Abstract
Background
Lipoprotein (a) [Lp(a)] is an independent risk factor for coronary artery disease (CAD). Recent studies have indicated that statins tend to increase Lp(a) levels by 10–20%. However, the association of statin-mediated increases in Lp(a) levels with CAD has not been determined.
Methods
This study included 488 patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI). Lp(a) levels were measured at baseline and 1 month after statin therapy. The study endpoints were major adverse cardiovascular events (MACE). Hazard ratios for the MACE were adjusted for potential confounder using Cox regression.
Results
After statin therapy, the mean level of Lp(a) increased by 19.3% from baseline. Lp(a) levels increased in 307 patients (62.9%) with a median elevation of 4.1 mg/dL. Patients with an increase in Lp(a) were at higher risk for MACE than those without an increase in Lp(a) (p = 0.044). Subgroup analyses revealed that a mild-to-moderate increase in Lp(a) was not associated with MACE, whereas there was a strong correlation between the highest quartile increase in Lp(a) (≥ 10.1 mg/dL) and MACE (HR = 2.29, 95%CI = 1.36–3.84, p = 0.002). This correlation was independent of baseline Lp(a) levels but not independent of on-statin Lp(a) levels.
Conclusions
Severe increases in Lp(a) following statin therapy raise the risk of MACE, but a mild-to-moderate increase in Lp(a) may not affect the cardiovascular prognosis of CAD patients. Even if the baseline Lp(a) levels are low, it is necessary to continue testing for Lp(a) concentration at least once after statin.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02932-y.
Keywords: Lipoprotein(a), Statin therapy, Coronary artery disease, Major adverse cardiovascular events
Introduction
Statin, an HMG-CoA reductase inhibitor, is overwhelmingly effective in lowering low density lipoprotein cholesterol (LDL-C) levels and reducing atherosclerotic cardiovascular disease (ASCVD). It has been recognized as the first-line treatment for primary and secondary prevention of cardiovascular disease (CVD) [1, 2]. An estimated 145.8 million people (2.6%) worldwide are taking statins [3], as for patients with coronary artery disease (CAD), statins are recommended to almost everyone [4]. In spite of this, the fact that statin therapy cannot eliminate residual risks and treat all the lipoproteins that cause atherosclerosis is increasingly accepted [5].
Lipoprotein (a) [Lp(a)] is formed by the covalent binding of apolipoprotein A to a low density lipoprotein (LDL)-like particle [6]. It has the effect of promoting arteriosclerosis, inflammation, calcification, and thrombosis, and is an independent risk factor for ASCVD [7, 8]. Different from other lipid components, the plasma concentration of Lp(a) is mainly regulated by LPA gene which encodes for apolipoprotein(a), and is less affected by external factors [9]. Results on Lp(a) changes after statin therapy are inconsistent. Some studies show a neutral effect [10], while more of them show the effect of increasing Lp(a) [11, 12]. Several large scale meta-analyses summarize the existing research data and indicate that statins increase Lp(a) levels by more than 10% [13, 14]. This has raised concerns as to whether the use of statins increase the cardiovascular risk related to Lp(a). To date, the clinical correlation between statin-mediated increases in Lp(a) levels and CVD has not been demonstrated [15]. The current study was undertaken to investigate the effect of increases in Lp(a) levels following statin therapy on cardiovascular prognosis in a secondary prevention population of CAD.
Patients and methods
Study subjects
This study consecutively enrolled 618 patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI) with drug-eluting stent implantation in the affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine from January 1 to December 31, 2017.
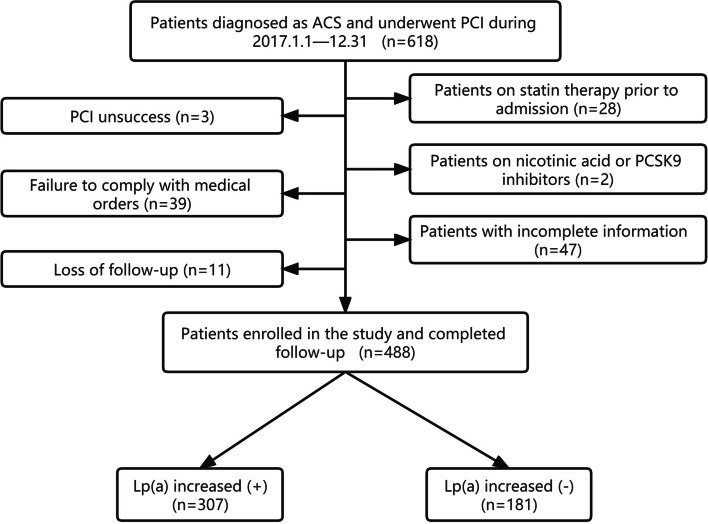
The following subjects were excluded: 1. patients on statin therapy prior to admission (n = 28); 2. patients with PCI unsuccess (n = 3); 3. patients with incomplete basic information or follow-up data (n = 47); 4. patients who discontinued or changed the medication without authorization during the follow-up (n = 39); 5. patients on nicotinic acid or PCSK9 inhibitors (n = 2); 6. patients who lost follow-up (n = 11). As shown in Fig. 1, 488 patients finally fulfilled the inclusion criteria and were classified as the study group. All patients in the study were treated according to the the standard protocols recommended by national guidelines [16, 17].
