Abstract
Purpose
We aimed to evaluate the performance of Chinese visceral adiposity index (CVAI), visceral adiposity index (VAI), lipid accumulation product (LAP), triglyceride glucose (TyG) as indices in screening abnormal glucose tolerance (AGT) in Chinese women with polycystic ovary syndrome (PCOS), using the oral glucose tolerance test (OGTT) as a reference test. In addition, we essentially compared the abilities of these indices with body mass index (BMI), waist circumference (WC), fasting plasma glucose (FPG).
Materials and methods
All 1113 PCOS patients evaluated in this study underwent OGTTs. The 2-h post-oral glucose load (2 h-PG) level was used to categorize subjects into two groups: those having AGT or normal glucose tolerance (NGT) levels.
Results
A statistically significant positive correlation between levels of 2 h-PG and FPG, BMI, WC, LAP, VAI, CVAI, TyG, (P < 0.05), was observed. The strongest correlation was found between the levels of 2 h-PG and CVAI (r = 0.47). The CVAI provided the highest area under the receiver-operating characteristic curve (AUC) for AGT, followed by LAP, BMI, TyG, VAI, WC, and FPG. The CVAI of 32.61 (with AUC: 0.76, sensitivity: 73%, specificity: 70%, positive preductive value (PPV): 0.41, negative predictive value (NPV): 0.90) was found to be the cut-off point for AGT in Chinese women with PCOS.
Conclusions
CVAI may not reliably detect AGT in Chinese women with PCOS. However, it is suitable as a first screening indicator to guide physicians to ordering OGTT.
Keywords: Abnormal glucose tolerance, Chinese visceral adiposity index, Visceral adiposity index, Lipid accumulation product, Triglyceride glucose index, Polycystic ovary syndrome
Introduction
Type 2 diabetes mellitus (T2DM), an established risk of cardiovascular disease, disability, and premature death, has become a major worldwide public health burden in the past decade [1, 2]. Impaired glucose tolerance (IGT), at an early stage in the natural history of T2DM, may remain underdiagnosed since it is usually asymptomatic, and its detection necessitates an oral glucose tolerance test (OGTT) [3]. Adoption of suitable lifestyle modifications or pharmacological interventions may delay or prevent deterioration of IGT to T2DM [3]. Therefore, a need for earlier detection of IGT has been significantly highlighted.
Polycystic ovary syndrome (PCOS), a very common endocrine disorder, impacts 5–10% of women in their reproductive age [4]. A more meaningful screening for abnormal glucose tolerance (AGT) is warranted in women with PCOS as they show an increased prevalence of disturbances of glucose metabolism compared with the general population and have shown characteristically postprandial abnormalities in glucose metabolism [5–7].
However, OGTT, considered the gold-standard in detecting AGT, is an inconvenient and time-consuming procedure. Therefore, it should have a simple and effective screening tool for AGT, especially in women with PCOS.
Studies have firmly established that both obesity and dyslipidemia are the traditional risk factors for T2DM because these two conditions can increase peripheral tissue insulin resistance (IR) [8]. Therefore, in general populations of different races, several obesity- and lipid-related indices, including visceral adiposity index (VAI) [9–11], lipid accumulation product (LAP) [9, 12, 13], and triglyceride Glucose index (TyG) [9, 11, 14], have been commonly suggested as promising surrogate indicators of both T2DM and prediabetes. Recently, the Chinese visceral adiposity index (CVAI), a tool developed to estimate visceral obesity of Chinese individuals, has been suggested as a better predictor of T2DM and prediabetes than any other obesity index for Chinese adults [1, 2, 15, 16].
To our knowledge, women with PCOS show more visceral fat accumulation than those without PCOS, even if they are of normal weight, and visceral adiposity is more associated with obesity-related metabolic abnormalities than subcutaneous or peripheral fat accumulation [17]. Therefore, the aforementioned indicators may have different implications for screening of AGT among the PCOS population than in the general population. Moreover, although there are many studies on the relationships of these indices with T2DM and prediabetes, however, the data establishing an association between these indices and AGT remains scarce [8, 12, 18], especially for the PCOS population [19, 20]. Thus, in this cross-sectional study, we aimed to evaluate the diagnostic performance of obesity and lipid-related indices as tools in screening AGT in Chinese women with PCOS, using the OGTT as our reference gold-standard method.
