Abstract
BACKGROUND
Spinal cysts in the interdural space are extremely rare and are not included in the standard classification of spinal meningeal cysts.
OBSERVATIONS
A 60-year-old female presented to our hospital with a spastic gait and numbness in both palms. Magnetic resonance imaging (MRI) revealed a spinal cyst from C4 to T4 compressing the spinal cord. Computed tomography myelography revealed a fistula at C4–5 and C5–6 that connected the cyst along the right C5 and C6 root sleeves. The cyst was located within the dura mater, and communication with the arachnoid space was achieved using a shunt tube. There was partial spastic gait amelioration after the procedure, but the patient experienced a relapse 2 months postoperation. A repeat procedure was performed without a shunt tube to allow greater communication between the cyst and the subarachnoid space. After this, marked improvement in gait function was observed, and MRI showed a significant reduction in cyst volume.
LESSONS
Interdural spinal meningeal cysts are rare. When the interdural cyst cannot be removed entirely, surgery may be appropriate for providing a shunt tube or establishing communication between the cyst and arachnoid space to maintain the circulation of cerebrospinal fluid collected in the cyst cavity.
Keywords: symptomatic, interdural cyst, cervical spine, thoracic spine
ABBREVIATIONS: CSF = cerebrospinal fluid, CTM = computed tomography myelography, MRI = magnetic resonance imaging, SMC = spinal meningeal cyst
Spinal meningeal cysts (SMCs) are commonly divided into two types: epidural and intradural cysts.1 However, a third type of SMC in the interdural space is extremely rare and barely considered, even though seven cases have been reported in the literature.2–6 Herein, we report the case of a cervicothoracic SMC in the interdural space and propose a revised classification.
Illustrative Case
A 60-year-old female presented to our hospital with numbness in both palms and a spastic gait that worsened in the afternoon. Her medical history included benign adult familial myoclonic epilepsy with no definite history of trauma prior to her visit to the hospital. Muscle strength had decreased slightly in the right deltoid muscle while tendon reflex findings in the bilateral upper and lower extremities were unremarkable.
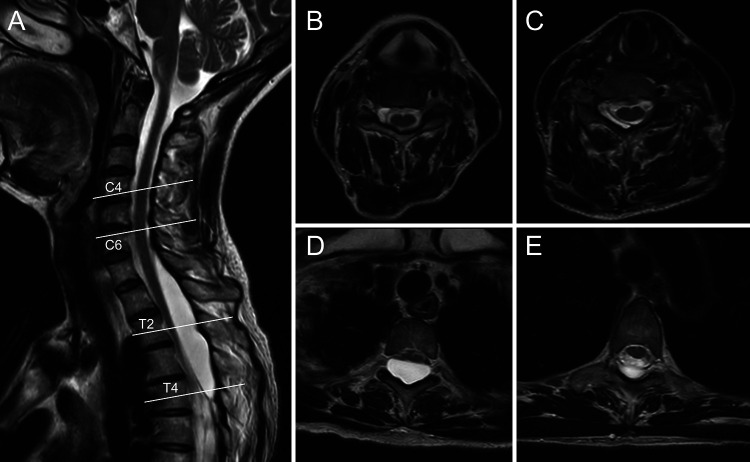
Magnetic resonance imaging (MRI) revealed a cystic lesion at the level of C4 to T4, compressing the spinal cord (Fig. 1). Computed tomography myelography (CTM) via lumbar puncture showed contrast medium accumulation in the cyst via the fistula along the C5 and C6 root sleeves and scalloping of the right pedicle and vertebral body at the C4 and C5 levels (Fig. 2). A differential diagnosis of an interdural or intradural spinal meningeal cyst was made based on MRI and CTM findings.
FIG. 1.
Initial spinal cord magnetic resonance (MR) images. Sagittal T2-weighted MR image (A) shows a cystic lesion from the C4 to T4 level, compressing the spinal cord. Axial T2-weighted MR images (B–E) at the level of the white lines in the sagittal image.
FIG. 2.
