Abstract
BACKGROUND
The computed tomography angiography (CTA) “spot sign” is a well-recognized radiographic marker in primary intracerebral hemorrhage (ICH). Although it has been demonstrated to represent an area of active hemorrhage or contrast extravasation, the exact pathophysiology remains unclear. Vascular mimics of the spot sign have been identified; however, those representing pseudoaneurysm and small vessel aneurysm have rarely been reported.
OBSERVATIONS
A 57-year-old female with a past medical history of hypertension and diabetes mellitus presented with 2 weeks of acute-onset, worsening headache. Computed tomography scanning showed a right interior frontal lobe intraparenchymal hemorrhage. CTA demonstrated a punctate focus of hyperattenuation within the hematoma, consistent with a spot sign, which corresponded to a distal anterior cerebral artery pseudoaneurysm on a cerebral angiogram. The patient subsequently underwent emergent resection of the pseudoaneurysm and hematoma evacuation without complications. Her postoperative course was unremarkable without acute concerns or residual symptoms at the 4-month follow-up.
LESSONS
The authors present a unique case of a distal anterior cerebral artery pseudoaneurysm presenting as a spot sign in a relatively young patient without underlying vascular disease. Given the need for emergent intervention, intracranial pseudoaneurysm is an important diagnosis to consider in the presence of a spot sign in atypical clinical presentations of primary ICH.
Keywords: aneurysm, pseudoaneurysm, spot sign, intracerebral hemorrhage
ABBREVIATIONS: CT = computed tomography, CTA = computed tomography angiography, ICH = intracerebral hemorrhage, MRI = magnetic resonance radiography, SICH = secondary intracranial hemorrhage
Intracerebral hemorrhage (ICH) comprises 10%–20% of all acute strokes and is associated with significant mortality, with a 50% survival rate at the 1-year follow-up.1 First described in 2007, the computed tomography angiography (CTA) “spot sign,” defined as one or more 1- to 2-mm foci of enhancement within the hematoma on CTA, has become a well-recognized predictor of hematoma expansion and poor clinical outcome.2,3 Although a spot sign has been demonstrated to represent a site of active hemorrhage and contrast extravasation,4 the exact underlying pathophysiology remains unclear. Studies have suggested it may represent a site of primary or secondary vessel disruption, a Charcot-Bouchard microaneurysm, or fibrin globe formation after arrested hemorrhage.2,4,5 In addition, vascular and nonvascular spot sign mimics have been identified, including tumor-related hematoma calcifications, microarteriovenous malformations, pseudoaneurysms in moyamoya disease, and partially thrombosed aneurysms.6
Pseudoaneurysm has been identified as a potential cause of spot sign since the spot sign was first described in 2007.2 However, to our knowledge, spot signs representing pseudoaneurysm and small vessel aneurysm have rarely been reported since then. Therefore, we present a case of a spot sign on initial CTA that was found to be a pseudoaneurysm on both cerebral angiogram and intraoperative examination. The aim of this brief report is to inform clinicians that a CTA spot sign in conjunction with an atypical clinical presentation could be indicative of an underlying pseudoaneurysm.
Illustrative Case
A 57-year-old African American female with a past medical history of hypertension and diabetes mellitus presented to an outside hospital with 2 weeks of a worsening headache. She had an acute-onset, sharp, holocephalic headache immediately after sneezing and feeling a “pop” in her head. After presenting to an outside hospital 1 week after symptom onset, she received standard migraine analgesia with minimal relief and was discharged home.
Over the next week, her headache worsened, ultimately prompting a computed tomography (CT) scan to be obtained, which showed a 5.1 × 1.8 × 3.3–cm intraparenchymal hematoma in the right interior frontal lobe (Fig. 1A). CTA showed no aneurysm; however, there were punctate foci of hyperattenuation within the hematoma, consistent with a spot sign (Fig. 2). Brain magnetic resonance imaging (MRI) with and without contrast and cerebral angiography were performed for further work-up of the hemorrhage. MRI confirmed a corresponding focus of enhancement within the medial aspect of the hematoma, which appeared to be stable (Fig. 1B). The cerebral angiogram demonstrated a 2.00 × 2.10–mm aneurysm of a distal branch of the anterior cerebral artery (Fig. 3), consistent with findings of a pseudoaneurysm.
FIG. 1.