Fig. 1.
Patient flow diagram
ACS was defined as acute chest patient occurring with or without persistent ST-segment elevation as well as positive, or negative in case of unstable angina, cardiac enzymes [18]. PCI unsuccess was defined as the residual stenosis diameter of target vessel ≥ 10%, TIMI blood flow less than Grade-III, or associated in-hospital major clinical complications [19].
Data collection
Patient characteristics were obtained from the hospital records. This included information such as age, sex, body mass index, smoking history, family history, comorbidities (hypertension, diabetes mellitus), use of secondary prevention medications (Antiplatelet agents, Lipid-lowering drugs, etc.), laboratory test results [LDL-C, Lp(a), creatinine, etc.] obtained before PCI, records of PCI and the results of blood lipids measurement after 1 month of statin therapy.
Follow-up and study endpoints
All patients received follow-up and repeated measurement of blood lipids at Hangzhou First People’s Hospital in the first month after discharge. The Lp(a) levels were measured by the same assay methodologies. Thereafter, they were followed up at our hospital or in private care clinics every 2–3 months. Endpoint events that occurred within 3 years in patients were collected through outpatient and inpatient records. For patients without an endpoint event record at 36 months after PCI, the occurrence of events was confirmed by telephone contact.
The study endpoints were major adverse cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction or ischemic stroke, hospitalization related to unstable angina and unplanned coronary revascularization.
Statistical analysis
Categorical variables were represented as number (%) and analyzed by chi-square test or Fisher’s exact test. Continuous variables that met the normal distribution were represented as mean ± S.D. and analyzed by t-test or variance analysis. Continuous variables without a normal distribution were represented as median (25th percentile,75th percentile) and analyzed by nonparametric test.
Lp(a) was converted into two variables [Lp(a) ≥ 50 mg/dL and log10-transformed Lp(a)]. The hazard ratio (HR) and 95% confidence interval (CI) for MACE were calculated respectively by Cox proportional-hazard regression models, which were used to compare the correlation of pre-and-post statin Lp(a) levels with MACE.
The cumulative MACE—free survival rates between Lp(a) increased ( +) group and Lp(a) increased (-) group were estimated via the Kaplan–Meier method, and the differences were analyzed by the log-rank test. The HR and 95%CI for MACE for each subgroup based on the quartile of the increase in Lp(a) were calculated via Cox proportional hazard models. Statistical analysis was performed using IBM SPSS Statistics, Version 25.0. (Armonk, NY), with a p value of < 0.05 considered significant.
Results
Effect of statins on Lp(a) levels
In this study, 457 (93.65%) patients had a lipid-lowering regimen consisting of statins alone, and 31 (6.35%) received statin plus ezetimibe. As shown in Table 1, the mean level of Lp(a) increased by approximately 19.3% in patients after LLT, a statistically significant difference (p = 0.036). Considering the possible effect of ezetimibe on Lp(a) [20], the changes of Lp(a) levels in patients taking ezetimibe and those not taking ezetimibe were compared. It showed that there was no significant difference between them (19.56% vs 19.30%, p = 0.972), so the influence of ezetimibe was ignored in subsequent steps.
Table 1.
Lp(a) levels at baseline and 1-month after LLT
| Lipid-lowering strategy | Baseline (mg/dL) | On-statin (mg/dL) | percent change |
|---|---|---|---|
| statin (n = 457) | 13.0 (8.0, 28.0) | 15.1 (8.3, 35.4) | +19.28% |
| statin + ezetimibe (n = 31) | 14.0 (9.0, 22.0) | 14.4 (9.0, 28.0) | +19.56% |
| Total (n = 488) | 13.0 (8.0, 27.0) | 15.1 (8.3, 35.1) | +19.30% |
On-statin: after 1 month of statin therapy
Table S1 describes the detailed use of statins. There was no obvious difference in the type and dose of statins used between the two groups.