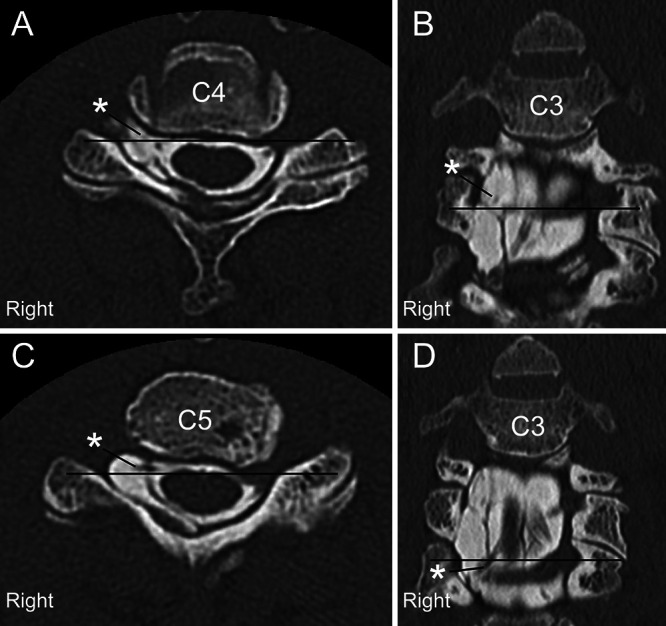
Axial (A and C) and coronal (B and D) CTM images at each level of the black line show contrast medium accumulation in the cyst via the fistula along the C5 and C6 right side root sleeves (asterisks) and scalloping of the right pedicle and vertebral body at the C4 and C5 levels.
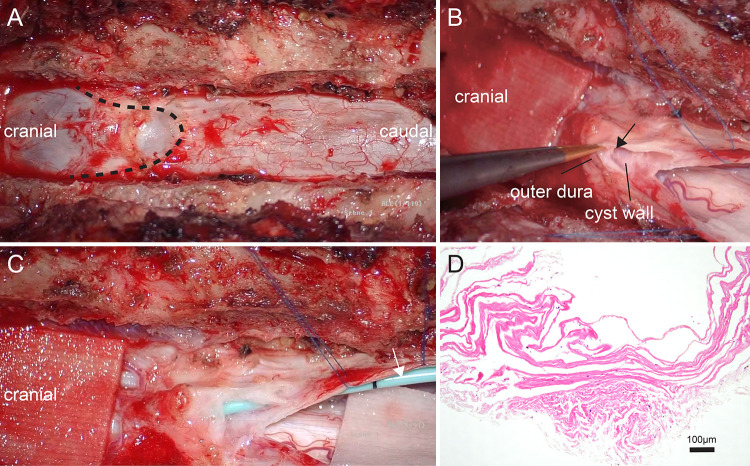
The patient underwent a right-sided C4–5 hemilaminectomy and C4–5 and C5–6 right laminoforaminotomy to expose the cyst. Dura mater thinning was observed as part of the intraoperative findings, as the cyst was located within the dural space. Thinning of the dura mater made closure of the fistula along the root sleeve difficult due to the high possibility of postoperative cerebrospinal fluid (CSF) leakage. Thus, we decided to approach from the caudal side of the cyst to allow communication with the arachnoid space. The patient underwent caudal T2 partial laminectomy and T3–6 laminectomy to allow communication between the cyst and arachnoid space. The cyst could be seen through the outer dura and was filled with CSF (Fig. 3A). The cyst was confirmed to be located within the dura mater (Fig. 3B), and part of its inner wall was sent for pathological analysis (Fig. 3D). Communication between the cyst and the arachnoid space was established using a shunt tube (Fig. 3C), and a watertight suture of the dura mater was performed. Histopathological examination of the cyst wall sample revealed fibroconnective tissue, further supporting the theory that it was located within the dura.
FIG. 3.
Intraoperative microscopic views showing the lower end of the cyst (broken line) and the lumen (black arrow) formed within the dura (A and B). A shunt tube (white arrow) was implanted between the cyst and the arachnoid space (C). Histopathological examination (hematoxylin and eosin) shows fibroconnective tissue, suggesting it to be dura (D).
Although numbness in both palms, which presented before surgery, did not change after the procedure, alleviation of the spastic gait was observed. The patient was discharged to home from the hospital on postoperative day 14. The patient experienced a relapse identical to the initial presentation 2 months postoperatively despite the partial improvement observed 1 month postoperatively. Since MRI revealed no significant changes in the cyst volume at the time of symptom relapse (Fig. 4A), shunt malfunction was suggested as the cause of the symptom recurrence. The repeat procedure was designed to create a bigger communication between the cyst and the subarachnoid space.