A: Noncontrast CT showing a right inferior frontal lobe intraparenchymal hemorrhage. B: MRI showing the spot sign (arrow).
FIG. 2.
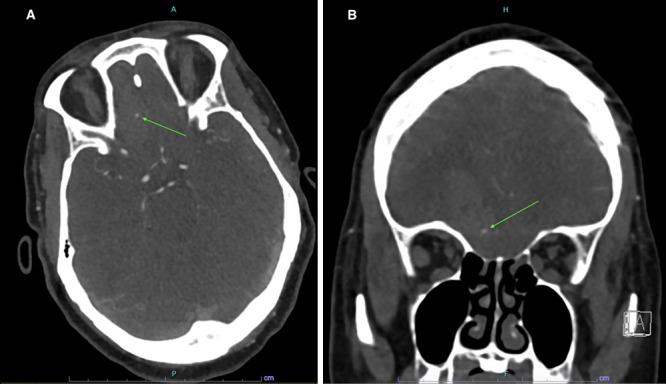
Axial (A) and coronal (B) CTA showing the spot sign (arrows).
FIG. 3.
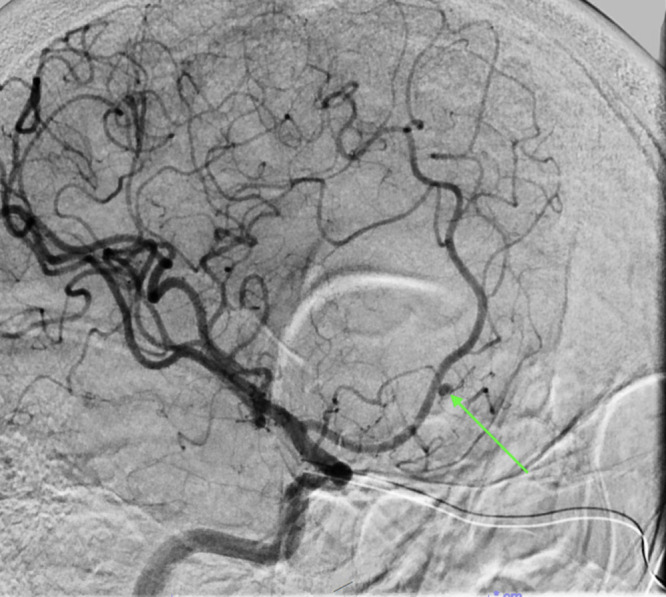
Cerebral angiogram showing a small distal anterior cerebral artery branch pseudoaneurysm (arrow).
Due to persistent symptoms and concerns for a risk of rerupture, the patient was taken urgently to the operating room for a stereotactic craniotomy with evacuation of the hematoma and exploration of the aneurysm. Once the superficial part of the hematoma was resected with suction, the pseudoaneurysm was visualized under the microscope. Because of its friable nature, it was resected with bipolar electrocautery while ensuring that the main anterior cerebral artery remained patent. An open approach was favored over an endovascular approach because there was concern that the vessel sacrifice that would have been performed endovascularly would have put the anterior cerebral artery more at risk. The aneurysm was located at a short distance off the main trunk of the anterior cerebral artery in a small vessel that had an acute angle off the main artery. The endovascular team felt it would be very difficult to catheterize that branch successfully. They also had reservations about the use of an embolization agent and having reflux into the anterior cerebral artery. The hematoma size was also felt to create some mass effect, and surgery provided an added benefit of normalizing the patient’s intracranial pressure. The patient tolerated the procedure well without intraoperative or postoperative complications. Repeat angiography showed no evidence of residual pseudoaneurysm 4 days after resection (Fig. 4A), and the patient was discharged on postoperative day 5. There were no acute concerns or residual symptoms at the 1-month and 4-month follow-up visits. Repeat head CT at the 4-month follow-up showed significant resorption of intraparenchymal hemorrhage and decreased cerebral edema (Fig. 4B).
FIG. 4.

A: Repeat cerebral angiography 4 days postoperatively with no residual aneurysm. B: Noncontrast CT at the 4-month follow-up.