Patient characteristics
The average age of patients was 65.9 ± 9.7 years, including 328 males (67.2%). After statin therapy, Lp(a) levels increased in 307 patients (62.9%) with a median elevation of 4.1 mg/dL, and 181 patients (37.1%) had no increase in Lp(a). Characteristics of the population are shown in Table 2. The number of diabetic mellitus patients in Lp(a) increased ( +) group was lower than that in Lp(a) increased (-) group (26.97% vs 38.67%, p = 0.049). The baseline Lp(a) levels were similar between the two groups [14.0 (8.0–29.0) vs 12.0 (7.0–22.0), p = 0.176], but significant differences were observed after statin therapy [20.7 (11.0–40.7) vs 10.0 (6.4–18.2), p < 0.001]. For other clinical features, there was no significant difference between the two groups (p > 0.05).
Table 2.
Baseline characteristics
| Lp(a) increased ( +) (n = 307) | Lp(a) increased (-) (n = 181) | p value | |
|---|---|---|---|
| Age[years] | 66.54 ± 9.49 | 64.85 ± 10.50 | 0.067 |
| Male [cases (%)] | 202 (65.80) | 126 (69.61) | 0.386 |
| BMI (kg/m2) | 24.08 ± 3.11 | 24.62 ± 3.12 | 0.067 |
| Diabetes mellitus [cases (%)] | 92 (26.97) | 70 (38.67) | 0.049 |
| Hypertension [cases (%)] | 229 (74.59) | 137 (75.69) | 0.787 |
| Smoking history [cases (%)] | 141 (45.93) | 84 (46.41) | 0.918 |
| Family history [cases (%)] | 28 (9.12) | 12 (6.63) | 0.333 |
| Creatinine (μmoI/L) | 87.91 ± 19.00 | 87.30 ± 17.12 | 0.726 |
| hs-CRP (mg/L) | 3.0 (1.0 – 25.0) | 3.0 (1.0 – 5.0) | 0.491 |
| Angiography data | |||
| Single-vessel lesiona [cases (%)] | 100 (32.57) | 48 (26.52) | 0.160 |
| Double-vessel lesions [cases (%)] | 79 (25.73) | 55 (30.39) | 0.266 |
| Triple-vessel lesions [cases (%)] | 127 (41.37) | 78 (43.09) | 0.709 |
| Left main lesion [cases (%)] | 27 (8.79) | 21 (11.60) | 0.314 |
| CTO [cases (%)] | 61 (19.87) | 37 (20.44) | 0.879 |
| Coronary stents (number) | 2.42 ± 1.53 | 2.53 ± 1.68 | 0.472 |
| Baseline blood lipids | 0.176 | ||
| TC (mmol/L) | 4.43 ± 1.67 | 4.45 ± 1.03 | 0.849 |
| LDL-C (mmol/L) | 2.57 ± 0.96 | 2.59 ± 0.82 | 0.820 |
| HDL-C (mmol/L) | 1.09 ± 0.27 | 1.08 ± 0.28 | 0.759 |
| Lp(a) mg/dL | 14.0 (8.0 – 29.0) | 12.0 (7.0 – 22.0) | 0.176 |
| TG (mmol/L) | 1.68 ± 1.41 | 1.68 ± 1.00 | 0.999 |
| On-statin blood lipids | |||
| TC (mmol/L) | 3.33 ± 0.73 | 3.31 ± 0.81 | 0.796 |
| LDL-C (mmol/L) | 1.65 ± 0.57 | 1.67 ± 0.50 | 0.654 |
| HDL-C (mmol/L) | 1.13 ± 1.00 | 1.01 ± 0.25 | 0.161 |
| Lp(a) mg/dL | 20.7 (11.0 – 40.7) | 10.0 (6.4 – 18.2) | < 0.001 |
| TG (mmol/L) | 1.31 ± 0.76 | 1.46 ± 0.78 | 0.031 |
| Medicine use | |||
