Abstract
We have recently demonstrated that the periodontopathogenic oral spirochete Treponema denticola possesses membrane-associated lipoproteins in addition to lipooligosaccharide (LOS). The aim of the present study was to test the potential of these oral spirochetal components to induce the production of inflammatory mediators by human macrophages, which in turn may stimulate tissue breakdown as observed in periodontal diseases. An enriched lipoprotein fraction (dLPP) from T. denticola ATCC 35404 obtained upon extraction of the treponemes with Triton X-114 was found to stimulate the production of nitric oxide (NO), tumor necrosis factor alpha (TNF-α), and interleukin-1 (IL-1) by mouse macrophages in a dose-dependent manner. Induction of NO by dLPP was at 25% of the levels obtained by Salmonella typhosa lipopolysaccharide (LPS) at similar concentrations, while IL-1 was produced at similar levels by both inducers. dLPP-mediated macrophage activation was unaffected by amounts of polymyxin B that neutralized the induction produced by S. typhosa LPS. dLPP also induced NO and TNF-α secretion from macrophages isolated from endotoxin-unresponsive C3H/HeJ mice to an extent similar to the stimulation produced in endotoxin-responsive mice. Purified T. denticola LOS also produced a concentration-dependent activation of NO and TNF-α in LPS-responsive and -nonresponsive mouse macrophages. However, macrophage activation by LOS was inhibited by polymyxin B. These results suggest that T. denticola lipoproteins and LOS may play a role in the inflammatory processes that characterize periodontal diseases.
Periodontal pathogens possess virulent factors that are able to induce the synthesis of inflammatory mediators by host cells (9). Tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) were found to be elevated in tissues involved in periodontal diseases and have been suggested to play a part in the accompanying tissue destruction (17, 18, 22, 23). Wahl et al. also reported increased NO levels in human inflamed periodontal tissues compared to those of uninflamed gingiva (38).
Treponema denticola, the oral spirochete associated with periodontal diseases (15, 32), was shown to possess various properties which enable it to damage periodontal tissues, e.g., motility, high proteolytic activity (12, 13, 28), adherence to epithelial cells, and cytotoxicity (36), as well as factors which may inhibit host cell functions (30). In addition, the proteases of this bacterium can cleave the IL-1β precursor with the generation of biologically active fragments (3). We have recently showed that T. denticola possesses membrane-associated lipoproteins and a lipooligosaccharide (LOS) (31). An enriched delipidated lipoprotein fraction from T. denticola was shown by us to trigger the production of oxygen radicals and induced lysozyme release from human polymorphonuclear neutrophils (31).
Lipoproteins from other spirochetes, such as Treponema pallidum and Borrelia burgdorferi, the etiological agents of syphilis and Lyme disease, have been isolated, and their roles as inflammatory mediators were recently studied (11, 25). It has been shown that spirochetal lipoproteins, covalently modified with fatty acids similarly to the Escherichia coli murine lipoprotein, may act as major immunogens (4) as well as potent in vitro activators of monocytes/macrophages (25), B lymphocytes, and endothelial cells (11, 27). Synthetic lipopeptides corresponding to the lipoproteins’ N-terminal domains were found to be responsible for the monocyte/macrophage activation (25). Furthermore, Norgard et al. (20) reported that both lipoproteins and their lipopeptide analogues induced dermal inflammatory reactions in vivo similar to those observed upon injection of whole spirochetes.
Oral spirochetes are normally found in low numbers in the gingival sulcus in direct contact with the epithelium. Their numbers increase drastically in diseased sites, where the pocket epithelial layer loses its attachment to the teeth and is characterized by microulcerations, irregular ridges, and discontinuous basal lamina (22). At this point, bacterial products may have deleterious effects on the underlying connective tissue (26) by activating host immune cells, which produce and release mediators that stimulate connective tissue breakdown. Diseased periodontal sites are characterized by a significant elevation in the number of pathogenic bacteria, including spirochetes, in the presence of dense inflammatory infiltrates. The aim of the present study was to investigate the capacity of T. denticola enriched lipoprotein fraction and LOS to activate secretory responses of macrophages.
MATERIALS AND METHODS
Bacteria.
T. denticola ATCC 35404 was obtained from the American Type Culture Collection, Rockville, Md., and cultured as described previously (28).
Preparation of detergent phase proteins.
