TIF 2047 kb)
Supp Figure 2: Genomic features of the Kdm6b locus. a Position of the Kdm6b locus relative to the topological associated domains (TAD), as defined by Dixon et al [34]. b The main genomic features are conserved at the mouse Kdm6b and human KDM6B loci, including the CpG island (CGI3) between exons 17 and 18. c Kdm6bos expression level in ES cells (n=3) and at D8 (n=3) and D12 (NP stage; n=3) of in vitro corticogenesis. Statistical significance was determined by one-way ANOVA (comparison of each differentiation point with ES cells). Data are presented as the mean ± SEM. There was no significant difference between ES and the differentiation points. d Data-mining analysis of H3K4me1, H3K27ac, and H3K4me3 at the Kdm6b locus in ES cells, whole brain, and NP cells. The GRO-seq signals obtained from ES cell are also shown. Signal are shown for Kdm6b sense (upper panel) and antisense (lower panel) transcription (TIF 3262 kb)