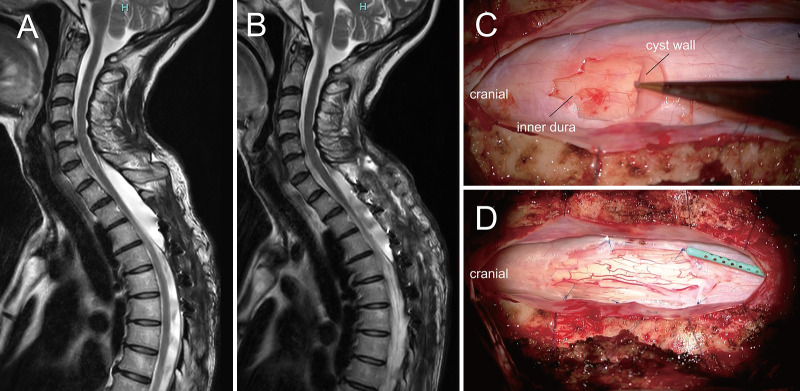
FIG. 4.
Postoperative sagittal T2-weighted magnetic resonance (MR) image (A) shows the shunt tube implanted in the cyst and no significant change in the volume of the cyst. Sagittal T2-weighted MR image (B) after the second procedure shows a significant decrease in the cyst volume. Intraoperative microscopic view of the second procedure shows the cyst wall and the inner layer of dura mater (C) and a bigger communication between the cyst cavity and the subarachnoid space (D).
The patient underwent caudal C7 partial laminectomy and T1–2 laminectomy to establish greater communication between the cyst cavity and the arachnoid space. Similar to findings from the initial procedure, the cyst was confirmed to be located within the dura mater. The cystic membrane was incised, and the inner layer of dura mater was identified (Fig. 4C). A bigger communication between the cyst cavity and the subarachnoid space was established by incision of the inner layer of the dura mater and the arachnoid membrane (Fig. 4D). The implanted shunt tube from the previous procedure was not removed, and a watertight suture of the dura mater was performed. Laminoplasty was performed from T1 to T3. A significant improvement in gait function was observed after the second procedure compared to the early stage of the initial procedure. MRI after the second procedure also revealed significant reduction in cyst volume (Fig. 4B). The patient was discharged home from the hospital on postoperative day 20. The patient did not experience a spastic gait relapse and had a good postoperative course 1 month after the second procedure.
Discussion
Observations
SMCs are classified into three conventional types based on intraoperative findings:1 extradural cysts without spinal nerve root fibers (type I), extradural cysts with spinal nerve root fibers (type II), and intradural cysts (type III). This classification is widely accepted. Although seven cases of interdural spinal cysts have been reported previously, some of these novel cases have only recently been described (Table 1).2–6 Herein, we also report this novel type of spinal cyst, which was not previously classified. Even though Sajjad et al.6 already suggested a revised classification that included SMCs, we further propose a modified classification that includes interdural cysts with and without communication with the arachnoid space via the fistula. Our proposed classification of SMCs is as follows: extradural meningeal cysts without spinal nerve root fibers (type IA), extradural meningeal cysts with spinal nerve root fibers (type IB), interdural meningeal cysts with communication with the arachnoid space via a fistula (type IIA), interdural meningeal cysts without communication with the arachnoid space via a fistula (type IIB), and intradural meningeal cysts (type III).
TABLE 1.
Literature review of interdural spinal cysts
| Authors & Year | Age (yrs) | Sex | Trauma | Location of Interdural Spinal Cyst | Communication Btwn Cyst & Arachnoid Space | Cyst Fluid | Procedure | Outcome |
|---|---|---|---|---|---|---|---|---|
| Done et al., 19844 |
6 |
M |
− |
L3–S3 |
+ |
CSF |
Opened cyst & communication btwn cyst & arachnoid space |
Partial improvement |
| Done et al., 19844 |
9 |
M |
− |
L5–S4 |
+ |
CSF |
Closed defect w/ flap & glue |
Partial improvement |
| Done et al., 19844 |
12 |
M |
− |
T12–L5 |
+ |
CSF |
Closed defect w/ flap & glue |
Complete improvement |
| Chen & Chen, 19963 |
18 |
M |
+ |
C3–5 |
Unknown |
CSF |
Opened cyst |
Partial improvement |
| Lee et al., 20105 |
72 |
M |
− |
L2–4 |
− |
Mucinous fluid |
Removal of all cystic component |
Partial improvement |
| Sajjad et al., 20156 |
40 |
F |
− |
T5 |
+ |
Unknown |
Opened cyst & occluded fistula |
Complete improvement |
| Aoun et al., 20192 |
37 |
M |
+ |
L4–5 |
− |
Motor-oil fluid |
Total cauterization |
Complete improvement |
| Present case | 60 | F | − | C5–T4 | + | CSF | Shunt & communication btwn cyst & arachnoid space | Partial improvement |
− = no; + = yes.