Extraction and phase separation with Triton X-114 was performed as described previously (31). The detergent phase proteins were delipidated by two washes of hexane-isopropanol (3:2 [vol/vol]) (24) and designated dLPP (delipidated lipoprotein). This solvent mixture was preferred to chloroform-methanol delipidation as it rendered homogeneous protein suspensions after resuspension of the lipoproteins in aqueous media. Where stated, dLPP was treated overnight at 37°C with proteinase K (Sigma) (1 μg of proteinase K/1.5 μg of dLPP) after the fraction was boiled for 10 min at 100°C.
Extraction and purification of LOS.
Extraction and purification of LOS was performed as described before (31) with the following modification. After submitting the methanol precipitate to lyophylization, we resuspended it in phosphate-buffered saline (PBS), boiled it for 10 min, and further treated it with proteinase K for 24 h at 37°C to hydrolyze any contaminating protein. This procedure was repeated twice. The extract was then submitted to hydrophobic chromatography as described previously (31). Biosynthetic labeling of the spirochetes and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously (31). After being labeled, LOS was purified as described above.
Care was taken to prevent contamination of the T. denticola components with endotoxin throughout the isolation procedures. Endotoxin-free water (Biological Industries) was used for the preparation of all media and buffers.
Preparation of mouse inflammatory macrophages.
A 1.5-ml quantity of 3% thioglycolate broth (Difco, Detroit, Mich.) was injected intraperitoneally into female mice 7 to 8 weeks of age. Four days after injection, the mice were sacrificed by cervical dislocation, and the peritoneum was washed with PBS. Macrophages were collected by aspiration of the PBS from the peritoneal cavity, washed twice, and counted with a hemocytometer. Cell viability was verified by the trypan blue exclusion technique and was found to be >95% in all experiments. The macrophages were suspended in RPMI 1640 medium (Biological Industries, Beit Ha-Emek, Israel) containing 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, and 5% fetal calf serum. The cells were plated in 24-well tissue culture plates (106 per well) and incubated for 60 min (37°C; 5% CO2). Nonadherent cells were removed by aspiration, and the remaining adherent cells were washed three times with PBS prior to being used in the subsequent experiments. More than 95% of the cells were nonspecific esterase positive.
NO2− accumulation.
NO2− accumulation was used as an indicator of NO production in the medium and was assayed by Gries reagent (5). Briefly, 100 μl of Gries reagent (1% sulfanilamide–0.1% naphthylethylene diamine dihydrochloride–2.5% H3PO4 [all from Sigma]) was added to 100 μl of each supernatant in triplicate wells of 96-well plates. The plates were read in a Vmax microplate reader (Molecular Devices, Palo Alto, Calif.) at 550 nm against a standard curve of NaNO2 (Sigma) in culture medium.
TNF-α measurement.
Mouse TNF-α was measured by enzyme-linked immunosorbent assay (ELISA) with an antibody pair from Pharmingen (San Diego, Calif.). ELISA plates (96-well) (Maxisorp; Nunc, Naperville, Ill.) were coated with anti-TNF-α monoclonal antibody in a coating buffer (carbonate-bicarbonate buffer, pH 9.6) followed by overnight incubation at 4°C. The wells were blocked overnight (4°C) with 3% bovine serum albumin in coating buffer and washed, and samples in duplicate were added and incubated overnight (4°C). A rat anti-TNF-α biotinylated antibody was added, followed by streptavidin-horseradish peroxidase conjugate (Jackson ImmunoResearch Laboratories, West Grove, Pa.). o-Phenylenediamine was used as the substrate. The reaction was terminated by the addition of 4 N sulfuric acid, and the optical density was read in a Vmax microplate reader at 490 to 650 nm.
IL-1 bioassay.
The assay used for measurement of IL-1 bioactivity was the modified lymphocyte activating factor assay as described earlier (2). Briefly, thymocytes from 1-month-old C3H/HeJ mice were dispersed into single-cell suspensions in complete RPMI. 2-Mercaptoethanol (50 μM), phytohemagglutinin (1 μg/ml final concentration) and 4 U of recombinant human IL-1β/ml (final concentration) were added to each well to establish a positive proliferation. The cells were plated at 5 × 106 well in 100 μl. The cultures were incubated at 37°C in 5% CO2 for 72 h with the addition of 1 μCi of well-tritiated thymidine (5 mCi/mmol) (ICN) for the last 12 h and harvested onto glass fiber strips (PHD cell harvester, Cambridge, Mass.). Scintillation fluid was added, and the vials were counted in quadruplicate in a beta counter.
Endotoxin analysis.
dLPP and the purified LOS were assayed for the presence of endotoxin activity by the Limulus amoebocyte lysate assay (kinetic turbidimetric method, performed by Biological Industries). Endotoxin activities of 0.05 and 0.36 ng/μg were found for dLPP and LOS, respectively.