Material and methods
Subjects and study design
A total of 1113 women with PCOS (aged 13–41 years) with complete medical records were recruited at the center for reproductive medicine and gynecological outpatient department, Xuzhou Central Hospital (outpatient consultation) from July 2012 to December 2020. Any medications known to affect sex hormone, glucose or lipid metabolism were discontinued for at least three months before the study.
PCOS diagnosis
PCOS adults (aged 20–41 years) were diagnosed when meeting at least two out of the following three criteria [4, 7] (PCOS adolescents (aged 13–19 years) required the presence of all three criteria) [21]: (i) olig- and/or anovulation (i.e. eight or fewer menstrual cycles in a year or menstrual cycles more than 35 days in length) (ii) clinical hyperandrogenism (i.e. acne or modified Ferriman–Gallwey scores ≥8) or biochemical hyperandrogenism (i.e. total testosterone (TT) ≥ 2.6 nmol/L, free testosterone (FT) ≥ 20.82 pmol/L); and (iii) polycystic ovaries (i.e. presence of ≥12 follicles in each ovary measuring 2–9 mm in diameter) and exclusion of related disorders (e.g. congenital adrenal hyperplasia, androgen-secreting tumours and Cushing’s syndrome, hyperprolactinemia, thyroid disorders).
AGT diagnosis
Glucose tolerance was determined using the following indicators [3]: normal fasting plasma glucose (FPG) (FPG < 6.1 mmol/L); impaired fasting glucose (IFG) = FPG ≥6.1 mmol/L but < 7.0 mmol/L; normal glucose tolerance (NGT) = 2-h post-oral glucose load (2 h-PG) < 7.8 mmol/L; IGT = 2 h-PG ≥7.8 mmol/L but < 11.1 mmol/L; and T2DM = FPG ≥ 7.0 mmol/L or 2 h-PG ≥11.1 mmol/L; prediabetes = IFG or IGT; AGT = 2 h-PG ≥ 7.8 mmol/L.
The formulas of indices
Indices were evaluated based on the formulas presented below [7]:
Demographic information and clinical measurements
All the recruited patients underwent 75-g OGTT and anthropometric measurements. All parameters were measured, as previously described [7, 22].
Fasting blood samples were obtained from PCOS patients between the first and fifth day of menstrual period/withdrawal bleeding. Prolactin and total testosterone (TT) were assessed by chemiluminescence immunometric assay (Beckman Unicel DxI 800). Free testosterone (FT) and thyroid-stimulating hormone were also measured using chemiluminescence immunometric assay (Snibe MAGLUMI 4000; Abbott Immulite 2000 analyzer). 17α-hydroxyprogesterone was measured using the ELISA method. Plasma glucose was measured using glucose oxidase method (Hitachi 7600 autoanalyzer). Plasma insulin was measured using chemiluminescence immunometric assay (Roche e601 analyzer). Total cholesterol (CHOL), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) were measured using enzymatic colorimetric method (Hitachi 7600 autoanalyzer).
Statistical analysis
The data were analyzed by the statistical software SPSS version 24.0 for Windows. We assessed the normality of the distribution of all continuous variables using the Kolmogorov–Smirnov test. As the variables were not normally distributed, continuous variables were described as median with 25th–75th percentile, and the differences between the groups were determined by the Mann–Whitney U test. The association between the 2 h-PG and FPG, obesity-and lipid-related indices were tested by Spearman’s rank correlations analysis. Receiver operating characteristic (ROC) curves and the area under ROC curves (AUCs (95%CI)) were used to assess the sensitivily and secpificity of each index in detecting AGT. The optimal cut-off value of each index was determined through the maximization of the Youden index (sensitivity + specificity − 1). Positive preductive value (PPV) was estimted by true positive cases (TP) and false positive cases (FP), and the formula is PPV = TP / (TP + FP). The negative predictive value (NPV) was assessed by ture negative cases (TN) and fasle negative cases (FN), and the formula is NPV = TN / (TN + FN). Differences in AUCs were assessed by the method described by Hanley and McNeil. P ≤ 0.05 (two-tailed) was considered statistically significant.
Results
In the overall study population, the prevalence of alterations in glucose metabolism was as follows: IFG 39/1113 (3.50%), IGT 202/1113 (18.15%), and T2DM 47/1113 (4.30%).