Discussion
Observations
The spot sign, characterized as a small area of contrast enhancement within the hematoma discontinuous from any adjacent vasculature,2 is present in 17%–56% of cases of primary ICH and serves as an independent predictor of hematoma expansion and poor clinical outcome.7 Current hypotheses for its origin include sites of active hemorrhage or contrast extravasation, fibrin globe formation after arrested hemorrhage, or Charcot-Bouchard microaneurysms.2,3 Dowlatshahi et al.4 demonstrated on dynamic CT that a spot sign in the early phases of ICH is a site of vessel rupture and contrast active extravasation, whereas in later phases, it represents a point of resolved hemorrhage consistent with the fibrin globe hypothesis. This suggests that there might be more than one underlying cause of spot sign, and it partially explains the varied positive predictive values for hematoma expansion reported in the literature (24%–79%).8–10
Because of its peripheral location in the hematoma and separation from adjacent vasculature on CTA, the spot sign is generally presumed to occur without underlying aneurysm or aneurysm-like vascular abnormalities.2 However, vascular spot sign mimics have been reported in both primary and secondary intracerebral hemorrhage (SICH), or ICH due to an underlying vascular abnormality.6,11–13 Gazzola et al.6 reported cases of aneurysm and pseudoaneurysm presenting as spot signs. However, both cases were excluded as true spot signs because of the subarachnoid location or extension of a linear density beyond the hematoma margin, which is not seen in any cases of spot sign.6 Similarly, de Jong et al.13 described a case of distal intracranial aneurysm masquerading as spot sign. Upon further imaging with dynamic four-dimensional CTA, the spot sign appeared to be connected to adjacent vasculature. In addition, Nicholson et al.11 reported multiple cases of “spot on a string sign,” a rare imaging finding of spot sign connected to a vessel, in a cohort of younger patients (average age 48 years) presenting with large-volume ICH. Two out of seven patients had underlying aneurysms corresponding to the “spot on a string” sign. Although Caton et al.12 previously compared imaging characteristics of patients with mycotic aneurysm versus spot sign–positive ICH and identified a “connecting vessel” as a unique feature for mycotic aneurysm, these two patients had no identifiable infection. Additional vascular causes of the spot sign reported in the literature include a 5-mm lenticulostriate aneurysm in an 81-year-old patient with a right basal ganglia ICH,5 a common location for hypertension-induced ICH. Another report documented an artery of Percheron pseudoaneurysm causing left thalamic ICH in a 50-year-old Japanese woman with moyamoya disease.14 In this case, alterations in blood flow and arterial wall integrity may have predisposed the patient to an aneurysm and/or aneurysm-like vascular malformation.15
Our patient has an atypical presentation for primary ICH. She presented at age 57, which is significantly younger than the mean age of 66 reported in a 2016 systemic review by Dowlatshahi et al.16 Furthermore, imaging was not acquired until she had worsening symptoms of over 2 weeks, whereas more than half of the patients with ICH underwent CTA within 4 hours of onset.16 The lobar location of the hemorrhage was also less common than typical locations in hypertension-induced ICH.7 In a 2017 multicenter INTERACT2 trail, Delcourt et al.7 reported the most common region of hemorrhage to the putamen/globus pallidus (56%) compared with cerebral lobes (14%). Although patients with SICH tend to be younger at presentation with a larger baseline hematoma,6,17 our patient did not present with subarachnoid hemorrhage and had no underlying vascular diseases (e.g., moyamoya disease). Given our patient’s atypical clinical presentation of primary ICH, further work-up, including MRI and angiography, was indicated to rule out underlying vascular malformations. On further imaging and intraoperative examination, the responsible lesion was found to be a ruptured pseudoaneurysm.
Intracranial pseudoaneurysm represents about 1% of all intracranial aneurysms and is thought to be due to an encapsulated hematoma in communication with the ruptured artery.18 The most common causes are trauma, iatrogenic and infectious; however, spontaneous cases have been reported.19,20 Despite accounting for only 1% of intracranial aneurysms, it has a high mortality rate, up to 20% in some reports.18 Given the high mortality rate and need for intervention, early diagnosis and management are necessary. Endovascular techniques such as coiling, stent coiling, parent artery occlusion, and glue embolization have been shown to be as effective as open surgery and have become the standard of care with few limitations.21 Surgery is typically performed in cases with difficult catheterization, failed endovascular therapy, presence of mass effect or acute hematoma requiring evacuation, or location at distal branches.21 Conservative management of the hematoma can be considered in selected cases;22 however, in this case, it was felt to be less optimal because there was some mass effect. Some perihematoma edema was also present on the CT scan in addition to the size of the hematoma. The hematoma was in the subcortical space but easily accessible with a small surgical risk. The decision for surgery was primarily driven by the reservations expressed by the endovascular team in this case. Considering the findings of Dowlatshahi et al. on dynamic CT, the spot sign in our patient’s case may represent fibrin globe formation after arrested hemorrhage from a pseudoaneurysm, because she presented 2 weeks after symptom onset and her hematoma was relatively stable on imaging. However, the spot sign could also represent rupture and active bleeding from a pseudoaneurysm in patients presenting with more acute onset of symptoms.