| Statin [cases (%)] | 307 (100.0) | 181 (100.0) | — |
| Ezetimibe [cases (%)] | 16 (5.21) | 15 (8.29) | 0.178 |
| β blocker [cases (%)] | 250 (81.43) | 151 (83.43) | 0.579 |
| ACEI/ARB [cases (%)] | 221 (71.99) | 139 (76.80) | 0.243 |
| Antiplatelet agent (at discharge) | |||
| DAPT [cases (%)] | 307 (100.0) | 181 (100.0) | — |
| Aspirin [cases (%)] | 307 (100.0) | 181 (100.0) | — |
| Clopidogrel [cases (%)] | 242 (78.83) | 136 (75.14) | 0.346 |
| Ticagrelor [cases (%)] | 65 (21.17) | 45 (28.86) | 0.346 |
| Antiplatelet agent (at the end of FUP) | |||
| DAPT [cases (%)] | 30 (9.77) | 12 (6.63) | 0.232 |
| SAPT [cases (%)] | 277 (90.23) | 169 (93.37) | 0.232 |
| Aspirin [cases (%)] | 268 (87.30) | 162 (89.50) | 0.467 |
| Clopidogrel [cases (%)] | 61 (19.87) | 28 (15.47) | 0.224 |
| Ticagrelor [cases (%)] | 8 (2.61) | 3 (1.66) | 0.495 |
Lp(a) increased ( +): patients with on-statin Lp(a) levels higher than baseline Lp(a) levels
Lp(a) increased (-): patients with on-statin Lp(a) levels less than or equal to baseline Lp(a) levels
BMI body mass index, hs-CRP high sensitivity C-reactive protein, TC total cholesterol, LDL-C low density lipoprotein cholesterol, HDL-C high density lipoprotein cholesterol, TG triglycerides, Lp(a) Lipoprotein (a), ACEI angiotensin converting enzyme inhibitor, ARB angiotensin-receptor blocker, DAPT dual antiplatelet therapy, SAPT single antiplatelet therapy, FUP follow up, CTO total coronary occlusion
aLesion was defined as coronary artery diameter stenosis of more than 50%
Association of pre-and-post statin Lp(a) levels with MACE
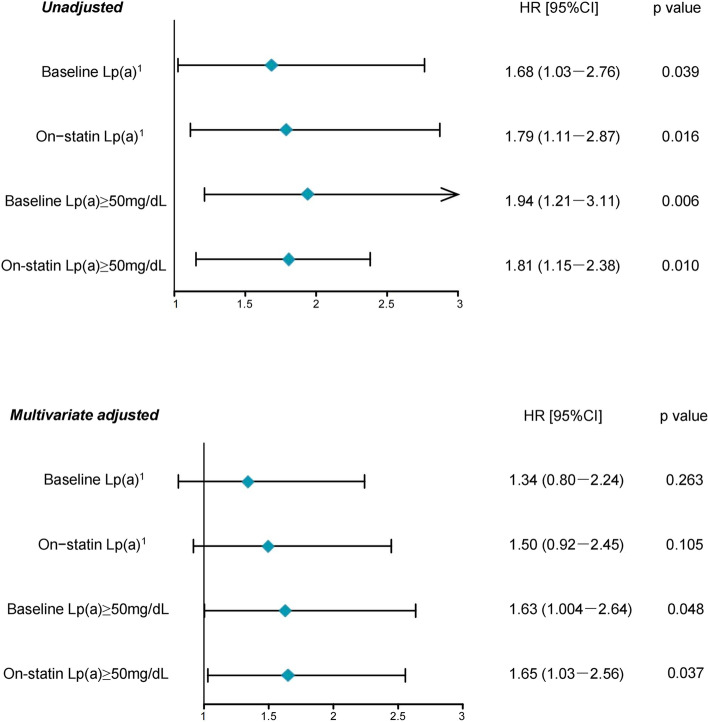
A total of 105 MACE (detailed in Table S2) were recorded during the 3-year follow-up period (average 31.4months). As shown in Figure 2, the association of pre-and-post statin Lp(a) levels with MACE remained unchanged substantially. Lp(a) levels ≥ 50mg/dL was an independent risk factor for MACE [Baseline: HR = 1.63, 95%CI =1.004-2.64, p = 0.048; On-statin: HR = 1.65, 95%CI =1.03-2.56, p = 0.037]. The log10-transformed Lp(a) was also associated with MACE [Baseline: HR = 1.68, 95%CI =1.03-2.76, p = 0.039; On-statin: HR = 1.79, 95%CI =1.11-2.87, p = 0.016], but the correlation became less significant after multi-factor adjustment [Baseline: HR = 1.34, 95%CI =0.80-2.24, p = 0.263; On-statin: HR = 1.50, 95%CI =0.92-2.45, p = 0.105].
Fig. 2.
Association of baseline and on-statin Lp(a) with MACE
Other risk factors for MACE are listed in Table 3. LDL-C and diabetes mellitus had a strong correlation with MACE, while age and hs-CRP were weak correlation factors for MACE.
Table 3.