Of the 47 women with T2DM, 5 were diagnosed based solely on FPG level, 34 on 2 h-PG, and 8 based on composite FPG and 2 h-PG. AGT was observed in 244 women (244/1113, 21.92%), the remaining 869 women (869/1113, 78.08%) demonstrated NGT.
As shown in Table 1, there were higher levels of age, BMI, WC, FPG, FIN, TG, LDL-c, CHOL, FT, LAP, VAI, CVAI, and TyG in subjects with AGT compared to those with NGT, while HDL-c was found lower in the same subjects (P < 0.05). No significant difference in TT(P > 0.05) was noticed.
Table 1.
Clinical and laboratory characteristics of PCOS patients with AGT and NGT (median [range])
| NGT(n = 869) | AGT(n = 244) | P | |
|---|---|---|---|
| Age (years) | 26.00 (22.00–29.00) | 29.00 (26.00–32.00) | < 0.001 |
| BMI (kg/m2) | 21.23 (19.22–24.14) | 25.41 (22.57–28.60) | < 0.001 |
| WC (cm) | 73.00 (68.00–81.00) | 84.00 (76.00–92.00) | < 0.001 |
| FPG (mmol/L) | 5.00 (4.70–5.30) | 5.30 (5.00–5.80) | < 0.001 |
| FIN (uU/ml) | 7.10(3.90–11.30) | 11.30 (7.45–17.40) | < 0.001 |
| TG (mmol/L) | 1.03(0.74–1.38) | 1.60(1.05–2.21) | < 0.001 |
| HDL-c (mmol/L) | 1.54(1.33–1.80) | 1.34(1.16–1.58) | < 0.001 |
| LDL-c (mmol/L) | 2.83(2.34–3.35) | 3.13(2.59–3.65) | < 0.001 |
| CHOL (mmol/L) | 4.75(4.30–5.44) | 5.21(4.51–5.73) | < 0.001 |
| LAP | 14.96 (7.70–30.40) | 41.36(21.80–65.14) | < 0.001 |
| VAI | 1.18 (0.78–1.85) | 2.17 (1.26–3.48) | < 0.001 |
| CVAI | 10.90 (−11.46–40.36) | 57.85 (33.90–84.25) | < 0.001 |
| TyG | 8.31(7.99–8.64) | 8.84(8.39–9.23) | < 0.001 |
| TT (nmol/L) | 2.26(1.65–2.90) | 2.18(1.62–2.88) | 0.189 |
| FT (pmol/L) | 10.48 (6.80–16.31) | 12.63(8.47–20.23) | 0.001 |
BMI body mass index, WC waist circumference, FPG fasting plasma glucose, FIN fasting insulin, TG triglycerides, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol, CHOL total cholesterol, LAP lipid accumulation product, VAI visceral adiposity index, CVAI Chinese visceral adiposity index, TyG triglyceride glucose Index, FT free testosterone, TT total testosterone
Table 2 presents the correlation coefficients between the 2 h-PG and each index. A significant positive correlation between the 2 h-PG and FPG, BMI, WC, LAP, VAI, CVAI, TyG (P < 0.05) was observed. The 2 h-PG and CVAI (r = 0.47) revealed the strongest correlation.
Table 2.
Spearman rank correlations between the 2 h-PG and FPG, obesity-and lipid-related indices
| r | p | |
|---|---|---|
| FPG | 0.38 | < 0.001 |
| CVAI | 0.47 | < 0.001 |
| VAI | 0.40 | < 0.001 |
| LAP | 0.44 | < 0.001 |
| TyG | 0.43 | < 0.001 |
| BMI | 0.41 | < 0.001 |
| WC | 0.38 | < 0.001 |
2 h-PG 2-h post-oral glucose load, FPG fasting plasma glucose, CVAI Chinese visceral adiposity index, VAI visceral adiposity index, LAP lipid accumulation product, TyG triglyceride glucose Index, BMI body mass index, WC waist circumference
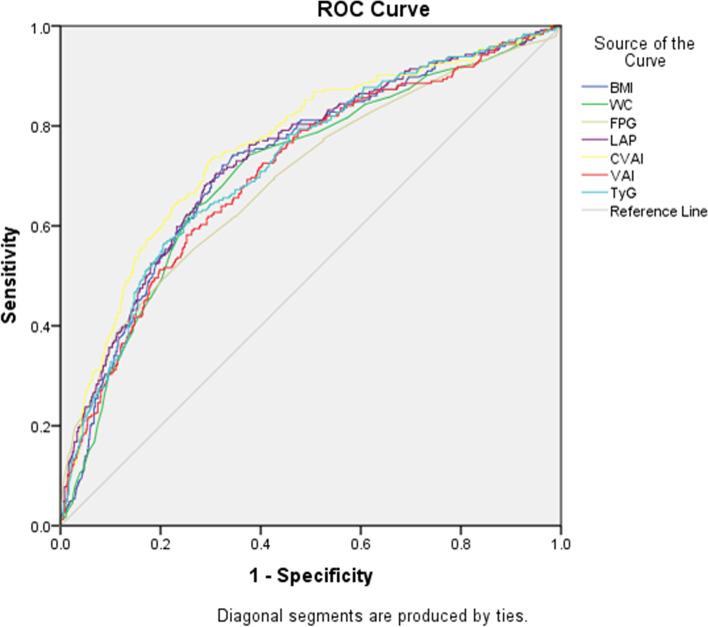
In this study, ROC curves analysis indicated that all indices could predict AGT and CVAI, showing the highest AUC, followed by LAP, BMI, TyG, VAI, WC, and FPG. The differences in AUC between CVAI with other indices were statistically significant (P < 0.05) (Table 3, Fig. 1).