Data analysis.
All experiments were carried out three to five times, except for IL-1 determination, which was carried out in two independent experiments. Each condition was maintained in four to six replicate wells of cell culture. Statistical significance between treatments was calculated by one-way analysis of variance. When the results showed a significant difference between groups, the significance was calculated by the Bonferroni t test. The level of significance was determined to be a P value of <0.05.
RESULTS
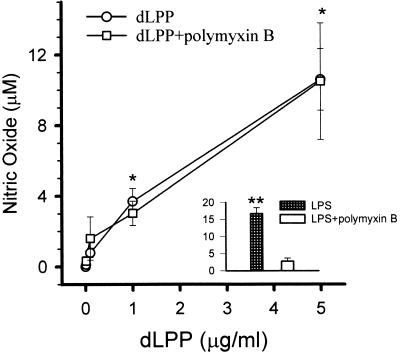
dLPP of T. denticola ATCC 35404 induced the production of NO in mouse macrophages in a concentration-dependent manner (Fig. 1). Maximal induction by dLPP was obtained at 5 μg/ml, a concentration which reached saturation. Salmonella typhosa lipopolysaccharide (LPS) was used as a positive control through all the experiments at a concentration of 1 μg/ml. Induction of NO by dLPP reached 25% of the levels obtained by LPS at similar concentrations. In order to ascertain that macrophage activation by dLPP was not due to contaminating endotoxin, macrophages were incubated with either LPS or dLPP in the presence of polymyxin B, which is known to form a stable complex with the lipid A region and neutralizes LPS activity (16). While NO production induced by S. typhosa LPS was reduced by 90% in the presence of polymyxin B, no effect on NO induction produced by T. denticola dLPP was observed (Fig. 1).
FIG. 1.
Stimulation of nitric oxide production by dLPP. Peritoneal mouse macrophages were incubated with increasing concentrations of dLPP in the presence or absence of polymyxin B (10 μg/ml). S. typhosa LPS (1 μg/ml) was used as a positive control. The supernatants were removed after 24 h and assayed for the presence of nitric oxide. The results are expressed as the mean ± standard deviation of four to six culture wells. ∗, P < 0.05 versus no dLPP; ∗∗, P < 0.05 versus LPS plus polymyxin B.
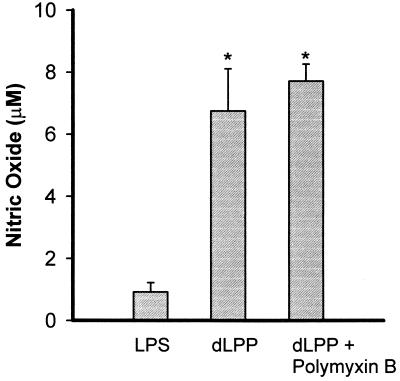
Induction of NO by LPS and dLPP was also examined in macrophages from LPS-nonresponsive C3H/HeJ mice (Fig. 2). These macrophages produced very low NO levels when incubated with 1 μg of LPS/ml, and the values were not different from the basal NO secretion. On the other hand, stimulation of C3H/HeJ macrophages with T. denticola dLPP (5 μg/ml) induced a sixfold enhancement of NO secretion and was not affected by the addition of polymyxin B.
FIG. 2.
Stimulation of nitric oxide production by C3H/HeJ (LPS-nonresponsive) mouse macrophages. The cells were incubated with dLPP (5 μg/ml) in the presence or absence of polymyxin B (10 mg/ml). S. typhosa LPS (1 μg/ml) was used as a control. The supernatants were removed after 24 h and assayed for the presence of nitric oxide. The results are expressed as the mean + standard deviation of four to six culture wells. ∗, significantly different from cells stimulated by LPS (P < 0.05). No significant difference was observed between stimulation with dLPP and stimulation with dLPP plus polymyxin B.
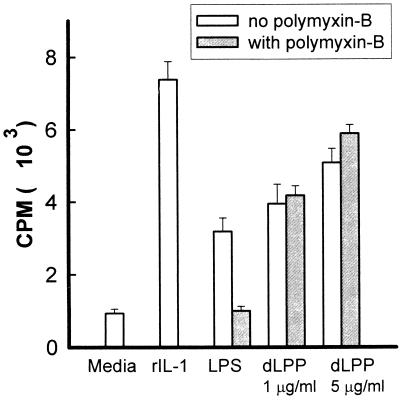
The effect of T. denticola dLPP on the production of IL-1 and TNF-α by mouse macrophages was also studied. LPS and dLPP at concentrations of 1 μg/ml induced the same level of secretion of IL-1 (Fig. 3).