Risk factors for MACE
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Age | 1.02 (1.003–1.05) | 0.023 | 1.02 (1.002–1.05) | 0.033 |
| hs-CRP | 1.03 (1.008–1.04) | 0.003 | 1.02 (1.003–1.04) | 0.020 |
| On-statin LDL-C | 1.47 (1.07–2.21) | 0.018 | 1.45 (1.05–2.01) | 0.024 |
| Diabetes mellitus | 1.73 (1.18–2.54) | 0.005 | 1.69 (1.15–2.49) | 0.008 |
hs-CRP high sensitivity C-reactive protein, LDL-C low density lipoprotein cholesterol
In Cox proportional risk model, Lp(a) was converted into continuous variable “Lp(a)1” and categorical variable “Lp(a) levels ≥ 50 mg/dL”. “Lp(a)1” = The log10-transformed Lp(a) level.
“Lp(a) ≥ 50 mg/dL” is generally considered a risk factor for CVD. In this study, 64 patients had baseline Lp(a) ≥ 50 mg/dL and 78 patients had on-statin Lp(a) ≥ 50 mg/dL.
Multivariate adjusted: The variables with p < 0.05 in univariate analysis were adjusted, including age, diabetes, LDL-C and hs-CRP. Univariate analysis is detailed in Table S3.
Effect of statin-mediated increases in Lp(a) levels on cardiovascular prognosis
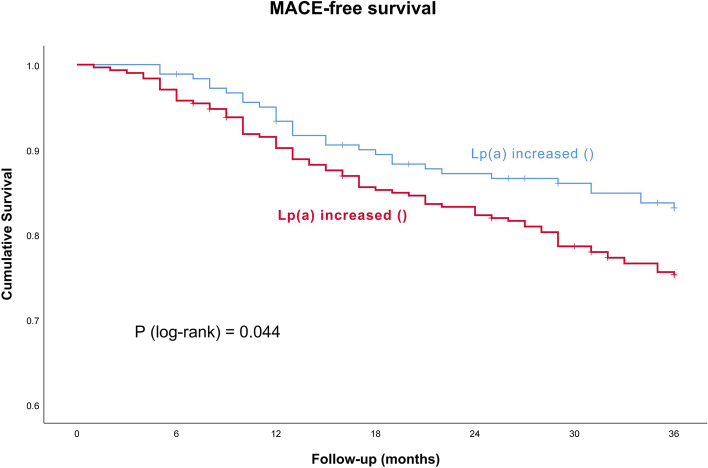
Of the 105 patients with MACE, 75(24.43%) were in the Lp(a) increased ( +) group and 30 (16.57%) were in the Lp(a) increased (-) group. Patients with an increase in Lp(a) had a higher incidence of MACE ( p = 0.044, Figs. 3).
Fig. 3.
Kaplan–Meier curve model for MACE. MACE = major adverse cardiovascular events
Then, patients with an increase in Lp(a) were divided into four groups based on the quartile of increases in Lp(a) levels. As shown in Table 4, patients in the highest quartile group had a significantly higher risk of MACE than patients without an increase in Lp(a) (HR = 2.29, 95CI = 1.36–3.84, p = 0.002). And there was no significant difference in MACE risk between other quartile groups and reference patients. (p > 0.05). After adjusting for baseline Lp(a) levels, the correlation did not change notablely. The highest quartile increase in Lp(a) is still closely related to MACE (HR = 2.00, 95CI = 1.18–3.42, p = 0.011). But this correlation disappeared in the model that adjusted for on-statin Lp(a) levels.
Table 4.
Correlation between magnitude of increase in Lp(a) levels and MACE
| Crude model | Adjusted model I | Adjusted model II | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Reference category (n = 181) ≤ 0 mg/dL | 1 | — | 1 | — | 1 | — |
| 1st quartile (n = 90) > 0-2 mg/dL | 1.57 (0.90–2.71) | 0.111 | 1.72 (0.98–3.02) | 0.058 | 1.63 (0.94–2.83) | 0.084 |
| 2nd quartile (n = 64) > 2–4.1 mg/dL | 1.59 (0.87–2.92) | 0.134 | 1.65 (0.90–3.02) | 0.109 | 1.56 (0.85–2.86) | 0.151 |
| 3rd quartile (n = 77) > 4.1–10.1 mg/dL | 0.78 (0.38–1.59) | 0.488 | 0.78 (0.38–1.59) | 0.485 | 0.73 (0.35–1.49) | 0.381 |
| 4th quartile (n = 76) > 10.1 mg/dL | 2.29 (1.36–3.84) | 0.002 | 2.00 (1.18–3.42) | 0.011 | 1.78 (0.97–3.28) | 0.062 |
Reference category: patients with an increase in Lp(a)
1st quartile: patients with an increase in Lp(a) level > 0-2 mg/dL
2nd quartile: > 2–4.1 mg/dL. 3rd quartile: > 4.1–10.1 mg/dL.4th quartile: > 10.1 mg/dL
Adjusted model I: adjusted for baseline Lp(a) levels
Adjusted model II:adjusted for on-statin Lp(a) levels
Discussion
This study has three major findings: 1) Statin therapy increases Lp(a) levels. The mean level of Lp(a) increased by approximately 19.3% in subjects after statin. 2) Lp(a) levels were associated with MACE, and hazard ratios for Lp(a) levels at both baseline and on-statin were comparable. 3) Patients with a severe increase in Lp(a) after statin therapy have a higher risk of MACE than those without an increase in Lp(a).