In previous reports, some researchers have hypothesized that interdural cyst formation might be caused by the destruction of the inner dura mater, resulting in a one-way valve that allows the CSF to accumulate in the interdural space.3,7 Furthermore, CSF might migrate from the subarachnoid space into the interstitial spaces of the dura via exiting nerves, exiting vessels, transcellular transport, and arachnoid granulations, indicating that CSF is always present in the dura in small amounts.8 Therefore, in the present case, the CSF may have flowed unidirectionally, and the cyst may have formed longitudinally within the dura mater due to a defect caused by weakness of the intradural membrane along the root sleeves. This hypothesis is supported by our radiographic, intraoperative, and histopathological findings. Additionally, the spastic gait, which worsened in the afternoon, can be explained by a gradual increase in fluid collection in the cyst over time. The marked amelioration of spastic gait was due to the establishment of communication between the cyst cavity and the arachnoid space.
Although the procedure aimed to establish communication between the interdural cyst and arachnoid space to relieve spinal cord compression, to the best of our knowledge, this study is the first to report such communication via a shunt tube (Table 1). In the present study, the procedure, which used a shunt tube, was performed to discharge the CSF collected within the dura mater and prevent spinal cord compression that further increases CSF collection. Total removal of the interdural cyst is the preferred procedure. However, when the interdural cyst cannot be removed entirely due to the presence of a fistula along the root sleeve (as in the current case), establishing a communication between the cyst and the arachnoid space using a shunt tube can be performed. This shunt method has the potential complication of shunt malfunction. In such cases, as in this present case, it is necessary to establish greater communication between the cyst cavity and the subarachnoid space to maintain CSF circulation.
Lessons
Interdural SMCs are rare. When the interdural cyst cannot be removed entirely, surgery may be appropriate for providing a shunt tube or establishing communication between the cyst and arachnoid space to maintain the circulation of CSF collected in the cyst cavity.
Acknowledgments
We would like to thank Editage for English language editing.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Kawasaki, Nakajima, Ioroi. Acquisition of data: Kawasaki, Nakajima. Analysis and interpretation of data: Kawasaki, Ioroi. Drafting the article: Kawasaki, Kobayashi. Critically revising the article: Maki. Reviewed submitted version of manuscript: Maki. Approved the final version of the manuscript on behalf of all authors: Kawasaki. Study supervision: Takayama.
References
- 1. Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988;68(3):366–377. doi: 10.3171/jns.1988.68.3.0366. [DOI] [PubMed] [Google Scholar]
- 2. Aoun SG, Plitt AR, El Ahmadieh TY, Al Tamimi M, Whitworth T. Traumatic lumbar interdural cyst with intradural expansion and compression of the cauda equina: case report and surgical video. Cureus. 2019;11(6):e4824. doi: 10.7759/cureus.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen HJ, Chen L. Traumatic interdural arachnoid cyst in the upper cervical spine. Case report. J Neurosurg. 1996;85(2):351–353. doi: 10.3171/jns.1996.85.2.0351. [DOI] [PubMed] [Google Scholar]
- 4. Done SL, Hayman LA, New PF, Davis KR, Chapman PH. Interdural cyst of the lumbosacral region. Neurosurgery. 1984;14(3):287–294. doi: 10.1227/00006123-198403000-00005. [DOI] [PubMed] [Google Scholar]
- 5. Lee JH, Jung TG, Kim HS, Jang JS, Lee SH. Symptomatic isolated lumbar interdural arachnoid cyst. Neurol Med Chir (Tokyo) 2010;50(11):1035–1038. doi: 10.2176/nmc.50.1035. [DOI] [PubMed] [Google Scholar]
- 6. Sajjad J, Yousaf I, Bermingham N, Kaar G. Interdural spinal cyst: a rare clinical entity. World Neurosurg. 2016;88:688.e9–688.e12. doi: 10.1016/j.wneu.2015.11.051. [DOI] [PubMed] [Google Scholar]
- 7. McCrum C, Williams B. Spinal extradural arachnoid pouches. Report of two cases. J Neurosurg. 1982;57(6):849–852. doi: 10.3171/jns.1982.57.6.0849. [DOI] [PubMed] [Google Scholar]
- 8. Sneyers B, Ramboer K. Spinal subdural hygroma. Acta Neurol Belg. 2021;121(2):311–319. doi: 10.1007/s13760-020-01558-1. [DOI] [PubMed] [Google Scholar]