Table 3.
ROC curves data for detecting AGT using each indicator
| AUC (95%CI) | Cut-off point | Sen | Spe | Youden index | PPV | NPV | P | |
|---|---|---|---|---|---|---|---|---|
| BMI | 0.73(0.69–0.76) | 22.70 | 0.72 | 0.68 | 0.40 | 0.39 | 0.90 | < 0.001 |
| WC | 0.71(0.67–0.75) | 80.50 | 0.64 | 0.73 | 0.37 | 0.40 | 0.88 | < 0.001 |
| FPG | 0.70(0.66–0.73) | 5.35 | 0.49 | 0.80 | 0.29 | 0.40 | 0.85 | 0.006 |
| LAP | 0.74(0.70–0.77) | 26.19 | 0.71 | 0.69 | 0.40 | 0.40 | 0.89 | 0.004 |
| CVAI | 0.76(0.72–0.79) | 32.61 | 0.73 | 0.70 | 0.43 | 0.41 | 0.90 | reference |
| VAI | 0.71(0.67–0.75) | 1.85 | 0.58 | 0.75 | 0.33 | 0.39 | 0.86 | 0.001 |
| TyG | 0.72(0.69–0.76) | 8.71 | 0.57 | 0.79 | 0.36 | 0.45 | 0.86 | 0.037 |
P: CVAI vs other indices
Sen Sensitivity, Spe Specificity, PPV positive preductive value, NPV negative predictive value, BMI body mass index, WC waist circumference, FPG fasting plasma glucose, LAP lipid accumulation product, CVAI Chinese visceral adiposity index, VAI visceral adiposity index, TyG triglyceride glucose Index
Fig. 1.
Receiver operating characteristic (ROC) curves for each indicator for detecting abnormal glucose tolerance (AGT) in Chinese women with Polycystic ovary syndrome (PCOS)
Discussion
Central obesity and visceral fat are major risk factors for IR. Visceral obesity causes IR by stimulating the formation of matabolic products derived from lipids, hormones, and cytokines [23, 24]. Therefore, various obesity and lipid-related indices have been proposed for detecting IR and prediabetes. In our study, we directly compared the performance of FPG, CVAI, VAI, LAP, TyG, WC, and BMI in detecting AGT and observed FPG demonstrated the lowest AUC value (0.70) and the lowest sensitivity (49%) among all the indices for detecting AGT. Thus, although FPG is an inexpensive assay and does not require mathematical calculations, it may not reliably detect AGT in our PCOS population, a finding consistent with previous studies [3, 5].
We next evaluate the abilities of six obesity- and lipid-related indices (CVAI, VAI, LAP, TyG, BMI, WC) as indicators of detecting AGT in PCOS patients. In line with 2 h-PG as criteria for AGT, our data demonstrated that CVAI outperformed other indices with a higher correlation coefficient and a larger AUC for AGT detection in Chinese women with PCOS.