Lp(a) is an emerging cardiovascular risk factor. Numerous studies have confirmed its correlation with the onset of coronary heart disease and the recurrence of adverse cardiovascular events. Emerging Risk Factors Collaboration reported that there are continuous, independent, and modest associations of Lp(a) concentration with risk of CAD and stroke. For per 3.5-fold higher than normal Lp(a) concentration, the risk of CAD increased by 13% [21]. Genome-wide association and Mendelian randomized trials showed that the variations at some loci of LPA gene were strongly associated with both an increased level of Lp(a) and an increased risk of CAD, proving the relationship between Lp(a) and CAD at the gene level [22–24]. Lp(a) accelerates the progression of low-attenuation coronary plaques (necrotic cores) [25]. As for patients with established cardiovascular disease, especially in young individuals, higher levels of Lp(a) are associated with an increased risk of MACE such as CAD death, myocardial infarction, and urgent revascularization [26–28].
Since Lp(a) level is mainly regulated by LPA gene and tends to be constant throughout life, measurement of Lp(a) after statin therapy is rarely performed in clinical practice, leading to a lack of recognition of statin-mediated increases in Lp(a) levels. However, adverse effects of statins on Lp(a) levels have been repeatedly observed in clinical trials. An ILLUMINATE trial showed that Lp(a) levels are positively and dose-dependently correlated with atorvastatin dosage [9]. De Boer et al. reported that statins are associated with approximately 1.1 mg/dL increases in Lp(a) levels compared to placebo, whereas high-intensity statins are associated with 2.6 mg/L increases in Lp(a) levels [29]. Several systematic reviews and meta-analyses most recently indicated that statins increase Lp(a) levels by 10–20% [13, 14]. In this study, statins increased the mean Lp(a) level by 19.6%, similar in extent to the current findings. The underlying mechanisms of statin-mediated increase in Lp(a) levels are not fully defined. It may be due to statin enhence LPA mRNA and apolipoprotein (a) synthesis and secretion. In addition, the increase in plasma PCSK9 protein after statin treatment may also be responsible for the increase in Lp(a) [14].
Do statin-mediated increases in Lp(a) levels cause additional cardiovascular risk? In a meta-analysis, baseline and on-statin Lp(a) levels ≥ 50 mg/dL are associated with a 1.35—and 1.42-fold increased risk of CVD, hazard ratios for high Lp(a) levels at both baseline and on statin are comparable [30]. The JUPITER trial aslo indicated that statin therapy does not significantly increase Lp(a)-associated CVD risk (HR: baseline vs On-statin = 1.18 vs. 1.27) [11]. And we come to the same result (shown in Fig. 2). However, previous studies only compared the overall association of pre-and-post statin Lp(a) levels with CVD/MACE in groups. This research is the first to explore the correlation between the increase of individual Lp(a) level and CVD/MACE after statin therapy, and proves that a significant increase in Lp(a) would raise the risk of MACE. In addition, this correlation is independent of baseline Lp(a), but dependent of on-statin Lp(a), suggesting the importance of repeated measurement of Lp(a) after statin therapy.
Based on the results, we consider it necessary to continue testing for Lp(a) levels at least once after statin therapy, even if the baseline Lp(a) levels are low. For patients with on-statin Lp(a) levels ≥ 50 mg/dL, PCSK9 inhibitors may be used as appropriate to reduce the residual cardiovascular risk [26, 31]. Several Lp(a) targeted therapies have entered into Phase II/III clinical trials and are believed to provide additional benefits to CVD patients in the near future [32–34]. However, statin use should not be hindered by fear of an increase in Lp(a). Because most patients have no or mild increase in Lp(a) after statin therapy, which does not increase the risk of cardiovascular events. Moreover, Statins play an irreplaceable role in reducing LDL-C, the chief culprit of atherosclerosis [35, 36]. Reducing LDL-C by 38.67 mg/dL results in cardiovascular benefits comparable to reducing Lp(a) by 67.5 mg/dL [37].