Lessons
We present a unique case of CT spot sign presenting as a distal anterior cerebral artery pseudoaneurysm. Pseudoaneurysm has been proposed as a mechanism for spot sign since Wada et al.2 first described it in 2007. However, few cases have been reported since then.13,14 Although multiple cases of pseudoaneurysm and aneurysm presenting as spot sign have been discussed, few cases are true spot signs upon further examination. To our knowledge, of the two reported cases of pseudoaneurysm, one case was excluded because the spot sign was connected to a vessel originating external to the hematoma. The other case was in a patient with preexisting moyamoya disease, a known vascular disease that predisposes to aneurysm formation.15
In contrast to the typical hypertensive ICH presentation, our patient presented at a relatively young age with a longer duration of symptoms without a history of anticoagulant use or neurological deficits. Furthermore, the lobar location of her hemorrhage is less common than typical subcortical areas seen in primary ICH and distinct from the other reported pseudoaneurysm case. Given her atypical presentation and the presence of the spot sign on CTA, further investigation was necessary. Subsequent cerebral angiogram and intraoperative examination found an anterior cerebral artery pseudoaneurysm, which was likely responsible for the lobar hemorrhage.
In the setting of unexpected clinical presentation, a spot sign on CTA may be due to underlying vascular pathologies, such as pseudoaneurysm. Given the risk of continued bleeding and rupture, immediate surgical intervention is required, and early diagnosis and management are necessary. Therefore, clinicians should be aware of pseudoaneurysm as a differential diagnosis in patients with ICH and potentially consider further imaging in patients with atypical presentations.
Disclosures
Dr. Coppens reported personal fees from NICO Corporation outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Coppens, Zhang, Prim. Acquisition of data: Huang, Zhang. Analysis and interpretation of data: Huang, Zhang, Prim. Drafting the article: all authors. Critically revising the article: Coppens, Huang, Zhang. Reviewed submitted version of manuscript: Coppens, Zhang. Approved the final version of the manuscript on behalf of all authors: Coppens. Administrative/technical/material support: Zhang. Study supervision: Prim.
References
- 1. Pinho J, Costa AS, Araújo JM, Amorim JM, Ferreira C. Intracerebral hemorrhage outcome: a comprehensive update. J Neurol Sci. 2019;398:54–66. doi: 10.1016/j.jns.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 2. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38(4):1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 3. Lei C, Geng J, Qi Z, Zhong L. Different criteria for defining “spot sign” in intracerebral hemorrhage show different abilities to predict hematoma expansion and clinical outcomes: a systematic review and meta-analysis. Neurosurg Rev. 2021;44(6):3059–3068. doi: 10.1007/s10143-021-01503-7. [DOI] [PubMed] [Google Scholar]
- 4. Dowlatshahi D, Wasserman JK, Momoli F, et al. Evolution of computed tomography angiography spot sign is consistent with a site of active hemorrhage in acute intracerebral hemorrhage. Stroke. 2014;45(1):277–280. doi: 10.1161/STROKEAHA.113.003387. [DOI] [PubMed] [Google Scholar]
- 5. Tan LA, Kasliwal MK, Johnson AK, Lopes DK. The “spot sign” secondary to a ruptured lenticulostriate artery aneurysm. Clin Imaging. 2014;38(4):508–509. doi: 10.1016/j.clinimag.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 6. Gazzola S, Aviv RI, Gladstone DJ, et al. Vascular and nonvascular mimics of the CT angiography “spot sign” in patients with secondary intracerebral hemorrhage. Stroke. 2008;39(4):1177–1183. doi: 10.1161/STROKEAHA.107.499442. [DOI] [PubMed] [Google Scholar]