CVAI, is known as Chinese VAI. VAI, was introduced to estimate visceral adipose function for the Caucasians and is an index established with the use of BMI, WC, TG, and HDL-c [10, 25]. In Caucasians, VAI was used as a valuable indicator of visceral adiposity and adipose tissue dysfunction to predict the risk for cardiovascular diseases and insulin resistance [25]. However, a previous study showed that VAI was poorly associated with adipose tissue area and has poor diagnostic performance to predict prediabetes and diabetes in Chinese [16]. In conformity with previous findings, we showed that the AUC of VAI for AGT was 0.71, which was lower than the AUC of CVAI (0.76), LAP (0.74), TyG (0.72), and BMI (0.73). Moreover, data based on correlation analyses also revealed similar tendencies. The main reason of the discrepancy may be ethnic difference and subject characteristic. VAI was developed initially for Caucasians. However, compared to equivalent Caucasians, Asian subjects have a greater proportion of body fat for a given BMI level [26] and are more prone to accumulate fat around the abdomen [27].
The CVAI index based on the combination of age and all the parameters of VAI (BMI, WC, TG and HDL-c) was developed to estimate visceral fat area for Chinese by Xia and colleagues [16]. In their study, among 6495 middle-aged and elderly Chinese, CVAI provided higher AUCs for the diagnosis of T2DM and prediabetes than BMI, WC and VAI [16]. Also, two prospective studies indicated that CVAI proved a better predictor of prediabetes and diabetes compared to BMI, WC, and VAI in the general Chinese population [15, 28]. However, no data are currently available establishing any association between CVAI and AGT. In our cohort of PCOS patients, CVAI provided the strongest correlation with 2 h-PG and the largest AUC for detecting AGT, thereby suggesting that CVAI might be a more useful indicator of AGT than LAP, TyG, BMI, VAI, and WC in Chinese women with PCOS. Although the calculation of CVAI requires more parameters and more complicated formulas, the parameters (age, BMI, WC, TG, and HDL-c) are easily available and low cost in clinical practice, and the complex algorithms can easily be performed by Tablets or Smartphone. In our study population, ROC curves analysis suggested a threshold value of 32.61 in CVAI for the detection of AGT (73% sensitivity, 70% specificity, 0.76 AUC, 0.41 PPV, 0.90 NPV).
LAP, a novel index based on a combination of WC and TG, was first introduced for the U.S. National Health and Nutrition Examination Survey [29]. Furthermore, it is proposed as a valuable marker indicating T2DM and prediabetes [9, 12]. However, few studies have explored the association of LAP with AGT. The results of previous studies indicated that LAP performed better than BMI in identifying abnormal glucose regulation in the young Korean women and provided higher AUC in estimating the risk for IGT than BMI and waist-to-hip ratio in Austrian PCOS women [12, 19], which was accordance with our results. However, these studies did not compare the utility of LAP with other novel obesity- and lipid-related indices. Our analysis showed that LAP performed superior to TyG, VAI, BMI, and WC, but inferior to CVAI in detecting AGT in Chinese PCOS population. According to the maximized Youden index, the LAP 26.19 (with AUC 0.74, sensitivity 71%, specificity 69%) was found to be the cut-off point for AGT in our study population, which was much higher than that in Korean PCOS women (12.98) [20]. This discrepancy could be partially due to ethnic difference.
TyG index, a composite indicator composed of TG and FPG, is a key predictor of T2DM and prediabetes in severl epidemiological studies. The TyG performed better than homeostasis model assessment of insulin resistance (HOMA-IR) and TG/HDL-c in predicting T2DM in Korean adults without initial T2DM [30]. A study conducted among 3307 elderly Colombian individuals suggested better discriminative power of the TyG index than BMI, WC, and VAI to predict prediabetes [11]. However, OGTT was not performed, and prediabetes was defined as a FPG of 100 to 125 mg/dL (5.6 to 7.0 mmol/L) in this study. In a prospective cohort study conducted on 4543 Chinese individuals without initial prediabetes or diabetes, Wen et al. reported that TyG provided a larger AUC for predicting prediabetes and isolated IGT than FPG, WC, and BMI [8]. However, this study did not compare the ability of TyG with other novel obesity- and lipid-related indices. In the current study, TyG was found inferior to CVAI and LAP for detecting AGT based on AUC and correlation coefficient.
Due to the fact that a plasma insulin assay is not yet available in all laboratories, has poor reproducibility and is costly [31], we do not evaluate the performance of the indices those using insulin for detecting AGT. Indeed, they do not perform better than obesity- and lipid-related indices studied in our report (data not shown).