Limitations
First, long-term outpatient follow-up were not all conducted in our hospital, some patients were followed up in private care clinics. We were unable to obtain accurate lipid measurement results and other clinical details for each patient after long-term statin therapy. Therefore, the prognostic implications of subsequent changes in Lp(a) levels cannot be determined. Second, the potential role of ezetimibe in the study is not analyzed. Third, this is a single-center study with a small sample size, and the results are susceptible to incidental factors. We emphasize that these results need to be verified in large-scale clinical studies.
Conclusions
Statins increase Lp(a) levels in some patients with CAD. Severe increases in Lp(a) following statin therapy raise the risk of MACE, whereas a mild-to-moderate increase in Lp(a) may not affect the cardiovascular prognosis of CAD patients. Statin use should not be hindered for fear of an increase in Lp(a), but it is necessary to continue testing for Lp(a) concentration at least once after statin therapy.
Supplementary Information
Additional file 1: Table S1. Statins used in study subjects. Table S2. Endpoint events for study subjects. Table S3. Univariate COX analysis of risk factors for MACE.
Acknowledgements
The authors express their gratitude to cardiac interventional therapy center of the affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicinefor their cooperation and assistance.
Abbreviations
- Lp(a)
Lipoprotein (a)
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- PCI
Percutaneous coronary intervention
- MACE
Major adverse cardiovascular events
- LDL-C
Low density lipoprotein cholesterol
- ASCVD
Atherosclerotic cardiovascular disease
- CVD
Cardiovascular disease
- LDL
Low density lipoprotein
- ACS
Acute coronary syndrome
- LLT
Lipid-lowering therapy
- HR
Hazard ratio
- CI
Confidence interval
- BMI
Body mass index
- hs-CRP
High sensitivity C-reactive protein
- TC
Total cholesterol
- HDL-C
High density lipoprotein cholesterol
- TG
Triglycerides
- ACEI
Angiotensin converting enzyme inhibitor
- ARB
Angiotensin-receptor blocker
- DAPT
Dual antiplatelet therapy
- SAPT
Single antiplatelet therapy
- FUP
Follow up
- CTO
Total coronary occlusion
- UA
Unstable angina
Authors’ contributions
LJZ contributed to the conception and design of the work. YLF contributed to the analysis and interpretation of the study data. BBG and XBJ contributed to patients follow-up. JMZ and YH contributed to data collection, LJZ drafted the manuscript. JYH critically revised the manuscript. All authors provided final approval and agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Funding
None.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol for the study was approved by the ethics committee of the affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from each participant at each examination phase.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blais JE, Wei Y, Yap KKW, Alwafi H, Ma TT, Brauer R, et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis. 2021;328:44–51. doi: 10.1016/j.atherosclerosis.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 5.Cho KI, Yu J, Hayashi T, Han SH, Koh KK. Strategies to Overcome Residual Risk During Statins Era. Circ J. 2019;83(10):1973–1979. doi: 10.1253/circj.CJ-19-0624. [DOI] [PubMed] [Google Scholar]
- 6.Kamstrup PR. Lipoprotein(a) and Cardiovascular Disease. Clin Chem. 2021;67(1):154–166. doi: 10.1093/clinchem/hvaa247. [DOI] [PubMed] [Google Scholar]
- 7.Duarte Lau F, Giugliano RP. Lipoprotein(a) and its Significance in Cardiovascular Disease: A Review. JAMA Cardiol. 2022;7(7):760–769. doi: 10.1001/jamacardio.2022.0987. [DOI] [PubMed] [Google Scholar]
- 8.Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Hanssen R, Gouni-Berthold I. Lipoprotein(a) Management: Pharmacological and Apheretic Treatment. Curr Med Chem. 2017;24(10):957–968. doi: 10.2174/0929867324666170112110928. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Li J, Ju J, Fan Y, Xu H. Effect of different types and dosages of statins on plasma lipoprotein(a) levels: A network meta-analysis. Pharmacol Res. 2021;163:105275. doi: 10.1016/j.phrs.2020.105275. [DOI] [PubMed] [Google Scholar]
- 11.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129(6):635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsenault BJ, Petrides F, Tabet F, Bao W, Hovingh GK, Boekholdt SM, et al. Effect of atorvastatin, cholesterol ester transfer protein inhibition, and diabetes mellitus on circulating proprotein subtilisin kexin type 9 and lipoprotein(a) levels in patients at high cardiovascular risk. J Clin Lipidol. 2018;12(1):130–136. doi: 10.1016/j.jacl.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Yeang C, Hung MY, Byun YS, Clopton P, Yang X, Witztum JL, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a) J Clin Lipidol. 2016;10(3):594–603. doi: 10.1016/j.jacl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–2284. doi: 10.1093/eurheartj/ehz310. [DOI] [PubMed] [Google Scholar]