FIG. 3.
Stimulation of IL-1 production by peritoneal mouse macrophages incubated with dLPP (1 and 5 μg per ml). S. typhosa LPS (1 μg/ml) and recombinant IL-1 (rIL-1) (1 ng/ml) were used as controls. The supernatants were removed after 24 h and assayed for the activity of IL-1. The results are expressed as the mean + standard deviation of four to six culture wells. A significant difference was observed between dLPP stimulation and control cells.
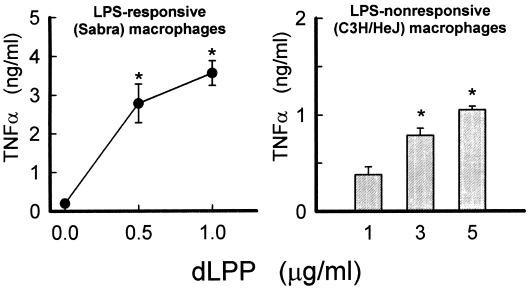
T. denticola dLPP induced the production of TNF-α in both LPS-responsive (Fig. 4, left panel) and LPS-nonresponsive (C3H/HeJ) (Fig. 4, right panel) macrophages in a concentration-dependent manner. The dLPP-mediated induction of both cytokines was not affected by the addition of polymyxin B (Fig. 3 and data not shown).
FIG. 4.
Stimulation of TNF-α production by responsive (left) and nonresponsive (right) mouse macrophages by increasing concentrations of dLPP. Peritoneal mouse macrophages were incubated with the indicated dLPP concentrations. The supernatants were removed after 24 h and assayed for the presence of TNF-α. The results are expressed as the mean + standard deviation of four to six culture wells. ∗, statistically significant differences from control cells with no stimulation (P < 0.05).
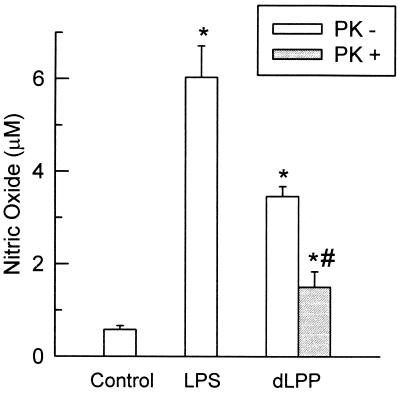
In order to find out whether structurally intact proteins contribute to the dLPP-induced macrophage activation, dLPP was submitted to degradation by proteinase K. As shown in Fig. 5, proteolytic degradation of dLPP prior to stimulation of the macrophages decreased the production of NO by 50%.
FIG. 5.
Stimulation of nitric oxide production by dLPP (5 μg/ml) before (−) and after (+) degradation with proteinase K (PK). S. typhosa LPS (1 μg/ml) was used as a positive control. The results are expressed as the mean + standard deviation of four to six culture wells. ∗, statistically significant difference from control cells; #, significantly lower than dLPP without PK.
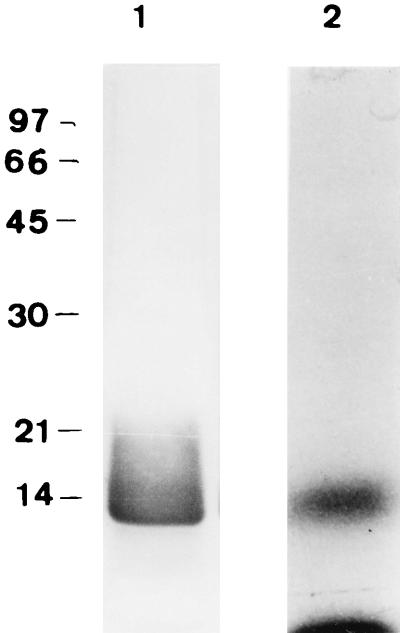
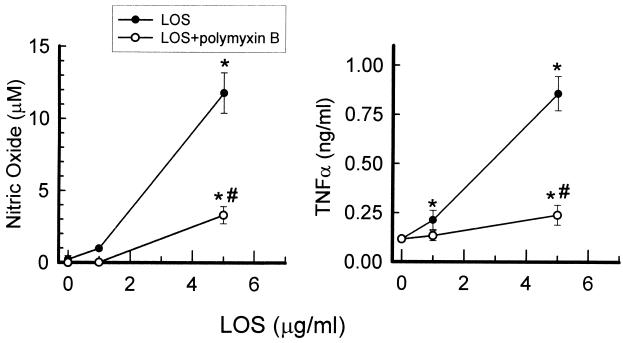
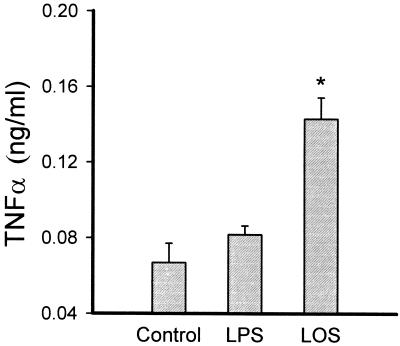
We have previously shown that T. denticola possesses an LOS molecule that can be extracted from the bacterium by the classical phenol-water method, followed by intensive proteolysis to degrade contaminating proteins, and purified by hydrophobic chromatography. SDS-PAGE of the purified LOS is shown in Fig. 6. The ability of the purified LOS to activate macrophages from LPS-responsive and LPS-nonresponsive mouse macrophages was further investigated. The LOS molecule induced the production of NO (Fig. 7, left panel) and TNF-α (Fig. 7, right panel) in a dose-dependent manner, but in contrast to dLPP, the LOS-mediated induction decreased by 80% in the presence of polymyxin B. Furthermore, LOS was able to induce TNF-α secretion from LPS-nonresponsive macrophages (Fig. 8).