Despite these relevant findings, it is important to point out the limitation of our study. All the subjects were recruited from Xuzhou Central Hospital, and only those with complete medical records. This population may not represent all the Chinese women with PCOS, with a possibility of bias in the results.
In conclusion, CVAI performed better than LAP, TyG, VAI, BMI, WC, and FPG for detecting AGT in Chinese women with PCOS. Due to the lower sensitivity and specificity, CVAI may not reliably detect AGT in our PCOS cohort. However, because of its good NPV, it is suitable as a first screening indicator to guide physicians to ordering OGTT. Furthermore, treatment plans (metformin or intense lifestyle interventions) could be used to prevent or slow the development of AGT in the women with elevated CVAI.
Acknowledgments
The authors thank all the subjects who participated in the study and the hospital staffs for their contribution in sample and data collection.
Abbreviations
- T2DM
type 2 diabetes mellitus
- IGT
impaired glucose tolerance
- OGTT
oral glucose tolerance test
- PCOS
polycystic ovary syndrome
- AGT
abnormal glucose tolerance
- IR
insulin resistance
- VAI
visceral adiposity index
- LAP
lipid accumulation product
- TyG
triglyceride Glucose index
- CVAI
Chinese visceral adiposity index
- TT
total testosterone
- FT
free testosterone
- FPG
fasting plasma glucose
- IFG
impaired fasting glucose
- NGT
normal glucose tolerance
- 2 h-PG
2-h post-oral glucose load
- CHOL
total cholesterol
- TG
triglycerides
- HDL-c
high-density lipoprotein cholesterol
- LDL-c
low-density lipoprotein cholesterol
- BMI
body mass index
- ROC
Receiver operating characteristic
- AUCs
ROC curves
- PPV
positive preductive value
- TP
true positive cases
- FP
false positive cases
- NPV
negative predictive value
- TN
ture negative cases
- FN
fasle negative cases
- HOMA-IR
homeostasis model assessment of insulin resistance
Authors’ contributions
QQ. Y performed the study, analyzed and interpreted the data, and drafted the paper. XN. Y collected and analyzed the data. YJ. C collected the data and revised the draft. JH. Z designed, performed the study, and revised the paper. All authors read and approved the final manuscript.
Funding
Design and data collection were supported by grants from National Natural Science Foundation of China (81571405), Jiangsu Province natural science foundation of China (BK20161169).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of Xuzhou Central Hospital. Written informed consent was obtained either from a legal guardian of each subject younger than 18 years old or from those subjects who were 18 years old or older.
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qianqian Yin and Xiaonan Yan contributed equally as the first authors.
Contributor Information
Yijuan Cao, Email: xzjj2002@126.com.
Jianhua Zheng, Email: zjh201207@163.com.
References
- 1.Han M, Qin P, Li Q, et al. Chinese visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab Res Rev. 2021;37(2):e3370. doi: 10.1002/dmrr.3370. [DOI] [PubMed] [Google Scholar]
- 2.Wei J, Liu X, Xue H, et al. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. 2019;11(7):1580. doi: 10.3390/nu11071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Yang D, Li L, et al. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum Reprod. 2006;21(8):2027–2032. doi: 10.1093/humrep/del142. [DOI] [PubMed] [Google Scholar]
- 4.Ni RM, Mo Y, Chen X, et al. Low prevalence of the metabolic syndrome but high occurrence of various metabolic disorders in Chinese women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161(3):411–418. doi: 10.1530/EJE-09-0298. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz-Flores AE, Luque-Ramírez M, Fernández-Durán E, et al. Diagnosis of disorders of glucose tolerance in women with polycystic ovary syndrome (PCOS) at a tertiary care center: fasting plasma glucose or oral glucose tolerance test? Metabolism. 2019;93:86–92. doi: 10.1016/j.metabol.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 7.Yin Q, Zheng J, Cao Y, et al. Evaluation of novel obesity and lipid-related indices as indicators for the diagnosis of metabolic syndrome and Premetabolic syndrome in Chinese women with polycystic ovary syndrome. Int J Endocrinol. 2021;2021:7172388. doi: 10.1155/2021/7172388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen J, Wang A, Liu G, et al. Elevated triglyceride-glucose (TyG) index predicts incidence of Prediabetes: a prospective cohort study in China. Lipids Health Dis. 2020;19(1):226. doi: 10.1186/s12944-020-01401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn N, Baumeister SE, Amann U, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. 