- 15.Pirillo A, Catapano AL. Statins increase Lp(a) plasma level: is this clinically relevant? Eur Heart J. 2020;41(24):2285–2287. doi: 10.1093/eurheartj/ehz505. [DOI] [PubMed] [Google Scholar]
- 16.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, ESC Scientific Document Group et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 17.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 18.Zdanyte M, Wrazidlo RW, Kaltenbach S, Groga-Bada P, Gawaz M, Geisler T, et al. Predicting 1-, 3- and 5-year outcomes in patients with coronary artery disease: A comparison of available risk assessment scores. Atherosclerosis. 2021;318:1–7. doi: 10.1016/j.atherosclerosis.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 20.Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M, Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group Effect of Ezetimibe Monotherapy on Plasma Lipoprotein(a) Concentrations in Patients with Primary Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Drugs. 2018;78(4):453–462. doi: 10.1007/s40265-018-0870-1. [DOI] [PubMed] [Google Scholar]
- 21.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 23.Guertin J, Kaiser Y, Manikpurage H, Perrot N, Bourgeois R, Couture C, et al. Sex-Specific Associations of Genetically Predicted Circulating Lp(a) (Lipoprotein(a)) and Hepatic LPA Gene Expression Levels With Cardiovascular Outcomes: Mendelian Randomization and Observational Analyses. Circ Genom Precis Med. 2021;14(4):e003271. doi: 10.1161/CIRCGEN.120.003271. [DOI] [PubMed] [Google Scholar]
- 24.Zeng L, Moser S, Mirza-Schreiber N, Lamina C, Coassin S, Nelson CP, et al. Cis-epistasis at the LPA locus and risk of cardiovascular diseases. Cardiovasc Res. 2022;118(4):1088–1102. doi: 10.1093/cvr/cvab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser Y, Daghem M, Tzolos E, Meah MN, Doris MK, Moss AJ, et al. Association of Lipoprotein(a) With Atherosclerotic Plaque Progression. J Am Coll Cardiol. 2022;79(3):223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139(12):1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi D, Wada H, Ogita M, Yasuda K, Nishio R, Takeuchi M, et al. Impact of Lipoprotein(a) as a Residual Risk Factor in Long-Term Cardiovascular Outcomes in Patients With Acute Coronary Syndrome Treated With Statins. Am J Cardiol. 2022;1(168):11–16. doi: 10.1016/j.amjcard.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Gragnano F, Fimiani F, Di Maio M, Cesaro A, Limongelli G, et al. Impact of lipoprotein(a) levels on recurrent cardiovascular events in patients with premature coronary artery disease. Intern Emerg Med. 2019;14(4):621–625. doi: 10.1007/s11739-019-02082-8. [DOI] [PubMed] [Google Scholar]
- 29.de Boer LM, Oorthuys AOJ, Wiegman A, Langendam MW, Kroon J, Spijker R, et al. Statin therapy and lipoprotein(a) levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(5):779–792. doi: 10.1093/eurjpc/zwab171. [DOI] [PubMed] [Google Scholar]
- 30.Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392(10155):1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 31.Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome. J Am Coll Cardiol. 2020;75(2):133–144. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 32.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. 2020;382(3):244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 33.Tsimikas S, Moriarty PM, Stroes ES. Emerging RNA Therapeutics to Lower Blood Levels of Lp(a): JACC Focus Seminar 2/4. J Am Coll Cardiol. 2021;77(12):1576–1589. doi: 10.1016/j.jacc.2021.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Cesaro A, Schiavo A, Moscarella E, Coletta S, Conte M, et al. Lipoprotein(a): a genetic marker for cardiovascular disease and target for emerging therapies. J Cardiovasc Med (Hagerstown) 2021;22(3):151–161. doi: 10.2459/JCM.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 35.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mert GÖ, Başaran Ö, Mert KU, Doğan V, Özlek B, et al. The reasons of poor lipid target attainment for secondary prevention in real life practice: Results from EPHESUS. Int J Clin Pract. 2019;73(9):1–9. doi: 10.1111/ijcp.13358. [DOI] [PubMed] [Google Scholar]
- 37.Lamina C, Kronenberg F, Lp(a)-GWAS-Consortium Estimation of the Required Lipoprotein(a)-Lowering Therapeutic Effect Size for Reduction in Coronary Heart Disease Outcomes: A Mendelian Randomization Analysis. JAMA Cardiol. 2019;4(6):575–579. doi: 10.1001/jamacardio.2019.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Statins used in study subjects. Table S2. Endpoint events for study subjects. Table S3. Univariate COX analysis of risk factors for MACE.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.