FIG. 6.
SDS-PAGE of the purified LOS of T. denticola ATCC 35404. Lane 1, silver stain; lane 2, autoradiography of the cis[9-3H]octadecanoic acid-labeled LOS.
FIG. 7.
Stimulation of nitric oxide (left) and TNF-α (right) production by mouse macrophages incubated with increasing amounts of LOS in the presence or absence of polymyxin B (10 μg/ml). The results are expressed as the mean ± standard deviation of four to six culture wells. ∗, significantly higher than control cells; #, significantly lower than cells stimulated with LOS alone.
FIG. 8.
Stimulation of TNF-α production by C3H/HeJ (LPS-nonresponsive) macrophages by LOS (5 μg/ml). S. typhosa LPS (1 μg/ml) was used as a control. The supernatants were removed after 24 h and assayed for the presence of nitric oxide. The results are expressed as the mean + standard deviation of four to six culture wells. ∗, significantly different from control cells and LPS-stimulated cells.
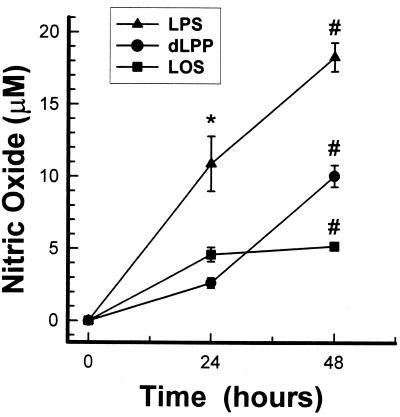
The kinetics of macrophage activation by dLPP and LOS were compared by measuring the NO induction of both stimulators after 24 and 48 h. Figure 9 shows kinetics of activation for the LOS different than that observed for dLPP. Maximal activation of NO by LOS was obtained after 24 h and then leveled off, while dLPP caused increased NO production throughout 48 h. S. typhosa LPS was used as a positive control and showed kinetics of activation similar to that of the T. denticola dLPP.
FIG. 9.
Kinetics of nitric oxide production by mouse macrophages stimulated with dLPP, LOS, and S. typhosa LPS. Peritoneal mouse macrophages were incubated with either dLPP (5 μg/ml), LOS (5 μg/ml), or S. typhosa LPS (1 μg/ml) for the indicated time intervals. The supernatants were removed and assayed for the presence of nitric oxide. The results are expressed as the mean ± standard deviation of four to six culture wells. ∗, significantly different from the LOS and dLPP group. The last two groups were not significantly different. #, All three groups were significantly different from each other.
DISCUSSION
Recent work in our laboratory has shown that T. denticola possesses membrane-associated lipoproteins and LOS (31). We also showed that an enriched lipoprotein fraction (dLPP) caused an enhanced luminol-dependent chemiluminescence effect and induced lyzozyme release by human polymorphonuclear leukocytes (31).