2019;9(1):9693. doi: 10.1038/s41598-019-46187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Wang F, Wang J, et al. Using different anthropometric indices to assess prediction ability of type 2 diabetes in elderly population: a 5 year prospective study. BMC Geriatr. 2018;18(1):218. doi: 10.1186/s12877-018-0912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramírez-Vélez R, Pérez-Sousa MÁ, González-Ruíz K, et al. Obesity- and lipid-related parameters in the identification of older adults with a high risk of Prediabetes according to the American Diabetes Association: an analysis of the 2015 health, well-being, and aging study. Nutrients. 2019;11(11):2654. doi: 10.3390/nu11112654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh JY, Sung YA, Lee HJ. The lipid accumulation product as a useful index for identifying abnormal glucose regulation in young Korean women. Diabet Med. 2013;30(4):436–442. doi: 10.1111/dme.12052. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, He S, Chen X. Capacity of different anthropometric measures to predict diabetes in a Chinese population in Southwest China: a 15-year prospective study. Diabet Med. 2019;36(10):1261–1267. doi: 10.1111/dme.14055. [DOI] [PubMed] [Google Scholar]
- 14.Tohidi M, Baghbani-Oskouei A, Ahanchi NS, et al. Fasting plasma glucose is a stronger predictor of diabetes than triglyceride-glucose index, triglycerides/high-density lipoprotein cholesterol, and homeostasis model assessment of insulin resistance: Tehran lipid and glucose study. Acta Diabetol. 2018;55(10):1067–1074. doi: 10.1007/s00592-018-1195-y. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Gong L, Li Q, et al. A novel visceral adiposity index for prediction of type 2 diabetes and pre-diabetes in Chinese adults: a 5-year prospective study. Sci Rep. 2017;7(1):13784. doi: 10.1038/s41598-017-14251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia MF, Chen Y, Lin HD, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. doi: 10.1038/srep38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring) 2013;21(8):1690–1694. doi: 10.1002/oby.20096. [DOI] [PubMed] [Google Scholar]
- 18.Malavazos AE, Cereda E, Ermetici F, et al. The "lipid accumulation product" is associated with 2-hour postload glucose outcomes in overweight/obese subjects with nondiabetic fasting glucose. Int J Endocrinol. 2015;2015:836941. doi: 10.1155/2015/836941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehr E, Gruber HJ, Giuliani A, et al. The lipid accumulation product is associated with impaired glucose tolerance in PCOS women. J Clin Endocrinol Metab. 2011;96(6):E986–E990. doi: 10.1210/jc.2011-0031. [DOI] [PubMed] [Google Scholar]
- 20.Jeong K, Park SJ, Jeon JH, Lee SR, Chung HW. Predictive markers for abnormal glucose intolerance in women with polycystic ovary syndrome. Minerva Med. 2016;107(4):185–193. [PubMed] [Google Scholar]
- 21.Ozler S, Oztas E, Tokmak A, et al. The association of thiol/disulphide homeostasis and lipid accumulation index with cardiovascular risk factors in overweight adolescents with polycystic ovary syndrome. Clin Endocrinol. 2016;84(4):516–523. doi: 10.1111/cen.12965. [DOI] [PubMed] [Google Scholar]
- 22.Hatch R, Rosenfield RL, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140(7):815–830. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 23.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 24.Moon HU, Ha KH, Han SJ, et al. The Association of Adiponectin and Visceral fat with insulin resistance and β-cell dysfunction. J Korean Med Sci. 2018;34(1):e7. doi: 10.3346/jkms.2019.34.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev. 2002;3(3):141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 27.Nazare JA, Smith JD, Borel AL, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with Cardiometabolic risk/intra-abdominal adiposity. Am J Clin Nutr. 2012;96(4):714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- 28.Xia MF, Lin HD, Chen LY, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: a prospective cohort study. Diabetes Metab Res Rev. 2018;34(7):e3048. doi: 10.1002/dmrr.3048. [DOI] [PubMed] [Google Scholar]
- 29.Kahn HS. The "lipid accumulation product" performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. doi: 10.1186/1471-2261-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SH, Kwon HS, Park YM, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju metabolic disease cohort (CMC) study. PLoS One. 2014;9(2):e90430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang B, Yang Y, Lee EY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes. 2017;41(5):789–792. doi: 10.1038/ijo.2017.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.