- 7. Delcourt C, Sato S, Zhang S, et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology. 2017;88(15):1408–1414. doi: 10.1212/WNL.0000000000003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li N, Wang Y, Wang W, et al. Contrast extravasation on computed tomography angiography predicts clinical outcome in primary intracerebral hemorrhage: a prospective study of 139 cases. Stroke. 2011;42(12):3441–3446. doi: 10.1161/STROKEAHA.111.623405. [DOI] [PubMed] [Google Scholar]
- 9. Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68(12):889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 11. Nicholson P, Gao M, Radovanovic I, et al. Not a string, not a tangle, not an aneurysm : emerging pattern of large parenchymal bleeding in younger patients associated with abnormal vessels on imaging. Clin Neuroradiol. 2021;31(3):653–659. doi: 10.1007/s00062-020-00944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caton MT, Jr, Wiggins WF, Nunez D. The “connecting vessel” sign: an imaging biomarker to differentiate ruptured infected (mycotic) intracranial aneurysm mimicking the CTA spot sign. Emerg Radiol. 2020;27(3):259–268. doi: 10.1007/s10140-020-01749-6. [DOI] [PubMed] [Google Scholar]
- 13. de Jong JP, Kluijtmans L, van Amerongen MJ, Prokop M, Boogaarts HD, Meijer FJA. “On the spot”: the use of four-dimensional computed tomography angiography to differentiate a true spot sign from a distal intracranial aneurysm. World Neurosurg. 2017;105:1037.e9–1037.e12. doi: 10.1016/j.wneu.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 14. Moughamian AJ, Morshed RA, Colorado RA, Liner Z, Cooke D, Hemphill JC. Teaching NeuroImages: artery of Percheron aneurysm masquerading as ICH spot sign. Neurology. 2017;89(6):e64–e65. doi: 10.1212/WNL.0000000000004212. [DOI] [PubMed] [Google Scholar]
- 15. Yeon JY, Kim JS, Hong SC. Incidental major artery aneurysms in patients with non-hemorrhagic moyamoya disease. Acta Neurochir (Wien) 2011;153(6):1263–1270. doi: 10.1007/s00701-011-0948-y. [DOI] [PubMed] [Google Scholar]
- 16. Dowlatshahi D, Brouwers HB, Demchuk AM, et al. Predicting intracerebral hemorrhage growth with the spot sign: the effect of onset-to-scan time. Stroke. 2016;47(3):695–700. doi: 10.1161/STROKEAHA.115.012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delgado Almandoz JE, Kelly HR, Schaefer PW, et al. CT angiography spot sign predicts in-hospital mortality in patients with secondary intracerebral hemorrhage. J Neurointerv Surg. 2012;4(6):442–447. doi: 10.1136/neurintsurg-2011-010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng Y, Lu Z, Shen J, Xu F. Intracranial pseudoaneurysms: evaluation and management. Front Neurol. 2020;11:582. doi: 10.3389/fneur.2020.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raper DMS, Rutledge WC, Winkler E, Abla AA. Spontaneous perforation of anterior choroidal artery with resultant pseudoaneurysm formation: unusual cause of subarachnoid hemorrhage. World Neurosurg. 2020;134:141–144. doi: 10.1016/j.wneu.2019.10.174. [DOI] [PubMed] [Google Scholar]
- 20. Gitto L, Richardson TE, Serinelli S, Diana F, Peschillo S, Domenicucci M. Massive intracranial bleeding due to the rupture of a rare spontaneous pseudoaneurysm of the middle cerebral artery in a pediatric patient: case report with clinical, radiological, and pathologic findings. Forensic Sci Med Pathol. 2019;15(3):474–480. doi: 10.1007/s12024-019-00122-5. [DOI] [PubMed] [Google Scholar]
- 21. Phogat V, Gandhi A, Srivastava T, Mishva K. Endovascular management of intracranial pseudoaneurysm: an institutional experience. J Cerebrovasc Endovasc Neurosurg. 2020;22(4):211–215. doi: 10.7461/jcen.2020.E2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turan N, Butler S, Larson TC, 3rd, Mason A. Nontraumatic, posterior circulation pseudoaneurysm of the basilar artery summit with complete spontaneous resolution: case report and literature review. Surg Neurol Int. 2017;8:50. doi: 10.4103/sni.sni_452_16. [DOI] [PMC free article] [PubMed] [Google Scholar]