The present results demonstrate that dLPP and the purified LOS of T. denticola 35404 are able to induce the synthesis of NO, IL-1, and TNF-α by murine macrophages. As Triton X-114 detergent phase extracts contain protein and nonprotein amphipathic molecules, it was important to determine the extent of macrophage activation by dLPP and LOS and to exclude the possibility that contaminating exogenous endotoxin was responsible for the macrophage activation. dLPP-mediated activation of mouse macrophages was unaffected by levels of polymyxin B that were found to neutralize the activity of LPS isolated from S. typhosa, as measured by the secretion of TNF-α, IL-1, and NO. The lack of LPS involvement was also substantiated by the observation that dLPP induced NO and TNF-α production in LPS-unresponsive C3H/HeJ mice while S. typhosa LPS (1 μg/ml) had no effect on these cells. The Limulus amoebocyte lysate assay indicated the presence of very low endotoxin activity in the dLPP fraction (0.05 ng/μg of dLPP), which may be due to the presence of the T. denticola LOS in this fraction. It is worth noting that concentrations of bacterial endotoxin over 0.1 μg/ml are needed to induce detectable NO secretion by macrophages (41); therefore, the endotoxin levels found in dLPP are too low to explain the considerable amounts of NO and cytokines secreted following activation by dLPP.
dLPP appears to differ from purified S. typhosa LPS in its relative ability to induce IL-1, TNF-α, and NO. dLPP was consistently more potent in inducing IL-1 than in inducing NO and TNF-α (Fig. 1, 3, and 4). NO production was induced by proteinase K-digested treponemal dLPP. These findings suggest that N-terminal lipopeptides may constitute the biologically functional portions of the molecules, similar to the murine lipoprotein of E. coli as well as to the spirochetal lipoproteins of T. pallidum and B. burgdorferi. Interestingly, proteinase K-degraded dLPP was unable to directly stimulate human polymorphonuclear leukocytes (31), in contrast to the undigested counterpart, suggesting that different molecules in dLPP are responsible for the polymorphonuclear leukocyte and macrophage stimulation.
T. denticola possesses an LOS of approximately 14 to 21 kDa, which runs as a broad band in SDS-PAGE. This LOS was purified from strain ATCC 35404 (31), but it is also present in T. denticola ATCC 33520 and the clinical isolate GM-1 (unpublished observations). Yotis et al. (40) have also isolated LOS from various strains of T. denticola, including ATCC 35404, using a similar extraction methodology. Although the chemical structure of this molecule was not elucidated, its chemical composition resembled some characteristics of LPS (40). On the other hand, Schultz et al. (29) have recently proposed that T. denticola LOS is a new type of outer membrane lipid. Their chemical analysis places this molecule chemically close to the lipoteichoic acids of gram-positive bacteria, while its biophysical membrane behavior is similar to that of the LPSs of gram-negative bacteria. It was therefore of interest to investigate its ability to activate macrophages and to assess its contribution to the dLPP-mediated macrophage activation due to its presence in the dLPP fraction. LOS stimulated the activation of NO and TNF-α in LPS-responsive and -nonresponsive mouse macrophages. Macrophage activation by LOS, similarly to LPS activation, was neutralized by polymyxin B, but in contrast to LPS, LOS was found to stimulate macrophages from LPS-nonresponsive mice. It is unlikely that activation of C3H/HeJ macrophages is due to contaminating proteins, as the LOS was submitted to repeated boiling and protease degradation before the chromatographic removal of the proteins.
On the other hand, although LOS is present in the dLPP fraction, its contribution to the overall dLPP-mediated macrophage activation does not seem to be significant. Silver-stained SDS-PAGE of dLPP (31) and densitometric analysis (not shown) showed that the LOS does not represent more than 5 to 6% of the total stain, and thus its concentration in dLPP concentrations effective for activation is very low. dLPP-mediated macrophage activation was not inhibited by polymyxin B, while LOS activation was significantly decreased by it. Furthermore, LOS and dLPP showed different activation kinetics (Fig. 9). Thus, it seems that T. denticola may induce macrophage activation by at least two types of molecules, LOS and a different component(s) of dLPP, probably lipoproteins.
Gram-negative bacteria and their products are considered to be the primary causes of periodontal disease (33). However, data accumulated over the last decade emphasize the important role of the host response, not only in protecting the tissues from the bacterial burden but also in the process of periodontal destruction (37). Bacterial products have the ability to stimulate host cells to secrete a wide variety of proinflammatory mediators, which have numerous functions, some of which result in soft tissue and bone destruction (17, 18, 23). Proinflammatory cytokines, such as TNF-α and IL-1β, are known inducers of bone resorption (17). These cytokines have been found in high levels in the gingival tissues and gingival crevicular fluids of patients with periodontal disease, and their presence has also been correlated with active bursts of periodontal disease activity (10, 21, 34, 35). In addition, antibodies to TNF-α and IL-1β were recently found to block experimental periodontal disease in a primate model (1). Taken together, the evidence suggests that these two proinflammatory cytokines are important mediators in the progression of periodontal destruction.
NO may be produced in large amounts and over long periods when inflammatory stimuli induce the nitric oxide synthetase of various host cells (8, 19). NO is an important mediator in the killing of intracellular parasites by macrophages (6, 7), and it is also involved in inflammatory conditions, such as autoimmune disorders (39) and streptococcal cell wall arthritis (14). In the latter condition, NO seems to contribute to bone erosion and cartilage destruction (14).
It has been reported that inflamed periodontal tissues generate increased nitrite levels compared to uninflamed gingival tissues (38). LPS from gram-negative periodontopathogenic bacteria as well as dLPP or LOS from oral spirochetes may contribute to the enhanced NO levels in the periodontal tissues. Nevertheless, no data are available on the possible role of NO in the elimination of periodontal bacteria or its contribution to the injury accompanying periodontitis.
Using monoclonal antibodies against T. denticola, which is part of the anaerobic flora found in periodontal pockets, Simonson et al. (32) established quantitative evidence for a positive relationship between the presence of this oral spirochete and severe periodontitis. These diseases are chronic inflammatory processes that ultimately lead to a continuous alveolar bone and periodontal connective tissue destruction. The data presented in this study show that oral treponemal lipoproteins and LOS may contribute to the chronic inflammatory periodontal process. T. denticola may attach to epithelial cells and induce loss of cell contacts and increased epithelial permeability (36). These effects may facilitate the access of spirochetal molecules, some of which are associated with outer sheath membrane blebs (28), to connective tissue macrophages and induce their activation.
ACKNOWLEDGMENT
This study was supported by the Chief Scientist of the Israeli Ministry of Health (grants to M.N.S., G.R., and L.S.).
REFERENCES
- 1.Assuma R, Oates T, Cochran D, Amar S, Graves D. IL-1/TNF account for most of the inflammatory response in experimental periodontitis. IADR abstract 1298. J Dent Res. 1997;76:176. [Google Scholar]
- 2.Barak V, Peritt D, Flechner I, Yanai P, Halperin T, Treves A J, Dinarello C A. The specific IL-1 inhibitor from the human M20 cell line is distinct from the IL-1 receptor antagonist. Lymphokine Cytokine Res. 1991;10:437–442. [PubMed] [Google Scholar]
- 3.Beausejour A, Deslauriers N, Grenier D. Activation of the interleukin-1b precursor by Treponema denticola: a potential role in chronic inflammatory periodontal diseases. Infect Immun. 1997;65:3199–3202. doi: 10.1128/iai.65.8.3199-3202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt M E B, Ridley S, Radolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 6.Evans T G, Thai L, Granger D L, Hibbs J B., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993;151:907–915. [PubMed] [Google Scholar]
- 7.Green S J, Meltzer M S, Hibbs J B, Jr, Nacy C A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine dependent killing mechanism. Immunology. 1990;144:278–283. [PubMed] [Google Scholar]
- 8.Hibbs J B, Jr, Taintor R R, Vavrin Z, Rachlin E M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 9.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 10.Honig J, Rordorf-Adam C, Siegmund C, Wiedermann W, Erard F. Increased interleukin-1 beta concentration in gingival tissue from periodontitis patients. J Periodontal Res. 1989;24:362–367. doi: 10.1111/j.1600-0765.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makinen K K, Makinen P L, Syed S A. Purification and substrate specificity of an endopeptidase from the human oral spirochete Treponema denticola ATCC 35405, active on furacryloyl-Leu-Gly-Pro-Ala and bradykinin. J Biol Chem. 1992;267:14285–14293. [PubMed] [Google Scholar]
- 13.Makinen K K, Makinen P L, Syed S A. An endo-active proline-specific oligopeptidase from Treponema denticola ATCC 35405: evidence of hydrolysis of human bioactive peptides. Infect Immun. 1994;62:4938–4947. doi: 10.1128/iai.62.11.4938-4947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCartney-Francis N, Allen J B, Mizel D E, Albina J E, Xie Q W, Nathan C F, Wahl S M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993;178:749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore W E C, Holdeman L V, Cato E P, Smibert R M, Burmeister J A, Ranney R R. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983;42:510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 17.Mundy G R. Inflammatory mediators and the destruction of bone. J Periodontal Res. 1991;26:213–217. doi: 10.1111/j.1600-0765.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 18.Mundy G R, Roodman G D, Bonewald L F, Yoneda T, Sabatini M. Effect of TNF and lymphotoxin on bone cells. In: Aggarwal B B, Vilcek J, editors. Tumor necrosis factors. Structure, function and mechanism of action. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 483–498. [PubMed] [Google Scholar]
- 19.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 20.Norgard M V, Riley B S, Richardson J A, Radolf J D. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect Immun. 1995;4:1507–1515. doi: 10.1128/iai.63.4.1507-1515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offenbacher S, Soskolne W A, Collins J G. Prostaglandins and other eicosanoids in gingival cervicular fluid as a markers of periodontal disease susceptibility and activity. In: Johnson N W, editor. Risk markers for oral diseases: periodontal disease. Cambridge, England: Cambridge University Press; 1991. pp. 313–337. [Google Scholar]
- 22.Page R C, Schroeder H E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Investig. 1976;33:235–249. [PubMed] [Google Scholar]
- 23.Page R C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 24.Radin N. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- 25.Radolph J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1994;154:2866–2877. [PubMed] [Google Scholar]
- 26.Ranney R R. Pathogenesis of periodontal disease. In: Klavan B, Genco R, Loe H, Page R, Stern I, Thorpe J, Barrington E, editors. International Conference on Research in the Biology of Periodontal Disease. Chicago, Ill: The College of Dentistry, University of Illinois; 1977. pp. 223–300. [Google Scholar]
- 27.Riley B S, Oppenheimer-Marks N, Hansen E J, Radolf J D, Norgard M V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992;165:484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- 28.Rosen G, Naor R, Rahamim E, Yishai R, Sela M N. Proteases of Treponema denticola outer sheath and extracellular vesicles. Infect Immun. 1995;63:3973–3979. doi: 10.1128/iai.63.10.3973-3979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz C P, Wolf V, Lange R, Mertens E, Wecke J, Naumann D, Zahringer U. Evidence for a new type of outer membrane lipid in oral spirochete Treponema denticola. J Biol Chem. 1998;273:15661–15666. doi: 10.1074/jbc.273.25.15661. [DOI] [PubMed] [Google Scholar]
- 30.Sela M N, Weinberg A, Borinsky R, Holt S C, Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by treponemal factors. Infect Immun. 1988;56:589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sela M N, Bolotin A, Naor R, Weinberg A, Rosen G. Lipoproteins of Treponema denticola. Their effect on human polymorphonuclear neutrophils. J Periodontal Res. 1997;32:455–466. doi: 10.1111/j.1600-0765.1997.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 32.Simonson L G, Goodman C H, Bial J J, Morton H E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988;56:726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 34.Stashenko P, Fujiyoshi P, Obernesser M N, Prostak L, Haffajee A D, Socransky S S. Levels of interleukin-1 beta in tissue from sites of active periodontal disease. J Clin Periodontol. 1991;18:548–554. doi: 10.1111/j.1600-051x.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 35.Stashenko P, Jundinski J J, Fujiyoshi P, Rynar J, Socransky S S. Tissue levels of bone-resorptive cytokines in periodontal disease. J Periodontol. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 36.Uitto V-J, Pan Y M, Leung W K, Larjva H, Ellen R P, Finlay B B, McBride B C. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63:3401–3410. doi: 10.1128/iai.63.9.3401-3410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Dyke T E, Lester M A, Shapira L. The role of host response in periodontal disease progression: implications for future treatment strategies. J Periodontol. 1993;64:792–806. doi: 10.1902/jop.1993.64.8s.792. [DOI] [PubMed] [Google Scholar]
- 38.Wahl S M, Hines K L, Imamichi T, Tian H, Shepheard S, McCartney-Francis N. Regulation of chronic inflammation. In: Genco R, Hamada S, Lehner T, McGhee J, Mergenhagen S, editors. Molecular pathogenesis of periodontal disease. Washington, D.C: ASM Press; 1994. pp. 183–190. [Google Scholar]
- 39.Weinberg J B, Granger D L, Pisetsky D S, Seldin M F, Misukonis M A, Mason S N, Pippen A M, Ruiz P, Wood E R, Gilkeson G S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-l-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yotis W W, Macaluso F, Gopalsami C. Immunochemical features of a macromolecule of Treponema denticola. J Basic Microbiol. 1995;35:255–268. doi: 10.1002/jobm.3620350411. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Morrison D C. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor α and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177:511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]